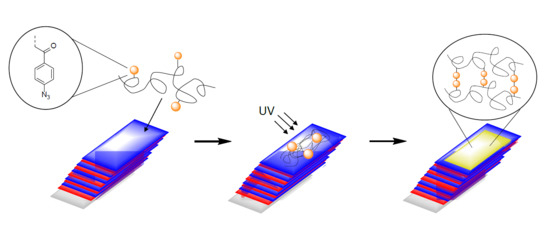
Chitosan–Azide Nanoparticle Coating as a Degradation Barrier in Multilayered Polyelectrolyte Drug Delivery Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Purification and Adjustment of the Degree of Acetylation (DA)
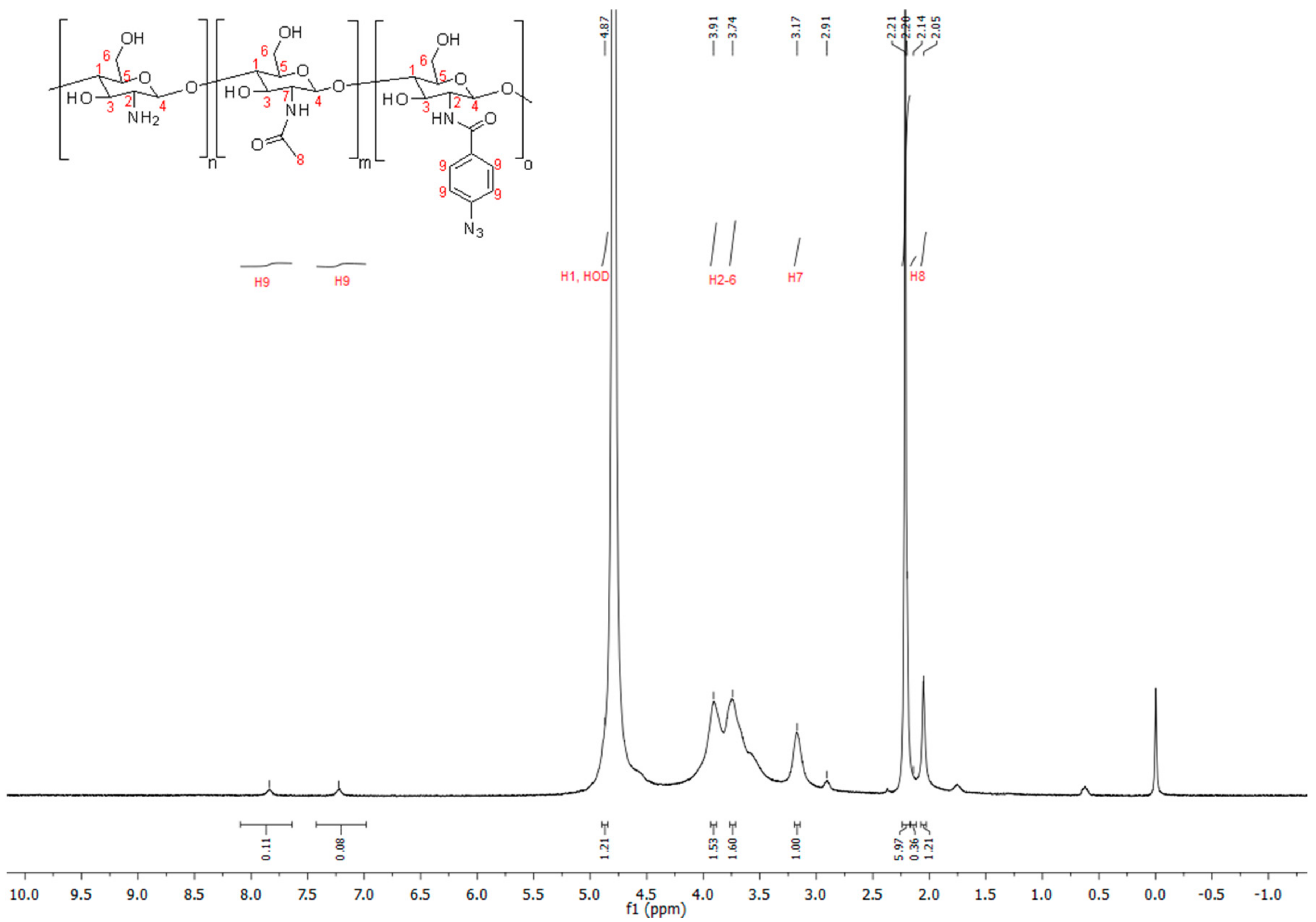
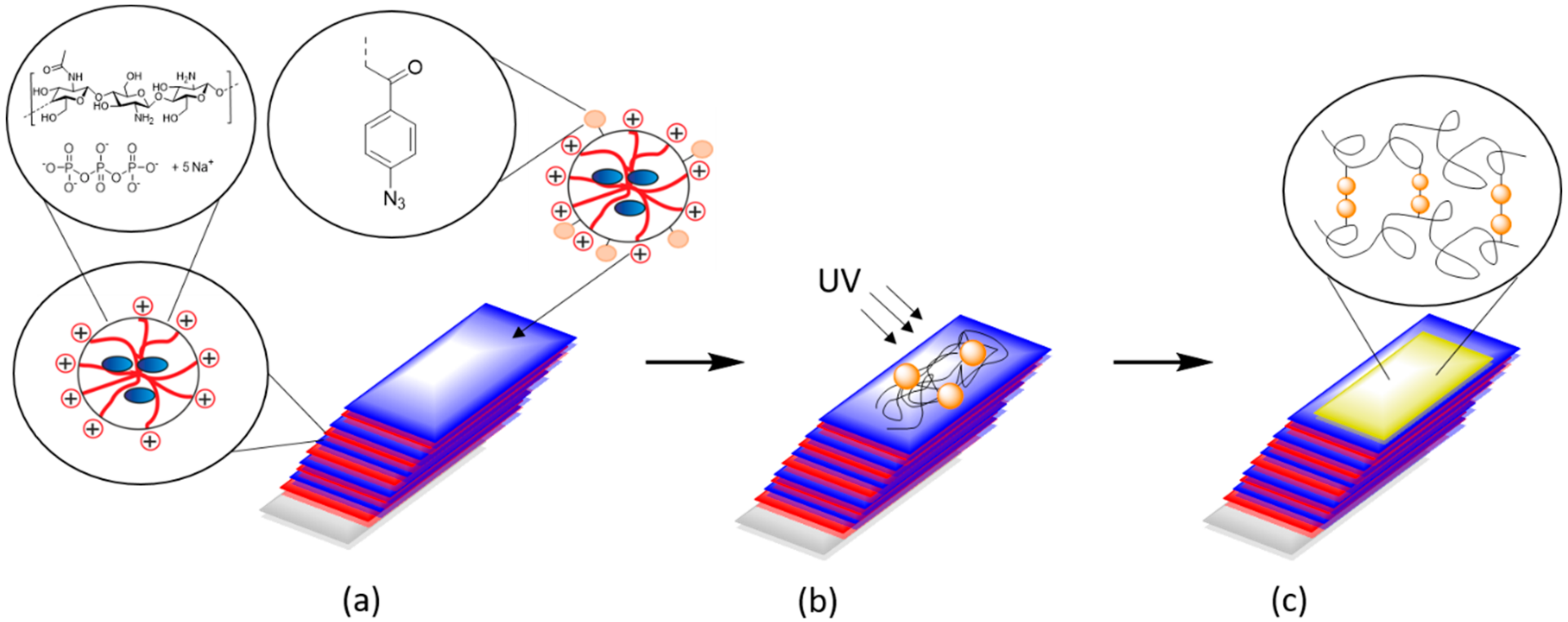
2.3. Synthesis of Chitosan-Aryl Azide
2.4. Multilayer Formation with Alginate (Alg), Chondroitin Sulfate (Cho) or Hyaluronic Acid (HA)
2.5. Preparation of CS–Az “Blocking Layer”
2.6. Degradation Studies for CS–Az Blocked Multilayers
2.7. Instruments
3. Results and Discussion
3.1. Polymer Synthesis
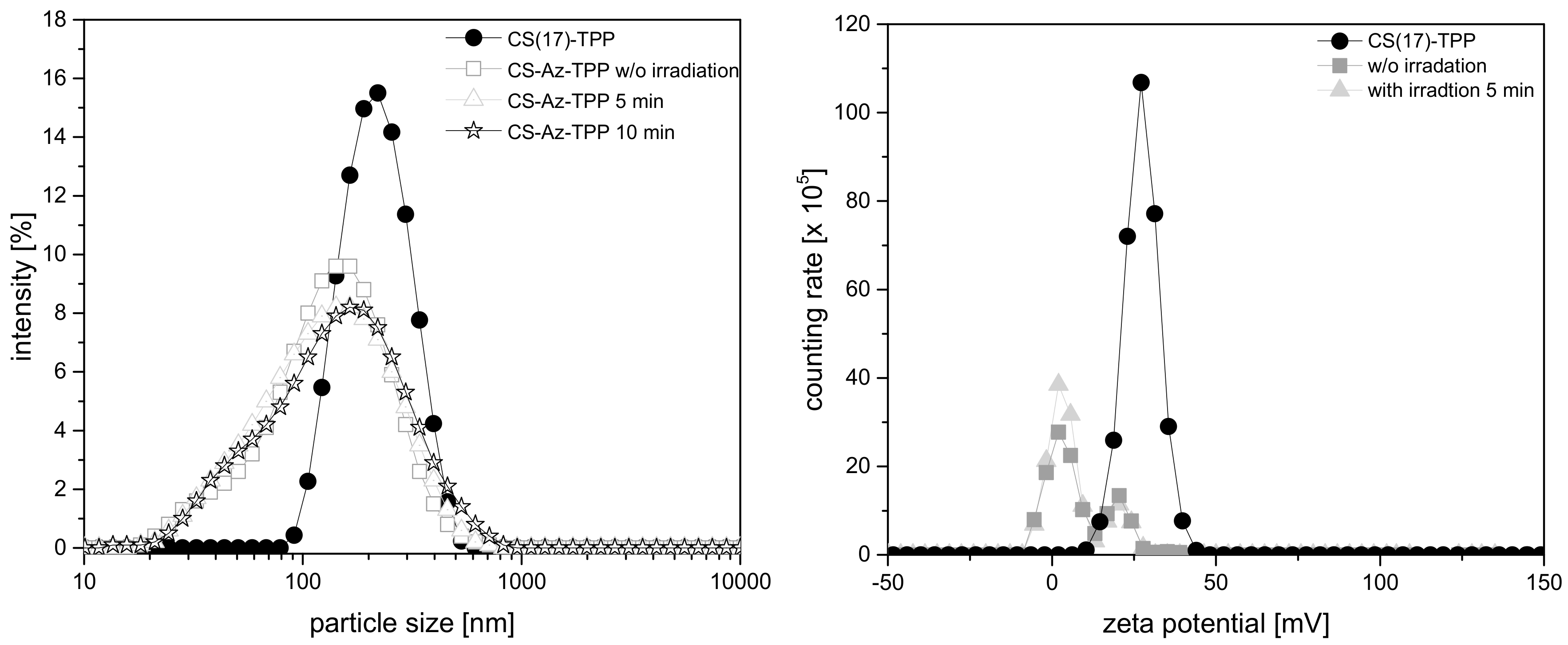
3.2. Preparation of Nanoparticles
3.3. Degradation Studies Nanoparticles
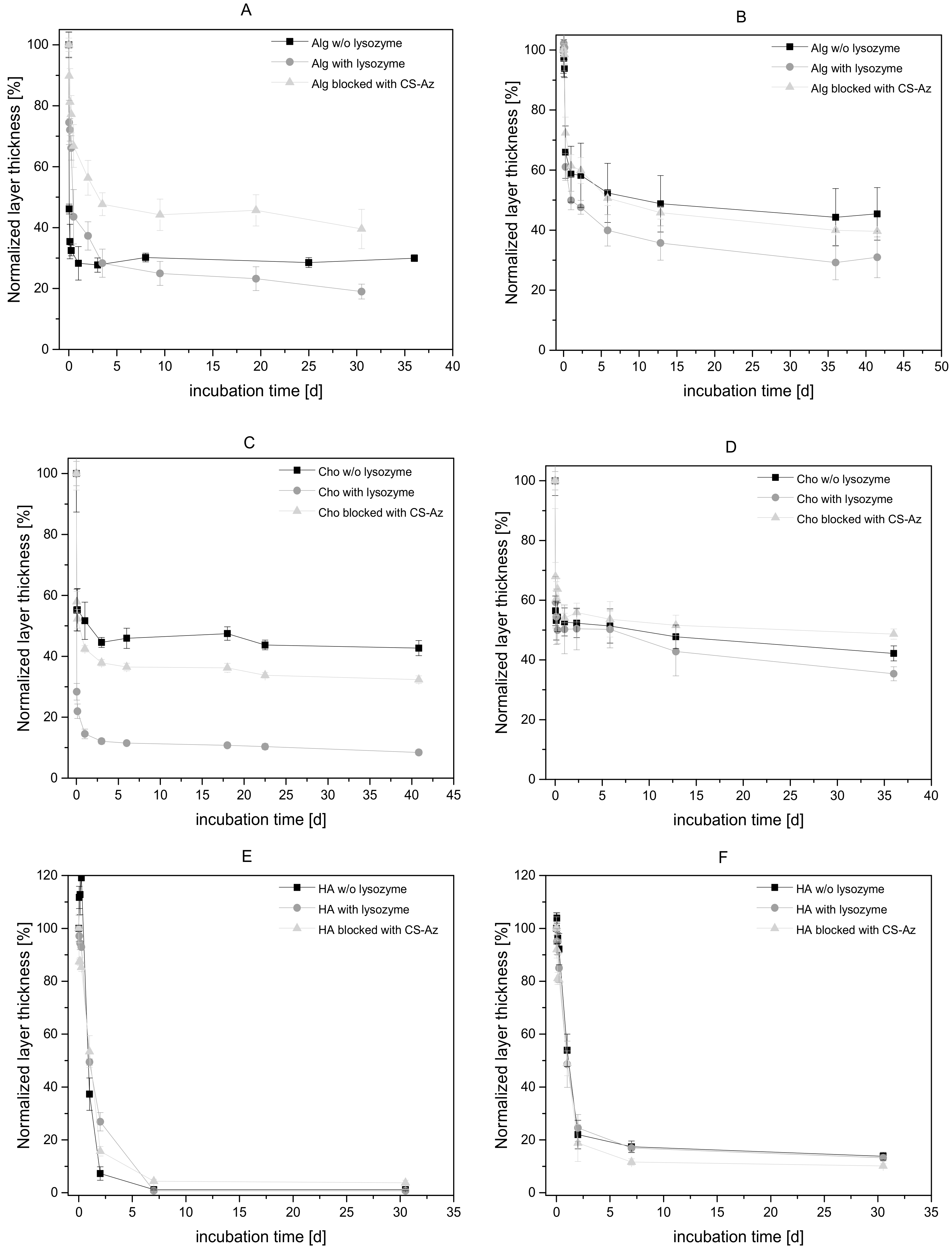
3.4. Degradation Study for CS–Az Blocked Multilayers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Langer, R.; Tirrell, D.A. Designing materials for biology and medicine. Nature 2004, 428, 487–492. [Google Scholar] [CrossRef] [PubMed]
- LaVan, D.A.; McGuire, T.; Langer, R. Small-scale systems for in vivo drug delivery. Nat. Biotechnol. 2003, 21, 1184–1191. [Google Scholar] [CrossRef] [PubMed]
- De Cassan, D.; Sydow, S.; Schmidt, N.; Behrens, P.; Roger, Y.; Hoffmann, A.; Glasmacher, B.; Menzel, H. Attachment of nanoparticulate drug-release systems on poly(ε-caprolactone) nanofibers via a graftpolymer as interlayer. Colloids Surf. B 2018, 163, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Sydow, S.; de Cassan, D.; Hänsch, R.; Gengenbach, T.R.; Easton, C.D.; Thissen, H.; Menzel, H. Layer-by-layer deposition of chitosan nanoparticles as drug-release coatings for PCL nanofibers. Biomater. Sci. 2018, 7, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Hsu, B.B.; Hagerman, S.R.; Hammond, P.T. Rapid and efficient sprayed multilayer films for controlled drug delivery. J. Appl. Polym. Sci. 2016, 133, 8833. [Google Scholar] [CrossRef]
- Anal, A.K.; Stevens, W.F. Chitosan-alginate multilayer beads for controlled release of ampicillin. Int. J. Pharm. 2005, 290, 45–54. [Google Scholar] [CrossRef]
- Hartmann, H.; Hossfeld, S.; Schlosshauer, B.; Mittnacht, U.; Pêgo, A.P.; Dauner, M.; Doser, M.; Stoll, D.; Krastev, R. Hyaluronic acid/chitosan multilayer coatings on neuronal implants for localized delivery of siRNA nanoplexes. J. Control Release 2013, 168, 289–297. [Google Scholar] [CrossRef]
- Zhao, Q.; Han, B.; Wang, Z.; Gao, C.; Peng, C.; Shen, J. Hollow chitosan-alginate multilayer microcapsules as drug delivery vehicle: Doxorubicin loading and in vitro and in vivo studies. Nanomedicine 2007, 3, 63–74. [Google Scholar] [CrossRef]
- Malkoch, M.; Vestberg, R.; Gupta, N.; Mespouille, L.; Dubois, P.; Mason, A.F.; Hedrick, J.L.; Liao, Q.; Frank, C.W.; Kingsbury, K.; et al. Synthesis of well-defined hydrogel networks using Click chemistry. Chem. Commun. 2006, 101, 2774. [Google Scholar] [CrossRef]
- Burdick, J.A.; Prestwich, G.D. Hyaluronic Acid Hydrogels for Biomedical Applications. Adv. Mater. 2011, 23, H41–H56. [Google Scholar] [CrossRef]
- Reddy, N.; Reddy, R.; Jiang, Q. Crosslinking biopolymers for biomedical applications. Trends Biotechnol. 2015, 33, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Williams, C.G.; Sun, D.D.N.; Wang, J.; Leong, K.; Elisseeff, J.H. Photocrosslinkable polysaccharides based on chondroitin sulfate. J. Biomed. Mater. Res. A 2003, 68A, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Poth, N.; Seiffart, V.; Gross, G.; Menzel, H.; Dempwolf, W. Biodegradable chitosan nanoparticle coatings on titanium for the delivery of BMP-2. Biomolecules 2015, 5, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Gan, Q.; Wang, T. Chitosan nanoparticle as protein delivery carrier--systematic examination of fabrication conditions for efficient loading and release. Colloids Surf. B 2007, 59, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Poth, N.F. Applikation von Nanopartikeln als Implantatbeschichtung zur Präsentation von biologisch aktivem BMP-2 an Implantatoberflächen. Ph.D. Thesis, Technische Universität Braunschweig, Braunschweig, Germany, 2015. [Google Scholar]
- Boudou, T.; Crouzier, T.; Ren, K.; Blin, G.; Picart, C. Multiple functionalities of polyelectrolyte multilayer films: New biomedical applications. Adv. Mater. 2010, 22, 441–467. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Dexter, A.F.; Middelberg, A.P.J. Biomolecular engineering at interfaces. Chem. Eng. Sci. 2006, 61, 989–1003. [Google Scholar] [CrossRef]
- Gan, Q.; Wang, T.; Cochrane, C.; McCarron, P. Modulation of surface charge, particle size and morphological properties of chitosan–TPP nanoparticles intended for gene delivery. Colloids Surf. B 2005, 44, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Freier, T.; Koh, H.S.; Kazazian, K.; Shoichet, M.S. Controlling cell adhesion and degradation of chitosan films by N-acetylation. Biomaterials 2005, 26, 5872–5878. [Google Scholar] [CrossRef] [PubMed]
- Hadler, C.; Wissel, K.; Brandes, G.; Dempwolf, W.; Reuter, G.; Lenarz, T.; Menzel, H. Photochemical coating of Kapton® with hydrophilic polymers for the improvement of neural implants. Mater. Sci. Eng. C 2017, 75, 286–296. [Google Scholar] [CrossRef]
- Kunishima, M.; Kawachi, C.; Hioki, K.; Terao, K.; Tani, S. Formation of carboxamides by direct condensation of carboxylic acids and amines in alcohols using a new alcohol- and water-soluble condensing agent: DMT-MM. Tetrahedron 2001, 57, 1551–1558. [Google Scholar] [CrossRef]
- Kunishima, M.; Kawachi, C.; Monta, J.; Terao, K.; Iwasaki, F.; Tani, S. 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methyl-morpholinium chloride: An efficient condensing agent leading to the formation of amides and esters. Tetrahedron 1999, 55, 13159–13170. [Google Scholar] [CrossRef]
- Sarwar, A.; Katas, H.; Samsudin, S.N.; Zin, N.M. Regioselective Sequential Modification of Chitosan via Azide-Alkyne Click Reaction: Synthesis, Characterization, and Antimicrobial Activity of Chitosan Derivatives and Nanoparticles. PLoS ONE 2015, 10, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Cheaburu-Yilmaz, C.N.; Karavana, S.Y.; Yilmaz, O. Functionalization of chitosan by click chemistry. In Proceedings of the AIP Conference Proceedings, Brasov, Romania, 27–29 June 2017; 1918, p. 020009. [Google Scholar] [CrossRef]
- Agnihotri, S.A.; Mallikarjuna, N.N.; Aminabhavi, T.M. Recent advances on chitosan-based micro- and nanoparticles in drug delivery. J. Control Release 2004, 100, 5–28. [Google Scholar] [CrossRef] [PubMed]
- Jonassen, H.; Kjoniksen, A.-L.; Hiorth, M. Stability of chitosan nanoparticles cross-linked with tripolyphosphate. Biomacromolecules 2012, 13, 3747–3756. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Saito, Y.; Yura, H.; Ishikawa, K.; Kurita, A.; Akaike, T.; Ishihara, M. Photocrosslinkable chitosan as a biological adhesive. J. Biomed. Mater. Res. 2000, 49, 289–295. [Google Scholar] [CrossRef]
- Mohamed, R.R.; Sabaa, M.W. Synthesis and characterization of antimicrobial crosslinked carboxymethyl chitosan nanoparticles loaded with silver. Int. J. Biol. Macromol. 2014, 69, 95–99. [Google Scholar] [CrossRef]
- Jameela, S.R.; Lakshmi, S.; James, N.R.; Jayakrishnan, A. Preparation and evaluation of photocrosslinkable chitosan as a drug delivery matrix. J. Appl. Polym. Sci. 2002, 86, 1873–1877. [Google Scholar] [CrossRef]
- Nordtveit, R.J.; Vårum, K.M.; Smidsrød, O. Degradation of partially N-acetylated chitosans with hen egg white and human lysozyme. Carbohydr. Polym. 1996, 29, 163–167. [Google Scholar] [CrossRef]
- Brouwer, J.; van Leeuwen-Herberts, T.; de Ruit, M.O.-V. Determination of lysozyme in serum, urine, cerebrospinal fluid and feces by enzyme immunoassay. Clin. Chim. Acta 1984, 142, 21–30. [Google Scholar] [CrossRef]
- Kean, T.; Thanou, M. Biodegradation, biodistribution and toxicity of chitosan. Adv. Drug Delivery Rev. 2010, 62, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Li, H.; Xiao, Q.; Liu, Y.; Zhu, J.; Du, Y. Water-solubility of chitosan and its antimicrobial activity. Carbohydr. Polym. 2006, 63, 367–374. [Google Scholar] [CrossRef]
- Tolle, C.; Riedel, J.; Mikolai, C.; Winkel, A.; Stiesch, M.; Wirth, D.; Menzel, H. Biocompatible Coatings from Smart Biopolymer Nanoparticles for Enzymatically Induced Drug Release. Biomolecules 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Koukaras, E.N.; Papadimitriou, S.A.; Bikiaris, D.N.; Froudakis, G.E. Insight on the formation of chitosan nanoparticles through ionotropic gelation with tripolyphosphate. Mol. Pharm. 2012, 9, 2856–2862. [Google Scholar] [CrossRef] [PubMed]
- Antunes, J.C.; Pereira, C.L.; Molinos, M.; Ferreira-da-Silva, F.; Dessì, M.; Gloria, A.; Ambrosio, L.; Gonçalves, R.M.; Barbosa, M.A. Layer-by-layer self-assembly of chitosan and poly(γ-glutamic acid) into polyelectrolyte complexes. Biomacromolecules 2011, 12, 4183–4195. [Google Scholar] [CrossRef] [PubMed]
- Crouzier, T.; Boudou, T.; Picart, C. Polysaccharide-based polyelectrolyte multilayers. Curr. Opin. Colloid Interface Sci. 2010, 15, 417–426. [Google Scholar] [CrossRef]
- Hernandez-Montelongo, J.; Lucchesi, E.G.; Gonzalez, I.; Macedo, W.A.A.; Nascimento, V.F.; Moraes, A.M.; Beppu, M.M.; Cotta, M.A. Hyaluronan/chitosan nanofilms assembled layer-by-layer and their antibacterial effect: A study using Staphylococcus aureus and Pseudomonas aeruginosa. Colloids Surf. B 2016, 141, 499–506. [Google Scholar] [CrossRef]
- Almodóvar, J.; Place, L.W.; Gogolski, J.; Erickson, K.; Kipper, M.J. Layer-by-layer assembly of polysaccharide-based polyelectrolyte multilayers: A spectroscopic study of hydrophilicity, composition, and ion pairing. Biomacromolecules 2011, 12, 2755–2765. [Google Scholar] [CrossRef]
- Bauer, S.V. Hydratation und Antifouling-Oberflächen: Modellsysteme auf Basis zwitterionischer SAMs und Polysaccharide. Ph.D. Thesis, Naturwissenschaftlich-Mathematischen Gesamtfakultät der Ruprecht-Karls-Universität Heidelberg, Heidelberg, Germany, 2014. [Google Scholar]
- Lawrie, G.; Keen, I.; Drew, B.; Chandler-Temple, A.; Rintoul, L.; Fredericks, P.; Grøndahl, L. Interactions between alginate and chitosan biopolymers characterized using FTIR and XPS. Biomacromolecules 2007, 8, 2533–2541. [Google Scholar] [CrossRef]
- Herman, E.S.; Lyon, L.A. Polyelectrolyte exchange and diffusion in microgel multilayer thin films. Colloid Polym. Sci. 2015, 293, 1535–1544. [Google Scholar] [CrossRef]
- Richert, L.; Lavalle, P.; Payan, E.; Shu, X.Z.; Prestwich, G.D.; Stoltz, J.-F.; Schaaf, P.; Voegel, J.-C.; Picart, C. Layer by Layer Buildup of Polysaccharide Films: Physical Chemistry and Cellular Adhesion Aspects. Langmuir 2004, 20, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Yi, H.; Wang, W.; Ma, X. The enzymatic degradation and swelling properties of chitosan matrices with different degrees of N-acetylation. Carbohydr. Res. 2005, 340, 2403–2410. [Google Scholar] [CrossRef] [PubMed]
- Nordtveit, R.J.; Vårum, K.M.; Smidsrød, O. Degradation of fully water-soluble, partially N-acetylated chitosans with lysozyme. Carbohydr. Polym. 1994, 23, 253–260. [Google Scholar] [CrossRef]
- Larsson, B.; Nilsson, M.; Tjälve, H. The binding of inorganic and organic cations and H+ to cartilage in vitro. Biochem. Pharmacol. 1981, 30, 2963–2970. [Google Scholar] [CrossRef]
- Tømmeraas, K.; Wahlund, P.-O. Poly-acid properties of biosynthetic hyaluronan studied by titration. Carbohydr. Polym. 2009, 77, 194–200. [Google Scholar] [CrossRef]
- Picart, C.; Mutterer, J.; Richert, L.; Luo, Y.; Prestwich, G.D.; Schaaf, P.; Voegel, J.-C.; Lavalle, P. Molecular basis for the explanation of the exponential growth of polyelectrolyte multilayers. Proc. Natl. Acad. Sci. USA 2002, 99, 12531–12535. [Google Scholar] [CrossRef] [PubMed]
- Lavalle, P.; Picart, C.; Mutterer, J.; Gergely, C.; Reiss, H.; Voegel, J.-C.; Senger, B.; Schaaf, P. Modeling the Buildup of Polyelectrolyte Multilayer Films Having Exponential Growth. J. Phys. Chem. B 2004, 108, 635–648. [Google Scholar] [CrossRef]


| Layer Thickness [nm] | CS–TPP + Alg | CS–TPP + Cho | CS–TPP + HA | |||
|---|---|---|---|---|---|---|
| CS(17) | CS(42) | CS(17) | CS(42) | CS(17) | CS(42) | |
| CS–TPP multilayer (10 layers) | 163 ± 11 | 143 ± 5 | 171 ± 8 | 240 ± 30 | 317 ± 8 | 312 ± 16 |
| CS–Az–TPP top–layer | 20 ± 3 | 23 ± 6 | 34 ± 19 | 42 ± 15 | 54 ± 15 | 23 ± 18 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sydow, S.; Aniol, A.; Hadler, C.; Menzel, H. Chitosan–Azide Nanoparticle Coating as a Degradation Barrier in Multilayered Polyelectrolyte Drug Delivery Systems. Biomolecules 2019, 9, 573. https://doi.org/10.3390/biom9100573
Sydow S, Aniol A, Hadler C, Menzel H. Chitosan–Azide Nanoparticle Coating as a Degradation Barrier in Multilayered Polyelectrolyte Drug Delivery Systems. Biomolecules. 2019; 9(10):573. https://doi.org/10.3390/biom9100573
Chicago/Turabian StyleSydow, Steffen, Armin Aniol, Christoph Hadler, and Henning Menzel. 2019. "Chitosan–Azide Nanoparticle Coating as a Degradation Barrier in Multilayered Polyelectrolyte Drug Delivery Systems" Biomolecules 9, no. 10: 573. https://doi.org/10.3390/biom9100573
APA StyleSydow, S., Aniol, A., Hadler, C., & Menzel, H. (2019). Chitosan–Azide Nanoparticle Coating as a Degradation Barrier in Multilayered Polyelectrolyte Drug Delivery Systems. Biomolecules, 9(10), 573. https://doi.org/10.3390/biom9100573