Ceramide Synthase 6: Comparative Analysis, Phylogeny and Evolution
Abstract
1. Introduction
2. Results and Discussion
2.1. Comparison of Vertebrate CerS6 Amino Acid Sequences and their Relation to Human CERS1–5 Sequences
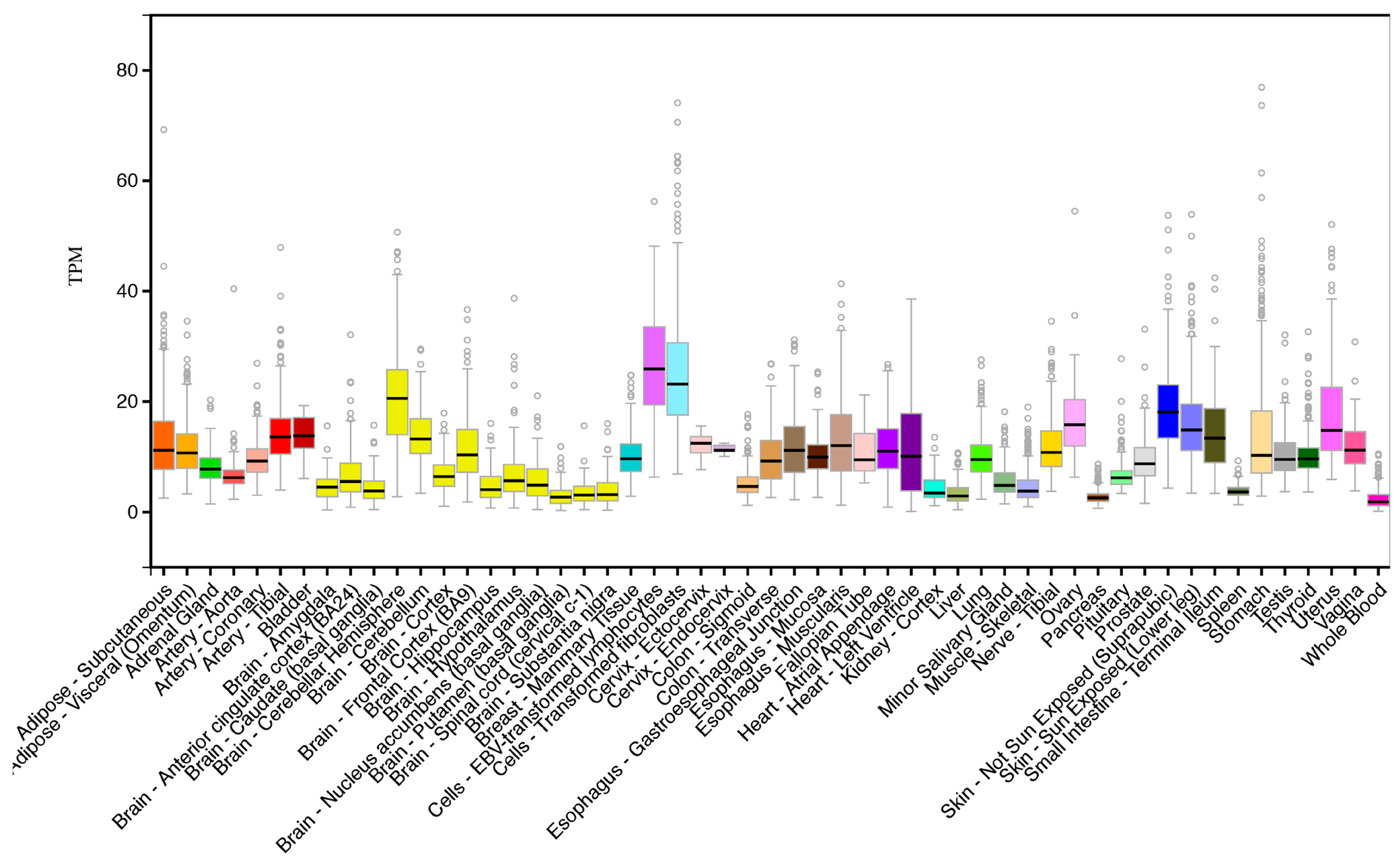
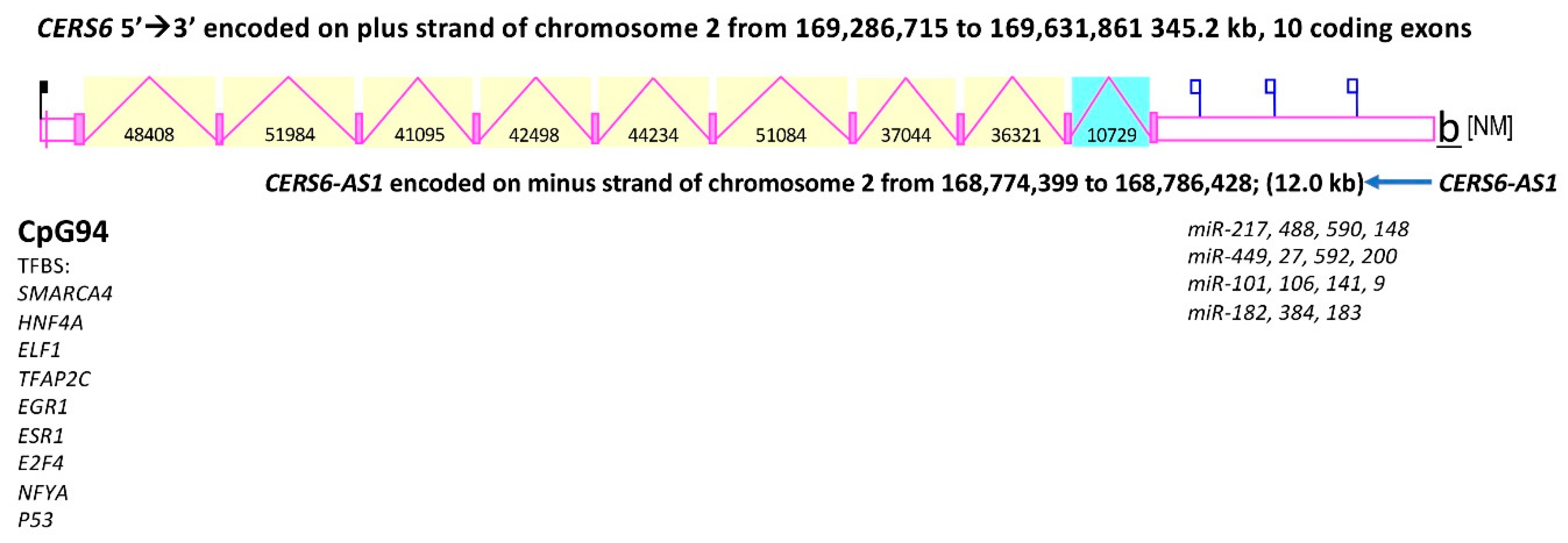
2.2. CERS6 Gene Expression
2.3. Comparative Analysis of Vertebrate CerS6 Genes and Human CERS1–5 Genes
2.4. Evolution of Vertebrate CerS1–6 Genes from the Invertebrate Lagr1 and Hyl1 Genes
3. Methods
3.1. CerS Gene and Protein Identification
3.2. Predicted Structures and Properties of Mammalian CerS6 Enzymes
3.3. Comparative Human Tissue CERS6 Gene Expression
3.4. Phylogeny Studies and Sequence Alignments
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Venkataraman, K.; Riebeling, C.; Bodennec, J.; Riezman, H.; Allegood, J.C.; Sullards, M.C.; Merrill, A.H., Jr.; Futerman, A.H. Upstream of growth and differentiation factor 1 (uog1), a mammalian homolog of the yeast longevity assurance gene 1 (LAG1), regulates N-stearoyl-sphinganine (C18-(dihydro)ceramide) synthesis in a fumonisin B1-independent manner in mammalian cells. J. Biol. Chem. 2002, 277, 35642–35649. [Google Scholar] [CrossRef] [PubMed]
- D’Mello, N.P.; Childress, A.M.; Franklin, D.S.; Kale, S.P.; Pinswasdi, C.; Jazwinski, S.M. Cloning and characterization of LAG1, a longevity-assurance gene in yeast. J. Biol. Chem. 1994, 269, 15451–15459. [Google Scholar] [PubMed]
- Guillas, I.; Kirchman, P.A.; Chuard, R.; Pfefferli, M.; Jiang, J.C.; Jazwinski, S.M.; Conzelmann, A. C26-CoA-dependent ceramide synthesis of Saccharomyces cerevisiae is operated by Lag1p and Lac1p. EMBO J. 2001, 20, 2655–2665. [Google Scholar] [CrossRef] [PubMed]
- Pewzner-Jung, Y.; Ben-Dor, S.; Futerman, A.H. When do Lasses (longevity assurance genes) become CerS (ceramide synthases)?: Insights into the regulation of ceramide synthesis. J. Biol. Chem. 2006, 281, 25001–25005. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.; Futerman, A.H. Mammalian ceramide synthases. IUBMB Life 2010, 62, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Schiffmann, S.; Ferreiros, N.; Birod, K.; Eberle, M.; Schreiber, Y.; Pfeilschifter, W.; Ziemann, U.; Pierre, S.; Scholich, K.; Grösch, S.; et al. Ceramide synthase 6 plays a critical role in the development of experimental autoimmune encephalomyelitis. J. Immunol. 2012, 188, 5723–5733. [Google Scholar] [CrossRef] [PubMed]
- Castro, B.M.; Prieto, M.; Silva, L.C. Ceramide: A simple sphingolipid with unique biophysical properties. Prog. Lipid Res. 2014, 54, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Hannun, Y.A.; Obeid, L.M. Many ceramides. J. Biol. Chem. 2011, 286, 27855–27862. [Google Scholar] [CrossRef] [PubMed]
- Ginkel, C.; Hartmann, D.; vom Dorp, K.; Zlomuzica, A.; Farwanah, H.; Eckhardt, M.; Sandhoff, R.; Degen, J.; Rabionet, M.; Dere, E.; et al. Ablation of neuronal ceramide synthase 1 in mice decreases ganglioside levels and expression of myelin-associated glycoprotein in oligodendrocytes. J. Biol. Chem. 2012, 287, 41888–41902. [Google Scholar] [CrossRef] [PubMed]
- Zigdon, H.; Kogot-Levin, A.; Park, J.W.; Goldschmidt, R.; Kelly, S.; Merrill, A.H., Jr.; Scherz, A.; Pewzner-Jung, Y.; Saada, A.; Futerman, A.H. Ablation of ceramide synthase 2 causes chronic oxidative stress due to disruption of the mitochondrial respiratory chain. J. Biol. Chem. 2013, 288, 4947–4956. [Google Scholar] [CrossRef] [PubMed]
- Park, W.J.; Brenner, O.; Kogot-Levin, A.; Saada, A.; Merrill, A.H., Jr.; Pewzner-Jung, Y.; Futerman, A.H. Development of pheochromocytoma in ceramide synthase 2 null mice. Endocr. Relat. Cancer 2015, 22, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, Y.; Kihara, A.; Igarashi, Y. LASS3 (longevity assurance homologue 3) is a mainly testis-specific (dihydro)ceramide synthase with relatively broad substrate specificity. Biochem. J. 2006, 398, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Jennemann, R.; Rabionet, M.; Gorgas, K.; Epstein, S.; Dalpke, A.; Rothermel, U.; Bayerle, A.; van der Hoeven, F.; Imgrund, S.; Kirsch, J.; et al. Loss of ceramide synthase 3 causes lethal skin barrier disruption. Hum. Mol. Genet. 2012, 21, 586–608. [Google Scholar] [CrossRef] [PubMed]
- Veret, J.; Coant, N.; Berdyshev, E.V.; Skobeleva, A.; Therville, N.; Bailbe, D.; Gorshkova, I.; Natarajan, V.; Portha, B.; Le Stunff, H. Ceramide synthase 4 and de novo production of ceramides with specific N-acyl chain lengths are involved in glucolipotoxicity-induced apoptosis of INS-1 β-cells. Biochem. J. 2011, 438, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Peters, F.; Vorhagen, S.; Brodesser, S.; Jakobshagen, K.; Brüning, J.C.; Niessen, C.M.; Krönke, M. Ceramide synthase 4 regulates stem cell homeostasis and hair follicle cycling. J. Investig. Dermatol. 2015, 135, 1501–1509. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, S.; Futerman, A.H. LASS5 is a bona fide dihydroceramide synthase that selectively utilizes palmitoyl-CoA as acyl donor. J. Biol. Chem. 2005, 280, 33735–33738. [Google Scholar] [CrossRef] [PubMed]
- Gosejacob, D.; Jäger, P.S.; Vom Dorp, K.; Frejno, M.; Carstensen, A.C.; Köhnke, M.; Degen, J.; Dörmann, P.; Hoch, M. Ceramide Synthase 5 Is Essential to Maintain C16:0-Ceramide Pools and Contributes to the Development of Diet-induced Obesity. J. Biol. Chem. 2016, 291, 6989–7003. [Google Scholar] [CrossRef] [PubMed]
- Weinmann, A.; Galle, P.R.; Teufel, A. LASS6, an additional member of the longevity assurance gene family. Int. J. Mol. Med. 2005, 16, 905–910. [Google Scholar] [CrossRef] [PubMed]
- Ebel, P.; Vom Dorp, K.; Petrasch-Parwez, E.; Zlomuzica, A.; Kinugawa, K.; Mariani, J.; Minich, D.; Ginkel, C.; Welcker, J.; Degen, J.; et al. Inactivation of ceramide synthase 6 in mice results in an altered sphingolipid metabolism and behavioral abnormalities. J. Biol. Chem. 2013, 288, 21433–21447. [Google Scholar] [CrossRef] [PubMed]
- Turpin, S.M.; Nicholls, H.T.; Willmes, D.M.; Mourier, A.; Brodesser, S.; Wunderlich, C.M.; Mauer, J.; Xu, E.; Hammerschmidt, P.; Brönneke, H.S.; et al. Obesity-induced CerS6-dependent C16:0 ceramide production promotes weight gain and glucose intolerance. Cell Metab. 2014, 20, 678–686. [Google Scholar] [CrossRef] [PubMed]
- Scheffel, M.J.; Helke, K.; Lu, P.; Bowers, J.S.; Ogretmen, B.; Garrett-Mayer, E.; Paulos, C.M.; Voelkel-Johnson, C. Adoptive Transfer of Ceramide Synthase 6 Deficient Splenocytes Reduces the Development of Colitis. Sci. Rep. 2017, 7, 15552. [Google Scholar] [CrossRef] [PubMed]
- Helke, K.; Angel, P.; Lu, P.; Garrett-Mayer, E.; Ogretmen, B.; Drake, R.; Voelkel-Johnson, C. Ceramide Synthase 6 Deficiency Enhances Inflammation in the DSS model of Colitis. Sci. Rep. 2018, 8, 1627. [Google Scholar] [CrossRef] [PubMed]
- Eberle, M.; Ebel, P.; Mayer, C.A.; Barthelmes, J.; Tafferner, N.; Ferreiros, N.; Ulshöfer, T.; Henke, M.; Foerch, C.; Männer de Bazo, A.; et al. Exacerbation of experimental autoimmune encephalomyelitis in ceramide synthase 6 knockout mice is associated with enhanced activation/migration of neutrophils. Immunol. Cell Biol. 2015, 93, 825–836. [Google Scholar] [CrossRef] [PubMed]
- Sofi, M.H.; Heinrichs, J.; Dany, M.; Nguyen, H.; Dai, M.; Bastian, D.; Schutt, S.; Wu, Y.; Daenthanasanmak, A.; Gencer, S.; et al. Ceramide synthesis regulates T cell activity and GVHD development. JCI Insight 2017, 2, 91701. [Google Scholar] [CrossRef] [PubMed]
- Hoeferlin, L.A.; Fekry, B.; Ogretmen, B.; Krupenko, S.A.; Krupenko, N.I. (2013) Folate stress induces apoptosis via p53-dependent de novo ceramide synthesis and up-regulation of ceramide synthase 6. J. Biol. Chem. 2013, 288, 12880–12890. [Google Scholar] [CrossRef] [PubMed]
- Fekry, B.; Jeffries, K.A.; Esmaeilniakooshkghazi, A.; Szulc, Z.M.; Knagge, K.J.; Kirchner, D.R.; Horita, D.A.; Krupenko, S.A.; Krupenko, N.I. C16-ceramide is a natural regulatory ligand of p53 in cellular stress response. Nat. Commun 2018, in press. [Google Scholar] [CrossRef]
- Schüll, S.; Günther, S.D.; Brodesser, S.; Seeger, J.M.; Tosetti, B.; Wiegmann, K.; Pongratz, C.; Diaz, F.; Witt, A.; Andree, M.; et al. Cytochrome c oxidase deficiency accelerates mitochondrial apoptosis by activating ceramide synthase 6. Cell Death Dis. 2015, 6, e1691. [Google Scholar] [CrossRef] [PubMed]
- Fekry, B.; Esmaeilniakooshkghazi, A.; Krupenko, S.A.; Krupenko, N.I. Ceramide Synthase 6 Is a Novel Target of Methotrexate Mediating Its Antiproliferative Effect in a p53-Dependent Manner. PLoS ONE 2016, 11, e0146618. [Google Scholar] [CrossRef] [PubMed]
- Tidhar, R.; Ben-Dor, S.; Wang, E.; Kelly, S.; Merrill, A.H., Jr.; Futerman, A.H. Acyl chain specificity of ceramide synthases is determined within a region of 150 residues in the Tram-Lag-CLN8 (TLC) domain. J. Biol. Chem. 2012, 287, 3197–3206. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, Y.; Kihara, A.; Igarashi, Y. Mammalian Lass6 and its related family members regulate synthesis of specific ceramides. Biochem. J. 2005, 390, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Krogh, A.; Larsson, B.; von Heijne, G.; Sonnhammer, E.L. Predicting transmembrane protein topology with a hidden Markov model: Application to complete genomes. J. Mol. Biol. 2001, 305, 567–580. [Google Scholar] [CrossRef] [PubMed]
- Marchler-Bauer, A.; Lu, S.; Anderson, J.B.; Chitsaz, F.; Derbyshire, M.K.; DeWeese-Scott, C.; Fong, J.H.; Geer, L.Y.; Geer, R.C.; Gonzales, N.R.; et al. CDD: A Conserved Domain Database for the functional annotation of proteins. Nucleic Acids Res. 2011, 39, D225–D229. [Google Scholar] [CrossRef] [PubMed]
- Mullen, T.D.; Hannun, Y.A.; Obeid, L.M. Ceramide synthases at the centre of sphingolipid metabolism and biology. Biochem. J. 2012, 441, 789–802. [Google Scholar] [CrossRef] [PubMed]
- Kageyama-Yahara, N.; Riezman, H. Transmembrane topology of ceramide synthase in yeast. Biochem. J. 2006, 398, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Spassieva, S.; Seo, J.G.; Jiang, J.C.; Bielawski, J.; Alvarez-Vasquez, F.; Jazwinski, S.M.; Hannun, Y.A.; Obeid, L.M. Necessary role for the Lag1p motif in (dihydro)ceramide synthase activity. J. Biol. Chem. 2006, 281, 33931–33938. [Google Scholar] [CrossRef] [PubMed]
- Mesika, A.; Ben-Dor, S.; Laviad, E.L.; Futerman, A.H. A new functional motif in Hox domain-containing ceramide synthases: Identification of a novel region flanking the Hox and TLC domains essential for activity. J. Biol. Chem. 2007, 282, 27366–27373. [Google Scholar] [CrossRef] [PubMed]
- Sassa, T.; Hirayama, T.; Kihara, A. Enzyme Activities of the Ceramide Synthases CERS2-6 Are Regulated by Phosphorylation in the C-terminal Region. J. Biol. Chem. 2016, 291, 7477–7487. [Google Scholar] [CrossRef] [PubMed]
- Muir, A.; Ramachandran, S.; Roelants, F.M.; Timmons, G.; Thorner, J. TORC2-dependent protein kinase Ypk1 phosphorylates ceramide synthase to stimulate synthesis of complex sphingolipids. eLife 2014, 3, e03779. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Corbacho, M.J.; Canals, D.; Adada, M.M.; Liu, M.; Senkal, C.E.; Yi, J.K.; Mao, C.; Luberto, C.; Hannun, Y.A.; Obeid, L.M. Tumor Necrosis Factor-α (TNFα)-induced Ceramide Generation via Ceramide Synthases Regulates Loss of Focal Adhesion Kinase (FAK) and Programmed Cell Death. J. Biol. Chem. 2015, 290, 25356–25373. [Google Scholar] [CrossRef] [PubMed]
- Thierry-Mieg, D.; Thierry-Mieg, J. AceView: A comprehensive cDNA-supported gene and transcripts annotation. Genome Biol. 2006, 7, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Sun, M.; Kent, W.J.; Huang, X.; Xie, H.; Wang, W.; Zhou, G.; Shi, R.Z.; Rowley, J.D. Over 20% of human transcripts might form sense-antisense pairs. Nucleic Acids Res. 2004, 32, 4812–4820. [Google Scholar] [CrossRef] [PubMed]
- Engström, P.G.; Suzuki, H.; Ninomiya, N.; Akalin, A.; Sessa, L.; Lavorgna, G.; Brozzi, A.; Luzi, L.; Tan, S.L.; Yang, L.; et al. Complex Loci in human and mouse genomes. PLoS Genet. 2006, 2, e47. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, S.; Henriksson, S.; Corcoran, M.; Mendez-Vidal, C.; Wiman, K.G.; Farnebo, M. Wrap53, a natural p53 antisense transcript required for p53 induction upon DNA damage. Mol. Cell 2009, 33, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Fekry, B.; Jeffries, K.A.; Esmaeilniakooshkghazi, A.; Ogretmen, B.; Krupenko, S.A.; Krupenko, N.I. CerS6 Is a Novel Transcriptional Target of p53 Protein Activated by Non-genotoxic Stress. J. Biol. Chem. 2016, 291, 16586–16596. [Google Scholar] [CrossRef] [PubMed]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef] [PubMed]
- Separovic, D.; Breen, P.; Joseph, N.; Bielawski, J.; Pierce, J.S.; VAN Burren, E.; Gudz, T.I. Ceramide synthase 6 knockdown suppresses apoptosis after photodynamic therapy in human head and neck squamous carcinoma cells. Anticancer Res. 2012, 32, 753–760. [Google Scholar] [PubMed]
- Kent, W.J.; Sugnet, C.W.; Furey, T.S.; Roskin, K.M.; Pringle, T.H.; Zahler, A.M.; Haussler, D. The human genome browser at UCSC. Genome Res. 2002, 12, 996–1006. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.M.; Di Tommaso, P.; Taly, J.F.; Notredame, C. Accurate multiple sequence alignment of transmembrane proteins with PSI-Coffee. BMC Bioinform. 2002, 13, S1. [Google Scholar] [CrossRef] [PubMed]
- Dereeper, A.; Guignon, V.; Blanc, G.; Audic, S.; Buffet, S.; Chevenet, F.; Dufayard, J.F.; Guindon, S.; Lefort, V.; Lescot, M.; et al. Phylogeny.fr: Robust phylogenetic analysis for the non-specialist. Nucleic Acids Res. 2008, 36, W465–W469. [Google Scholar] [CrossRef] [PubMed]
- Dereeper, A.; Audic, S.; Claverie, J.M.; Blanc, G. BLAST-EXPLORER helps you building datasets for phylogenetic analysis. BMC Evol. Biol. 2010, 10, 8. [Google Scholar] [CrossRef] [PubMed]
- Sievers, F.; Higgins, D.G. Clustal Omega for making accurate alignments of many protein sequences. Protein Sci. 2018, 27, 135–145. [Google Scholar] [CrossRef] [PubMed]
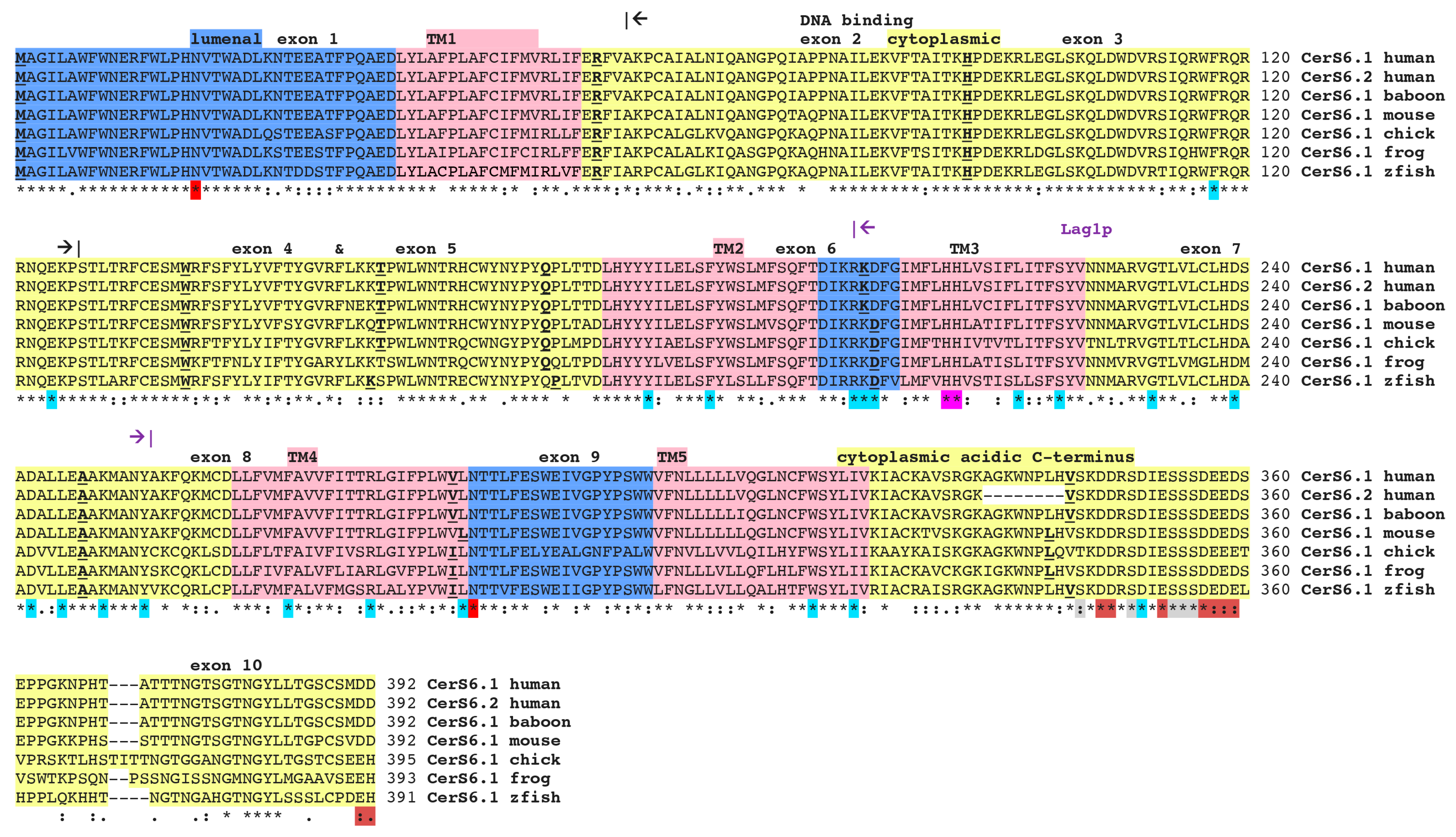
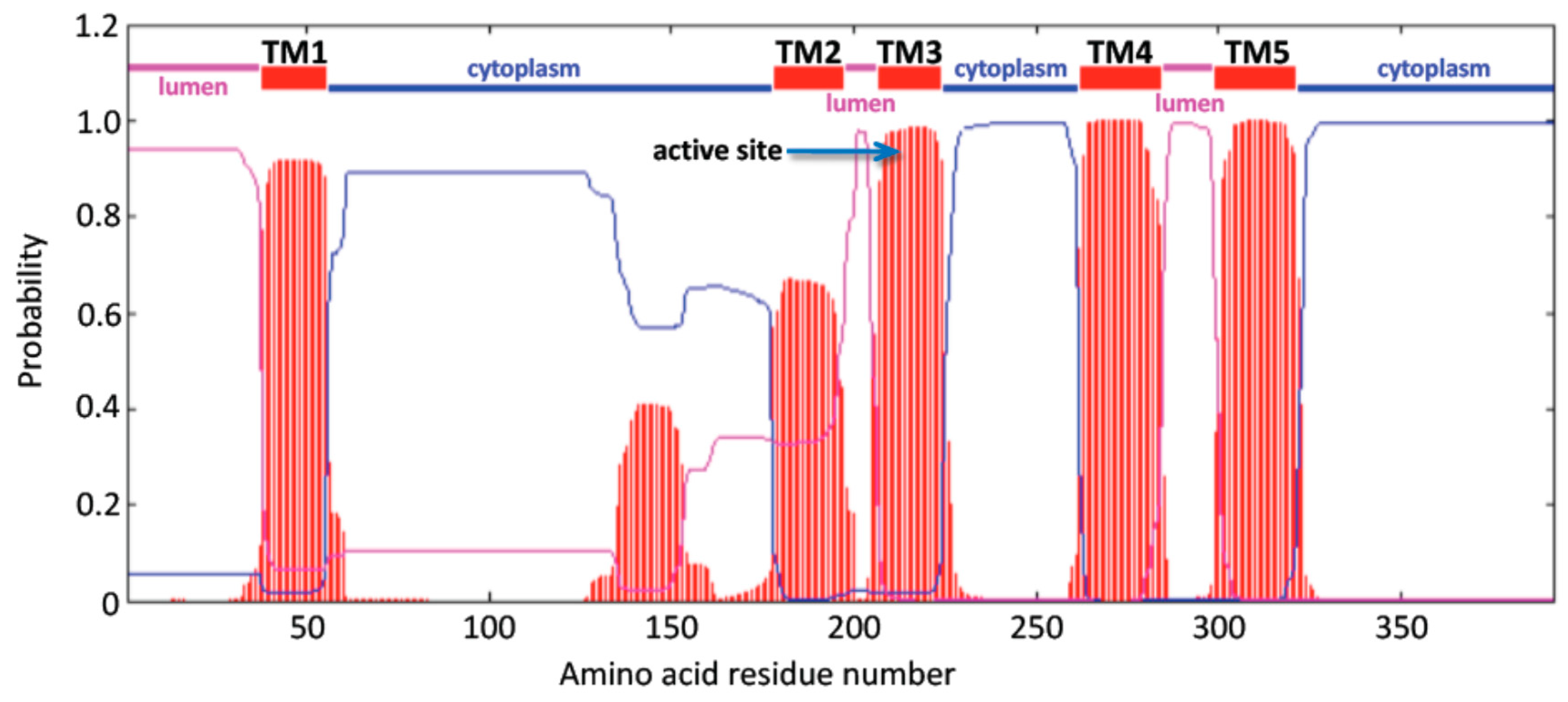
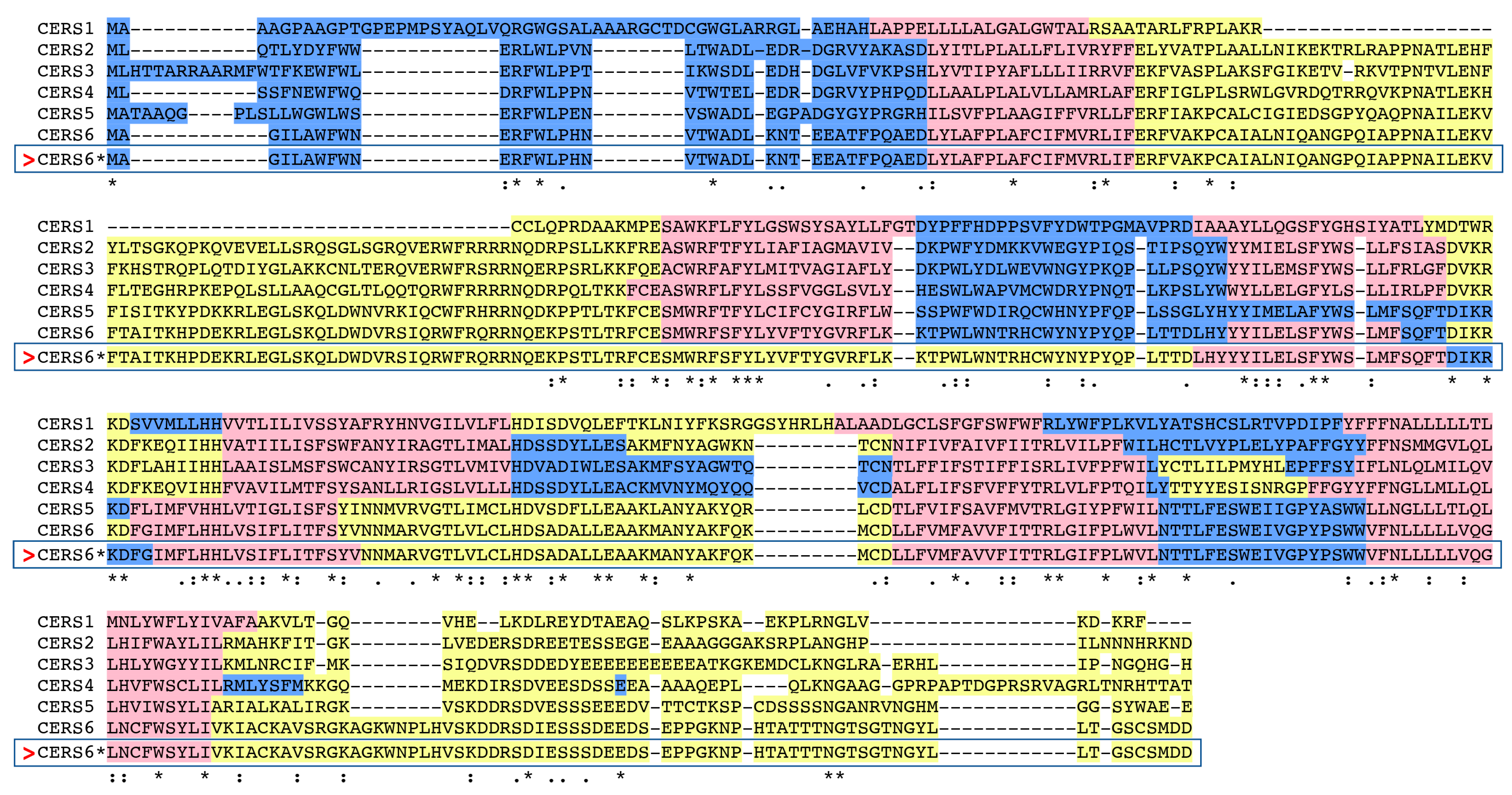

| Gene | Organism | Species | Chromosome ^ location | Coding Exons (strand) | Gene Size bps | GenBank ID * | UNIPROT ID | Amino Acids | Subunit MW (pI) | Similarity to Human CerS6, % |
|---|---|---|---|---|---|---|---|---|---|---|
| CERS6 | Human | Homo Sapiens | 2:168,456,249–168,775,134 | 10 (+strand) | 313,211 | NM_001256126 | Q6ZMG9-2 | 392 | 45,794 (8.0) | 100 |
| CerS6 | Chimp | Pan troglodytes | 2B:172,816,690–173,137,732 | 10 (+strand) | 321,043 | * XP_001154789 | H2RCH7 | 392 | 45,794 (8.0) | 100 |
| CerS6 | Gorilla | Gorilla gorilla | 2B:60,802,122–61,119,872 | 10 (+strand) | 317,751 | * XP_004032792 | A0A2I2YTR2 | 392 | 45,794 (8.0) | 100 |
| CerS6 | Gibbon | Nomascus leucogenys | 22A:58,027,154–58,354,057 | 10 (+strand) | 321,406 | * XP_004092010 | G1QX90 | 392 | 45,767 (8.0) | 99.7 |
| CerS6 | Baboon | Papio anubis | 12:30,381,688–30,694,736 | 10 (+strand) | 313,049 | * XP_017802114 | A0A2I3NGN0 | 392 | 45,824 (7.1) | 99.9 |
| CerS6 | Rhesus monkey | Macaca mulatta | 12:55,419,737–55,731,333 | 10 (+strand) | 311,597 | * XP_014965738 | > H9Z7B4 | 392 | 45,824 (8.0) | 99.5 |
| CerS6 | Green monkey | Chlorocebus sabaeus | 10:53,972,990–54,294,221 | 10 (+strand) | 321,232 | * XP_007963450 | A0A0D9RS37 | 392 | 45,797 (8.0) | 99.2 |
| CerS6 | Marmoset | Callithrix jacchus | 6:49,862,083–50,206,291 | 10 (−strand) | 344,209 | * XP_002749346 | F7I442 | 392 | 45,812 (8.0) | 99.0 |
| CerS6 | Mouse lemur | Microcebus murinus | ^ KQ055119v1:198,292–459,448 | 10 (+strand) | 261,157 | * XP_012637321 | na | 392 | 45,736 (7.1) | 99.0 |
| CerS6 | Mouse | Mus musculus | 2:68,861,636–69,108,591 | 10 (+strand) | 246,956 | NM_001347161 | H3BL08 | 392 | 45,717 (8.0) | 95.4 |
| CerS6 | Rat | Rattus norvegicus | 3:55,094,637–55,338,445 | 10 (+strand) | 242,941 | * XP_006224534 | F1LTP8 | 392 | 45,749 (8.0) | 95.2 |
| CerS6 | Horse | Equus caballus | 18:48,176,490–48,465,346 | 10 (+strand) | 288,857 | NM_001301183 | > F7BVU2 | 392 | 45,773 (8.0) | 97.4 |
| CerS6 | Dog | Canis familiaris | 36:13,308,109–13,602,677 | 10 (+strand) | 294,569 | > * XP_025315848 | F1P6P4 | 391 | 45,585 (8.0) | 95.4 |
| CerS6 | Opossum | Monodelphis domestica | 4:177,901,296–178,232,211 | 10 (+strand) | 330,304 | * XP_001375412 | K7E682 | 396 | 46,206 (8.5) | 90.0 |
| CerS6 | Chicken | Gallus gallus | 7:18,442,947–18,552,421 | 10 (−strand) | 109,475 | * XP_025008400 | E1C4X9 | 387 | 45,079 (8.50) | 77.6 |
| CerS6 | Lizard | Anolis carolinensis | 1:245,388,446–245,518,415 | 10 (−strand) | 129,970 | * XP_008124100 | G1K9E6 | 386 | 44,737 (8.8) | 75.5 |
| CerS6 | Frog | Xenopus tropicalis | 9:63,975,031–64,070,810 | 10 (+strand) | 93,133 | * XP_004917763 | >F6ZS80 | 393 | 46,096 (8.5) | 78.8 |
| CerS6 | Zebrafish | Danio rerio | 9:49,172,637–49,239,755 | 10 (−strand) | 67,119 | * XP_693283 | F1QPF3 | 391 | 45,860 (8.6) | 79.5 |
| Gene | Organism | Species | Chromosome ^ Location | Coding Exons (strand) | Gene Size bps | GenBank ID * | UNIPROT KD | Amino Acids | Subunit MW (pI) |
|---|---|---|---|---|---|---|---|---|---|
| CERS1 | Human | H. sapiens | 19:18,868,552-18,896,072 | 7 (−strand) | 27,593 | NP_067090 | P27544 | 350 | 39,536 (9.2) |
| CerS1 | Mouse | M. musculus | 8:70,315,775–70,331,587 | 7 (+strand) | 15,812 | NP_619588 | P27545 | 350 | 40,100 (8.7) |
| CerS1 | Chicken | Gallus gallus | 28:3,502,179–3,512,634 | 7 (+strand) | 10,456 | NP_001264694 | F1N9S0 | 354 | 41,235 (9.2) |
| CerS1 | Zebrafish | D. rerio | 22:4,791,880–4,847,724 | 7 (+strand) | 55,845 | * XP_009294228 | F1Q5B1 | 358 | 42,229 (5.5) |
| CERS2 | Human | H. sapiens | 1:150,965,173–150,975,003 | 10 (−strand) | 9830 | NM_022075 | Q96G23 | 380 | 44,876 (9.0) |
| CerS2 | Mouse | M. musculus | 3:95,320,063–95,322,789 | 10 (+strand) | 2727 | NM_001320492 | Q924Z4 | 380 | 45,024 (8.9) |
| CerS2 | Chicken | G. gallus | 25:2,627,873–2,630,268 | 10 (−strand) | 2396 | * XP_003642714 | > A0A1D5PKE9 | 377 | 44,621 (8.9) |
| CerS2 | Zebrafish | D. rerio | 16:1,360,026–1,370,907 | 10 (+strand) | 10,882 | * XP_693668 | Q90YY7 | 383 | 45,191 (8.6) |
| CERS3 | Human | H. sapiens | 15:100,400,395–100,544,286 | 10 (−strand) | 143,892 | NM_001290342 | Q8IU89 | 383 | 46,316 (7.6) |
| CerS3 | Mouse | M. musculus | 7:66,733,234–66,823,692 | 10 (+strand) | 90,459 | * XP_011249179 | Q1A3B0 | 383 | 46,081 (8.3) |
| CerS3 | Chicken | G. gallus | 10:17,297,468–17,326,587 | 10 (−strand) | 29,120 | * XP_424275 | F1N8P2 | 380 | 45,302 (8.9) |
| CerS3 | Zebrafish | D. rerio | 7:9,904,627–9,961,727 | 10 (+strand) | 35,391 | * XP_002662790 | A0A140LFW4 | 380 | 45,066 (7.2) |
| CERS4 | Human | H. sapiens | 19:8,209,353–8,262,421 | 10 (+strand) | 50,069 | NM_024552 | Q9HA82 | 394 | 46,399 (9.2) |
| CerS4 | Mouse | M. musculus | 8:4,493,405–4,526,079 | 10 (+strand) | 32,675 | NM_026058 | Q9D6J1 | 393 | 46,017 (8.7) |
| CerS4 | Chicken | G. gallus | 28:100,066–130,610 | 10 (−strand) | 30,545 | * XP_015155290 | A0A1D5P159 | 398 | 47,408 (8.5) |
| CerS4 | Zebrafish | D. rerio | 22:4,707,655–4,733,106 | 10 (+strand) | 25,452 | NM_153670 | Q90YY6 | 406 | 47,636 (8.4) |
| CERS5 | Human | H. sapiens | 12:50,129,798–50,167,422 | 10 (−strand) | 37,625 | NM_147190 | Q8N5B7 | 392 | 45,752 (8.2) |
| CerS5 | Mouse | M. musculus | 15:99,735,592–99,772,515 | 10 (−strand) | 36,924 | NM_028015 | Q9D6K9 | 414 | 48,167 (8.4) |
| CerS5 | Chicken | G. gallus | ^268,433–285,586 | 10 (+strand) | 17,154 | * XP_424486 | A0A1D5NZ43 | 391 | 45,516 (8.9) |
| CerS5 | Zebrafish | D. rerio | 22:5,758,094–5,821,733 | 10 (−strand) | 63,640 | NM_199628 | A8E7D4 | 387 | 45,541 (9.2) |
| LAGR1 | Nematode | Caenorhabditis elegans | I:13,724,671–13,731,990 | 7 (−strand) | 7317 | NM_061002 | Q9XWE9 | 360 | 42,743 (8.5) |
| HYL1 | Nematode | C. elegans | IV:8,540,663–8,544,055 | 8 (+strand) | 2958 | NM_069058 | G5ED45 | 368 | 43,851 (8.9) |
| LAC1 | Yeast | Saccharomyces cerevisiae | XI:427,295–428,551 | 1 | 1256 | NM_001179574 | P28496 | 418 | 48,992 (9.5) |
| Human CerSes | CerS1, Identity (%) | CerS2, Identity (%) | CerS3, Identity (%) | CerS4, Identity (%) | CerS5, Identity (%) | CerS6, Identity (%) |
|---|---|---|---|---|---|---|
| CerS1 | 100 | 27 | 25 | 31 | 27 | 29 |
| CerS2 | 27 | 100 | 53 | 49 | 41 | 41 |
| CerS3 | 25 | 53 | 100 | 45 | 40 | 39 |
| CerS4 | 31 | 49 | 45 | 100 | 43 | 42 |
| CerS5 | 27 | 41 | 40 | 43 | 100 | 63 |
| CerS6 | 19 | 41 | 39 | 42 | 63 | 100 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holmes, R.S.; Barron, K.A.; Krupenko, N.I. Ceramide Synthase 6: Comparative Analysis, Phylogeny and Evolution. Biomolecules 2018, 8, 111. https://doi.org/10.3390/biom8040111
Holmes RS, Barron KA, Krupenko NI. Ceramide Synthase 6: Comparative Analysis, Phylogeny and Evolution. Biomolecules. 2018; 8(4):111. https://doi.org/10.3390/biom8040111
Chicago/Turabian StyleHolmes, Roger S., Keri A. Barron, and Natalia I. Krupenko. 2018. "Ceramide Synthase 6: Comparative Analysis, Phylogeny and Evolution" Biomolecules 8, no. 4: 111. https://doi.org/10.3390/biom8040111
APA StyleHolmes, R. S., Barron, K. A., & Krupenko, N. I. (2018). Ceramide Synthase 6: Comparative Analysis, Phylogeny and Evolution. Biomolecules, 8(4), 111. https://doi.org/10.3390/biom8040111




