Insights into the Pathophysiology of Scheuermann’s Kyphosis: From Structural Deformities to Genetic Predisposition and Underlying Signalling Pathways
Abstract
1. Introduction
2. Pathophysiology of Structural Deformities
2.1. Mechanical Factors
2.2. Vertebral Body Wedging and Anterior Longitudinal Ligament
2.3. Schmorl’sNodes and Intervertebral Discs
3. Bone Metabolism-Related Factors
4. Genetic Foundation
5. Endochondral Ossification
6. Molecular Signalling
7. Limitations of the Study
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaur, S.; Lalam, R. Scheuermann’s Disease. Semin. Musculoskelet. Radiol. 2023, 27, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Bezalel, T.; Carmeli, E.; Kalichman, L. Scheuermann’s Disease: Radiographic Pathomorphology and Association with Clinical Features. Asian Spine J. 2019, 13, 86–95. [Google Scholar] [CrossRef]
- Sørensen, K.H. Scheuermann’s Juvenile Kyphosis: Clinical Appearances, Radiography, Aetiology, and Prognosis. Med. J. Bone Jt. Surg.-Br. Med. J. Bone Jt. Surg.-Br. Vol. 1965, 47 B, 203. [Google Scholar]
- Sachs, B.; Bradford, D.; Winter, R.; Lonstein, J.; Moe, J.; Willson, S. Scheuermann kyphosis. Follow-Up of Milwaukee-brace treatment. J. Bone Jt. Surg. Am. 1987, 69, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Makurthou, A.A.; Oei, L.; El Saddy, S.; Breda, S.J.; Castano-Betancourt, M.C.; Hofman, A.; van Meurs, J.B.; Uitterlinden, A.G.; Rivadeneira, F.; Oei, E.H. Scheuermann disease: Evaluation of radiological criteria and population prevalence. Spine 2013, 38, 1690–1694. [Google Scholar] [CrossRef] [PubMed]
- Armbrecht, G.; Felsenberg, D.; Ganswindt, M.; Lunt, M.; Kaptoge, S.K.; Abendroth, K.; Aroso, A.; Banzer, D.; Bhalla, A.K.; Dequeker, J.; et al. Vertebral Scheuermann’s disease in Europe: Prevalence, geographic variation and radiological correlates in men and women aged 50 and over. Osteoporos Int. 2015, 26, 2509–2519. [Google Scholar] [CrossRef]
- Wenger, D.R.; Frick, S.L. Scheuermann kyphosis. Spine 1999, 24, 2630–2639. [Google Scholar] [CrossRef]
- Paajanen, H.; Alanen, A.; Erkintalo, M.; Salminen, J.J.; Katevuo, K. Disc degeneration in Scheuermann disease. Skeletal. Radiol. 1989, 18, 523–526. [Google Scholar] [CrossRef]
- Blumenthal, S.L.; Roach, J.; Herring, J.A. Lumbar Scheuermann’s. A clinical series and classification. Spine 1987, 12, 929–932. [Google Scholar] [CrossRef]
- Kaspiris, A.; Spyrou, I.; Panagopoulos, F.; Marougklianis, V.; Pelantis, P.; Vavourakis, M.; Sakellariou, E.; Lianou, I.; Ntourantonis, D.; Repantis, T.; et al. Surgical Strategies and Challenges in Scheuermann’s Kyphosis: A Comprehensive Review. J. Clin. Med. 2025, 14, 4276. [Google Scholar] [CrossRef]
- Scheuermann, H.W. The classic: Kyphosis dorsalis juvenilis. Clin. Orthop. Relat. Res. 1977, 128, 5–7. [Google Scholar] [CrossRef]
- Bick, E.M.; Copel, J.W. Longitudinal growth of the human vertebra; a contribution to human osteogeny. J. Bone Jt. Surg. Am. 1950, 32, 803–814. [Google Scholar] [CrossRef]
- Alexander, C.J. Scheuermann’s disease. Skelet. Radiol. 1977, 1, 209–221. [Google Scholar] [CrossRef]
- Sebaaly, A.; Farjallah, S.; Kharrat, K.; Kreichati, G.; Daher, M. Scheuermann’s kyphosis: Update on pathophysiology and surgical treatment. EFORT Open Rev. 2022, 7, 782–791. [Google Scholar] [CrossRef] [PubMed]
- Wood, K.B.; Melikian, R.; Villamil, F. Adult Scheuermann kyphosis: Evaluation, management, and new developments. J. Am. Acad. Orthop. Surg. 2012, 20, 113–121. [Google Scholar] [CrossRef]
- Scoles, P.V.; Latimer, B.M.; DigIovanni, B.F.; Vargo, E.; Bauza, S.; Jellema, L.M. Vertebral alterations in Scheuermann’s kyphosis. Spine 1991, 16, 509–515. [Google Scholar] [CrossRef]
- van Linthoudt, D.; Revel, M. Similar radiologic lesions of localized Scheuermann’s disease of the lumbar spine in twin sisters. Spine 1994, 19, 987–989. [Google Scholar] [CrossRef] [PubMed]
- Dommisse, G.F. The vulnerable, rapidly growing thoracic spine of the adolescent. S. Afr. Med. J. 1990, 78, 211–213. [Google Scholar] [PubMed]
- Lambrinudi, C. Adolescent and Senile Kyphosis. Br. Med. J. 1934, 2, 800–820. [Google Scholar] [CrossRef]
- Sward, L.; Hellstrom, M.; Jacobsson, B.; Nyman, R.; Peterson, L. Disc degeneration and associated abnormalities of the spine in elite gymnasts. A magnetic resonance imaging study. Spine 1991, 16, 437–443. [Google Scholar] [CrossRef]
- Endler, M.; Haber, P.; Hofner, W. Spinal deformities and their mechanopathology in oarsmen (author’s transl). Z. Orthop. Ihre. Grenzgeb. 1980, 118, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Revel, M.; Andre-Deshays, C.; Roudier, R.; Roudier, B.; Hamard, G.; Amor, B. Effects of repetitive strains on vertebral end plates in young rats. Clin. Orthop. Relat. Res. 1992, 279, 303–309. [Google Scholar] [CrossRef]
- Wassmann, K. Kyphosis juvenilis Scheuermann--an occupational disorder. Acta Orthop. Scand. 1951, 21, 65–74. [Google Scholar] [CrossRef]
- Ogden, J.A. Skeletal Injury in the Child, 3rd ed.; Springer: New York, NY, USA, 2000; pp. xxii–1198. [Google Scholar]
- de Mauroy, J.; Weiss, H.; Aulisa, A.; Aulisa, L.; Brox, J.; Durmala, J.; Fusco, C.; Grivas, T.; Hermus, J.; Kotwicki, T.; et al. 7th SOSORT consensus paper: Conservative treatment of idiopathic & Scheuermann’s kyphosis. Scoliosis 2010, 5, 9. [Google Scholar] [CrossRef]
- Aulisa, A.G.; Falciglia, F.; Giordano, M.; Mastantuoni, G.; Poscia, A.; Guzzanti, V. Conservative treatment in Scheuermann’s kyphosis: Comparison between lateral curve and variation of the vertebral geometry. Scoliosis Spinal Disord. 2016, 11, 33. [Google Scholar] [CrossRef]
- Aulisa, A.G.; Marsiolo, M.; Calogero, V.; Giordano, M.; Falciglia, F. Long-term outcome after brace treatment of Scheuermann’s kyphosis: An observational controlled cohort study. Eur. J. Phys. Rehabil. Med. 2023, 59, 529–534. [Google Scholar] [CrossRef]
- Wang, S.; Xu, L.; Wang, M.; Qiu, Y.; Zhu, Z.; Wang, B.; Sun, X. Contribution of postoperative vertebral remodeling to reversal of vertebral wedging and prevention of correction loss in patients with adolescent Scheuermann’s kyphosis. J. Neurosurg. Spine 2021, 35, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Banse, X.; Devogelaer, J.P.; Munting, E.; Delloye, C.; Cornu, O.; Grynpas, M. Inhomogeneity of human vertebral cancellous bone: Systematic density and structure patterns inside the vertebral body. Bone 2001, 28, 563–571. [Google Scholar] [CrossRef]
- Bruno, A.G.; Burkhart, K.; Allaire, B.; Anderson, D.E.; Bouxsein, M.L. Spinal Loading Patterns From Biomechanical Modeling Explain the High Incidence of Vertebral Fractures in the Thoracolumbar Region. J. Bone Miner Res. 2017, 32, 1282–1290. [Google Scholar] [CrossRef]
- Fotiadis, E.; Grigoriadou, A.; Kapetanos, G.; Kenanidis, E.; Pigadas, A.; Akritopoulos, P.; Samoladas, E. The role of sternum in the etiopathogenesis of Scheuermann disease of the thoracic spine. Spine 2008, 33, E21–E24. [Google Scholar] [CrossRef]
- Lowe, T.G. Scheuermann’s kyphosis. Neurosurg. Clin. N. Am. 2007, 18, 305–315. [Google Scholar] [CrossRef]
- Gokce, E.; Beyhan, M. Radiological imaging findings of scheuermann disease. World J. Radiol. 2016, 8, 895–901. [Google Scholar] [CrossRef]
- Hurtado-Aviles, J.; Roca-Gonzalez, J.; Santonja-Medina, F. Hypothesis about an existent biomechanical cause-effect relationship between Scheuermann’s kyphosis and scoliosis. Med. Hypotheses 2015, 85, 94–98. [Google Scholar] [CrossRef]
- Peleg, S.; Dar, G.; Steinberg, N.; Masharawi, Y.; Hershkovitz, I. Sacral orientation and Scheuermann’s kyphosis. Springerplus 2016, 5, 141. [Google Scholar] [CrossRef]
- Digiovanni, B.F.; Scoles, P.V.; Latimer, B.M. Anterior extension of the thoracic vertebral bodies in Scheuermann’s kyphosis. An anatomic study. Spine 1989, 14, 712–716. [Google Scholar] [CrossRef]
- Tyrakowski, M.; Janusz, P.; Mardjetko, S.; Kotwicki, T.; Siemionow, K. Comparison of radiographic sagittal spinopelvic alignment between skeletally immature and skeletally mature individuals with Scheuermann’s disease. Eur. Spine J. 2015, 24, 1237–1243. [Google Scholar] [CrossRef]
- Peleg, S.; Dar, G.; Steinberg, N.; Peled, N.; Hershkovitz, I.; Masharawi, Y. Sacral orientation revisited. Spine 2007, 32, E397–E404. [Google Scholar] [CrossRef] [PubMed]
- Roussouly, P.; Gollogly, S.; Berthonnaud, E.; Dimnet, J. Classification of the normal variation in the sagittal alignment of the human lumbar spine and pelvis in the standing position. Spine 2005, 30, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Jansen, R.C.; van Rhijn, L.W.; van Ooij, A. Predictable correction of the unfused lumbar lordosis after thoracic correction and fusion in Scheuermann kyphosis. Spine 2006, 31, 1227–1231. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Qiu, Y.; Xu, L.; Liu, Z.; Wang, Z.; Sha, S.; Zhu, Z. Sagittal spinopelvic alignment in adolescents associated with Scheuermann’s kyphosis: A comparison with normal population. Eur. Spine J. 2014, 23, 1420–1426. [Google Scholar] [CrossRef]
- Loder, R.T. The sagittal profile of the cervical and lumbosacral spine in Scheuermann thoracic kyphosis. J. Spinal Disord. 2001, 14, 226–231. [Google Scholar] [CrossRef]
- Cahill, P.J.; Steiner, C.D.; Dakwar, E.; Trobisch, P.D.; Harms Study, G.; Lonner, B.S.; Newton, P.O.; Shah, S.A.; Sponseller, P.D.; Shufflebarger, H.L.; et al. Sagittal Spinopelvic Parameters in Scheuermann’s Kyphosis: A Preliminary Study. Spine Deform. 2015, 3, 267–271. [Google Scholar] [CrossRef]
- Lonner, B.S.; Newton, P.; Betz, R.; Scharf, C.; O’Brien, M.; Sponseller, P.; Lenke, L.; Crawford, A.; Lowe, T.; Letko, L.; et al. Operative management of Scheuermann’s kyphosis in 78 patients: Radiographic outcomes, complications, and technique. Spine 2007, 32, 2644–2652. [Google Scholar] [CrossRef] [PubMed]
- Hosman, A.J.; de Kleuver, M.; Anderson, P.G.; van Limbeek, J.; Langeloo, D.D.; Veth, R.P.; Slot, G.H. Scheuermann kyphosis: The importance of tight hamstrings in the surgical correction. Spine 2003, 28, 2252–2259. [Google Scholar] [CrossRef] [PubMed]
- Skalli, W.; Zeller, R.D.; Miladi, L.; Bourcereau, G.; Savidan, M.; Lavaste, F.; Dubousset, J. Importance of pelvic compensation in posture and motion after posterior spinal fusion using CD instrumentation for idiopathic scoliosis. Spine 2006, 31, E359–E366. [Google Scholar] [CrossRef]
- Gum, J.L.; Asher, M.A.; Burton, D.C.; Lai, S.M.; Lambart, L.M. Transverse plane pelvic rotation in adolescent idiopathic scoliosis: Primary or compensatory? Eur. Spine J. 2007, 16, 1579–1586. [Google Scholar] [CrossRef] [PubMed]
- Cil, A.; Yazici, M.; Uzumcugil, A.; Kandemir, U.; Alanay, A.; Alanay, Y.; Acaroglu, R.E.; Surat, A. The evolution of sagittal segmental alignment of the spine during childhood. Spine 2005, 30, 93–100. [Google Scholar] [CrossRef]
- Vedantam, R.; Lenke, L.G.; Keeney, J.A.; Bridwell, K.H. Comparison of standing sagittal spinal alignment in asymptomatic adolescents and adults. Spine 1998, 23, 211–215. [Google Scholar] [CrossRef]
- Mac-Thiong, J.M.; Labelle, H.; Roussouly, P. Pediatric sagittal alignment. Eur. Spine J. 2011, 20, 586–590. [Google Scholar] [CrossRef]
- Stagnara, P.; De Mauroy, J.C.; Dran, G.; Gonon, G.P.; Costanzo, G.; Dimnet, J.; Pasquet, A. Reciprocal angulation of vertebral bodies in a sagittal plane: Approach to references for the evaluation of kyphosis and lordosis. Spine 1982, 7, 335–342. [Google Scholar] [CrossRef]
- Voutsinas, S.A.; MacEwen, G.D. Sagittal profiles of the spine. Clin. Orthop. Relat. Res. 1986, 210, 235–242. [Google Scholar] [CrossRef]
- Farrell, B.M.; Kuo, C.C.; Tang, J.A.; Phan, S.; Buckley, J.M.; Kondrashov, D.G. Scheuermann kyphosis in nonhuman primates. Spine 2012, 37, E1432–E1437. [Google Scholar] [CrossRef]
- Palazzo, C.; Sailhan, F.; Revel, M. Scheuermann’s disease: An update. Jt. Bone Spine 2014, 81, 209–214. [Google Scholar] [CrossRef]
- Mallet, J.; Rey, J.C.; Raimbeau, G.; Senly, G. Scheuermann’s disease. Spinal growth dystrophy. Rev. Prat 1984, 34, 29–39. [Google Scholar] [PubMed]
- Lowe, T.G. Scheuermann’s disease. Orthop. Clin. N. Am. 1999, 30, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Bradford, D.S.; Moe, J.H. Scheuermann’s juvenile kyphosis. A histologic study. Clin. Orthop. Relat. Res. 1975, 110, 45–53. [Google Scholar] [CrossRef]
- Neumann, P.; Keller, T.S.; Ekstrom, L.; Perry, L.; Hansson, T.H.; Spengler, D.M. Mechanical properties of the human lumbar anterior longitudinal ligament. J. Biomech. 1992, 25, 1185–1194. [Google Scholar] [CrossRef]
- Pintar, F.A.; Yoganandan, N.; Myers, T.; Elhagediab, A.; Sances, A., Jr. Biomechanical properties of human lumbar spine ligaments. J. Biomech. 1992, 25, 1351–1356. [Google Scholar] [CrossRef]
- Hukins, D.W.; Kirby, M.C.; Sikoryn, T.A.; Aspden, R.M.; Cox, A.J. Comparison of structure, mechanical properties, and functions of lumbar spinal ligaments. Spine 1990, 15, 787–795. [Google Scholar] [CrossRef]
- Kirby, M.C.; Sikoryn, T.A.; Hukins, D.W.; Aspden, R.M. Structure and mechanical properties of the longitudinal ligaments and ligamentum flavum of the spine. J. Biomed. Eng. 1989, 11, 192–196. [Google Scholar] [CrossRef]
- Aspden, R.M. Review of the functional anatomy of the spinal ligaments and the lumbar erector spinae muscles. Clin. Anat. 1992, 5, 372–387. [Google Scholar] [CrossRef]
- Tkaczuk, H. Tensile properties of human lumbar longitudinal ligaments. Acta Orthop. Scand. 1968, 39, 111. [Google Scholar] [CrossRef]
- Panjabi, M.M.; Goel, V.K.; Takata, K. Physiologic strains in the lumbar spinal ligaments. An in vitro biomechanical study 1981 Volvo Award in Biomechanics. Spine 1982, 7, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Wolny, R.; Wiczenbach, T.; Andrzejewska, A.J.; Spodnik, J.H. Mechanical response of human thoracic spine ligaments under quasi-static loading: An experimental study. J. Mech. Behav. Biomed. Mater. 2024, 151, 106404. [Google Scholar] [CrossRef]
- Chazal, J.; Tanguy, A.; Bourges, M.; Gaurel, G.; Escande, G.; Guillot, M.; Vanneuville, G. Biomechanical properties of spinal ligaments and a histological study of the supraspinal ligament in traction. J. Biomech. 1985, 18, 167–176. [Google Scholar] [CrossRef]
- Palanca, M.; Ruspi, M.L.; Cristofolini, L.; Liebsch, C.; Villa, T.; Brayda-Bruno, M.; Galbusera, F.; Wilke, H.J.; La Barbera, L. The strain distribution in the lumbar anterior longitudinal ligament is affected by the loading condition and bony features: An in vitro full-field analysis. PLoS ONE 2020, 15, e0227210. [Google Scholar] [CrossRef] [PubMed]
- Myklebust, J.B.; Pintar, F.; Yoganandan, N.; Cusick, J.F.; Maiman, D.; Myers, T.J.; Sances, A., Jr. Tensile strength of spinal ligaments. Spine 1988, 13, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Neumann, P.; Ekstrom, L.A.; Keller, T.S.; Perry, L.; Hansson, T.H. Aging, vertebral density, and disc degeneration alter the tensile stress-strain characteristics of the human anterior longitudinal ligament. J. Orthop. Res. 1994, 12, 103–112. [Google Scholar] [CrossRef]
- Jin, Z.W.; Song, K.J.; Lee, N.H.; Nakamura, T.; Fujimiya, M.; Murakami, G.; Cho, B.H. Contribution of the anterior longitudinal ligament to ossification and growth of the vertebral body: An immunohistochemical study using the human fetal lumbar vertebrae. Surg. Radiol. Anat. 2011, 33, 11–18. [Google Scholar] [CrossRef]
- Birnbaum, K.; Siebert, C.H.; Hinkelmann, J.; Prescher, A.; Niethard, F.U. Correction of kyphotic deformity before and after transection of the anterior longitudinal ligament—A cadaver study. Arch. Orthop. Trauma Surg. 2001, 121, 142–147. [Google Scholar] [CrossRef]
- Mok, F.P.; Samartzis, D.; Karppinen, J.; Luk, K.D.; Fong, D.Y.; Cheung, K.M. ISSLS prize winner: Prevalence, determinants, and association of Schmorl nodes of the lumbar spine with disc degeneration: A population-based study of 2449 individuals. Spine 2010, 35, 1944–1952. [Google Scholar] [CrossRef]
- Dar, G.; Peleg, S.; Masharawi, Y.; Steinberg, N.; May, H.; Hershkovitz, I. Demographical aspects of Schmorl nodes: A skeletal study. Spine 2009, 34, E312–E315. [Google Scholar] [CrossRef] [PubMed]
- Swischuk, L.E.; John, S.D.; Allbery, S. Disk degenerative disease in childhood: Scheuermann’s disease, Schmorl’s nodes, and the limbus vertebra: MRI findings in 12 patients. Pediatr. Radiol. 1998, 28, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Dar, G.; Masharawi, Y.; Peleg, S.; Steinberg, N.; May, H.; Medlej, B.; Peled, N.; Hershkovitz, I. Schmorl’s nodes distribution in the human spine and its possible etiology. Eur. Spine J. 2010, 19, 670–675. [Google Scholar] [CrossRef]
- Pfirrmann, C.W.; Resnick, D. Schmorl nodes of the thoracic and lumbar spine: Radiographic-pathologic study of prevalence, characterization, and correlation with degenerative changes of 1650 spinal levels in 100 cadavers. Radiology 2001, 219, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.L.; Murtagh, F.R.; Arrington, J.A.; Stallworth, D. Relationship of Schmorl’s nodes to vertebral body endplate fractures and acute endplate disk extrusions. AJNR Am. J. Neuroradiol. 2000, 21, 276–281. [Google Scholar] [PubMed]
- Edgren, W.; Vainio, S. Osteochondrosis juvenilis lumbalis. Acta Chir. Scand. Suppl. 1957, 227, 1–47. [Google Scholar]
- Peng, B.; Wu, W.; Hou, S.; Shang, W.; Wang, X.; Yang, Y. The pathogenesis of Schmorl’s nodes. J. Bone Jt. Surg. Br. 2003, 85, 879–882. [Google Scholar] [CrossRef]
- Wang, Y.X.J. Schmorl’s node of primarily developmental cause and Schmorl’s node of primarily acquired cause: Two related yet different entities. Quant Imaging Med. Surg. 2023, 13, 4044–4049. [Google Scholar] [CrossRef]
- Fahey, V.; Opeskin, K.; Silberstein, M.; Anderson, R.; Briggs, C. The pathogenesis of Schmorl’snodes in relation to acute trauma. An autopsy study. Spine 1998, 23, 2272–2275. [Google Scholar] [CrossRef]
- Gungor, O.; Gezer, N.S.; Ozdamarlar, U.; Balci, A. The effect of bone mineral density on development of Schmorl’s nodes in young patients. Acta Orthop. Traumatol. Turc. 2020, 54, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Reimers, E.; Mas-Pascual, M.; Arnay-De-La-Rosa, M.; Velasco-Vazquez, J.; Santolaria-Fernandez, F. Schmorl nodes: Lack of relationship between degenerative changes and osteopenia. Radiology 2002, 222, 293–294. [Google Scholar] [CrossRef] [PubMed]
- Samartzis, D.; Mok, F.P.S.; Karppinen, J.; Fong, D.Y.T.; Luk, K.D.K.; Cheung, K.M.C. Classification of Schmorl’s nodes of the lumbar spine and association with disc degeneration: A large-scale population-based MRI study. Osteoarthr. Cartil. 2016, 24, 1753–1760. [Google Scholar] [CrossRef]
- Williams, F.M.; Manek, N.J.; Sambrook, P.N.; Spector, T.D.; Macgregor, A.J. Schmorl’s nodes: Common, highly heritable, and related to lumbar disc disease. Arthritis Rheum. 2007, 57, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Lonner, B.S.; Toombs, C.S.; Mechlin, M.; Ciavarra, G.; Shah, S.A.; Samdani, A.F.; Sponseller, P.; Shufflebarger, H.L.; Betz, R.R.; Yaszay, B.; et al. MRI Screening in Operative Scheuermann Kyphosis: Is it Necessary? Spine Deform. 2017, 5, 124–133. [Google Scholar] [CrossRef]
- Guo, X.; Chen, Z.; Liu, N.; Guo, Z.; Qi, Q.; Li, W.; Zeng, Y.; Sun, C. Comparison between two types of “Scheuermann disease-like people”: Thoracolumbar disc herniation patients and healthy volunteers with radiological signs of Scheuermann’s disease. Chin. Med. J. 2014, 127, 3862–3866. [Google Scholar] [CrossRef]
- Wang, Y.; Videman, T.; Battie, M.C. Lumbar vertebral endplate lesions: Prevalence, classification, and association with age. Spine 2012, 37, 1432–1439. [Google Scholar] [CrossRef]
- Wan, C.; Shen, X.; Wu, X.; Yu, C.; Shao, Y.; Zhang, R.; Shang, J.; Li, J.; Zhang, Y.; Li, Y. Assessing the biomechanics of scheuermann’s kyphosis affected thoracolumbar spine in forward flexion at the tissue-level using a finite element model. Sci. Rep. 2025, 15, 27408. [Google Scholar] [CrossRef]
- Liu, N.; Chen, Z.; Qi, Q.; Shi, Z. The relationship of symptomatic thoracolumbar disc herniation and Scheuermann’s disease. Eur. Spine J. 2014, 23, 1059–1066. [Google Scholar] [CrossRef]
- Gille, O.; Soderlund, C.; Razafimahandri, H.J.; Mangione, P.; Vital, J.M. Analysis of hard thoracic herniated discs: Review of 18 cases operated by thoracoscopy. Eur. Spine J. 2006, 15, 537–542. [Google Scholar] [CrossRef]
- Biedert, R.M.; Friederich, N.F.; Gruhl, C. Sacral osseous destruction in a female gymnast: Unusual manifestation of Scheuermann’s disease? Knee Surg. Sports Traumatol. Arthrosc. 1993, 1, 110–112. [Google Scholar] [CrossRef]
- Lopez, R.A.; Burke, S.W.; Levine, D.B.; Schneider, R. Osteoporosis in Scheuermann’s disease. Spine 1988, 13, 1099–1103. [Google Scholar] [CrossRef] [PubMed]
- Wahner, H.W.; Dunn, W.L.; Brown, M.L.; Morin, R.L.; Riggs, B.L. Comparison of dual-energy x-ray absorptiometry and dual photon absorptiometry for bone mineral measurements of the lumbar spine. Mayo Clin. Proc. 1988, 63, 1075–1084. [Google Scholar] [CrossRef]
- Gilsanz, V.; Gibbens, D.T.; Carlson, M.; King, J. Vertebral bone density in Scheuermann disease. J. Bone Jt. Surg. Am. 1989, 71, 894–897. [Google Scholar] [CrossRef]
- Ashton, L.A.; Stephen, J.; Nabavi-Tabrizi, A.; Bleasel, J.; Briody, J. Osteoporosis: A possible aetiological factor in the development of Scheuermann’s disease. J. Orthop. Surg. 2001, 9, 15–17. [Google Scholar] [CrossRef]
- Popko, J.; Konstantynowicz, J.; Kossakowski, D.; Kaczmarski, M.; Piotrowska-Jastrzebska, J. Assessment of bone density in children with Scheuermann’s disease. Rocz. Akad. Med. Bialymst. 1997, 42, 245–250. [Google Scholar]
- Li, N.; Li, X.M.; Xu, L.; Sun, W.J.; Cheng, X.G.; Tian, W. Comparison of QCT and DXA: Osteoporosis Detection Rates in Postmenopausal Women. Int. J. Endocrinol. 2013, 2013, 895474. [Google Scholar] [CrossRef]
- Gilsanz, V.; Gibbens, D.T.; Roe, T.F.; Carlson, M.; Senac, M.O.; Boechat, M.I.; Huang, H.K.; Schulz, E.E.; Libanati, C.R.; Cann, C.C. Vertebral bone density in children: Effect of puberty. Radiology 1988, 166, 847–850. [Google Scholar] [CrossRef]
- Gaude, M.; Chapurlat, R.; Pialat, J.B.; Szulc, P. Long term prognosis of Scheuermann’s disease: The association with fragility fracture—The MINOS cohort. Bone 2018, 117, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Skogland, L.B.; Steen, H.; Trygstad, O. Spinal deformities in tall girls. Acta Orthop. Scand. 1985, 56, 155–157. [Google Scholar] [CrossRef] [PubMed]
- Hershkovich, O.; Friedlander, A.; Gordon, B.; Arzi, H.; Derazne, E.; Tzur, D.; Shamiss, A.; Afek, A. Association between body mass index, body height, and the prevalence of spinal deformities. Spine J. 2014, 14, 1581–1587. [Google Scholar] [CrossRef]
- Fotiadis, E.; Kenanidis, E.; Samoladas, E.; Christodoulou, A.; Akritopoulos, P.; Akritopoulou, K. Scheuermann’s disease: Focus on weight and height role. Eur. Spine J. 2008, 17, 673–678. [Google Scholar] [CrossRef]
- Murray, P.M.; Weinstein, S.L.; Spratt, K.F. The natural history and long-term follow-up of Scheuermann kyphosis. J. Bone Jt. Surg. Am. 1993, 75, 236–248. [Google Scholar] [CrossRef]
- Bradford, D.S.; Brown, D.M.; Moe, J.H.; Winter, R.B.; Jowsey, J. Scheuermann’s kyphosis: A form of osteoporosis? Clin. Orthop. Relat. Res. 1976, 10–15. [Google Scholar] [CrossRef]
- Ippolito, E.; Ponseti, I.V. Juvenile kyphosis: Histological and histochemical studies. J. Bone Jt. Surg. Am. 1981, 63, 175–182. [Google Scholar] [CrossRef]
- Lüdemann, W.; Heary, R.F.; Albert, T.J. Spinal Deformities: The Essentials. Child’s Nerv. Syst. 2015, 31, 631–632. [Google Scholar] [CrossRef]
- Aufdermaur, M. Juvenile kyphosis (Scheuermann’s disease): Radiography, histology, and pathogenesis. Clin. Orthop. Relat. Res. 1981, 154, 166–174. [Google Scholar] [CrossRef]
- Rappaport, R.; Forest, M.G.; Bayard, F.; Duval-Beaupere, G.; Blizzard, R.M.; Migeon, C.J. Plasma androgens and LH in scoliotic patients with premature pubarche. J. Clin. Endocrinol. Metab. 1974, 38, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Chokshi, S.N.; Mudiganty, S.A.-O.; Clement, R.C., 3rd.; Accousti, W. Vitamin D deficiency in Scheuermann’s disease is associated with increased adverse outcomes. SICOT-J 2024, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- Frost, H.M. Bone’s mechanostat: A 2003 update. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 2003, 275, 1081–1101. [Google Scholar] [CrossRef]
- Frost, H.M. Bone “mass” and the “mechanostat”: A proposal. Anat. Rec. 1987, 219, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Schoenau, E.; Fricke, O.; Rauch, F. The regulation of bone development as a biological system. Homo 2003, 54, 113–118. [Google Scholar] [CrossRef]
- Schoenau, E. From mechanostat theory to development of the “Functional Muscle-Bone-Unit”. J. Musculoskelet. Neuronal. Interact 2005, 5, 232–238. [Google Scholar]
- Lerebours, C.; Buenzli, P.R. Towards a cell-based mechanostat theory of bone: The need to account for osteocyte desensitisation and osteocyte replacement. J. Biomech. 2016, 49, 2600–2606. [Google Scholar] [CrossRef]
- Hughes, J.M.; Castellani, C.M.; Popp, K.L.; Guerriere, K.I.; Matheny, R.W., Jr.; Nindl, B.C.; Bouxsein, M.L. The Central Role of Osteocytes in the Four Adaptive Pathways of Bone’s Mechanostat. Exerc. Sport Sci. Rev. 2020, 48, 140–148. [Google Scholar] [CrossRef]
- Fricke, O.; Schoenau, E. The ‘Functional Muscle-Bone Unit’: Probing the relevance of mechanical signals for bone development in children and adolescents. Growth Horm. IGF Res. 2007, 17, 1–9. [Google Scholar] [CrossRef]
- Rauch, F.; Bailey, D.A.; Baxter-Jones, A.; Mirwald, R.; Faulkner, R. The ‘muscle-bone unit’ during the pubertal growth spurt. Bone 2004, 34, 771–775. [Google Scholar] [CrossRef]
- Axenovich, T.I.; Zaidman, A.M.; Zorkoltseva, I.V.; Kalashnikova, E.V.; Borodin, P.M. Segregation analysis of Scheuermann disease in ninety families from Siberia. Am. J. Med. Genet. 2001, 100, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Zaidman, A.M.; Zaidman, M.N.; Strokova, E.L.; Korel, A.V.; Kalashnikova, E.V.; Rusova, T.V.; Mikhailovsky, M.V. The mode of inheritance of Scheuermann’s disease. Biomed. Res. Int. 2013, 2013, 973716. [Google Scholar] [CrossRef] [PubMed]
- Damborg, F.; Engell, V.; Andersen, M.; Kyvik, K.O.; Thomsen, K. Prevalence, concordance, and heritability of Scheuermann kyphosis based on a study of twins. J. Bone Jt. Surg. Am. 2006, 88, 2133–2136. [Google Scholar] [CrossRef]
- Damborg, F.; Engell, V.; Nielsen, J.; Kyvik, K.O.; Andersen, M.O.; Thomsen, K. Genetic epidemiology of Scheuermann’s disease. Acta Orthop. 2011, 82, 602–605. [Google Scholar] [CrossRef] [PubMed]
- Kewalramani, L.S.; Riggins, R.S.; Fowler, W.M., Jr. Scheuermann’s kyphoscoliosis associated with Charcot-Marie-Tooth syndrome. Arch. Phys. Med. Rehabil. 1976, 57, 391–397. [Google Scholar]
- Halal, F.; Gledhill, R.B.; Fraser, C. Dominant inheritance of Scheuermann’s juvenile kyphosis. Am. J. Dis. Child. 1978, 132, 1105–1107. [Google Scholar] [CrossRef]
- McKenzie, L.; Sillence, D. Familial Scheuermann disease: A genetic and linkage study. J. Med. Genet. 1992, 29, 41–45. [Google Scholar] [CrossRef]
- Findlay, A.; Conner, A.N.; Connor, J.M. Dominant inheritance of Scheuermann’s juvenile kyphosis. J. Med. Genet. 1989, 26, 400–403. [Google Scholar] [CrossRef]
- Graat, H.C.; van Rhijn, L.W.; Schrander-Stumpel, C.T.; van Ooij, A. Classical Scheuermann disease in male monozygotic twins: Further support for the genetic etiology hypothesis. Spine 2002, 27, E485–E487. [Google Scholar] [CrossRef]
- Gustavel, M.; Beals, R.K. Scheuermann’s disease of the lumbar spine in identical twins. AJR Am. J. Roentgenol. 2002, 179, 1078–1079. [Google Scholar] [CrossRef]
- Bjersand, A.J. Juvenile kyphosis in identical twins. AJR Am. J. Roentgenol. 1980, 134, 598–599. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.J. Idiopathic thoracic kyphosis in identical twins. J. Bone Jt. Surg. Br. 1990, 72, 144. [Google Scholar] [CrossRef] [PubMed]
- Al Kaissi, A.; Marrakchi, Z.; Nassib, N.M.; Hofstaetter, J.; Grill, F.; Ganger, R.; Kircher, S.G. Craniosynostosis, Scheuermann’s disease, and intellectual disability resembling Shprintzen-Goldberg syndrome: A report on a family over 4 generations: Case report. Medicine 2017, 96, e6199. [Google Scholar] [CrossRef]
- Dai, Y.; Li, Y.; Li, P.; Li, L.; Tu, Z.; Wang, B. Familial lumbar Scheuermann disease with idiopathic scoliosis in China: First case report. Medicine 2017, 96, e7100. [Google Scholar] [CrossRef]
- Al Kaissi, A.; Laccone, F.; Karner, C.; Ganger, R.; Klaushofer, K.; Grill, F. Hip dysplasia and spinal osteochondritis (Scheuermann’s disease) in a girl with type II manifesting collagenopathy. Orthopade 2013, 42, 963–968. [Google Scholar] [CrossRef]
- Lopponen, T.; Korkko, J.; Lundan, T.; Seppanen, U.; Ignatius, J.; Kaariainen, H. Childhood-onset osteoarthritis, tall stature, and sensorineural hearing loss associated with Arg75-Cys mutation in procollagen type II gene (COL2A1). Arthritis Rheum. 2004, 51, 925–932. [Google Scholar] [CrossRef]
- Karppinen, J.; Paakko, E.; Paassilta, P.; Lohiniva, J.; Kurunlahti, M.; Tervonen, O.; Nieminen, P.; Goring, H.H.; Malmivaara, A.; Vanharanta, H.; et al. Radiologic phenotypes in lumbar MR imaging for a gene defect in the COL9A3 gene of type IX collagen. Radiology 2003, 227, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Paassilta, P.; Lohiniva, J.; Goring, H.H.; Perala, M.; Raina, S.S.; Karppinen, J.; Hakala, M.; Palm, T.; Kroger, H.; Kaitila, I.; et al. Identification of a novel common genetic risk factor for lumbar disk disease. JAMA 2001, 285, 1843–1849. [Google Scholar] [CrossRef] [PubMed]
- Annunen, S.; Paassilta, P.; Lohiniva, J.; Perala, M.; Pihlajamaa, T.; Karppinen, J.; Tervonen, O.; Kroger, H.; Lahde, S.; Vanharanta, H.; et al. An allele of COL9A2 associated with intervertebral disc disease. Science 1999, 285, 409–412. [Google Scholar] [CrossRef] [PubMed]
- Esapa, C.T.; Hough, T.A.; Testori, S.; Head, R.A.; Crane, E.A.; Chan, C.P.; Evans, H.; Bassett, J.H.; Tylzanowski, P.; McNally, E.G.; et al. A mouse model for spondyloepiphyseal dysplasia congenita with secondary osteoarthritis due to a Col2a1 mutation. J. Bone Miner Res. 2012, 27, 413–428. [Google Scholar] [CrossRef]
- Oei, L.; Estrada, K.; Duncan, E.L.; Christiansen, C.; Liu, C.T.; Langdahl, B.L.; Obermayer-Pietsch, B.; Riancho, J.A.; Prince, R.L.; van Schoor, N.M.; et al. Genome-wide association study for radiographic vertebral fractures: A potential role for the 16q24 BMD locus. Bone 2014, 59, 20–27. [Google Scholar] [CrossRef]
- Wang, S.; Wang, X.; Teng, X.; Li, S.; Zhang, H.; Shan, Z.; Li, Y. Lumbar Scheuermann’s disease found in a patient with osteogenesis imperfecta (OI) caused by a heterozygous mutation in COL1A2 (c.4048G > A): A case report. BMC Musculoskelet. Disord. 2021, 22, 525. [Google Scholar] [CrossRef]
- Iba, K.; Durkin, M.E.; Johnsen, L.; Hunziker, E.; Damgaard-Pedersen, K.; Zhang, H.; Engvall, E.; Albrechtsen, R.; Wewer, U.M. Mice with a targeted deletion of the tetranectin gene exhibit a spinal deformity. Mol. Cell. Biol. 2001, 21, 7817–7825. [Google Scholar] [CrossRef]
- Iram, S.; Rahman, S.; Choi, I.; Kim, J. Insight into the function of tetranectin in human diseases: A review and prospects for tetranectin-targeted disease treatment. Heliyon 2024, 10, e23512. [Google Scholar] [CrossRef]
- Juneja, S.C.; Vonica, A.; Zeiss, C.; Lezon-Geyda, K.; Yatsula, B.; Sell, D.R.; Monnier, V.M.; Lin, S.; Ardito, T.; Eyre, D.; et al. Deletion of Mecom in mouse results in early-onset spinal deformity and osteopenia. Bone 2014, 60, 148–161. [Google Scholar] [CrossRef]
- Lowe, T.G.; Line, B.G. Evidence based medicine: Analysis of Scheuermann kyphosis. Spine 2007, 32, S115–S119. [Google Scholar] [CrossRef]
- Ippolito, E.; Bellocci, M.; Montanaro, A.; Ascani, E.; Ponseti, I.V. Juvenile kyphosis: An ultrastructural study. J. Pediatr. Orthop. 1985, 5, 315–322. [Google Scholar] [CrossRef]
- Aufdermaur, M.; Spycher, M. Pathogenesis of osteochondrosis juvenilis Scheuermann. J. Orthop. Res. 1986, 4, 452–457. [Google Scholar] [CrossRef]
- Nielsen, L.W.; Høgedal, P.; Arnbjerg, J.; Jensen, H.E. Juvenile kyphosis in pigs. A spontaneous model of Scheuermann’s kyphosis. Acta Pathol. Microbiol. Immunol. Scand. 2005, 113, 702–707. [Google Scholar] [CrossRef]
- Faubel, R.; Westendorf, C.; Bodenschatz, E.; Eichele, G. Cilia-based flow network in the brain ventricles. Science 2016, 353, 176–178. [Google Scholar] [CrossRef]
- Cantaut-Belarif, Y.; Sternberg, J.R.; Thouvenin, O.; Wyart, C.; Bardet, P.L. The Reissner Fiber in the Cerebrospinal Fluid Controls Morphogenesis of the Body Axis. Curr. Biol. 2018, 28, 2479–2486. [Google Scholar] [CrossRef] [PubMed]
- Sternberg, J.R.; Prendergast, A.E.; Brosse, L.; Cantaut-Belarif, Y.; Thouvenin, O.; Orts-Del’Immagine, A.; Castillo, L.; Djenoune, L.; Kurisu, S.; McDearmid, J.R.; et al. Pkd2l1 is required for mechanoception in cerebrospinal fluid-contacting neurons and maintenance of spine curvature. Nat. Commun. 2018, 9, 3804. [Google Scholar] [CrossRef] [PubMed]
- Bohm, U.L.; Prendergast, A.; Djenoune, L.; Nunes Figueiredo, S.; Gomez, J.; Stokes, C.; Kaiser, S.; Suster, M.; Kawakami, K.; Charpentier, M.; et al. CSF-contacting neurons regulate locomotion by relaying mechanical stimuli to spinal circuits. Nat. Commun. 2016, 7, 10866. [Google Scholar] [CrossRef] [PubMed]
- Orts-Del’immagine, A.; Wanaverbecq, N.; Tardivel, C.; Tillement, V.; Dallaporta, M.; Trouslard, J. Properties of subependymal cerebrospinal fluid contacting neurones in the dorsal vagal complex of the mouse brainstem. J. Physiol. 2012, 590, 3719–3741. [Google Scholar] [CrossRef] [PubMed]
- Orts-Del’Immagine, A.; Kastner, A.; Tillement, V.; Tardivel, C.; Trouslard, J.; Wanaverbecq, N. Morphology, distribution and phenotype of polycystin kidney disease 2-like 1-positive cerebrospinal fluid contacting neurons in the brainstem of adult mice. PLoS ONE 2014, 9, e87748. [Google Scholar] [CrossRef] [PubMed]
- Marie-Hardy, L.; Slimani, L.; Messa, G.; El Bourakkadi, Z.; Prigent, A.; Sayetta, C.; Koeth, F.; Pascal-Moussellard, H.; Wyart, C.; Cantaut-Belarif, Y. Loss of CSF-contacting neuron sensory function is associated with a hyper-kyphosis of the spine reminiscent of Scheuermann’s disease. Sci. Rep. 2023, 13, 5529. [Google Scholar] [CrossRef]
- Boswell, C.W.; Ciruna, B. Understanding Idiopathic Scoliosis: A New Zebrafish School of Thought. Trends Genet. 2017, 33, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Abul-Kasim, K.; Schlenzka, D.; Selariu, E.; Ohlin, A. Spinal epidural lipomatosis: A common imaging feature in Scheuermann disease. J. Spinal Disord. Tech. 2012, 25, 356–361. [Google Scholar] [CrossRef]
- Yasuda, T.; Suzuki, K.; Kawaguchi, Y.; Seki, S.; Makino, H.; Watanabe, K.; Hori, T.; Yamagami, T.; Kanamori, M.; Kimura, T. Clinical and imaging characteristics in patients undergoing surgery for lumbar epidural lipomatosis. BMC Musculoskelet. Disord. 2018, 19, 66. [Google Scholar] [CrossRef]
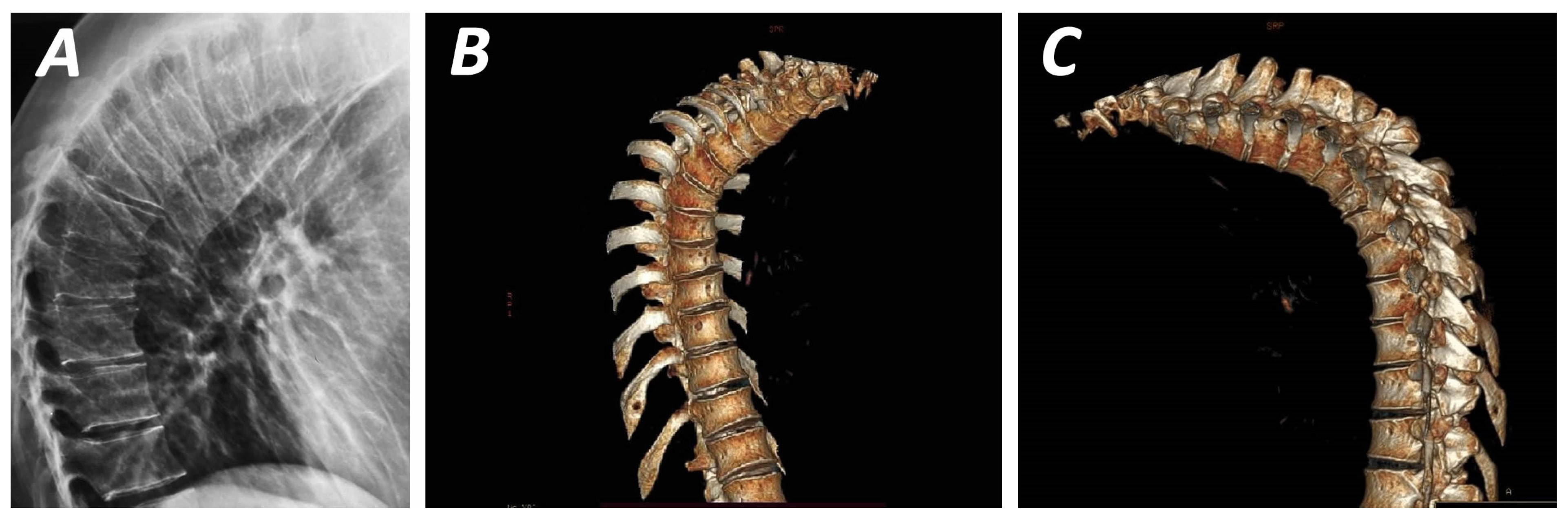
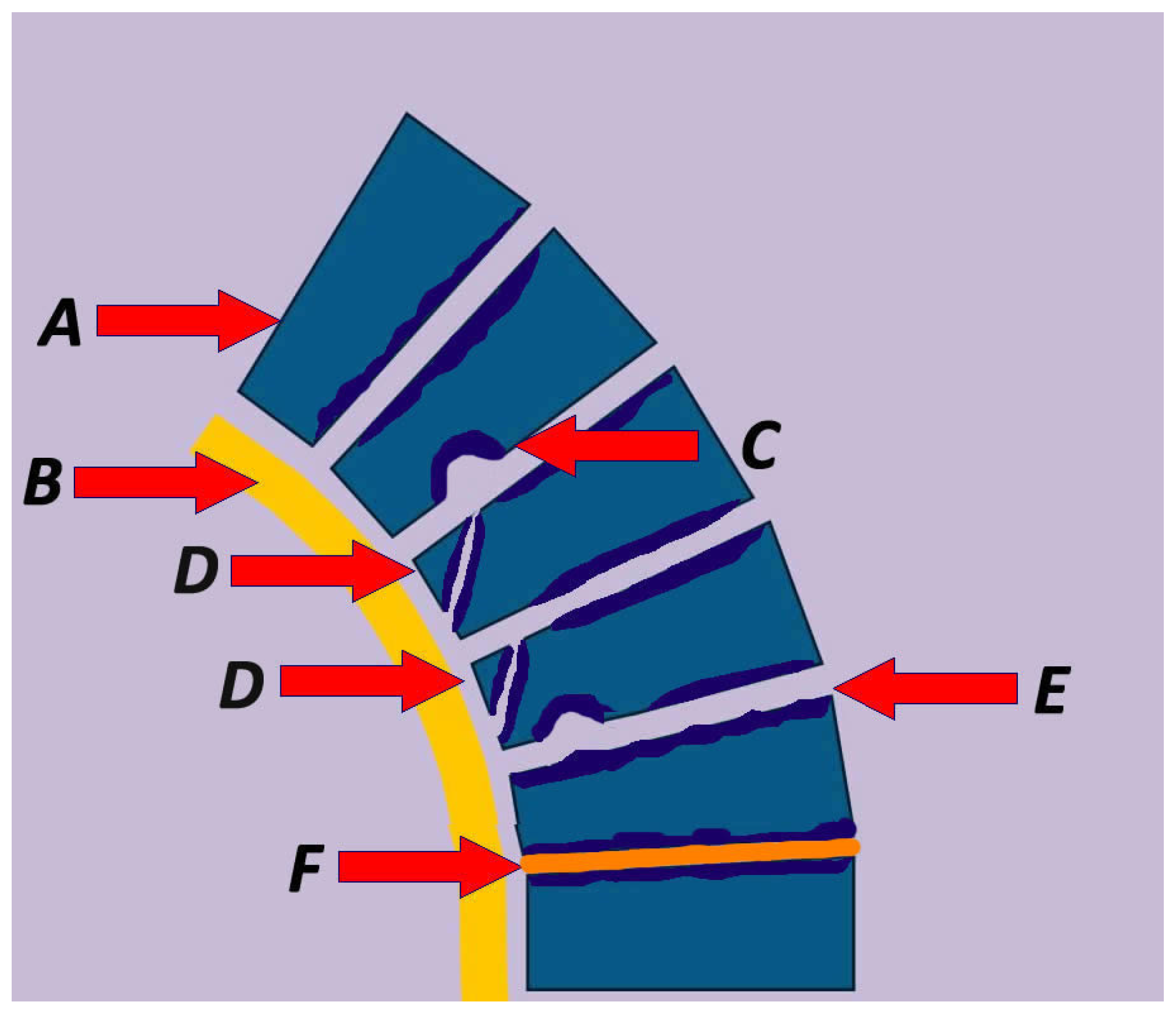
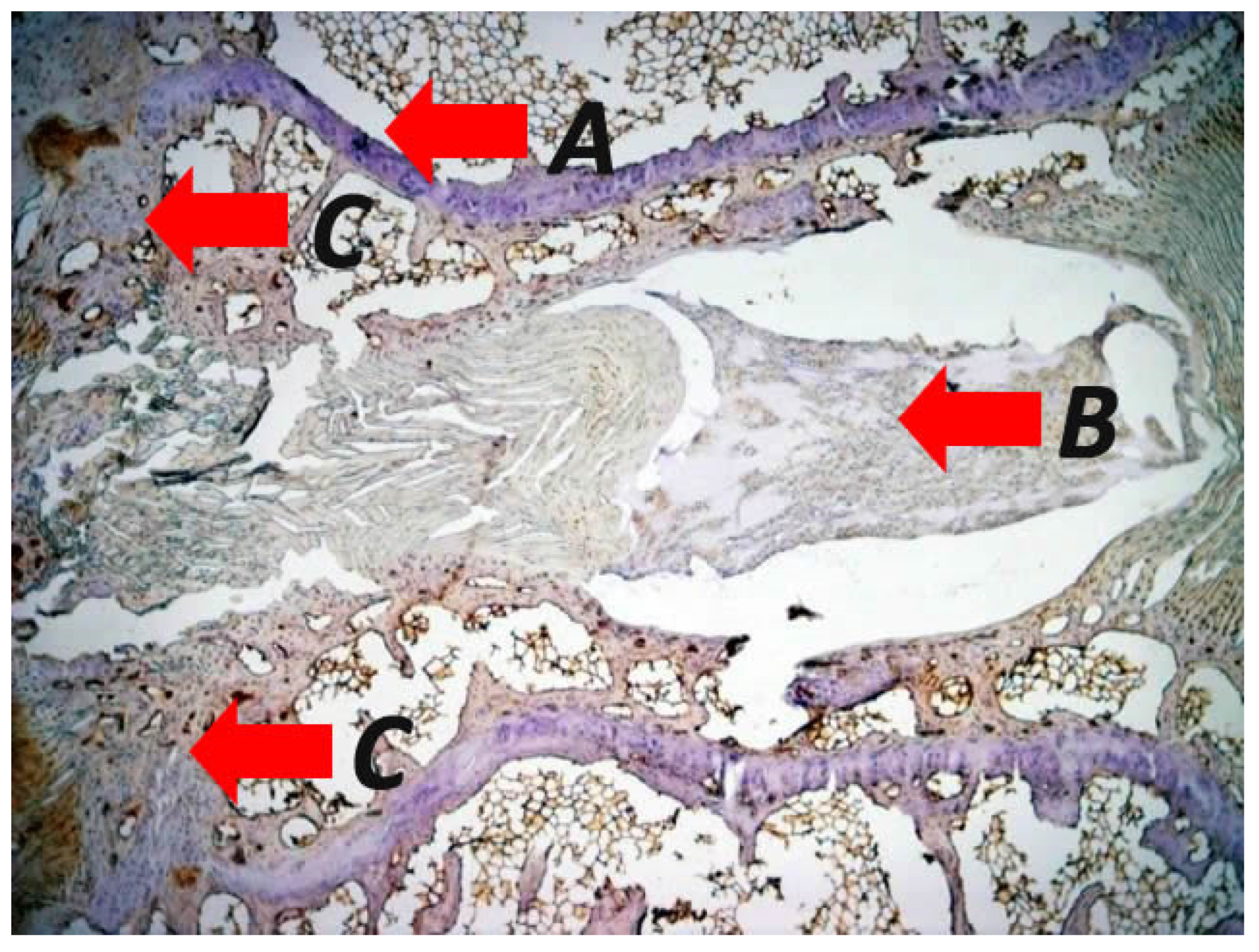
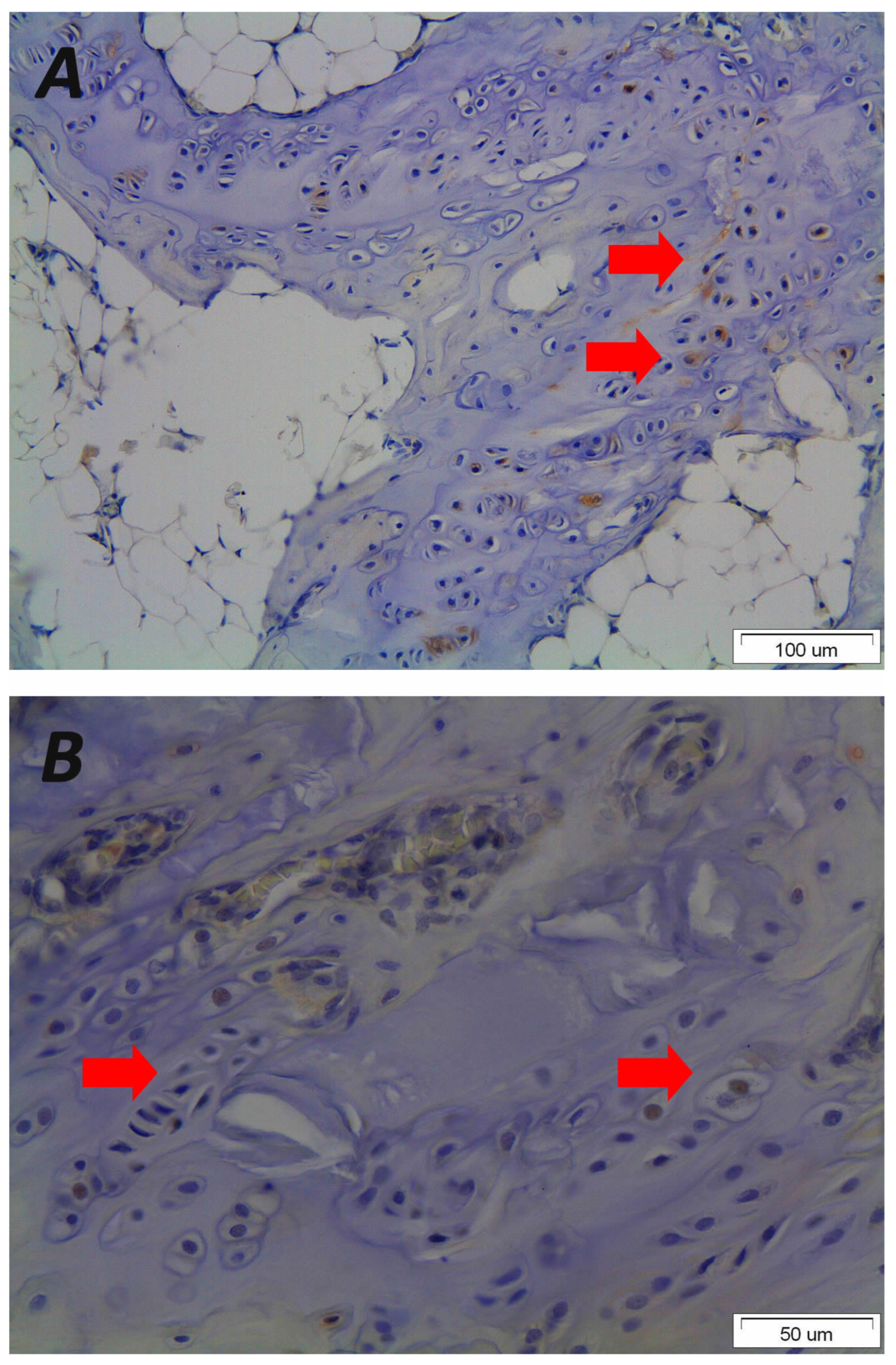
| Study | Number of Patients (N) | Age (Mean) | Male/Female Ratio | Diagnosis | Mean Kyphosis | Intervention | Follow Up (Months) | Outcome |
|---|---|---|---|---|---|---|---|---|
| Peleg et al., 2016 [35] | SK Group: 183 Control group: 185 normal skeletons | N/A | N/A | Sorensen’s criteria | N/A | Cadaveric study/Radiographic parameter analysis | N/A | SAO: SK group: 44.44 ± 9.7°, Control group: 50 ± 9.9° (p < 0.01). Sacrum more horizontally inclined in SK patients. |
| Tyrakowski et al., 2015 [37] | Total N: 66 Group 1: skeletally mature SK: 33 Group 2: skeletally immature SK: 33 | Skeletally mature 22.7 (16.1–47.4) Skeletally immature: 14.1 (11–16.3) | Skeletally mature: 2 Skeletally immature: 3.12 | Sorensen’s criteria | Skeletally mature: 56° (3–81) Skeletally immature: 57.8° (13–96) | Radiographic parameter analysis/No intervention | N/A | Skeletally mature pts: PI: 39.4 ± 8.9 PT: 7.3 ± 9.4 SS: 32.1 ± 9.2 Skeletally immature pts: PI: 36.7 ± 8.1 PT: 3.8 ± 7.5 SS: 32.8 ± 9.2 No significant difference between PI, PT, SS, LL SK patients have lower PI and SS than non- patients. |
| Jansen et al., 2006 [40] | 30 | 28 | 1.3 | Sach’s Criteria (TK > 45° and at least one wedged vertebra ≥ 5°) | 80° | PSF: -Harrington rods: 5 Pts -Cotrel-Dubousset 25 Pts Anterior release: 29 Pts | 12 | Maximum kyphosis (±SD): preop: 80° (±7) postop: 47° (±9) Maximum lordosis (±SD): preop: 72° (±12) postop: 59° (±11) Mean L5-S1, SS unchanged pre- and postoperatively Avg correction of kyphosis: 2.3x Avg correction of lordosis Postoperative correlation of kyphosis versus lordosis (R = 0.591, p = 0.001) |
| Jiang et al., 2014 [41] | Total N: 115 SK Pts: 55 (subdivided in Thoracic Kyphosis and Thoracolumbar kyphosis subgroups) Healthy controls: N:60 | SK Group: 14.2 (10–18) Control group: 14.2 (11–18) | SK Group: 3.23 Control group: N/A | Sorensen’s criteria | 45.6° ± 24.3 | Radiographic parameter analysis/No intervention | N/A | SK group: PI: 32° ± 10.8 Control group: 45° ± 10.8 (p: 0.001) SK group: PT: 0.2° ± 11.0 Control group: 11° ± 9.2 (p: 0.001) SK Thoracic Kyphosis subgroup: TK strongly correlated with LL (r = −0.792, p\0.001) and PT (r = 0.551, p = 0.008). PI related to PT (r = 0.514, p = 0.014) and SS (r = 0.564, p = 0.006). No correlations were found between LL and SS. For thoracolumbar kyphosis group: LL correlated with SS (r = −0.641, p\0.001) and PI (r = −0.365, p = 0.037). |
| Loder, 2001 [42] | N:34 | 15.5 | 1.12 | Sorensen’s criteria | 65° ± 12 | Radiographic parameter analysis/No intervention (Preoperative Radiographs) | N/A | Cervical lordosis correlated with LL (Cobb angle, r2 = 0.17, p = 0.024) No correlations between cervical lordosis and TK or sacral inclination. |
| Cahill et al., 2015 [43] | Total N: 97 SK group: N:47 Control group: N:50 (from database) | SK group: 16.1 Control group: 13.5 | SK group: 2.61 Control group: 0.28 | N/A | 65.5° ± 13.4 | Radiographic parameter analysis (Multi-centre study)/Retrospective Preoperative radiographic parameter analysis | 24 | SK group: PI: 41.8° ± 12.0, PT: 7.3° ± 8.1, SS: 34.5° ± 9.5 Control group: PI: 45.5° ± 8.5, PT: 8.4 ° ± 6.7, SS: 37.1° ± 8.5 Above results not statistically significant different between studied populations. SK group: LL 66.3° ± 12.9 Control group LL: 55.1° ± 11.9 (p < 0.001) T5-12 kyphosis and C7-SVA correlated with LL (p < 0.05) PI directly correlated with LL in both groups (p!0.005) Greatest Cobb kyphosis in SK did not significantly correlate with PI or LL. |
| Lonner et al., 2007 [44] | N:78 | 16.7 (9–27) | 7.54 | Sorensen’s criteria | 78.8° | Pts divided as follows: -Combined Anterior and Posterior Surgery: 42 Pts -PSF: 36 Pts (Multi-centre study) Radiographic parameter analysis | 34.8 ± 16.8 | TK: Whole group preop: 78.8° ± 11.6 (55–106), final follow-up: 51.4° ± 12.3 (27–82) Correlation of LL and PI both before surgery (p < 0.001) and at follow-up (p = 0.000). LL correlated with TK at final follow-up (p < 0.02) No correlation between LL and TK before surgery (p = 0.23). TK did not correlate with PI. LL statistically significant increase in both groups, between the first postoperative visit and final follow-up. Whole group LL: Postop: 46.5 ± 12.3, Follow-up 51.7 ± 13.8 (p = 0.053) |
| Hosman et al., 2003 [45] | Total N: 33 Group 1: Pts w/tight hamstrings N:16 Group 2: Pts w/nonlight hamstrings N:17 | Group 1: 24.9 Group 2: 26.6 | N/A | N/A | Group 1: 78.1° Group 2: 79.3° | Surgical correction and fusion, Radiographic parameter analysis | 54 (24–98.4) | Kyphosis correction: Group 1: 27.2°, Group 2: 22.2° (p: 0.14) LL Reduction: Group 1: −10.3°, Group 2: −10° (p: 0.93) SS shift: Group 1: 0.19°, Group 2:6.3° (p: 0.0001) Preoperatively sagittal imbalanced: statistically non-significant between groups Postoperatively sagittal imbalanced: Group 1: 8/16, Group 2: 1/16 (0.036) Group 2: Greater pelvic and lumbar ROM compared to group 1 |
| Study/Country | Number of Patients (N) | Age Mean and Range (in Years) | Mean Kyphosis (in Degrees) | Method | Site of Measurement | Results |
|---|---|---|---|---|---|---|
| Lopez et al., 1988, United States [93] | SK group n = 10 Control group n = 7 Total n = 17 | SK:16 (14–19) Control group:16 | 64° (35–85°) Control group: no spinal pathology | Dual Photon Absorptiometry/Single Photon Absorptiometry | Right femoral neck, L2–L4/Distal radius | Mean BMD: SK group 0.975, Control group 1.130 (p: 0.025) (DPA) Femoral neck BMD: SK group 1.00, Control Group 1.22 (p < 0.005) (DPA) Subgroup with kyphosis > 45° (n = 7) BMD Lspine: 0.913 (p < 0.005), BMD femoral neck: 0.983 (p < 0.005) (DPA) Distal radius BMD: SK group: 0.689, Control group: 0.748. Not reaching statistical significance (SPA). |
| Gilsanz et al., 1989, United States [95] | SK group n = 20 Control group n = 20 Total n = 40 | SK group: 15.6 ± 1.9 Control group: 15.6 ± 2.1 | 56°(45–79) Control group: no spinal deformity | Quantitative CT | L-Spine | SK Group: 199.2 ± 18.7 mg/cm3 of mineral equivalent Control Group 193.7 ± 17.4 mg/cm3 of mineral equivalent. Not significantly different. |
| Ashton et al., 2001, Australia [96] | SK pts = 12 | SK group: 14.08 (11–16) Control group: data from 335 pts 14.5 | N/A | DEXA | L2–L4 | Mean Lumbar Z-score 1.55 (0.0–3.9) |
| Popko et al., 1997, Poland [97] | SK n= 24 | N/A. Control group: population database | N/A | DEXA | L2–L4 | In 9/24 SK pts lower Total Skeleton BMD and L2–L4 BMD compared to population data. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Kaspiris, A.; Spyrou, I.; Marougklianis, V.; Afrati, S.R.; Sakellariou, E.; Varsamos, I.; Karampinas, P.; Vasiliadis, E.; Pneumaticos, S.G. Insights into the Pathophysiology of Scheuermann’s Kyphosis: From Structural Deformities to Genetic Predisposition and Underlying Signalling Pathways. Biomolecules 2026, 16, 56. https://doi.org/10.3390/biom16010056
Kaspiris A, Spyrou I, Marougklianis V, Afrati SR, Sakellariou E, Varsamos I, Karampinas P, Vasiliadis E, Pneumaticos SG. Insights into the Pathophysiology of Scheuermann’s Kyphosis: From Structural Deformities to Genetic Predisposition and Underlying Signalling Pathways. Biomolecules. 2026; 16(1):56. https://doi.org/10.3390/biom16010056
Chicago/Turabian StyleKaspiris, Angelos, Ioannis Spyrou, Vasileios Marougklianis, Spyridoula Roberta Afrati, Evangelos Sakellariou, Iordanis Varsamos, Panagiotis Karampinas, Elias Vasiliadis, and Spiros G. Pneumaticos. 2026. "Insights into the Pathophysiology of Scheuermann’s Kyphosis: From Structural Deformities to Genetic Predisposition and Underlying Signalling Pathways" Biomolecules 16, no. 1: 56. https://doi.org/10.3390/biom16010056
APA StyleKaspiris, A., Spyrou, I., Marougklianis, V., Afrati, S. R., Sakellariou, E., Varsamos, I., Karampinas, P., Vasiliadis, E., & Pneumaticos, S. G. (2026). Insights into the Pathophysiology of Scheuermann’s Kyphosis: From Structural Deformities to Genetic Predisposition and Underlying Signalling Pathways. Biomolecules, 16(1), 56. https://doi.org/10.3390/biom16010056








