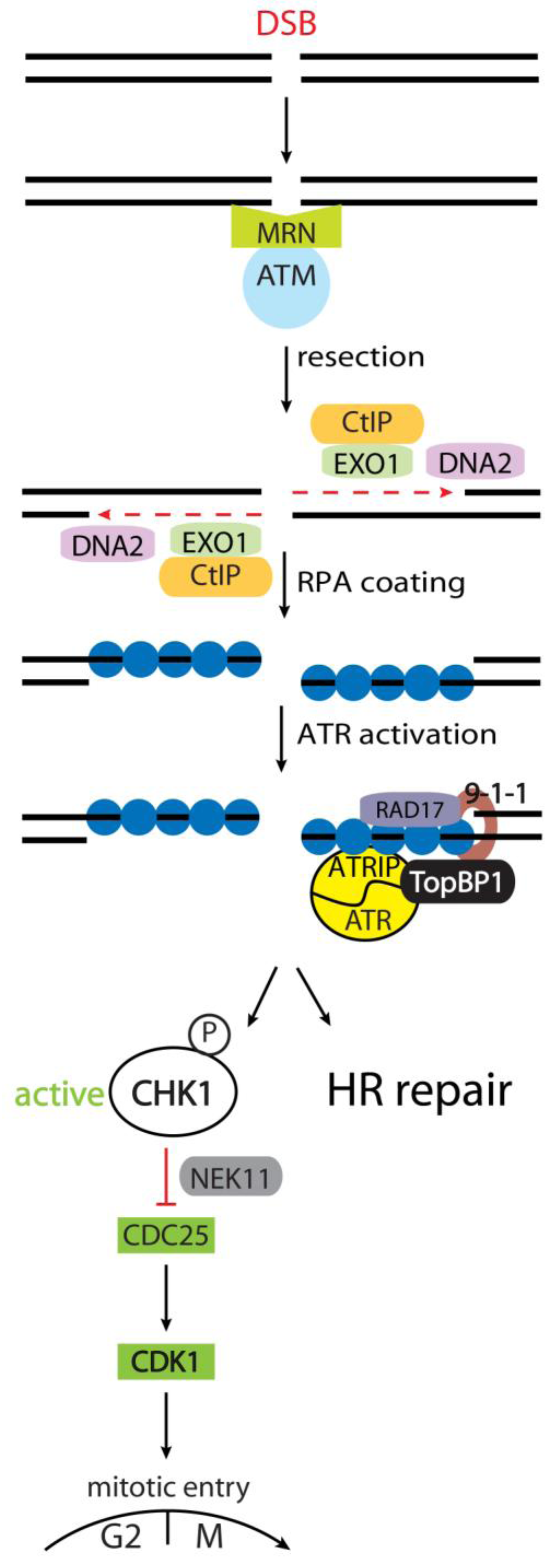
Correction: Kousholt, A.N. et al. Pathways for Genome Integrity in G2 Phase of the Cell Cycle. Biomolecules 2012, 2, 579-607
References
- Kousholt, A.N.; Menzel, T.; Sørensen, C.S. Pathways for Genome Integrity in G2 Phase of the Cell Cycle. Biomolecules 2012, 2, 579–607. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kousholt, A.N.; Menzel, T.; Sørensen, C.S. Correction: Kousholt, A.N. et al. Pathways for Genome Integrity in G2 Phase of the Cell Cycle. Biomolecules 2012, 2, 579-607. Biomolecules 2013, 3, 72-74. https://doi.org/10.3390/biom3010072
Kousholt AN, Menzel T, Sørensen CS. Correction: Kousholt, A.N. et al. Pathways for Genome Integrity in G2 Phase of the Cell Cycle. Biomolecules 2012, 2, 579-607. Biomolecules. 2013; 3(1):72-74. https://doi.org/10.3390/biom3010072
Chicago/Turabian StyleKousholt, Arne Nedergaard, Tobias Menzel, and Claus Storgaard Sørensen. 2013. "Correction: Kousholt, A.N. et al. Pathways for Genome Integrity in G2 Phase of the Cell Cycle. Biomolecules 2012, 2, 579-607" Biomolecules 3, no. 1: 72-74. https://doi.org/10.3390/biom3010072
APA StyleKousholt, A. N., Menzel, T., & Sørensen, C. S. (2013). Correction: Kousholt, A.N. et al. Pathways for Genome Integrity in G2 Phase of the Cell Cycle. Biomolecules 2012, 2, 579-607. Biomolecules, 3(1), 72-74. https://doi.org/10.3390/biom3010072



