Membrane Permeabilities of Ascorbic Acid and Ascorbate
Abstract
1. Introduction
2. Materials and Methods
2.1. Planar Lipid Bilayer
2.2. Ascorbic Acid
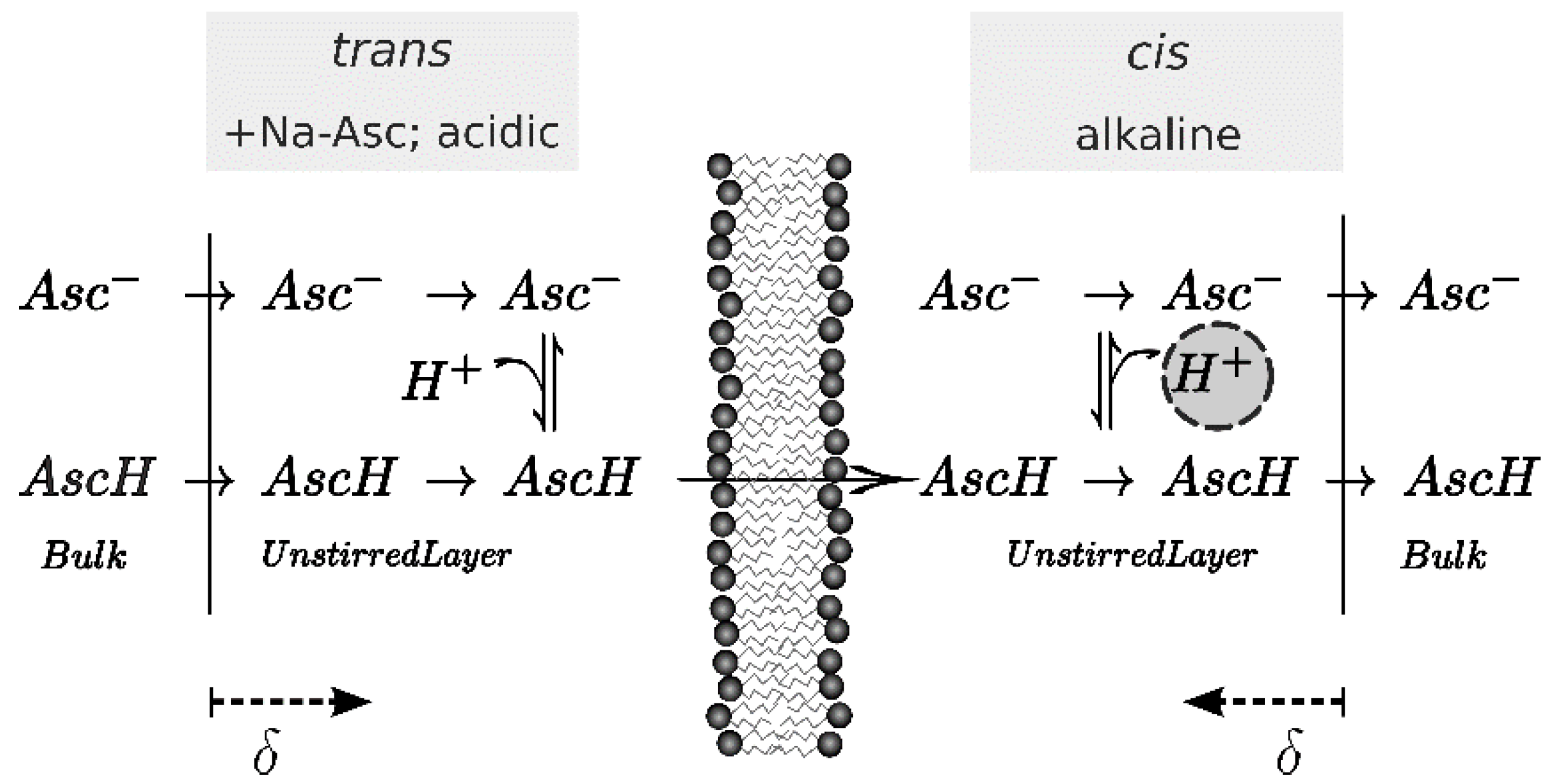
2.3. Scanning Electrochemical Microscopy
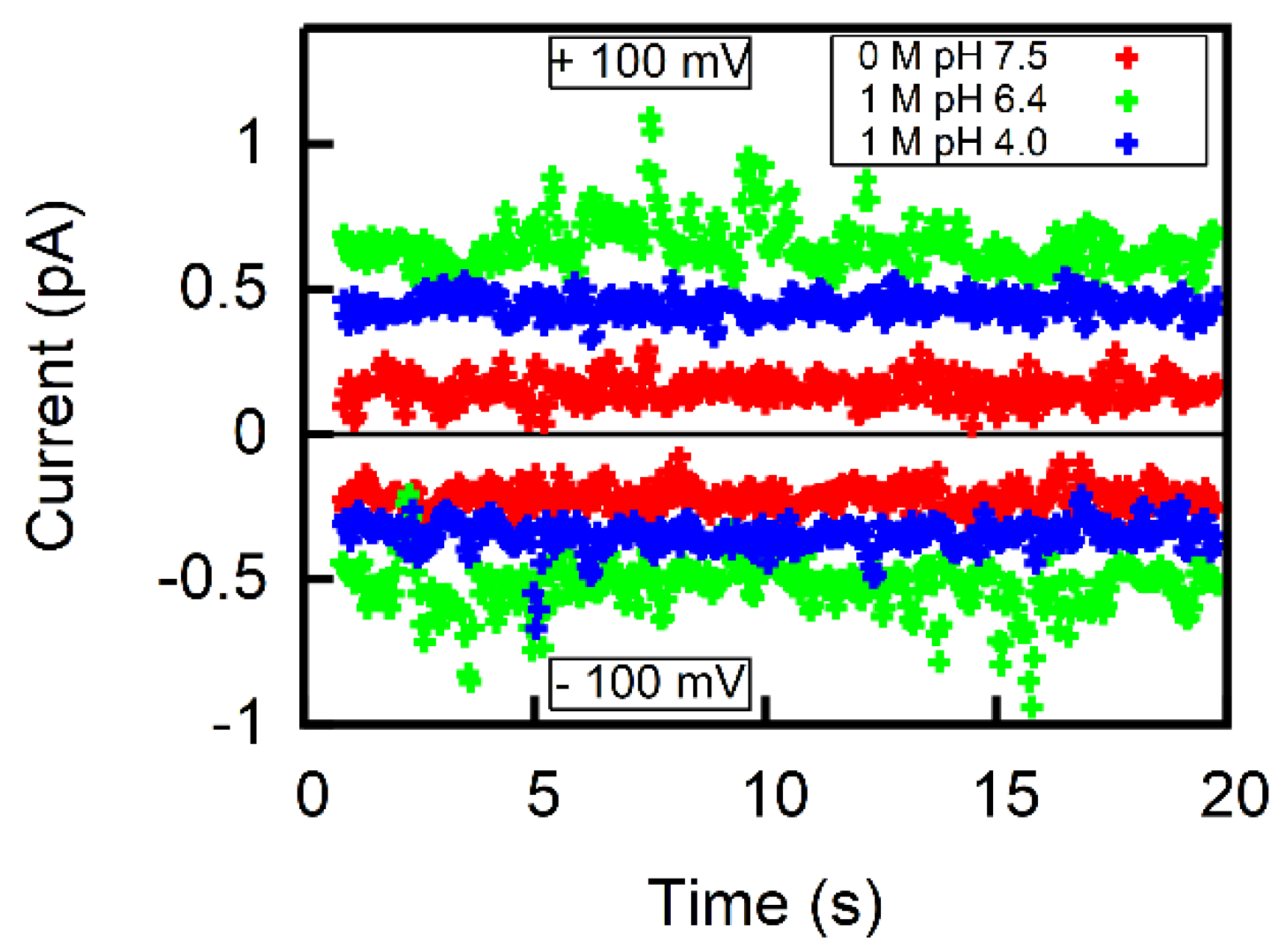
2.4. Calculation of P− from Membrane Conductivity Measurements
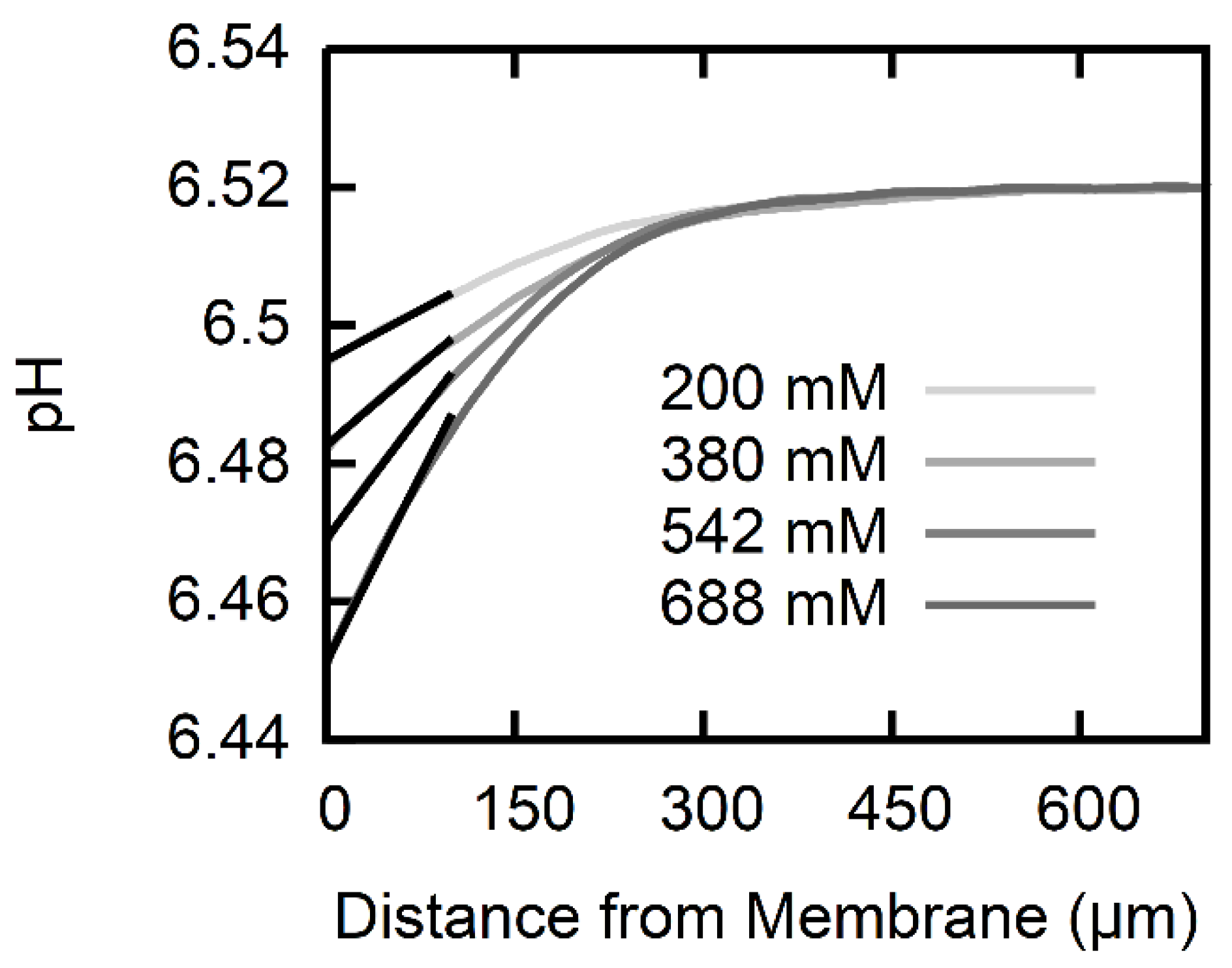
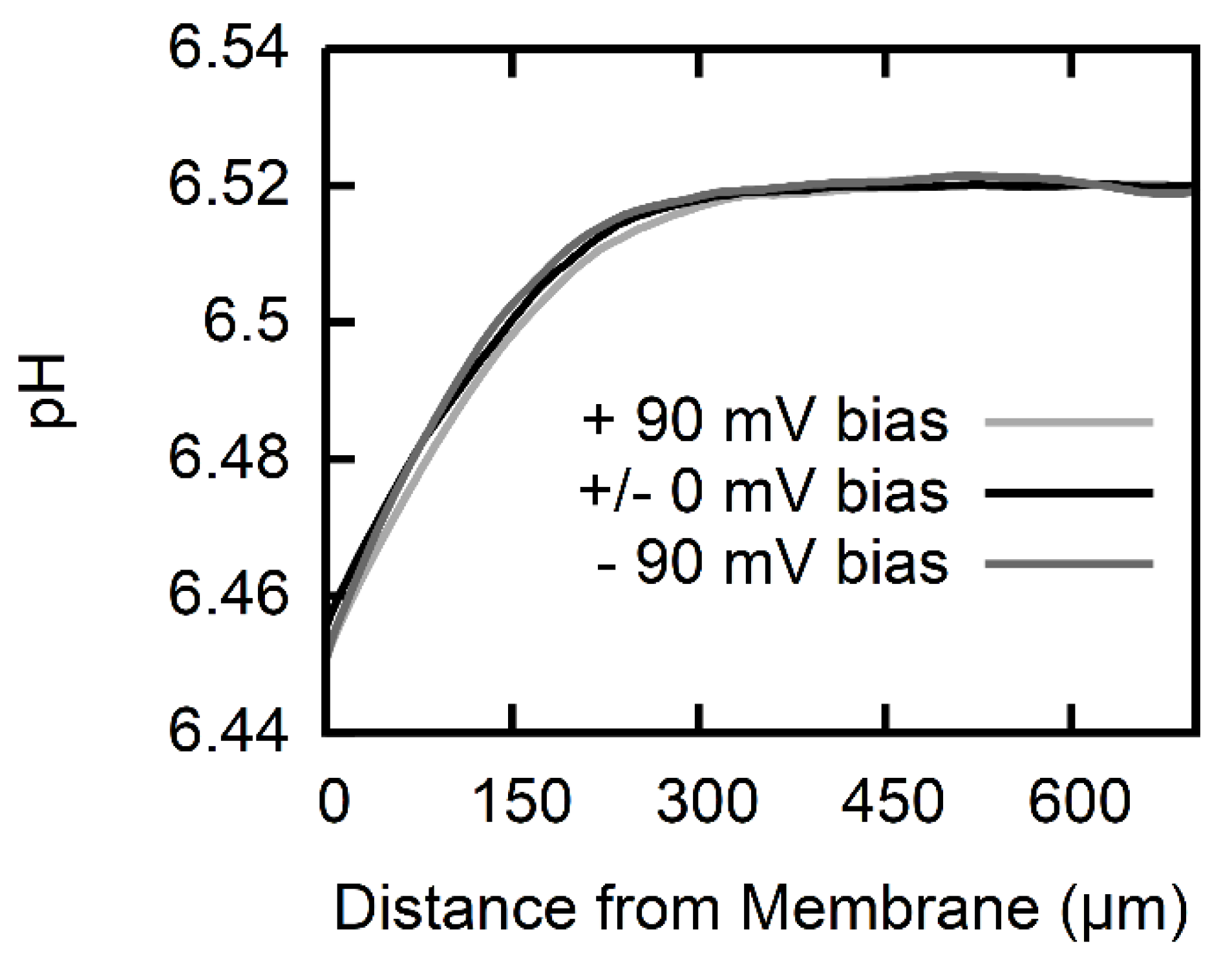
2.5. Calculation of Permeability from pH Profiles in the Unstirred Layers
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hediger, M.A. New view at C. Nat. Med. 2002, 8, 445–446. [Google Scholar] [CrossRef] [PubMed]
- Tsukaguchi, H.; Tokui, T.; Mackenzie, B.; Berger, U.V.; Chen, X.Z.; Wang, Y.; Brubaker, R.F.; Hediger, M.A. A family of mammalian Na+-dependent L-ascorbic acid transporters. Nature 1999, 399, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Corpe, C.P.; Tu, H.; Eck, P.; Wang, J.; Faulhaber-Walter, R.; Schnermann, J.; Margolis, S.; Padayatty, S.; Sun, H.; Wang, Y.; et al. Vitamin C transporter Slc23a1 links renal reabsorption, vitamin C tissue accumulation, and perinatal survival in mice. J. Clin. Investig. 2010, 120, 1069–1083. [Google Scholar] [CrossRef] [PubMed]
- Windholz, M. The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals, 10th ed.; Merck & Co.: Rahway, NJ, USA, 1983; p. 2179. [Google Scholar]
- Saparov, S.M.; Antonenko, Y.N.; Pohl, P. A new model of weak acid permeation through membranes revisited: Does overton still rule? Biophys. J. 2006, 90, L86–L88. [Google Scholar] [CrossRef] [PubMed]
- Antonenko, Y.N.; Denisov, G.A.; Pohl, P. Weak acid transport across bilayer lipid membrane in the presence of buffers. Theoretical and experimental pH profiles in the unstirred layers. Biophys. J. 1993, 64, 1701–1710. [Google Scholar] [CrossRef]
- Missner, A.; Kugler, P.; Antonenko, Y.N.; Pohl, P. Passive transport across bilayer lipid membranes: Overton continues to rule. Proc. Natl. Acad. Sci. USA 2008, 105, E123. [Google Scholar] [CrossRef] [PubMed]
- Shamim, M.; Khoo, S. Some physical properties of aqueous L-ascorbic acid solutions. Aust. J. Chem. 1979, 32, 2293–2295. [Google Scholar] [CrossRef]
- Sapper, H.; Roth, K.D.; Lohmann, W. The diffusion of L(+)-ascorbic acid across DPPC vesicle membranes. J. Microencapsul. 1985, 2, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Schreier-Muccillo, S.; Marsh, D.; Smith, I.C. Monitoring the permeability profile of lipid membranes with spin probes. Arch. Biochem. Biophys. 1976, 172, 1–11. [Google Scholar] [CrossRef]
- Oldendorf, W.H. Lipid Solubility and Drug Penetration of the Blood Brain Barrier. Exp. Biol. Med. 1974, 147, 813–816. [Google Scholar] [CrossRef]
- Mathai, J.C.; Missner, A.; Kügler, P.; Saparov, S.M.; Zeidel, M.L.; Lee, J.K.; Pohl, P. Membrane Transport of Hydrogen Sulfide: No Facilitator Required. Biophys. J. 2010, 98. [Google Scholar] [CrossRef]
- Antonenko, Y.N.; Pohl, P.; Denisov, G.A. Permeation of ammonia across bilayer lipid membranes studied by ammonium ion selective microelectrodes. Biophys. J. 1997, 72, 2187–2195. [Google Scholar] [CrossRef]
- Mueller, P.; Rudin, D.O.; Tien, H.T.; Wescott, W.C. Reconstitution of cell membrane structure in vitro and its transformation into an excitable system. Nature 1962, 194, 979–980. [Google Scholar] [CrossRef] [PubMed]
- Buettner, G.R. In the absence of catalytic metals ascorbate does not autoxidize at pH 7: Ascorbate as a test for catalytic metals. J. Biochem. Biophys. Methods 1988, 16, 27–40. [Google Scholar] [CrossRef]
- Pohl, P.; Saparov, S.M. Solvent drag across gramicidin channels demonstrated by microelectrodes. Biophys. J. 2000, 78, 2426–2434. [Google Scholar] [CrossRef]
- Antonenko, Y.N.; Bulychev, A.A. Measurements of Local pH Changes near Bilayer Lipid-Membrane by Means of a pH Microelectrode and a Protonophore-Dependent Membrane-Potential. Comparison of the Methods. Biochim. Biophys. Acta 1991, 1070, 279–282. [Google Scholar] [CrossRef]
- Hodgkin, A.L.; Katz, B. The effect of sodium ions on the electrical activity of giant axon of the squid. J. Physiol. 1949, 108, 37–77. [Google Scholar] [CrossRef] [PubMed]
- Hille, B. Ionic Channels of Excitable Membranes; Sinauer Associates: Sunderland, MA, USA, 1984. [Google Scholar]
- Goldman, D.E. Potential, Impedance, and Rectification in Membranes. J. Gen. Physiol. 1943, 27, 37–60. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, A. Weak-acid uncouplers of oxidative phosphorylation. Mechanism of action on thin lipid membranes. Biochim. Biophys. Acta 1970, 205, 1–6. [Google Scholar] [CrossRef]
- Lide, D. Handbook of Chemistry and Physics, 72nd ed.; CRC Press: Boca Raton, FL, USA, 1991–1992. [Google Scholar]
- Marshall, W.L.; Franck, E.U. Ion Product of Water Substance, 0 °C–1000 °C, 1–10,000 Bars New International Formulation and Its Background. J. Phys. Chem. Ref. Data 1981, 10, 295–304. [Google Scholar] [CrossRef]
- Robinson, D.; Anderson, J.E.; Lin, J.L. Measurement of Diffusion-Coefficients of Some Indoles and Ascorbic-Acid by Flow-Injection Analysis. J. Phys. Chem. 1990, 94, 1003–1005. [Google Scholar] [CrossRef]
- Donoian, H.C.; Kegeles, G. Diffusion of Beta-Alanine in Water at 25 Degrees. J. Am. Chem. Soc. 1961, 83, 255–259. [Google Scholar] [CrossRef]
- Edwards, O.W.; Huffman, E.O. Diffusion of Aqueous Solutions of Phosphoric Acid at 25-Degrees. J. Phys. Chem. 1959, 63, 1830–1833. [Google Scholar] [CrossRef]
- Pohl, P.; Saparov, S.M.; Antonenko, Y.N. The effect of a transmembrane osmotic flux on the ion concentration distribution in the immediate membrane vicinity measured by microelectrodes. Biophys. J. 1997, 72, 1711–1718. [Google Scholar] [CrossRef]
- Gutsmann, T.; Heimburg, T.; Keyser, U.; Mahendran, K.R.; Winterhalter, M. Protein reconstitution into freestanding planar lipid membranes for electrophysiological characterization. Nat. Protoc. 2015, 10, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Gutknecht, J.; Tosteson, D.C. Diffusion of weak acids across lipid bilayer membranes: Effects of chemical reactions in the unstirred layers. Science 1973, 182, 1258–1261. [Google Scholar] [CrossRef] [PubMed]
- Wood, R.E.; Wirth, F.P., Jr.; Morgan, H.E. Glucose permeability of lipid bilayer membranes. Biochim. Biophys. Acta 1968, 163, 171–178. [Google Scholar] [CrossRef]
- Hauser, H.; Oldani, D.; Phillips, M.C. Mechanism of ion escape from phosphatidylcholine and phosphatidylserine single bilayer vesicles. Biochemistry 1973, 12, 4507–4517. [Google Scholar] [CrossRef] [PubMed]
- Rashin, A.A.; Honig, B. Reevaluation of the Born model of ion hydration. J. Phys. Chem. 1985, 89, 5588–5593. [Google Scholar] [CrossRef]
- Lomize, A.L.; Pogozheva, I.D.; Mosberg, H.I. Anisotropic solvent model of the lipid bilayer. 2. Energetics of insertion of small molecules, peptides, and proteins in membranes. J. Chem. Inf. Model. 2011, 51, 930–946. [Google Scholar] [CrossRef] [PubMed]
- Honig, B.H.; Hubbell, W.L.; Flewelling, R.F. Electrostatic interactions in membranes and proteins. Annu. Rev. Biophys. Biophys. Chem 1986, 15, 163–193. [Google Scholar] [CrossRef] [PubMed]
- De Gier, J.; Mandersloot, J.G.; Hupkes, J.V.; McElhaney, R.N.; Van Beek, W.P. On the mechanism of non-electrolyte permeation through lipid bilayers and through biomembranes. Biochim. Biophys. Acta 1971, 233, 610–618. [Google Scholar] [CrossRef]
- Flewelling, R.F.; Hubbell, W.L. Hydrophobic ion interactions with membranes. thermodynamic analysis of tetraphenylphosphonium binding to vesicles. Biophys. J. 1986, 49, 531–540. [Google Scholar] [CrossRef]
- Missner, A.; Pohl, P. 110 years of the Meyer-Overton rule: Predicting membrane permeability of gases and other small compounds. ChemPhysChem 2009, 10, 1405–1414. [Google Scholar] [CrossRef] [PubMed]
- Nusair, N.A.; Mayo, D.J.; Dorozenski, T.D.; Cardon, T.B.; Inbaraj, J.J.; Karp, E.S.; Newstadt, J.P.; Grosser, S.M.; Lorigan, G.A. Time-resolved EPR immersion depth studies of a transmembrane peptide incorporated into bicelles. Biochim. Biophys. Acta 2012, 1818, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Kornberg, R.D.; McConnell, H.M. Inside-outside transitions of phospholipids in vesicle membranes. Biochemistry 1971, 10, 1111–1120. [Google Scholar] [PubMed]
- Walter, A.; Gutknecht, J. Permeability of small nonelectrolytes through lipid bilayer membranes. J. Membr. Biol. 1986, 90, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Bharate, S.S.; Kumar, V.; Vishwakarma, R.A. Determining Partition Coefficient (Log P), Distribution Coefficient (Log D) and Ionization Constant (pKa) in Early Drug Discovery. Comb. Chem. High Throughput Screen. 2016, 19, 461–469. [Google Scholar] [CrossRef]
- Petrauskas, A.A.; Kolovanov, E.A. ACD/Log P method description. Perspect. Drug Discov. Des. 2000, 19, 99–116. [Google Scholar] [CrossRef]
- Klopman, G.; Namboodiri, K.; Schochet, M. Simple Method of Computing the Partition-Coefficient. J. Comput. Chem. 1985, 6, 28–38. [Google Scholar] [CrossRef]
- Ulrich, N.; Endo, S.; Brown, T.N.; Watanabe, N.; Bronner, G.; Abraham, M.H.; Goss, K.U. UFZ-LSER Database V 3.2 [Internet]. Available online: http://www.ufz.de/lserd (accessed on 7 June 2018).
- Sprunger, L.M.; Achi, S.S.; Acree, W.E.; Abraham, M.H. Development of correlations for describing solute transfer into acyclic alcohol solvents based on the Abraham model and fragment-specific equation coefficients. Fluid Phase Equilib. 2010, 288, 139–144. [Google Scholar] [CrossRef]
- Helander, H.F.; Fandriks, L. Surface area of the digestive tract—Revisited. Scand. J. Gastroenterol. 2014, 49, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Waring, A.J.; Drake, I.M.; Schorah, C.J.; White, K.L.; Lynch, D.A.; Axon, A.T.; Dixon, M.F. Ascorbic acid and total vitamin C concentrations in plasma, gastric juice, and gastrointestinal mucosa: Effects of gastritis and oral supplementation. Gut 1996, 38, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Jacob, R.A.; Sotoudeh, G. Vitamin C function and status in chronic disease. Nutr. Clin. Care 2002, 5, 66–74. [Google Scholar] [CrossRef] [PubMed]
- McCoy, G.D.; Rosenkranz, H.S.; Klopman, G. Non-mutagenic carcinogens are primarily hydrophobic. Carcinogenesis 1990, 11, 1111–1117. [Google Scholar] [CrossRef] [PubMed]
- Dörwald, F.Z. Lead Optimization for Medicinal Chemists: Pharmacokinetic Properties of Functional Groups and Organic Compounds; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
| Species | Symbol | Index i | Di (10−10 m²/s) | pK | Equilibrium Constant (Ki) |
|---|---|---|---|---|---|
| Protons | 1 | 93.1 [6] | 14 [23] | ||
| Hydroxide anions | 2 | 52.6 [6] | 14 [23] | ||
| Ascorbic acid, ascorbate | , | 8, 7 | 5.97 [24] | 4.17 [4] | |
| β-alanine zwitterion and cation | , | 3, 4 | 9.36 [25] | 3.63 [4] | |
| Hydrogen phosphate, dihydrogen phosphate | , | 5, 6 | 10.41 [26] | 7.21 [22] |
| Na-l-Ascorbate Gradient (mM) | USL Width | AscH Membrane Permeability P (cm/s) |
|---|---|---|
| 200 | 260 | |
| 380 | 245 | |
| 542 | 215 | |
| 688 | 195 | |
| Average | ||
| Year and Source | Solvents | Method | Log K | K | Log P (log cm/s) | P (cm/s) |
|---|---|---|---|---|---|---|
| 1974 [11] | Water/olive oil | Shake-flask method and radioactively labeled AscH/, fraction of radioactivity in both phases | −2.34 | 0.0046 | −3.33 | |
| Ibidem with correction for uncharged fraction | 1.04 | 10.96 | −0.3 | 0.49 | ||
| 1990 [49] | Water/octanole | Calculated with quantitative structure-activity relation [43] | −2.0482 | 0.0089 | −4.55 | 2.8 × 10−5 |
| 2012 [50] | Water/octanole | Calculated with quantitative structure-activity relation [42] | −2.41 | 0.0039 | −4.97 | |
| 2016 [41] | Water/octanole | HPLC-based assay | −2.67 | 0.0021 | −5.27 | 5.3 × 10−6 |
| 2017 [44] | Water/octanole | Calculated with Linear Solvation Energy Relationship (LSER) approach | −2.61 | 0.0025 | −5.2 | |
| 2017 [44] | Water/olive oil | Calculated with LSER | −4.4 | 0.00004 | −5.7 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hannesschlaeger, C.; Pohl, P. Membrane Permeabilities of Ascorbic Acid and Ascorbate. Biomolecules 2018, 8, 73. https://doi.org/10.3390/biom8030073
Hannesschlaeger C, Pohl P. Membrane Permeabilities of Ascorbic Acid and Ascorbate. Biomolecules. 2018; 8(3):73. https://doi.org/10.3390/biom8030073
Chicago/Turabian StyleHannesschlaeger, Christof, and Peter Pohl. 2018. "Membrane Permeabilities of Ascorbic Acid and Ascorbate" Biomolecules 8, no. 3: 73. https://doi.org/10.3390/biom8030073
APA StyleHannesschlaeger, C., & Pohl, P. (2018). Membrane Permeabilities of Ascorbic Acid and Ascorbate. Biomolecules, 8(3), 73. https://doi.org/10.3390/biom8030073






