Heme Dissociation from Myoglobin in the Presence of the Zwitterionic Detergent N,N-Dimethyl-N-Dodecylglycine Betaine: Effects of Ionic Liquids
Abstract
1. Introduction
2. Materials and Methods
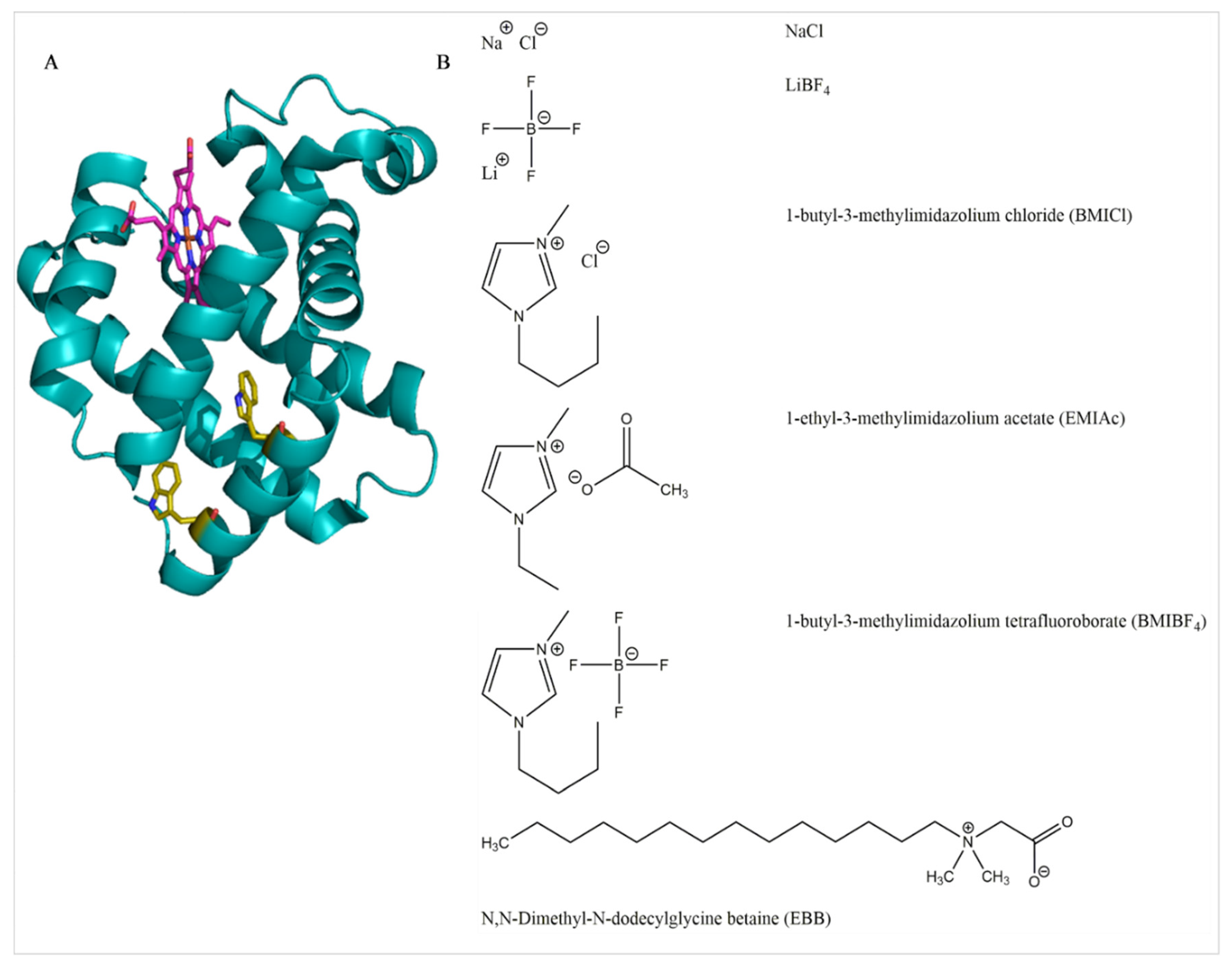
2.1. Materials
2.2. Sample Preparation
2.3. Critical Micelle Concentration Determination
2.4. Absorbance Measurement
2.5. Fluorescence Spectroscopy
2.6. Circular Dichroism Spectroscopy
2.7. Calculation of Gibbs Free Energy of Dissociation
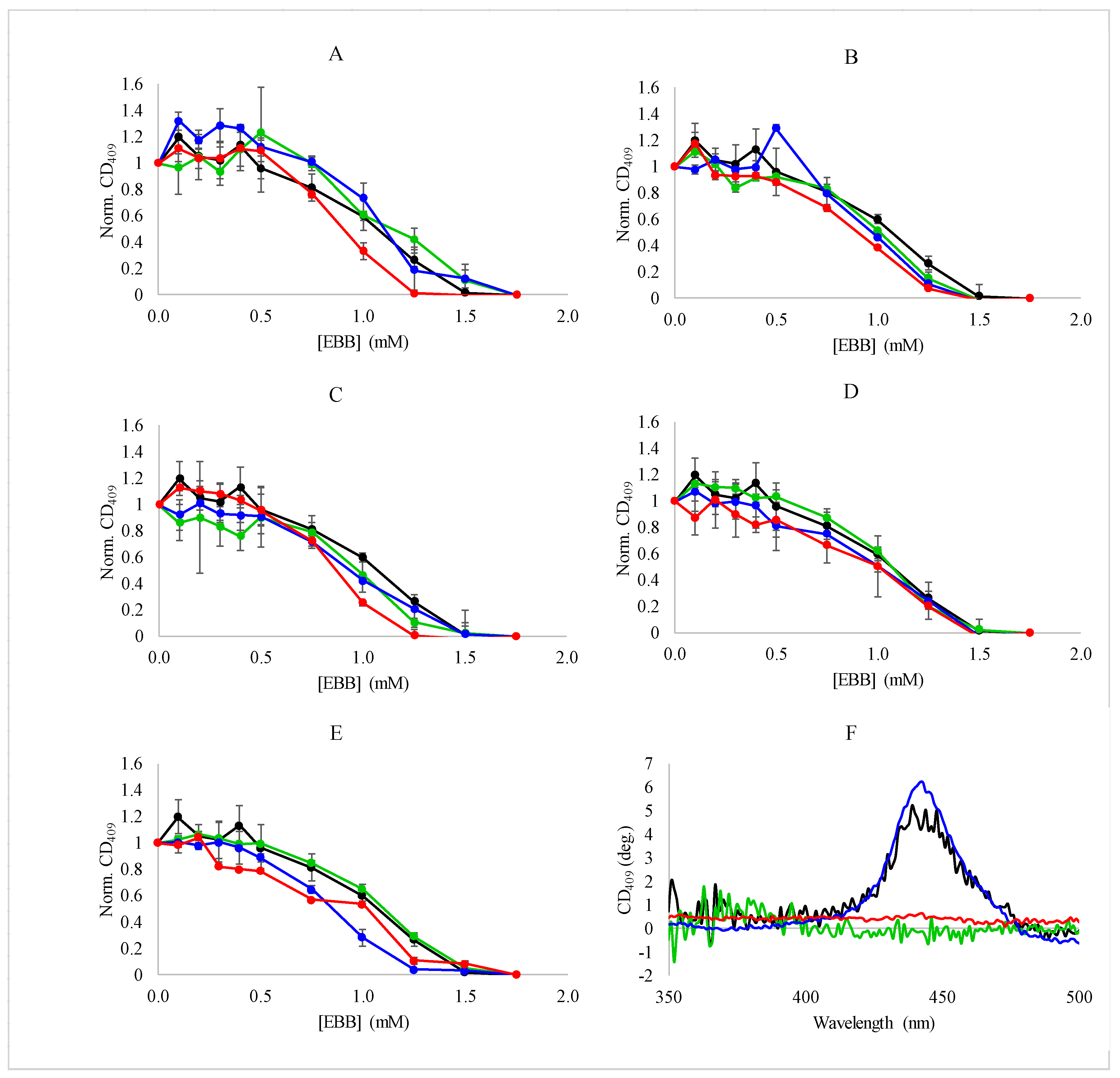
3. Results
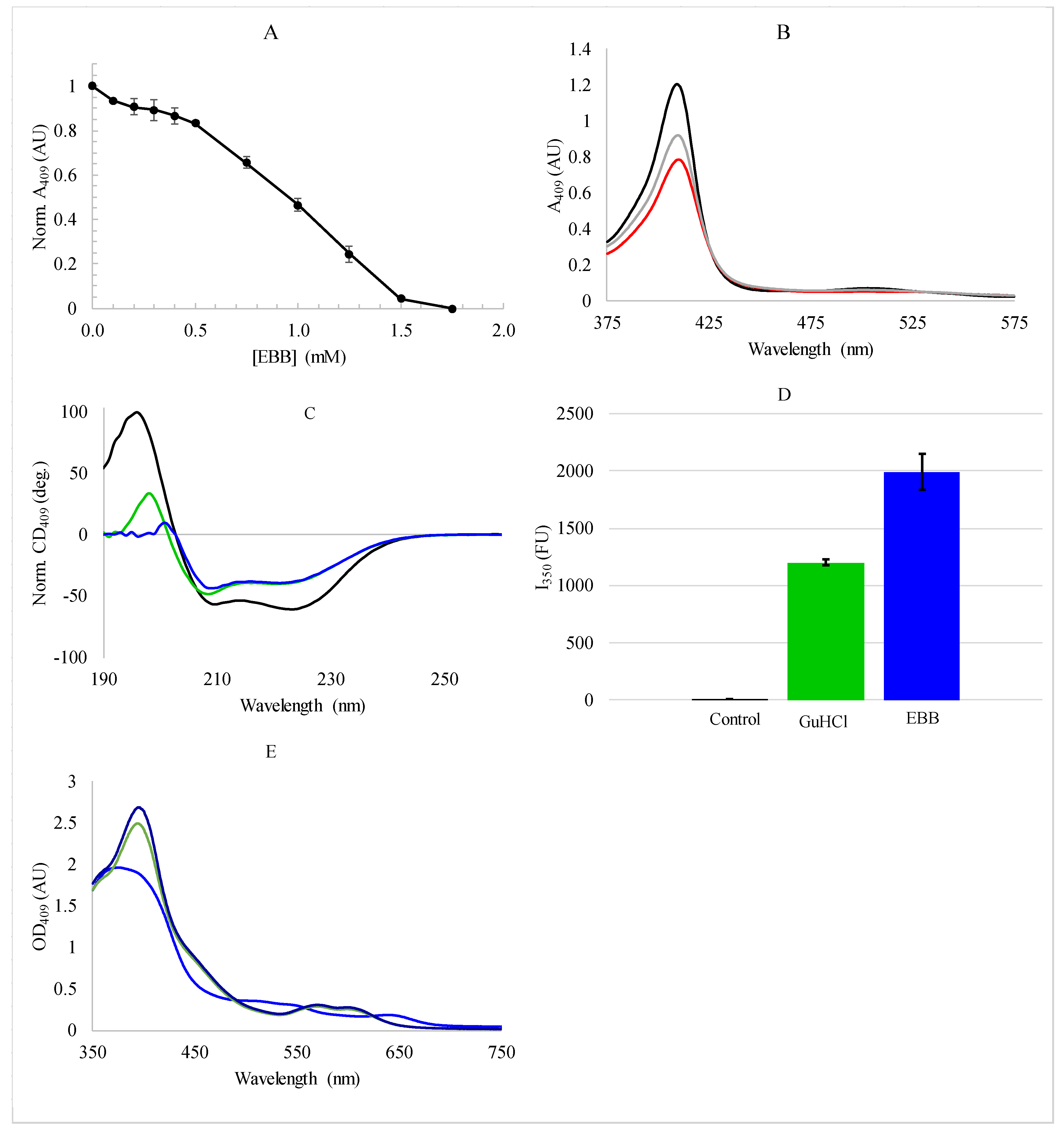
3.1. Protein Denaturation and Heme Dissociation by Empigen BB
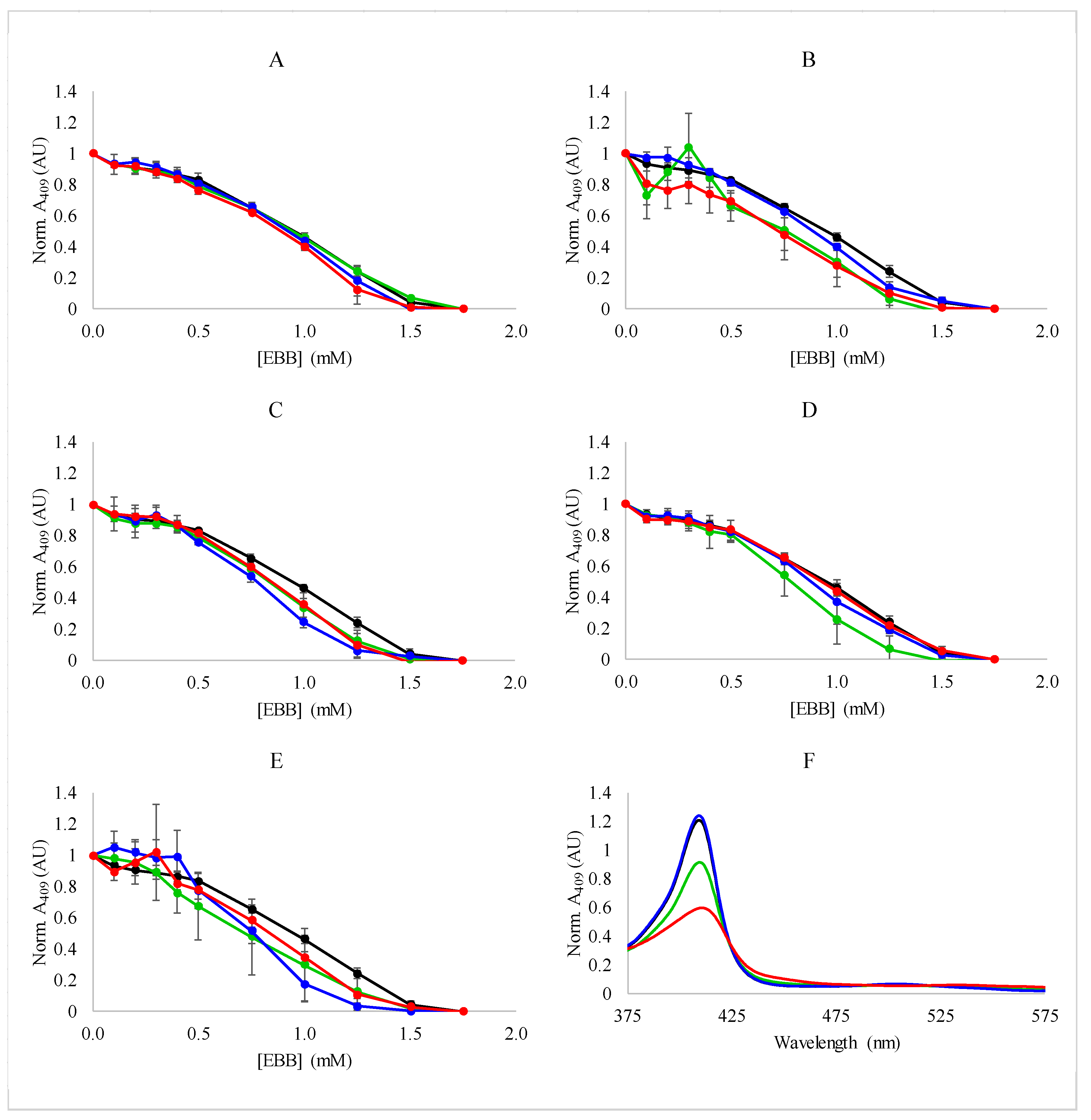
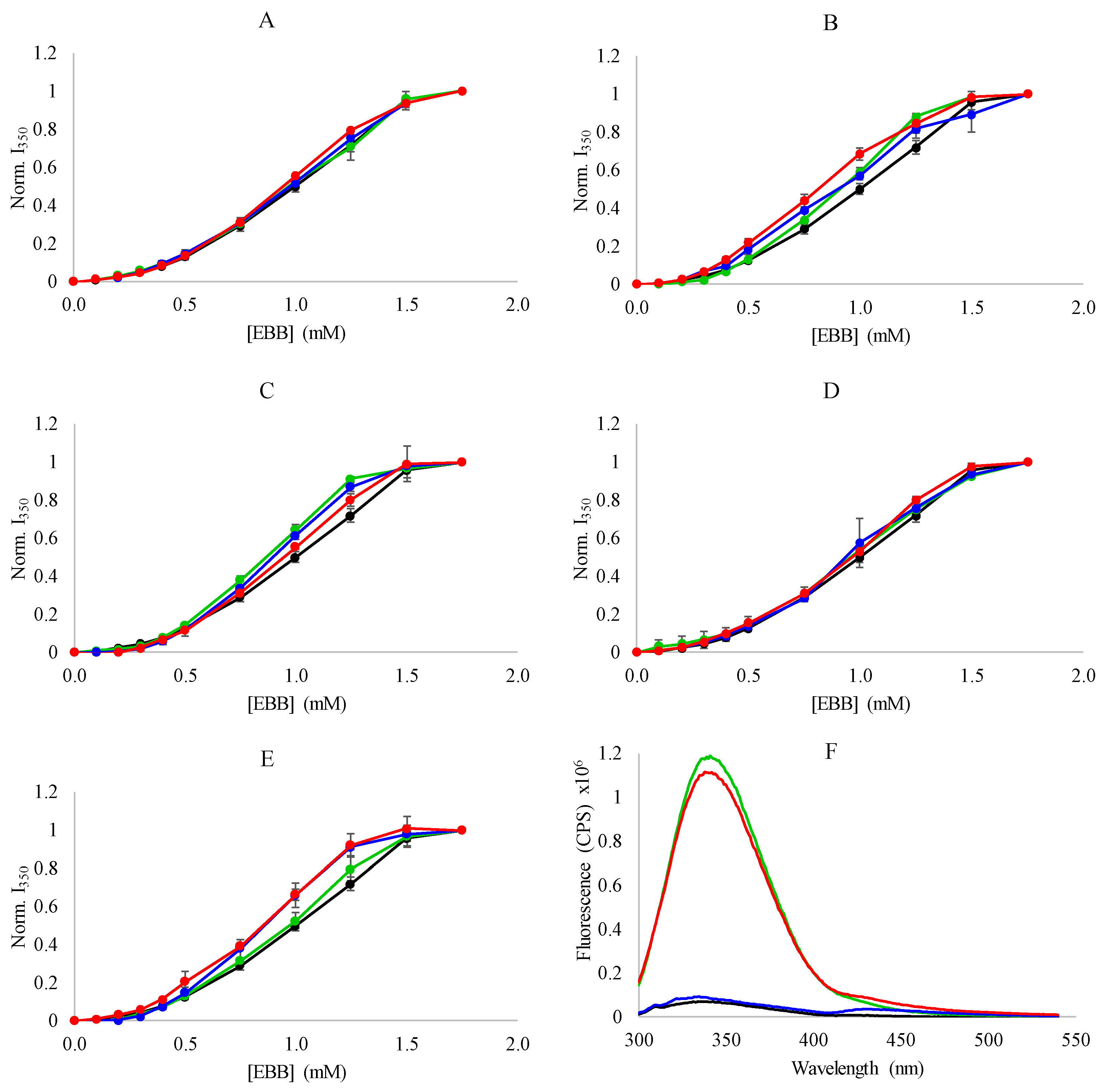
3.2. Spectroscopic Analysis of Heme Dissociation from Myoglobin with Ionic Liquids
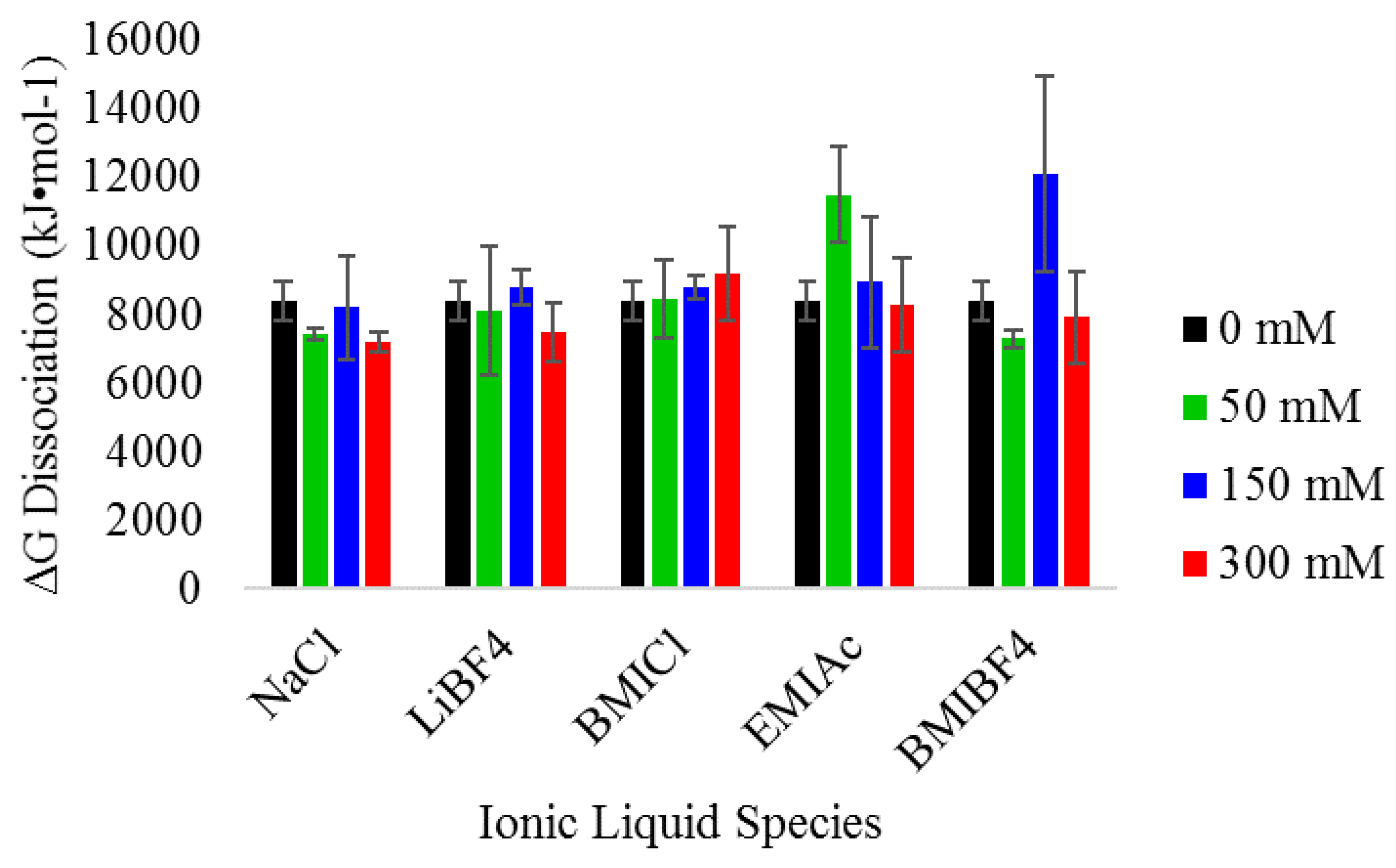
3.3. Thermodynamic Analysis of Heme Dissociation from Myoglobin
3.4. Critical Micelle Concentration Determination
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Woolhead, C.A.; McCormick, P.J.; Johnson, A.E. Nascent Membrane and Secretory Proteins Differ in FRET-Detected Folding Far inside the Ribosome and in Their Exposure to Ribosomal Proteins. Cell 2004, 116, 725–736. [Google Scholar] [CrossRef]
- Camilloni, C.; Guerini Rocco, A.; Eberini, I.; Gianazza, E.; Broglia, R.A.; Tiana, G. Urea and Guanidinium Chloride Denature Protein L in Different Ways in Molecular Dynamics Simulations. Biophys. J. 2008, 94, 4654–4661. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.K.; Rösgen, J.; Englander, S.W. Urea, but not guanidinium, destabilizes proteins by forming hydrogen bonds to the peptide group. Proc. Natl. Acad. Sci. USA 2009, 106, 2595–2600. [Google Scholar] [CrossRef] [PubMed]
- Monera, O.D.; Kay, C.M.; Hodges, R.S. Protein denaturation with guanidine hydrochloride or urea provides a different estimate of stability depending on the contributions of electrostatic interactions. Protein Sci. 1994, 3, 1984–1991. [Google Scholar] [CrossRef] [PubMed]
- Andersen, K.K.; Westh, P.; Otzen, D.E. Global Study of Myoglobin−Surfactant Interactions. Langmuir 2008, 24, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Bhuyan, A.K. On the mechanism of SDS-induced protein denaturation. Biopolymers 2010, 93, 186–199. [Google Scholar] [CrossRef] [PubMed]
- Otzen, D.E. Protein Unfolding in Detergents: Effect of Micelle Structure, Ionic Strength, pH, and Temperature. Biophys. J. 2002, 83, 2219–2230. [Google Scholar] [CrossRef]
- Almarza, J.; Rincon, L.; Bahsas, A.; Brito, F. Molecular Mechanism for the Denaturation of Proteins by Urea. Biochemistry 2009, 48, 7608–7613. [Google Scholar] [CrossRef] [PubMed]
- Jha, S.K.; Marqusee, S. Kinetic evidence for a two-stage mechanism of protein denaturation by guanidinium chloride. Proc. Natl. Acad. Sci. USA 2014, 111, 4856–4861. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Borrell, K.L.; Cancglin, C.; Stinger, B.L.; DeFrates, K.G.; Caputo, G.A.; Wu, C.; Vaden, T.D. An Experimental and Molecular Dynamics Study of Red Fluorescent Protein mCherry in Novel Aqueous Amino Acid Ionic Liquids. J. Phys. Chem. B 2017, 121, 4823–4832. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Tang, S.K.Y.; Mace, C.R.; Whitesides, G.M. Denaturation of Proteins by SDS and Tetraalkylammonium Dodecyl Sulfates. Langmuir 2011, 27, 11560–11574. [Google Scholar] [CrossRef] [PubMed]
- Rath, A.; Glibowicka, M.; Nadeau, V.G.; Chen, G.; Deber, C.M. Detergent binding explains anomalous SDS-PAGE migration of membrane proteins. Proc. Natl. Acad. Sci. USA 2009, 106, 1760–1765. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, J.A.; Tanford, C. The gross conformation of protein-sodium dodecyl sulfate complexes. J. Biol. Chem. 1970, 245, 5161–5165. [Google Scholar] [PubMed]
- Aube, A.; Campbell, S.; Schmitzer, A.R.; Claing, A.; Masson, J.F. Ultra-low fouling methylimidazolium modified surfaces for the detection of HER2 in breast cancer cell lysates. Analyst 2017, 142, 2343–2353. [Google Scholar] [CrossRef] [PubMed]
- Du, P.; Liu, S.; Wu, P.; Cai, C. Preparation and characterization of room temperature ionic liquid/single-walled carbon nanotube nanocomposites and their application to the direct electrochemistry of heme-containing proteins/enzymes. Electrochim. Acta 2007, 52, 6534–6547. [Google Scholar] [CrossRef]
- Liu, G.; Zhong, R.; Hu, R.; Zhang, F. Applications of ionic liquids in biomedicine. Biophys. Rev. Lett. 2012, 7, 121–134. [Google Scholar] [CrossRef]
- Nordwald, E.M.; Armstrong, G.S.; Kaar, J.L. NMR-Guided Rational Engineering of an Ionic-Liquid-Tolerant Lipase. ACS Catal. 2014, 4, 4057–4064. [Google Scholar] [CrossRef]
- Nordwald, E.M.; Kaar, J.L. Mediating Electrostatic Binding of 1-Butyl-3-methylimidazolium Chloride to Enzyme Surfaces Improves Conformational Stability. J. Phys. Chem. B 2013, 117, 8977–8986. [Google Scholar] [CrossRef] [PubMed]
- Riduan, S.N.; Zhang, Y. Imidazolium salts and their polymeric materials for biological applications. Chem. Soc. Rev. 2013, 42, 9055–9070. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Kang, T.S. Ionic Liquid Surfactant Mediated Structural Transitions and Self-Assembly of Bovine Serum Albumin in Aqueous Media: Effect of Functionalization of Ionic Liquid Surfactants. J. Phys. Chem. B 2015, 119, 10573–10585. [Google Scholar] [CrossRef] [PubMed]
- van Rantwijk, F.; Sheldon, R.A. Biocatalysis in Ionic Liquids. Chem. Rev. 2007, 107, 2757–2785. [Google Scholar] [CrossRef] [PubMed]
- Zakrewsky, M.; Lovejoy, K.S.; Kern, T.L.; Miller, T.E.; Le, V.; Nagy, A.; Goumas, A.M.; Iyer, R.S.; Del Sesto, R.E.; Koppisch, A.T.; et al. Ionic liquids as a class of materials for transdermal delivery and pathogen neutralization. Proc. Natl. Acad. Sci. USA 2014, 111, 13313–13318. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Venkatesu, P. A comparative study of myoglobin stability in the presence of Hofmeister anions of ionic liquids and ionic salts. Process Biochem. 2014, 49, 2158–2169. [Google Scholar] [CrossRef]
- Kumar, A.; Venkatesu, P. Does the stability of proteins in ionic liquids obey the Hofmeister series? Int. J. Biol. Macromol. 2014, 63, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Schröder, C. Proteins in Ionic Liquids: Current Status of Experiments and Simulations. Top. Curr. Chem. 2017, 375, 25. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z. Hofmeister effects: An explanation for the impact of ionic liquids on biocatalysis. J. Biotechnol. 2009, 144, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cremer, P.S. Interactions between macromolecules and ions: The Hofmeister series. Curr. Opin. Chem. Biol. 2006, 10, 658–663. [Google Scholar] [CrossRef] [PubMed]
- Erbeldinger, M.; Mesiano, A.J.; Russell, A.J. Enzymatic Catalysis of Formation of Z-Aspartame in Ionic Liquid−An Alternative to Enzymatic Catalysis in Organic Solvents. Biotechnol. Prog. 2000, 16, 1129–1131. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, V.W.; Pfaendtner, J. Destabilization of Human Serum Albumin by Ionic Liquids Studied Using Enhanced Molecular Dynamics Simulations. J. Phys. Chem. B 2016, 120, 12079–12087. [Google Scholar] [CrossRef] [PubMed]
- Maurya, J.K.; Mir, M.U.H.; Singh, U.K.; Maurya, N.; Dohare, N.; Patel, S.; Ali, A.; Patel, R. Molecular investigation of the interaction between ionic liquid type gemini surfactant and lysozyme: A spectroscopic and computational approach. Biopolymers 2015, 103, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, V.; Polavarapu, P.L. First Room Temperature Chiral Anionic Liquid Forming Micelles and Reverse Micelles. J. Phys. Chem. B 2017, 121, 1629–1639. [Google Scholar] [CrossRef] [PubMed]
- Satish, L.; Millan, S.; Sahoo, H. Spectroscopic insight into the interaction of bovine serum albumin with imidazolium-based ionic liquids in aqueous solution. Luminescence 2017, 32, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Shipovskov, S.; Gunaratne, H.Q.N.; Seddon, K.R.; Stephens, G. Catalytic activity of laccases in aqueous solutions of ionic liquids. Green Chem. 2008, 10, 806–810. [Google Scholar] [CrossRef]
- Singh, G.; Singh, G.; Kang, T.S. Effect of alkyl chain functionalization of ionic liquid surfactants on the complexation and self-assembling behavior of polyampholyte gelatin in aqueous medium. Phys. Chem. Chem. Phys. 2016, 18, 25993–26009. [Google Scholar] [CrossRef] [PubMed]
- Yee, P.; Shah, J.K.; Maginn, E.J. State of Hydrophobic and Hydrophilic Ionic Liquids in Aqueous Solutions: Are the Ions Fully Dissociated? J. Phys. Chem. B 2013, 117, 12556–12566. [Google Scholar] [CrossRef] [PubMed]
- Madeira Lau, R.; Van Rantwijk, F.; Seddon, K.R.; Sheldon, R.A. Lipase-Catalyzed Reactions in Ionic Liquids. Organ. Lett. 2000, 2, 4189–4191. [Google Scholar] [CrossRef]
- Hekmat, D.; Hebel, D.; Joswig, S.; Schmidt, M.; Weuster-Botz, D. Advanced protein crystallization using water-soluble ionic liquids as crystallization additives. Biotechnol. Lett. 2007, 29, 1703–1711. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, X.; Dan, Y.; Feng, J.; Ge, L.; Zhang, M. The crystallization of lysozyme in the system of ionic liquid [BMIm][BF4]-water. Cryst. Res. Technol. 2008, 43, 1062–1068. [Google Scholar] [CrossRef]
- Weingartner, H.; Cabrele, C.; Herrmann, C. How ionic liquids can help to stabilize native proteins. Phys. Chem. Chem. Phys. 2012, 14, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.; Liu, M.; Chen, S.; Chen, X.; Wang, J. New Insight into Molecular Interactions of Imidazolium Ionic Liquids with Bovine Serum Albumin. J. Phys. Chem. B 2011, 115, 12306–12314. [Google Scholar] [CrossRef] [PubMed]
- Fiebig, O.C.; Mancini, E.; Caputo, G.; Vaden, T.D. Quantitative evaluation of myoglobin unfolding in the presence of guanidinium hydrochloride and ionic liquids in solution. J. Phys. Chem. B 2014, 118, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.C.; Hanna, S.L.; DeFrates, K.G.; Fiebig, O.C.; Vaden, T.D. Kinetics and mass spectrometric measurements of myoglobin unfolding in aqueous ionic liquid solutions. Int. J. Biol. Macromol. 2016, 85, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Lowthert, L.A.; Ku, N.O.; Liao, J.; Coulombe, P.A.; Omary, M.B. Empigen BB: A Useful Detergent for Solubilization and Biochemical Analysis of Keratins. Biochem. Biophys. Res. Commun. 1995, 206, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Savva, C.G.; Dewey, J.S.; Deaton, J.; White, R.L.; Struck, D.K.; Holzenburg, A.; Young, R. The holin of bacteriophage lambda forms rings with large diameter. Mol. Microbiol. 2008, 69, 784–793. [Google Scholar] [CrossRef] [PubMed]
- Kraus, D. Consolidated data analysis and presentation using an open-source add-in for the Microsoft Excel spreadsheet software. Med. Writ. 2014, 23, 25–28. [Google Scholar] [CrossRef]
- Zhang, C.; Yu, A.; Lu, R. Cationic effect of imidazolium-based ionic liquid on the stability of myoglobin. Process Biochem. 2017, 58, 181–185. [Google Scholar] [CrossRef]
- Moriyama, Y.; Takeda, K. Critical Temperature of Secondary Structural Change of Myoglobin in Thermal Denaturation up to 130 °C and Effect of Sodium Dodecyl Sulfate on the Change. J. Phys. Chem. B 2010, 114, 2430–2434. [Google Scholar] [CrossRef] [PubMed]
- Gryczynski, Z.; Lubkowski, J.; Bucci, E. Heme-Protein Interactions in Horse Heart Myoglobin at Neutral pH and Exposed to Acid Investigated by Time-resolved Fluorescence in the Pico- to Nanosecond Time Range. J. Biol. Chem. 1995, 270, 19232–19237. [Google Scholar] [CrossRef] [PubMed]
- Vivian, J.T.; Callis, P.R. Mechanisms of Tryptophan Fluorescence Shifts in Proteins. Biophys. J. 2001, 80, 2093–2109. [Google Scholar] [CrossRef]
- Beyaz, A.; Oh, W.S.; Reddy, V.P. Ionic liquids as modulators of the critical micelle concentration of sodium dodecyl sulfate. Colloids Surf. B Biointerfaces 2004, 35, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, A.; London, E. Fluorimetric determination of critical micelle concentration avoiding interference from detergent charge. Anal. Biochem. 1984, 139, 408–412. [Google Scholar] [CrossRef]
- Dutkiewicz, E.; Jakubowska, A. Effect of electrolytes on the physicochemical behaviour of sodium dodecyl sulphate micelles. Colloid Polym. Sci. 2002, 280, 1009–1014. [Google Scholar] [CrossRef]
- Tang, Q.; Kalsbeck, W.A.; Bocian, D.F. Acid-induced transformations of myoglobin. II. Effect of ionic strength on the free energy and formation rate of the 426-nm absorbing deoxyheme intermediate. Biospectroscopy 1997, 3, 17–29. [Google Scholar] [CrossRef]
- Stigter, D.; Alonso, D.O.; Dill, K.A. Protein stability: Electrostatics and compact denatured states. Proc. Natl. Acad. Sci. USA 1991, 88, 4176–4180. [Google Scholar] [CrossRef] [PubMed]
- Barrick, D.; Baldwin, R.L. The molten globule intermediate of apomyoglobin and the process of protein folding. Protein Sci. 1993, 2, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Moosavi-Movahedi, A.A.; Chamani, J.; Goto, Y.; Hakimelahi, G.H. Formation of the Molten Globule-Like State of Cytochrome C Induced by n-Alkyl Sulfates at Low Concentrations. J. Biochem. 2003, 133, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Naeem, A.; Khan, R.H. Characterization of molten globule state of cytochrome C at alkaline, native and acidic pH induced by butanol and SDS. Int. J. Biochem. Cell Biol. 2004, 36, 2281–2292. [Google Scholar] [CrossRef] [PubMed]
- Blauer, G.; Harmatz, D.; Zvilichovsky, B. Effect of cationic detergents on ferrimyoglobin. FEBS Lett. 1973, 34, 344–346. [Google Scholar] [CrossRef]
- Dohnal, J.C.; Garvin, J.E. The interaction of dodecyl and tetradecyl sulfate with proteins during polyacrylamide gel electrophoresis. Biochim. Biophys. Acta (BBA) Protein Struct. 1979, 576, 393–403. [Google Scholar] [CrossRef]
- Wasylewski, Z. Protein-cationic detergent interaction. Fourier transform infrared and laser Raman spectroscopic studies on the interaction between proteins and dodecylpyridinium bromide. Acta Biochim. Pol. 1979, 26, 205–214. [Google Scholar] [PubMed]
- Andersen, K.K.; Otzen, D.E.; Andersen, K.K.; Otzen, D.E. Denaturation of α-lactalbumin and myoglobin by the anionic biosurfactant rhamnolipid. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2014, 1844, 2338–2345. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.H.; Petersen, S.V.; Andersen, K.K.; Enghild, J.J.; Damhus, T.; Otzen, D. Stable intermediates determine proteins’ primary unfolding sites in the presence of surfactants. Biopolymers 2009, 91, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, H.G.; Madsen, J.K.; Andersen, K.K.; Vosegaard, T.; Deen, G.R.; Otzen, D.E.; Pedersen, J.S. Myoglobin and α-Lactalbumin Form Smaller Complexes with the Biosurfactant Rhamnolipid than with SDS. Biophys. J. 2017, 113, 2621–2633. [Google Scholar] [CrossRef] [PubMed]
- Ey, P.L.; Ferber, E. Calf thymus alkaline phosphatase. II. Interaction with detergents. Biochim. Biophys. Acta (BBA) Enzymol. 1977, 480, 163–177. [Google Scholar] [CrossRef]
- Jennings, R.; Quasim, T.; Sharrard, R.M.; Hockley, D.; Potter, C.W. Zwitterionic detergent solubilisation of HSV-1 surface antigens. Arch. Virol. 1988, 98, 137–153. [Google Scholar] [CrossRef] [PubMed]
- Sreerama, N.; Woody, R.W. Estimation of Protein Secondary Structure from Circular Dichroism Spectra: Comparison of CONTIN, SELCON, and CDSSTR Methods with an Expanded Reference Set. Anal. Biochem. 2000, 287, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Constantinescu, D.; Weingärtner, H.; Herrmann, C. Protein Denaturation by Ionic Liquids and the Hofmeister Series: A Case Study of Aqueous Solutions of Ribonuclease A. Angew. Chem. Int. Ed. 2007, 46, 8887–8889. [Google Scholar] [CrossRef] [PubMed]
- Scholtz, J.M.; York, E.J.; Stewart, J.M.; Baldwin, R.L. A neutral, water-soluble, alpha-helical peptide: The effect of ionic strength on the helix-coil equilibrium. J. Am. Chem. Soc. 1991, 113, 5102–5104. [Google Scholar] [CrossRef]
- Baker, G.A.; Pandey, S.; Pandey, S.; Baker, S.N. A new class of cationic surfactants inspired by N-alkyl-N-methyl pyrrolidinium ionic liquids. Analyst 2004, 129, 890–892. [Google Scholar] [CrossRef] [PubMed]
- Mester, P.; Wagner, M.; Rossmanith, P. Ionic liquids designed as chaotrope and surfactant for use in protein chemistry. Sep. Purif. Technol. 2012, 97, 211–215. [Google Scholar] [CrossRef]
- Smirnova, N.A.; Safonova, E.A. Ionic liquids as surfactants. Russ. J. Phys. Chem. A 2010, 84, 1695–1704. [Google Scholar] [CrossRef]
- Lange, C.; Patil, G.; Rudolph, R. Ionic liquids as refolding additives: N′-alkyl and N′-(ω-hydroxyalkyl) N-methylimidazolium chlorides. Protein Sci. 2005, 14, 2693–2701. [Google Scholar] [CrossRef] [PubMed]
- Summers, C.A.; Flowers, R.A.; Summers, C.A.; Flowers, R.A. Protein renaturation by the liquid organic salt ethylammonium nitrate. Protein Sci. 2000, 9, 2001–2008. [Google Scholar] [CrossRef] [PubMed]
| Lower Linear Intersection (mM) | Inflection Point (mM) | Upper Linear Intersection (mM) | |
|---|---|---|---|
| NaCl | |||
| 0 mM | 0.38 ± 0.10 | 1.48 ± 0.63 | 1.76 ± 0.82 |
| 50 mM | 0.39 ± 0.16 | 1.34 ± 0.57 | 1.51 ± 0.21 |
| 100 mM | 0.42 ± 0.12 | 1.26 ± 0.73 | 1.94 ± 0.23 |
| 150 mM | 0.32 ± 0.08 | 1.15 ± 0.46 | 1.89 ± 0.25 |
| 300 mM | 0.28 ± 0.08 | 0.95 ± 0.17 | 2.22 ± 0.12 |
| LiBF4 | |||
| 0 mM | 0.46 ± 0.03 | 2.61 ± 0.58 | 2.42 ± 0.36 |
| 50 mM | 0.35 ± 0.10 | 2.24 ± 0.54 | 2.52 ± 0.38 |
| 100 mM | 0.51 ± 0.14 | 1.94 ± 0.12 | 3.14 ± 0.66 |
| 150 mM | 0.50 ± 0.17 | 1.80 ± 0.38 | 2.91 ± 0.96 |
| 300 mM | 0.29 ± 0.06 | 1.80 ± 0.99 | 2.22 ± 0.34 |
| BMIBF4 | |||
| 0 mM | 0.42 ± 0.04 | 2.19 ± 0.63 | 3.05 ± 0.91 |
| 50 mM | 0.36 ± 0.92 | 1.91 ± 0.74 | 3.06 ± 0.24 |
| 100 mM | 0.25 ± 0.34 | 1.51 ± 0.16 | 2.86 ± 0.57 |
| 150 mM | 0.31 ± 0.06 | 1.46 ± 0.32 | 2.06 ± 0.83 |
| 300 mM | 0.31 ± 0.06 | 1.92 ± 0.54 | 2.24 ± 0.65 |
| EMIAc | |||
| 0 mM | 0.37 ± 0.05 | 1.57 ± 0.46 | 2.93 ± 0.23 |
| 50 mM | 0.37 ± 0.05 | 2.08 ± 0.67 | 2.65 ± 0.43 |
| 100 mM | 0.42 ± 0.11 | 1.94 ± 0.46 | 2.47 ± 0.73 |
| 150 mM | 0.45 ± 0.10 | 2.12 ± 0.92 | 2.82 ± 0.56 |
| 300 mM | 0.51 ± 0.07 | 1.67 ± 0.26 | 2.34 ± 0.46 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kohn, E.M.; Lee, J.Y.; Calabro, A.; Vaden, T.D.; Caputo, G.A. Heme Dissociation from Myoglobin in the Presence of the Zwitterionic Detergent N,N-Dimethyl-N-Dodecylglycine Betaine: Effects of Ionic Liquids. Biomolecules 2018, 8, 126. https://doi.org/10.3390/biom8040126
Kohn EM, Lee JY, Calabro A, Vaden TD, Caputo GA. Heme Dissociation from Myoglobin in the Presence of the Zwitterionic Detergent N,N-Dimethyl-N-Dodecylglycine Betaine: Effects of Ionic Liquids. Biomolecules. 2018; 8(4):126. https://doi.org/10.3390/biom8040126
Chicago/Turabian StyleKohn, Eric M., Joshua Y. Lee, Anthony Calabro, Timothy D. Vaden, and Gregory A. Caputo. 2018. "Heme Dissociation from Myoglobin in the Presence of the Zwitterionic Detergent N,N-Dimethyl-N-Dodecylglycine Betaine: Effects of Ionic Liquids" Biomolecules 8, no. 4: 126. https://doi.org/10.3390/biom8040126
APA StyleKohn, E. M., Lee, J. Y., Calabro, A., Vaden, T. D., & Caputo, G. A. (2018). Heme Dissociation from Myoglobin in the Presence of the Zwitterionic Detergent N,N-Dimethyl-N-Dodecylglycine Betaine: Effects of Ionic Liquids. Biomolecules, 8(4), 126. https://doi.org/10.3390/biom8040126






