Therapeutic Chemical Screen Identifies Phosphatase Inhibitors to Reconstitute PKB Phosphorylation and Cardiac Contractility in ILK-Deficient Zebrafish
Abstract
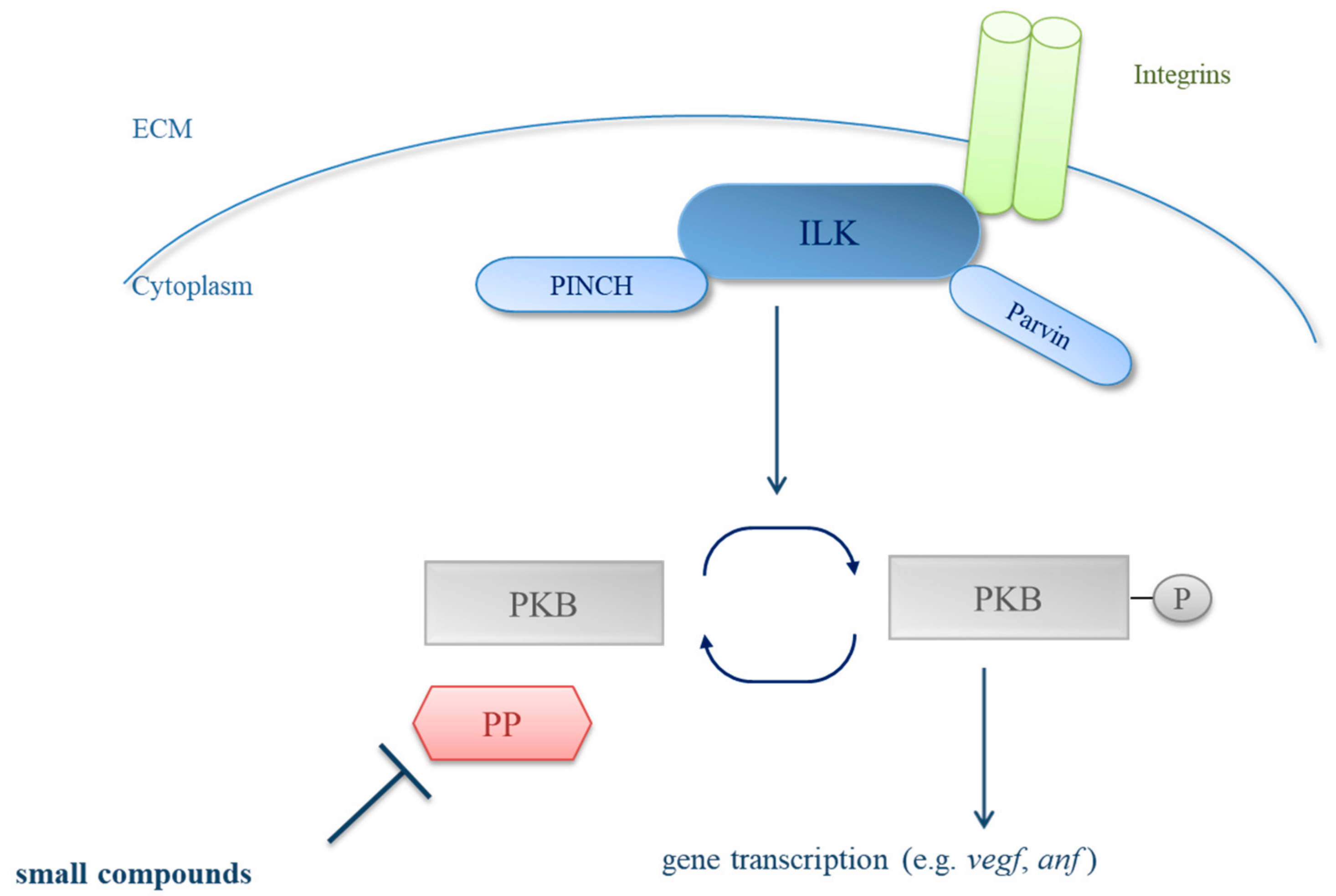
1. Introduction
2. Material and Methods
2.1. Zebrafish Strains
2.2. Genotyping, Western Blot Analysis, and RNA In Situ Hybridization
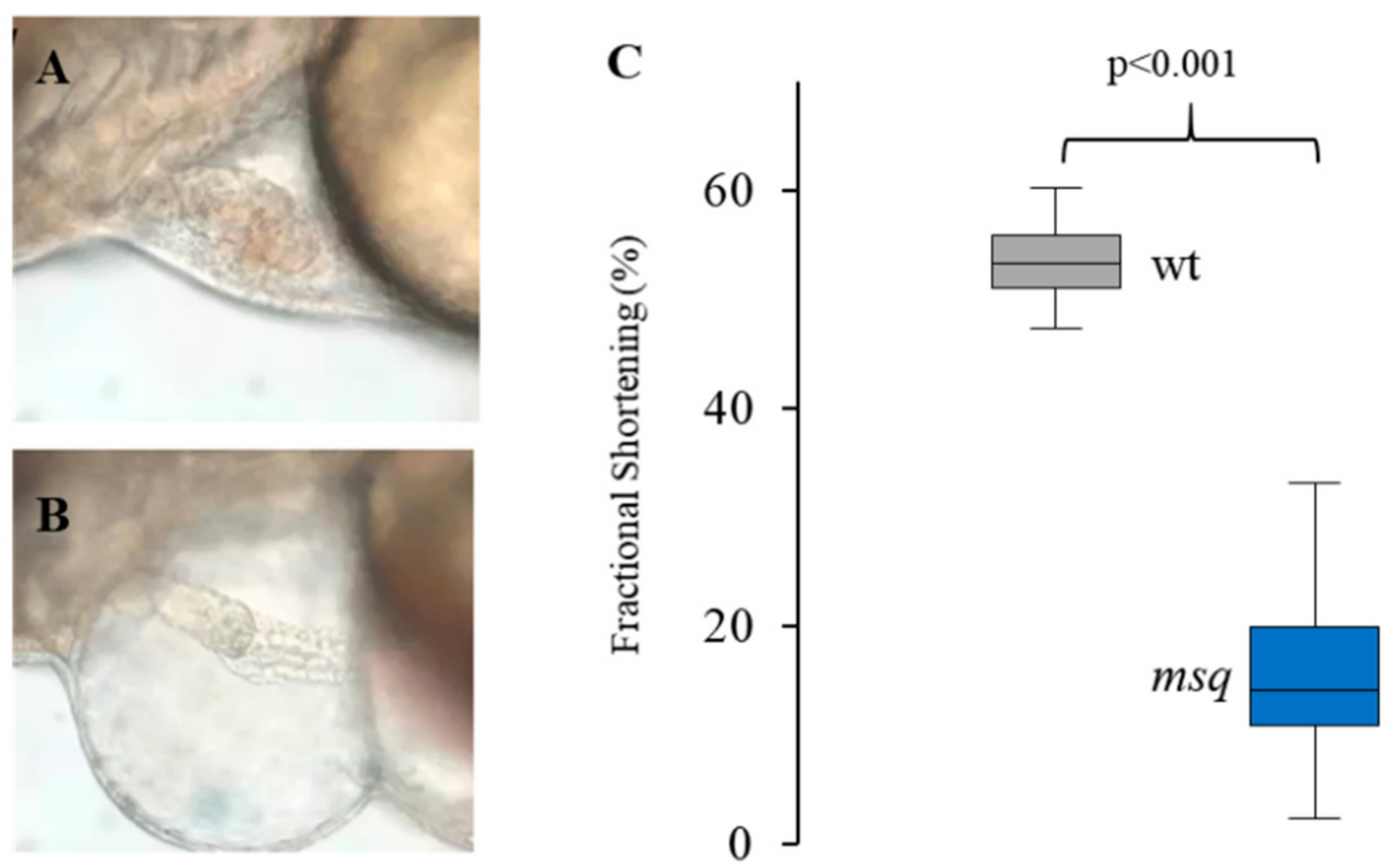
2.3. Small Compound Screen and Functional Assessment in Main Squeeze Embryos
2.4. Statistical Analysis
3. Results
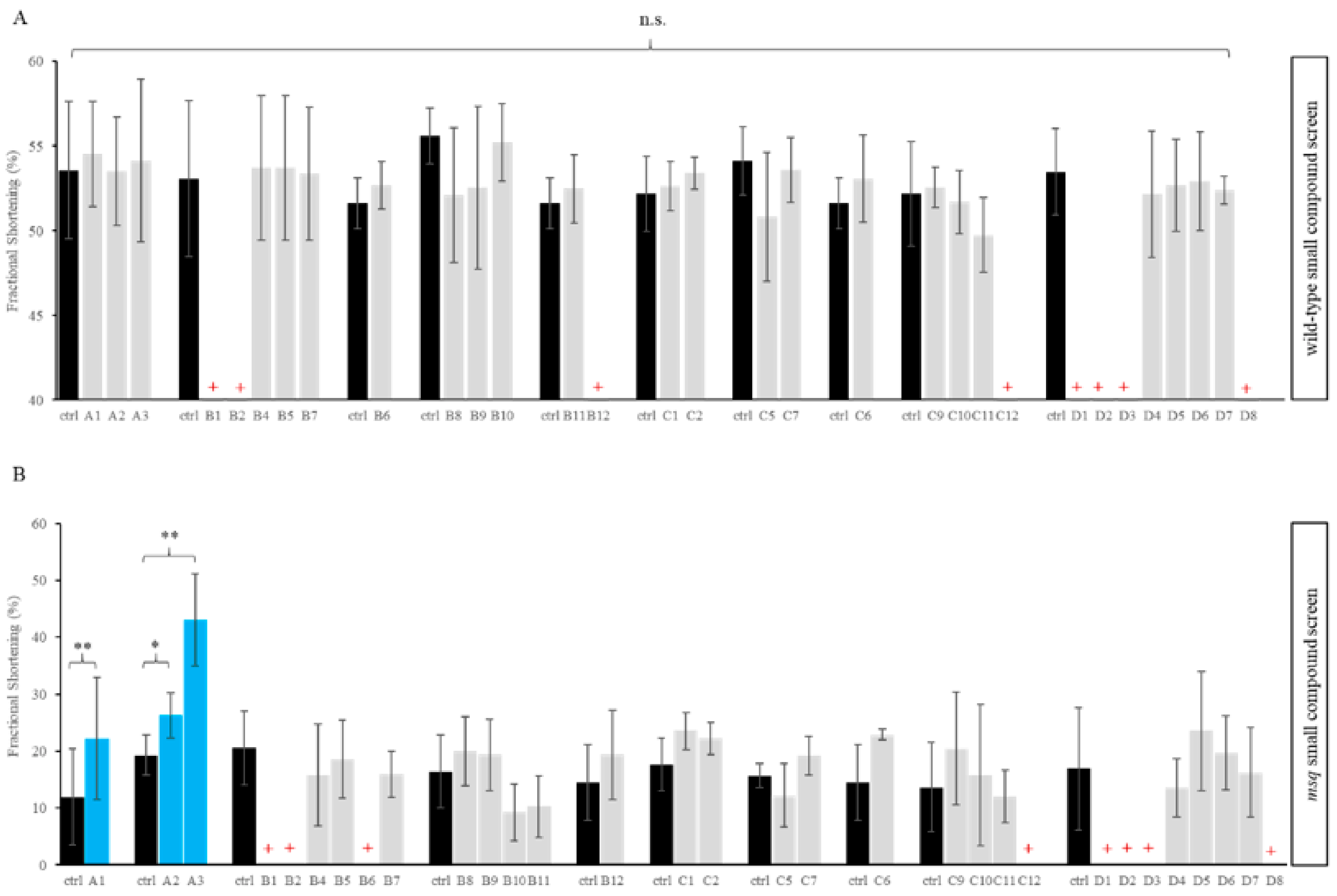
3.1. Primary Small Compound Screen Identifies Phosphatase Inhibitors Restoring Cardiac Contractility in msq Zebrafish
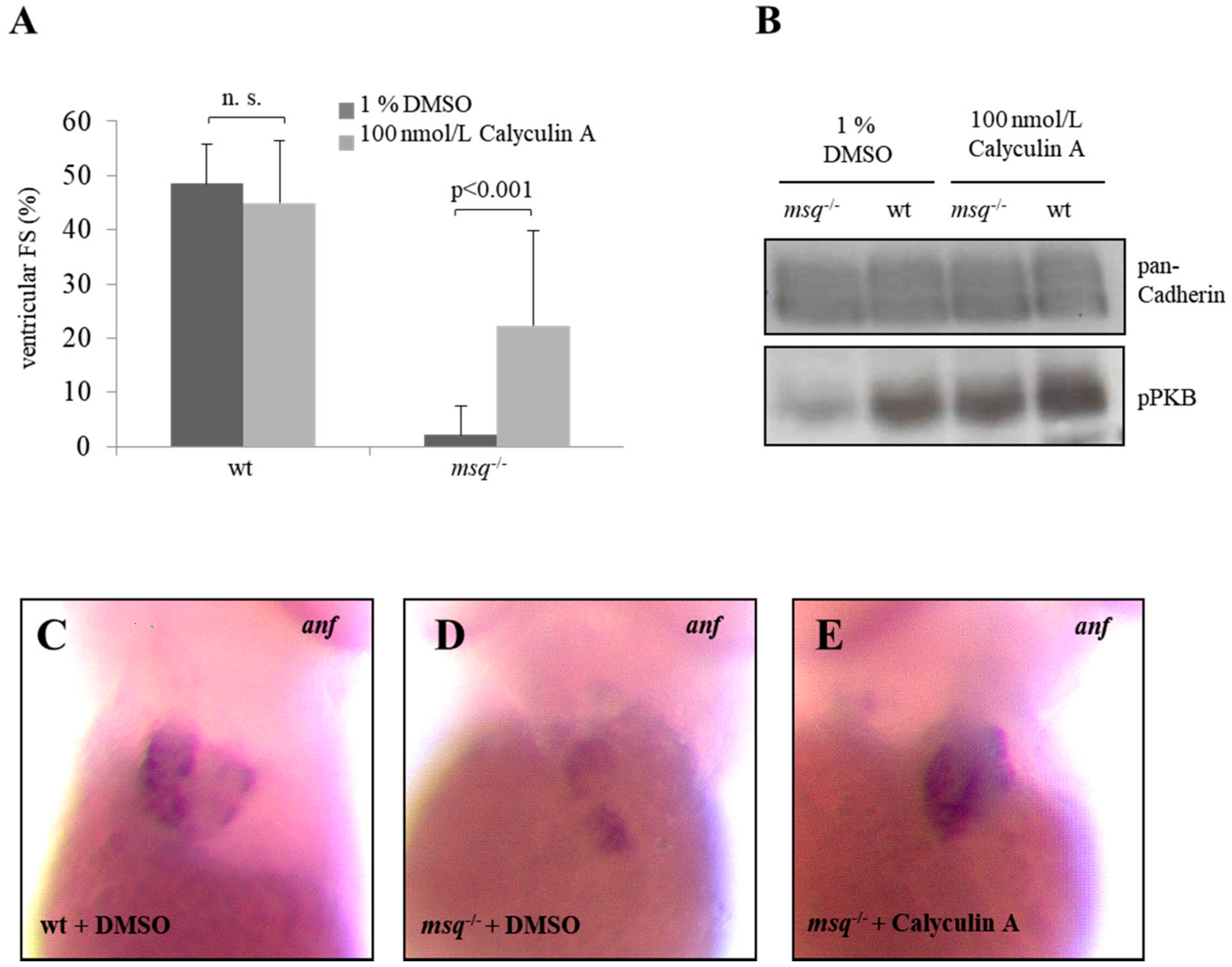
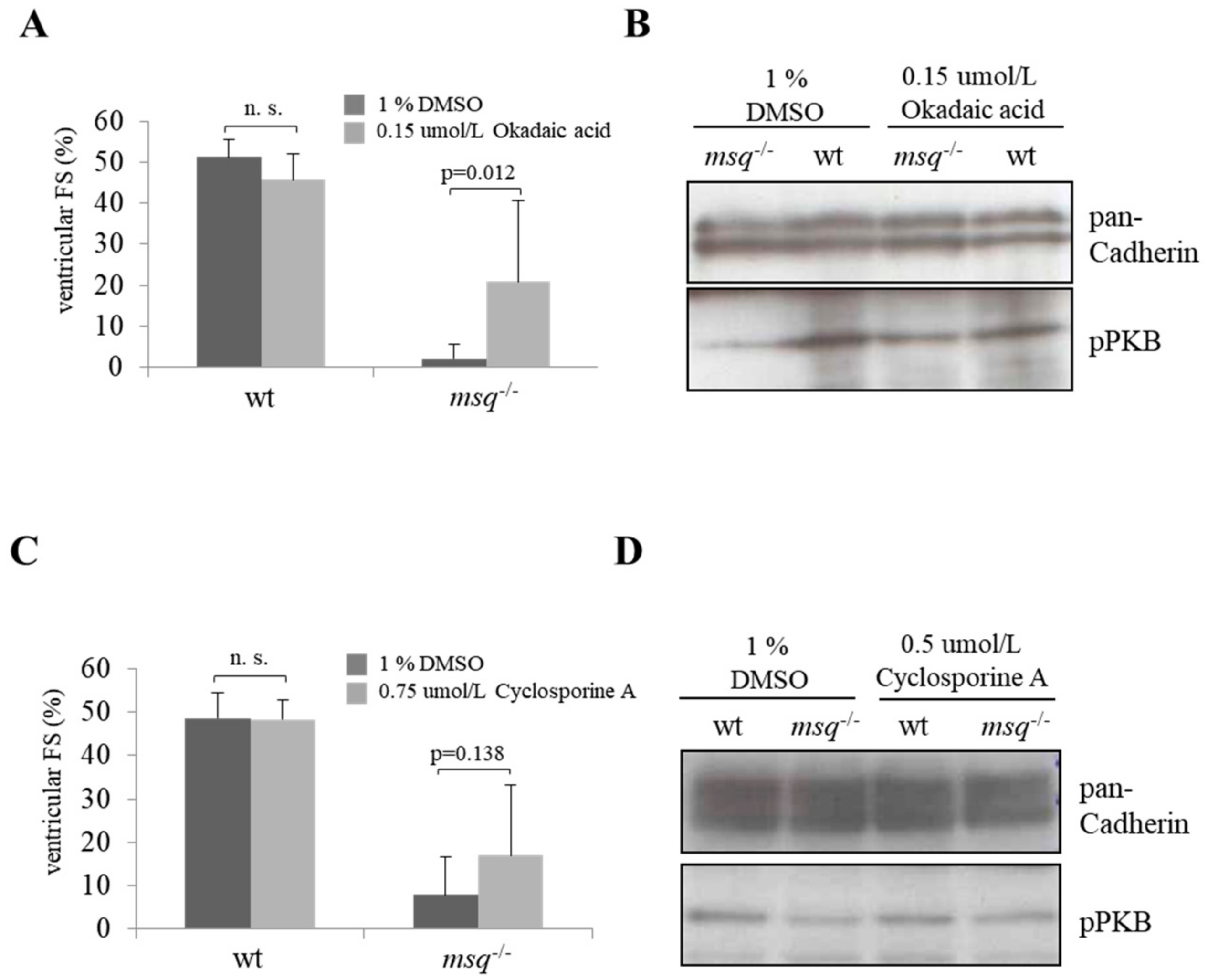
3.2. Calyculin A and Okadaic Acid Reconstitute Cardiac Contractility in msq Cardiomyopathy via Restored PKB Phosphorylation
3.3. Cyclosporine A is a Poor Modulator of Ventricular Fractional Shortening and PKB Phosphorylation in msq Mutants
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Plate Location | Name | Target | Lethality |
|---|---|---|---|
| A1 | Calyculin A | PP1 and PP2A | |
| A2 | Cyclosporin A | PP2A | |
| A3 | Okaid acid | PP1 and PP2A | |
| B1 | Cantharidic acid | PP1 and PP2A | wt, msq |
| B2 | Cantharidin | PP1 and PP2A | wt, msq |
| B3 | Endothall | PP2A | |
| B4 | Benzylphosphonic acid | Tyrosine phosphatases | |
| B5 | L-p-Bromotetramisole oxalate | Tyrosine phosphatases | |
| B6 | RK-682 | Tyrosine phosphatases | msq |
| B7 | RWJ-60475 | CD45 tyrosine phosphatase | |
| B8 | RWJ-60475 (AM)3 | CD45 tyrosine phosphatase (cell permeable) | |
| B9 | Levamisole HCl | Mammalian alkaline phosphatase | |
| B10 | Tetramisole HCl | Mammalian alkaline phosphatase | |
| B11 | Cypermethrin | Calcineurin (PP2B) | |
| B12 | Deltamethrin | Calcineurin (PP2B) | wt |
| C1 | Fenvalerate | Calcineurin (PP2B) | |
| C2 | Tyrphostin 8 | Calcineurin (PP2B) | |
| C5 | BN-82002 | ||
| C6 | Shikonin | ||
| C7 | NSC-663284 | CDC25 | |
| C9 | Pentamidine | PRL1 | |
| C10 | BVT-948 | Tyrosine phosphatases | |
| C11 | 1-(2-Bromobenzyloxy)-4-bromo-2-benzylidene rhodanine | PRL3 | |
| C12 | Alexidine·2HCl | PTPMT1 | wt, msq |
| D1 | 9,10-Phenanthrenequinone | CD45 tyrosine phosphatase | wt, msq |
| D2 | BML-260 | JSP-1 | wt, msq |
| D3 | Sanguinarine chloride | PP2C | wt, msq |
| D4 | BML-267 | PTP1B | |
| D5 | BML-267 Ester | PTP1B (cell permeable) | |
| D6 | OBA | Tyrosine phosphatases | |
| D7 | OBA Ester | Tyrosine phosphatases (cell permeable) | |
| D8 | Gossypol | Calcineurin (PP2B) | wt, msq |
References
- McKenna, W.J.; Maron, B.J.; Thiene, G. Classification, Epidemiology, and Global Burden of Cardiomyopathies. Circ. Res. 2017, 121, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Meder, B.; Katus, H.A. Clinical and genetic aspects of hypertrophic and dilated cardiomyopathy. Der Internist 2012, 53, 408–414, 417–418. [Google Scholar] [CrossRef] [PubMed]
- Masarone, D.; Kaski, J.P.; Pacileo, G.; Elliott, P.M.; Bossone, E.; Day, S.M.; Limongelli, G. Epidemiology and Clinical Aspects of Genetic Cardiomyopathies. Heart Fail. Clin. 2018, 14, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Thiene, G.; Corrado, D.; Basso, C. Revisiting definition and classification of cardiomyopathies in the era of molecular medicine. Eur. Heart J. 2008, 29, 144–146. [Google Scholar] [CrossRef] [PubMed]
- Haas, J.; Frese, K.S.; Peil, B.; Kloos, W.; Keller, A.; Nietsch, R.; Feng, Z.; Muller, S.; Kayvanpour, E.; Vogel, B.; et al. Atlas of the clinical genetics of human dilated cardiomyopathy. Eur. Heart J. 2015, 36, 1123–1135. [Google Scholar] [CrossRef] [PubMed]
- Knoll, R.; Hoshijima, M.; Chien, K. Cardiac mechanotransduction and implications for heart disease. J. Mol. Med. 2003, 81, 750–756. [Google Scholar] [CrossRef] [PubMed]
- Knoll, R.; Postel, R.; Wang, J.; Kratzner, R.; Hennecke, G.; Vacaru, A.M.; Vakeel, P.; Schubert, C.; Murthy, K.; Rana, B.K.; et al. Laminin-α4 and integrin-linked kinase mutations cause human cardiomyopathy via simultaneous defects in cardiomyocytes and endothelial cells. Circulation 2007, 116, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Hoshijima, M. Mechanical stress-strain sensors embedded in cardiac cytoskeleton: Z disk, titin, and associated structures. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H1313–H1325. [Google Scholar] [CrossRef] [PubMed]
- White, D.E.; Coutu, P.; Shi, Y.F.; Tardif, J.C.; Nattel, S.; St Arnaud, R.; Dedhar, S.; Muller, W.J. Targeted ablation of ILK from the murine heart results in dilated cardiomyopathy and spontaneous heart failure. Genes Dev. 2006, 20, 2355–2360. [Google Scholar] [CrossRef] [PubMed]
- Bendig, G.; Grimmler, M.; Huttner, I.G.; Wessels, G.; Dahme, T.; Just, S.; Trano, N.; Katus, H.A.; Fishman, M.C.; Rottbauer, W. Integrin-linked kinase, a novel component of the cardiac mechanical stretch sensor, controls contractility in the zebrafish heart. Genes Dev. 2006, 20, 2361–2372. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Huang, Y.; Zhang, Y.; Hua, Y.; Wu, C. A new focal adhesion protein that interacts with integrin-linked kinase and regulates cell adhesion and spreading. J. Cell Biol. 2001, 153, 585–598. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Wu, C. ILK: A pseudokinase in the center stage of cell-matrix adhesion and signaling. Curr. Opin. Cell Biol. 2012, 24, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Legate, K.R.; Montanez, E.; Kudlacek, O.; Fassler, R. ILK, PINCH and parvin: The tIPP of integrin signalling. Nat. Rev. Mol. Cell Biol. 2006, 7, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Meier, R.; Thelen, M.; Hemmings, B.A. Inactivation and dephosphorylation of protein kinase Bα (PKBα) promoted by hyperosmotic stress. EMBO J. 1998, 17, 7294–7303. [Google Scholar] [CrossRef] [PubMed]
- Meder, B.; Huttner, I.G.; Sedaghat-Hamedani, F.; Just, S.; Dahme, T.; Frese, K.S.; Vogel, B.; Kohler, D.; Kloos, W.; Rudloff, J.; et al. PINCH proteins regulate cardiac contractility by modulating integrin-linked kinase-protein kinase B signaling. Mol. Cell. Biol. 2011, 31, 3424–3435. [Google Scholar] [CrossRef] [PubMed]
- Melchior, C.; Kreis, S.; Janji, B.; Kieffer, N. Promoter characterization and genomic organization of the gene encoding integrin-linked kinase 1. Biochim. Biophys. Acta 2002, 1575, 117–122. [Google Scholar] [CrossRef]
- Sakai, T.; Li, S.; Docheva, D.; Grashoff, C.; Sakai, K.; Kostka, G.; Braun, A.; Pfeifer, A.; Yurchenco, P.D.; Fassler, R. Integrin-linked kinase (ILK) is required for polarizing the epiblast, cell adhesion, and controlling actin accumulation. Genes Dev. 2003, 17, 926–940. [Google Scholar] [CrossRef] [PubMed]
- Hannigan, G.E.; Leung-Hagesteijn, C.; Fitz-Gibbon, L.; Coppolino, M.G.; Radeva, G.; Filmus, J.; Bell, J.C.; Dedhar, S. Regulation of cell adhesion and anchorage-dependent growth by a new β 1-integrin-linked protein kinase. Nature 1996, 379, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Mackinnon, A.C.; Qadota, H.; Norman, K.R.; Moerman, D.G.; Williams, B.D. C. elegans PAT-4/ILK functions as an adaptor protein within integrin adhesion complexes. Curr. Biol. 2002, 12, 787–797. [Google Scholar] [CrossRef]
- Fukuda, K.; Knight, J.D.; Piszczek, G.; Kothary, R.; Qin, J. Biochemical, proteomic, structural, and thermodynamic characterizations of integrin-linked kinase (ILK): Cross-validation of the pseudokinase. J. Biol. Chem. 2011, 286, 21886–21895. [Google Scholar] [CrossRef] [PubMed]
- Spomer, W.; Pfriem, A.; Alshut, R.; Just, S.; Pylatiuk, C. High-throughput screening of zebrafish embryos using automated heart detection and imaging. J. Lab. Autom. 2012, 17, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Pylatiuk, C.; Sanchez, D.; Mikut, R.; Alshut, R.; Reischl, M.; Hirth, S.; Rottbauer, W.; Just, S. Automatic zebrafish heartbeat detection and analysis for zebrafish embryos. Zebrafish 2014, 11, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Pott, A.; Rottbauer, W.; Just, S. Functional genomics in zebrafish as a tool to identify novel antiarrhythmic targets. Curr. Med. Chem. 2014, 21, 1320–1329. [Google Scholar] [CrossRef] [PubMed]
- Rottbauer, W.; Just, S.; Wessels, G.; Trano, N.; Most, P.; Katus, H.A.; Fishman, M.C. VEGF-PLCγ1 pathway controls cardiac contractility in the embryonic heart. Genes Dev. 2005, 19, 1624–1634. [Google Scholar] [CrossRef] [PubMed]
- Dawson, J.F.; Holmes, C.F. Molecular mechanisms underlying inhibition of protein phosphatases by marine toxins. Front. Biosci. 1999, 4, D646–D658. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Ming, M.; He, T.C.; He, Y.Y. Immunosuppressive cyclosporin A activates AKT in keratinocytes through PTEN suppression: Implications in skin carcinogenesis. J. Biol. Chem. 2010, 285, 11369–11377. [Google Scholar] [CrossRef] [PubMed]
- Honkanen, R.E.; Codispoti, B.A.; Tse, K.; Boynton, A.L.; Honkanan, R.E. Characterization of natural toxins with inhibitory activity against serine/threonine protein phosphatases. Toxicon 1994, 32, 339–350. [Google Scholar] [CrossRef]
- Tu, S.; Chi, N.C. Zebrafish models in cardiac development and congenital heart birth defects. Differentiation 2012, 84, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Meder, B.; Laufer, C.; Hassel, D.; Just, S.; Marquart, S.; Vogel, B.; Hess, A.; Fishman, M.C.; Katus, H.A.; Rottbauer, W. A single serine in the carboxyl terminus of cardiac essential myosin light chain-1 controls cardiomyocyte contractility in vivo. Circ. Res. 2009, 104, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, H.; Martin, B.L.; Brautigan, D.L.; Karaki, H.; Ozaki, H.; Kato, Y.; Fusetani, N.; Watabe, S.; Hashimoto, K.; Uemura, D. Calyculin A and okadaic acid: Inhibitors of protein phosphatase activity. Biochem. Biophys. Res. Commun. 1989, 159, 871–877. [Google Scholar] [CrossRef]
- Verbinnen, I.; Ferreira, M.; Bollen, M. Biogenesis and activity regulation of protein phosphatase 1. Biochem. Soc. Trans. 2017, 45, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Baskaran, R.; Velmurugan, B.K. Protein phosphatase 2A as therapeutic targets in various disease models. Life Sci. 2018. [Google Scholar] [CrossRef] [PubMed]
- Meguro, T.; Hong, C.; Asai, K.; Takagi, G.; McKinsey, T.A.; Olson, E.N.; Vatner, S.F. Cyclosporine attenuates pressure-overload hypertrophy in mice while enhancing susceptibility to decompensation and heart failure. Circ. Res. 1999, 84, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Lessman, C.A. The developing zebrafish (Danio rerio): A vertebrate model for high-throughput screening of chemical libraries. Birth Defects Res. C Embryo Today 2011, 93, 268–280. [Google Scholar] [CrossRef] [PubMed]
- Eder, A.; Vollert, I.; Hansen, A.; Eschenhagen, T. Human engineered heart tissue as a model system for drug testing. Adv. Drug Deliv. Rev. 2016, 96, 214–224. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pott, A.; Shahid, M.; Köhler, D.; Pylatiuk, C.; Weinmann, K.; Just, S.; Rottbauer, W. Therapeutic Chemical Screen Identifies Phosphatase Inhibitors to Reconstitute PKB Phosphorylation and Cardiac Contractility in ILK-Deficient Zebrafish. Biomolecules 2018, 8, 153. https://doi.org/10.3390/biom8040153
Pott A, Shahid M, Köhler D, Pylatiuk C, Weinmann K, Just S, Rottbauer W. Therapeutic Chemical Screen Identifies Phosphatase Inhibitors to Reconstitute PKB Phosphorylation and Cardiac Contractility in ILK-Deficient Zebrafish. Biomolecules. 2018; 8(4):153. https://doi.org/10.3390/biom8040153
Chicago/Turabian StylePott, Alexander, Maryam Shahid, Doreen Köhler, Christian Pylatiuk, Karolina Weinmann, Steffen Just, and Wolfgang Rottbauer. 2018. "Therapeutic Chemical Screen Identifies Phosphatase Inhibitors to Reconstitute PKB Phosphorylation and Cardiac Contractility in ILK-Deficient Zebrafish" Biomolecules 8, no. 4: 153. https://doi.org/10.3390/biom8040153
APA StylePott, A., Shahid, M., Köhler, D., Pylatiuk, C., Weinmann, K., Just, S., & Rottbauer, W. (2018). Therapeutic Chemical Screen Identifies Phosphatase Inhibitors to Reconstitute PKB Phosphorylation and Cardiac Contractility in ILK-Deficient Zebrafish. Biomolecules, 8(4), 153. https://doi.org/10.3390/biom8040153





