Endiandric Acid Derivatives and Other Constituents of Plants from the Genera Beilschmiedia and Endiandra (Lauraceae)
Abstract
:1. Introduction
2. Chemical Constituents
2.1. Endiandric Acid Derivatives from Beilschmiedia and Endiandra

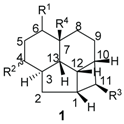
2.1.1. Endiandric Acid Derivatives with an 13 Carbon Atoms Fused Tetracyclic Ring System (Table 1)
| Compounds | R1 | R2 | R3 | R4 | Unsaturation | Sources | Ref. |
|---|---|---|---|---|---|---|---|
| Endiandric acid A (3) | Phenyl | H | CH2COOH | H | Δ4,5, Δ8,9 | Leaves, B. obstusifolia, B. tooram, E. introrsa | [35,36,37,38,39,40] |
| Endiandric acid B (4) | Phenyl | H | CH2CH=CHCOOH | H | Δ4,5, Δ8,9 | Leaves, B. jonesii, E. introrsa, B. tooram | [40] |
| 3'',4''-methylenedioxy Endiandric acid A (5) | 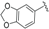 | H | CH2COOH | H | Δ4,5, Δ8,9 | Leaves, B. oligandra, stem bark, B. manii, | [40,44] |
| 3'',4''-methylenedioxy Endiandric acid A methyl ester (6) |  | H | CH2COOMe | H | Δ4,5, Δ8,9 | Synthesis, methylation of B. oligandra extract | [40] |
| Endiandric acid H (7) |  | α-OH | CH2COOH | H | Δ5,6, Δ8,9 | Stem, B. fulva | [53,54] |
| Beilschmiedic acid A (8) | COOH | β-OH |  | H | Δ5,6, Δ8,9 | Leave, Beilschmiedia spp | [17,48] |
| Beilschmiedic acid B (9) | COOH | β-OH |  | OH | Δ5,6, Δ8,9 | Leaves, Beilschmiedia spp; Bark, B. anacardioides | [17] |
| Beilschmiedic acid C (10) | COOH | α-OH |  | H | Δ5,6, Δ8,9 | Leaves, Beilschmiedia spp; Bark, B. anacardioides | [17,48] |
| Beilschmiedic acid D (11) | COOH | H | 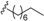 | H | Δ5,6, Δ8,9 | Bark, B. anacardioides | [20] |
| Beilschmiedic acid E (12) | COOH | H | 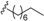 | H | Δ4,5, Δ8,9 | Bark, B. anacardioides | [20] |
| Beilschmiedic acid F (13) | 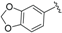 | =O | CH2COOH | H | Δ5,6, Δ8,9 | Bark, B. anacardioides | [19] |
| Beilschmiedic acid H (14) | COOH | α-OH |  | H | Δ5,6, Δ8,9 | Leave , Beilschmiedia spp | [48] |
| Beilschmiedic acid I (15) | COOH | β-OH |  | H | Δ5,6, Δ8,9 | Leaves, Beilschmiedia spp | [48] |
| Beilschmiedic acid J (16) | COOH | H | 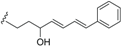 | H | Δ5,6, Δ8,9 | Leaves, Beilschmiedia spp | [48] |
| Beilschmiedic acid K (17) | COOH | α-OH |  | H | Δ5,6, Δ8,9 | Leaves, Beilschmiedia spp | [48] |
| Beilschmiedic acid M (18) | COOH | α-OH | 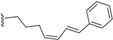 | H | Δ5,6, Δ8,9 | Leaves, Beilschmiedia spp | [48] |
| Beilschmiedic acid L (19) | COOH | α-OH |  | H | Δ5,6, Δ8,9 | Leaves, Beilschmiedia spp | [48] |
| Beilschmiedic acid N (20) | COOH | α-OH | 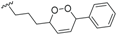 | H | Δ5,6, Δ8,9 | Leaves, Beilschmiedia spp | [48] |
| Beilschmiedic acid O (21) | COOH | α-OH | 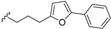 | H | Δ5,6, Δ8,9 | Leaves, Beilschmiedia spp | [48] |
| Erythrophloin A (22) | COOMe | H | 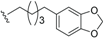 | H | Δ4,5, Δ8,9 | Roots, B. erythrophloia | [41] |
| Erythrophloin B (23) | COOMe | H | 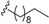 | H | Δ4,5, Δ8,9 | Roots, B. erythrophloia | [41] |
| Erythrophloin C (24) | COOMe | H |  | H | Δ4,5, Δ8,9 | Roots, B. erythrophloia | [41] |
| Erythrophloin D (25) | COOMe | H | 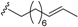 | H | Δ4,5, Δ8,9 | Roots, B. erythrophloia | [41] |
| Erythrophloin E (26) | COOH | H |  | H | Δ4,5, Δ8,9 | Roots, B. erythrophloia | [41] |
| Erythrophloin F (27) | COOH | H | 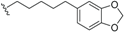 | H | Δ4,5, Δ8,9 | Roots, B. erythrophloia | [41] |
| Tsangibeilin A (28) | COOH | H | 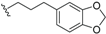 | H | Δ4,5, Δ8,9 | Roots, B. tsangii | [23] |
| Tsangibeilin B (29) | COOH | H |  | H | Δ4,5, Δ8,9 | Roots, B. tsangii; Bark, B. cryptocaryoides | [34,41,52] |
| Tsangibeilin C (30) | COOH | =O | 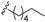 | H | Δ5,6, Δ8,9 | Roots, B. tsangii | [22] |
| Tsangibeilin D (31) | COOH | =O | 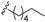 | OH | Δ5,6, Δ8,9 | Roots, B. tsangii | [22] |
| Endiandramide A (32) | CONHCH2-ipr | H | 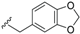 | H | Δ45, Δ8,9 | Roots, B. tsangii | [23] |
| Cryptobeilic acid A (33) | COOH | β-OH | 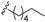 | H | Δ5,6, Δ8,9 | Bark, B. cryptocaryoides | [52] |
| Cryptobeilic acid B (34) | COOH | α-OH | 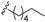 | H | Δ5,6, Δ8,9 | Bark, B. cryptocaryoides | [52] |
| Cryptobeilic acid C (35) | COOH | =O | 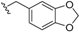 | H | Δ5,6, Δ8,9 | Bark, B. cryptocaryoides | [52] |
| Cryptobeilic acid D (36) | COOH | H | 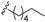 | H | Δ4,5, Δ8,9 | Bark, B. cryptocaryoides | [52] |
| Ferrugineic acid A (37) | COOH | H | 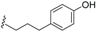 | H | Δ4,5, Δ8,9 | Leaves, flowers; B. ferruginea | [24] |
| Ferrugineic acid B (38) | COOH | H | 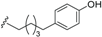 | H | Δ4,5, Δ8,9 | Leaves, flowers, B. ferruginea | [24] |
| Ferrugineic acid C (39) | COOH | H |  | H | Δ4,5, Δ8,9 | Leaves, flowers, B. ferruginea | [24] |
| Ferrugineic acid D (40) | COOH | H | 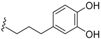 | H | Δ4,5, Δ8,9 | Leaves, flowers, B. ferruginea | [24] |
| Ferrugineic acid E (41) | COOH | H | 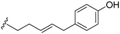 | H | Δ4,5, Δ8,9 | Leaves, flowers, B. ferruginea | [24] |
| Ferrugineic acid F (42) | COOH | α-OH | 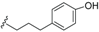 | H | Δ5,6, Δ8,9 | Leaves, flowers, B. ferruginea | [24] |
| Ferrugineic acid G (43) | COOH | β-OH | 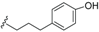 | H | Δ5,6, Δ8,9 | B. ferruginea | [24] |
| Ferrugineic acid H (44) | COOH | =O | 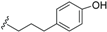 | H | Δ5,6, Δ8,9 | Leaves, flowers, B. ferruginea | [24] |
| Ferrugineic acid I (45) | COOH | α-OH | 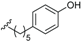 | H | Δ5,6, Δ8,9 | B. ferruginea | [24] |
| Ferrugineic acid J (46) | COOH | β-OH | 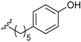 | H | Δ5,6, Δ8,9 | Leaves, flowers, B. ferruginea | [24] |
| Kingianic acid F (47) | COOH | H | 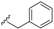 | H | Δ4,5, Δ8,9 | Bark, E. kingiana | [34] |
| Kingianic acid G (48) | COOH | α-OH |  | H | Δ5,6, Δ8,9 | Bark, E. kingiana | [34] |
| Endiandric acid (49) | COOH | H |  | H | Δ5,6, Δ8,9 | Bark, E. kingiana | [34] |
2.1.2. Endiandric Acid Derivatives with an 11 Carbon Atoms Fused Tetracylic Ring System (Table 2)
| Compounds | R1 | R2 | Sources | Ref. |
|---|---|---|---|---|
| Endiandric acid C (50) | COOH |  | B. tooram, B. oligandra, E. jonessi, E. introsa | [38,40] |
| Endiandric acid I (51) | COOH |  | Root, B. erythrophloia | [49] |
| Endiandric acid J (52) | COOH | 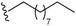 | Root, B. erythrophloia | [49] |
| Beicyclone A (53) | COCH3 | 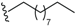 | Root, B. erythrophloia | [41] |
| Endiandric acid K (54) | COOH |  | Roots, B. tsangii | [23] |
| Endiandric acid L (55) | CH=CHCOOH | 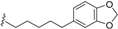 | Roots of B. tsangii | [23] |
| Endiandric acid M (56) | CH=CH-COOH |  | Roots of B. tsangii, stem bark E. kingiana | [23,34] |
| Endiandramide B (57) | CONHCH2iPr |  | B. tsangii | [23] |
| Ferrugineic acid K (58) | CH=CH-COOH | 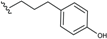 | B. ferruginea | [24] |
| Kingianic acid A (59) | COOH |  | Stem bark, E. kingiana | [34] |
| Kingianic acid B (60) | COOH |  | Stem bark, E. kingiana | [34] |
| Kingianic acid C (61) | COOH | 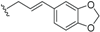 | Stem bark, E. kingiana | [34] |
| Kingianic acid D (62) | COOH |  | Stem bark, E. kingiana | [34] |
| Kingianic acid E (63) | 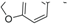 | -CH2COOH | Stem bark, E. kingiana | [34] |
2.1.3. Other Endiandric Acid Derivatives
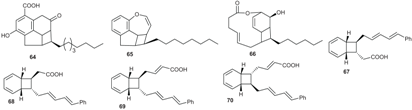
2.1.4. Biosynthesis of Endiandric Derivatives
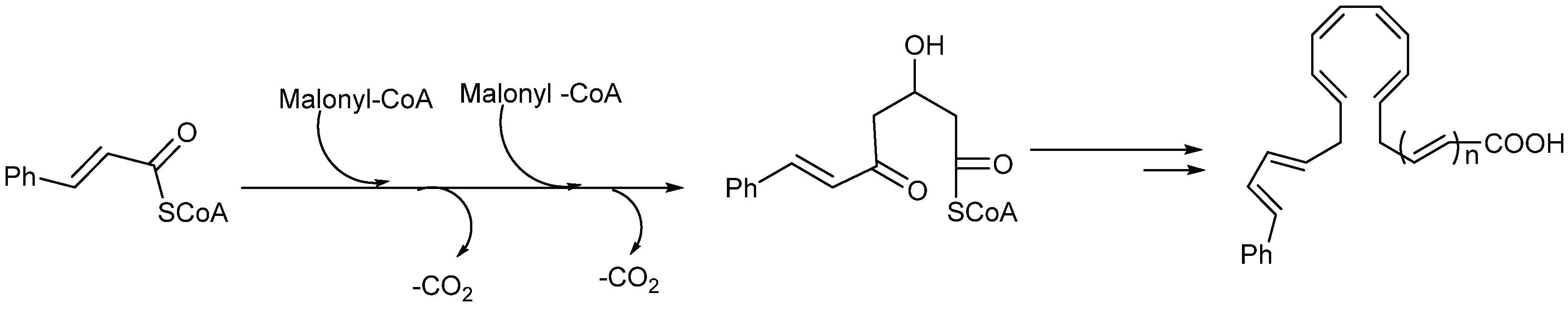
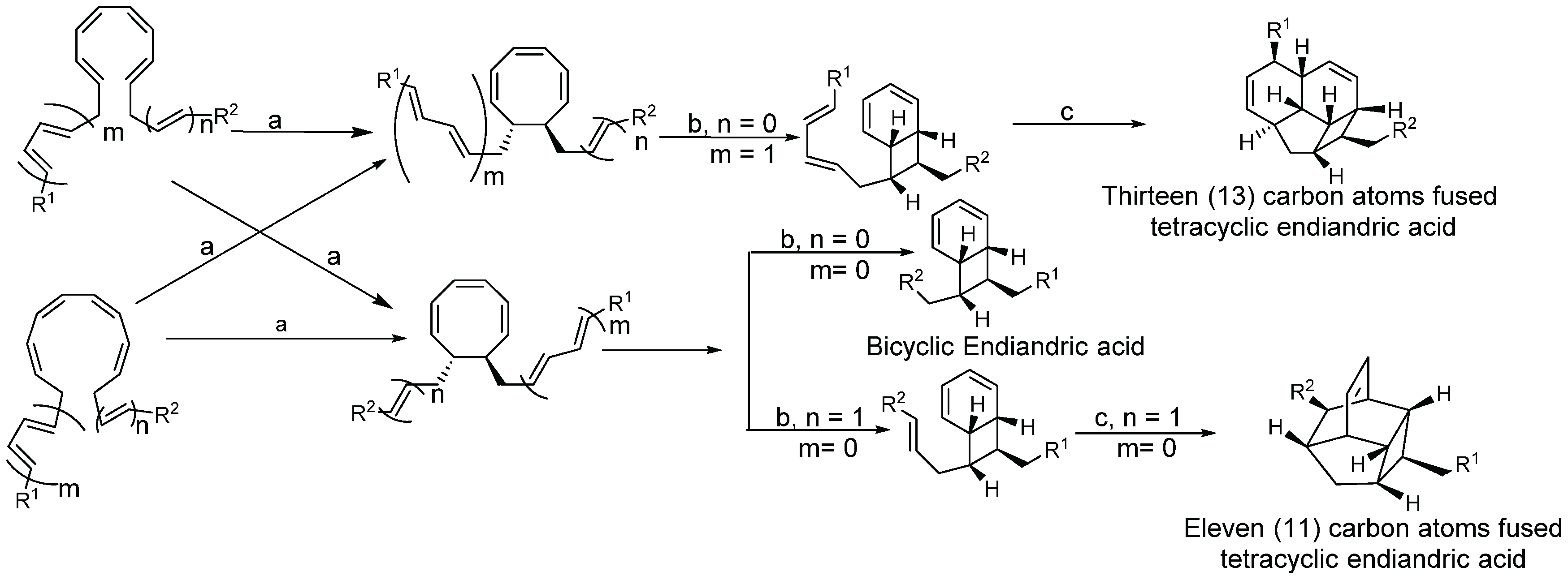
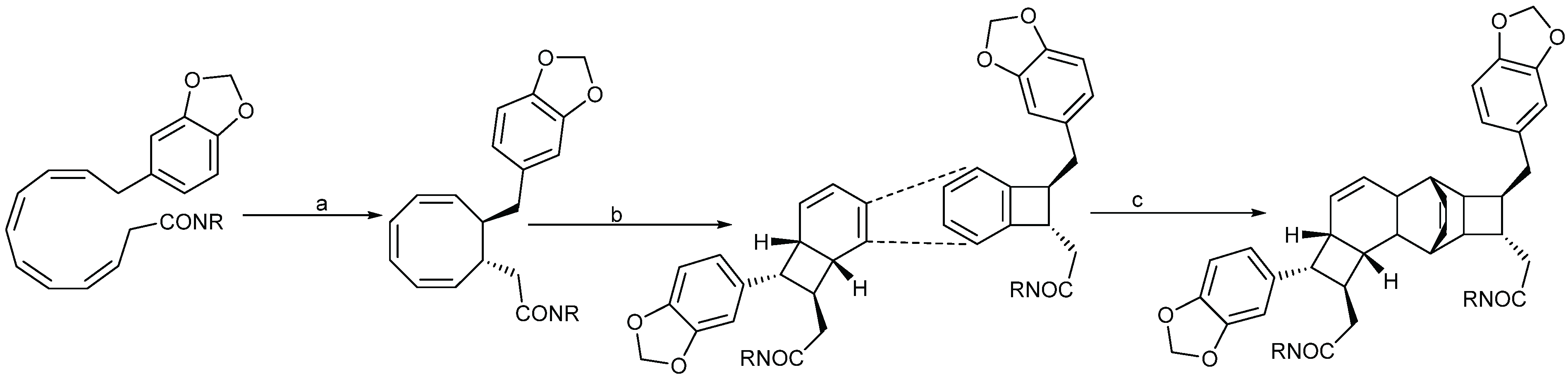
2.1.5. Spectroscopic Characterization
2.1.5.1. Mass Spectra
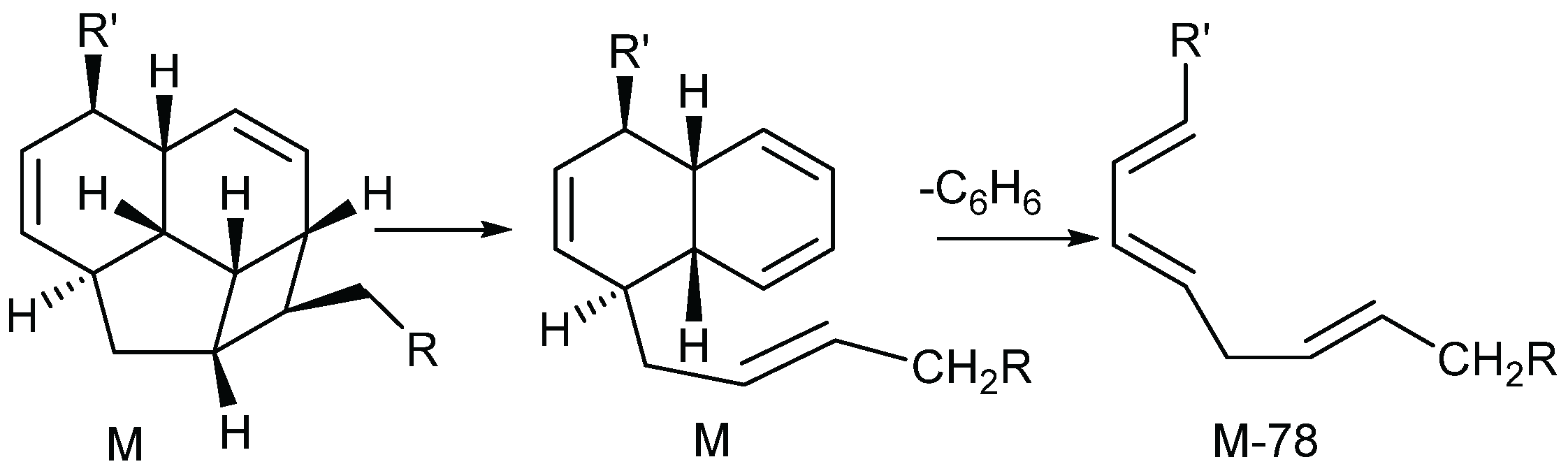
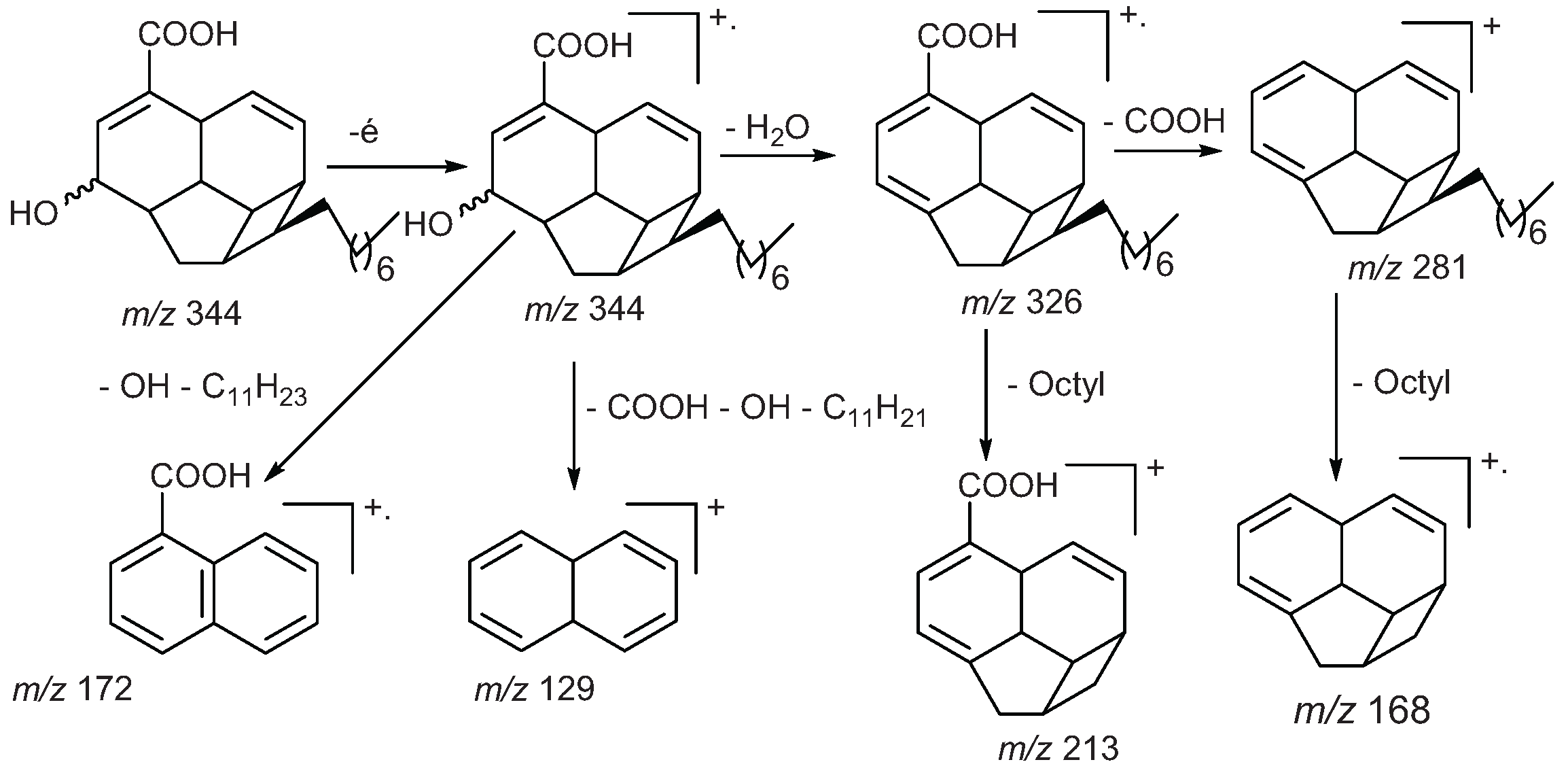
2.1.5.2. NMR Spectra
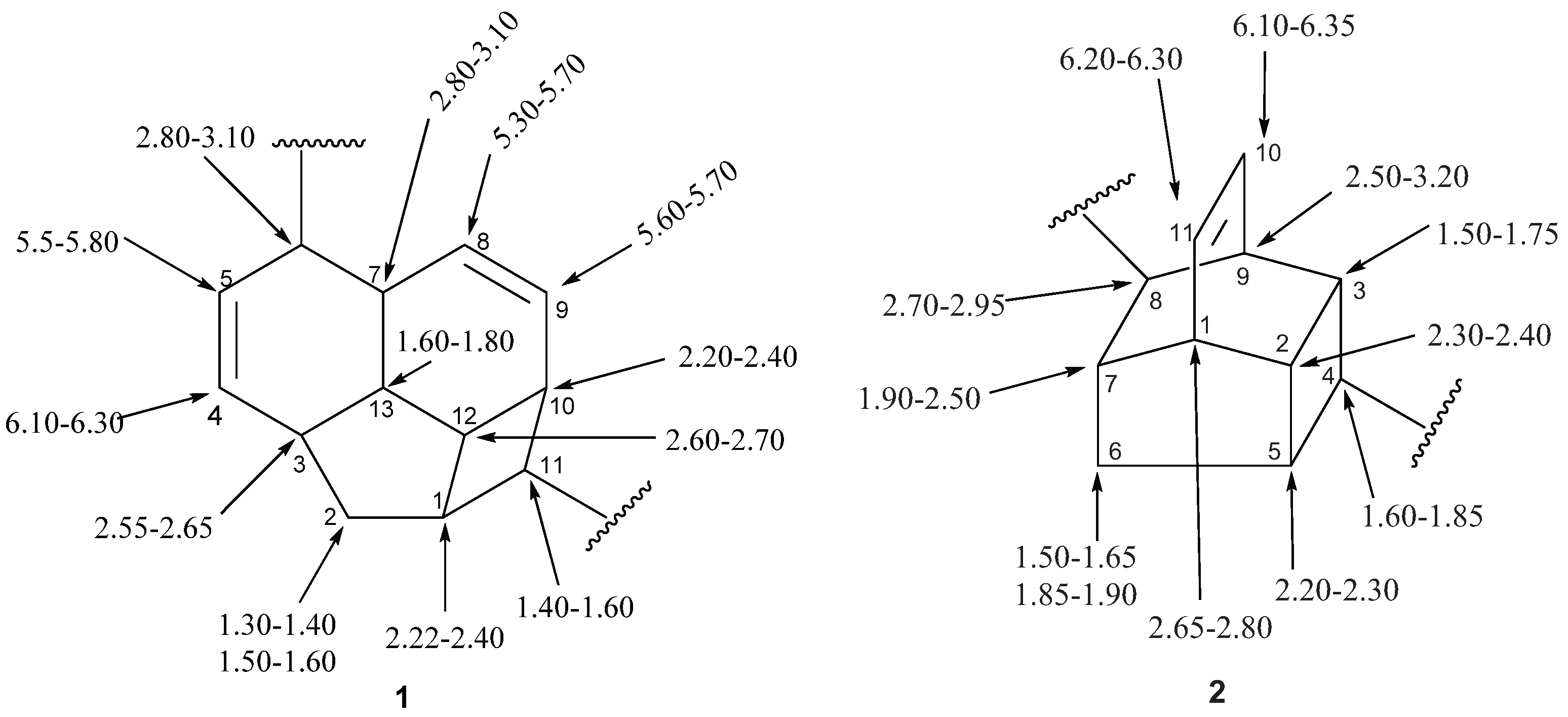
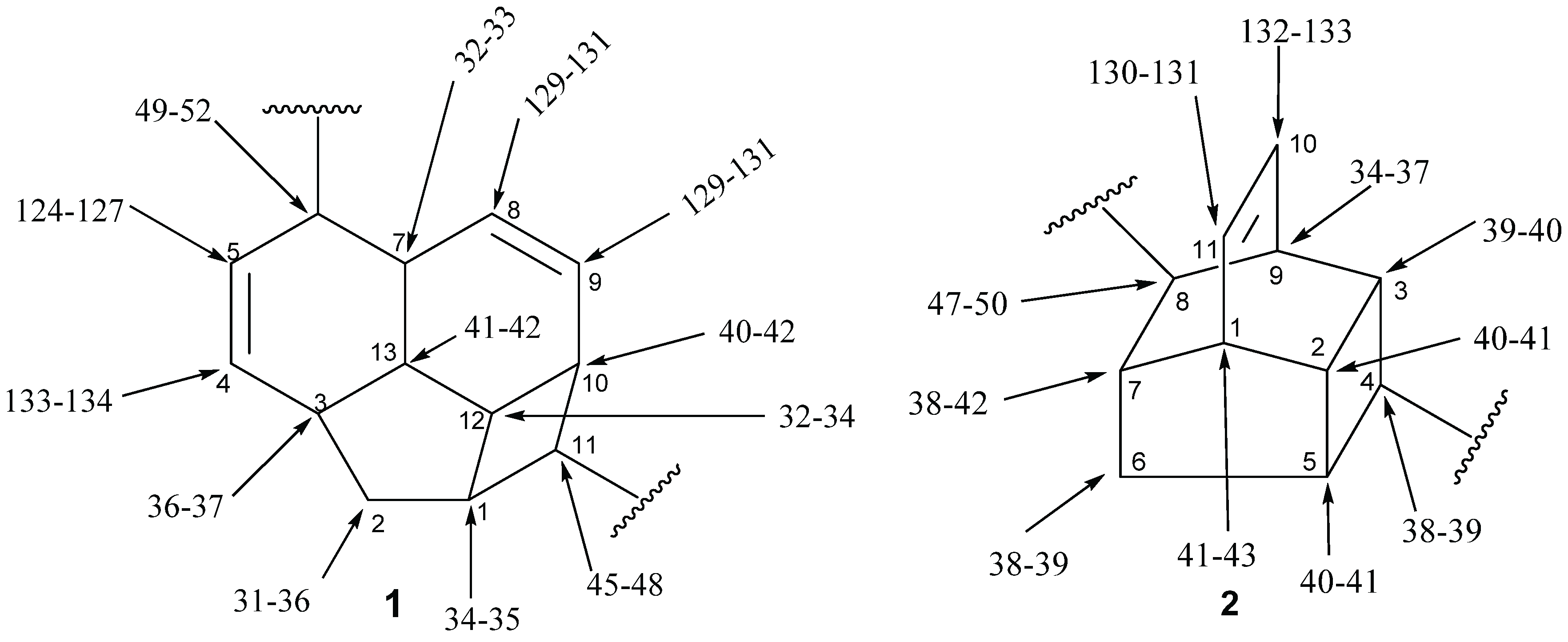
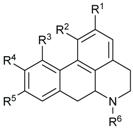
2.2. Alkaloids and Amides
| Compounds | R1 | R2 | R3 | R4 | R5 | R6 | Sources | Ref. |
|---|---|---|---|---|---|---|---|---|
| (+)-Predicentrine (73) | OH | OMe | H | OMe | OMe | Me | B. podagrica | [66] |
| Boldine (74) | OH | OMe | H | OMe | OH | Me | B. alloiophylla, B. kunstleri | [29] |
| Norpredicentrine (75) | OH | OMe | H | OMe | OMe | H | B. podagrica | [66] |
| (+)-Isocorydine (76) | OMe | OMe | OH | OMe | H | Me | B. podagrica | [66] |
| (+)-Glaucine (77) | OMe | OMe | H | OMe | OMe | Me | B. podagrica | [66] |
| (+)-N-methylindcarpine (78) | OH | OMe | OH | OMe | H | Me | B. podagrica | [66] |
| (+)-Laurelliptine (79) | OMe | OH | H | OMe | OH | H | B. podagrica | [67,68] |
| (+)-Isoboldine (80) | OMe | OH | H | OMe | OH | Me | B. alloiophylla, B. tawa | [29,46] |
| 2-Hydroxy-9-methoxy aporphine (81) | OH | H | H | H | OMe | Me | B. alloiophylla | [29] |
| (+)-Laurotetanine (82) | OMe | OMe | H | OMe | OH | H | B. alloiophylla, B. kunstleri | [29,31] |
| (−)-Asimilobine (83) | OH | OMe | H | H | H | H | B. alloiophylla | [29] |
| (+)-Norboldine (84) | OH | OMe | H | OMe | OH | H | B. kunstleri | [31] |
| (+)-Cassithicine (85) | O-CH2- | H | OMe | OH | Me | B. kunstleri | [31] | |
| Nornuciferine (86) | OMe | OMe | H | H | H | H | B. kunstleri | [31] |
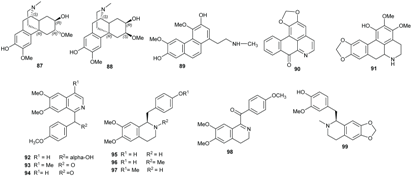
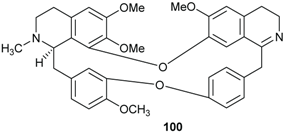

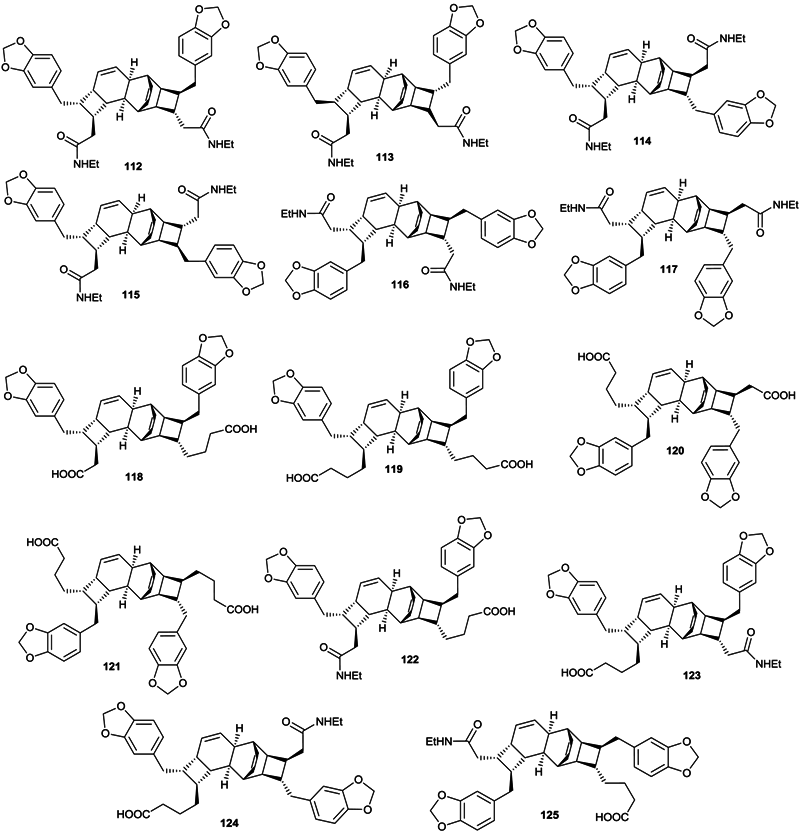
2.3. Lignans and Neolignans
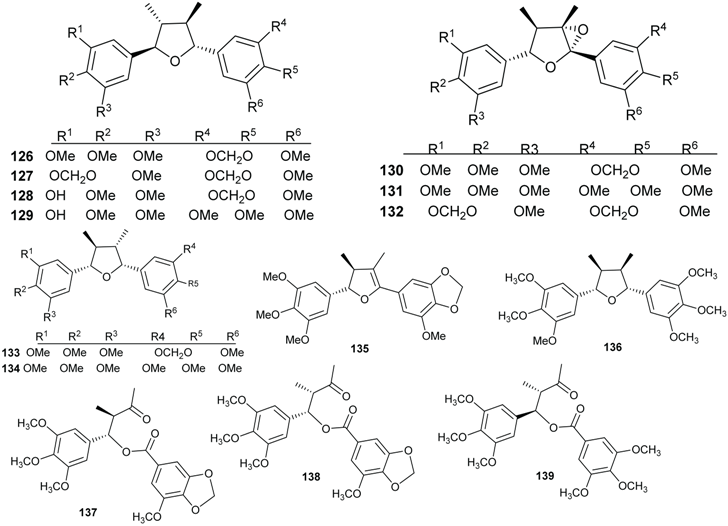
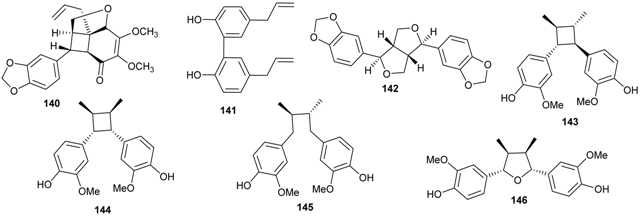
2.4. Flavonoids and Chalcones
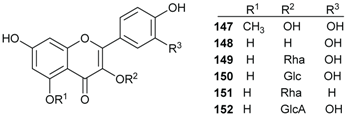
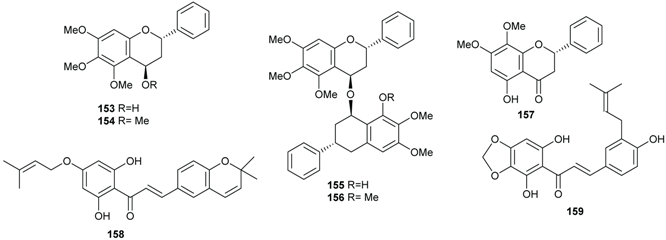
2.5. Benzene Derivatives
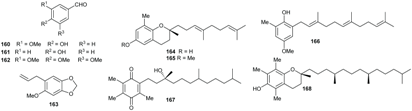
2.6. Terpenoids
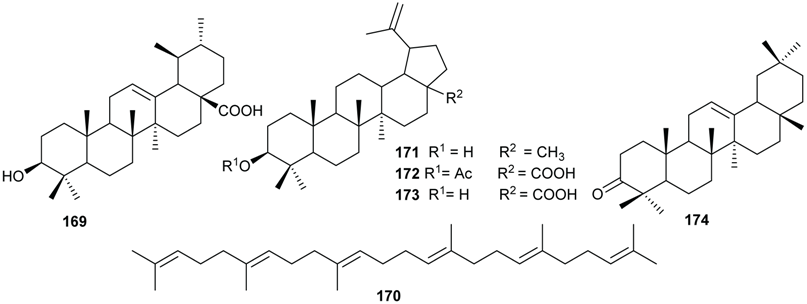

| Species | Major Constituents |
|---|---|
| B. miersii | Leaf oil: Germacrene D (185, 24.8%), α-terpinene (186, 10%), γ-curcumene (187, 9.6%), 1-octen-3-yl acetate (188, 8.2%), (E)-β-ocimene (189, 6.4%) [51] |
| B. tarairie | Leaf oil: α-terpinene (186, 17.8%), β-pinene (190, 9.4%), germacrene D (185) [75] |
| B. brenesii | Leaf oil: germacrene D (185, 19.3, (E)-caryophyllene (191, 13.4%), 2-undecanone (192, 12.8%), α-copaene (183, 9.0), trans-2-hexanal (193, 8.8%) [76] |
| B. costaricensis | Leaf oil: α-bisabolol (194, 72.1%), Cis 2-hexenol (195, 5.2%), α-terpinene (186, 3.0%) [76] |
| B. alloiophylla | Leaf oil: germacrene D (185, (18.9), cis-β-ocimene (196, 18.8%), β-pinene (190, 3.0%), trans-β-ocimene (189, 9.3%), bicyclogermacrene (197, 9.1%) [77] |
| B. talaranensis | Leaf oil: germacrene D (185, 54.9%), β-caryophyllene (191, 14.8%), α-terpinene (186, 3.6%) [77] |
| B. madang | leaf oil: δ-cadinene (198, 17.0%), β-caryophyllene (191, 10.3%), α-cubebene (199, 11.3%), and α-cadinol (200, 5.8%) [78]; bark oil: δ-cadinene (195, 20.5%), β-caryophyllene (191, 6.7%), α-cubebene (199, 15.6%), and α-cadinol (200, 10.6%) [78] |
| B."chancho chancho" | Leaf oil: β-caryophyllene (191, 16.6%), bicyclogermacrene (197, 14.1%), β-pinene (190, 7.7%), germacrene D (185, 6.6%), δ-cadinene (198, 6.1%) [79] |
| B. pendula | leaf oil: β-pinene (190, 10.4%), β-caryophyllene (191, 8.6%), c (201, 7.9%) and bicyclogermacrene (197, 7.2%) [80]; branch oil: β-caryophyllene (191, 17.3%), β-selinene (202, 9.1%), bicyclogermacrene (197, 8.9%), α-cadinol (200, 5.8%) and spathulenol (203, 4.6%) [80] |
| B. erythrophloia | Leaf oil: β-caryophyllene (191, 22.6%), α-humulene (204, 21.9%), terpinen-4-ol (205, 5.3%), cis-β-ocimene (196, 5.1%), sabinene (206, 5.0%), limonene (207, 4.5%) [81] |

2.7. Cyanoglycosides
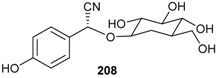
2.8. Other Metabolites

3. Biological Activities
3.1. Anticancer and Cytotoxic Activities
| Compound | Bcl-xL/Bak Binding Affinity | Mcl-1/Bid Binding Affinity | ||
|---|---|---|---|---|
| % at 100 μM | Ki μM | % at 100 μM | Ki μM | |
| Tsangibeilin B (29) | 26 ± 2.5 | ND | 81 ± 2.4 | ND |
| Ferrugineic acid A (37) | 22 ± 2 | >100 | 0 | 14 ± 33 |
| Ferrugineic acid B (38) | 60 ± 6 | 19.2 ± 1.6 | 85 ±2 | 12.0 ±5.0 |
| Ferrugineic acid C (39) | 93 ± 3 | 12 ± 0.2 | 82 ± 2 | 13.0 ± 5.0 |
| Ferrugineic acid D (40) | 39 ± 3 | >100 | 82 ± 2 | 5.2 ± 0.2 |
| Ferrugineic acid E (41) | 20 ± 1 | ND | 14.3 ± 3 | ND |
| Ferrugineic acid F (42) | 7 ± 1 | ND | 0 | ND |
| Ferrugineic acid G (43) | 17 ± 1 | ND | 3 ± 1 | ND |
| Ferrugineic acid I (45) | 35 ± 1 | ND | 7 ± 2 | ND |
| Ferrugineic acid J (46) | 58 ± 7 | 19.4 ± 3 | 81 ± 3 | 5.9 ± 0.5 |
| Kingianic acid F (47) | 22 ± 2.9 | ND | 80 ± 0.7 | ND |
| Kingianic acid G (48) | 19 ± 1.6 | ND | 47 ± 2.9 | ND |
| Kingianic acid A (54) | 21 ± 1.8 | ND | 36 ± 2.3 | ND |
| Endiandric acid M (56) | 10 ± 0.5 | ND | 39 ± 2.9 | ND |
| Kingianic acid C (61) | 25 ±1.7 | ND | 75 ± 1.1 | ND |
| Kingianic acid E (63) | 1 ± 0.8 | ND | 8 ± 5.5 | ND |
| U-Bak (Ki) | 0.0012 ± 10−3 | ND | ||
| U-Bid (Ki) | 0.016 ± 0.002 | |||
| ABT-737 (Ki) | 57 ± 10 nM | 47 ± 22 nM | ||
| Compound | Bcl-xL Ki | ||
|---|---|---|---|
| Racemic mixture | (−) Enantiomer | (+) Enantiomer | |
| Kingianin A (112) | 213 ± 83 | 60 ± 1.5 | >300 |
| Kingianin B (113) | >300 | ||
| Kingianin C (114) | >300 | ||
| Kingianin D (115) | >300 | ||
| Kingianin E (116) | >300 | ||
| Kingianin F (117) | 231 ± 47 | ||
| Kingianin G (118) | 2 ± 0 | 1.0 ± 0.2 | 5.0 ± 1.0 |
| Kingianin H (119) | 18 ± 7 | 4.0 ± 0.4 | 27.0 ± 0.6 |
| Kingianin I (120) | 18 ± 3 | 12.0 ± 1.1 | 16.0 ± 2.2 |
| Kingianin J (121) | 29 ± 6 | 9.0 ± 0.2 | 25.0 ± 3.2 |
| Kingianin K (122) | 80 ± 36 | 6.0 ± 0.2 | 112 ± 45 |
| Kingianin L (123) | 36 ± 11 | 4.0 ± 0.1 | 71.0 ± 10 |
| Kingianin M (124) | 236 ± 34 | ||
| Kingianin N (125) | 177 ± 9 | ||
| Unlabeled Bak (BH3) | 0.90 ± 0.27 | ||
3.2. Antimalaria Activity
3.3. Anti-Asthmatic and Other Anti-Inflammatory Activities
3.4. Antimicrobial Activity
3.5. Other Activities
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nishida, S. Revision of Beilschmiedia (Lauraceae) in the neotropics. Ann. Mo. Bot. Gard. 1999, 86, 657–701. [Google Scholar] [CrossRef]
- Liao, J.-C. Lauraceae: Flora of Taiwan, 2nd ed.; Editorial Committee of the Flora of Taiwan: Taipei, Taiwan, 1996; Volume 2, pp. 433–499. [Google Scholar]
- Ng, F.S.P. Tree Flora of Malaya; Longman: Kuala Lumpur, Malaysia, 1989; p. 4. [Google Scholar]
- Henry, T.A. The Plant Alkaloids, 4th ed.; J.A. Churchill Ltd.: London, UK, 1949; p. 317. [Google Scholar]
- Perry, L.M.; Metzgr, J. Medicinal Plants East and Southest Asia: Attributed Properties and Uses; MIT Press: Cambridge, MA, USA, 1980; Volume 20, pp. 201–202. [Google Scholar]
- Fouilloy, R. Flore du Cameroun (Lauracées, Myristicaées et Momimiacées); Muséum National d’Histoire Naturelle: Paris, France, 1974; Volume 18, p. 8. [Google Scholar]
- Van der Werff, H. A synopsis of the genus Beilschmiedia (Lauraceae) in Madagascar. Adansonia 2003, 3, 77–92. [Google Scholar]
- Nkeng-Efouet, A.P. Phytochemicals from Beilschmiedia Anacardioides and Their Biological Significance: Phytochemicals—A Global Perspective of Their Role in Nutrition and Health; Rao, V., Ed.; InTech: Rijeka, Croatia, 2012; pp. 167–186. [Google Scholar]
- Burkill, I.H. A Dictionary of the Economic Products of the Malay Peninsular, 2nd ed.Government of Malaya and Singapore: Kuala Lumpur, Malaysia, 1966; p. 1773.
- Maberley, D.J. Mabberley’s Plant-Book: A Portable Dictionary of Plants, Their Classification and Uses, 3rd ed.; Cambridge University Press: Cambridge, UK, 2008; p. 1021. [Google Scholar]
- Maberley, D.J. The plant-book: A Portable Dictionary of the Vascular Plants, 2nd ed.; Cambridge University Press: Cambridge, UK, 1997; p. 858. [Google Scholar]
- Kitagawa, I.; Minagawa, K.; Zhang, R.S.; Hori, K.; Doi, M.; Inoue, M.I.; Ishida, T.; Kimura, M.; Uji, T.; Shibuya, H. Dehatrine, an antimalarial bisbenzylisoquinoline alkaloid from the Indonesian medicinal plant Beilschmiedia madang, isolated as a mixture of two rotational isomers. Chem. Pharm. Bull. 1993, 41, 997–999. [Google Scholar] [CrossRef] [PubMed]
- Suarez, L.E.C.; Vargas, O.E.B. New chalcones from Beilschmiedia tovarensis. Rev. Colomb. Quim. 2005, 34, 35–41. [Google Scholar]
- Iwu, M.M. Handbook of African Medicinal Plants; CRC Press: BocaRaton, FL, USA, 1993; p. 435. [Google Scholar]
- Tchiegang, C.; Parmentier, M. Chemical composition and nutritional evaluation of two Cameroonian soup thickeners: Belschmiedia jacques felexii and Belschmiedia anacardiodes. J. Food Sci. Tech. 2008, 45, 187–189. [Google Scholar]
- Sahoré, A.D.; Koffi, L.B. Technical sheet of Beisclmiedia mannii (Lauraceae) seed preparation in Ivory Coast. J. Pharm. Sci. Innov. 2013, 2, 62–64. [Google Scholar] [CrossRef]
- Chouna, J.R.; Nkeng-Efouet, P.A.; Lenta, B.N.; Devkota, K.P.; Neumann, B.; Stammler, H.G.; Kimbu, S.F.; Sewald, N. Antibacterial endiandric acid derivatives from Beilschmiedia anacardioides. Phytochemistry 2009, 70, 684–688. [Google Scholar] [CrossRef]
- Lenta, B.N.; Tantangmo, F.; Devkota, K.P.; Wansi, J.D.; Chouna, J.R.; Soh, R.C.F.; Neumann, B.; Stammler, H.-G.; Tsamo, E.; Sewald, N. Bioactive constituents of the stem bark of Beilschmiedia zenkeri. J. Nat. Prod. 2009, 72, 2130–2134. [Google Scholar] [CrossRef] [PubMed]
- Chouna, J.R.; Nkeng-Efouet, P.A.; Lenta, B.N.; Wansi, D.J.; Neumann, B.; Stammler, H.-G.; Fon Kimbu, S.; Sewald, N. Beilschmiedic acids F and G, other endiandric Acid derivatives from Beilschmiedia anacardioides. Helv. Chim. Acta 2011, 94, 1071–1076. [Google Scholar] [CrossRef]
- Chouna, J.R.; Nkeng-Efouet, P.A.; Lenta, B.N.; Wansi, J.D.; Fon Kimbu, S.; Sewald, N. Endiandric acid derivatives from the stem bark of Beilschmiedia anacardioides. Phytochem. Lett. 2010, 3, 13–16. [Google Scholar] [CrossRef]
- Harborne, J.B.; Mendez, J. Flavonoids of Beilschmiedia miersii. Phytochemistry 1969, 8, 763–764. [Google Scholar] [CrossRef]
- Huang, Y.-T.; Chang, H.-S.; Wang, G.-J.; Lin, C.-H.; Chen, I.-S. Secondary metabolites from the roots of Beilschmiedia tsangii and their anti-inflammatory activities. Int. J. Mol. Sci. 2012, 13, 16430–16443. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-T.; Chang, H.-S.; Wang, G.-J.; Cheng, M.-J.; Chen, C.-H.; Yang, Y.-J.; Chen, I.-S. Anti-inflammatory endiandric acid analogues from the roots of Beilschmiedia tsangii. J. Nat. Prod. 2011, 74, 1875–1880. [Google Scholar] [CrossRef] [PubMed]
- Apel, C.; Geny, C.; Dumontet, V.; Birlirakis, N.; Roussi, F.; Pham, V.C.; Huong, D.T.M.; Nguyen, V.H.; Chau, V.M.; Litaudon, M. Endiandric acid analogues from Beilschmiedia ferruginea as dual inhibitors of Bcl-xL/Bak and Mcl-1/Bid interactions. J. Nat. Prod. 2014, 77, 1430–1437. [Google Scholar] [CrossRef]
- Chen, J.-J.; Chou, E.-T.; Duh, C.-Y.; Yang, S.-Z.; Chen, I.-S. New cytotoxic tetrahydrofuran- and dihydrofuran-type lignans from the stem of Beilschmiedia tsangii. Planta Med. 2006, 72, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-J.; Chou, E.-T.; Peng, C.-F.; Chen, I.-S.; Yang, S.-Z.; Huang, H.-Y. Novel epoxyfuranoid lignans and antitubercular constituents from the leaves of Beilschmiedia tsangii. Planta Med. 2007, 73, 567–571. [Google Scholar] [CrossRef]
- Kumamoto, J.; Scora, R.W. Structure of sarisan, an isomer of myristicin, isolated from the leaf oil of Beilschmiedia miersii. J. Agric. Food Chem. 1970, 18, 544–545. [Google Scholar] [CrossRef]
- Lenta, B.N.; Chouna, J.R.; Nkeng-Efouet, P.A.; Fon Kimbu, S.; Tsamo, E.; Sewald, N. Obscurine: A new cyclostachine acid derivative from Beilschmiedia obscura. Nat. Prod. Commun. 2011, 6, 1591–1592. [Google Scholar] [PubMed]
- Mollataghi, A.; Coudiere, E.; Hadi, A.H.A.; Mukhtar, M.R.; Awang, K.; Litaudon, M.; Ata, A. Anti-acetylcholinesterase, anti-α-glucosidase, anti-leishmanial and anti-fungal activities of chemical constituents of Beilschmiedia species. Fitoterapia 2012, 83, 298–302. [Google Scholar] [CrossRef]
- Mollataghi, A.; Hadi, A.H.A.; Awang, K.; Mohamad, J.; Litaudon, M.; Mukhtar, M.R. (+)-Kunstlerone, a new antioxidant neolignan from the leaves of Beilschmiedia kunstleri Gamble. Molecules 2011, 16, 6582–6590. [Google Scholar] [CrossRef]
- Mollataghi, A.; Hadi, A.H.; Cheah, S.-C. (−)-Kunstleramide, a new antioxidant and cytotoxic dienamide from the bark of Beilschmiedia kunstleri Gamble. Molecules 2012, 17, 4197–4208. [Google Scholar] [CrossRef]
- Davis, R.A.; Barnes, E.C.; Longden, J.; Avery, V.M.; Healy, P.C. Isolation, structure elucidation and cytotoxic evaluation of endiandrin B from the Australian rain forest plant Endiandra anthropophagorum. Bioorg. Med. Chem. 2009, 17, 1387–1392. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.A.; Carroll, A.R.; Duffy, S.; Avery, V.M.; Guymer, G.P.; Forster, P.I.; Quinn, R.J. Endiandrin A, a potent glucocorticoid receptor binder isolated from the Australian plant Endiandra anthropophagorum. J. Nat. prod. 2007, 70, 1118–1121. [Google Scholar] [CrossRef] [PubMed]
- Azmi, M.N.; Geny, C.; Leverrier, A.; Litaudon, M.; Dumontet, V.; Birlirakis, N.; Gueritte, F.; Leong, K.H.; Abd, H.; Siti, N.; et al. Kingianic acids A–G, endiandric acid analogues from Endiandra kingiana. Molecules 2014, 19, 1732–1747. [Google Scholar] [CrossRef]
- Bandaranayake, W.M.; Banfield, J.E.; Black, D.S.C.; Fallon, G.D.; Gatehouse, B.M. Endiandric acid, a novel carboxylic acid from Endiandra introrsa (Lauraceae): X-ray structure determination. J. Chem. Soc. Chem. Comm. 1980, 4, 162–163. [Google Scholar] [CrossRef]
- Bandaranayake, W.M.; Banfield, J.E.; Black, D.S.C.; Fallon, G.D.; Gatehouse, B.M. Constituents of Endiandra species. I. Endiandric acid, a novel carboxylic acid from Endiandra introrsa (Lauraceae), and a derived lactone. Aust. J. Chem. 1981, 34, 1655–1667. [Google Scholar] [CrossRef]
- Bandaranayake, W.M.; Banfield, J.E.; Black, D.S.C. Constituents of Endiandra species. II. (E)-4-(6'-phenyltetracyclo[5.4.2.03,13.010,12]trideca-4',8'-dien-11'-yl)but-2-enoic acid from Endiandra introsa (Lauraceae). Aust. J. Chem. 1982, 35, 557–565. [Google Scholar] [CrossRef]
- Bandaranayake, W.M.; Banfield, J.E.; Black, D.S.C.; Fallon, G.D.; Gatehouse, B.M. Constituents of Endiandra species. III. 4-[(E,E)-5'-Phenylpenta-2',4'-dien-1'-yl]tetracyclo[5.4.0.02.5.03.9]undec-10-ene-8-carboxylic acid from Endiandra introrsa (Lauraceae). Aust. J. Chem 1982, 35, 567–579. [Google Scholar] [CrossRef]
- Banfield, J.E.; Black, D.S.C.; Johns, S.R.; Willing, R.I. Constituents of Endiandra species. IV. Isolation of 2-(8'-[(E,E)-5"-phenylpenta-2",4"-dien-1"-yl]bicyclo[4.2.0]octa-2',4'-dien-7'-yl)acetic acid, a biogenetically predicted metabolite of Endiandra introrsa (Lauraceae) and its structure determination by means of 1D and 2D NMR spectroscopy. Aust. J. Chem. 1982, 35, 2247–2256. [Google Scholar] [CrossRef]
- Banfield, J.E.; Black, D.S.C.; Collins, D.J.; Hyland, B.P.M.; Lee, J.J.; Pranowo, S.R. Constituents of some species of Beilschmiedia and Endiandra (Lauraceae): New endiandric acid and benzopyran derivatives isolated from B. oligandra. Aust. J. Chem. 1994, 47, 587–607. [Google Scholar] [CrossRef]
- Yang, P.-S.; Cheng, M.-J.; Peng, C.-F.; Chen, J.-J.; Chen, I.-S. Endiandric acid analogues from the roots of Beilschmiedia erythrophloia. J. Nat. Prod. 2009, 72, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Leverrier, A.; Dau, M.E.T.H.; Retailleau, P.; Awang, K.; Gueritte, F.; Litaudon, M. Kingianin A: A new natural pentacyclic compound from Endiandra Kingiana. Org. Lett. 2010, 12, 3638–3641. [Google Scholar] [CrossRef] [PubMed]
- Leverrier, A.; Awang, K.; Gueritte, F.; Litaudon, M. Pentacyclic polyketides from Endiandra kingiana as inhibitors of the Bcl-xL/Bak interaction. Phytochemistry 2011, 72, 1443–1452. [Google Scholar] [CrossRef] [PubMed]
- Mpetga, S.D.J. Contribution à L´étude Phytochimique D´une Plante Médicinale du Cameroun: Beilschmiedia mannii (Lauracee). Ph.D. Thesis, Université de Dschang, Dschang, Cameroon, 2005. [Google Scholar]
- Pudjiastuti, P.; Mukhtar, M.R.; Hadi, A.H.A.; Saidi, N.; Morita, H.; Litaudon, M.; Awang, K. 6,7-Dimethoxy-4-methylisoquinolinyl)-(4'-methoxyphenyl)-methanone, a new benzylisoquinoline alkaloid from Beilschmiedia brevipes. Molecules 2010, 15, 2339–2346. [Google Scholar] [CrossRef]
- Russell, G.B.; Fraser, J.G. Identification of isoboldine as the major alkaloid form berries of Beilschmiedia tawa. New Zeal. J. Sci. 1969, 12, 694–695. [Google Scholar]
- Tillequin, F.; Koch, M.; Pusset, J.; Chauviere, G. Two new morphinane alkaloids from Beilschmiedia oreophila Schlechter (Lauraceae). Heterocycles 1985, 23, 1357–1361. [Google Scholar] [CrossRef]
- Williams, R.B.; Martin, S.M.; Hu, J.-F.; Norman, V.L.; Goering, M.G.; Loss, S.; O’Neil-Johnson, M.; Eldridge, G.R.; Starks, C.M. Cytotoxic and antibacterial beilschmiedic acids from a Gabonese species of Beilschmiedia. J. Nat. Prod. 2012, 75, 1319–1325. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.-S.; Cheng, M.-J.; Chen, J.-J.; Chen, I.-S. Two new endiandric acid analogs, a new benzopyran, and a new benzenoid from the root of Beilschmiedia erythrophloia. Helv. Chim. Acta 2008, 91, 2130–2138. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, Z.; Feng, J.; Feng, G.; Zhang, X. Bioactivity of sarisan against Musca domestica L. and Culex pipiens pallens. J. Chin. J. Pest. Sci. 2005, 71, 85–87. [Google Scholar]
- Li, Y.; Jiang, G.; Wang, H. On analysis of bactericidal ability and effective component of four garden trees. J. Southwest. China Norm. Univ. Nat. Sci. 2014, 39, 29–34. [Google Scholar]
- Talontsi, F.M.; Lamshöft, M.; Bauer, J.O.; Razakarivony, A.A.; Andriamihaja, B.; Strohmann, C.; Spiteller, M. Antibacterial and antiplasmodial constituents of Beilschmiedia cryptocaryoides. J. Nat. Prod. 2013, 76, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Eder, C.; Kogler, H.; Haag-Richter, S. New Fused Tetracyclic Compounds, e.g., Endiandric Acid H Obtained from the Plant Beilschmieda fulva, are C-maf and NFAT Inhibitors Useful for Treating Allergy or Asthma. German Patent DE 10235624 A1, 19 February 2004. [Google Scholar]
- Eder, C.; Kogler, H.; Haag-Richter, S. Endiandric Acid H and Its Derivatives, Process for Their Preparation and Use Thereof. U.S. Patent US007019028B2, 28 March 2006. [Google Scholar]
- Nicolaou, K.C.; Petasis, N.A.; Zipkin, R.E.; Uenishi, J. The endiandric acid cascade. Electrocyclizations in organic synthesis. 1 stepwise, stereocontrolled total synthesis of endiandric acids A and B. J. Am. Chem. Soc. 1982, 104, 5555–5557. [Google Scholar]
- Reynolds, W.F.; Enriquez, R.G. Choosing the best pulse sequences, acquisition parameters, postacquisition processing strategies, and probes for natural product structure elucidation by NMR spectroscopy. J. Nat. Prod. 2002, 65, 221–244. [Google Scholar] [CrossRef] [PubMed]
- Bross-Walch, N.; Kühn, T.; Moskau, D.; Zerb, O. Strategies and tools for structure determination of natural products using modern methods of NMR spectroscopy. Chem. Biodivers. 2005, 2, 147–177. [Google Scholar] [CrossRef] [PubMed]
- Coy Barrera, E.D.; Cuca Suárez, L.E. In vitro inhibitory activities of Lauraceae aporphine alkaloids. Nat. Prod. Commun. 2010, 5, 383–386. [Google Scholar] [PubMed]
- Omar, H.; Hashim, N.M.; Zajmi, A.; Nordin, N.; Abdelwahab, S.I.; Azizan, A.H.S.; Hadi, A.H.A.; Ali, H.M. Aporphine alkaloids from the leaves of Phoebe grandis (Nees) Mer. (Lauraceae) and their cytotoxic and antibacterial activities. Molecules 2013, 18, 8994–9009. [Google Scholar]
- Nasrullah, A.A.; Zahari, A.; Mohamad, J.; Awang, K. Antiplasmodial alkaloids from the bark of Cryptocarya nigra (Lauraceae). Molecules 2013, 18, 8009–8017. [Google Scholar] [CrossRef] [PubMed]
- Stermitz, F.R.; Castro, O.C. Pentasubstituted aporphine alkaloids from Phoebe molicella. J. Nat. Prod. 1983, 46, 913–916. [Google Scholar] [CrossRef]
- Custódio, D.L.; da Veiga, J.V.F. Lauraceae alkaloids. RSC Adv. 2014, 4, 21864–21890. [Google Scholar] [CrossRef]
- Pabon, L.C.; Cuca, L.E. Aporphine alkaloids from Ocotea macrophylla (Lauraceae). Quim Nova 2010, 33, 875–879. [Google Scholar] [CrossRef]
- Chen, J.; Gao, K.; Liu, T.; Zhao, H.; Wang, J.; Wu, H.; Liu, B.; Wang, W. Aporphine alkaloids: A kind of alkaloids’ extract source, chemical constitution and pharmacological actions in different botany. Asian J. Chem. 2013, 25, 10015–10027. [Google Scholar]
- Tschesche, R.; Welzel, P.; Legler, G. Structure of certain tetrasubstituted aporphine alkaloids. Tetrahedron Lett. 1965, 8, 445–450. [Google Scholar] [CrossRef]
- Johns, S.R.; Lamberton, J.A.; Sioumis, A.A.; Tweeddale, H.J. New aporphine alkaloids from Beilschmiedia podagrica. Aust. J. Chem. 1969, 22, 1277–1281. [Google Scholar] [CrossRef]
- Clezy, P.S.; Gellert, E.; Lau, D.Y.K.; Nichol, A.W. The alkaloids of Beilschmiedia elliptica. Aust. J. Chem. 1966, 19, 135–142. [Google Scholar] [CrossRef]
- Clezy, P.S.; Nichol, A.W.; Gellert, E. Structures of laurelliptine, a new aporphine alkaloid, and thalicmidine. Experientia 1968, 19, 1–2. [Google Scholar] [CrossRef]
- Chen, J.-J.; Kuo, W.-L.; Sung, P.-J.; Chen, I.-S.; Cheng, M.-J.; Lim, Y.-P.; Liao, H.-R.; Chang, T.-H.; Wei, D.-C.; Chen, J.-Y. Beilschamide, a new amide, and cytotoxic constituents of Beilschmiedia erythrophloia. Chem. Nat. Compd. 2015, 51, 302–305. [Google Scholar] [CrossRef]
- Monte Neto, R.L.; Sousa, L.M.; Dias, C.S.; Filho, J.M.; Oliveira, M.R. Yangambin cytotoxicity: A pharmacologically active lignan obtained from Ocotea duckei Vattimo (Lauraceae). Z. Naturforsch C. 2008, 63, 681–686. [Google Scholar] [CrossRef]
- Filho da Silva, A.A.; Costa, E.S.; Cunha, W.R.; Silva, M.L.A.; Nanayakkara, N.P.D.; Bastos, J.K. In vitro antileishmanial and antimalarial activities of tetrahydrofuran lignans isolated from Nectandra megapotamica (Lauraceae). Phytother. Res. 2008, 22, 1307–1310. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.J.; Chen, W.; Luo, Y.P.; Li, G.P.; Yang, X.D.; Li, L. Lignans and ketonic compounds from Litsea chinpingensis (Lauraceae). Biochem. Syst. Ecol. 2009, 37, 696–698. [Google Scholar] [CrossRef]
- Yang, J.Y. A Flavonoid Study of the Lauraceae. Master Thesis, The University of British Columbia, Vancouver, Canada, 1998. [Google Scholar]
- Gomez-Garibay, F.; Calderon, J.S.; Quijano, L.; Tallez, O.; Olivares, M.D.S.; Rios, T. An unusual prenyl biflavanol from Tephrosia tepicana. Phytochemistry 1997, 46, 1285–1287. [Google Scholar] [CrossRef]
- Scora, R.W.; Scora, P.E. Essential leaf oil of Persea subgenus, Eriodaphne and closely related Perseoid genera. J. Essent. Oil Res. 2001, 13, 37–42. [Google Scholar] [CrossRef]
- Agius, B.R.; Setzer, M.C.; Stokes, S.L.; Walker, T.M.; Haber, W.A.; Setzer, W.N. Composition and bioactivity of essential oils of Lauraceae from Monteverde, Costa Rica. Int. J. Essent. Oil Ther. 2007, 1, 167–171. [Google Scholar]
- Setzer, W.N.; Haber, W.A. Leaf essential oil composition of five species of Beilschmiedia from Monteverde, Costa Rica. Nat. Prod. Commun. 2007, 2, 79–83. [Google Scholar]
- Salleh, W.M.; Ahmad, F.; Yen, K.H. Chemical compositions and biological activities of the essential oils of Beilschmiedia madang Blume (Lauraceae). Arch. Pharm. Res. 2014, 38, 485–493. [Google Scholar] [CrossRef]
- Wright, B.S.; Bansal, A.; Moriarity, D.M.; Takaku, S.; Setzer, W.N. Cytotoxic leaf essential oils from neotropical Lauraceae: Synergistic effects of essential oil components. Nat. Prod. Commun. 2007, 2, 1241–1244. [Google Scholar]
- Chaverri, C.; Ciccio, J.F. Essential oils from Beilschmiedia pendula (Sw.) Hemsl. (Lauraceae) from Costa Rica. J. Essent. Oil Res. 2010, 22, 259–262. [Google Scholar] [CrossRef]
- Su, Y.C.; Ho, C.L. Composition and in vitro cytotoxic activities of the leaf essential oil of Beilschmiedia erythrophloia from Taiwan. Nat. Prod. Commun. 2013, 8, 143–144. [Google Scholar]
- Miller, R.E.; Tuck, K.L. Reports on the distribution of aromatic cyanogenic glycosides in Australian tropical rainforest tree species of the Lauraceae and Sapindaceae. Phytochemistry 2013, 92, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.S.Y. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Canc. Res. 2011. [Google Scholar] [CrossRef]
- Davids, M.S.; Letai, A. Targeting the B-cell lymphoma/leukemia 2 family in cancer. J. Clin. Oncol. 2012, 30, 3127–3135. [Google Scholar] [CrossRef] [PubMed]
- Lessene, G.; Czabotar, P.E.; Colman, P.M. BCL-2 family antagonists for cancer therapy. Nat. Rev. Drug. Discov. 2008, 7, 989–1000. [Google Scholar] [CrossRef] [PubMed]
- Jeng, P.S.; Cheng, E.H. Cancer therapeutics: Pulling the plug on BCL-XL. Nat. Chem. Biol. 2013, 9, 351–352. [Google Scholar] [CrossRef] [PubMed]
- Placzek, W.J.; Wei, J.; Kitada, S.; Zhai, D.; Reed, J.C.; Pellecchia, M. A survey of the anti-apoptotic Bcl-2 subfamily expression in cancer types provides a platform to predict the efficacy of Bcl-2 antagonists in cancer therapy. Cell. Death Dis. 2010, 1, e40. [Google Scholar] [CrossRef] [PubMed]
- Kuete, V.; Tankeo, S.B.; Saeed, M.E.M.; Wiench, B.; Tane, P.; Efferth, T. Cytotoxicity and modes of action of five Cameroonian medicinal plants against multi-factorial drug resistance of tumor cells. J. Ethnopharmacol. 2014, 153, 207–219. [Google Scholar] [CrossRef] [PubMed]
- WHO. World Malaria Report 2014; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Clark, C.; Key, S.W. WHO releases revised facts on Malaria. Malar. Wkly. 1996, 15, 7–9. [Google Scholar]
- Gessler, M.C.; Nkunya, M.H.H.; Mwasumbi, L.B.; Henrich, M.; Tanner, M. Screening Tanzanian medicinal plants for antimalarial activity. Acta Trop. 1994, 56, 65–77. [Google Scholar] [CrossRef]
- Ziegler, H.L.; Stærk, D.; Christensen, J.; Hviid, L.; Hägerstrand, H.; Jaroszewski, J.W. In vitro Plasmodium falciparum drug sensitivity assay: Inhibition of parasite growth by incorporation of stomatocytogenic amphiphiles into the erythrocyte membrane. Antimicrob. Agents Chemother. 2002, 46, 1441–1446. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Cohn, L. Th2 cells and GATA-3 in asthma: New insights into the regulation of airway inflammation. J. Clin. Invest 1999, 104, 985–993. [Google Scholar]
- Hamid, Q.; Tulic, M.K.; Liu, M.C.; Moqbel, R. Inflammatory cells in asthma: Mechanisms and implications for therapy. J. Allergy Clin. Immunol. 2003, 111, S12–S17. [Google Scholar]
- Kuntzsch, D.; Bergann, T.; Dames, P.; Fromm, A.; Fromm, M.; Davis, R.A.; Melzig, M.F.; Schulzke, J.D. The plant-derived glucocorticoid receptor agonist endiandrin a acts as co-stimulator of colonic epithelial sodium channels (ENaC) via SGK-1 and MAPKs. PLoS ONE 2012, 7, e49426. [Google Scholar] [CrossRef] [PubMed]
- Pujols, L.; Mullol, J.; Torrego, A.; Picado, C. Glucocorticoid receptors in human airways. Allergy 2004, 59, 1042–1052. [Google Scholar] [CrossRef]
- Bamberger, C.M.; Schulte, H.M.; Chrousos, G.P. Molecular determinants of glucocorticoid receptor function and tissue sensitivity to glucocorticoids. Endocr. Rev. 1996, 17, 245–261. [Google Scholar] [CrossRef]
- Tanaka, H.; Yoshikawa, N.; Shimizu, N.; Morimoto, C. Selective modulation of glucocorticoid receptor function toward development of novel anti-inflammation: Lessons from a phenyl-pyrazolo-steroid cortivazol. Mod. Rheumatol. 2004, 14, 347–355. [Google Scholar] [CrossRef]
- Park, J.; Lee, J.; Jung, E.; Park, Y.; Kim, K.; Park, B.; Jung, K.; Park, E.; Kim, J.; Park, D. In vitro antibacterial and anti-inflammatory effects of honokiol and magnolol against Propioni bacterium sp. Eur. J. Pharm. 2004, 496, 189–195. [Google Scholar] [CrossRef]
- Fankam, A.G.; Kuiate, J.R.; Kuete, V. Antibacterial activities of Beilschmiedia obscura and six other Cameroonian medicinal plants against multi-drug resistant Gram-negative phenotypes. BMC Complem. Altern. Med. 2014. [Google Scholar] [CrossRef]
- Paulo, M.D.Q.; Barbosa-Filho, J.M.; Lima, E.O.; Maia, R.F.; Barbosa, R.D.C.; Kaplan, M.A. Antimicrobial activity of benzylisoquinoline alkaloids from Annona salzmanii D.C. J. Ethnopharmacol. 1992, 36, 39–41. [Google Scholar] [CrossRef]
- Setzer, W.N.; Stokes, S.L.; Penton, A.F.; Takaku, S.; Haber, W.A.; Hansell, E.; Caffrey, C.R.; McKerrow, J.H. Cruzain inhibitory activity of leaf essential oils of neotropical Lauraceae and essential oil components. Nat. Prod. Commun. 2007, 2, 1203–1210. [Google Scholar]
- McGrath, M.E.; EaKin, A.E.; Engel, J.C.; McKerrow, J.H.; Craik, C.S.; Fletterick, R.J. The crystal structure of cruzain: A therapeutic target for Chagas’ disease. J. Mol. Biol. 1995, 247, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Steverding, D.; Tyler, K.M. Novel antitrypanosomal agents. Expert Opin. Investig. Drugs 2005, 14, 939–955. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lenta, B.N.; Chouna, J.R.; Nkeng-Efouet, P.A.; Sewald, N. Endiandric Acid Derivatives and Other Constituents of Plants from the Genera Beilschmiedia and Endiandra (Lauraceae). Biomolecules 2015, 5, 910-942. https://doi.org/10.3390/biom5020910
Lenta BN, Chouna JR, Nkeng-Efouet PA, Sewald N. Endiandric Acid Derivatives and Other Constituents of Plants from the Genera Beilschmiedia and Endiandra (Lauraceae). Biomolecules. 2015; 5(2):910-942. https://doi.org/10.3390/biom5020910
Chicago/Turabian StyleLenta, Bruno Ndjakou, Jean Rodolphe Chouna, Pepin Alango Nkeng-Efouet, and Norbert Sewald. 2015. "Endiandric Acid Derivatives and Other Constituents of Plants from the Genera Beilschmiedia and Endiandra (Lauraceae)" Biomolecules 5, no. 2: 910-942. https://doi.org/10.3390/biom5020910
APA StyleLenta, B. N., Chouna, J. R., Nkeng-Efouet, P. A., & Sewald, N. (2015). Endiandric Acid Derivatives and Other Constituents of Plants from the Genera Beilschmiedia and Endiandra (Lauraceae). Biomolecules, 5(2), 910-942. https://doi.org/10.3390/biom5020910