Functional Analysis of the Glucuronyltransferases GlcAT-P and GlcAT-S of Drosophila melanogaster: Distinct Activities towards the O-linked T-antigen
Abstract
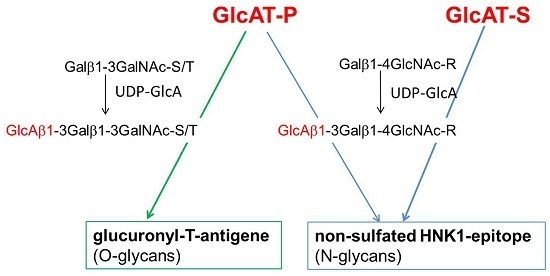
:1. Introduction
2. Results and Discussion
2.1. Establishing Epitope Specificities of Monoclonal Anti-GlcA Antibodies
2.2. Recombinant Expression of dGlcAT-P and dGlcAT-S in Drosophila S2-Cells
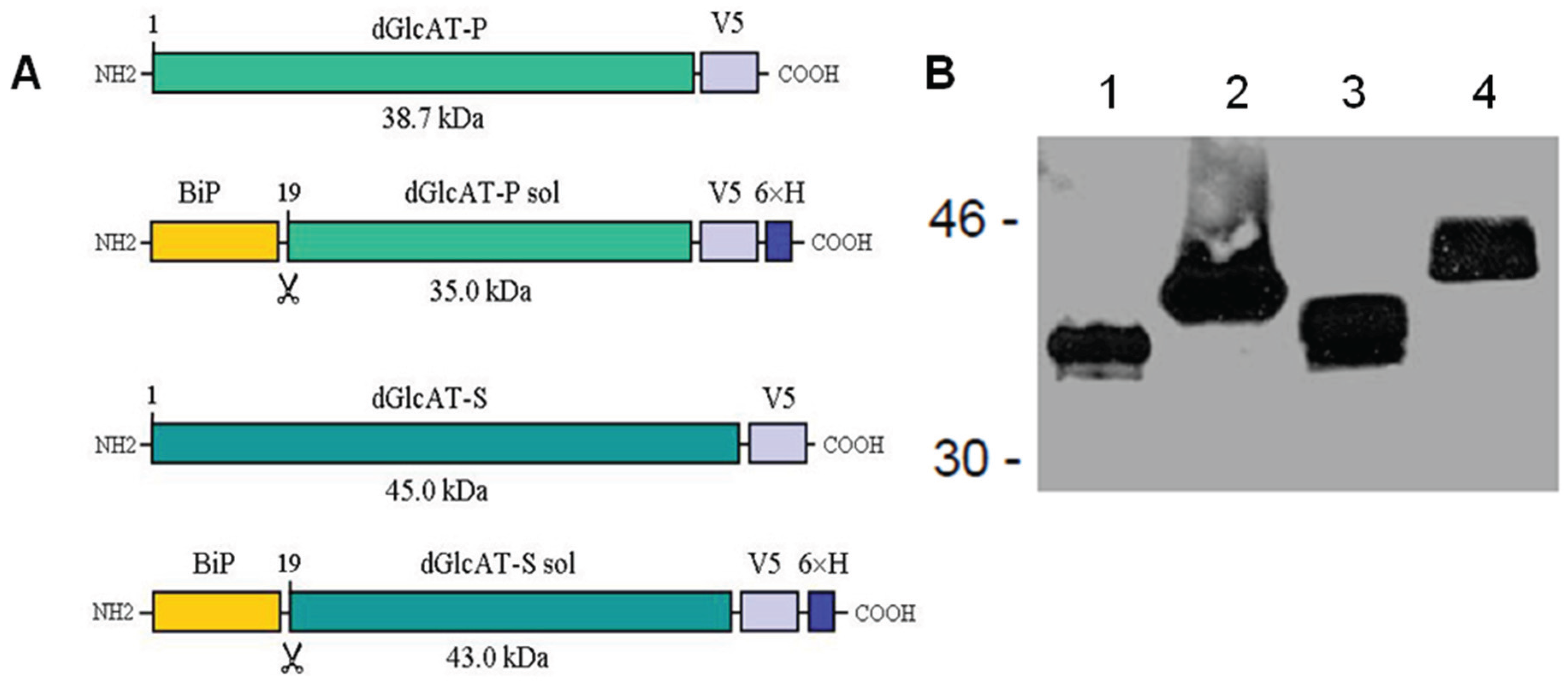
2.3. In Vivo Glucuronylation Studies
2.3.1. Glucuronylation of O-linked Chains
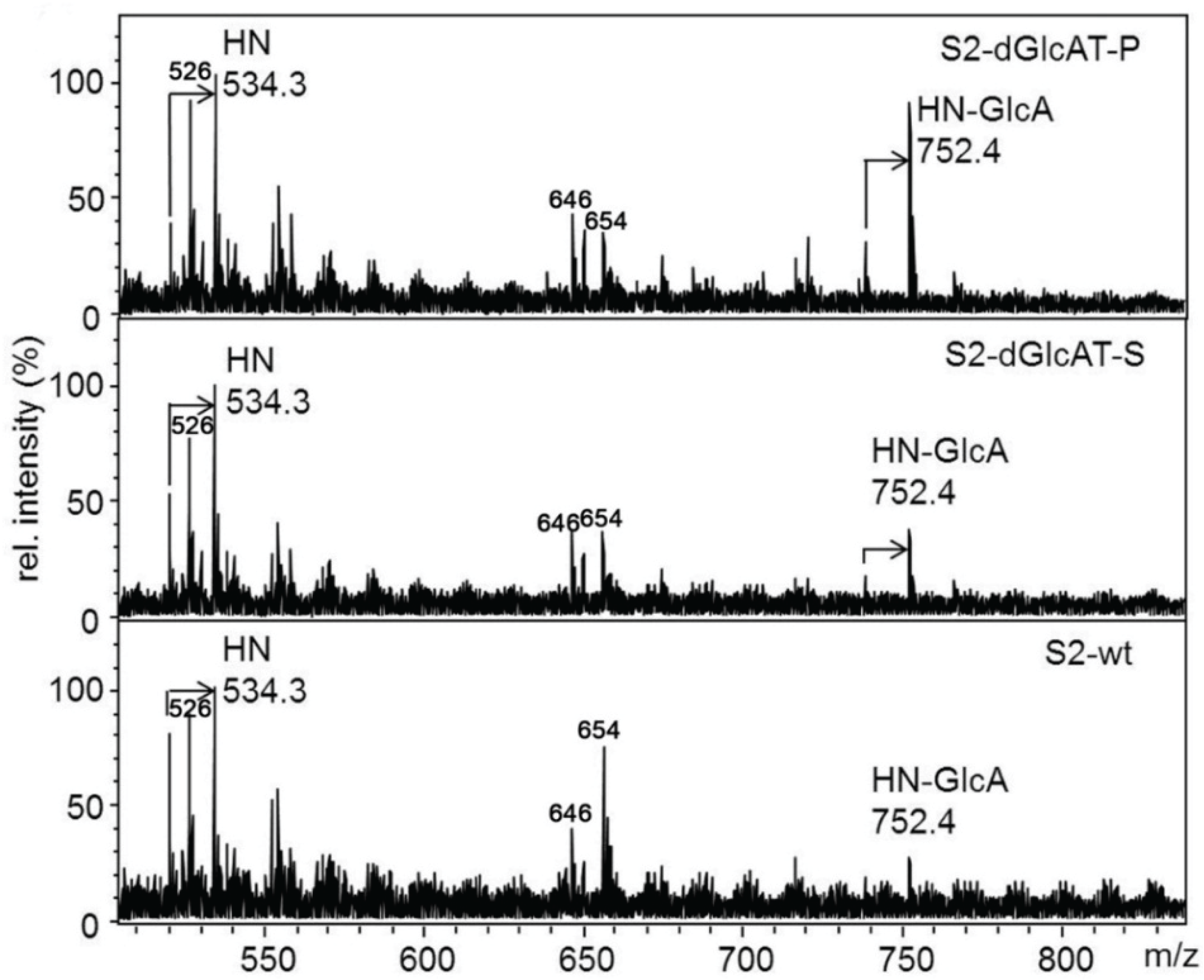
2.3.2. Glucuronylation of N-Linked Chains

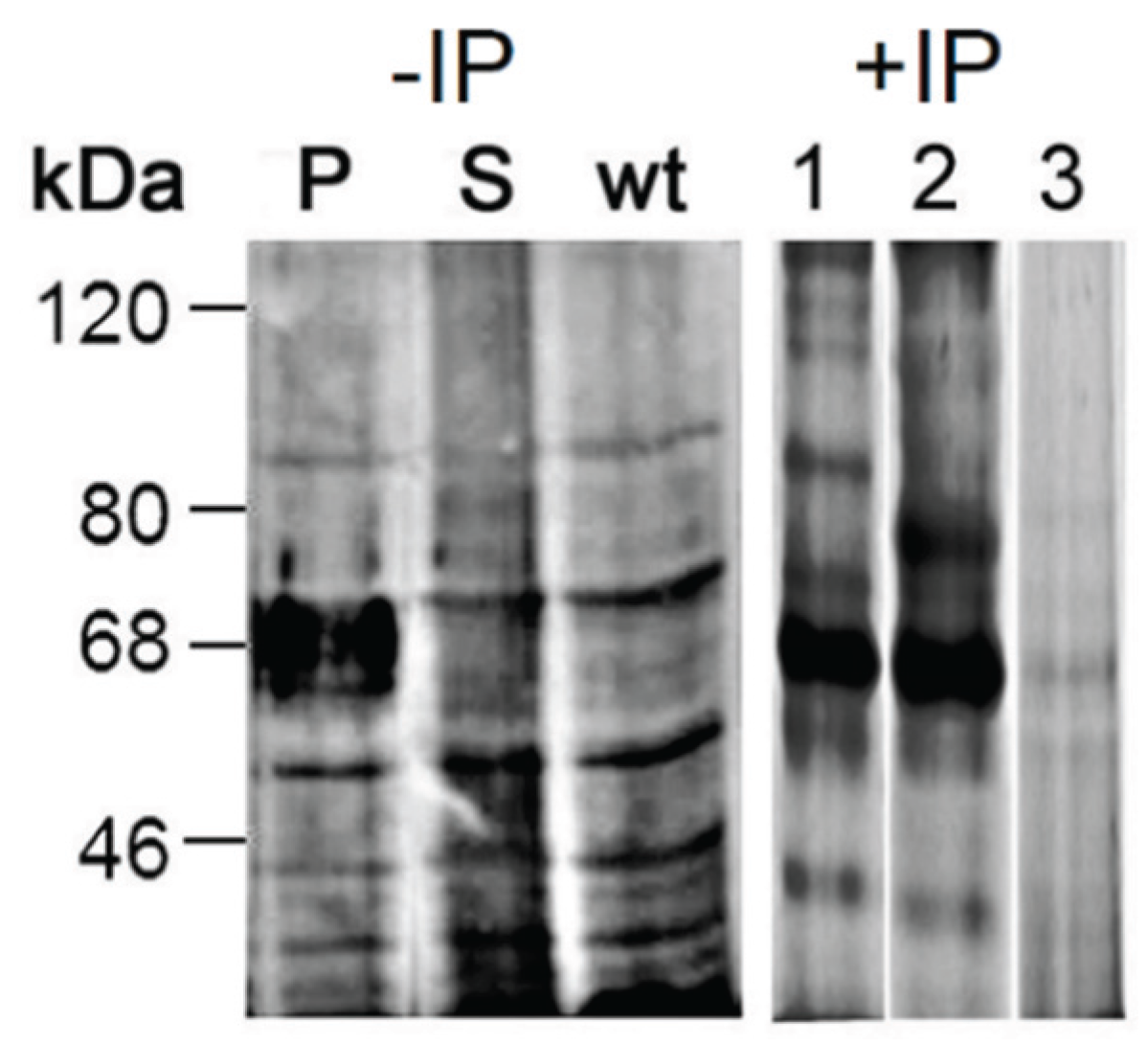
2.3.3. Proteomic Identification of Glucuronic Acid-Modified Proteins
| Protein | Identified in Experiment IP (+/−) | Reported Localization | Accession No. (NCBI) |
|---|---|---|---|
| calnexin | + | M, ER | gi|2213427 |
| CD98 heavy chain | + | M | gi|17945866 |
| CG2918, HSP70 family | +/− | EC, M | gi|20128923 |
| ERp60 | +/− | ER | gi|45551086 |
| glycoprotein 93 | +/− | EC | gi|21357739 |
| heat shock protein 60 | +/− | MI | gi|33636453 |
| heat shock protein 83 | +/− | CP | gi|17647529 |
| heat shock protein cognate 1 | − | MT | gi|17647515 |
| heat shock protein cognate 4 | +/− | N, CP, EC | gi|17737967 |
| heat shock protein cognate 71 | +/− | ER | gi|157667 |
| heat shock protein cognate 72 | +/− | ER, EC | gi|157658 |
| Hexosaminidase 2 | + | M, EC | gi|17933586 |
| Inos | +/− | CP | gi|17137626 |
| oligosaccharide transferase | + | M | gi|19922486 |
| peptidase S28 | + | EC, M | gi|20129649 |
| protein disulfide isomerase | +/− | ER, EC | gi|17647799 |
| Scavenger receptor class C | + | M | gi|984515 |
| Ugt58Fa glycosyltransferase | + | M | gi|22024248 |
| Ugt86Da glycosyltransferase | + | M | gi|21357701 |
| vacuolar H[+]-ATPase | + | M, CP | gi|17136796 |
2.4. In Vitro-Analysis of the Recombinantly Expressed Glucuronyltransferases
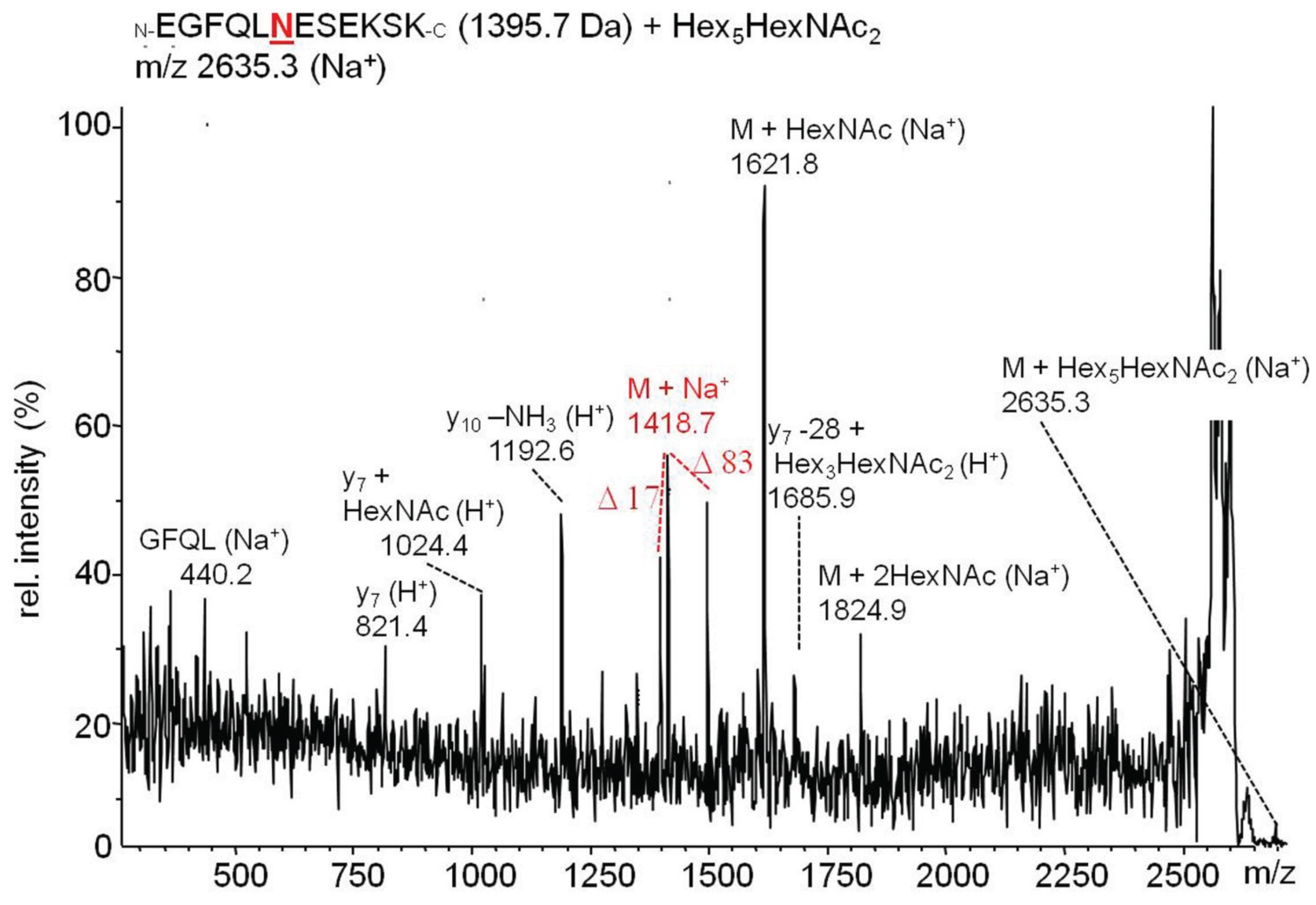
2.4.1. In Vitro-Synthesis of the Non-Sulfated HNK1-Epitope
2.4.2. In Vitro-Synthesis of the Glucuronyl-T Antigen
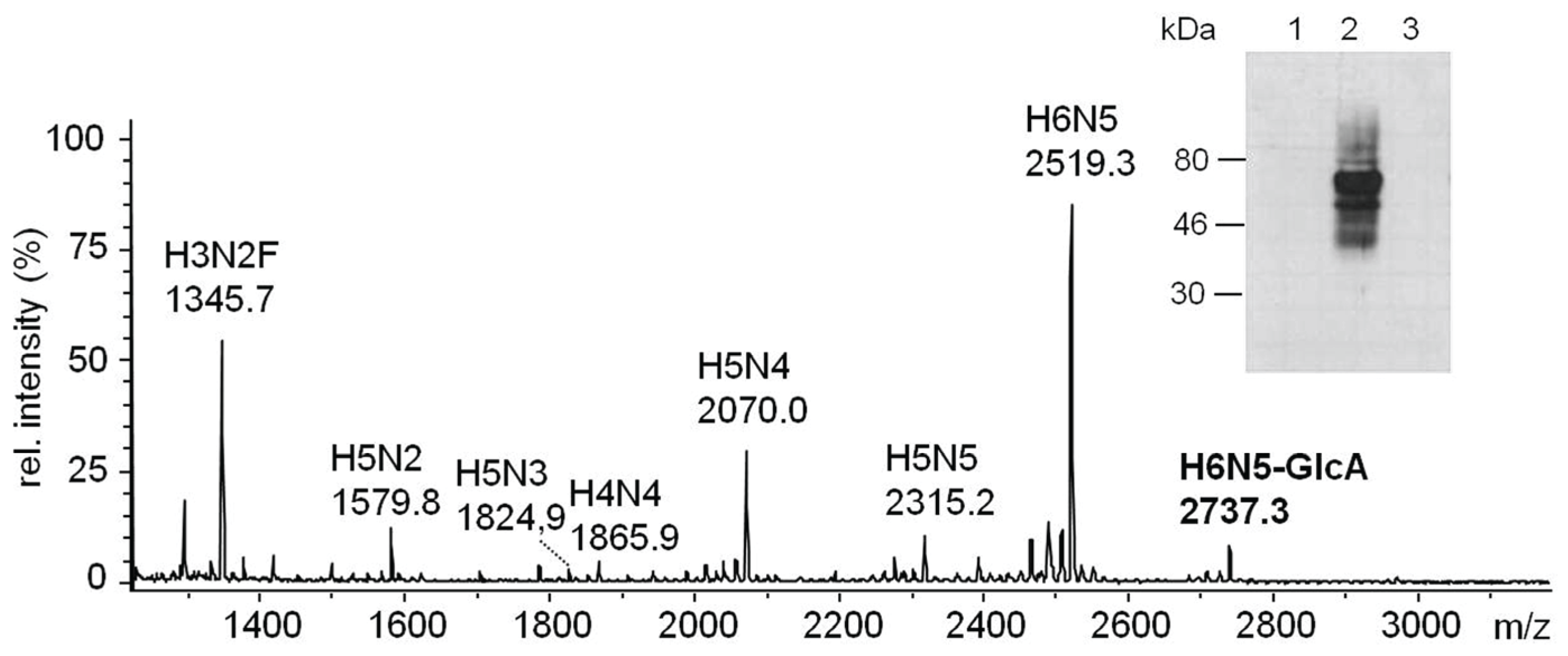
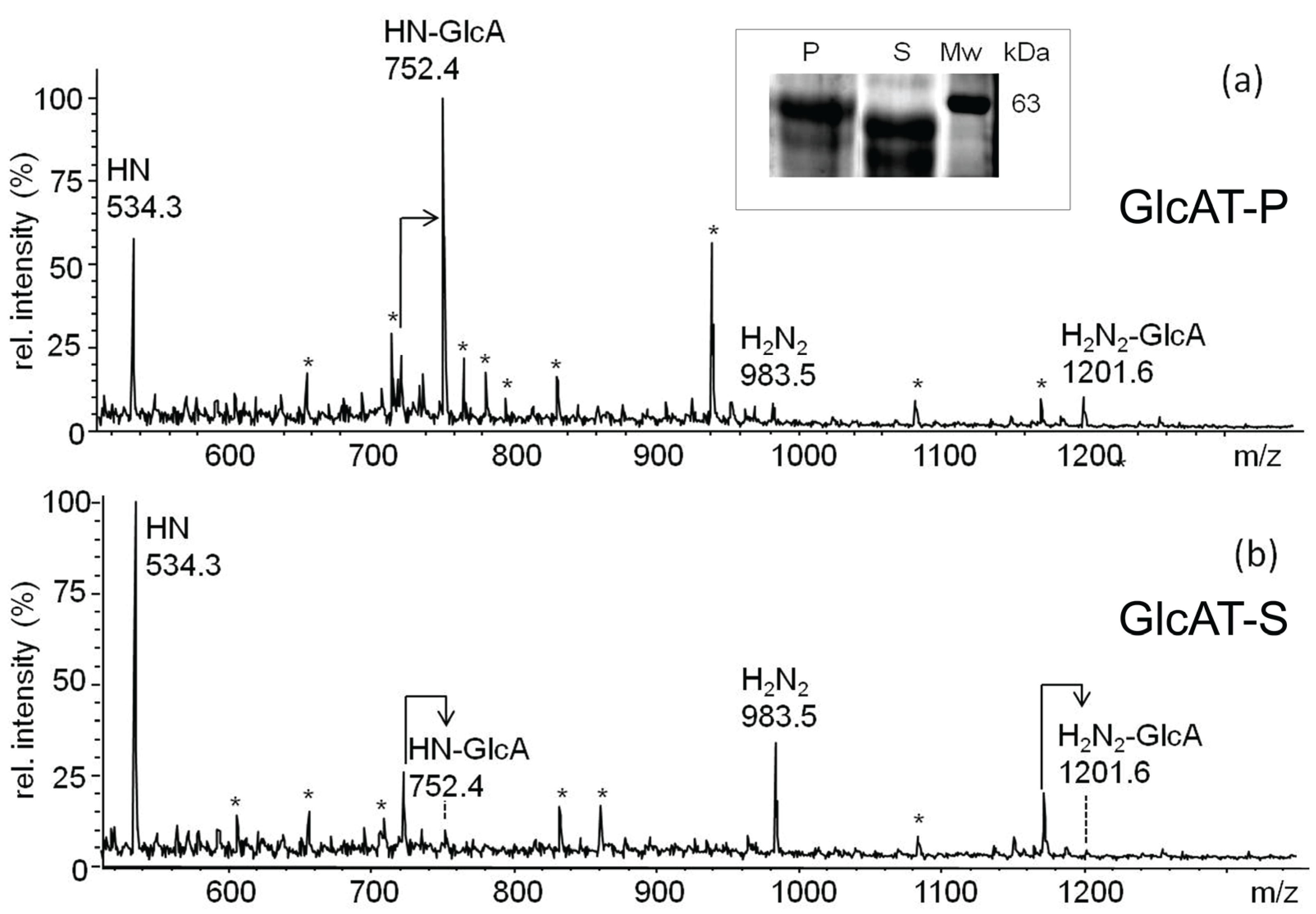
2.5. Recombinant Expression of dGlcAT-P and dGlcAT-S in CHO-Lec2-Cells
2.5.1. Coexpression of Glycosylation Probes in CHO-Lec2-Cells
2.5.2. In Vivo Analysis of dGlcAT-P
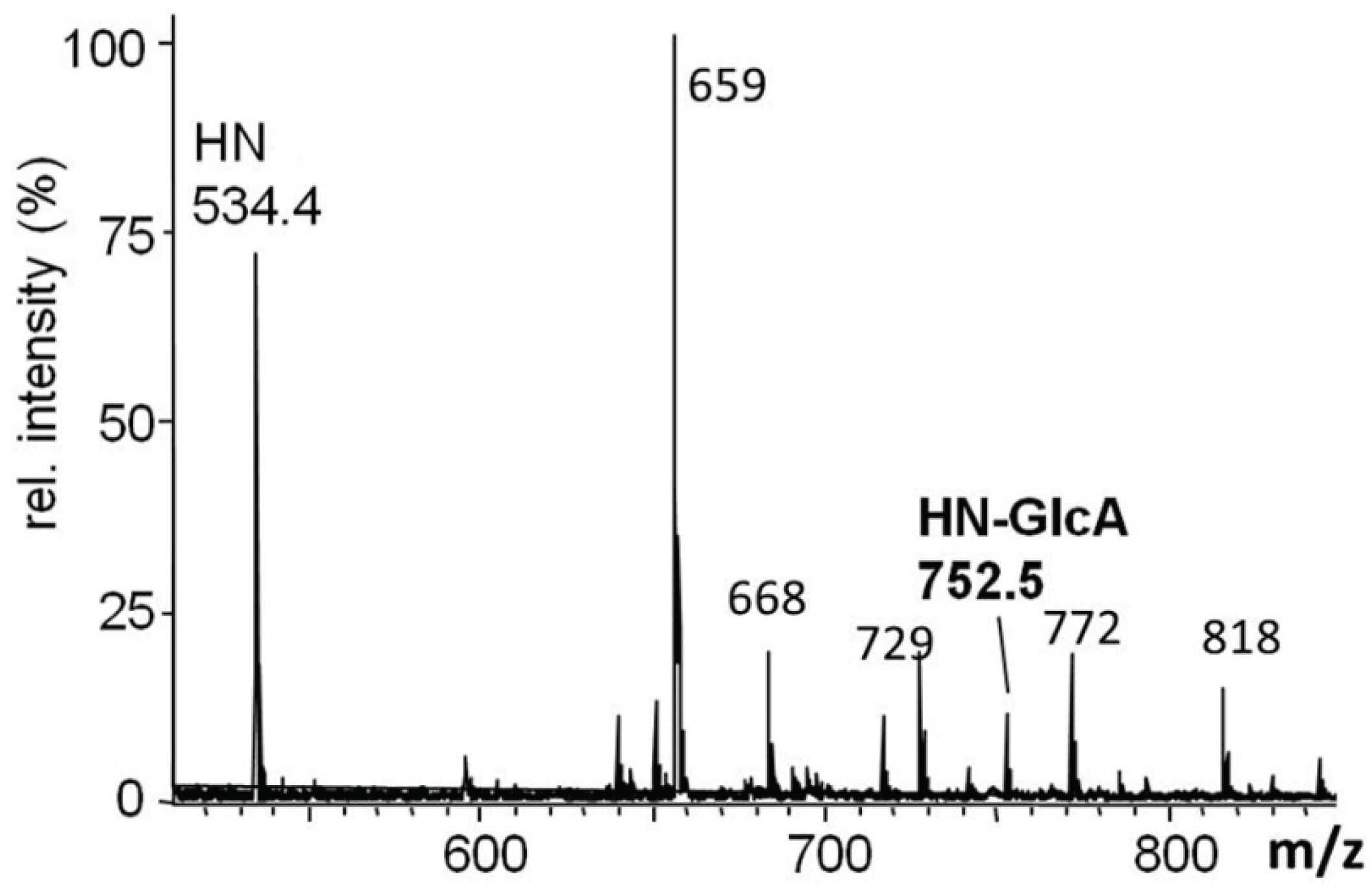
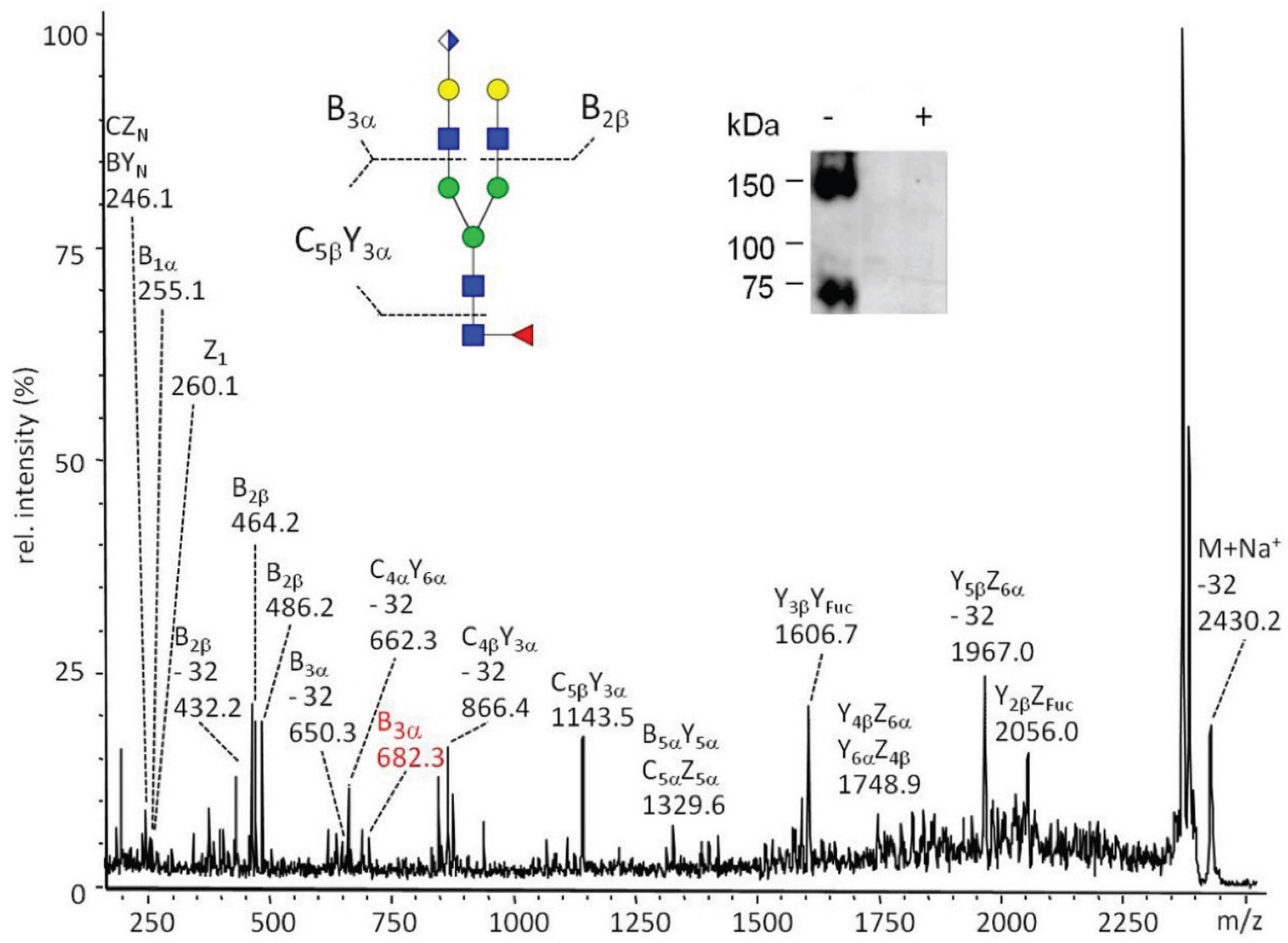
2.5.3. In vivo Analysis of dGlcAT-S
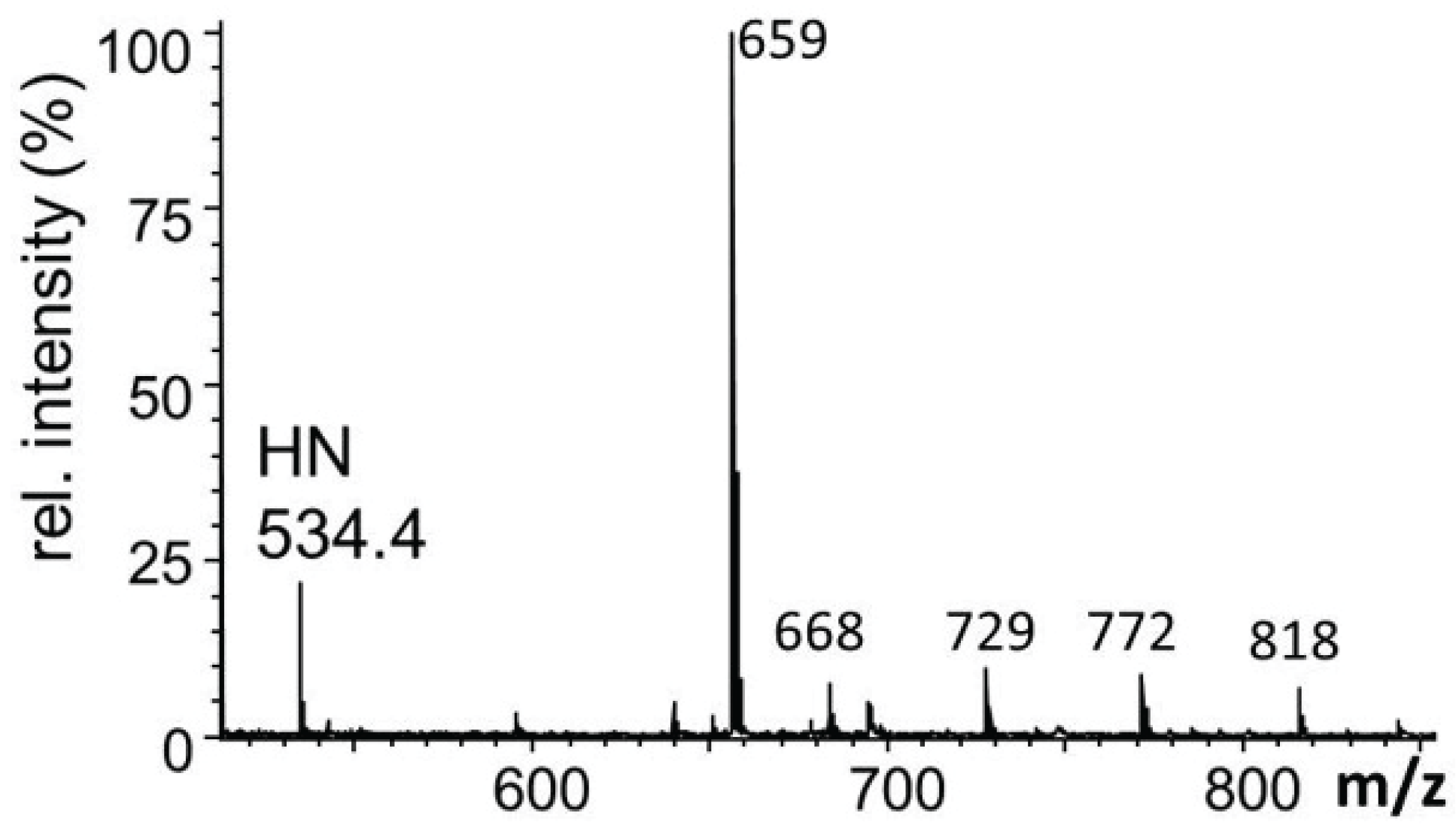
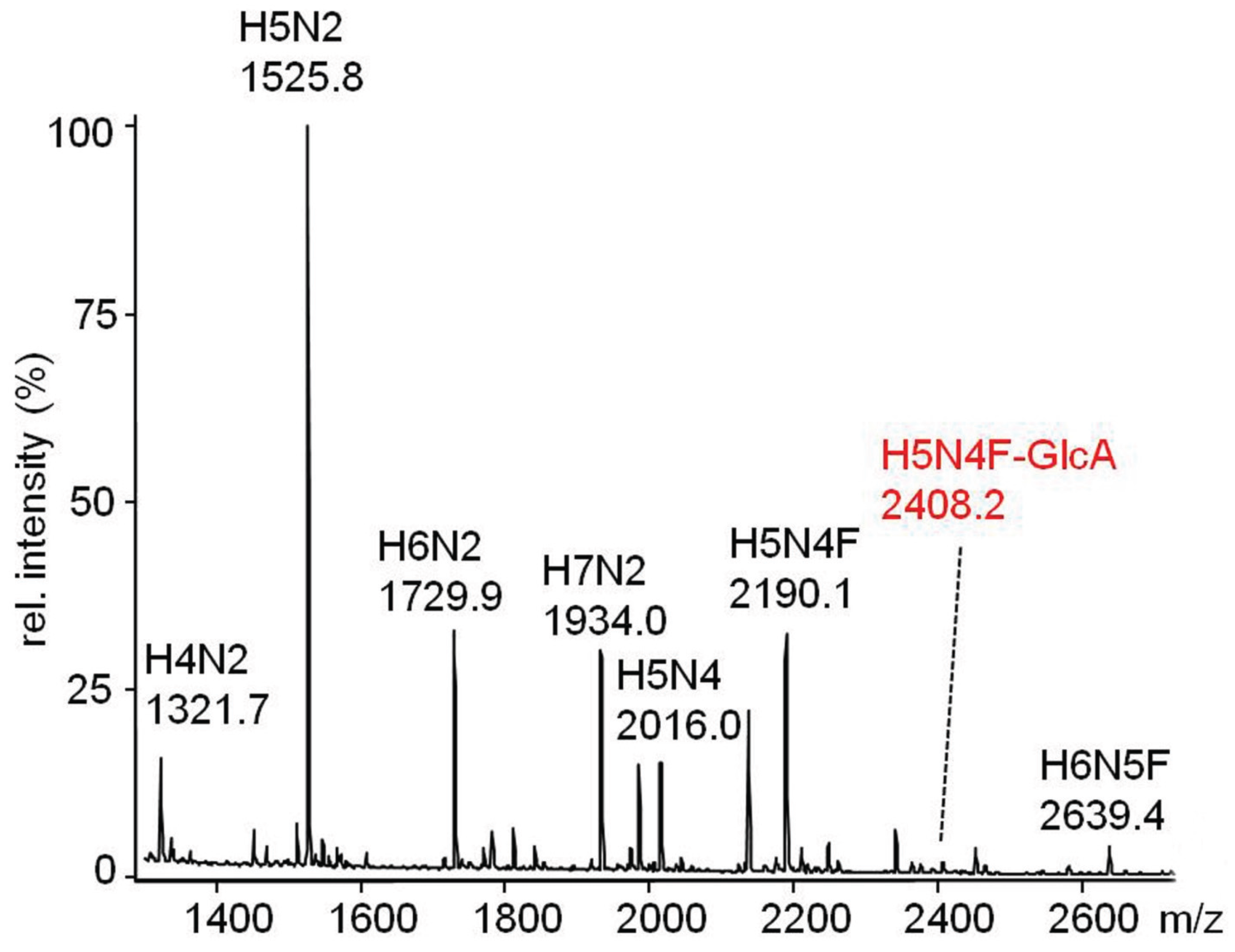
3. Experimental Section
3.1. Materials
3.2. Cell Culture
3.3. Expression and Isolation of Recombinant Glycosylation Probes
3.4. Cloning of a New Vector Series for Stable Drosophila Cell Transfection
3.5. Recombinant Expression of the Glucuronyltransferases
3.5.1. Drosophila GlcAT-P
3.5.2. Drosophila GlcAT-S
3.5.3. CHO-Lec2
3.6. Immunofluorescence
3.7. Purification of the Recombinant Glucuronyltransferases from Cell Culture Supernatant
3.8. Enzymatic Digestions
3.9. In-Vitro Glucuronyltransferase Assay
3.10. Purification of Monoclonal Antibodies
3.11. SDS-Polyacrylamide Gel Electrophoresis (SDS-PAGE) and Western Blot
3.12. Immunoprecipitation Experiments
3.13. Protein Identification by Mass Spectrometric Proteomics
3.14. Glycan Analysis of Cell Lysates
3.15. Glycan Derivatisation for MS
3.16. Glycan and Glycopeptide Analysis by MALDI-TOF-MS/MS
4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Aoki, K.; Porterfield, M.; Lee, S.; Dong, B.; Nguyen, K.; McGlamry, K.H.; Tiemeyer, M. The diversity of O-linked glycans expressed during Drosophila melanogaster development reflects stage- and tissue-specific requirements for cell signaling. J. Biol. Chem. 2008, 283, 30385–30400. [Google Scholar] [CrossRef] [PubMed]
- Breloy, I.; Schwientek, T.; Lehr, S.; Hanisch, F.G. Glucuronic acid can extend O-linked core 1 glycans, but it contributes only weakly to the negative surface charge of Drosophila melanogaster Schneider-2 cells. FEBS Lett. 2008, 582, 1593–1598. [Google Scholar] [CrossRef] [PubMed]
- Dennis, R.D.; Martini, R.; Schachner, M. Expression of carbohydrate epitopes L2/HNK-1 and L3 in the larva and imago of Drosophila melanogaster and Calliphora vicina. Cell Tissue Res. 1991, 265, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Morita, I.; Kizuka, Y.; Kakuda, S.; Oka, S. Expression and function of the HNK-1 carbohydrate. J. Biochem. 2008, 143, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, M.; Yu, R.K. The expression and functions of glycoconjugates in neural stem cells. Glycobiology 2007, 17, 57R–74R. [Google Scholar] [CrossRef] [PubMed]
- Aoki, K.; Tiemeyer, M. The glycomics of glycan glucuronylation in Drosophila melanogaster. Methods Enzymol. 2010, 480, 297–321. [Google Scholar] [PubMed]
- Kim, B.T.; Tsuchida, K.; Lincecum, J.; Kitagawa, H.; Bernfield, M.; Sugahara, K. Identification and characterization of three Drosophila melanogaster glucuronyltransferases responsible for the synthesis of the conserved glycosaminoglycan-protein linkage region of proteoglycans. Two novel homologs exhibit broad specificity toward oligosaccharides from proteoglycans, glycoproteins, and glycosphingolipids. J. Biol. Chem. 2003, 278, 9116–9124. [Google Scholar] [PubMed]
- Inoue, M.; Kato, K.; Matsuhashi, H.; Kizuka, Y.; Kawasaki, T.; Oka, S. Distributions of glucuronyltransferases, GlcAT-P and GlcAT-S, and their target substrate, the HNK-1 carbohydrate epitope in the adult mouse brain with or without a targeted deletion of theGlcAT-P gene. Brain Res. 2007, 1179, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Terayama, K.; Oka, S.; Seiki, T.; Miki, Y.; Nakamura, A.; Kozutsumi, Y.; Takio, K.; Kawasaki, T. Cloning and functional expression of a novel glucuronyltransferase involved in the biosynthesis of the carbohydrate epitope HNK-1. Proc. Natl. Acad. Sci. USA 1997, 94, 6093–6098. [Google Scholar] [CrossRef] [PubMed]
- Tagawa, H.; Kizuka, Y.; Ikeda, T.; Itoh, S.; Kawasaki, N.; Kurihara, H.; Onozato, M.L.; Tojo, A.; Sakai, T.; Kawasaki, T.; et al. A non-sulfated form of the HNK-1 carbohydrate is expressed in mouse kidney. J. Biol. Chem. 2005, 280, 23876–23883. [Google Scholar] [CrossRef] [PubMed]
- Obata, K.; Tanaka, H. Molecular differentiation of the otic vesicle and neural tube in the chick embryo demonstrated by monoclonal antibodies. Neurosci. Res. 1988, 6, 131–142. [Google Scholar] [CrossRef]
- Turano, C.; Coppari, S.; Altieri, F.; Ferraro, A. Proteins of the PDI family: Unpredicted non-ER locations and functions. J. Cell. Physiol. 2002, 193, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Bello, C.; Farbiarz, K.; Möller, J.F.; Becker, C.F.; Schwientek, T. A quantitative and site-specific chemoenzymatic glycosylation approach for PEGylated MUC1 peptides. Chem. Sci. 2014, 5, 1634–1641. [Google Scholar] [CrossRef]
- Schwientek, T.; Mandel, U.; Roth, U.; Müller, S.; Hanisch, F.G. A serial lectin approach to the mucin-type O-glycoproteome of Drosophila melanogaster S2 cells. Proteomics 2007, 7, 3264–3277. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.J.; Rylett, C.M.; Keen, J.N.; Audsley, N.; Sajid, M.; Shirras, A.D.; Isaac, R.E. Proteomic identification of Drosophila melanogaster male accessory gland proteins, including a pro-cathepsin and a soluble γ-glutamyl transpeptidase. Proteome Sci. 2006, 4, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Stanley, P. Chinese hamster ovary cell mutants with multiple glycosylation defects for production of glycoproteins with minimal carbohydrate heterogeneity. Mol. Cell. Biol. 1989, 9, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Bechtel, M.; Keller, M.V.; Bloch, W.; Sasaki, T.; Boukamp, P.; Zaucke, F.; Paulsson, M.; Nischt, R. Different domains in nidogen-1 and nidogen-2 drive basement membrane formation in skin organotypic cocultures. FASEB J. 2012, 26, 3637–3648. [Google Scholar] [CrossRef] [PubMed]
- Breloy, I.; Schwientek, T.; Gries, B.; Razawi, H.; Macht, M.; Albers, C.; Hanisch, F.G. Initiation of mammalian O-mannosylation in vivo is independent of a consensus sequence and controlled by peptide regions within and upstream of the α-dystroglycan mucin domain. J. Biol. Chem. 2008, 283, 18832–18840. [Google Scholar] [CrossRef] [PubMed]
- Groma, G.; Grskovic, I.; Schael, S.; Ehlen, H.; Wagener, R.; Fosang, A.; Aszodi, A.; Paulsson, M.; Brachvogel, B.; Zaucke, F. Matrilin-4 is processed by ADAMTS-5 in late Golgi vesicles present in growth plate chondrocytes of defined differentiation state. Matrix Biol. 2011, 30, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Selman, M.; Hemayatkar, M.; Deelder, A.M.; Wuhrer, M. Cotton HILIC SPE Microtips for Microscale Purification and Enrichment of Glycans and Glycopeptides. Anal. Chem. 2011, 83, 2492–2499. [Google Scholar] [CrossRef] [PubMed]
- Breloy, I.; Pacharra, S.; Aust, C.; Hanisch, F.G. A sensitive gel-based global O-glycomics approach reveals high levels of mannosyl glycans in the high mass region of the mouse brain proteome. Biol. Chem. 2012, 393, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Bonar, D.; Hanisch, F.G. Trefoil factor family domains represent highly efficient conformational determinants for N-linked N,N'-di-N-acetyllactosediamine (LacdiNAc) synthesis. J. Biol. Chem. 2014, 289, 29677–29690. [Google Scholar] [CrossRef] [PubMed]
- Ciucanu, I.; Kerek, F. A simple and rapid method for the permethylation of carbohydrates. Carbohydr. Res. 1984, 131, 209–217. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Breloy, I.; Schwientek, T.; Althoff, D.; Holz, M.; Koppen, T.; Krupa, A.; Hanisch, F.-G. Functional Analysis of the Glucuronyltransferases GlcAT-P and GlcAT-S of Drosophila melanogaster: Distinct Activities towards the O-linked T-antigen. Biomolecules 2016, 6, 8. https://doi.org/10.3390/biom6010008
Breloy I, Schwientek T, Althoff D, Holz M, Koppen T, Krupa A, Hanisch F-G. Functional Analysis of the Glucuronyltransferases GlcAT-P and GlcAT-S of Drosophila melanogaster: Distinct Activities towards the O-linked T-antigen. Biomolecules. 2016; 6(1):8. https://doi.org/10.3390/biom6010008
Chicago/Turabian StyleBreloy, Isabelle, Tilo Schwientek, Deborah Althoff, Marvin Holz, Tim Koppen, Angelika Krupa, and Franz-Georg Hanisch. 2016. "Functional Analysis of the Glucuronyltransferases GlcAT-P and GlcAT-S of Drosophila melanogaster: Distinct Activities towards the O-linked T-antigen" Biomolecules 6, no. 1: 8. https://doi.org/10.3390/biom6010008
APA StyleBreloy, I., Schwientek, T., Althoff, D., Holz, M., Koppen, T., Krupa, A., & Hanisch, F.-G. (2016). Functional Analysis of the Glucuronyltransferases GlcAT-P and GlcAT-S of Drosophila melanogaster: Distinct Activities towards the O-linked T-antigen. Biomolecules, 6(1), 8. https://doi.org/10.3390/biom6010008