Optimizing Extracellular Vesicles for Cardiac Repair Post-Myocardial Infarction: Approaches and Challenges
Abstract
1. Introduction
2. Basic Mechanisms: EVs Biology and MI Pathophysiology
3. Therapeutic Effects of EVs in Cardiac Repair
4. Engineering Strategies for Enhanced EV Efficacy
4.1. Optimizing Donor-Cell Sources
4.2. Preconditioning Donor Cells
4.2.1. Hypoxic Preconditioning
4.2.2. Drug/Molecular Preconditioning
4.2.3. Genetic Engineering of Donor Cells
4.3. Molecular and Surface Engineering of EVs
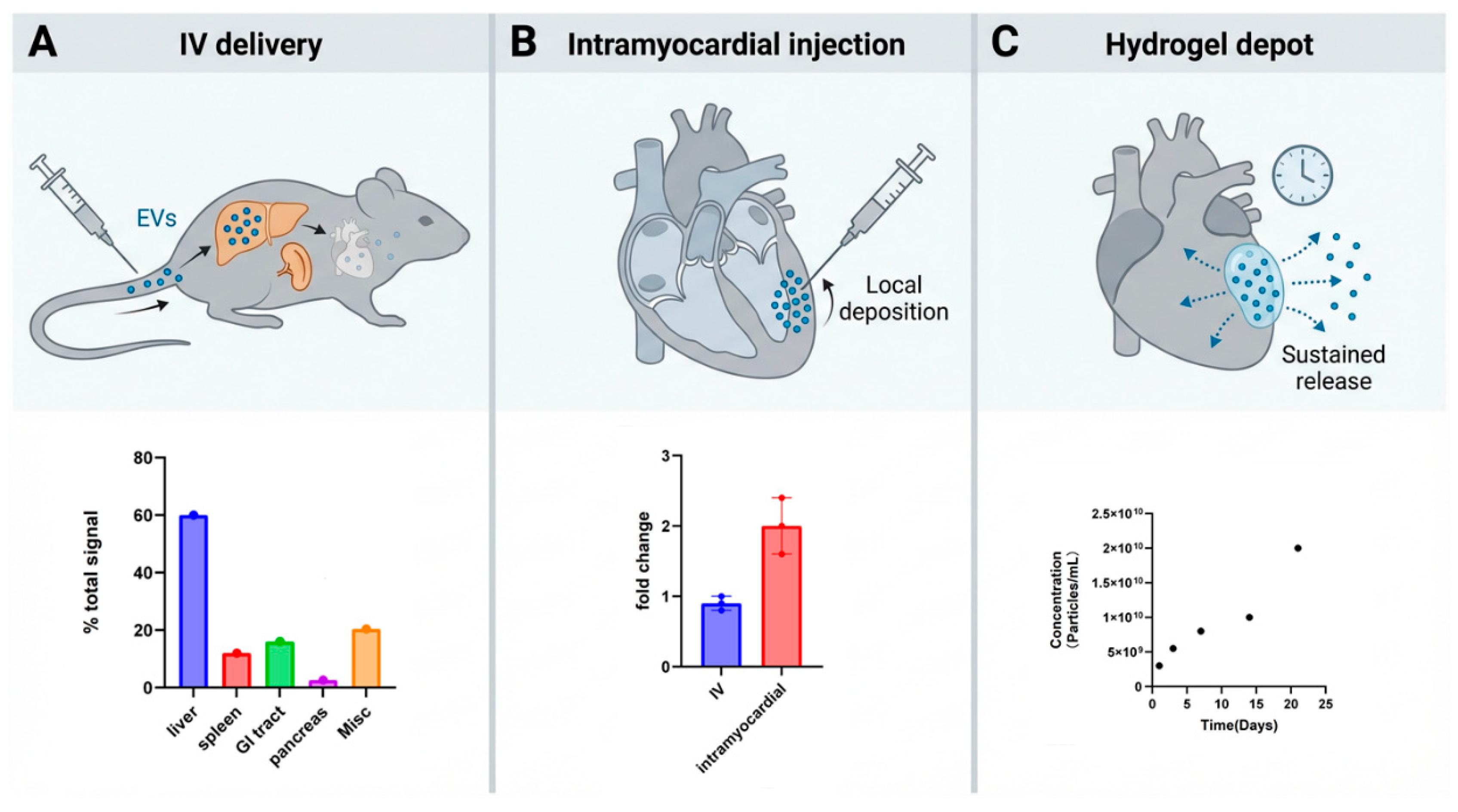
5. Carrier Engineering to Enhance EV Delivery
5.1. Biomaterial Carriers for Localized EV Delivery
5.2. Synthetic and Hybrid EV Carrier Systems
6. Manufacturing and Quality Control of Clinical-Grade EVs
6.1. Scalable Production Methods
6.2. Advanced Purification Technologies
6.3. Robust Quality Control (QC) Frameworks
7. Challenges and Future Prospects
7.1. Challenges of Quality Control
7.2. In Vivo Targeting and Biodistribution
7.3. Immunogenicity and Safety Assessment
7.4. Clinical Bottlenecks
7.5. Clinical Translation: Registered Interventional Studies in Cardiovascular Disease
8. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Johansson, S.; Rosengren, A.; Young, K.; Jennings, E. Mortality and morbidity trends after the first year in survivors of acute myocardial infarction: A systematic review. BMC Cardiovasc. Disord. 2017, 17, 53. [Google Scholar] [CrossRef]
- Butler, J.; Hammonds, K.; Talha, K.M.; Alhamdow, A.; Bennett, M.M.; Bomar, J.V.A.; A Ettlinger, J.; Traba, M.M.; Priest, E.L.; Schmedt, N.; et al. Incident heart failure and recurrent coronary events following acute myocardial infarction. Eur. Hear. J. 2025, 46, 1540–1550. [Google Scholar] [CrossRef]
- Rashid, M.; Abramov, D.; Naseer, M.U.; Van Spall, H.G.C.; Ahmed, F.Z.; Lawson, C.; Dafaalla, M.; Kontopantelis, E.; O Mohamed, M.; Petrie, M.C.; et al. 15-Year trends, predictors, and outcomes of heart failure hospitalization complicating first acute myocardial infarction in the modern percutaneous coronary intervention era. Eur. Hear. J. Open 2025, 5, oeaf013. [Google Scholar] [CrossRef]
- Cung, T.-T.; Morel, O.; Cayla, G.; Rioufol, G.; Garcia-Dorado, D.; Angoulvant, D.; Bonnefoy-Cudraz, E.; Guérin, P.; Elbaz, M.; Delarche, N.; et al. Cyclosporine before PCI in Patients with Acute Myocardial Infarction. N. Engl. J. Med. 2015, 373, 1021–1031. [Google Scholar] [CrossRef]
- Hausenloy, D.J.; Yellon, D.M. Targeting Myocardial Reperfusion Injury—The Search Continues. N. Engl. J. Med. 2015, 373, 1073–1075. [Google Scholar] [CrossRef]
- Hausenloy, D.J.; Kharbanda, R.K.; Møller, U.K.; Ramlall, M.; Aarøe, J.; Butler, R.; Bulluck, H.; Clayton, T.; Dana, A.; Dodd, M.; et al. Effect of remote ischaemic conditioning on clinical outcomes in patients with acute myocardial infarction (CONDI-2/ERIC-PPCI): A single-blind randomised controlled trial. Lancet 2019, 394, 1415–1424. [Google Scholar] [CrossRef] [PubMed]
- Qian, G.; Zhang, Y.; Ma, Z.; Yang, R.; A, X.; Tian, J.; Li, P.; Zhang, H.; Ma, X.; Zhao, L.; et al. Long-term prognostic role of persistent microvascular obstruction determined by cardiac magnetic resonance for ST-segment elevation myocardial infarction. Am. Hear. J. 2025, 290, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Yin, Q.; Yang, Y.; Miao, H.; Han, S.; Chi, Q.; Lv, H.; Lu, Y.; Zhou, Y. Integrating angio-IMR and CMR-assessed microvascular obstruction for improved risk stratification of STEMI patients. Sci. Rep. 2025, 15, 5470. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.G.-E.; Cheng, K.; Marbán, E. Exosomes as Critical Agents of Cardiac Regeneration Triggered by Cell Therapy. Stem Cell Rep. 2014, 2, 606–619. [Google Scholar] [CrossRef]
- Gallet, R.; Dawkins, J.; Valle, J.; Simsolo, E.; De Couto, G.; Middleton, R.; Tseliou, E.; Luthringer, D.; Kreke, M.; Smith, R.R.; et al. Exosomes secreted by cardiosphere-derived cells reduce scarring, attenuate adverse remodelling, and improve function in acute and chronic porcine myocardial infarction. Eur. Heart J. 2017, 38, 201–211. [Google Scholar] [CrossRef]
- Barile, L.; Lionetti, V.; Cervio, E.; Matteucci, M.; Gherghiceanu, M.; Popescu, L.M.; Torre, T.; Siclari, F.; Moccetti, T.; Vassalli, G. Extracellular vesicles from human cardiac progenitor cells inhibit cardiomyocyte apoptosis and improve cardiac function after myocardial infarction. Cardiovasc. Res. 2014, 103, 530–541. [Google Scholar] [CrossRef]
- Mentkowski, K.I.; Lang, J.K. Exosomes Engineered to Express a Cardiomyocyte Binding Peptide Demonstrate Improved Cardiac Retention in Vivo. Sci. Rep. 2019, 9, 10041. [Google Scholar] [CrossRef]
- Rogers, R.G.; Ciullo, A.; Marbán, E.; Ibrahim, A.G. Extracellular Vesicles as Therapeutic Agents for Cardiac Fibrosis. Front. Physiol. 2020, 11, 479. [Google Scholar] [CrossRef] [PubMed]
- de Castilla, P.E.M.; Tong, L.; Huang, C.; Sofias, A.M.; Pastorin, G.; Chen, X.; Storm, G.; Schiffelers, R.M.; Wang, J.-W. Extracellular vesicles as a drug delivery system: A systematic review of preclinical studies. Adv. Drug Deliv. Rev. 2021, 175, 113801. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, W.; Ali, S.R.; Takeda, K.; Vahl, T.P.; Zhu, D.; Hong, Y.; Cheng, K. Extracellular vesicle therapeutics for cardiac repair. J. Mol. Cell. Cardiol. 2024, 199, 12–32. [Google Scholar] [CrossRef]
- Kang, M.; Jordan, V.; Blenkiron, C.; Chamley, L.W. Biodistribution of extracellular vesicles following administration into animals: A systematic review. J. Extracell. Vesicles 2021, 10, e12085. [Google Scholar] [CrossRef]
- Foglio, E.; Pellegrini, L.; Russo, M.A.; Limana, F. HMGB1-Mediated Activation of the Inflammatory-Reparative Response Following Myocardial Infarction. Cells 2022, 11, 216. [Google Scholar] [CrossRef] [PubMed]
- Ciortan, L.; Macarie, R.D.; Barbu, E.; Naie, M.L.; Mihaila, A.C.; Serbanescu, M.; Butoi, E. Cross-Talk Between Neutrophils and Macrophages Post-Myocardial Infarction: From Inflammatory Drivers to Therapeutic Targets. Int. J. Mol. Sci. 2025, 26, 10575. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Anzai, A.; Katsumata, Y.; Matsuhashi, T.; Ito, K.; Endo, J.; Yamamoto, T.; Takeshima, A.; Shinmura, K.; Shen, W.; et al. Temporal dynamics of cardiac immune cell accumulation following acute myocardial infarction. J. Mol. Cell. Cardiol. 2013, 62, 24–35. [Google Scholar] [CrossRef]
- Luther, K.M.; Haar, L.; McGuinness, M.; Wang, Y.; Lynch, T.L.L., IV; Phan, A.; Song, Y.; Shen, Z.; Gardner, G.; Kuffel, G.; et al. Exosomal miR-21a-5p mediates cardioprotection by mesenchymal stem cells. J. Mol. Cell. Cardiol. 2018, 119, 125–137. [Google Scholar] [CrossRef]
- Cambier, L.; De Couto, G.; Ibrahim, A.; Echavez, A.K.; Valle, J.; Liu, W.; Kreke, M.; Smith, R.R.; Marbán, L.; Marbán, E. Y RNA fragment in extracellular vesicles confers cardioprotection via modulation of IL-10 expression and secretion. EMBO Mol. Med. 2017, 9, 337–352. [Google Scholar] [CrossRef]
- Guo, H.; Li, Z.; Xiao, B.; Huang, R. M2 macrophage-derived exosomes promote angiogenesis and improve cardiac function after myocardial infarction. Biol. Direct 2024, 19, 43. [Google Scholar] [CrossRef]
- Li, D.; Zhao, Y.; Zhang, C.; Wang, F.; Zhou, Y.; Jin, S. Plasma Exosomes at the Late Phase of Remote Ischemic Pre-conditioning Attenuate Myocardial Ischemia-Reperfusion Injury Through Transferring miR-126a-3p. Front. Cardiovasc. Med. 2021, 8, 736226. [Google Scholar] [CrossRef] [PubMed]
- Shafei, S.; Khanmohammadi, M.; Ghanbari, H.; Nooshabadi, V.T.; Tafti, S.H.A.; Rabbani, S.; Kasaiyan, M.; Basiri, M.; Tavoosidana, G. Effectiveness of exosome mediated miR-126 and miR-146a delivery on cardiac tissue regeneration. Cell Tissue Res. 2022, 390, 71–92. [Google Scholar] [CrossRef] [PubMed]
- Wiklander, O.P.B.; Nordin, J.Z.; O’Loughlin, A.; Gustafsson, Y.; Corso, G.; Mäger, I.; Vader, P.; Lee, Y.; Sork, H.; Seow, Y.; et al. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J. Extracell. Vesicles 2015, 4, 26316. [Google Scholar] [CrossRef]
- Christianson, H.C.; Svensson, K.J.; van Kuppevelt, T.H.; Li, J.-P.; Belting, M. Cancer cell exosomes depend on cell-surface heparan sulfate proteoglycans for their internalization and functional activity. Proc. Natl. Acad. Sci. USA 2013, 110, 17380–17385. [Google Scholar] [CrossRef]
- Hoshino, A.; Costa-Silva, B.; Shen, T.-L.; Rodrigues, G.; Hashimoto, A.; Mark, M.T.; Molina, H.; Kohsaka, S.; Di Giannatale, A.; Ceder, S.; et al. Tumour exosome integrins determine organotropic metastasis. Nature 2015, 527, 329–335. [Google Scholar] [CrossRef]
- Cedillo-Servin, G.; Louro, A.F.; Gamelas, B.; Meliciano, A.; Zijl, A.; Alves, P.M.; Malda, J.; Serra, M.; Castilho, M. Microfiber-reinforced hydrogels prolong the release of human induced pluripotent stem cell-derived extracellular vesicles to promote endothelial migration. Mater. Sci. Eng. C 2023, 155, 213692. [Google Scholar] [CrossRef]
- Chen, C.W.; Wang, L.L.; Zaman, S.; Gordon, J.; Arisi, M.F.; Venkataraman, C.M.; Chung, J.J.; Hung, G.; Gaffey, A.C.; Spruce, L.A.; et al. Sustained release of endothelial progenitor cell-derived extracellular vesicles from shear-thinning hydrogels improves angiogenesis and promotes function after myocardial infarction. Cardiovasc. Res. 2018, 114, 1029–1040. [Google Scholar] [CrossRef]
- Charles, C.J.; Li, R.R.; Yeung, T.; Mazlan, S.M.I.; Lai, R.C.; de Kleijn, D.P.V.; Lim, S.K.; Richards, A.M. Systemic Mesenchymal Stem Cell-Derived Exosomes Reduce Myocardial Infarct Size: Characterization With MRI in a Porcine Model. Front. Cardiovasc. Med. 2020, 7, 601990. [Google Scholar] [CrossRef] [PubMed]
- Bian, S.; Zhang, L.; Duan, L.; Wang, X.; Min, Y.; Yu, H. Extracellular vesicles derived from human bone marrow mesenchymal stem cells promote angiogenesis in a rat myocardial infarction model. J. Mol. Med. 2013, 92, 387–397. [Google Scholar] [CrossRef]
- Sun, J.; Shen, H.; Shao, L.; Teng, X.; Chen, Y.; Liu, X.; Yang, Z.; Shen, Z. HIF-1α overexpression in mesenchymal stem cell-derived exosomes mediates cardioprotection in myocardial infarction by enhanced angiogenesis. Stem Cell Res. Ther. 2020, 11, 373. [Google Scholar] [CrossRef]
- Zhu, W.; Sun, L.; Zhao, P.; Liu, Y.; Zhang, J.; Zhang, Y.; Hong, Y.; Zhu, Y.; Lu, Y.; Zhao, W.; et al. Macrophage migration inhibitory factor facilitates the therapeutic efficacy of mesenchymal stem cells derived exosomes in acute myocardial infarction through upregulating miR-133a-3p. J. Nanobiotechnol. 2021, 19, 61. [Google Scholar] [CrossRef]
- Li, H.; Wang, L.; Ma, T.; Liu, Z.; Gao, L. Exosomes secreted by endothelial cells derived from human induced pluripotent stem cells improve recovery from myocardial infarction in mice. Stem Cell Res. Ther. 2023, 14, 278. [Google Scholar] [CrossRef]
- Ning, H.; Chen, H.; Deng, J.; Xiao, C.; Xu, M.; Shan, L.; Yang, C.; Zhang, Z. Exosomes secreted by FNDC5-BMMSCs protect myocardial infarction by anti-inflammation and macrophage polarization via NF-κB signaling pathway and Nrf2/HO-1 axis. Stem Cell Res. Ther. 2021, 12, 519. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Chen, Y.; Chen, Y.; Meng, Q.; Sun, J.; Shao, L.; Yu, Y.; Huang, H.; Hu, Y.; Yang, Z.; et al. MicroRNA-132, Delivered by Mesenchymal Stem Cell-Derived Exosomes, Promote Angiogenesis in Myocardial Infarction. Stem Cells Int. 2018, 2018, 3290372. [Google Scholar] [CrossRef]
- Santoso, M.R.; Ikeda, G.; Tada, Y.; Jung, J.; Vaskova, E.; Sierra, R.G.; Gati, C.; Goldstone, A.B.; von Bornstaedt, D.; Shukla, P.; et al. Exosomes From Induced Pluripotent Stem Cell–Derived Cardiomyocytes Promote Autophagy for Myocardial Repair. J. Am. Hear. Assoc. 2020, 9, e014345. [Google Scholar] [CrossRef]
- Minghua, W.; Zhijian, G.; Chahua, H.; Qiang, L.; Minxuan, X.; Luqiao, W.; Weifang, Z.; Peng, L.; Biming, Z.; Lingling, Y.; et al. Plasma exosomes induced by remote ischaemic preconditioning attenuate myocardial ischaemia/reperfusion injury by transferring miR-24. Cell Death Dis. 2018, 9, 320. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Guo, D.; Liu, G.; Chen, G.; Hang, M.; Jin, M. Exosomes from MiR-126-Overexpressing Adscs Are Therapeutic in Relieving Acute Myocardial Ischaemic Injury. Cell. Physiol. Biochem. 2017, 44, 2105–2116. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Chang, S.; Xu, R.; Chen, L.; Song, X.; Wu, J.; Qian, J.; Zou, Y.; Ma, J. Hypoxia-challenged MSC-derived exosomes deliver miR-210 to attenuate post-infarction cardiac apoptosis. Stem Cell Res. Ther. 2020, 11, 224. [Google Scholar] [CrossRef]
- Zhu, L.-P.; Tian, T.; Wang, J.-Y.; He, J.-N.; Chen, T.; Pan, M.; Xu, L.; Zhang, H.-X.; Qiu, X.-T.; Li, C.-C.; et al. Hypoxia-elicited mesenchymal stem cell-derived exosomes facilitates cardiac repair through miR-125b-mediated prevention of cell death in myocardial infarction. Theranostics 2018, 8, 6163–6177. [Google Scholar] [CrossRef]
- Pham, T.P.; Boon, R.A. Exosomes and non-coding RNA, the healers of the heart? Cardiovasc. Res. 2019. [Google Scholar] [CrossRef]
- Ning, Y.; Huang, P.; Chen, G.; Xiong, Y.; Gong, Z.; Wu, C.; Xu, J.; Jiang, W.; Li, X.; Tang, R.; et al. Atorvastatin-pretreated mesenchymal stem cell-derived extracellular vesicles promote cardiac repair after myocardial infarction via shifting macrophage polarization by targeting microRNA-139-3p/Stat1 pathway. BMC Med. 2023, 21, 96. [Google Scholar] [CrossRef]
- Xiong, Y.; Tang, R.; Xu, J.; Jiang, W.; Gong, Z.; Zhang, L.; Ning, Y.; Huang, P.; Xu, J.; Chen, G.; et al. Tongxinluo-pretreated mesenchymal stem cells facilitate cardiac repair via exosomal transfer of miR-146a-5p targeting IRAK1/NF-κB p65 pathway. Stem Cell Res. Ther. 2022, 13, 289. [Google Scholar] [CrossRef] [PubMed]
- Pu, Y.; Li, C.; Qi, X.; Xu, R.; Dong, L.; Jiang, Y.; Gong, Q.; Wang, D.; Cheng, R.; Zhang, C.; et al. Extracellular Vesicles from NMN Preconditioned Mesenchymal Stem Cells Ameliorated Myocardial Infarction via miR-210-3p Promoted Angiogenesis. Stem Cell Rev. Rep. 2023, 19, 1051–1066. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, C.; Zhang, J.; Jiao, Z.; Dong, N.; Wang, G.; Wang, Z.; Wang, L. Localized injection of miRNA-21-enriched extracellular vesicles effectively restores cardiac function after myocardial infarction. Theranostics 2019, 9, 2346–2360. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Wang, Q.; Zhang, J.; Sun, L.; Hong, X.; Du, W.; Duan, R.; Jiang, J.; Ji, Y.; Wang, H.; et al. Exosomes derived from mir-214-3p overexpressing mesenchymal stem cells promote myocardial repair. Biomater. Res. 2023, 27, 77. [Google Scholar] [CrossRef]
- Gong, Z.-T.; Xiong, Y.-Y.; Ning, Y.; Tang, R.-J.; Xu, J.-Y.; Jiang, W.-Y.; Li, X.-S.; Zhang, L.-L.; Chen, C.; Pan, Q.; et al. Nicorandil-Pretreated Mesenchymal Stem Cell-Derived Exosomes Facilitate Cardiac Repair After Myocardial Infarction via Promoting Macrophage M2 Polarization by Targeting miR-125a-5p/TRAF6/IRF5 Signaling Pathway. Int. J. Nanomed. 2024, 19, 2005–2024. [Google Scholar] [CrossRef] [PubMed]
- Ciullo, A.; Biemmi, V.; Milano, G.; Bolis, S.; Cervio, E.; Fertig, E.T.; Gherghiceanu, M.; Moccetti, T.; Camici, G.G.; Vassalli, G.; et al. Exosomal Expression of CXCR4 Targets Cardioprotective Vesicles to Myocardial Infarction and Improves Outcome after Systemic Administration. Int. J. Mol. Sci. 2019, 20, 468. [Google Scholar] [CrossRef]
- Xiao, Z.; Li, S.; Wu, X.; Chen, X.; Yan, D.; He, J. GATA-4 overexpressing BMSC-derived exosomes suppress H/R-induced cardiomyocyte ferroptosis. iScience 2024, 27, 110784. [Google Scholar] [CrossRef]
- He, J.-G.; Li, H.-R.; Han, J.-X.; Li, B.-B.; Yan, D.; Li, H.-Y.; Wang, P.; Luo, Y. GATA-4-expressing mouse bone marrow mesenchymal stem cells improve cardiac function after myocardial infarction via secreted exosomes. Sci. Rep. 2018, 8, 9047. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.-Y.; Kim, H.; Mun, D.; Yun, N.; Joung, B. Co-delivery of curcumin and miRNA-144-3p using heart-targeted extracellular vesicles enhances the therapeutic efficacy for myocardial infarction. J. Control. Release 2021, 331, 62–73. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, B.; Zhang, L.; Xu, X.; Cheng, Q.; Wang, Y.; Li, Y.; Jiang, R.; Duan, S.; Zhang, L. Engineered nanovesicles mediated cardiomyocyte survival and neovascularization for the therapy of myocardial infarction. Colloids Surf. B Biointerfaces 2024, 243, 114135. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Zhao, Z.; Meng, Q.; Yu, Y.; Sun, J.; Yang, Z.; Chen, Y.; Li, J.; Ma, T.; et al. Engineered Exosomes With Ischemic Myocardium-Targeting Peptide for Targeted Therapy in Myocardial Infarction. J. Am. Hear. Assoc. 2018, 7, e008737. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.; Su, G.; Zhang, Y.; Wang, Z.; Jia, Y.; Yu, Q.; Shen, Z.; Zhang, Y.; Yu, Y. Neutrophil-derived apoptotic body membranes-fused exosomes targeting treatment for myocardial infarction. Regen. Biomater. 2024, 12, rbae145. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Zhu, D.; Huang, K.; Caranasos, T.G. Minimally invasive delivery of a hydrogel-based exosome patch to prevent heart failure. J. Mol. Cell. Cardiol. 2022, 169, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Chen, Z.; Zhao, Y.; Fan, F.; Xiong, W.; Song, S.; Yin, Y.; Hu, J.; Yang, K.; Yang, L.; et al. Mononuclear phagocyte system blockade using extracellular vesicles modified with CD47 on membrane surface for myocardial infarction reperfusion injury treatment. Biomaterials 2021, 275, 121000. [Google Scholar] [CrossRef]
- Xia, L.-X.; Xiao, Y.-Y.; Jiang, W.-J.; Yang, X.-Y.; Tao, H.; Mandukhail, S.R.; Qin, J.-F.; Pan, Q.-R.; Zhu, Y.-G.; Zhao, L.-X.; et al. Exosomes derived from induced cardiopulmonary progenitor cells alleviate acute lung injury in mice. Acta Pharmacol. Sin. 2024, 45, 1644–1659. [Google Scholar] [CrossRef]
- Gao, L.; Wang, L.; Wei, Y.; Krishnamurthy, P.; Walcott, G.P.; Menasché, P.; Zhang, J. Exosomes secreted by hiPSC-derived cardiac cells improve recovery from myocardial infarction in swine. Sci. Transl. Med. 2020, 12, eaay1318. [Google Scholar] [CrossRef]
- Kim, S.-C.; Stice, J.P.; Chen, L.; Jung, J.S.; Gupta, S.; Wang, Y.; Baumgarten, G.; Trial, J.; Knowlton, A.A. Extracellular Heat Shock Protein 60, Cardiac Myocytes, and Apoptosis. Circ. Res. 2009, 105, 1186–1195. [Google Scholar] [CrossRef]
- Malik, Z.A.; Kott, K.S.; Poe, A.J.; Kuo, T.; Chen, L.; Ferrara, K.W.; Knowlton, A.A. Cardiac myocyte exosomes: Stability, HSP60, and proteomics. Am. J. Physiol. Circ. Physiol. 2013, 304, H954–H965. [Google Scholar] [CrossRef]
- Qiao, L.; Hu, S.; Liu, S.; Zhang, H.; Ma, H.; Huang, K.; Li, Z.; Su, T.; Vandergriff, A.; Tang, J.; et al. microRNA-21-5p dysregulation in exosomes derived from heart failure patients impairs regenerative potential. J. Clin. Investig. 2019, 129, 2237–2250. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.; Wang, C. Mesenchymal stem cell-derived exosomal mir-21-5p inhibits YAP1 expression and improves outcomes in myocardial infarction. BMC Cardiovasc. Disord. 2024, 24, 547. [Google Scholar] [CrossRef]
- Vandergriff, A.; Huang, K.; Shen, D.; Hu, S.; Hensley, M.T.; Caranasos, T.G.; Qian, L.; Cheng, K. Targeting regenerative exosomes to myocardial infarction using cardiac homing peptide. Theranostics 2018, 8, 1869–1878. [Google Scholar] [CrossRef]
- He, L.; He, T.; Xing, J.; Zhou, Q.; Fan, L.; Liu, C.; Chen, Y.; Wu, D.; Tian, Z.; Liu, B.; et al. Bone marrow mesenchymal stem cell-derived exosomes protect cartilage damage and relieve knee osteoarthritis pain in a rat model of osteoarthritis. Stem Cell Res. Ther. 2020, 11, 276. [Google Scholar] [CrossRef]
- Wong, K.L.; Zhang, S.; Wang, M.; Ren, X.; Afizah, H.; Lai, R.C.; Lim, S.K.; Lee, E.H.; Hui, J.H.P.; Toh, W.S. Intra-Articular Injections of Mesenchymal Stem Cell Exosomes and Hyaluronic Acid Improve Structural and Mechanical Properties of Repaired Cartilage in a Rabbit Model. Arthrosc. J. Arthrosc. Relat. Surg. 2020, 36, 2215–2228.e2. [Google Scholar] [CrossRef]
- Gharehchelou, B.; Mehrarya, M.; Sefidbakht, Y.; Uskoković, V.; Suri, F.; Arjmand, S.; Maghami, F.; Siadat, S.O.R.; Karima, S.; Vosough, M. Mesenchymal stem cell-derived exosome and liposome hybrids as transfection nanocarriers of Cas9-GFP plasmid to HEK293T cells. PLoS ONE 2025, 20, e0315168. [Google Scholar] [CrossRef]
- Villa, C.; Secchi, V.; Macchi, M.; Tripodi, L.; Trombetta, E.; Zambroni, D.; Padelli, F.; Mauri, M.; Molinaro, M.; Oddone, R.; et al. Magnetic-field-driven targeting of exosomes modulates immune and metabolic changes in dystrophic muscle. Nat. Nanotechnol. 2024, 19, 1532–1543. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Wang, X.; Li, Z.; Zhu, D.; Cores, J.; Wang, Z.; Li, J.; Mei, X.; Cheng, X.; Su, T.; et al. Platelet membrane and stem cell exosome hybrids enhance cellular uptake and targeting to heart injury. Nano Today 2021, 39, 101210. [Google Scholar] [CrossRef] [PubMed]
- Haraszti, R.A.; Miller, R.; Stoppato, M.; Sere, Y.Y.; Coles, A.; Didiot, M.-C.; Wollacott, R.; Sapp, E.; Dubuke, M.L.; Li, X.; et al. Exosomes Produced from 3D Cultures of MSCs by Tangential Flow Filtration Show Higher Yield and Improved Activity. Mol. Ther. 2018, 26, 2838–2847. [Google Scholar] [CrossRef] [PubMed]
- Ulpiano, C.; Salvador, W.; Franchi-Mendes, T.; Huang, M.-C.; Lin, Y.-H.; Lin, H.-T.; Rodrigues, C.A.V.; Fernandes-Platzgummer, A.; Cabral, J.M.S.; Monteiro, G.A.; et al. Continuous collection of human mesenchymal-stromal-cell-derived extracellular vesicles from a stirred tank reactor operated under xenogeneic-free conditions for therapeutic applications. Stem Cell Res. Ther. 2025, 16, 210. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.A.; Goberdhan, D.C.; O’Driscoll, L.; Théry, C.; Witwer, K.W. MISEV2023: An updated guide to EV research and applications. J. Extracell. Vesicles 2024, 13, e12416. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Onodi, Z.; Pelyhe, C.; Terezia Nagy, C.; Brenner, G.B.; Almasi, L.; Kittel, A.; Mancek-Keber, M.; Ferdinandy, P.; Buzas, E.I.; Giricz, Z. Isolation of High-Purity Extracellular Vesicles by the Combination of Iodixanol Density Gradient Ultracentrifugation and Bind-Elute Chromatography From Blood Plasma. Front. Physiol. 2018, 9, 1479. [Google Scholar] [CrossRef]
- Böing, A.N.; van der Pol, E.; Grootemaat, A.E.; Coumans, F.A.W.; Sturk, A.; Nieuwland, R. Single-step isolation of extracellular vesicles by size-exclusion chromatography. J. Extracell. Vesicles 2014, 3, 23430. [Google Scholar] [CrossRef]
- Busatto, S.; Vilanilam, G.; Ticer, T.; Lin, W.-L.; Dickson, D.W.; Shapiro, S.; Bergese, P.; Wolfram, J. Tangential Flow Filtration for Highly Efficient Concentration of Extracellular Vesicles from Large Volumes of Fluid. Cells 2018, 7, 273. [Google Scholar] [CrossRef]
- Gámez-Valero, A.; Monguió-Tortajada, M.; Carreras-Planella, L.; La Franquesa, M.; Beyer, K.; Borràs, F.E. Size-Exclusion Chromatography-based isolation minimally alters Extracellular Vesicles’ characteristics compared to precipitating agents. Sci. Rep. 2016, 6, 33641. [Google Scholar] [CrossRef]
- Ströhle, G.; Gan, J.; Li, H. Affinity-based isolation of extracellular vesicles and the effects on downstream molecular analysis. Anal. Bioanal. Chem. 2022, 414, 7051–7067. [Google Scholar] [CrossRef]
- Meggiolaro, A.; Moccia, V.; Brun, P.; Pierno, M.; Mistura, G.; Zappulli, V.; Ferraro, D. Microfluidic Strategies for Extracellular Vesicle Isolation: Towards Clinical Applications. Biosensors 2022, 13, 50. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Freitas, D.; Kim, H.S.; Fabijanic, K.; Li, Z.; Chen, H.; Mark, M.T.; Molina, H.; Martin, A.B.; Bojmar, L.; et al. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat. Cell Biol. 2018, 20, 332–343. [Google Scholar] [CrossRef]
- Yan, L.; Wu, X. Exosomes produced from 3D cultures of umbilical cord mesenchymal stem cells in a hollow-fiber bioreactor show improved osteochondral regeneration activity. Cell Biol. Toxicol. 2019, 36, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Takov, K.; Yellon, D.M.; Davidson, S.M. Comparison of small extracellular vesicles isolated from plasma by ultracentrifugation or size-exclusion chromatography: Yield, purity and functional potential. J. Extracell. Vesicles 2019, 8, 1560809. [Google Scholar] [CrossRef]
- Nordin, J.Z.; Lee, Y.; Vader, P.; Mäger, I.; Johansson, H.J.; Heusermann, W.; Wiklander, O.P.B.; Hällbrink, M.; Seow, Y.; Bultema, J.J.; et al. Ultrafiltration with size-exclusion liquid chromatography for high yield isolation of extracellular vesicles preserving intact biophysical and functional properties. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 879–883. [Google Scholar] [CrossRef]
- Ter-Ovanesyan, D.; Gilboa, T.; Budnik, B.; Nikitina, A.; Whiteman, S.; Lazarovits, R.; Trieu, W.; Kalish, D.; Church, G.M.; Walt, D.R. Improved isolation of extracellular vesicles by removal of both free proteins and lipoproteins. eLife 2023, 12, e86394. [Google Scholar] [CrossRef]
- Xu, R.; Greening, D.W.; Zhu, H.-J.; Takahashi, N.; Simpson, R.J. Extracellular vesicle isolation and characterization: Toward clinical application. J. Clin. Investig. 2016, 126, 1152–1162. [Google Scholar] [CrossRef]
- Wu, M.; Chen, C.; Wang, Z.; Bachman, H.; Ouyang, Y.; Huang, P.-H.; Sadovsky, Y.; Huang, T.J. Separating extracellular vesicles and lipoproteins via acoustofluidics. Lab A Chip 2019, 19, 1174–1182. [Google Scholar] [CrossRef]
- Li, M.; Soder, R.; Abhyankar, S.; Home, T.; Pathak, H.; Shen, X.; Godwin, A.K.; Abdelhakim, H. Large-scale manufacturing of immunosuppressive extracellular vesicles for human clinical trials. Cytotherapy 2025, 27, 1219–1228. [Google Scholar] [CrossRef]
- Gobin, J.; Muradia, G.; Mehic, J.; Westwood, C.; Couvrette, L.; Stalker, A.; Bigelow, S.; Luebbert, C.C.; Bissonnette, F.S.-D.; Johnston, M.J.W.; et al. Hollow-fiber bioreactor production of extracellular vesicles from human bone marrow mesenchymal stromal cells yields nanovesicles that mirrors the immuno-modulatory antigenic signature of the producer cell. Stem Cell Res. Ther. 2021, 12, 127. [Google Scholar] [CrossRef] [PubMed]
- Powsner, E.H.; Kronstadt, S.M.; Nikolov, K.; Aranda, A.; Jay, S.M. Mesenchymal stem cell extracellular vesicle vascularization bioactivity and production yield are responsive to cell culture substrate stiffness. Bioeng. Transl. Med. 2025, 10, e10743. [Google Scholar] [CrossRef] [PubMed]
- Garcia, S.G.; Clos-Sansalvador, M.; Sanroque-Muñoz, M.; Pan, L.; Franquesa, M. Functional and potency assays for mesenchymal stromal cell–extracellular vesicles in kidney disease. Curr. Opin. Physiol. 2024, 38, 100746. [Google Scholar] [CrossRef]
- Kobayashi, H.; Shiba, T.; Yoshida, T.; Bolidong, D.; Kato, K.; Sato, Y.; Mochizuki, M.; Seto, T.; Kawashiri, S.; Hanayama, R. Precise analysis of single small extracellular vesicles using flow cytometry. Sci. Rep. 2024, 14, 7465. [Google Scholar] [CrossRef]
- Liu, H.; Tian, Y.; Xue, C.; Niu, Q.; Chen, C.; Yan, X. Analysis of extracellular vesicle DNA at the single-vesicle level by nano-flow cytometry. J. Extracell. Vesicles 2022, 11, e12206. [Google Scholar] [CrossRef]
- Mizenko, R.R.; Brostoff, T.; Rojalin, T.; Koster, H.J.; Swindell, H.S.; Leiserowitz, G.S.; Wang, A.; Carney, R.P. Tetraspanins are unevenly distributed across single extracellular vesicles and bias sensitivity to multiplexed cancer biomarkers. J. Nanobiotechnol. 2021, 19, 250. [Google Scholar] [CrossRef]
- Ashique, S.; Anand, K. Radiolabelled Extracellular Vesicles as Imaging Modalities for Precise Targeted Drug Delivery. Pharmaceutics 2023, 15, 1426. [Google Scholar] [CrossRef] [PubMed]
- Ibanez, B.; Aletras, A.H.; Arai, A.E.; Arheden, H.; Bax, J.; Berry, C.; Bucciarelli-Ducci, C.; Croisille, P.; Dall’Armellina, E.; Dharmakumar, R.; et al. Cardiac MRI Endpoints in Myocardial Infarction Experimental and Clinical Trials: JACC Scientific Expert Panel. J. Am. Coll. Cardiol. 2019, 74, 238–256. [Google Scholar] [CrossRef]
- Patel, S.; Schmidt, K.F.; Farhoud, M.; Zi, T.; Jang, S.C.; Dooley, K.; Kentala, D.; Dobson, H.; Economides, K.; Williams, D.E. In vivo tracking of [89Zr]Zr-labeled engineered extracellular vesicles by PET reveals organ-specific biodistribution based upon the route of administration. Nucl. Med. Biol. 2022, 112–113, 20–30. [Google Scholar] [CrossRef]
- Hikita, T.; Miyata, M.; Watanabe, R.; Oneyama, C. In vivo imaging of long-term accumulation of cancer-derived exosomes using a BRET-based reporter. Sci. Rep. 2020, 10, 16616. [Google Scholar] [CrossRef]
- Leal, A.C.; Mizurini, D.M.; Gomes, T.; Rochael, N.C.; Saraiva, E.M.; Dias, M.S.; Werneck, C.C.; Sielski, M.S.; Vicente, C.P.; Monteiro, R.Q. Tumor-Derived Exosomes Induce the Formation of Neutrophil Extracellular Traps: Implications For The Establishment of Cancer-Associated Thrombosis. Sci. Rep. 2017, 7, 6438. [Google Scholar] [CrossRef] [PubMed]
- Almeida, V.H.; Rondon, A.M.R.; Gomes, T.; Monteiro, R.Q. Novel Aspects of Extracellular Vesicles as Mediators of Cancer-Associated Thrombosis. Cells 2019, 8, 716. [Google Scholar] [CrossRef]
- Sun, L.; Ji, Y.; Chi, B.; Xiao, T.; Li, C.; Yan, X.; Xiong, X.; Mao, L.; Cai, D.; Zou, A.; et al. A 3D culture system improves the yield of MSCs-derived extracellular vesicles and enhances their therapeutic efficacy for heart repair. Biomed. Pharmacother. 2023, 161, 114557. [Google Scholar] [CrossRef] [PubMed]
- Bauer, F.N.; Tertel, T.; Stambouli, O.; Wang, C.; Dittrich, R.; Staubach, S.; Börger, V.; Hermann, D.M.; Brandau, S.; Giebel, B. CD73 activity of mesenchymal stromal cell-derived extracellular vesicle preparations is detergent-resistant and does not correlate with immunomodulatory capabilities. Cytotherapy 2022, 25, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Mizenko, R.R.; Feaver, M.; Bozkurt, B.T.; Lowe, N.; Nguyen, B.; Huang, K.; Wang, A.; Carney, R.P. A critical systematic review of extracellular vesicle clinical trials. J. Extracell. Vesicles 2024, 13, e12510. [Google Scholar] [CrossRef] [PubMed]
- Giebel, B.; Lim, S.K. Overcoming challenges in MSC-sEV therapeutics: Insights and advances after a decade of research. Cytotherapy 2025, 27, 843–848. [Google Scholar] [CrossRef] [PubMed]
- Manno, M.; Bongiovanni, A.; Margolis, L.; Bergese, P.; Arosio, P. The physico-chemical landscape of extracellular vesicles. Nat. Rev. Bioeng. 2024, 3, 68–82. [Google Scholar] [CrossRef]
- Assistance Publique-Hôpitaux de Paris. Treatment of Non-Ischemic Dilated Cardiomyopathies by Intravenous Infusions of the Extracellular Vesicle-Enriched Secretome of Cardiovascular Progenitor Cells; Clinical Trial Registration NCT05774509; Clinicaltrials.gov, 2023. Available online: https://clinicaltrials.gov/study/NCT05774509 (accessed on 17 December 2025).
- McLeod, C.J. Safety Evaluation of Intracoronary Infusion of Extracellular Vesicles in Patients Following Coronary Stent Implantation (EV-CSI); Clinical Trial Registration NCT04327635; Clinicaltrials.gov, 2025. Available online: https://clinicaltrials.gov/study/NCT04327635 (accessed on 17 December 2025).
- tafti, A. Co-Transplantation of Mesenchymal Stem Cell Derived Exosomes and Autologous Mitochondria for Patients Candidate for CABG Surgery with EF < 25% (Clinical Trial Phase); Clinical Trial Registration NCT05669144; Clinicaltrials.gov, 2022. Available online: https://clinicaltrials.gov/study/NCT05669144 (accessed on 17 December 2025).
- Kumamoto University. Effect of Plasma Derived Exosomes on Intractable Cutaneous Wound Healing: Prospective Trial; Clinical Trial Registration NCT02565264; Clinicaltrials.gov, 2020. Available online: https://clinicaltrials.gov/study/NCT02565264 (accessed on 17 December 2025).
| Exosome Source | Optimization Strategy | Administration Route | Experimental Model | Main Therapeutic Outcomes | Ref. |
|---|---|---|---|---|---|
| EPC-EVs | shear-thinning hydrogel–based local delivery; sustained release/retention | IM (local myocardial injection) | Rat MI | enhanced angiogenesis; improved cardiac function; increased EV retention | [29] |
| BMMSC-EVs | source-cell genetic engineering (FNDC5 overexpression); anti-inflammation/macrophage polarization | IM (infarcted heart injection) | Mouse MI | attenuated inflammation/apoptosis; promoted M2 polarization; improved post-MI cardiac function | [35] |
| MSC-EVs | exogenous cargo loading (miR-132) via electroporation | IM (ischemic heart/peri-infarct delivery) | Mouse MI | enhanced neovascularization; preserved cardiac function; pro-angiogenic effect | [36] |
| ADSC-EVs | exogenous cargo loading (miR-126 + miR-146a mimics); hydrogel encapsulation (alginate derivative) for sustained release | local myocardial delivery (hydrogel-based) | Rat MI | reduced infarct size; reduced fibrosis; enhanced angiogenesis | [24] |
| iCM-EVs | delivery matrix optimization (Matrigel/PBS carrier) to improve retention of injected exosomes | IM (peri-infarct, multi-site injection) | Mouse MI | improved cardiac function/viability; reduced cardiomyocyte apoptosis; improved myocardial repair | [37] |
| Plasma-EVs | donor preconditioning (RIPC-induced exosomes); therapeutic miRNA transfer (miR-24) | direct myocardial injection | Rat I/R injury | reduced apoptosis; reduced infarct size; cardioprotection | [38] |
| ADSC-EVs | source-cell genetic engineering (miR-126 overexpression); pro-angiogenic enhancement | IV (tail vein) | Rat AMI | decreased inflammation/fibrosis; increased angiogenesis; reduced myocardial injury | [39] |
| MSC-EVs | hypoxia preconditioning; cargo enrichment via donor-cell engineering (miR-210 OE, mechanistic validation) | IM (peri-infarct/border zone) | rat MI (LAD ligation) | reduced infarct size; improved cardiac function; attenuated apoptosis | [40] |
| MSC-EVs | hypoxia preconditioning; surface conjugation with IMT cardiac-targeting peptide (enhanced targeting/retention) | IV (tail vein) | mouse MI (coronary ligation) | improved cardiac function; reduced cardiomyocyte death; enhanced myocardial targeting | [41] |
| MSC-EVs | donor-cell drug pretreatment (atorvastatin); lncRNA H19 upregulation/enrichment | IM (border zone) | rat AMI (LAD ligation) | improved cardiac function; reduced infarct size/apoptosis; enhanced angiogenesis; reduced inflammation | [42] |
| BM-MSC-EVs | donor-cell drug pretreatment (atorvastatin); cargo modulation (miR-139-3p mimic/inhibitor via transfection) | IM (border zone, multi-point) | rat AMI (LAD ligation) | improved LVEF/LVFS; promoted M2 macrophage polarization; enhanced cardiac repair | [43] |
| MSC-EVs | donor-cell pretreatment (Tongxinluo); miR-146a-5p–associated cardioprotection | IM (border zone, multi-point) | rat AMI (LAD ligation) | improved cardiac function; reduced infarct size; anti-apoptotic/anti-inflammatory; enhanced repair | [44] |
| hUCMSC-EVs | donor-cell pretreatment (NMN); EV optimization with miR-210-3p enrichment/functional dependence | IM (peri-infarct/border zone) | rat MI | improved cardiac function; reduced LV remodelling/fibrosis; enhanced angiogenesis; reduced apoptosis | [45] |
| HEK293T-EVs | genetic engineering for miR-21 enrichment; localized delivery strategy | local IM (infarct area) | rat MI | restored cardiac function; reduced injury/remodelling; pro-survival/pro-repair effects | [46] |
| HuMSC-EVs | donor-cell genetic engineering (miR-214 OE) for therapeutic cargo enrichment | IM (border zone) | rat MI | improved cardiac function; reduced infarct size; enhanced repair | [47] |
| MSC-EVs | drug preconditioning (nicorandil); pro-repair cargo shift (e.g., miR-125a-5p upregulation) | IM (border zone) | Rat AMI (coronary ligation) | improved LVEF/LVFS; reduced infarct size; reduced fibrosis/inflammation; enhanced angiogenesis | [48] |
| CPC-EVs | donor-cell genetic engineering (CXCR4 overexpression) to improve homing/efficacy | IV (systemic) | Rat myocardial I/R | reduced infarct size; improved cardiac function; enhanced cardiac retention | [49] |
| BMSC-EVs | genetic engineering (GATA-4 overexpression) | IV (tail vein) | Mouse myocardial I/R | reduced infarct area; improved LVEF/LVFS; attenuated ferroptosis | [50] |
| BMSC-EVs | genetic engineering (GATA-4 overexpression) | IM (post-MI injection) | Mouse MI | improved cardiac function; reduced apoptosis; enhanced angiogenesis | [51] |
| HEK293-EVs | surface targeting peptide (CTP) via LAMP2b display; cargo loading (curcumin); miRNA loading (miR-144-3p); co-delivery | IV | Mouse MI | enhanced heart accumulation; reduced apoptosis; improved therapeutic efficacy; improved cardiac function | [52] |
| UCMSC-EVs | cargo loading (PLGF); surface conjugation (CHP, covalent); engineered nanovesicles | IV (systemic) | Mouse MI | improved cardiomyocyte survival; improved cardiac repair/function | [53] |
| MSC-EVs | surface display of ischemic myocardium-targeting peptide (CSTSMLKAC; IMTP) | IV (tail vein) | Mouse MI | enhanced myocardial retention; improved EF/FS recovery; attenuated remodelling | [54] |
| MSC-EVs | biomimetic membrane fusion (neutrophil apoptotic body membrane; NAM) | IV (tail vein) | Mouse MI | reduced fibrosis; improved cardiac function; enhanced repair | [55] |
| MSC-EVs | hydrogel-based delivery (HA ExoGel); minimally invasive intrapericardial administration (local/retentive delivery) | intrapericardial | Rat pressure-overload HF (TAC) | reduced fibrosis; improved cardiac function; attenuated remodelling | [56] |
| MSC-EVs | CD47 surface display (MSC CD47 overexpression) to reduce MPS clearance and prolong circulation; miR-21a loading via electroporation (two-step engineered EVs) | IV (tail vein) | Mouse myocardial I/R injury (LAD ligation/reperfusion) | prolonged circulation; increased cardiac accumulation; reduced cardiomyocyte apoptosis; attenuated inflammatory infiltration; improved cardiac function recovery | [57] |
| GWIT-iCPP-Exo | Source-cell reprogramming via transcription factor overexpression (GLI1/WNT2/ISL1/TBX5); dose strategy (Exo-low vs. Exo-high) | Intratracheal instillation | LPS-induced mouse ALI | dose-dependent attenuation of lung inflammation; improved endothelial function; restored capillary endothelium and epithelial barrier | [58] |
| Isolation Method | Principle And Typical Use | Purity (Main Contaminants) | Yield/Cost/Scalability | Impact on EV Integrity and Function | Ref. |
|---|---|---|---|---|---|
| Differential ultracentrifugation (dUC) | Sequential low- to high-speed centrifugation to pellet EVs by sedimentation; classical method for cell culture supernatants and some biofluids. | Purity: low–moderate; co-isolation of proteins, lipoproteins, protein/RNA aggregates and mixed EV subtypes. | Yield: medium. Cost: low consumables but requires an ultracentrifuge. Scalability: batch-based, time-consuming for large volumes. | High g-forces and long spins can induce aggregation and partial membrane damage, potentially affecting biodistribution and biological activity. | [73] |
| Density-gradient ultracentrifugation (DG-UC) | Flotation or sedimentation of EVs through sucrose or iodixanol gradients to separate them from particles of different buoyant densities; often used for plasma/serum when high purity is required. | Purity: generally higher than dUC; improved separation from soluble proteins and part of lipoproteins, but overlapping-density species can remain. | Yield: lower than dUC due to narrower density window and handling losses. Cost: higher (gradient media, tubes, time). Scalability: technically demanding, low–medium throughput. | Gentler than hard pelleting; typically preserves EV markers reasonably well, although long ultracentrifugation still poses some risk to integrity. | [74] |
| Size-exclusion chromatography (SEC) | Separation by hydrodynamic size on porous matrices (e.g., Sepharose CL-2B); EVs elute in early/void fractions, smaller proteins and many soluble contaminants enter the pores. | Purity: high for soluble proteins; many free proteins and some lipoproteins are efficiently removed, though very small EVs and some lipoprotein particles may overlap. | Yield: moderate, with some dilution and column-binding losses. Cost: columns and buffers moderate; columns often reusable. Scalability: good standardization; throughput can be increased by parallel columns or automation. | Low shear and no extreme g-forces; generally maintains EV morphology, surface markers and cargo, suitable for functional and omics analyses. | [75] |
| Ultrafiltration/tangential-flow filtration (UF/TFF) | Membrane-based retention of EVs above a defined pore size or MWCO; used to concentrate EVs and often combined with SEC or chromatography for further purification. | Purity: moderate when used alone; removes very large particles and small solutes, but protein aggregates and non-EV nanoparticles can be retained and co-concentrated. | Yield: high and compatible with large volumes. Cost: higher equipment cost but economical for scale. Scalability: excellent; attractive for GMP-scale EV production. | Shear at the membrane can be kept relatively low; when optimized, UF/TFF preserves EV size distribution and protein composition better than dUC. | [76] |
| Polymer-based precipitation (e.g., PEG; ExoQuick-like) | Hydrophilic polymers (typically PEG) reduce EVs solubility and promote precipitation of EVs together with other macromolecular complexes; convenient, no specialized equipment. | Purity: relatively low; strong co-precipitation of lipoproteins, protein complexes and polymer; often requires additional clean-up for sensitive downstream analyses. | Yield: high particle counts (EVs plus contaminants). Cost: PEG itself inexpensive; commercial kits can be relatively costly per sample. Scalability: simple for many small samples but less convenient for very large volumes. | EVs are usually morphologically intact, but residual polymer and altered protein corona can influence uptake and biological activity; additional purification is recommended for functional studies. | [77] |
| Immunoaffinity capture | Antibody-coated beads, plates or columns capture EVs expressing specific surface antigens (e.g., CD9, CD63, CD81, EpCAM); used for highly specific enrichment or phenotyping of defined EV subsets. | Purity: very high for the targeted EVs subpopulation; strong depletion of unrelated proteins and EVs, but strong selection bias toward marker-positive vesicles. | Yield: low–moderate and limited to antigen-positive EVs; capacity determined by antibody surface and antigen expression levels. Cost: high due to antibodies and matrices. Scalability: mainly suitable for analytical/diagnostic applications rather than bulk production. | Binding and elution can alter surface epitopes and cargo; harsh elution or on-bead lysis prevents re-use of intact EVs in functional assays, so gentle conditions are required if functional integrity is important. | [78] |
| Microfluidic-based isolation (emerging) | Lab-on-chip devices that isolate EVs directly from small volumes of biofluids via on-chip immunocapture, size-based filtration or acoustofluidic separation; often integrate capture, washing and detection. | Purity: medium–high, depending on design. Immunocapture chips provide highly enriched subpopulations; acoustofluidic chips deplete cells and platelets and enrich small EVs, though some lipoproteins/protein aggregates may remain. | Yield: moderate and sufficient for diagnostics, limited by chip capacity and channel fouling. Cost: devices may be costly to fabricate, but per-sample reagent cost is low. Scalability: good for parallel processing of many small samples; currently less suited for large therapeutic batches. | Typically low shear and short residence time, preserving EVs morphology and cargo; strong surface interactions and narrow channels can introduce selection bias and potentially alter surface proteins. | [79] |
| Asymmetric-flow field-flow fractionation (AF4; emerging) | Separation of nanoparticles in a thin channel using a cross-flow field perpendicular to laminar flow; EVs and non-vesicular nanoparticles are resolved by hydrodynamic size and diffusion. Used for high-resolution fractionation and characterization of EVs subpopulations and non-vesicular nanoparticles. | Purity: very high analytical purity and resolution when combined with pre-enrichment (e.g., UC, SEC); efficient separation of EVs from many protein aggregates and lipoproteins and resolution of distinct EVs subsets and non-membranous nanoparticles. | Yield: good particle recovery in collected fractions but mainly analytical rather than preparative at present. Cost: high instrumentation and expertise requirements. Scalability: limited for bulk production; powerful for detailed biophysical and omics studies. | Label-free, low-shear separation without hard pelleting; EVs integrity is generally well preserved. AF4 has revealed size- and compositionally distinct EVs subsets and exomeres with different organ biodistribution and potential biological functions. | [80] |
| Registry ID | Product/EV Source | Indication/Population | Phase and Design | Route and Regimen | Primary Endpoint(s) | Key Exploratory Endpoints | Ref |
|---|---|---|---|---|---|---|---|
| NCT05774509 | EV-enriched secretome from iPSC-derived cardiovascular progenitor cells | HFrEF (non-ischemic DCM) | Phase I, single-group, open-label | IV, 3 infusions; dose-escalation | Serious adverse events | Potency/bioactivity assays; immunologic measures; longer-term follow-up signals | [105] |
| NCT04327635 | EV-containing biological drug (PEP) | PCI ± stent placement | Early-phase, sequential assignment, open-label | Intracoronary, single infusion post-PCI | DLT/MTD (safety/tolerability) | CMR scar size; CMR ejection fraction; alloimmune response | [106] |
| NCT05669144 | MSC-derived exosomes (±mitochondria) | Recent Q-wave MI; CABG candidates; very low LVEF | Phase I/II, randomized, parallel, quadruple-masked | Intracoronary + intramyocardial | EF; allergic reactions | Viability imaging; NYHA class; biomarkers (per protocol) | [107] |
| NCT02565264 | Plasma-derived exosomes | Intractable cutaneous ulcers (vascular-complication context) | Interventional, single-group, open-label | Topical daily × 28 days | Ulcer size metrics | Pain score | [108] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Huang, Y.; Li, H.; Xiong, J.; Wang, X.; Lv, J.; Xiong, N.; Liu, Q.; Yin, L.; Wang, Z.; Wang, Y. Optimizing Extracellular Vesicles for Cardiac Repair Post-Myocardial Infarction: Approaches and Challenges. Biomolecules 2026, 16, 58. https://doi.org/10.3390/biom16010058
Huang Y, Li H, Xiong J, Wang X, Lv J, Xiong N, Liu Q, Yin L, Wang Z, Wang Y. Optimizing Extracellular Vesicles for Cardiac Repair Post-Myocardial Infarction: Approaches and Challenges. Biomolecules. 2026; 16(1):58. https://doi.org/10.3390/biom16010058
Chicago/Turabian StyleHuang, Yanling, Han Li, Jinjie Xiong, Xvehua Wang, Jiaxi Lv, Ni Xiong, Qianyi Liu, Lihui Yin, Zhaohui Wang, and Yan Wang. 2026. "Optimizing Extracellular Vesicles for Cardiac Repair Post-Myocardial Infarction: Approaches and Challenges" Biomolecules 16, no. 1: 58. https://doi.org/10.3390/biom16010058
APA StyleHuang, Y., Li, H., Xiong, J., Wang, X., Lv, J., Xiong, N., Liu, Q., Yin, L., Wang, Z., & Wang, Y. (2026). Optimizing Extracellular Vesicles for Cardiac Repair Post-Myocardial Infarction: Approaches and Challenges. Biomolecules, 16(1), 58. https://doi.org/10.3390/biom16010058







