Koumine Promotes ROS Production to Suppress Hepatocellular Carcinoma Cell Proliferation Via NF-κB and ERK/p38 MAPK Signaling
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Cell Culture
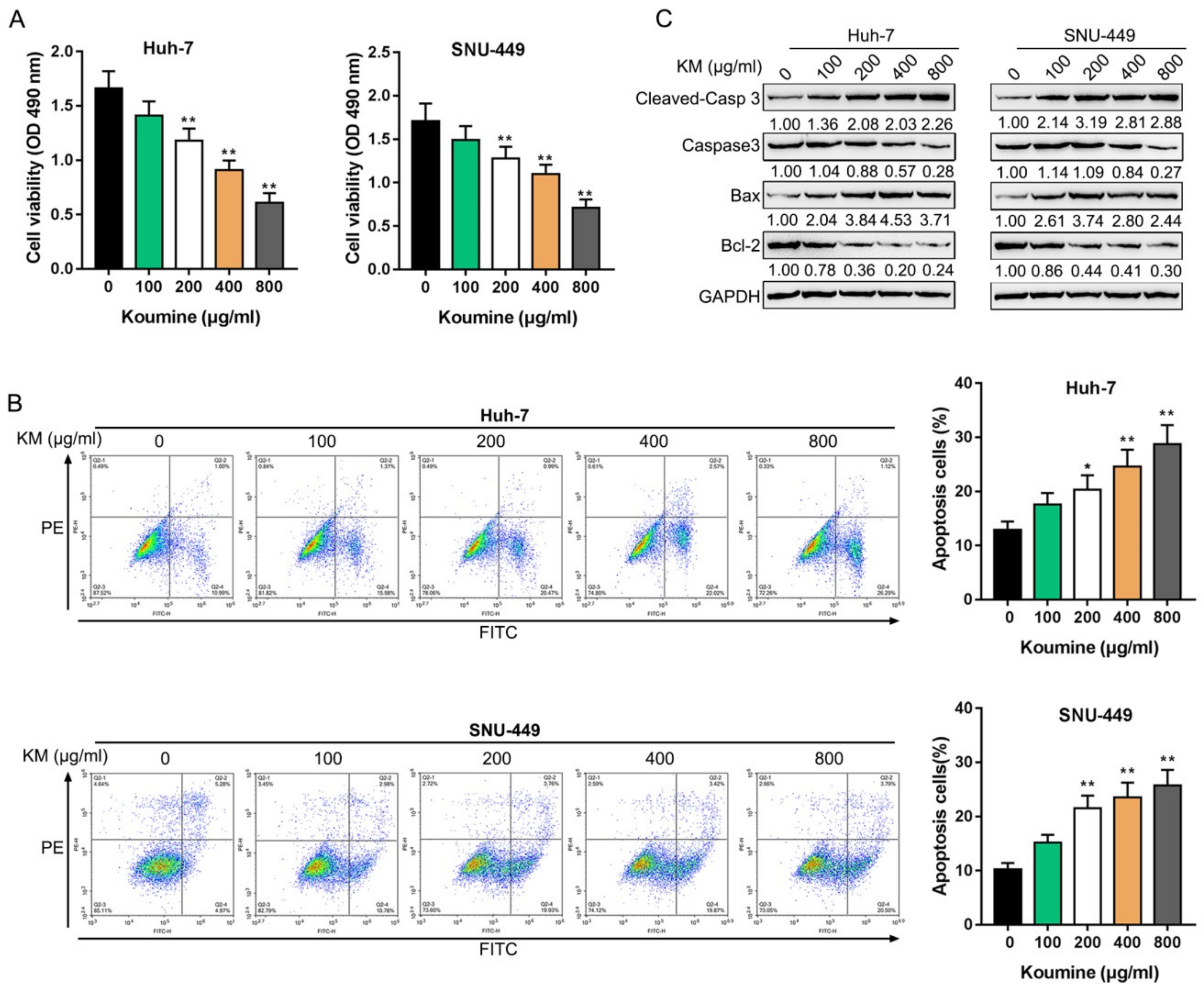
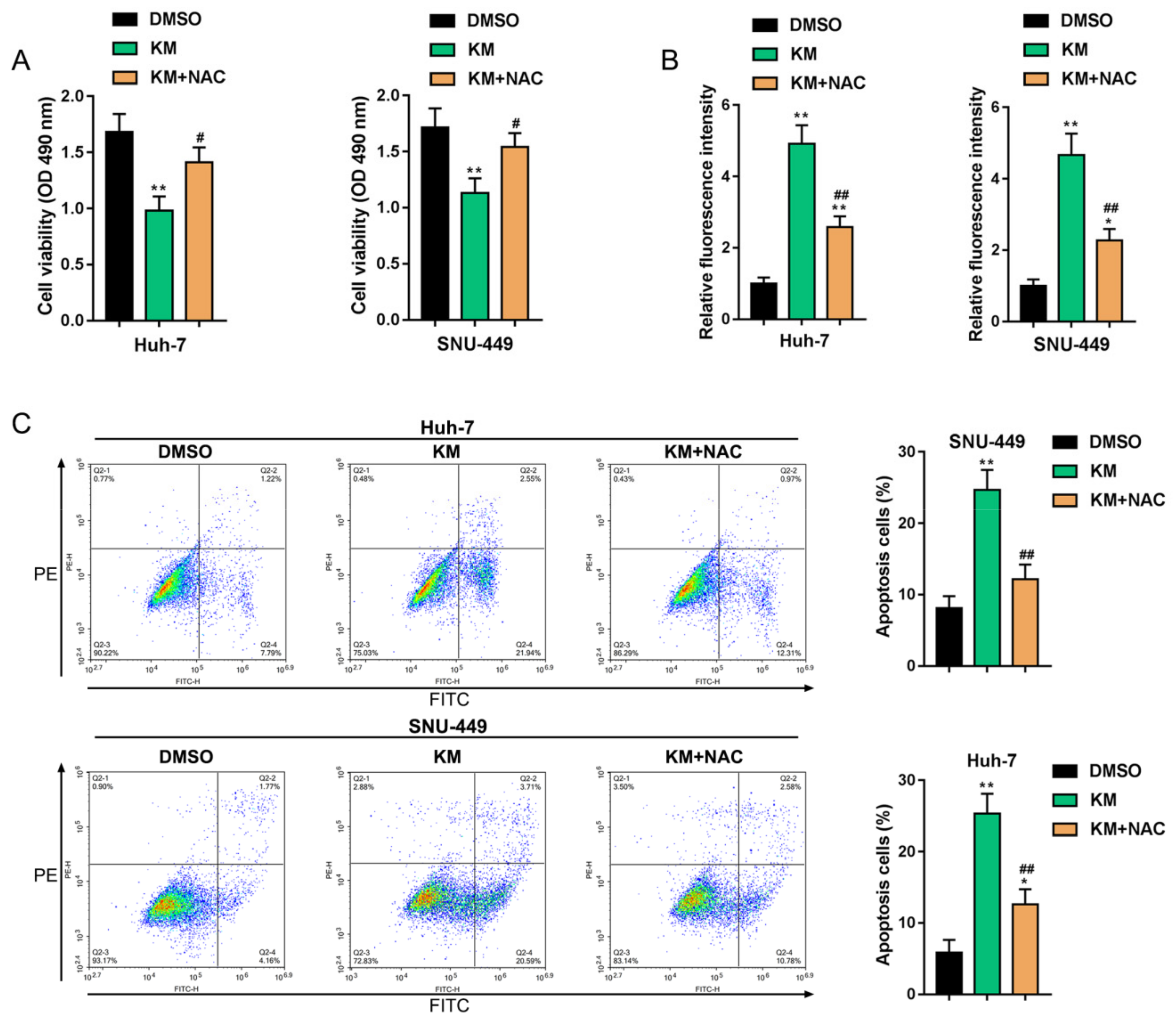
2.2. Cell Viability Determined by MTT Assays
2.3. Cell Apoptosis Determined by Flow Cytometry
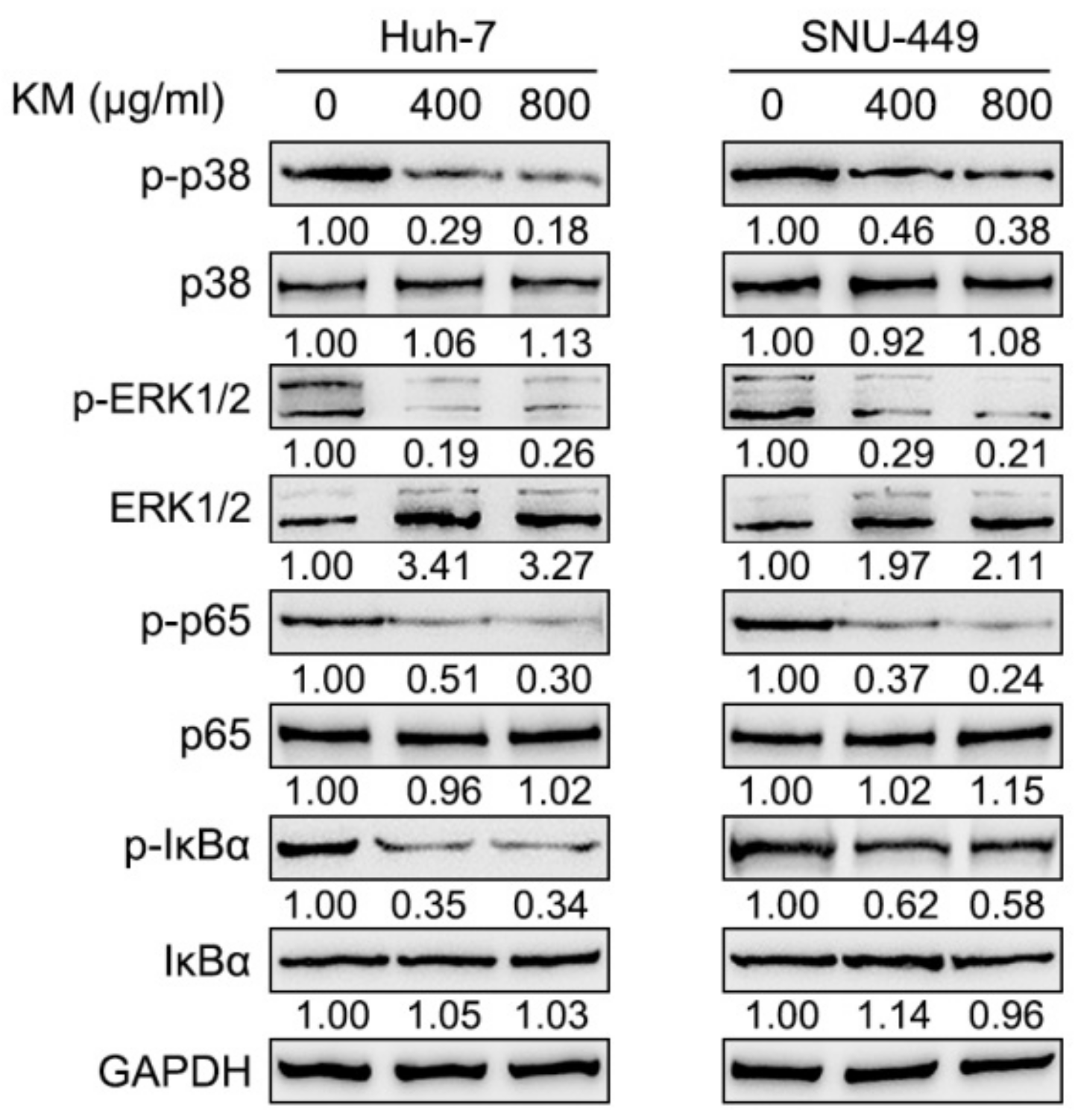
2.4. Immunoblotting
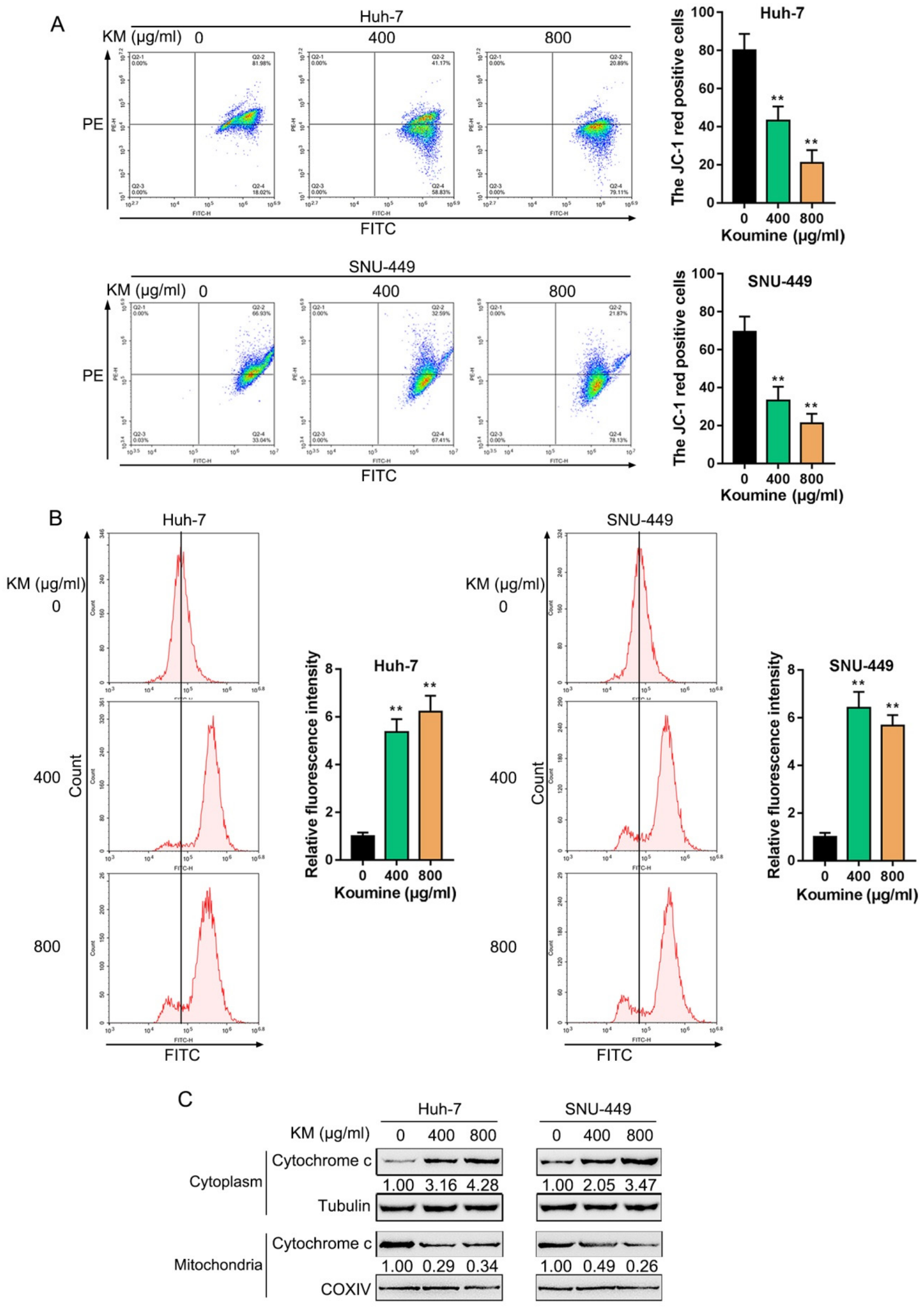
2.5. Mitochondrial Membrane Potential (ΔΨm) Assay
2.6. Determination of the Intracellular ROS
2.7. Data Process and Statistical Analysis
3. Results
3.1. The Killing Effects of Koumine upon Hepatocellular Carcinoma Cells
3.2. Koumine Induced Mitochondrial Dysfunction and ROS Production in HCC Cells
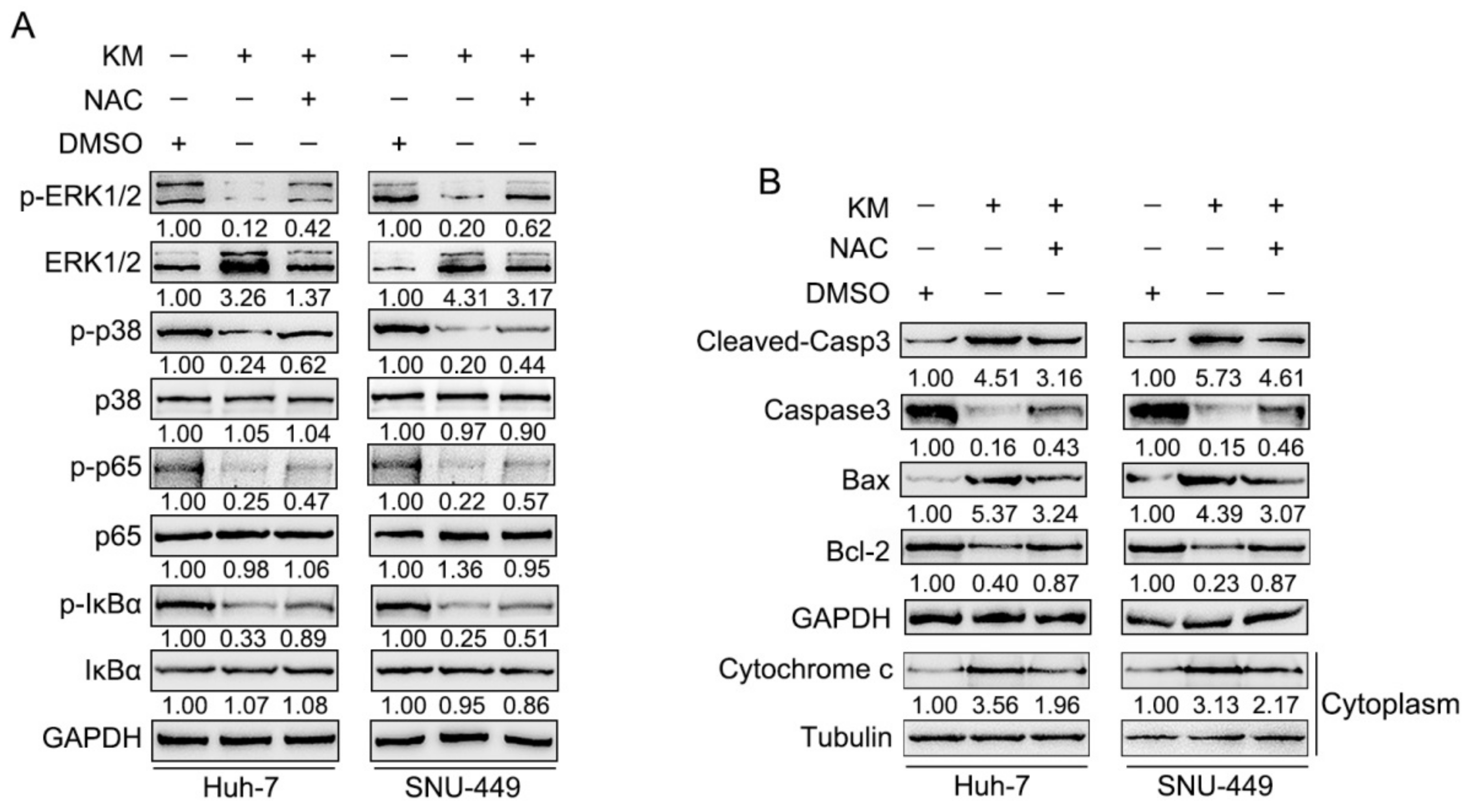
3.3. Koumine Inhibits NF-κB and ERK/p38 MAPK Signaling Pathways within HCC Cells
3.4. Koumine Modulated HCC Cell Apoptosis and ERK/p38 MAPK and NF-κB Signaling Activation via Producing Excessive ROS
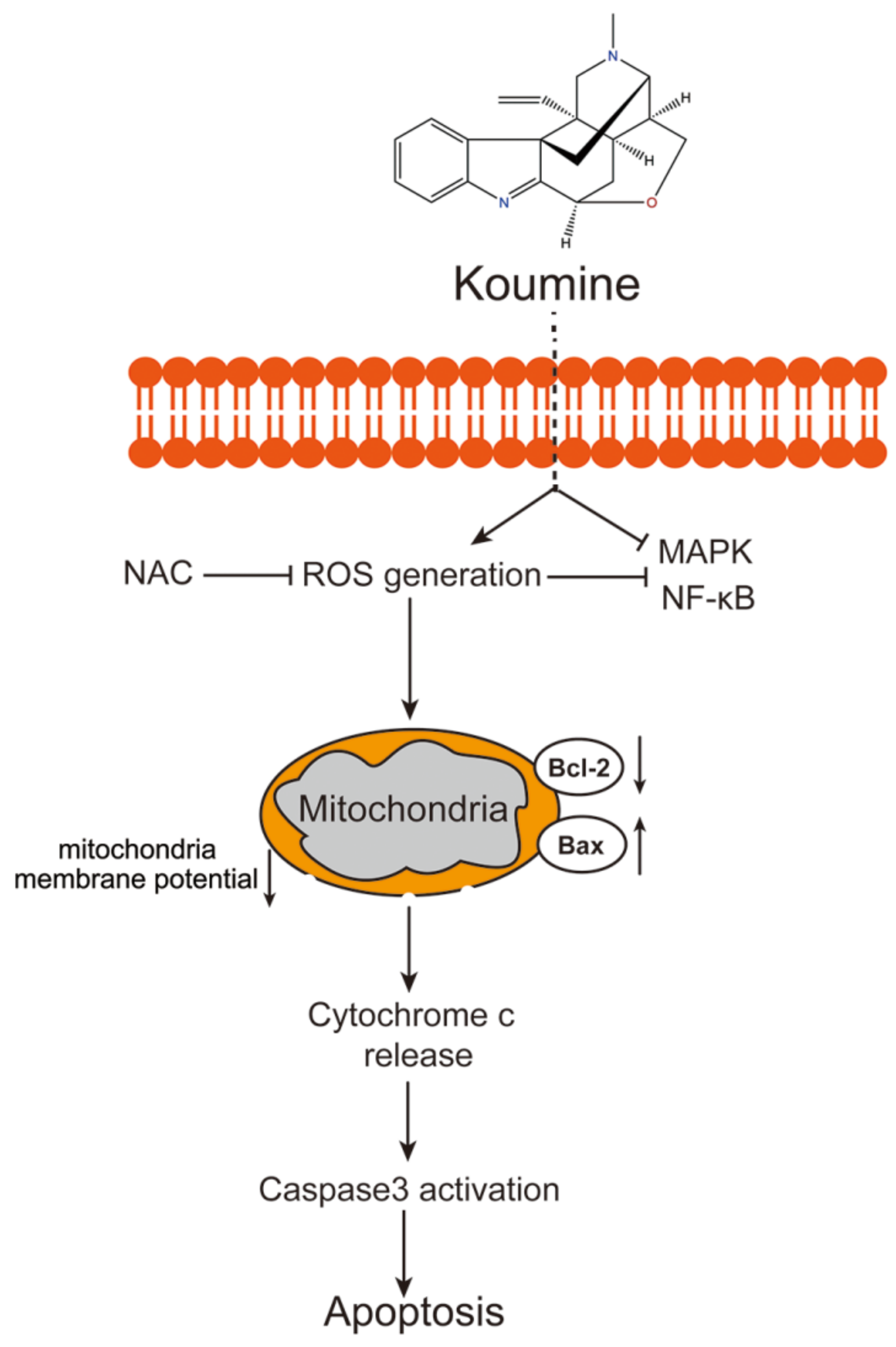
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Janevska, D.; Chaloska-Ivanova, V.; Janevski, V. Hepatocellular Carcinoma: Risk Factors, Diagnosis and Treatment. OAMJMS 2015, 3, 732–736. [Google Scholar] [CrossRef] [PubMed]
- Bosch, F.X.; Ribes, J.; Diaz, M.; Cleries, R. Primary liver cancer: Worldwide incidence and trends. Gastroenterology 2004, 127, S5–S16. [Google Scholar] [CrossRef] [PubMed]
- Petruzziello, A. Epidemiology of Hepatitis B Virus (HBV) and Hepatitis C Virus (HCV) Related Hepatocellular Carcinoma. Open Virol. J. 2018, 12, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Levi, F.; Lucchini, F.; Negri, E.; La Vecchia, C. Continuing declines in cancer mortality in the European Union. Ann. Oncol. 2007, 18, 593–595. [Google Scholar] [CrossRef] [PubMed]
- La Vecchia, C.; Negri, E.; Pelucchi, C. The rise and fall in primary liver cancer mortality in Italy. Dig. Liver Dis. 2002, 34, 169–171. [Google Scholar] [CrossRef]
- Montalto, G.; Cervello, M.; Giannitrapani, L.; Dantona, F.; Terranova, A.; Castagnetta, L.A. Epidemiology, risk factors, and natural history of hepatocellular carcinoma. Ann. N. Y. Acad. Sci. 2002, 963, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Marra, M.; Sordelli, I.M.; Lombardi, A.; Lamberti, M.; Tarantino, L.; Giudice, A.; Stiuso, P.; Abbruzzese, A.; Sperlongano, R.; Accardo, M.; et al. Molecular targets and oxidative stress biomarkers in hepatocellular carcinoma: An overview. J. Transl. Med. 2011, 9, 171. [Google Scholar] [CrossRef] [PubMed]
- Storz, P. Reactive oxygen species in tumor progression. Front. Biosci. 2005, 10, 1881–1896. [Google Scholar] [CrossRef]
- Piccoli, C.; Scrima, R.; Quarato, G.; D’Aprile, A.; Ripoli, M.; Lecce, L.; Boffoli, D.; Moradpour, D.; Capitanio, N. Hepatitis C virus protein expression causes calcium-mediated mitochondrial bioenergetic dysfunction and nitro-oxidative stress. Hepatology 2007, 46, 58–65. [Google Scholar] [CrossRef]
- Jordan, R.; Wang, L.; Graczyk, T.M.; Block, T.M.; Romano, P.R. Replication of a cytopathic strain of bovine viral diarrhea virus activates PERK and induces endoplasmic reticulum stress-mediated apoptosis of MDBK cells. J. Virol. 2002, 76, 9588–9599. [Google Scholar] [CrossRef]
- Tardif, K.D.; Mori, K.; Siddiqui, A. Hepatitis C virus subgenomic replicons induce endoplasmic reticulum stress activating an intracellular signaling pathway. J. Virol. 2002, 76, 7453–7459. [Google Scholar] [CrossRef] [PubMed]
- Waris, G.; Tardif, K.D.; Siddiqui, A. Endoplasmic reticulum (ER) stress: Hepatitis C virus induces an ER-nucleus signal transduction pathway and activates NF-kappaB and STAT-3. Biochem. Pharmacol. 2002, 64, 1425–1430. [Google Scholar] [CrossRef]
- Lee, T.H.; Tai, D.I.; Cheng, C.J.; Sun, C.S.; Lin, C.Y.; Sheu, M.J.; Lee, W.P.; Peng, C.Y.; Wang, A.H.; Tsai, S.L. Enhanced nuclear factor-kappa B-associated Wnt-1 expression in hepatitis B- and C-related hepatocarcinogenesis: Identification by functional proteomics. J. Biomed. Sci. 2006, 13, 27–39. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Marshall, C. How do small GTPase signal transduction pathways regulate cell cycle entry? Curr. Opin. Cell Biol. 1999, 11, 732–736. [Google Scholar] [CrossRef]
- Calvisi, D.F.; Pinna, F.; Pellegrino, R.; Sanna, V.; Sini, M.; Daino, L.; Simile, M.M.; De Miglio, M.R.; Frau, M.; Tomasi, M.L.; et al. Ras-driven proliferation and apoptosis signaling during rat liver carcinogenesis is under genetic control. Int. J. Cancer 2008, 123, 2057–2064. [Google Scholar] [CrossRef] [PubMed]
- Feo, F.; De Miglio, M.R.; Simile, M.M.; Muroni, M.R.; Calvisi, D.F.; Frau, M.; Pascale, R.M. Hepatocellular carcinoma as a complex polygenic disease. Interpretive analysis of recent developments on genetic predisposition. Biochim. Biophys. Acta 2006, 1765, 126–147. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.L.; Su, Y.P.; Liu, M.; Xu, Y.; Yang, J.; Liao, K.J.; Yu, C.X. Medicinal plants of the genus Gelsemium (Gelsemiaceae, Gentianales)—A review of their phytochemistry, pharmacology, toxicology and traditional use. J. Ethnopharmacol. 2014, 152, 33–52. [Google Scholar] [CrossRef]
- Liu, M.; Huang, H.H.; Yang, J.; Su, Y.P.; Lin, H.W.; Lin, L.Q.; Liao, W.J.; Yu, C.X. The active alkaloids of Gelsemium elegans Benth. are potent anxiolytics. Psychopharmacology 2013, 225, 839–851. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.L.; He, S.D.; Lin, S.M.; Hong, L.M.; Chen, W.Q.; Xu, Y.; Yang, J.; Li, S.P.; Yu, C.X. Koumine Attenuates Neuroglia Activation and Inflammatory Response to Neuropathic Pain. Neural Plast. 2018, 2018, 9347696. [Google Scholar] [CrossRef]
- Shoaib, R.M.; Zhang, J.Y.; Mao, X.F.; Wang, Y.X. Gelsemine and koumine, principal active ingredients of Gelsemium, exhibit mechanical antiallodynia via spinal glycine receptor activation-induced allopregnanolone biosynthesis. Biochem. Pharmacol. 2019, 161, 136–148. [Google Scholar] [CrossRef]
- Jin, G.L.; Yang, J.; Chen, W.Q.; Wang, J.; Qiu, H.Q.; Xu, Y.; Yu, C.X. The analgesic effect and possible mechanisms by which koumine alters type II collagen-induced arthritis in rats. J. Nat. Med. 2019, 73, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, Y.; Gao, B.; Luo, D.; Wen, Y.; Ma, X. Apoptotic Effect of Koumine on Human Breast Cancer Cells and the Mechanism Involved. Cell Biochem. Biophys. 2015, 72, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Chi, D.B.; Li, L.; Sun, L.S.; Ma, W.F. Koumine-induced apoptosis of human colonic adenocarcinoma cells: The cell biological mechanism. Nan Fang Yi Ke Da Xue Xue Bao 2007, 27, 994–997. [Google Scholar] [PubMed]
- Huang, R.Z.; Jin, L.; Wang, C.G.; Xu, X.J.; Du, Y.; Liao, N.; Ji, M.; Liao, Z.X.; Wang, H.S. A pentacyclic triterpene derivative possessing polyhydroxyl ring A suppresses growth of HeLa cells by reactive oxygen species-dependent NF-kappaB pathway. Eur. J. Pharmacol. 2018, 838, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Matias, F.B.; Wu, J.; Liang, Z.; Sun, Z. Koumine Attenuates Lipopolysaccaride-Stimulated Inflammation in RAW264.7 Macrophages, Coincidentally Associated with Inhibition of NF-kappaB, ERK and p38 Pathways. Int. J. Mol. Sci. 2016, 17, 430. [Google Scholar] [CrossRef]
- Ng, Y.W.; Say, Y.H. Palmitic acid induces neurotoxicity and gliatoxicity in SH-SY5Y human neuroblastoma and T98G human glioblastoma cells. PeerJ 2018, 6, e4696. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Deng, H.; Zhao, Y.; Li, C.; Liang, Y. LncRNA XIST/miR-34a axis modulates the cell proliferation and tumor growth of thyroid cancer through MET-PI3K-AKT signaling. J. Exp. Clin. Cancer Res. 2018, 37, 279. [Google Scholar] [CrossRef]
- Zheng, K.M.; Zhang, J.; Zhang, C.L.; Zhang, Y.W.; Chen, X.C. Curcumin inhibits appoptosin-induced apoptosis via upregulating heme oxygenase-1 expression in SH-SY5Y cells. Acta Pharmacol. Sin. 2015, 36, 544–552. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Han, S.; Xiang, H.; Peng, Y.; Wu, Y.; Pan, S.; Zhang, Y.; Ruan, J. RY10-4 Inhibits the Proliferation of Human Hepatocellular Cancer HepG2 Cells by Inducing Apoptosis In Vitro and In Vivo. PLoS ONE 2016, 11, e0151679. [Google Scholar] [CrossRef][Green Version]
- Ling, M.; Li, Y.; Xu, Y.; Pang, Y.; Shen, L.; Jiang, R.; Zhao, Y.; Yang, X.; Zhang, J.; Zhou, J.; et al. Regulation of miRNA-21 by reactive oxygen species-activated ERK/NF-kappaB in arsenite-induced cell transformation. Free Radic. Biol. Med. 2012, 52, 1508–1518. [Google Scholar] [CrossRef]
- Zhu, D.; Shen, Z.; Liu, J.; Chen, J.; Liu, Y.; Hu, C.; Li, Z.; Li, Y. The ROS-mediated activation of STAT-3/VEGF signaling is involved in the 27-hydroxycholesterol-induced angiogenesis in human breast cancer cells. Toxicol. Lett. 2016, 264, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Lei, L.S.; Chi, D.B. Antineoplastic effect of koumine in mice bearing H22 solid tumor. Nan Fang Yi Ke Da Xue Xue Bao 2009, 29, 1851–1852. [Google Scholar] [PubMed]
- Chi, D.B.; Lei, L.S.; Jin, H.; Pang, J.X.; Jiang, Y.P. Study of koumine-induced apoptosis of human colon adenocarcinoma LoVo cells in vitro. Di Yi Jun Yi Da Xue Xue Bao 2003, 23, 911–913. [Google Scholar] [PubMed]
- Coulthard, L.R.; White, D.E.; Jones, D.L.; McDermott, M.F.; Burchill, S.A. p38(MAPK): Stress responses from molecular mechanisms to therapeutics. Trends Mol. Med. 2009, 15, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Michaelidis, B.; Hatzikamari, M.; Antoniou, V.; Anestis, A.; Lazou, A. Stress activated protein kinases, JNKs and p38 MAPK, are differentially activated in ganglia and heart of land snail Helix lucorum (L.) during seasonal hibernation and arousal. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2009, 153, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Watson, J.L.; Greenshields, A.; Hill, R.; Hilchie, A.; Lee, P.W.; Giacomantonio, C.A.; Hoskin, D.W. Curcumin-induced apoptosis in ovarian carcinoma cells is p53-independent and involves p38 mitogen-activated protein kinase activation and downregulation of Bcl-2 and survivin expression and Akt signaling. Mol. Carcinog. 2010, 49, 13–24. [Google Scholar] [CrossRef]
- Rosini, P.; De Chiara, G.; Lucibello, M.; Garaci, E.; Cozzolino, F.; Torcia, M. NGF withdrawal induces apoptosis in CESS B cell line through p38 MAPK activation and Bcl-2 phosphorylation. Biochem. Biophys. Res. Commun. 2000, 278, 753–759. [Google Scholar] [CrossRef][Green Version]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.L.; Forner, A.; et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef]
- Chen, T.C.; Chien, C.C.; Wu, M.S.; Chen, Y.C. Evodiamine from Evodia rutaecarpa induces apoptosis via activation of JNK and PERK in human ovarian cancer cells. Phytomedicine 2016, 23, 68–78. [Google Scholar] [CrossRef]
- Yang, J.; Wu, L.J.; Tashiro, S.; Onodera, S.; Ikejima, T. Nitric oxide activated by p38 and NF-κB facilitates apoptosis and cell cycle arrest under oxidative stress in evodiamine-treated human melanoma A375-S2 cells. Free Radic. Res. 2008, 42, 1–11. [Google Scholar] [CrossRef]
- Wang, C.; Wang, M.W.; Tashiro, S.; Onodera, S.; Ikejima, T. Evodiamine induced human melanoma A375-S2 cell death partially through interleukin 1 mediated pathway. Biol. Pharm. Bull. 2005, 28, 984–989. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, Z.; Liang, Z.; Yi, J.; Chen, X.; Li, R.; Wu, J.; Sun, Z. Koumine Promotes ROS Production to Suppress Hepatocellular Carcinoma Cell Proliferation Via NF-κB and ERK/p38 MAPK Signaling. Biomolecules 2019, 9, 559. https://doi.org/10.3390/biom9100559
Yuan Z, Liang Z, Yi J, Chen X, Li R, Wu J, Sun Z. Koumine Promotes ROS Production to Suppress Hepatocellular Carcinoma Cell Proliferation Via NF-κB and ERK/p38 MAPK Signaling. Biomolecules. 2019; 9(10):559. https://doi.org/10.3390/biom9100559
Chicago/Turabian StyleYuan, Zhihang, Zengenni Liang, Jine Yi, Xiaojun Chen, Rongfang Li, Jing Wu, and Zhiliang Sun. 2019. "Koumine Promotes ROS Production to Suppress Hepatocellular Carcinoma Cell Proliferation Via NF-κB and ERK/p38 MAPK Signaling" Biomolecules 9, no. 10: 559. https://doi.org/10.3390/biom9100559
APA StyleYuan, Z., Liang, Z., Yi, J., Chen, X., Li, R., Wu, J., & Sun, Z. (2019). Koumine Promotes ROS Production to Suppress Hepatocellular Carcinoma Cell Proliferation Via NF-κB and ERK/p38 MAPK Signaling. Biomolecules, 9(10), 559. https://doi.org/10.3390/biom9100559



