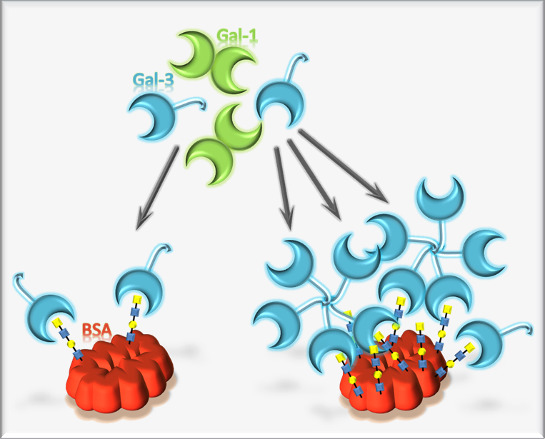
Galectin Binding to Neo-Glycoproteins: LacDiNAc Conjugated BSA as Ligand for Human Galectin-3
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemo-Enzymatic Synthesis of LacNAc-LacNAc and LacDiNAc-LacNAc
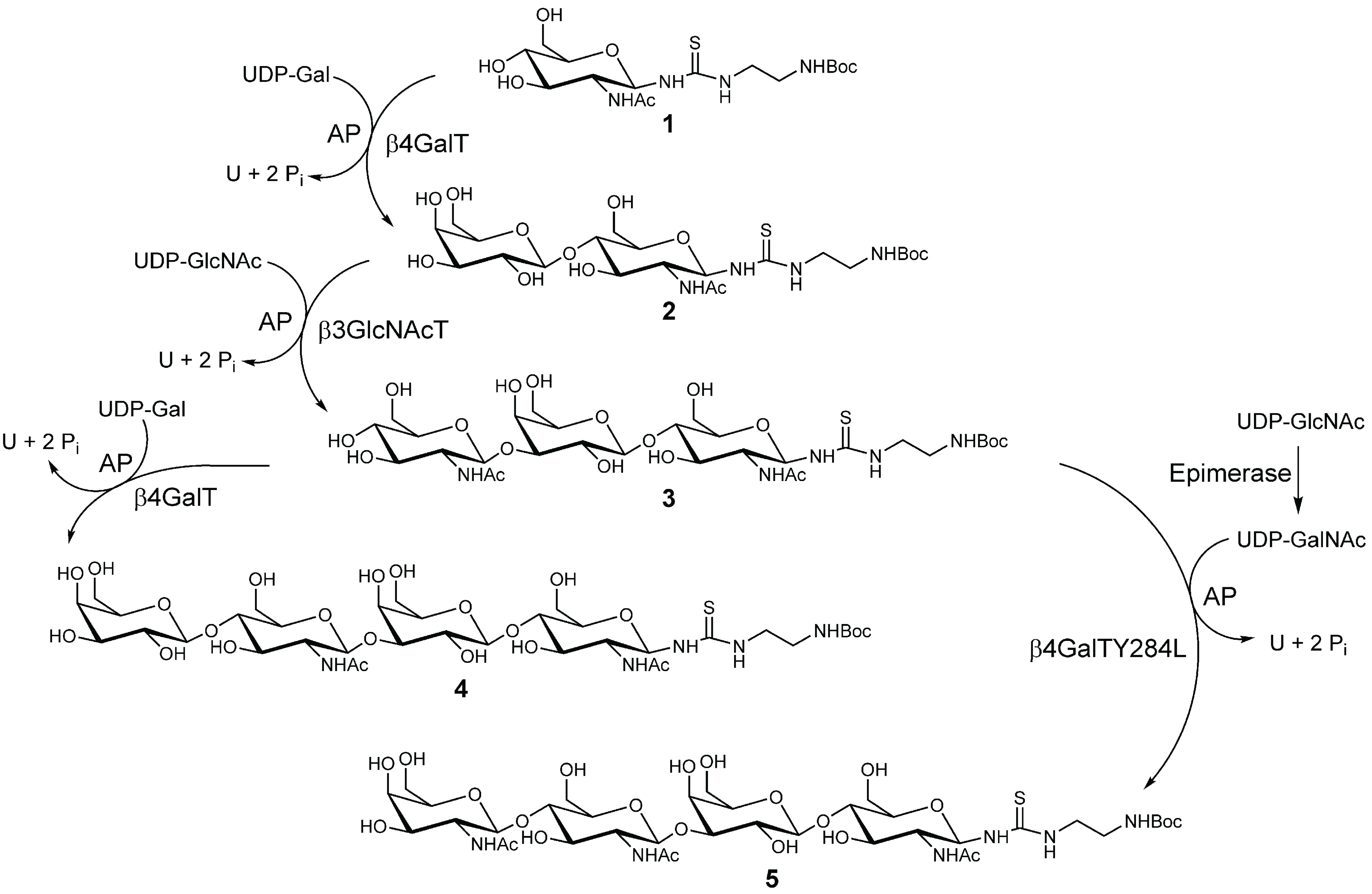
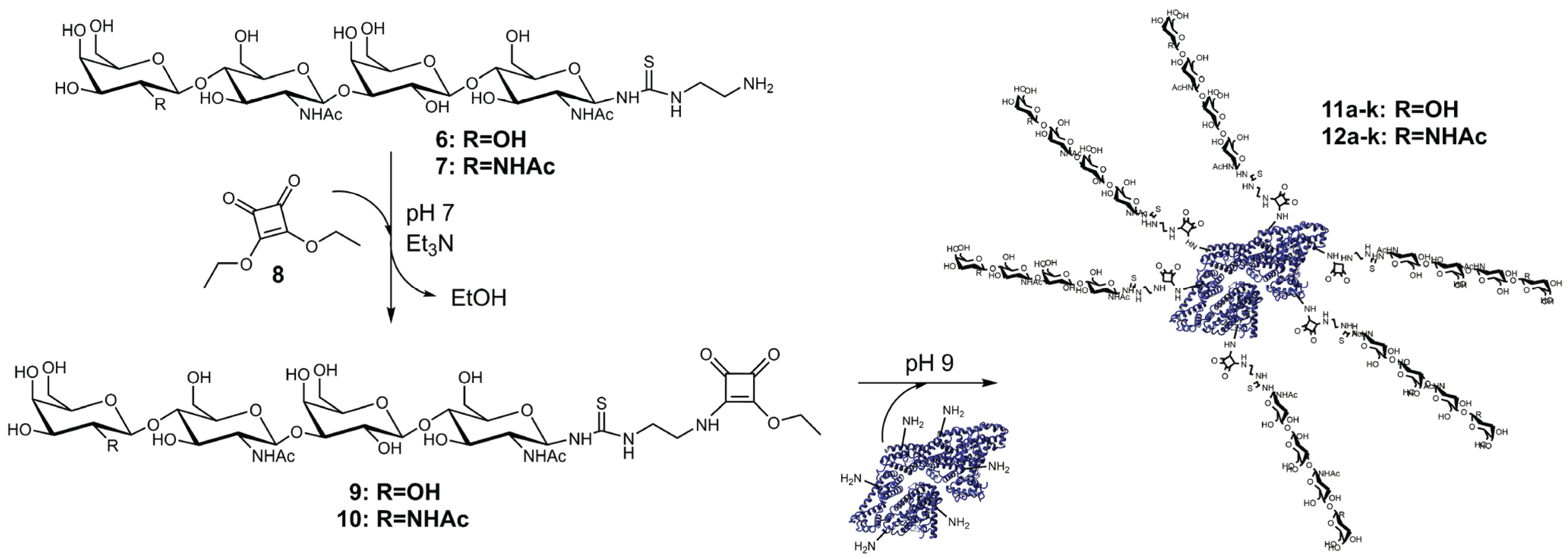
2.2. Synthesis and Purification of Squaric Acid Monoamide Esters
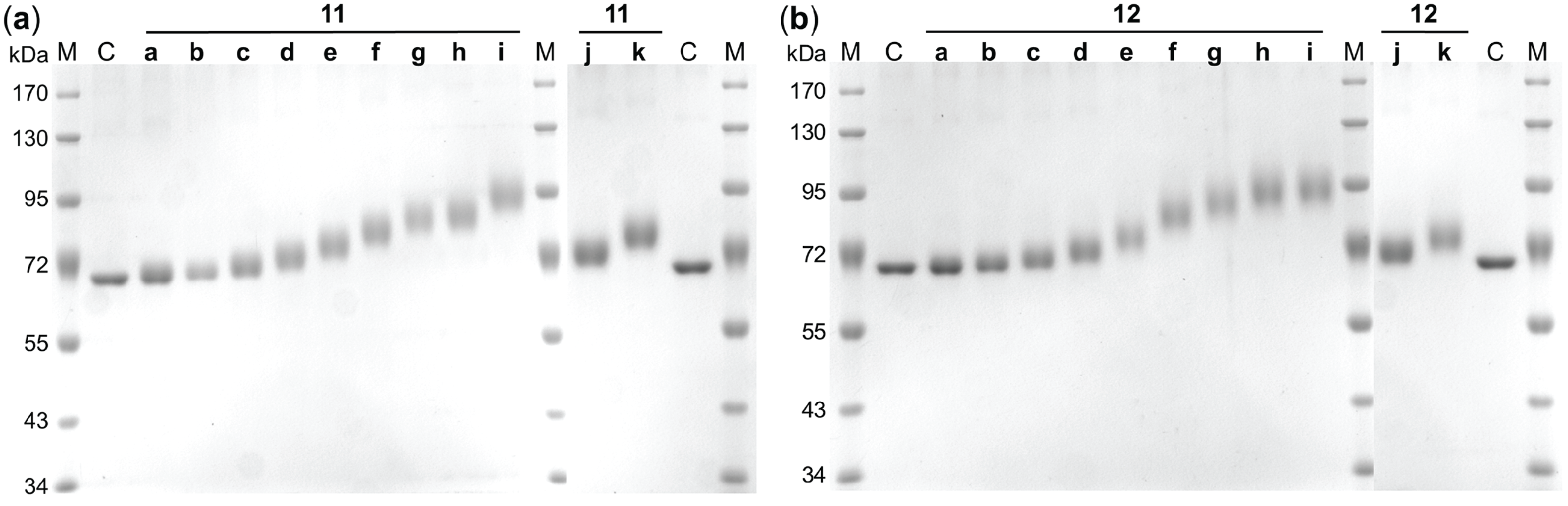
2.3. Synthesis and Analysis of Neo-Glycoproteins
| Compound | Unmodified lysine residues [mol/mol BSA] | Amidated lysine residues [mol/mol BSA] | MW [kDa] | Compound | Unmodified lysine residues [mol/mol BSA] | Amidated lysine residues [mol/mol BSA] | MW [kDa] |
|---|---|---|---|---|---|---|---|
| BSA | 60.0 ± 0.027 | 0.0 | 66.4 | BSA | 60.0 ± 0.027 | 0.0 | 66.4 |
| 11a | 58.4 ± 0.016 | 1.6 | 67.9 | 12a | 58.3 ± 0.012 | 1.7 | 68.1 |
| 11b | 56.2 ± 0.005 | 3.8 | 70.0 | 12b | 57.8 ± 0.005 | 2.2 | 68.6 |
| 11c | 53.9 ± 0.013 | 6.1 | 72.2 | 12c | 54.1 ± 0.012 | 5.9 | 72.2 |
| 11d | 50.0 ± 0.040 | 10.0 | 75.9 | 12d | 51.2 ± 0.012 | 8.8 | 75.1 |
| 11e | 45.6 ± 0.015 | 14.4 | 80.0 | 12e | 45.9 ± 0.046 | 14.1 | 80.3 |
| 11f | 40.6 ± 0.027 | 19.4 | 84.7 | 12f | 38.7 ± 0.091 | 21.3 | 87.4 |
| 11g | 35.8 ± 0.079 | 24.2 | 89.3 | 12g | 35.6 ± 0.115 | 24.4 | 90.4 |
| 11h | 34.0 ± 0.009 | 26.0 | 90.9 | 12h | 35.0 ± 0.066 | 25.0 | 91.0 |
| 11i | 31.0 ± 0.130 | 29.0 | 93.8 | 12i | 32.5 ± 0.097 | 27.5 | 93.5 |
| 11j | 52.5 ± 0.012 | 7.5 | 73.5 | 12j | 52.6 ± 0.018 | 7.4 | 73.7 |
| 11k | 42.2 ± 0.084 | 17.8 | 83.2 | 12k | 42.0 ± 0.060 | 18.0 | 84.1 |
| Compound | TNBSA ΔMW [kDa] | SDS-PAGE ΔMW [kDa] | Compound | TNBSA ΔMW [kDa] | SDS-PAGE ΔMW [kDa] |
|---|---|---|---|---|---|
| BSA | 0.0 | 0.0 | BSA | 0.0 | 0.0 |
| 11a | 1.5 | 1.3 | 12a | 1.7 | 1.5 |
| 11b | 3.6 | 3.0 | 12b | 2.2 | 2.5 |
| 11c | 5.8 | 5.0 | 12c | 5.8 | 4.6 |
| 11d | 9.4 | 8.2 | 12d | 8.7 | 7.3 |
| 11e | 13.6 | 12.1 | 12e | 13.9 | 11.7 |
| 11f | 18.3 | 17.4 | 12f | 21.0 | 19.0 |
| 11g | 22.8 | 22.4 | 12g | 24.0 | 22.2 |
| 11h | 24.5 | 24.3 | 12h | 24.6 | 25.5 |
| 11i | 27.3 | 27.6 | 12i | 27.1 | 28.2 |
| 11j | 7.1 | 7.0 | 12j | 7.3 | 7.1 |
| 11k | 16.8 | 15.0 | 12k | 17.7 | 15.6 |
2.4. Comparison of Galectin-3 and Galectin-1 Binding to Neo-Glycoproteins
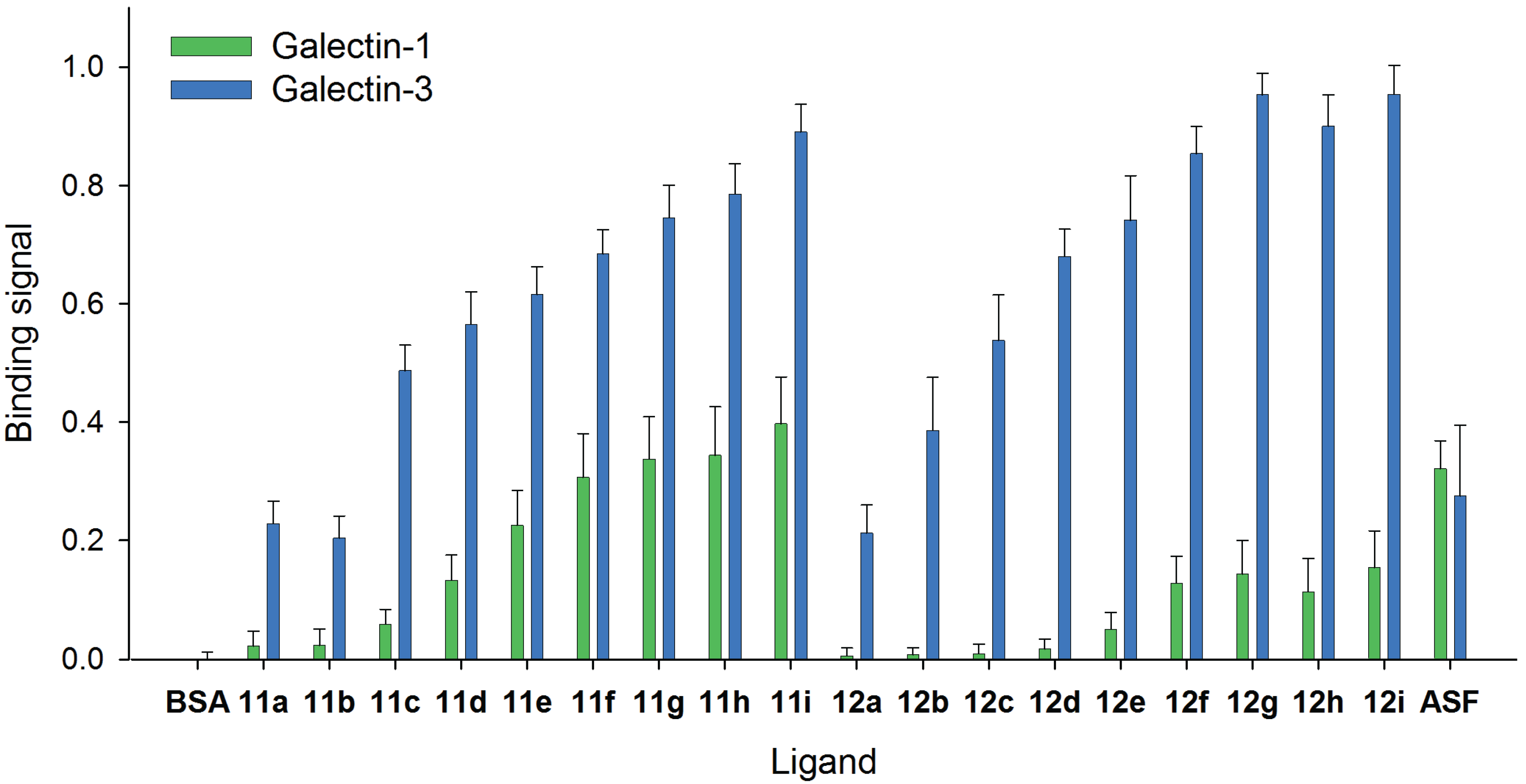
2.5. Galectin-3 Binding to Neo-Glycoproteins at Different Galectin Concentrations
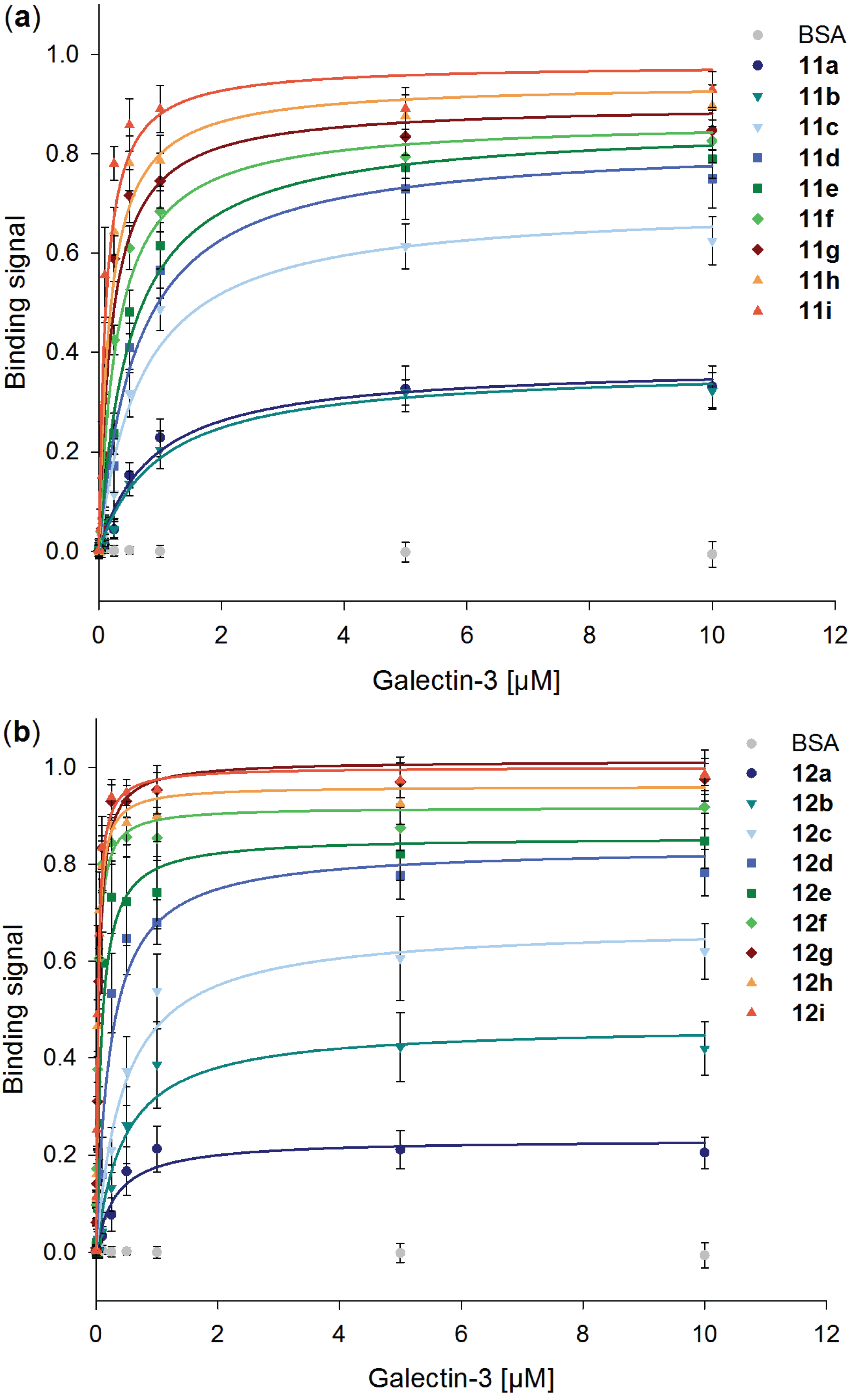
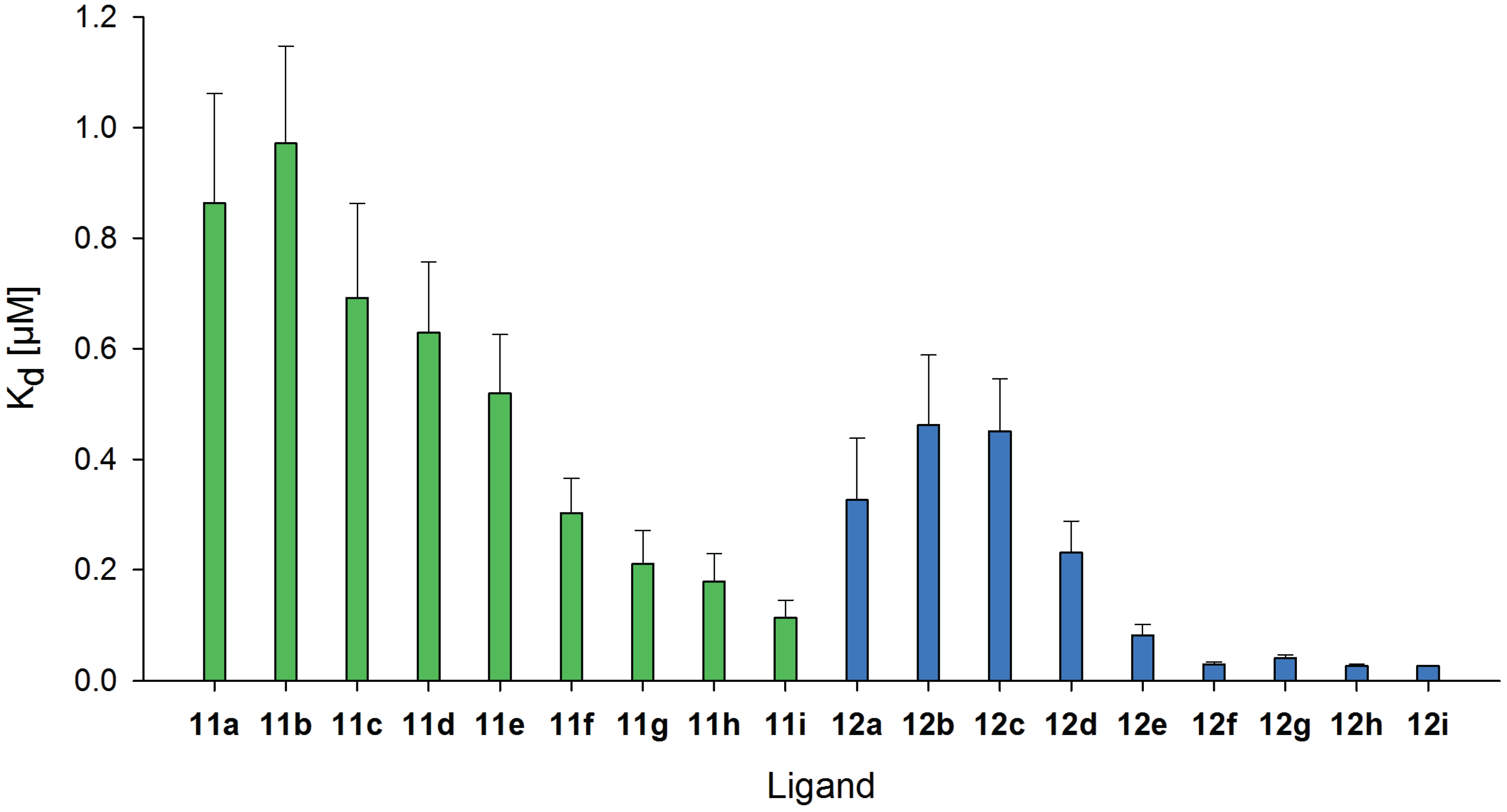
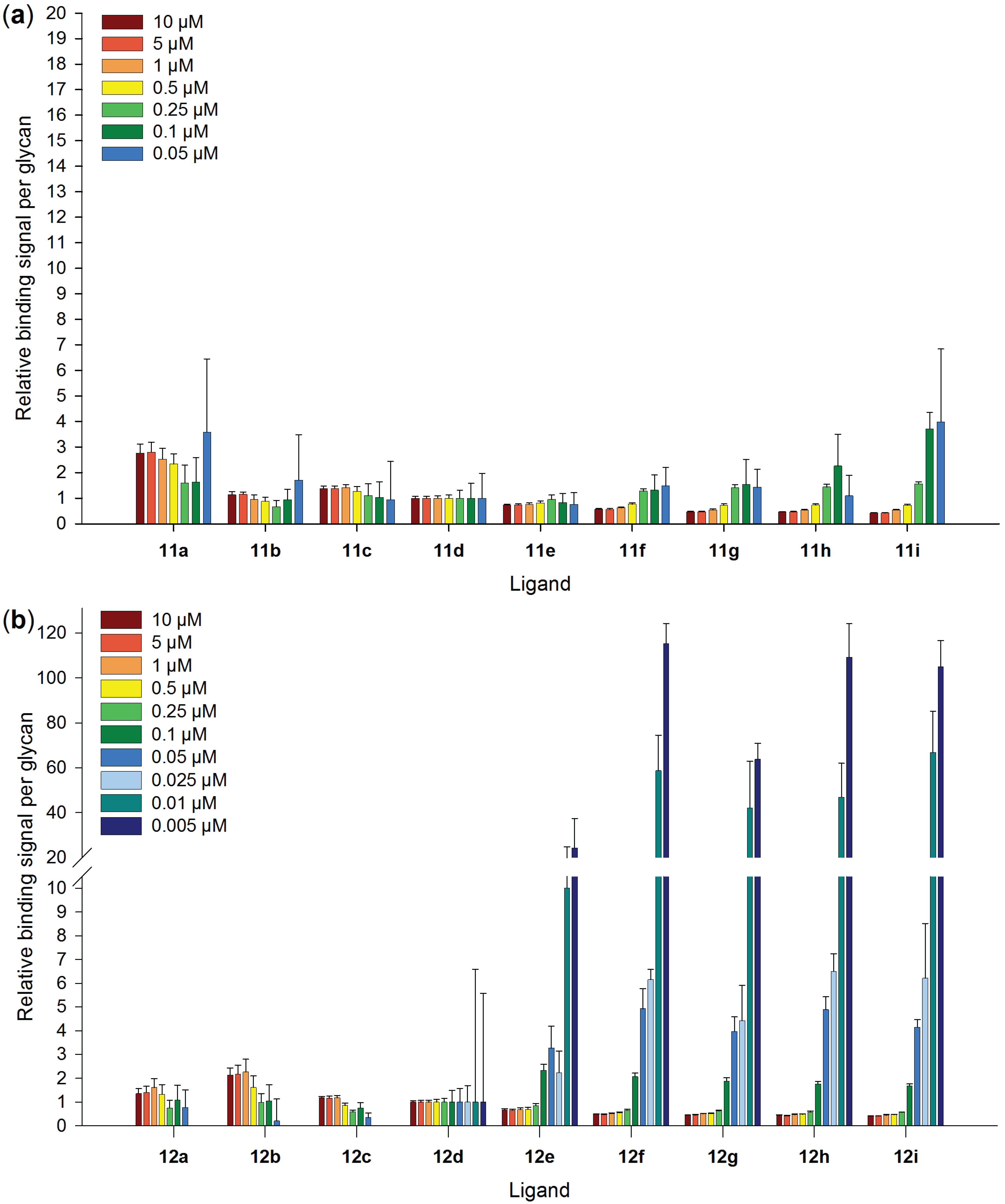
2.6. Inhibitory Potency of Neo-Glycoproteins
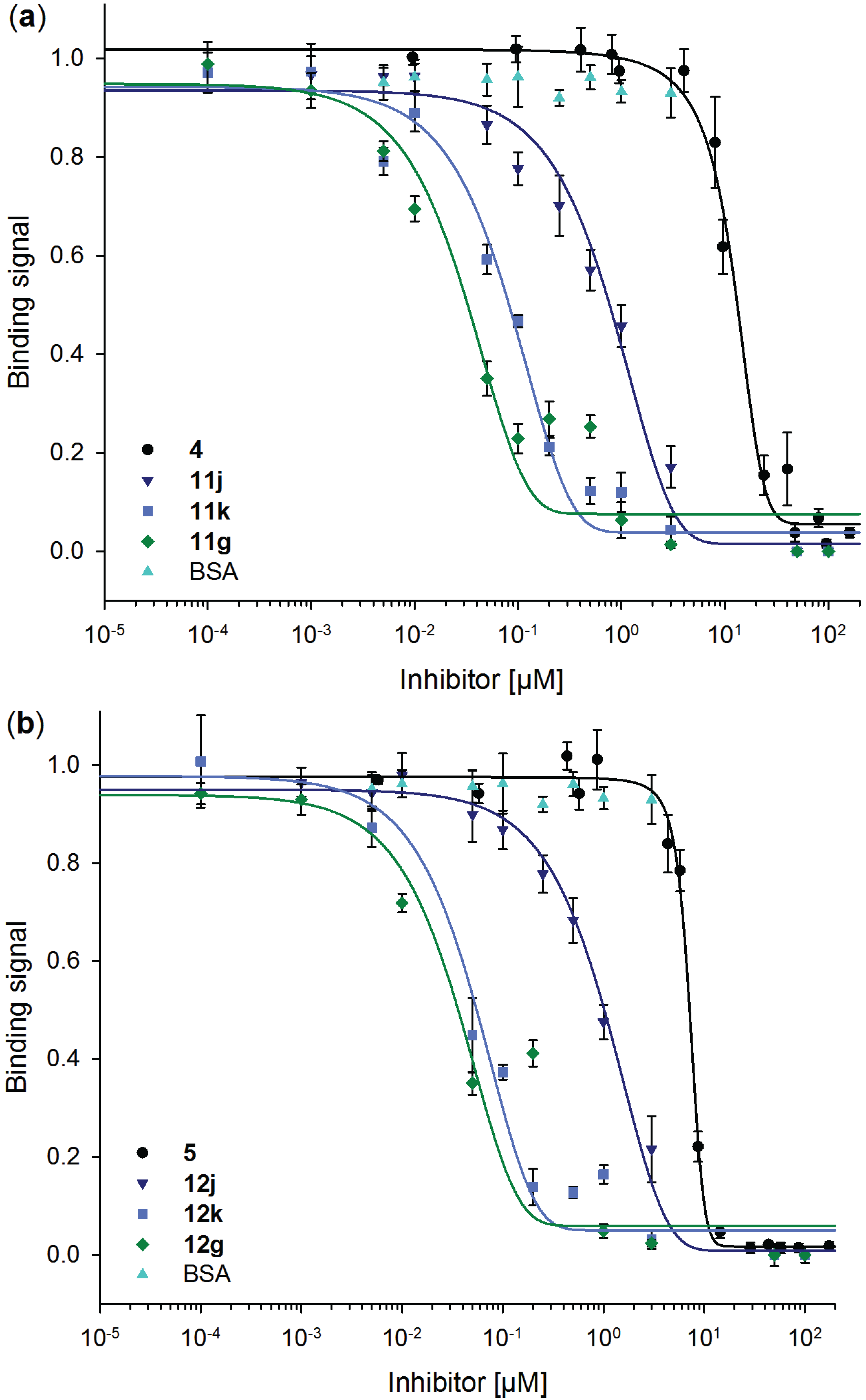
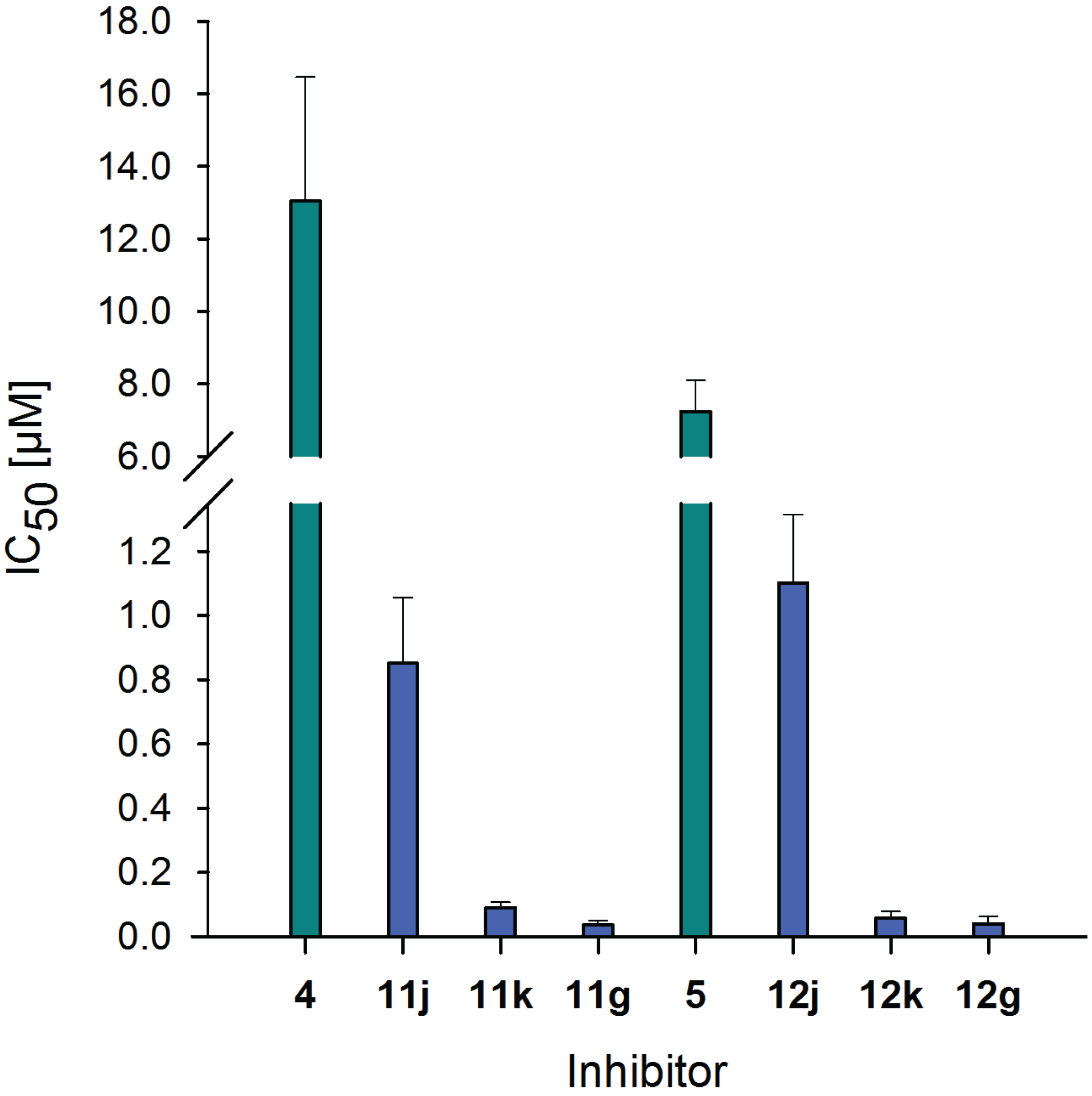
| Compound | IC50 [µM] | Relative potency | Relative potency per glycan |
|---|---|---|---|
| 4 | 13.04 ± 3.43 | 1.0 ± 0.26 | 1.0 ± 0.26 |
| 11j | 0.85 ± 0.21 | 15.3 ± 3.14 | 2.0 ± 0.42 |
| 11k | 0.09 ± 0.02 | 145.2 ± 2.45 | 8.2 ± 0.14 |
| 11g | 0.04 ± 0.01 | 367.8 ± 5.12 | 15.2 ± 0.21 |
| 5 | 7.23 ± 0.87 | 1.0 ± 0.12 | 1.0 ± 0.12 |
| 12j | 1.10 ± 0.22 | 6.6 ± 1.42 | 0.9 ± 0.19 |
| 12k | 0.06 ± 0.02 | 127.1 ± 2.56 | 7.1 ± 0.15 |
| 12g | 0.04 ± 0.02 | 182.0 ± 4.06 | 7.5 ± 0.17 |
3. Experimental Section
3.1. Production of Recombinant Enzymes
3.2. Chemo-Enzymatic Synthesis of Glycans (4 and 5)
3.3. Synthesis of Compounds 9 and 10
3.4. Synthesis of Neo-Glycoproteins Based on BSA
3.5. Analysis of Neo-Glycoproteins
3.6. Expression and Purification of Recombinant Galectins
3.7. Galectin Binding Assays on Neo-Glycoproteins
3.8. Inhibition of Galectin Binding with Neo-Glycoproteins
4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Varki, A. Biological roles of oligosaccharides: All of the theories are correct. Glycobiology 1993, 3, 97–130. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-Y.; Takahashi, M.; Gu, J.-G.; Miyoshi, E.; Matsumoto, A.; Kitazume, S.; Taniguchi, N. Functional roles of N-glycans in cell signaling and cell adhesion in cancer. Cancer Sci. 2008, 99, 1304–1310. [Google Scholar] [CrossRef] [PubMed]
- Boscher, C.; Dennis, J.W.; Nabi, I.R. Glycosylation, galectins and cellular signaling. Curr. Opin. Cell Biol. 2011, 23, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Fred Brewer, C. Binding and cross-linking properties of galectins. BBA Gen. Subj. 2002, 1572, 255–262. [Google Scholar] [CrossRef]
- Funasaka, T.; Raz, A.; Nangia-Makker, P. Galectin-3 in angiogenesis and metastasis. Glycobiology 2014, 24, 886–891. [Google Scholar] [CrossRef] [PubMed]
- Lau, K.S.; Dennis, J.W. N-glycans in cancer progression. Glycobiology 2008, 18, 750–760. [Google Scholar] [CrossRef] [PubMed]
- Noorjahan, P. Role of galectins in wound healing. In Galectins and Disease Implications for Targeted Therapeutics; ACS: Washington, DC, USA, 2012; Volume 1115, pp. 415–432. [Google Scholar]
- Schattner, M. Platelets and galectins. Ann. Transl. Med. 2014, 2. [Google Scholar] [CrossRef]
- Lundquist, J.J.; Toone, E.J. The cluster glycoside effect. Chem. Rev. 2002, 102, 555–578. [Google Scholar] [CrossRef] [PubMed]
- Kiessling, L.L.; Young, T.; Gruber, T.D.; Mortell, K.H. Multivalency in protein-carbohydrate recognition. In Glycoscience; Fraser-Reid, B., Tatsuta, K., Thiem, J., Eds.; Springer: Berlin, Heidelberg, Germany, 2008; pp. 2483–2523. [Google Scholar]
- Pieters, R.J. Maximising multivalency effects in protein-carbohydrate interactions. Org. Biomol. Chem. 2009, 7, 2013–2025. [Google Scholar] [CrossRef] [PubMed]
- Pieters, R.J.; Arnusch, C.J.; Breukink, E. Membrane permeabilization by multivalent anti-microbial peptides. Protein Pept. Lett. 2009, 16, 736–742. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, A.; Jimenez-Barbero, J.; Casnati, A.; de Castro, C.; Darbre, T.; Fieschi, F.; Finne, J.; Funken, H.; Jaeger, K.-E.; Lahmann, M.; et al. Multivalent glycoconjugates as anti-pathogenic agents. Chem. Soc. Rev. 2013, 42, 4709–4727. [Google Scholar] [CrossRef] [PubMed]
- Peri, F. Clustered carbohydrates in synthetic vaccines. Chem. Soc. Rev. 2013, 42, 4543–4556. [Google Scholar] [CrossRef] [PubMed]
- Branson, T.R.; Turnbull, W.B. Bacterial toxin inhibitors based on multivalent scaffolds. Chem. Soc. Rev. 2013, 42, 4613–4622. [Google Scholar] [CrossRef] [PubMed]
- Chabre, Y.M.; Roy, R. Multivalent glycoconjugate syntheses and applications using aromatic scaffolds. Chem. Soc. Rev. 2013, 42, 4657–4708. [Google Scholar] [CrossRef] [PubMed]
- Marradi, M.; Chiodo, F.; Garcia, I.; Penades, S. Glyconanoparticles as multifunctional and multimodal carbohydrate systems. Chem. Soc. Rev. 2013, 42, 4728–4745. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.; Ortiz Mellet, C.; Garcia Fernandez, J.M. Cyclodextrin-based multivalent glycodisplays: Covalent and supramolecular conjugates to assess carbohydrate-protein interactions. Chem. Soc. Rev. 2013, 42, 4746–4773. [Google Scholar] [CrossRef] [PubMed]
- Hatano, K.; Matsuoka, K.; Terunuma, D. Carbosilane glycodendrimers. Chem. Soc. Rev. 2013, 42, 4574–4598. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Star, A.; Vidal, S. Sweet carbon nanostructures: Carbohydrate conjugates with carbon nanotubes and graphene and their applications. Chem. Soc. Rev. 2013, 42, 4532–4542. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.C.; Grünstein, D.; Lai, C.-H.; Seeberger, P.H. Glycosylated nanoscale surfaces: Preparation and applications in medicine and molecular biology. Chem. Eur. J. 2013, 19, 3794–3800. [Google Scholar] [CrossRef] [PubMed]
- Sansone, F.; Casnati, A. Multivalent glycocalixarenes for recognition of biological macromolecules: Glycocalyx mimics capable of multitasking. Chem. Soc. Rev. 2013, 42, 4623–4639. [Google Scholar] [CrossRef] [PubMed]
- Cecioni, S.; Imberty, A.; Vidal, S. Glycomimetics versus multivalent glycoconjugates for the design of high affinity lectin ligands. Chem. Rev. 2014, 115, 525–561. [Google Scholar] [CrossRef] [PubMed]
- Bojarová, P.; Rosencrantz, R.R.; Elling, L.; Křen, V. Enzymatic glycosylation of multivalent scaffolds. Chem. Soc. Rev. 2013, 42, 4774–4797. [Google Scholar] [CrossRef] [PubMed]
- Ercolani, G.; Schiaffino, L. Allosteric, chelate, and interannular cooperativity: A mise AU point. Angew. Chem. Int. Ed. 2011, 50, 1762–1768. [Google Scholar] [CrossRef] [PubMed]
- Hunter, C.A.; Anderson, H.L. What is cooperativity? Angew. Chem. Int. Ed. 2009, 48, 7488–7499. [Google Scholar] [CrossRef] [PubMed]
- Maierhofer, C.; Rohmer, K.; Wittmann, V. Probing multivalent carbohydrate-lectin interactions by an enzyme-linked lectin assay employing covalently immobilized carbohydrates. Bioorg. Med. Chem. 2007, 15, 7661–7676. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.; Lee, Y. Affinity enhancement by multivalent lectin-carbohydrate interaction. Glycoconj. J. 2000, 17, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Profit, A.A.; Lee, T.R.; Lawrence, D.S. Bivalent inhibitors of protein tyrosine kinases. J. Am. Chem. Soc. 1999, 121, 280–283. [Google Scholar] [CrossRef]
- Schaschke, N.; Matschiner, G.; Zettl, F.; Marquardt, U.; Bergner, A.; Bode, W.; Sommerhoff, C.P.; Moroder, L. Bivalent inhibition of human β-tryptase. Chem. Biol. 2001, 8, 313–327. [Google Scholar] [CrossRef]
- Rao, J.; Lahiri, J.; Isaacs, L.; Weis, R.M.; Whitesides, G.M. A trivalent system from vancomycin·d-Ala-d-Ala with higher affinity than avidin·biotin. Science 1998, 280, 708–711. [Google Scholar] [CrossRef] [PubMed]
- Blackmore, P.F.; Eisoldt, S. The neoglycoprotein mannose-bovine serum albumin, but not progesterone, activates T-type calcium channels in human spermatozoa. Mol. Hum. Reprod. 1999, 5, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.S.; Ahn, H.S.; Gye, M.C. Fucosyl neoglycoprotein binds to mouse epididymal spermatozoa and inhibits sperm binding to the egg zona pellucida. Andrologia 2013, 45, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.X.; Kim, H.-Y.; Dass, C. Probing three-dimensional structure of bovine serum albumin by chemical cross-linking and mass spectrometry. J. Am. Soc. Mass Spectrom. 2004, 15, 1237–1247. [Google Scholar] [CrossRef] [PubMed]
- Luyai, A.; Lasanajak, Y.; Smith, D.F.; Cummings, R.D.; Song, X. Facile preparation of fluorescent neoglycoproteins using p-nitrophenyl anthranilate as a heterobifunctional linker. Bioconjug. Chem. 2009, 20, 1618–1624. [Google Scholar] [CrossRef] [PubMed]
- Unverzagt, C.; André, S.; Seifert, J.; Kojima, S.; Fink, C.; Srikrishna, G.; Freeze, H.; Kayser, K.; Gabius, H.-J. Structure-activity profiles of complex biantennary glycans with core fucosylation and with/without additional α2,3/α2,6 sialylation: Synthesis of neoglycoproteins and their properties in lectin assays, cell binding, and organ uptake. J. Med. Chem. 2002, 45, 478–491. [Google Scholar] [CrossRef] [PubMed]
- Gabius, H.J.; Bardosi, A. Neoglycoproteins as tools in glycohistochemistry. Prog. Histochem. Cytochem. 1991, 22, 1–63. [Google Scholar] [CrossRef]
- Stowell, C.P.; Lee, Y.C. Neoglycoproteins the preparation and application of synthetic glycoproteins. In Advances in Carbohydrate Chemistry and Biochemistry; Tipson, R.S., Derek, H., Eds.; Academic Press: Waltham, MA, USA, 1980; Volume 37, pp. 225–281. [Google Scholar]
- Kerekgyarto, M.; Fekete, A.; Szurmai, Z.; Kerekgyarto, J.; Takacs, L.; Kurucz, I.; Guttman, A. Neoglycoproteins as carbohydrate antigens: Synthesis, analysis, and polyclonal antibody response. Electrophoresis 2013, 34, 2379–2386. [Google Scholar] [CrossRef] [PubMed]
- Barondes, S.H.; Cooper, D.N.; Gitt, M.A.; Leffler, H. Galectins. Structure and function of a large family of animal lectins. J. Biol. Chem. 1994, 269, 20807–20810. [Google Scholar] [PubMed]
- Leffler, H.; Carlsson, S.; Hedlund, M.; Qian, Y.; Poirier, F. Introduction to galectins. Glycoconj. J. 2002, 19, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Griffioen, A.W.; Thijssen, V.L. Galectins in tumor angiogenesis. Ann. Transl. Med. 2014, 2. [Google Scholar] [CrossRef]
- Rabinovich, G.A.; van Kooyk, Y.; Cobb, B.A. Glycobiology of immune responses. Ann. NY Acad. Sci. 2012, 1253, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.-T.; Rabinovich, G.A. Galectins as modulators of tumour progression. Nat. Rev. Cancer 2005, 5, 29–41. [Google Scholar] [CrossRef] [PubMed]
- D’Haene, N.; Maris, C.; Rorive, S.; Decaestecker, C.; le Mercier, M.; Salmon, I. Galectins and neovascularization in central nervous system tumors. Glycobiology 2014, 24, 892–898. [Google Scholar] [CrossRef] [PubMed]
- Compagno, D.; Gentilini, L.D.; Jaworski, F.M.; Pérez, I.G.; Contrufo, G.; Laderach, D.J. Glycans and galectins in prostate cancer biology, angiogenesis and metastasis. Glycobiology 2014, 24, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Le Mercier, M.; Fortin, S.; Mathieu, V.; Kiss, R.; Lefranc, F. Galectins and gliomas. Brain Pathol. 2010, 20, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, S.; Oka, N.; Raz, A. On the role of galectin-3 in cancer apoptosis. Apoptosis 2005, 10, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Dumic, J.; Dabelic, S.; Flögel, M. Galectin-3: An open-ended story. BBA Gen. Subj. 2006, 1760, 616–635. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, G.; Iacobini, C.; Pesce, C.M.; Menini, S. Galectin-3: An emerging all-out player in metabolic disorders and their complications. Glycobiology 2015, 25, 136–150. [Google Scholar] [CrossRef] [PubMed]
- Hakon, L.; Ulf, J.N. Low-molecular weight inhibitors of galectins. In Galectins and Disease Implications for Targeted Therapeutics; ACS: Washington, DC, USA, 2012; Volume 1115, pp. 47–59. [Google Scholar]
- Stowell, S.R.; Arthur, C.M.; Mehta, P.; Slanina, K.A.; Blixt, O.; Leffler, H.; Smith, D.F.; Cummings, R.D. Galectin-1,-2, and-3 exhibit differential recognition of sialylated glycans and blood group antigens. J. Biol. Chem. 2008, 283, 10109–10123. [Google Scholar] [CrossRef] [PubMed]
- Téllez-Sanz, R.; Garcia-Fuentes, L.; Vargas-Berenguel, A. Human galectin-3 selective and high affinity inhibitors. Present state and future perspectives. Curr. Med. Chem. 2013, 20, 2979–2990. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Gabius, H.J.; Andre, S.; Kaltner, H.; Sabesan, S.; Roy, R.; Liu, B.C.; Macaluso, F.; Brewer, C.F. Galectin-3 precipitates as a pentamer with synthetic multivalent carbohydrates and forms heterogeneous cross-linked complexes. J. Biol. Chem. 2004, 279, 10841–10847. [Google Scholar] [CrossRef] [PubMed]
- Lepur, A.; Salomonsson, E.; Nilsson, U.J.; Leffler, H. Ligand induced galectin-3 protein self-association. J. Biol. Chem. 2012, 287, 21751–21756. [Google Scholar] [CrossRef] [PubMed]
- Dam, T.K.; Gabius, H.-J.; André, S.; Kaltner, H.; Lensch, M.; Brewer, C.F. Galectins bind to the multivalent glycoprotein asialofetuin with enhanced affinities and a gradient of decreasing binding constants. Biochemistry 2005, 44, 12564–12571. [Google Scholar] [CrossRef] [PubMed]
- Goodman, C.K.; Wolfenden, M.L.; Nangia-Makker, P.; Michel, A.K.; Raz, A.; Cloninger, M.J. Multivalent scaffolds induce galectin-3 aggregation into nanoparticles. Beilstein J. Org. Chem. 2014, 10, 1570–1577. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xue, X.C.; Jin, X.F.; Wang, L.J.; Sha, Y.L.; Li, Z.J. Synthesis of multivalent n-acetyl lactosamine modified quantum dots for the study of carbohydrate and galectin-3 interactions. Tetrahedron 2012, 68, 7148–7154. [Google Scholar] [CrossRef]
- Wolfenden, M.; Cousin, J.; Nangia-Makker, P.; Raz, A.; Cloninger, M. Glycodendrimers and modified elisas: Tools to elucidate multivalent interactions of galectins 1 and 3. Molecules 2015, 20, 7059–7096. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, T.K.; Honing, H.; Franke, N.; van Remoortere, A.; Schiphorst, W.; Liu, F.T.; Deelder, A.M.; Cummings, R.D.; Hokke, C.H.; van Die, I. Lacdinac-glycans constitute a parasite pattern for galectin-3-mediated immune recognition. J. Immunol. 2004, 173, 1902–1907. [Google Scholar] [CrossRef] [PubMed]
- Hirano, K.; Matsuda, A.; Shirai, T.; Furukawa, K. Expression of lacdinac group on N-glycans among human tumors is complex. Biomed. Res. Int. 2014. [CrossRef] [PubMed]
- Do, K.-Y.; Do, S.; Cummings, R.D. Differential expression of lacdinac sequences (GalNAcβ1-4GlcNAc-R) in glycoproteins synthesized by chinese hamster ovary and human 293 cells. Glycobiology 1997, 7, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Kenny, D.T.; Skoog, E.C.; Lindén, S.K.; Struwe, W.B.; Rudd, P.M.; Karlsson, N.G. Presence of terminal N-acetylgalactosamineβ1-4N-acetylglucosamine residues on O-linked oligosaccharides from gastric MUC5AC: Involvement in helicobacter pylori colonization? Glycobiology 2012, 22, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Rossez, Y.; Gosset, P.; Boneca, I.G.; Magalhaes, A.; Ecobichon, C.; Reis, C.A.; Cieniewski-Bernard, C.; Joncquel Chevalier Curt, M.; Leonard, R.; Maes, E.; et al. The lacdinac-specific adhesin laba mediates adhesion of helicobacter pylori to human gastric mucosa. J. Infect. Dis. 2014, 210, 1286–1295. [Google Scholar] [CrossRef] [PubMed]
- Šimonová, A.; Kupper, C.E.; Böcker, S.; Müller, A.; Hofbauerová, K.; Pelantová, H.; Elling, L.; Křen, V.; Bojarová, P. Chemo-enzymatic synthesis of lacdinac dimers of varying length as novel galectin ligands. J. Mol. Catal. B Enzym. 2014, 101, 47–55. [Google Scholar] [CrossRef]
- Kupper, C.E.; Böcker, S.; Liu, H.L.; Adamzyk, C.; van de Kamp, J.; Recker, T.; Lethaus, B.; Jahnen-Dechent, W.; Neuss, S.; Müller-Newen, G.; et al. Fluorescent SNAP-Tag galectin fusion proteins as novel tools in glycobiology. Curr. Pharm. Des. 2013, 19, 5457–5467. [Google Scholar] [CrossRef] [PubMed]
- Rech, C.; Rosencrantz, R.R.; Křenek, K.; Pelantová, H.; Bojarová, P.; Römer, C.E.; Hanisch, F.-G.; Křen, V.; Elling, L. Combinatorial one-pot synthesis of poly-N-acetyllactosamine oligosaccharides with leloir-glycosyltransferases. Adv. Synth. Catal. 2011, 353, 2492–2500. [Google Scholar] [CrossRef]
- Kamath, V.P.; Diedrich, P.; Hindsgaul, O. Use of diethyl squarate for the coupling of oligosaccharide amines to carrier proteins and characterization of the resulting neoglycoproteins by MALDI-TOF mass spectrometry. Glycoconj. J. 1996, 13, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Tietze, L.F.; Arlt, M.; Beller, M.; gl üsenkamp, K.-H.; Jähde, E.; Rajewsky, M.F. Anticancer agents, 15. Squaric acid diethyl ester: A new coupling reagent for the formation of drug biopolymer conjugates. Synthesis of squaric acid ester amides and diamides. Chem. Ber. 1991, 124, 1215–1221. [Google Scholar] [CrossRef]
- Tietze, L.F.; Schroeter, C.; Gabius, S.; Brinck, U.; Goerlach-Graw, A.; Gabius, H.J. Conjugation of p-aminophenyl glycosides with squaric acid diester to a carrier protein and the use of the neoglycoprotein in the histochemical detection of lectins. Bioconjug. Chem. 1991, 2, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.W.; Asnani, A.; Auzanneau, F.I. Synthesis of a BSA-Lex glycoconjugate and recognition of Lex analogues by the anti-Lex monoclonal antibody sh1: The identification of a non-cross reactive analogue. Bioorg. Med. Chem. 2010, 18, 7174–7185. [Google Scholar] [CrossRef] [PubMed]
- Wurm, F.R.; Klok, H.A. Be squared: Expanding the horizon of squaric acid-mediated conjugations. Chem. Soc. Rev. 2013, 42, 8220–8236. [Google Scholar] [CrossRef] [PubMed]
- Komarova, B.S.; Orekhova, M.V.; Tsvetkov, Y.E.; Beau, R.; Aimanianda, V.; Latge, J.P.; Nifantiev, N.E. Synthesis of a pentasaccharide and neoglycoconjugates related to fungal alpha-(1→3)-glucan and their use in the generation of antibodies to trace aspergillus fumigatus cell wall. Chemistry 2015, 21, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Jahouh, F.; Hou, S.J.; Kovac, P.; Banoub, J.H. Determination of glycation sites by tandem mass spectrometry in a synthetic lactose-bovine serum albumin conjugate, a vaccine model prepared by dialkyl squarate chemistry. Rapid. Commun. Mass Spectrom. 2012, 26, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Jahouh, F.; Xu, P.; Vann, W.F.; Kovac, P.; Banoub, J.H. Mapping the glycation sites in the neoglycoconjugate from hexasaccharide antigen of vibrio cholerae, serotype ogawa and the recombinant tetanus toxin C-fragment carrier. J. Mass Spectrom. 2013, 48, 1083–1090. [Google Scholar] [CrossRef] [PubMed]
- Sauerzapfe, B.; Namdjou, D.J.; Schumacher, T.; Linden, N.; Křenek, K.; Křen, V.; Elling, L. Characterization of recombinant fusion constructs of human β1,4-galactosyltransferase 1 and the lipase pre-propeptide from staphylococcus hyicus. J. Mol. Catal. B Enzym. 2008, 50, 128–140. [Google Scholar] [CrossRef]
- Logan, S.M.; Altman, E.; Mykytczuk, O.; Brisson, J.R.; Chandan, V.; Schur, M.J.; St Michael, F.; Masson, A.; Leclerc, S.; Hiratsuka, K.; et al. Novel biosynthetic functions of lipopolysaccharide rfaj homologs from helicobacter pylori. Glycobiology 2005, 15, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Kupper, C.E.; Rosencrantz, R.R.; Henssen, B.; Pelantová, H.; Thönes, S.; Drozdova, A.; Křen, V.; Elling, L. Chemo-enzymatic modification of poly-N-acetyllactosamine (LacNAc) oligomers and N,N-diacetyllactosamine (LacDINAc) based on galactose oxidase treatment. Beilstein J. Org. Chem. 2012, 8, 712–725. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.J.; Saksena, R.; Kovac, P. Preparation of glycoconjugates by dialkyl squarate chemistry revisited. Carbohydr. Res. 2008, 343, 196–210. [Google Scholar] [CrossRef] [PubMed]
- Cayot, P.; Tainturier, G. The quantification of protein amino groups by the trinitrobenzenesulfonic acid method: A reexamination. Anal. Biochem. 1997, 249, 184–200. [Google Scholar] [CrossRef] [PubMed]
- Roth, Z.; Yehezkel, G.; Khalaila, I. Identification and quantification of protein glycosylation. Int. J. Carbohydr. Chem. 2012. [Google Scholar] [CrossRef]
- Ahmad, N.; Gabius, H.J.; Kaltner, H.; Andre, S.; Kuwabara, I.; Liu, F.T.; Oscarson, S.; Norberg, T.; Brewer, C.F. Thermodynamic binding studies of cell surface carbohydrate epitopes to galectins-1,-3, and-7: Evidence for differential binding specificities. Can. J. Chem. 2002, 80, 1096–1104. [Google Scholar] [CrossRef]
- Rapoport, E.M.; Andre, S.; Kurmyshkina, O.V.; Pochechueva, T.V.; Severov, V.V.; Pazynina, G.V.; Gabius, H.J.; Bovin, N.V. Galectin-loaded cells as a platform for the profiling of lectin specificity by fluorescent neoglycoconjugates: A case study on galectins-1 and-3 and the impact of assay setting. Glycobiology 2008, 18, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Stowell, S.R.; Dias-Baruffi, M.; Penttila, L.; Renkonen, O.; Nyame, A.K.; Cummings, R.D. Human galectin-1 recognition of poly-N-acetyllactosamine and chimeric polysaccharides. Glycobiology 2004, 14, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Leppänen, A.; Stowell, S.; Blixt, O.; Cummings, R.D. Dimeric galectin-1 binds with high affinity to alpha 2,3-sialylated and non-sialylated terminal N-acetyllactosamine units on surface-bound extended glycans. J. Biol. Chem. 2005, 280, 5549–5562. [Google Scholar] [CrossRef] [PubMed]
- Di Virgilio, S.; Glushka, J.; Moremen, K.; Pierce, M. Enzymatic synthesis of natural and C-13 enriched linear poly-N-acetyllactosamines as ligands for galectin-1. Glycobiology 1999, 9, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Qun, Z.; Cummings, R.D. L-14 lectin recognition of laminin and its promotion of in vitro cell adhesion. Arch. Biochem. Biophys. 1993, 300, 6–17. [Google Scholar] [CrossRef]
- Wang, H.; Huang, W.; Orwenyo, J.; Banerjee, A.; Vasta, G.R.; Wang, L.X. Design and synthesis of glycoprotein-based multivalent glyco-ligands for influenza hemagglutinin and human galectin-3. Bioorg. Med. Chem. 2013, 21, 2037–2044. [Google Scholar] [CrossRef] [PubMed]
- André, S.; Sansone, F.; Kaltner, H.; Casnati, A.; Kopitz, J.; Gabius, H.J.; Ungaro, R. Calix N arene-based glycoclusters: Bioactivity of thiourea-linked galactose/lactose moieties as inhibitors of binding of medically relevant lectins to a glycoprotein and cell-surface glycoconjugates and selectivity among human adhesion/growth-regulatory galectins. ChemBioChem 2008, 9, 1649–1661. [Google Scholar] [PubMed]
- André, S.; Grandjean, C.; Gautier, F.M.; Bernardi, S.; Sansone, F.; Gabius, H.J.; Ungaro, R. Combining carbohydrate substitutions at bioinspired positions with multivalent presentation towards optimising lectin inhibitors: Case study with calixarenes. Chem. Commun. 2011, 47, 6126–6128. [Google Scholar] [CrossRef] [PubMed]
- André, S.; Pieters, R.J.; Vrasidas, I.; Kaltner, H.; Kuwabara, I.; Liu, F.T.; Liskamp, R.M.; Gabius, H.J. Wedgelike glycodendrimers as inhibitors of binding of mammalian galectins to glycoproteins, lactose maxiclusters, and cell surface glycoconjugates. ChemBioChem 2001, 2, 822–830. [Google Scholar] [CrossRef]
- Song, L.; Tang, J.W.; Owusu, L.; Sun, M.Z.; Wu, J.; Zhang, J. Galectin-3 in cancer. Clin. Chim. Acta 2014, 431, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Saussez, S.; Lorfevre, F.; Lequeux, T.; Laurent, G.; Chantrain, G.; Vertongen, F.; Toubeau, G.R.; Decaestecker, C.; Kiss, R. The determination of the levels of circulating galectin-1 and -3 in HNSCC patients could be used to monitor tumor progression and/or responses to therapy. Oral. Oncol. 2008, 44, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Sakaki, M.; Oka, N.; Nakanishi, R.; Yamaguchi, K.; Fukumori, T.; Kanayama, H.O. Serum level of galectin-3 in human bladder cancer. J. Med. Invest. 2008, 55, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Iurisci, I.; Tinari, N.; Natoli, C.; Angelucci, D.; Cianchetti, E.; Iacobelli, S. Concentrations of galectin-3 in the sera of normal controls and cancer patients. Clin. Cancer Res. 2000, 6, 1389–1393. [Google Scholar] [PubMed]
- Johnson, K.D.; Glinskii, O.V.; Mossine, V.V.; Turk, J.R.; Mawhinney, T.P.; Anthony, D.C.; Henry, C.J.; Huxley, V.H.; Glinsky, G.V.; Pienta, K.J.; et al. Galectin-3 as a potential therapeutic target in tumors arising from malignant endothelia. Neoplasia 2007, 9, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Sörme, P.; Arnoux, P.; Kahl-Knutsson, B.; Leffler, H.; Rini, J.M.; Nilsson, U.J. Structural and thermodynamic studies on cation-Pi interactions in lectin-ligand complexes: High-affinity galectin-3 inhibitors through fine-tuning of an arginine-arene interaction. J. Am. Chem. Soc. 2005, 127, 1737–1743. [Google Scholar] [CrossRef] [PubMed]
- Nangia-Makker, P.; Hogan, V.; Honjo, Y.; Baccarini, S.; Tait, L.; Bresalier, R.; Raz, A. Inhibition of human cancer cell growth and metastasis in nude mice by oral intake of modified citrus pectin. J. Natl. Cancer Inst. 2002, 94, 1854–1862. [Google Scholar] [CrossRef] [PubMed]
- Iurisci, I.; Cumashi, A.; Sherman, A.A.; Tsvetkov, Y.E.; Tinari, N.; Piccolo, E.; D’Egidio, M.; Adamo, V.; Natoli, C.; Rabinovich, G.A.; et al. Synthetic inhibitors of galectin-1 and -3 selectively modulate homotypic cell aggregation and tumor cell apoptosis. Anticancer Res. 2009, 29, 403–410. [Google Scholar] [PubMed]
- Michel, A.K.; Nangia-Makker, P.; Raz, A.; Cloninger, M.J. Lactose-functionalized dendrimers arbitrate the interaction of galectin-3/muc1 mediated cancer cellular aggregation. ChemBioChem 2014, 15, 2106–2112. [Google Scholar] [CrossRef] [PubMed]
- Prasanphanich, N.S.; Song, X.; Heimburg-Molinaro, J.; Luyai, A.E.; Lasanajak, Y.; Cutler, C.E.; Smith, D.F.; Cummings, R.D. Intact reducing glycan promotes the specific immune response to lacto-n-neotetraose-bsa neoglycoconjugates. Bioconjug. Chem. 2015, 26, 559–571. [Google Scholar] [CrossRef] [PubMed]
- Mencke, A.J.; Wold, F. Neoglycoproteins. Preparation and in vivo clearance of serum albumin derivatives containing ovalbumin oligosaccharides. J. Biol. Chem. 1982, 257, 14799–14805. [Google Scholar] [PubMed]
- Vrasidas, I.; Andre, S.; Valentini, P.; Bock, C.; Lensch, M.; Kaltner, H.; Liskamp, R.M.; Gabius, H.J.; Pieters, R.J. Rigidified multivalent lactose molecules and their interactions with mammalian galectins: A route to selective inhibitors. Org. Biomol. Chem. 2003, 1, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Parera Pera, N.; Branderhorst, H.M.; Kooij, R.; Maierhofer, C.; van der Kaaden, M.; Liskamp, R.M.; Wittmann, V.; Ruijtenbeek, R.; Pieters, R.J. Rapid screening of lectins for multivalency effects with a glycodendrimer microarray. ChemBioChem 2010, 11, 1896–1904. [Google Scholar] [CrossRef] [PubMed]
- Sauerzapfe, B.; Krenek, K.; Schmiedel, J.; Wakarchuk, W.W.; Pelantova, H.; Kren, V.; Elling, L. Chemo-enzymatic synthesis of poly-N-acetyllactosamine (poly-LacNAc) structures and their characterization for CGL2-galectin-mediated binding of ecm glycoproteins to biomaterial surfaces. Glycoconj. J. 2009, 26, 141–159. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.F. Removal of fatty acids from serum albumin by charcoal treatment. J. Biol. Chem. 1967, 242, 173–181. [Google Scholar] [PubMed]
- Witten, K.G.; Rech, C.; Eckert, T.; Charrak, S.; Richtering, W.; Elling, L.; Simon, U. Glyco-DNA-gold nanoparticles: Lectin-mediated assembly and dual-stimuli response. Small 2011, 7, 1954–1960. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Böcker, S.; Laaf, D.; Elling, L. Galectin Binding to Neo-Glycoproteins: LacDiNAc Conjugated BSA as Ligand for Human Galectin-3. Biomolecules 2015, 5, 1671-1696. https://doi.org/10.3390/biom5031671
Böcker S, Laaf D, Elling L. Galectin Binding to Neo-Glycoproteins: LacDiNAc Conjugated BSA as Ligand for Human Galectin-3. Biomolecules. 2015; 5(3):1671-1696. https://doi.org/10.3390/biom5031671
Chicago/Turabian StyleBöcker, Sophia, Dominic Laaf, and Lothar Elling. 2015. "Galectin Binding to Neo-Glycoproteins: LacDiNAc Conjugated BSA as Ligand for Human Galectin-3" Biomolecules 5, no. 3: 1671-1696. https://doi.org/10.3390/biom5031671
APA StyleBöcker, S., Laaf, D., & Elling, L. (2015). Galectin Binding to Neo-Glycoproteins: LacDiNAc Conjugated BSA as Ligand for Human Galectin-3. Biomolecules, 5(3), 1671-1696. https://doi.org/10.3390/biom5031671