Effect of Adipose-Derived Stem Cells and Their Exo as Adjunctive Therapy to Nonsurgical Periodontal Treatment: A Histologic and Histomorphometric Study in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
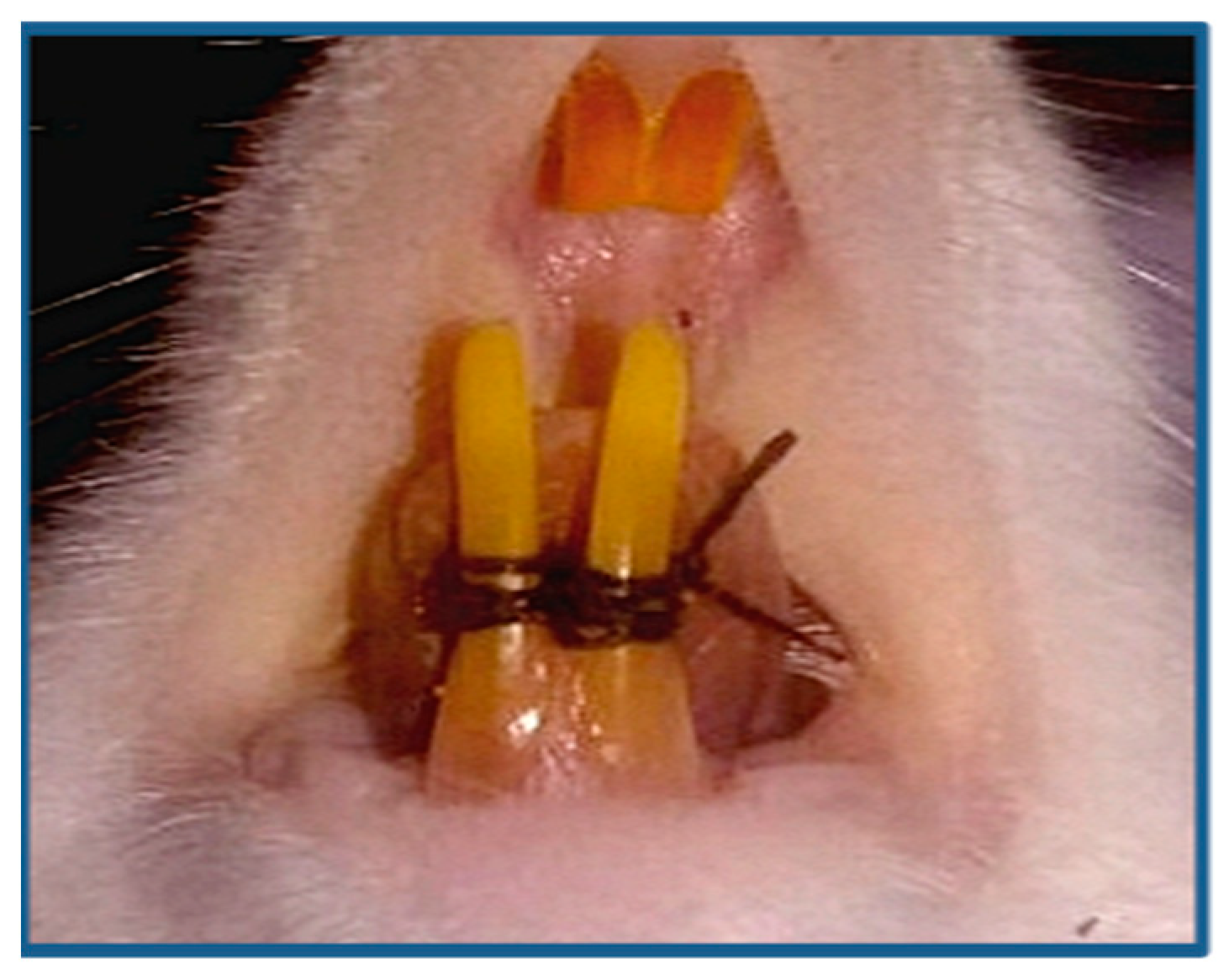
2.2. Induction of Periodontitis
2.3. Adipose-Derived Stem/Stromal Cells and Their Exo. Preparation
2.4. Study Groups
2.5. Histologic and Histochemical Preparation
2.6. Histomorphometric and Statistical Analysis
3. Results
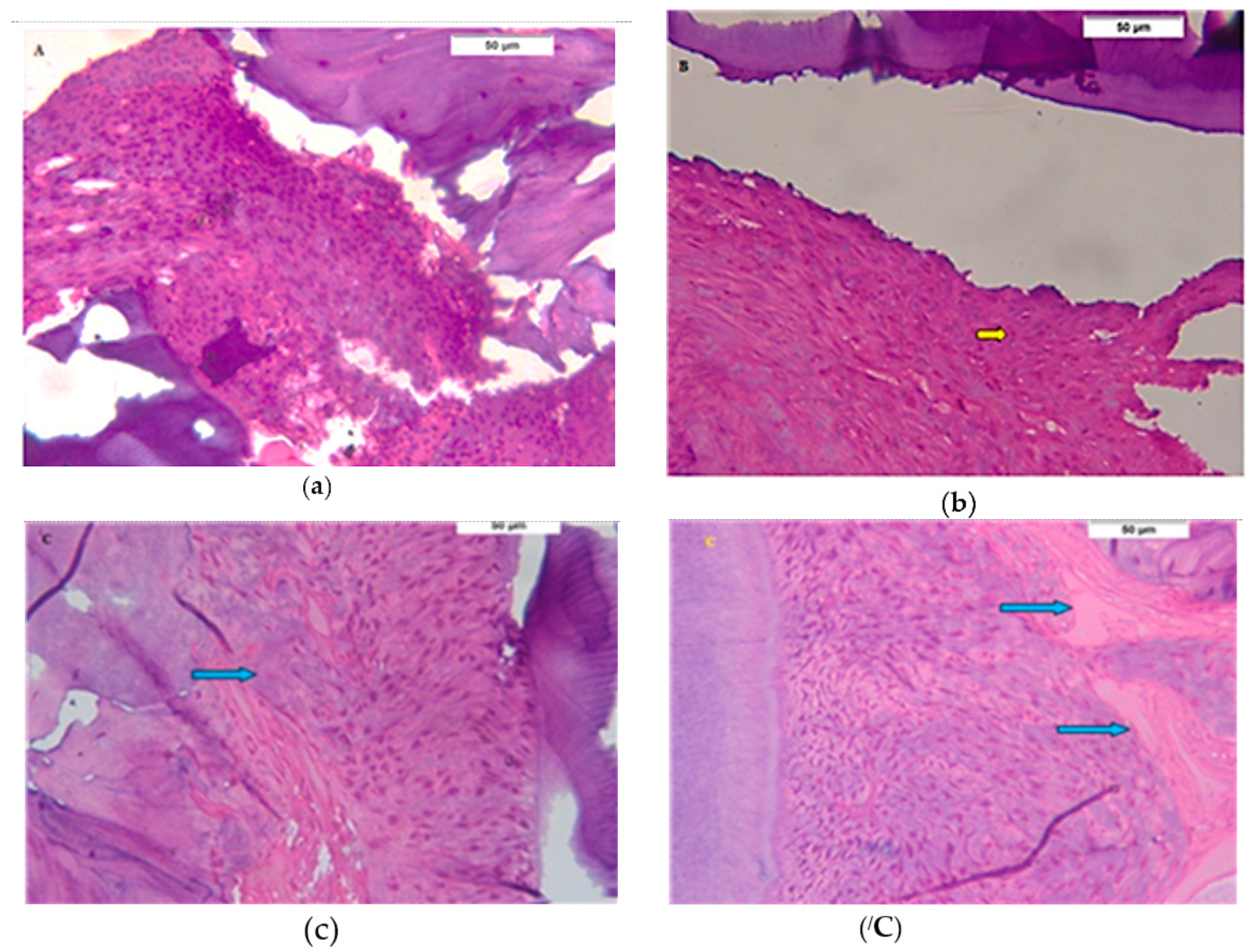
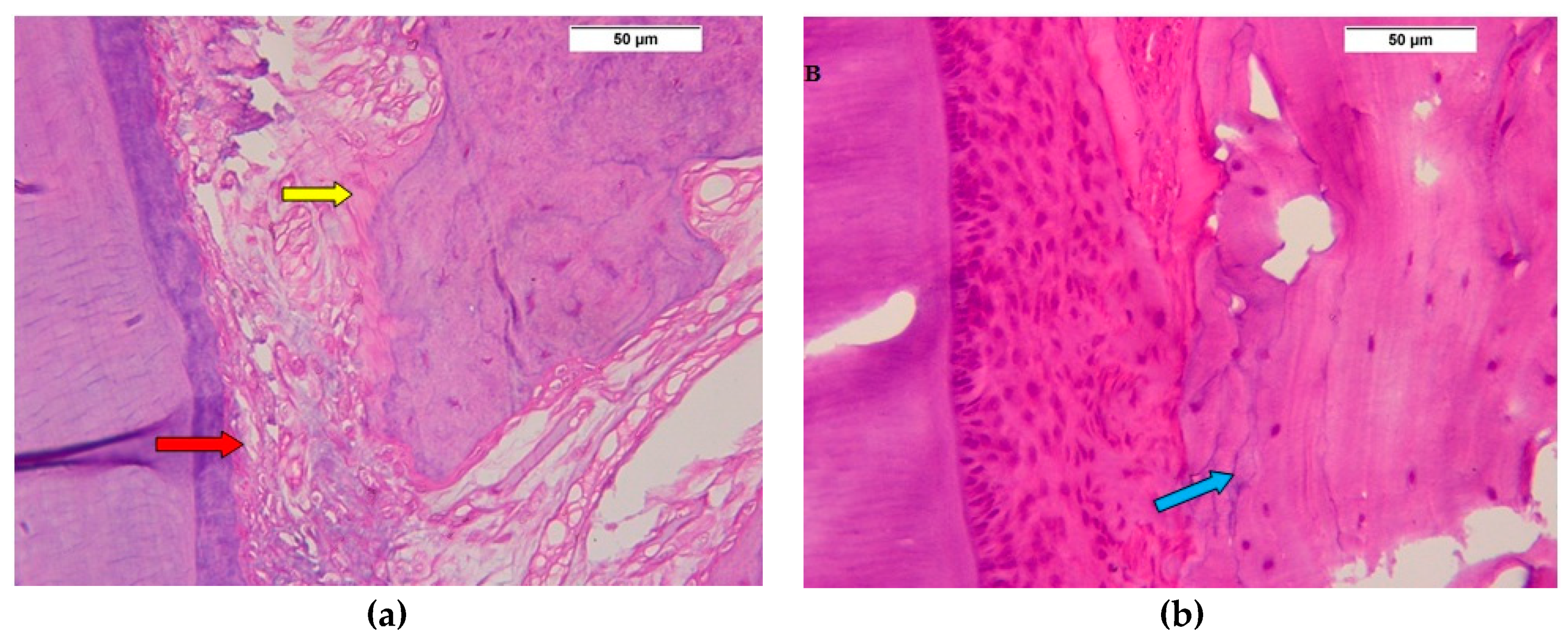
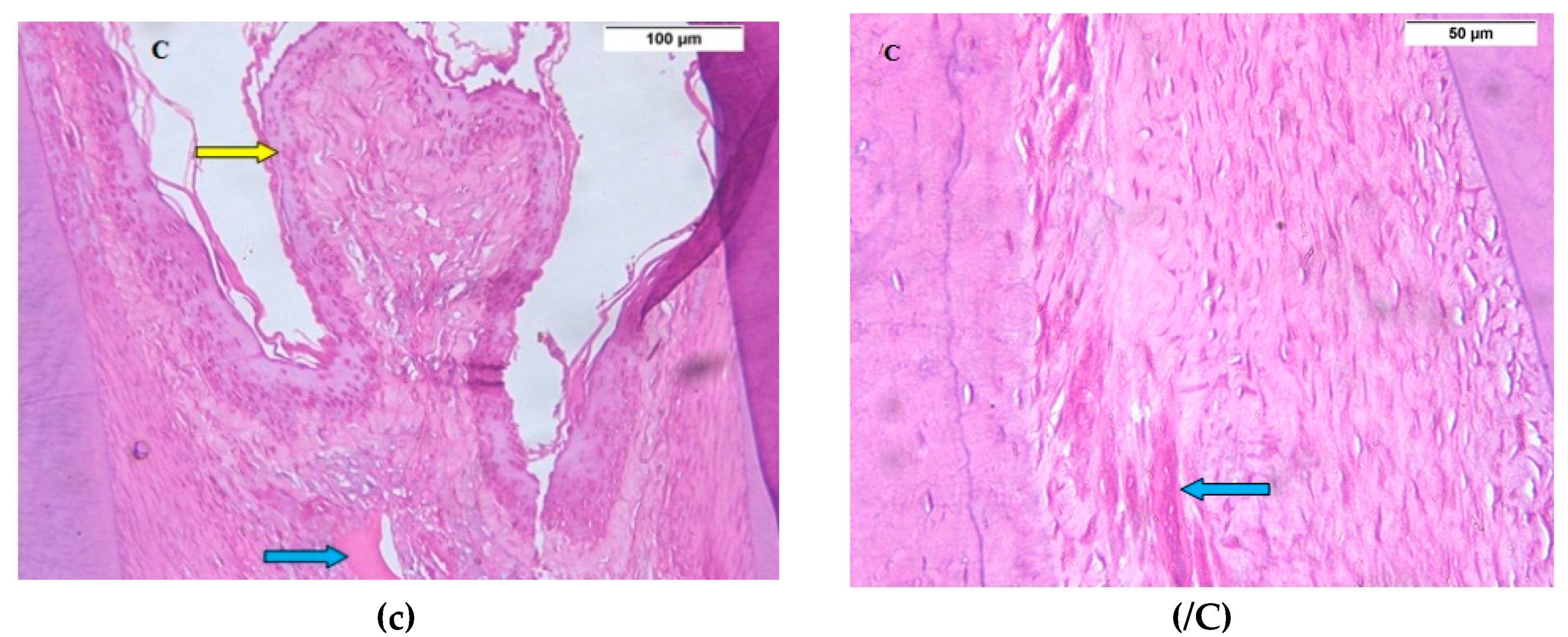
3.1. Descriptive Histologic Results
3.2. Histochemical and Histomorphometric Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hajishengallis, G. Periodontitis: From microbial immune subversion to systemic inflammation. Nat. Rev. Immunol. 2015, 15, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Pihlstrom, B.L.; Michalowicz, B.S.; Johnson, N.W. Periodontal diseases. Lancet 2005, 366, 1809–1820. [Google Scholar] [CrossRef]
- Albandar, J.M. Periodontal disease surveillance. J. Periodontol. 2007, 78, 1179–1181. [Google Scholar] [CrossRef] [PubMed]
- Greenwell, H. Committee on Research, Science and Therapy, American Academy of Periodontology. Position paper: Guidelines for periodontal therapy. Periodontol 2001, 72, 1624–1628. [Google Scholar] [CrossRef]
- Alpiste-Illueca, F.M.; Buitrago-Vera, P.; de Grado-Cabanilles, P.; Fuenmayor-Fernandez, V.; Gil-Loscos, F.J. Periodontal regeneration in clinical practice. Med. Oral Patol. Oral Cir. Bucal 2006, 11, E382–E392. [Google Scholar]
- Hughes, F.J.; Ghuman, M.; Talal, A. Periodontal regeneration: A challenge for the tissue engineer? Proc. Inst. Mech. Eng. H. 2010, 224, 1345–1358. [Google Scholar] [CrossRef]
- Tanwar, J.; Hungund, S.A.; Dodani, K. Nonsurgical periodontal therapy: A review. J. Oral Res. Rev. 2016, 8, 39–44. [Google Scholar] [CrossRef]
- Dabra, S.; Chhina, K.; Nitin Soni, N.; Bhatnagar, R. Tissue engineering in periodontal regeneration: A brief review. Dent. Res. J. (Isfahan) 2012, 9, 671–680. [Google Scholar]
- Kocan, B.; Maziarz, A.; Tabarkiewicz, J.; Ochiya, T.; Banaś-Ząbczyk, A. Trophic Activity and Phenotype of Adipose Tissue-Derived Mesenchymal Stem Cells as a Background of Their Regenerative Potential. Stem Cells Int. 2017, 2017, 1653254. [Google Scholar] [CrossRef]
- Trofin, E.; Monsarrat, P.; Kémoun, P. Cell therapy of periodontium: From animal to human? Front. Physiol. 2013, 4, 325. [Google Scholar] [CrossRef]
- Tassi, S.A.; Sergio, N.Z.; Misawa, M.Y.O; Villar, C.C. Efficacy of stem cells on periodontal regeneration: Systematic review of pre-clinical studies. J. Periodont. Res. 2017, 52, 793–812. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Takahashi, Y. Possibility of Exosome-Based Therapeutics and Challenges in Production of Exosomes Eligible for Therapeutic Application. Biol. Pharm. Bull. 2018, 41, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Vizoso, F.J.; Eiro, N.; Cid, S.; Schneider, J.; Perez-Fernandez, R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int. J. Mol. Sci. 2017, 25, 1852. [Google Scholar] [CrossRef] [PubMed]
- Derkus, B.; Emregul, K.; Emregul, E. A new approach in stem cell research Exosomes: Their mechanism of action via cellular pathways. Cell Biol. Int. 2017, 41, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Shan, Z.; Ma, P.; Wang, S.; Fan, Z. Allogeneic Bone Marrow Mesenchymal Stem Cell Transplantation for Periodontal Regeneration. J. Dent. Res. 2014, 93, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Ionel, A.; Lucaciu, O.; Moga, M.; Buhatel, D.; Ilea, A.; Tabaran, F.; Catoi, C.; Berce, C.; Toader, S.; Campian, R.S. Periodontal disease induced in Wistar rats—Experimental study. HVM Bioflux 2015, 7, 90–95. [Google Scholar]
- Nassar, W.; El-Ansary, M.; Sabry, D.; Mostafa, M.A.; Temraz, M.; Saad, E.K.; Saad, A.N.; Essa, W.; Adel, H. Umbilical cord mesenchymal stem cells derived extracellular vesicles can safely ameliorate the progression of chronic kidney diseases. Biomater. Res. 2016, 5, 21. [Google Scholar] [CrossRef] [PubMed]
- Graves, D.T.; Kang, J.; Andriankaja, O.; Wada, K.; Rossa, C., Jr. Animal models to study host-bacteria interactions involved in periodontitis. Front. Oral Biol. 2012, 15, 117–132. [Google Scholar] [CrossRef] [PubMed]
- Jordan, H.V. Rodent model systems in periodontal disease research. J. Dent. Res. 1971, 50, 236–242. [Google Scholar] [CrossRef]
- Polimeni, G.V.; Xiropaidis, A.; Wikesjo, U.E. Biology and principles of periodontal wound healing/regeneration. Periodontology 2000, 2006, 30–47. [Google Scholar] [CrossRef] [PubMed]
- Angelo, A.; Barone, L.; Khalifian, S.; Lee, A.; Brandacher, G. Immunomodulatory Effects of Adipose-Derived Stem Cells: Fact or Fiction? BioMed. Res. Int. 2013, 2013, 383685. [Google Scholar]
- Cosenza, S.; Toupet, K.; Maumus, M.; Luz-Crawford, P.; Blanc-Brude, O.; Jorgensen, C.; Noël, D. Mesenchymal stem cells-derived exosomes are more immunosuppressive than microparticles in inflammatory arthritis. Theranostics 2018, 8, 1399–1410. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, J.O. Histometric study of healing of periodontal tissues in rats after surgical injury. II. Healing events of alveolar bone, periodontal ligaments and cementum. Odontol. Revy 1976, 27, 131–144. [Google Scholar] [PubMed]
- Bosshardt, D.; Sculean, A. Does periodontal tissue regeneration really work? Periodontology 2000, 2009, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Sabry, D.; Mohamed, E.; Elmenofy, H.; El Ghaffar, K.A. Evaluation of Regenerative Potential of Adipose: Derived Stem Cells and Autogenous Bone Graft in Treatment of Periodontal Defects. Ain Shams Dent. J. 2014, 17, 29–35. [Google Scholar] [CrossRef]
- Bassir, S.; Wisitrasameewong, W.; Raanan, J.; Ghaffarigarakani, S.; Chung, J.; Freire, C.; Andrada, M.L.; Intini, G. Potential for Stem Cell-Based Periodontal Therapy. J. Cell Physiol. 2016, 231, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Lemaitre, M.; Monsarrat, P.; Blasco-baque, V.; Loubières, P.; Burcelin, R.; Casteilla, L.; Planat-Bénard, V.; Kémoun, P. Periodontal Tissue Regeneration Using Syngeneic Adipose-Derived Stromal Cells in a Mouse Model. Stem Cells Trans. Med. 2017, 6, 656–665. [Google Scholar] [CrossRef]
- Salgado, A.J.; Reis, R.L.; Sousa, N.J.; Gimble, J.M. Adipose tissue derived stem cells secretome: Soluble factors and their roles in regenerative medicine. Curr. Stem Cell Res. Therapy 2010, 5, 103–110. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, H.; Cho, H.; Bae, Y.; Suh, K.; Jung, J. Direct comparison of human mesenchymal stem cells derived from adipose tissues and bone marrow in mediating neovascularization in response to vascular ischemia. Cell. Physiol. Biochem. 2007, 20, 867–876. [Google Scholar] [CrossRef]
- Rehman, J.; Traktuev, D.; Li, J.; Merfeld-Clauss, S.; Temm-Grove, C.J.; Bovenkerk, J.E.; Pell, C.L.; Johnstone, B.H.; Considine, R.V. March KLSecretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation 2004, 109, 1292–1298. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Wang, J.; Zhou, X.; Xiong, Z.; Zhao, J.; Yu, R.; Huang, F.; Zhang, H.; Chen, L. Exosomes derived from human adipose mensenchymal stem cells accelerates cutaneous wound healing via optimizing the characteristics of fibroblasts. Sci. Rep. 2016, 6, 32993. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Rao, S.H.; Wang, Z.H.; Cao, J.; Tan, Y.; Luo, J.; Li, H.; Zhang, W.; Chen, C.H.; Xie, H. Exosomes from human umbilical cord blood accelerate cutaneous wound healing through miR-21-3p mediated promotion of angiogenesis and fibroblast function. Theranostics 2018, 8, 169–184. [Google Scholar] [CrossRef] [PubMed]


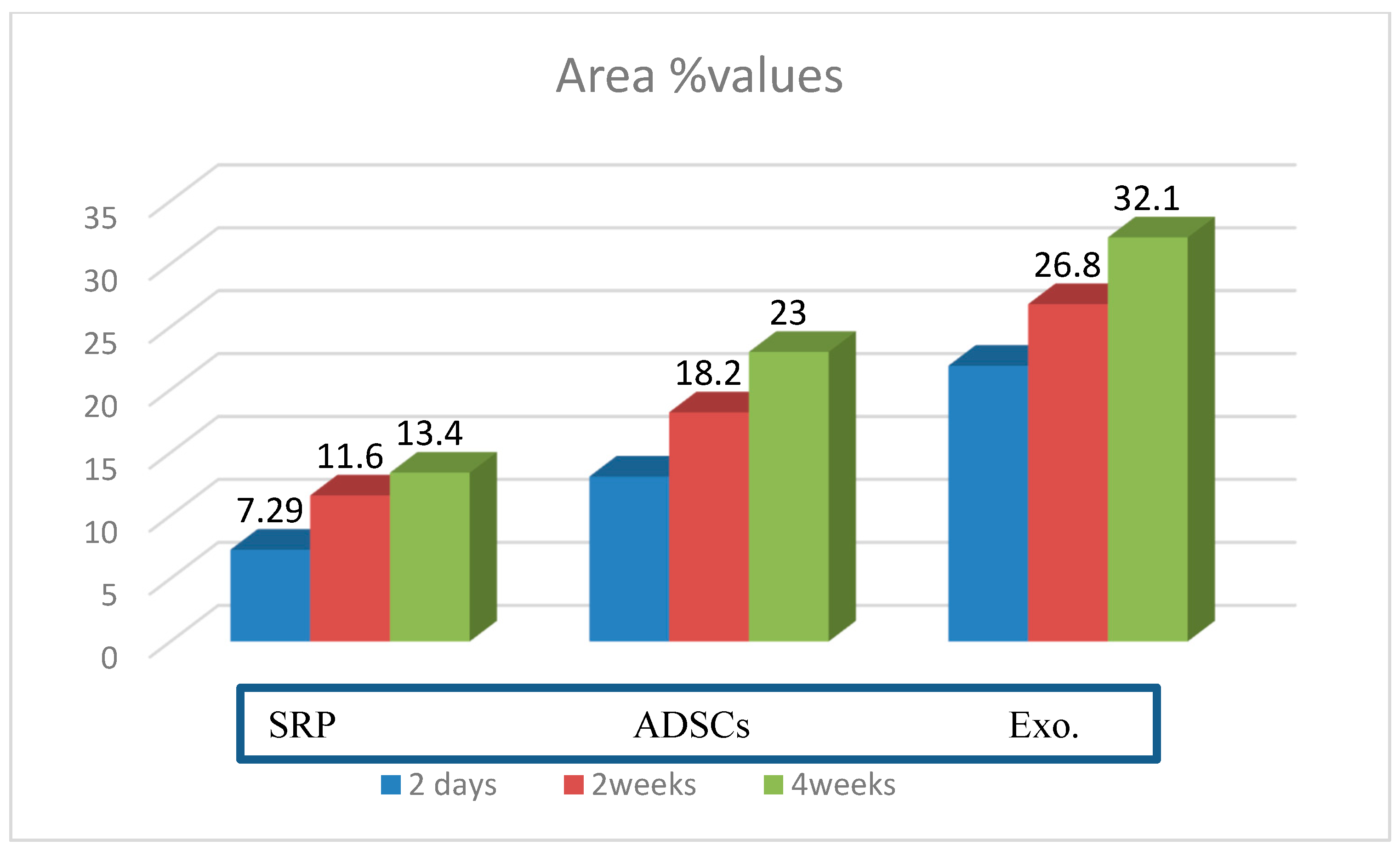
| SRP Group | ADSC Group | Exo. Group | p-Value | |
|---|---|---|---|---|
| 2 days | 7.29 ± 1.34 | 13.1 ± 2.81 | 21.9 ± 1.97 | p = 0.001 * |
| 2 weeks | 11.6 ± 1.14 | 18.2 ± 1.38 | 26.8 ± 2.12 | p = 0.001 * |
| 4 weeks | 13.4 ± 2.30 | 23.0 ± 2.85 | 32.1 ± 3.5 | p = 0.001 * |
| Groups | SRP Group | ADSC Group | Exo. Group |
|---|---|---|---|
| SRP group | − | p = 0.0001 * | p = 0.0005 * |
| ADSC group | p = 0.0001 * | − | p = 0.0001 * |
| Exo. group | p = 0.0005 * | p = 0.0001 * | − |
| Groups | SRP Group | ADSC Group | Exo. Group |
|---|---|---|---|
| SRP group | − | p = 0.0001 * | p = 0.0001 * |
| ADSC group | p = 0.0001 * | − | p = 0.0001 * |
| Exo. group | p = 0.0001 * | p = 0.0001 * | − |
| Groups | SRP Group | ADSC Group | Exo. Group |
|---|---|---|---|
| SRP group | − | p = 0.0006 * | p = 0.0001 * |
| ADSC group | p = 0.0006 * | − | p = 0.0010 * |
| Exo. group | p = 0.0001 * | p = 0.0010 * | − |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammed, E.; Khalil, E.; Sabry, D. Effect of Adipose-Derived Stem Cells and Their Exo as Adjunctive Therapy to Nonsurgical Periodontal Treatment: A Histologic and Histomorphometric Study in Rats. Biomolecules 2018, 8, 167. https://doi.org/10.3390/biom8040167
Mohammed E, Khalil E, Sabry D. Effect of Adipose-Derived Stem Cells and Their Exo as Adjunctive Therapy to Nonsurgical Periodontal Treatment: A Histologic and Histomorphometric Study in Rats. Biomolecules. 2018; 8(4):167. https://doi.org/10.3390/biom8040167
Chicago/Turabian StyleMohammed, Ebtehal, Eman Khalil, and Dina Sabry. 2018. "Effect of Adipose-Derived Stem Cells and Their Exo as Adjunctive Therapy to Nonsurgical Periodontal Treatment: A Histologic and Histomorphometric Study in Rats" Biomolecules 8, no. 4: 167. https://doi.org/10.3390/biom8040167
APA StyleMohammed, E., Khalil, E., & Sabry, D. (2018). Effect of Adipose-Derived Stem Cells and Their Exo as Adjunctive Therapy to Nonsurgical Periodontal Treatment: A Histologic and Histomorphometric Study in Rats. Biomolecules, 8(4), 167. https://doi.org/10.3390/biom8040167




