TRIC-A Loss Sensitizes the Heart to β-Adrenergic Stress and Drives Cardiomyocyte Death and Fibrosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. In Vivo β-Adrenergic Stimulation
2.3. In Vivo Angiotensin II and Phenylephrine Treatment
2.4. Isolation of Ventricular Cardiomyocytes and Cardiac Fibroblasts
2.5. Ca2+ Imaging in Cardiomyocytes and Cardiac Fibroblasts
2.6. Transmission Electron Microscopy
2.7. Serum Troponin T Assay
2.8. Evans Blue Dye Uptake
2.9. Histology and Fibrosis Quantification
2.10. Quantitative RT-PCR
2.11. Western Blotting and Immunostaining
2.12. Statistical Analysis
3. Results
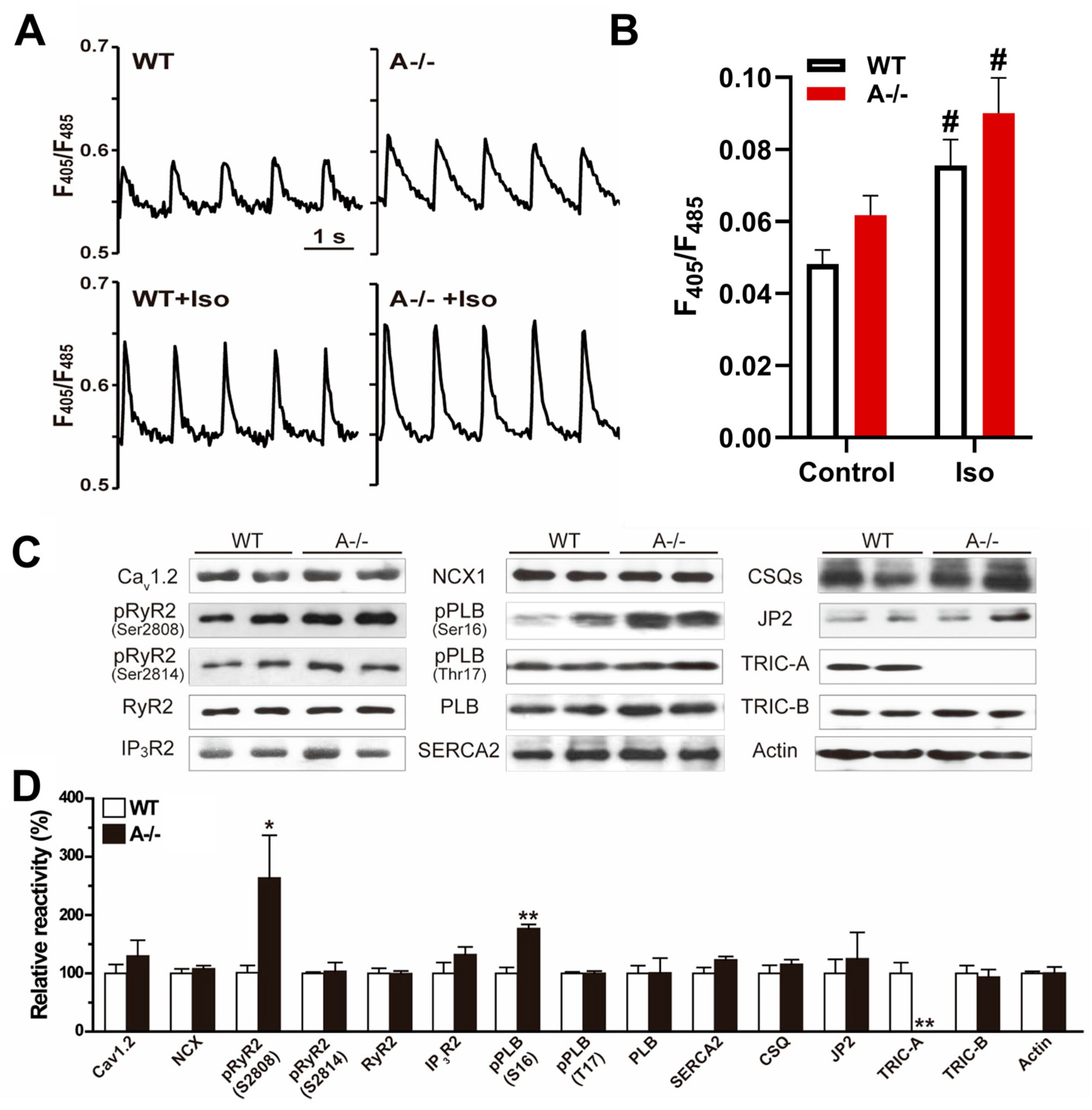
3.1. TRIC-A Deficiency Leads to SR Ca2+ Overload and Baseline Hyperphosphorylation of RyR2 and Phospholamban
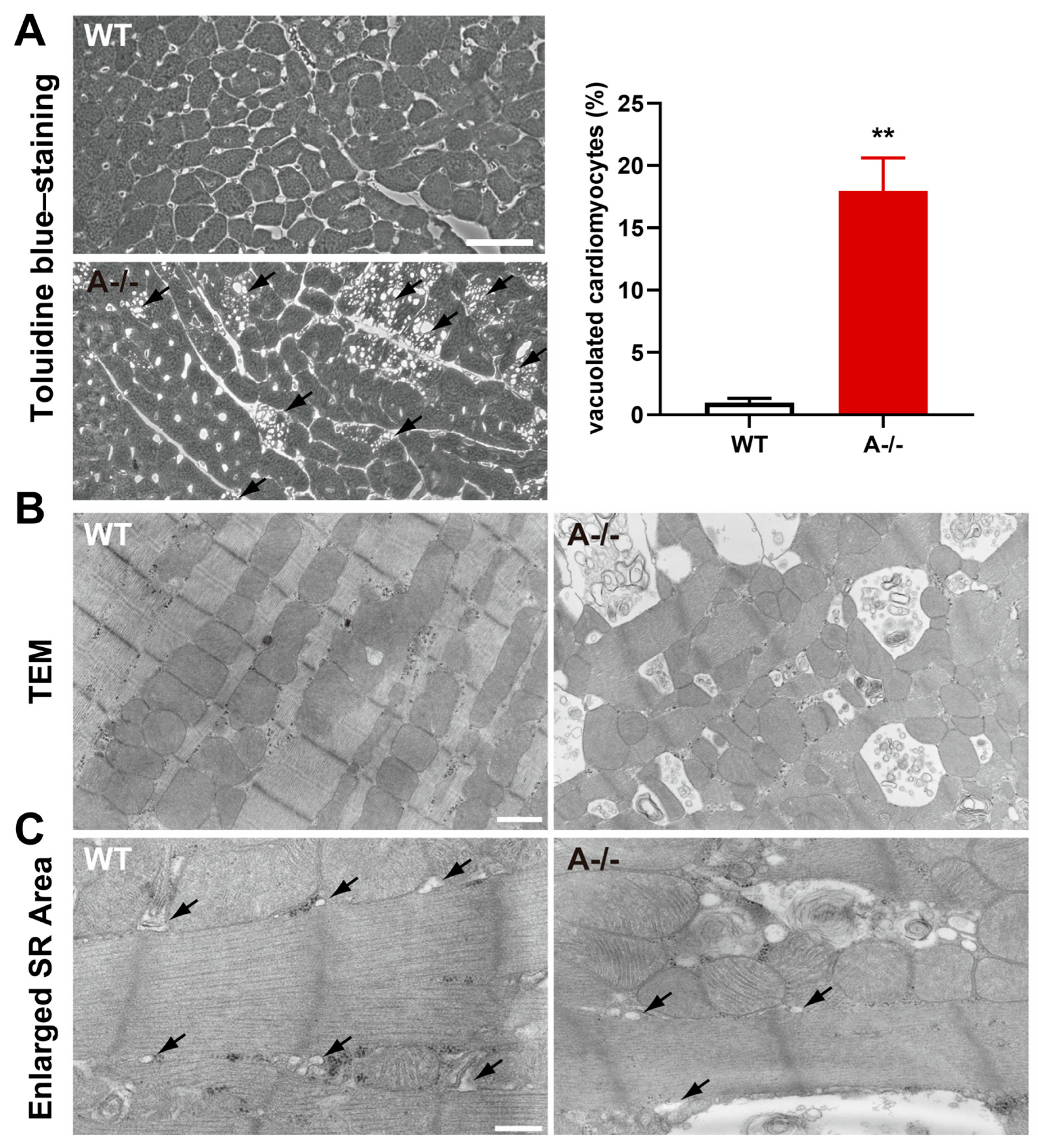
3.2. Acute β-Adrenergic Stimulation Provokes Mitochondrial Injury in TRIC-A Deficient Hearts
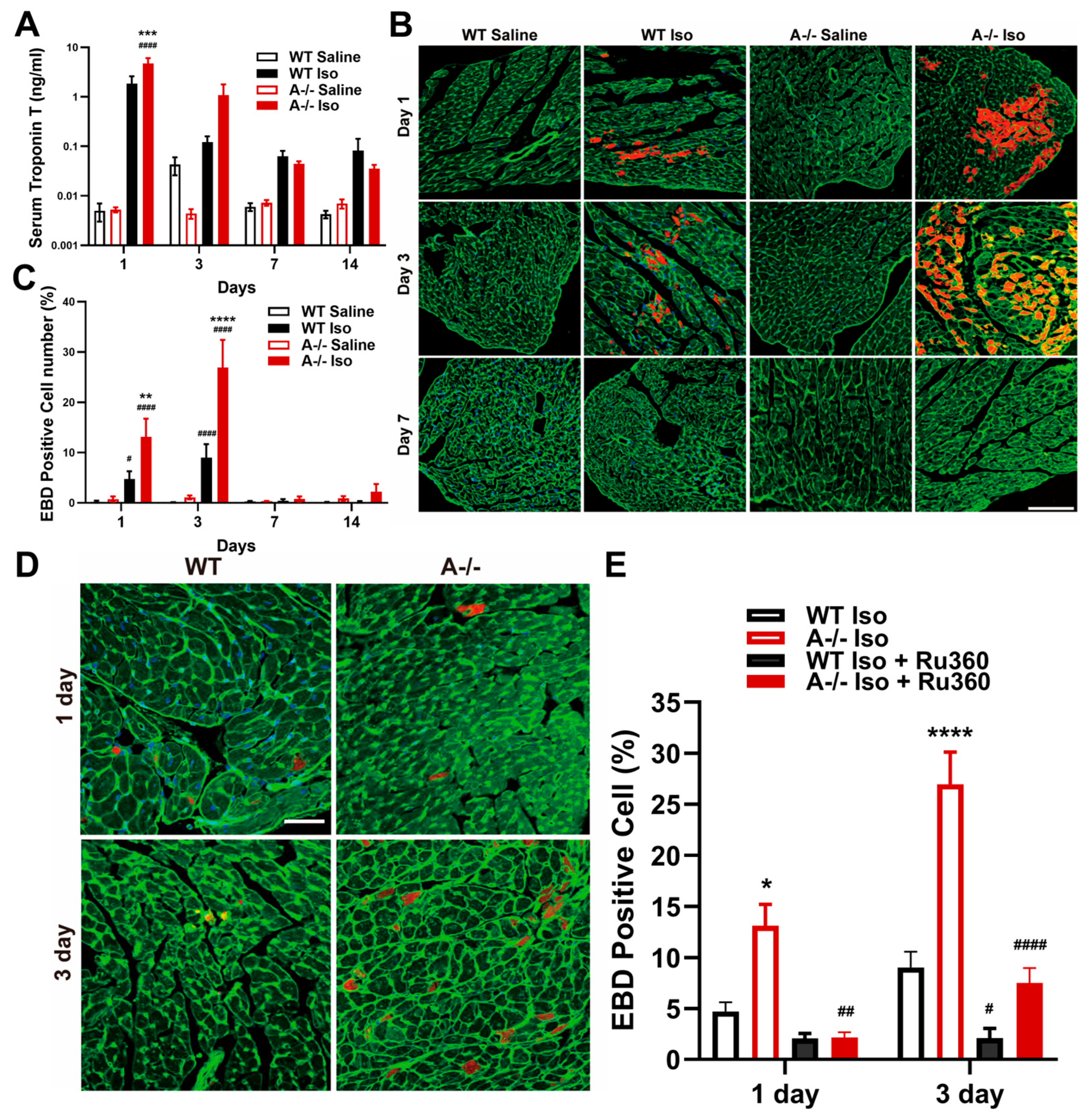
3.3. Cardiomyocyte Death with Loss of Membrane Integrity Follows Mitochondrial Injury
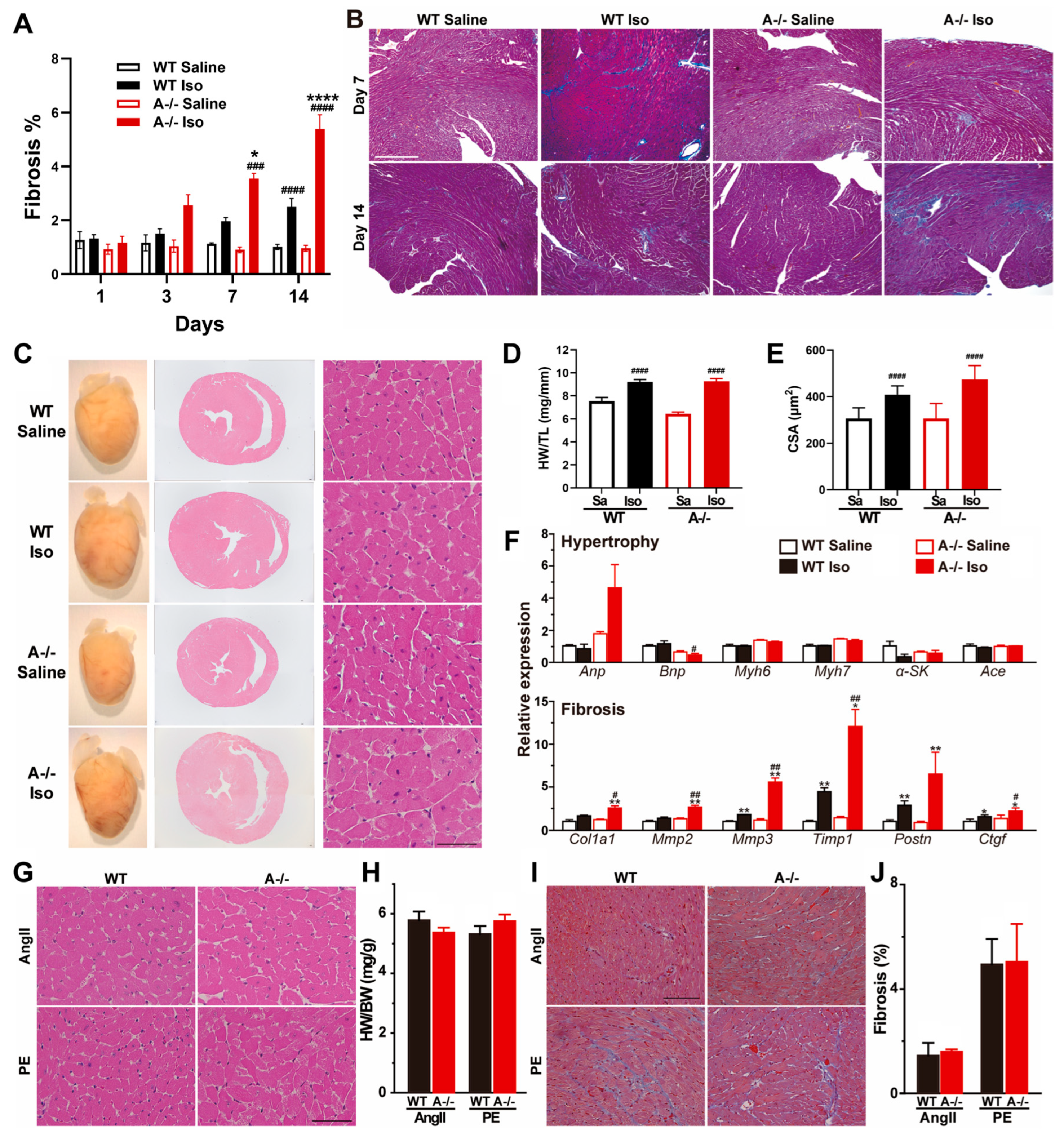
3.4. Sustained β-Adrenergic Stimulation Drives Fibrotic Remodeling in TRIC-A Deficient Hearts
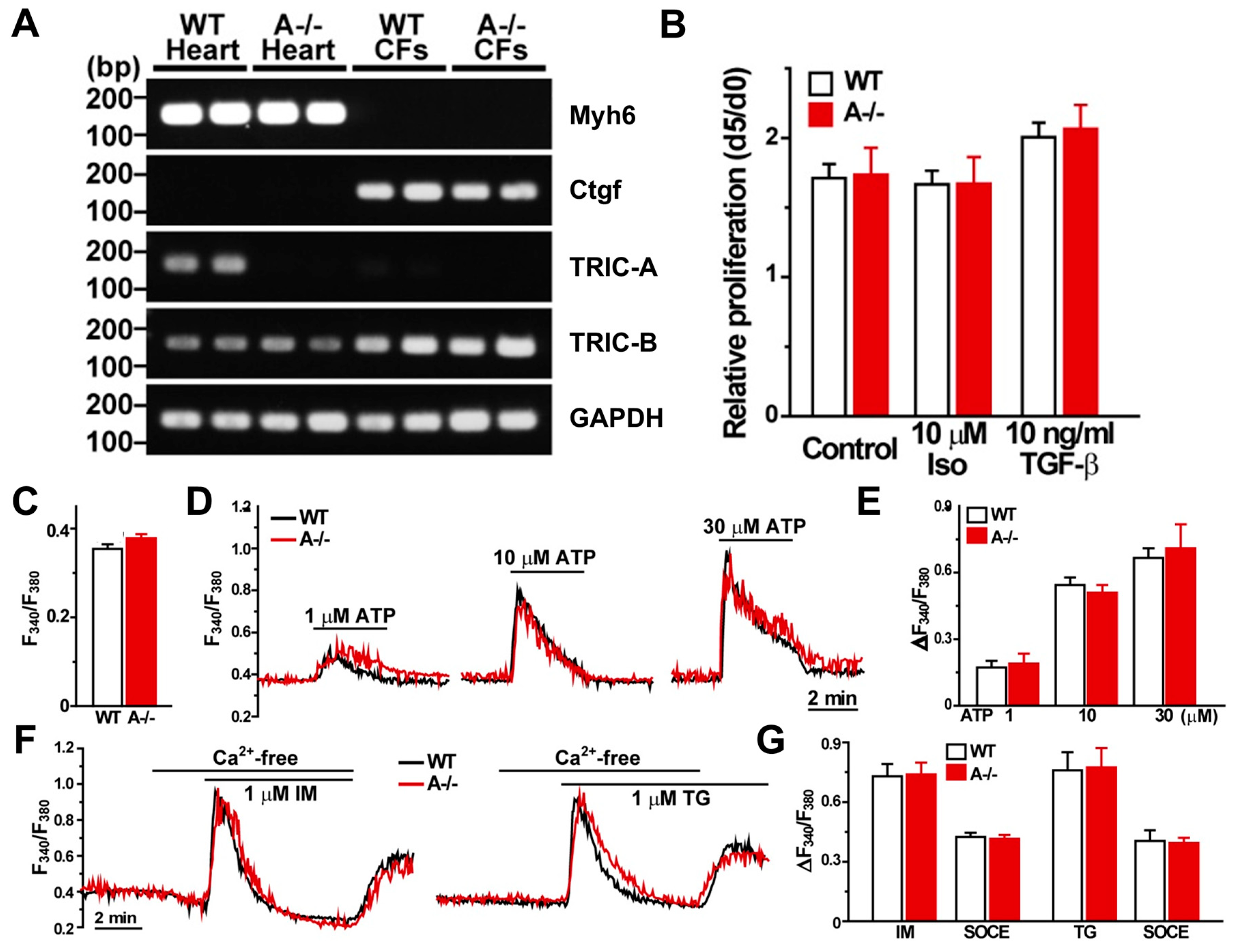
3.5. Cardiac Fibroblasts Lack TRIC-A Expression and Exhibit Normal Proliferation and Ca2+ Handling
4. Discussion
4.1. TRIC-A as a Dual Regulator of SR Ca2+ Release
4.2. β-Adrenergic Stimulation Exposes a Latent Vulnerability in TRIC-A Deficiency
4.3. Necrosis-Driven Fibrosis as the Dominant Remodeling Pathway
4.4. Implications for Cardiac Physiology and Disease
4.5. Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AngII | Angiotensin II |
| ATP | Adenosine triphosphate |
| BDM | 2,3-Butanedione monoxime |
| CaMKII | Ca2+/calmodulin-dependent protein kinase II |
| CFs | Cardiac fibroblasts |
| CSQ/CSQs | Calsequestrin |
| EBD | Evans blue dye |
| ECLIA | Electrochemiluminescence immunoassay |
| ER | Endoplasmic reticulum |
| HE | Hematoxylin and eosin |
| HEPES | 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid |
| IM | Ionomycin |
| ISO | Isoproterenol |
| JP2 | Junctophilin-2 |
| MCU | Mitochondrial Ca2+ uniporter |
| MT | Masson trichrome |
| NCX/NCX1 | Na+/Ca2+ exchanger (isoform 1) |
| PBS | Phosphate-buffered saline |
| PKA | Protein kinase A |
| PLB | Phospholamban |
| PVDF | Polyvinylidene fluoride |
| RT-PCR | Reverse transcription polymerase chain reaction |
| RyR2 | Ryanodine receptor 2 |
| SOCE | Store-operated Ca2+ entry |
| SOICR | Store-overload-induced Ca2+ release |
| SR | Sarcoplasmic reticulum |
| TEM | Transmission electron microscopy |
| TG | Thapsigargin |
| TMEM38A | Transmembrane protein 38A |
| TMEM38B | Transmembrane protein 38B |
| TRIC | Trimeric intracellular cation channel |
| TRIC-A | Trimeric intracellular cation channel A (TMEM38A) |
| TRIC-B | Trimeric intracellular cation channel B (TMEM38B) |
| WT | Wild type |
References
- Bers, D.M. Cardiac excitation-contraction coupling. Nature 2002, 415, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Hegyi, B.; Bailey, L.R.J.; Mira Hernandez, J.; Ko, C.Y.; Shen, E.Y.; Bossuyt, J.; Davis, J.M.; Bers, D.M. Excitation-contraction coupling, cardiomyocyte electrophysiology, and transcriptome profiles in two HFpEF murine models: Etiology and sex-dependent differences. Am. J. Physiol. Heart Circ. Physiol. 2026, 330, H348–H366. [Google Scholar] [CrossRef]
- Wang, J.; Yang Bennett, D.S.; Echard, E.J.; Chen, B.; Ciampa, G.; Zhao, W.; Shi, Q.; Yoon, J.Y.; Weiss, R.M.; Grueter, C.E.; et al. Junctophilin-2 Regulates Store-Operated Calcium Entry to Drive Cardiac Fibroblast Activation, Fibrotic Repair, and Angiogenesis After Myocardial Infarction. Circulation 2025, 152, 699–716. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, T.; Zhao, J.; Zhu, H.; Tan, X.; Chen, J.; Zhang, Z.; Shen, L.; Lu, S. Calcium handling remodeling in dilated cardiomyopathy: From molecular mechanisms to targeted therapies. Channels 2025, 19, 2519545. [Google Scholar] [CrossRef]
- Sutanto, H.; Lyon, A.; Lumens, J.; Schotten, U.; Dobrev, D.; Heijman, J. Cardiomyocyte calcium handling in health and disease: Insights from in vitro and in silico studies. Prog. Biophys. Mol. Biol. 2020, 157, 54–75. [Google Scholar] [CrossRef] [PubMed]
- Mattiazzi, A.; Jaquenod De Giusti, C.; Valverde, C.A. CaMKII at the crossroads: Calcium dysregulation, and post-translational modifications driving cell death. J. Physiol. 2025, 1–17. [Google Scholar] [CrossRef]
- Eisner, D.A.; Caldwell, J.L.; Kistamas, K.; Trafford, A.W. Calcium and Excitation-Contraction Coupling in the Heart. Circ. Res. 2017, 121, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Janicek, R.; Agarwal, H.; Gomez, A.M.; Egger, M.; Ellis-Davies, G.C.R.; Niggli, E. Local recovery of cardiac calcium-induced calcium release interrogated by ultra-effective, two-photon uncaging of calcium. J. Physiol. 2021, 599, 3841–3852. [Google Scholar] [CrossRef]
- Blatter, L.A.; Kanaporis, G.; Martinez-Hernandez, E.; Oropeza-Almazan, Y.; Banach, K. Excitation-contraction coupling and calcium release in atrial muscle. Pflug. Arch. 2021, 473, 317–329. [Google Scholar] [CrossRef]
- Marchena, M.; Echebarria, B. Influence of the tubular network on the characteristics of calcium transients in cardiac myocytes. PLoS ONE 2020, 15, e0231056. [Google Scholar] [CrossRef]
- Fink, R.H.; Stephenson, D.G. Ca2+-movements in muscle modulated by the state of K+-channels in the sarcoplasmic reticulum membranes. Pflug. Arch. 1987, 409, 374–380. [Google Scholar] [CrossRef]
- Berti, C.; Zsolnay, V.; Shannon, T.R.; Fill, M.; Gillespie, D. Sarcoplasmic reticulum Ca2+, Mg2+, K+, and Cl− concentrations adjust quickly as heart rate changes. J. Mol. Cell Cardiol. 2017, 103, 31–39. [Google Scholar] [CrossRef]
- Yazawa, M.; Ferrante, C.; Feng, J.; Mio, K.; Ogura, T.; Zhang, M.; Lin, P.-H.; Pan, Z.; Komazaki, S.; Kato, K. TRIC channels are essential for Ca2+ handling in intracellular stores. Nature 2007, 448, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Kasuya, G.; Hiraizumi, M.; Maturana, A.D.; Kumazaki, K.; Fujiwara, Y.; Liu, K.; Nakada-Nakura, Y.; Iwata, S.; Tsukada, K.; Komori, T.; et al. Crystal structures of the TRIC trimeric intracellular cation channel orthologues. Cell Res. 2016, 26, 1288–1301. [Google Scholar] [CrossRef]
- Yamazaki, D.; Tabara, Y.; Kita, S.; Hanada, H.; Komazaki, S.; Naitou, D.; Mishima, A.; Nishi, M.; Yamamura, H.; Yamamoto, S.; et al. TRIC-A channels in vascular smooth muscle contribute to blood pressure maintenance. Cell Metab. 2011, 14, 231–241. [Google Scholar] [CrossRef]
- Zsolnay, V.; Fill, M.; Gillespie, D. Sarcoplasmic Reticulum Ca2+ Release Uses a Cascading Network of Intra-SR and Channel Countercurrents. Biophys. J. 2018, 114, 462–473. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Maturana, A.D. Effects of aging on calcium channels in skeletal muscle. Front. Mol. Biosci. 2025, 12, 1558456. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, D.; Komazaki, S.; Nakanishi, H.; Mishima, A.; Nishi, M.; Yazawa, M.; Yamazaki, T.; Taguchi, R.; Takeshima, H. Essential role of the TRIC-B channel in Ca2+ handling of alveolar epithelial cells and in perinatal lung maturation. Development 2009, 136, 2355–2361. [Google Scholar] [CrossRef]
- Zhou, X.; Park, K.H.; Yamazaki, D.; Lin, P.H.; Nishi, M.; Ma, Z.; Qiu, L.; Murayama, T.; Zou, X.; Takeshima, H.; et al. TRIC-A Channel Maintains Store Calcium Handling by Interacting with Type 2 Ryanodine Receptor in Cardiac Muscle. Circ. Res. 2020, 126, 417–435. [Google Scholar] [CrossRef]
- Zhou, X.; Li, A.; Lin, P.H.; Zhou, J.; Ma, J. TRIC-A regulates intracellular Ca2+ homeostasis in cardiomyocytes. Pflug. Arch. 2021, 473, 547–556. [Google Scholar] [CrossRef]
- Zhao, X.; Yamazaki, D.; Park, K.H.; Komazaki, S.; Tjondrokoesoemo, A.; Nishi, M.; Lin, P.; Hirata, Y.; Brotto, M.; Takeshima, H.; et al. Ca2+ overload and sarcoplasmic reticulum instability in tric-a null skeletal muscle. J. Biol. Chem. 2010, 285, 37370–37376. [Google Scholar] [CrossRef]
- Cabral, W.A.; Ishikawa, M.; Garten, M.; Makareeva, E.N.; Sargent, B.M.; Weis, M.; Barnes, A.M.; Webb, E.A.; Shaw, N.J.; Ala-Kokko, L.; et al. Absence of the ER Cation Channel TMEM38B/TRIC-B Disrupts Intracellular Calcium Homeostasis and Dysregulates Collagen Synthesis in Recessive Osteogenesis Imperfecta. PLoS Genet. 2016, 12, e1006156. [Google Scholar] [CrossRef] [PubMed]
- Lv, F.; Xu, X.J.; Wang, J.Y.; Liu, Y.; Asan; Wang, J.W.; Song, L.J.; Song, Y.W.; Jiang, Y.; Wang, O.; et al. Two novel mutations in TMEM38B result in rare autosomal recessive osteogenesis imperfecta. J. Hum. Genet. 2016, 61, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Volodarsky, M.; Markus, B.; Cohen, I.; Staretz-Chacham, O.; Flusser, H.; Landau, D.; Shelef, I.; Langer, Y.; Birk, O.S. A deletion mutation in TMEM38B associated with autosomal recessive osteogenesis imperfecta. Hum. Mutat. 2013, 34, 582–586. [Google Scholar] [CrossRef] [PubMed]
- Rubinato, E.; Morgan, A.; D’Eustacchio, A.; Pecile, V.; Gortani, G.; Gasparini, P.; Faletra, F. A novel deletion mutation involving TMEM38B in a patient with autosomal recessive osteogenesis imperfecta. Gene 2014, 545, 290–292. [Google Scholar] [CrossRef]
- Shaheen, R.; Alazami, A.M.; Alshammari, M.J.; Faqeih, E.; Alhashmi, N.; Mousa, N.; Alsinani, A.; Ansari, S.; Alzahrani, F.; Al-Owain, M.; et al. Study of autosomal recessive osteogenesis imperfecta in Arabia reveals a novel locus defined by TMEM38B mutation. J. Med. Genet. 2012, 49, 630–635. [Google Scholar] [CrossRef]
- Robson, M.I.; de Las Heras, J.I.; Czapiewski, R.; Le Thanh, P.; Booth, D.G.; Kelly, D.A.; Webb, S.; Kerr, A.R.W.; Schirmer, E.C. Tissue-Specific Gene Repositioning by Muscle Nuclear Membrane Proteins Enhances Repression of Critical Developmental Genes during Myogenesis. Mol. Cell 2016, 62, 834–847. [Google Scholar] [CrossRef]
- Meinke, P.; Kerr, A.R.W.; Czapiewski, R.; de Las Heras, J.I.; Dixon, C.R.; Harris, E.; Kolbel, H.; Muntoni, F.; Schara, U.; Straub, V.; et al. A multistage sequencing strategy pinpoints novel candidate alleles for Emery-Dreifuss muscular dystrophy and supports gene misregulation as its pathomechanism. EBioMedicine 2020, 51, 102587. [Google Scholar] [CrossRef]
- Le Thanh, P.; Meinke, P.; Korfali, N.; Srsen, V.; Robson, M.I.; Wehnert, M.; Schoser, B.; Sewry, C.A.; Schirmer, E.C. Immunohistochemistry on a panel of Emery-Dreifuss muscular dystrophy samples reveals nuclear envelope proteins as inconsistent markers for pathology. Neuromuscul. Disord. 2017, 27, 338–351. [Google Scholar] [CrossRef]
- Jiang, D.; Xiao, B.; Yang, D.; Wang, R.; Choi, P.; Zhang, L.; Cheng, H.; Chen, S.R. RyR2 mutations linked to ventricular tachycardia and sudden death reduce the threshold for store-overload-induced Ca2+ release (SOICR). Proc. Natl. Acad. Sci. USA 2004, 101, 13062–13067. [Google Scholar] [CrossRef]
- Yoshida, A.; Takahashi, M.; Imagawa, T.; Shigekawa, M.; Takisawa, H.; Nakamura, T. Phosphorylation of ryanodine receptors in rat myocytes during beta-adrenergic stimulation. J. Biochem. 1992, 111, 186–190. [Google Scholar] [CrossRef] [PubMed]
- De Lucia, C.; Eguchi, A.; Koch, W.J. New Insights in Cardiac beta-Adrenergic Signaling During Heart Failure and Aging. Front. Pharmacol. 2018, 9, 904. [Google Scholar] [CrossRef] [PubMed]
- Izem-Meziane, M.; Djerdjouri, B.; Rimbaud, S.; Caffin, F.; Fortin, D.; Garnier, A.; Veksler, V.; Joubert, F.; Ventura-Clapier, R. Catecholamine-induced cardiac mitochondrial dysfunction and mPTP opening: Protective effect of curcumin. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H665–H674. [Google Scholar] [CrossRef]
- Shen, X.; van den Brink, J.; Bergan-Dahl, A.; Kolstad, T.R.; Norden, E.S.; Hou, Y.; Laasmaa, M.; Aguilar-Sanchez, Y.; Quick, A.P.; Espe, E.K.S.; et al. Prolonged beta-adrenergic stimulation disperses ryanodine receptor clusters in cardiomyocytes and has implications for heart failure. eLife 2022, 11, e77725. [Google Scholar] [CrossRef]
- Su, M.; Gao, F.; Yuan, Q.; Mao, Y.; Li, D.L.; Guo, Y.; Yang, C.; Wang, X.H.; Bruni, R.; Kloss, B.; et al. Structural basis for conductance through TRIC cation channels. Nat. Commun. 2017, 8, 15103. [Google Scholar] [CrossRef]
- Yang, H.; Hu, M.; Guo, J.; Ou, X.; Cai, T.; Liu, Z. Pore architecture of TRIC channels and insights into their gating mechanism. Nature 2016, 538, 537–541. [Google Scholar] [CrossRef]
- Ou, X.; Guo, J.; Wang, L.; Yang, H.; Liu, X.; Sun, J.; Liu, Z. Ion- and water-binding sites inside an occluded hourglass pore of a trimeric intracellular cation (TRIC) channel. BMC Biol. 2017, 15, 31. [Google Scholar] [CrossRef]
- Wang, X.H.; Su, M.; Gao, F.; Xie, W.; Zeng, Y.; Li, D.L.; Liu, X.L.; Zhao, H.; Qin, L.; Li, F.; et al. Structural basis for activity of TRIC counter-ion channels in calcium release. Proc. Natl. Acad. Sci. USA 2019, 116, 4238–4243. [Google Scholar] [CrossRef]
- Li, A.; Zhou, X.; Park, K.H.; Yi, J.; Li, X.; Ko, J.K.; Chen, Y.; Nishi, M.; Yamazaki, D.; Takeshima, H.; et al. TRIC-A Facilitates Sarcoplasmic Reticulum-Mitochondrial Ca2+ Signaling Crosstalk in Cardiomyocytes. Cells 2025, 14, 1579. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Rabinovitch, P.S. Protocol for Isolation of Cardiomyocyte from Adult Mouse and Rat. Bio Protoc. 2022, 12, e4412. [Google Scholar] [CrossRef]
- Qian, L.; Huang, Y.; Spencer, C.I.; Foley, A.; Vedantham, V.; Liu, L.; Conway, S.J.; Fu, J.D.; Srivastava, D. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature 2012, 485, 593–598. [Google Scholar] [CrossRef]
- Ullrich, N.D.; Valdivia, H.H.; Niggli, E. PKA phosphorylation of cardiac ryanodine receptor modulates SR luminal Ca2+ sensitivity. J. Mol. Cell Cardiol. 2012, 53, 33–42. [Google Scholar] [CrossRef]
- Janicek, R.; Camors, E.M.; Potenza, D.M.; Fernandez-Tenorio, M.; Zhao, Y.; Dooge, H.C.; Loaiza, R.; Alvarado, F.J.; Egger, M.; Valdivia, H.H.; et al. Dual ablation of the RyR2-Ser2808 and RyR2-Ser2814 sites increases propensity for pro-arrhythmic spontaneous Ca2+ releases. J. Physiol. 2024, 602, 5179–5201. [Google Scholar] [CrossRef]
- Uchinoumi, H.; Yang, Y.; Oda, T.; Li, N.; Alsina, K.M.; Puglisi, J.L.; Chen-Izu, Y.; Cornea, R.L.; Wehrens, X.H.T.; Bers, D.M. CaMKII-dependent phosphorylation of RyR2 promotes targetable pathological RyR2 conformational shift. J. Mol. Cell Cardiol. 2016, 98, 62–72. [Google Scholar] [CrossRef]
- Sepulveda, M.; Burgos, J.I.; Ciocci Pardo, A.; Gonzalez Arbelaez, L.; Mosca, S.; Vila Petroff, M. CaMKII-dependent ryanodine receptor phosphorylation mediates sepsis-induced cardiomyocyte apoptosis. J. Cell Mol. Med. 2020, 24, 9627–9637. [Google Scholar] [CrossRef]
- Valverde, C.A.; Aguero, R.; Wehrens, X.; Vila Petroff, M.; Mattiazzi, A.; Gonano, L.A. RyR2 phosphorylation at serine-2814 increases cardiac tolerance to arrhythmogenic Ca2+ alternans in mice. J. Mol. Cell Cardiol. 2025, 200, 40–44. [Google Scholar] [CrossRef]
- Wegener, A.D.; Jones, L.R. Phosphorylation-induced mobility shift in phospholamban in sodium dodecyl sulfate-polyacrylamide gels. Evidence for a protein structure consisting of multiple identical phosphorylatable subunits. J. Biol. Chem. 1984, 259, 1834–1841. [Google Scholar] [CrossRef] [PubMed]
- Tada, M.; Kirchberger, M.A.; Katz, A.M. Phosphorylation of a 22,000-dalton component of the cardiac sarcoplasmic reticulum by adenosine 3′:5′-monophosphate-dependent protein kinase. J. Biol. Chem. 1975, 250, 2640–2647. [Google Scholar] [CrossRef]
- Cleary, S.R.; Teng, A.C.T.; Kongmeneck, A.D.; Fang, X.; Phillips, T.A.; Cho, E.E.; Smith, R.A.; Karkut, P.; Makarewich, C.A.; Kekenes-Huskey, P.M.; et al. Dilated cardiomyopathy variant R14del increases phospholamban pentamer stability, blunting dynamic regulation of calcium. J. Biol. Chem. 2025, 301, 108118. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Zhu, W.Z.; Xiao, B.; Brochet, D.X.; Chen, S.R.; Lakatta, E.G.; Xiao, R.P.; Cheng, H. Ca2+/calmodulin kinase II-dependent phosphorylation of ryanodine receptors suppresses Ca2+ sparks and Ca2+ waves in cardiac myocytes. Circ. Res. 2007, 100, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Kirichok, Y.; Krapivinsky, G.; Clapham, D.E. The mitochondrial calcium uniporter is a highly selective ion channel. Nature 2004, 427, 360–364. [Google Scholar] [CrossRef]
- Ying, W.L.; Emerson, J.; Clarke, M.J.; Sanadi, D.R. Inhibition of mitochondrial calcium ion transport by an oxo-bridged dinuclear ruthenium ammine complex. Biochemistry 1991, 30, 4949–4952. [Google Scholar] [CrossRef] [PubMed]
- Promila, L.; Sarkar, K.; Guleria, S.; Rakshit, A.; Rathore, M.; Singh, N.C.; Khan, S.; Tomar, M.S.; Ammanathan, V.; Barthwal, M.K.; et al. Mitochondrial calcium uniporter regulates human fibroblast-like synoviocytes invasion via altering mitochondrial dynamics and dictates rheumatoid arthritis pathogenesis. Free Radic. Biol. Med. 2025, 234, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, L.; Xuan, J.; Chen, T.; Du, Y.; Qiao, H.; Zhang, S.; Sun, Z.; Wang, J.; Niu, R. Fluoride induces spermatocyte apoptosis by IP3R1/MCU-mediated mitochondrial calcium overload through MAMs. J. Hazard. Mater. 2025, 489, 137514. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Park, K.H.; Yamazaki, D.; Zhou, X.; Komazaki, S.; Zhao, C.; Nishi, M.; Zhou, J.; Takeshima, H.; Ma, J. TRIC-A Loss Sensitizes the Heart to β-Adrenergic Stress and Drives Cardiomyocyte Death and Fibrosis. Biomolecules 2026, 16, 181. https://doi.org/10.3390/biom16020181
Park KH, Yamazaki D, Zhou X, Komazaki S, Zhao C, Nishi M, Zhou J, Takeshima H, Ma J. TRIC-A Loss Sensitizes the Heart to β-Adrenergic Stress and Drives Cardiomyocyte Death and Fibrosis. Biomolecules. 2026; 16(2):181. https://doi.org/10.3390/biom16020181
Chicago/Turabian StylePark, Ki Ho, Daiju Yamazaki, Xinyu Zhou, Shinji Komazaki, Chengzhu Zhao, Miyuki Nishi, Jingsong Zhou, Hiroshi Takeshima, and Jianjie Ma. 2026. "TRIC-A Loss Sensitizes the Heart to β-Adrenergic Stress and Drives Cardiomyocyte Death and Fibrosis" Biomolecules 16, no. 2: 181. https://doi.org/10.3390/biom16020181
APA StylePark, K. H., Yamazaki, D., Zhou, X., Komazaki, S., Zhao, C., Nishi, M., Zhou, J., Takeshima, H., & Ma, J. (2026). TRIC-A Loss Sensitizes the Heart to β-Adrenergic Stress and Drives Cardiomyocyte Death and Fibrosis. Biomolecules, 16(2), 181. https://doi.org/10.3390/biom16020181








