Mitochondrial Reactive Oxygen Species: A Unifying Mechanism in Long COVID and Spike Protein-Associated Injury: A Narrative Review
Abstract
1. Introduction
| Viral Infection | Mitochondrial Alteration | Mechanism/Effect | Functional Consequence | Reference |
|---|---|---|---|---|
| HIV | Enhanced fission; loss of ΔΨm; mtDNA depletion; reduced ATP-linked respiration | DRP1 upregulation; interaction with mPTP; ETC impairment | Mitochondrial fragmentation, apoptosis, and reduced energy production; neuropathy/myopathy | [20,23] |
| HCV | Enhanced fission; disruption of Ca2+ homeostasis; impaired ETC (complex I inhibition); mtDNA damage/depletion | DRP-1 increase; altered Ca2+ signaling; ROS generation | Mitochondrial fragmentation, oxidative stress, shift to glycolysis (Warburg effect), energy imbalance, and HIF-1α stabilization; depression, neurobehavioral dysfunction | [20,23] |
| HBV | Enhanced fission; disruption of Ca2+ homeostasis; loss of ΔΨm; mtDNA deletion/depletion | DRP-1 upregulation; VDAC/ANT interactions; apoptosis induction | Mitochondrial injury, apoptosis, progression of fibrosis/cirrhosis, fatigue, depression | [20,23] |
| EBV | Enhanced fission | DRP-1 activation | Increased mitochondrial fragmentation, linked to oncogenesis | [23] |
| SARS-CoV | Induces fusion; degradation of fission molecules | Degradation of DRP1 by ORF9b, mitochondrial fusion/elongation, and suppression of MAVS signaling | A more stable mitochondrial network supporting viral replication increases ROS; similar post-viral sequelae of SARS-CoV-2 | [24] |
| SARS-CoV-2 | Fusion, inefficient bioenergetics, increased mROS, mtDNA reduction (circulating and cellular), and immune evasion | ORF9b fusion induction and fission molecule degradation; OXPHOS inhibition → mROS → HIF-1α stabilization; ORF10-mediated MAVS inhibition; depletion of mtDNA in microglia and blood cells | Increase in glycolysis to fuel replication; chronic persistence/long COVID reservoirs; reduced mtDNA linked to higher mortality; attenuated IFN hyperinflammation via DAMP signaling; “long COVID” | [19,23] |
| Influenza Virus | Fusion and biogenesis; loss of ΔΨm; mtDNA release; MAVS inhibition | M2 → OPA1/MFN1-2 upregulation; PB1-F2 → ΔΨm dissipation, Cyt C release; M2 viroporin activity → mtDNA release | Increased mitochondrial number; apoptosis; immune suppression; activation of inflammasomes; cytokine storm. Fatigue, depression, encephalopathy | [20,23,25] |
| HSV-1 | mtDNA depletion; Disruption of Ca2+ homeostasis; reduced ATP; ROS generation | UL12.5 causes mtDNA degradation; oxidative stress induction | Reduced respiration, impaired bioenergetics; depression | [20,23] |
| HTLV-1 | Loss of ΔΨm; disruption of Ca2+ homeostasis | Alters inner membrane ion homeostasis | Induction of apoptosis via Cyt C release | [23] |
| CMV | Anti-apoptotic (prevents ΔΨm loss); Disruption of Ca2+ homeostasis; metabolic reprogramming | vMIA localizes to mitochondria; recruits BAX | Apoptosis inhibition to prolong infection; Warburg shift | [23] |
| HHV-8 | Decreased mitochondrial biogenesis; suppressed OXPHOS; increased lactate production; disruption of Ca2+ homeostasis | Viral proteins (VGPCR, LANA, vCyclin, vFLIP) stabilize HIF-1a, upregulating glycolytic enzymes (PKM2, PDK1) and glucose transporters | Warburg shift → promotes survival, proliferation, and tumorigenesis of infected cells | [23,26] |
| HPV | Inhibition of apoptosis | Downregulates BAX-dependent pathways (via E6/E7) | Prevention of host cell death → persistence of infection | [23] |
| Encephalomyocarditis virus | mtDNA release | Viroporin 2B-mediated disturbance of mitochondrial membranes; MAVS-dependent translocation of mtDNA into the cytosol; activates NLRP3 | mtDNA leakage into cytosol → immune activation and inflammation | [23,25] |
2. Disambiguating Long COVID and Post-Acute COVID-19 Vaccination Syndrome
3. Sources and Functional Roles of ROS in Macrophages
4. Mitochondrial ROS in Antiviral Innate Immunity
5. Mitochondrial Stress and MAVS Dysregulation
6. Mitochondrial Dysfunction and Redox Signaling in Long COVID
6.1. Long COVID as a Mitochondrial Disorder
6.2. Viral Hijack and Metabolic Reprogramming
6.3. Immune Activation and the Vicious Cycle of Injury
6.4. Organ System Impact and Clinical Correlates
6.5. Biomarkers of Redox Imbalance and Mitochondrial Injury
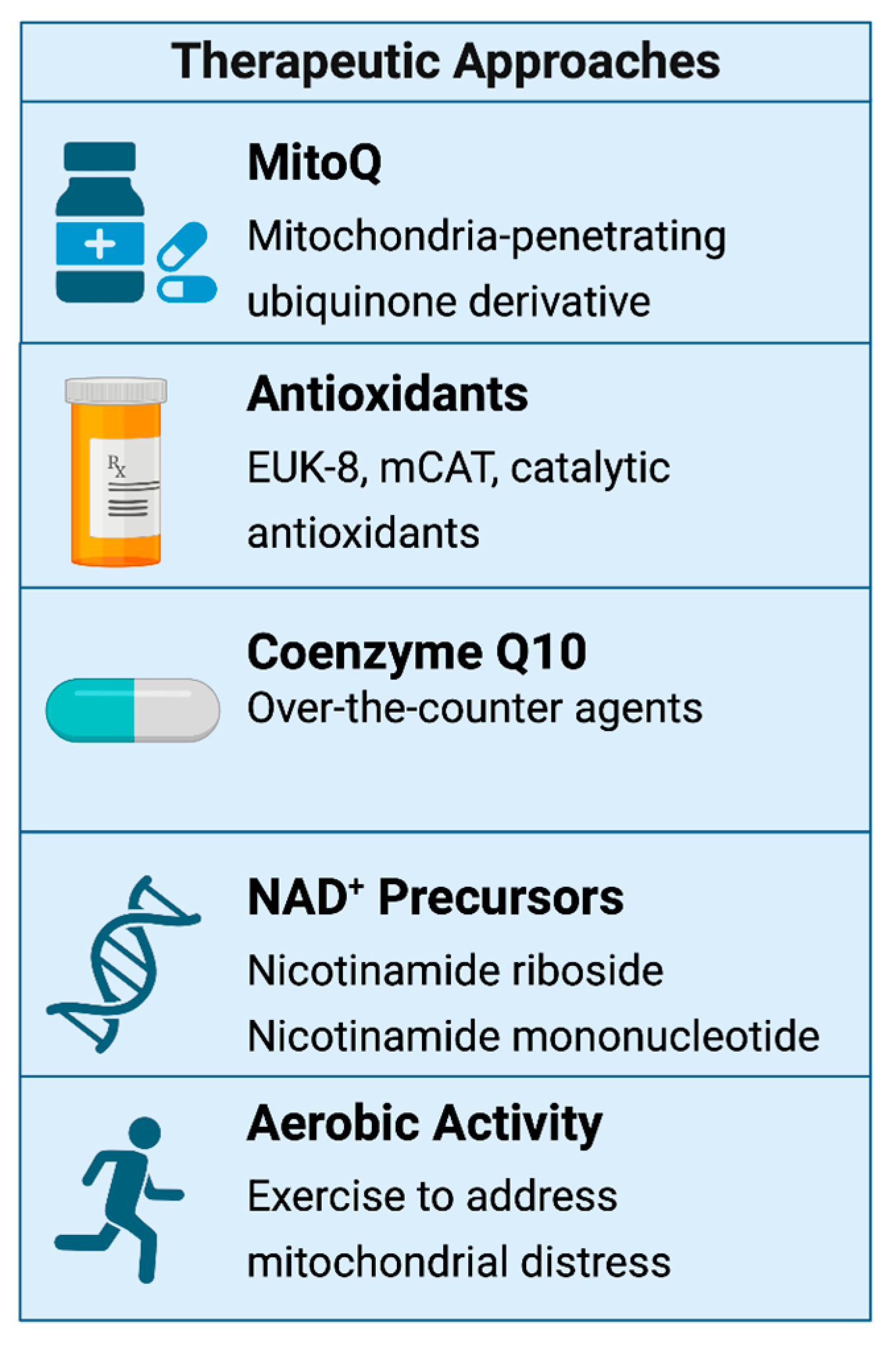
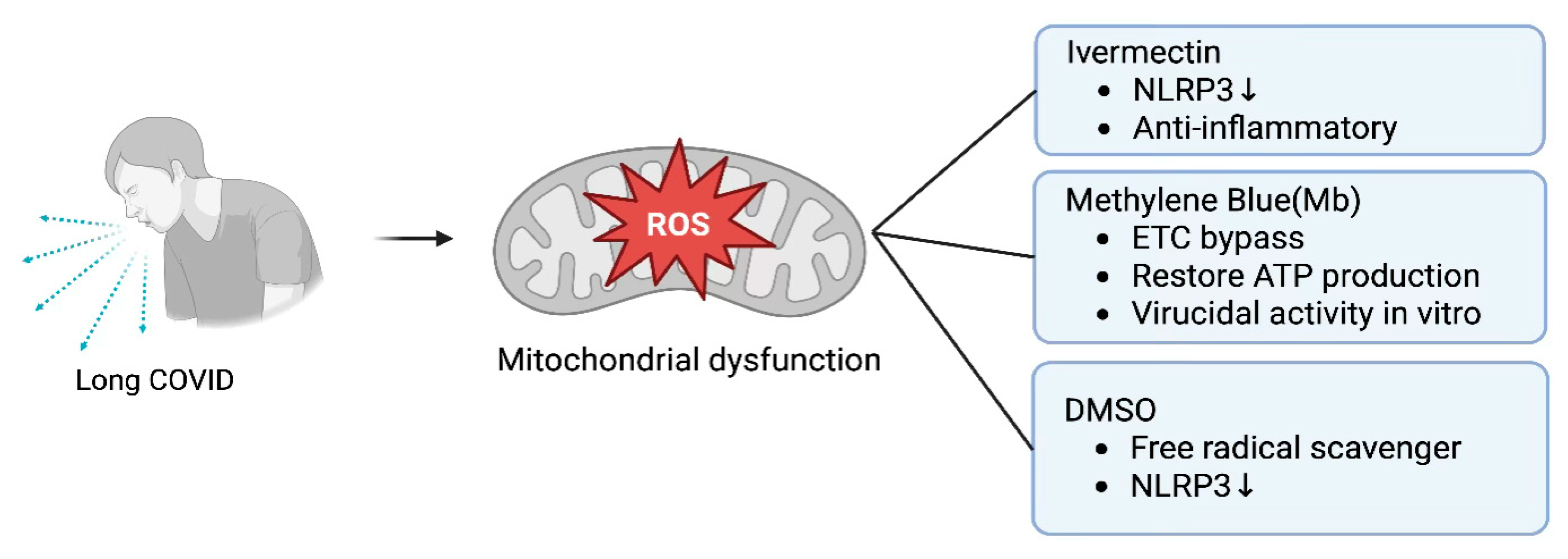
6.6. Therapeutic Interventions: Mitochondrial Resuscitation Targeting Mitochondrial Dysfunction
6.7. Exercise and Mitochondrial Rehabilitation
6.8. Translational Tools and Precision Medicine Outlook
6.9. Mitochondrial Complications from Spike Protein
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hickie, I.; Davenport, T.; Wakefield, D.; Vollmer-Conna, U.; Cameron, B.; Vernon, S.D.; Reeves, W.C.; Lloyd, A. Post-Infective and Chronic Fatigue Syndromes Precipitated by Viral and Non-Viral Pathogens: Prospective Cohort Study. BMJ 2006, 333, 575. [Google Scholar] [CrossRef]
- Tryfonos, A.; Pourhamidi, K.; Jörnåker, G.; Engvall, M.; Eriksson, L.; Elhallos, S.; Asplund, N.; Mandić, M.; Sundblad, P.; Sepic, A.; et al. Functional Limitations and Exercise Intolerance in Patients With Post-COVID Condition: A Randomized Crossover Clinical Trial. JAMA Netw. Open 2024, 7, e244386. [Google Scholar] [CrossRef]
- Zuo, Y.; Yalavarthi, S.; Shi, H.; Gockman, K.; Zuo, M.; Madison, J.A.; Blair, C.N.; Weber, A.; Barnes, B.J.; Egeblad, M.; et al. Neutrophil Extracellular Traps in COVID-19. JCI Insight 2020, 5, e138999. [Google Scholar] [CrossRef]
- Mukherjee, A.; Ghosh, K.K.; Chakrabortty, S.; Gulyás, B.; Padmanabhan, P.; Ball, W.B. Mitochondrial Reactive Oxygen Species in Infection and Immunity. Biomolecules 2024, 14, 670. [Google Scholar] [CrossRef]
- Pérez, S.E.; Gooz, M.; Maldonado, E.N. Mitochondrial Dysfunction and Metabolic Disturbances Induced by Viral Infections. Cells 2024, 13, 1789. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Liu, R.; Lan, W.; Liu, S. The Essential Role of Mitochondrial Dynamics in Viral Infections. Int. J. Mol. Sci. 2025, 26, 1955. [Google Scholar] [CrossRef]
- Purandare, N.; Ghosalkar, E.; Grossman, L.I.; Aras, S. Mitochondrial Oxidative Phosphorylation in Viral Infections. Viruses 2023, 15, 2380. [Google Scholar] [CrossRef]
- Wanderoy, S.; Hees, J.T.; Klesse, R.; Edlich, F.; Harbauer, A.B. Kill One or Kill the Many: Interplay between Mitophagy and Apoptosis. Biol. Chem. 2020, 402, 73–88. [Google Scholar] [CrossRef]
- Hung, C.-M.; Lombardo, P.S.; Malik, N.; Brun, S.N.; Hellberg, K.; Van Nostrand, J.L.; Garcia, D.; Baumgart, J.; Diffenderfer, K.; Asara, J.M.; et al. AMPK/ULK1-Mediated Phosphorylation of Parkin ACT Domain Mediates an Early Step in Mitophagy. Sci. Adv. 2021, 7, eabg4544. [Google Scholar] [CrossRef] [PubMed]
- Changaei, M.; Azimzadeh Tabrizi, Z.; Karimi, M.; Kashfi, S.A.; Koochaki Chahardeh, T.; Hashemi, S.M.; Soudi, S. From Powerhouse to Modulator: Regulating Immune System Responses through Intracellular Mitochondrial Transfer. Cell Commun. Signal. 2025, 23, 232. [Google Scholar] [CrossRef] [PubMed]
- Brand, M.D. Mitochondrial Generation of Superoxide and Hydrogen Peroxide as the Source of Mitochondrial Redox Signaling. Free. Radic. Biol. Med. 2016, 100, 14–31. [Google Scholar] [CrossRef]
- Fleury, C.; Mignotte, B.; Vayssière, J.-L. Mitochondrial Reactive Oxygen Species in Cell Death Signaling. Biochimie 2002, 84, 131–141. [Google Scholar] [CrossRef]
- Fan, P.; Xie, X.-H.; Chen, C.-H.; Peng, X.; Zhang, P.; Yang, C.; Wang, Y.-T. Molecular Regulation Mechanisms and Interactions Between Reactive Oxygen Species and Mitophagy. DNA Cell Biol. 2019, 38, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Checa, J.; Aran, J.M. Reactive Oxygen Species: Drivers of Physiological and Pathological Processes. J. Inflamm. Res. 2020, 13, 1057–1073. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wang, Y.; Zhang, J.; Hu, C.; Jiang, J.; Li, Y.; Peng, Z. ROS-Induced Lipid Peroxidation Modulates Cell Death Outcome: Mechanisms behind Apoptosis, Autophagy, and Ferroptosis. Arch. Toxicol. 2023, 97, 1439–1451. [Google Scholar] [CrossRef] [PubMed]
- Silwal, P.; Kim, J.K.; Kim, Y.J.; Jo, E.-K. Mitochondrial Reactive Oxygen Species: Double-Edged Weapon in Host Defense and Pathological Inflammation During Infection. Front. Immunol. 2020, 11, 1649. [Google Scholar] [CrossRef]
- Ward, C.; Schlichtholz, B. Post-Acute Sequelae and Mitochondrial Aberration in SARS-CoV-2 Infection. Int. J. Mol. Sci. 2024, 25, 9050. [Google Scholar] [CrossRef]
- Crook, H.; Raza, S.; Nowell, J.; Young, M.; Edison, P. Long COVID—Mechanisms, Risk Factors, and Management. BMJ 2021, 374, n1648. [Google Scholar] [CrossRef]
- Molnar, T.; Lehoczki, A.; Fekete, M.; Varnai, R.; Zavori, L.; Erdo-Bonyar, S.; Simon, D.; Berki, T.; Csecsei, P.; Ezer, E. Mitochondrial Dysfunction in Long COVID: Mechanisms, Consequences, and Potential Therapeutic Approaches. GeroScience 2024, 46, 5267–5286. [Google Scholar] [CrossRef]
- Park, E.-S.; Shin, C.Y.; Jeon, S.J.; Ham, B.-J. Is There Such a Thing as Post-Viral Depression?: Implications for Precision Medicine. Biomol. Ther. 2024, 32, 659–684. [Google Scholar] [CrossRef]
- Cornwell, W.K.; Levine, B.D.; Baptiste, D.; Bhave, N.; Desai, S.; Dineen, E.; Durstenfeld, M.; Edward, J.; Huang, M.; Jacobsen, R.; et al. Exercise Intolerance and Response to Training in Patients With Postacute Sequelae of SARS-CoV2 (Long COVID): A Scientific Statement From the American Heart Association. Circulation 2025, 152, e50–e62. [Google Scholar] [CrossRef]
- Halma, M.; Varon, J. Breaking the Silence: Recognizing Post-Vaccination Syndrome. Heliyon 2025, 11, e43478. [Google Scholar] [CrossRef]
- Gay, L.; Desquiret-Dumas, V.; Nagot, N.; Rapenne, C.; Van De Perre, P.; Reynier, P.; Molès, J. Long-term Persistence of Mitochondrial Dysfunctions after Viral Infections and Antiviral Therapies: A Review of Mechanisms Involved. J. Med. Virol. 2024, 96, e29886. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.-S.; Qi, H.-Y.; Boularan, C.; Huang, N.-N.; Abu-Asab, M.; Shelhamer, J.H.; Kehrl, J.H. SARS-Coronavirus Open Reading Frame-9b Suppresses Innate Immunity by Targeting Mitochondria and the MAVS/TRAF3/TRAF6 Signalosome. J. Immunol. Baltim. Md 1950 2014, 193, 3080–3089. [Google Scholar] [CrossRef]
- Moriyama, M.; Koshiba, T.; Ichinohe, T. Influenza A Virus M2 Protein Triggers Mitochondrial DNA-Mediated Antiviral Immune Responses. Nat. Commun. 2019, 10, 4624. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, C.; Wang, Y.; Wei, F.; Cai, Q. KSHV Reprogramming of Host Energy Metabolism for Pathogenesis. Front. Cell. Infect. Microbiol. 2021, 11, 621156. [Google Scholar] [CrossRef] [PubMed]
- Krumholz, H.M.; Sawano, M.; Wu, Y.; Shah, R.; Zhou, T.; Arun, A.S.; Khosla, P.; Kaleem, S.; Vashist, A.; Bhattacharjee, B.; et al. Comparative Analysis of Long COVID and Post-Vaccination Syndrome: A Cross-Sectional Study of Clinical Symptoms and Machine Learning-Based Differentiation. medRix 2025. [Google Scholar] [CrossRef]
- Fatima, S.; Ismail, M.; Ejaz, T.; Shah, Z.; Fatima, S.; Shahzaib, M.; Jafri, H.M. Association between Long COVID and Vaccination: A 12-Month Follow-up Study in a Low- to Middle-Income Country. PLoS ONE 2023, 18, e0294780. [Google Scholar] [CrossRef] [PubMed]
- Anderer, S. Vaccines Lowered Risk of Long COVID in US Veterans, but Not Completely. JAMA 2024, 332, 781. [Google Scholar] [CrossRef]
- Hedberg, P.; Van Der Werff, S.D.; Nauclér, P. The Effect of COVID-19 Vaccination on the Risk of Persistent Post–COVID-19 Condition: Cohort Study. J. Infect. Dis. 2025, 231, e941–e944. [Google Scholar] [CrossRef]
- Notarte, K.I.; Catahay, J.A.; Velasco, J.V.; Pastrana, A.; Ver, A.T.; Pangilinan, F.C.; Peligro, P.J.; Casimiro, M.; Guerrero, J.J.; Gellaco, M.M.L.; et al. Impact of COVID-19 Vaccination on the Risk of Developing Long-COVID and on Existing Long-COVID Symptoms: A Systematic Review. eClinicalMedicine 2022, 53, 101624. [Google Scholar] [CrossRef] [PubMed]
- Strain, W.D.; Sherwood, O.; Banerjee, A.; Van Der Togt, V.; Hishmeh, L.; Rossman, J. The Impact of COVID Vaccination on Symptoms of Long COVID: An International Survey of People with Lived Experience of Long COVID. Vaccines 2022, 10, 652. [Google Scholar] [CrossRef]
- Grady, C.B.; Bhattacharjee, B.; Silva, J.; Jaycox, J.; Lee, L.W.; Silva Monteiro, V.; Sawano, M.; Massey, D.; Caraballo, C.; Gehlhausen, J.R.; et al. Impact of COVID-19 Vaccination on Symptoms and Immune Phenotypes in Vaccine-Naïve Individuals with Long COVID. Commun. Med. 2025, 5, 163. [Google Scholar] [CrossRef]
- Chow, N.K.N.; Tsang, C.Y.W.; Chan, Y.H.; Telaga, S.A.; Ng, L.Y.A.; Chung, C.M.; Yip, Y.M.; Cheung, P.P.-H. The Effect of Pre-COVID and Post-COVID Vaccination on Long COVID: A Systematic Review and Meta-Analysis. J. Infect. 2024, 89, 106358. [Google Scholar] [CrossRef]
- Asadi-Pooya, A.A.; Nemati, M.; Shahisavandi, M.; Nemati, H.; Karimi, A.; Jafari, A.; Nasiri, S.; Mohammadi, S.S.; Rahimian, Z.; Bayat, H.; et al. How Does COVID-19 Vaccination Affect Long-COVID Symptoms? PLoS ONE 2024, 19, e0296680. [Google Scholar] [CrossRef]
- Quach, T.C.; Miglis, M.G.; Tian, L.; Bonilla, H.; Yang, P.C.; Grossman, L.; Paleru, A.; Xin, V.; Tiwari, A.; Shafer, R.W.; et al. Post-COVID-19 Vaccination and Long COVID: Insights from Patient-Reported Data. Vaccines 2024, 12, 1427. [Google Scholar] [CrossRef]
- Halma, M.; Vottero, P.; Thorp, J.; Peers, T.; Tuszynski, J.; Marik, P. The Possible Mechanistic Basis of Individual Susceptibility to Spike Protein Injury. Adv. Virol. 2025, 2025, 7990876. [Google Scholar] [CrossRef] [PubMed]
- Selvakumar, J.; Havdal, L.B.; Drevvatne, M.; Brodwall, E.M.; Lund Berven, L.; Stiansen-Sonerud, T.; Einvik, G.; Leegaard, T.M.; Tjade, T.; Michelsen, A.E.; et al. Prevalence and Characteristics Associated With Post-COVID-19 Condition Among Nonhospitalized Adolescents and Young Adults. JAMA Netw. Open 2023, 6, e235763. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Chiu, C.-H.; Chen, C.-J. Neurological and Psychiatric Aspects of Long COVID among Vaccinated Healthcare Workers: An Assessment of Prevalence and Reporting Biases. J. Microbiol. Immunol. Infect. 2025, S1684-1182(25)00125-2. [Google Scholar] [CrossRef]
- Platschek, B.; Boege, F. The Post-Acute COVID-19-Vaccination Syndrome in the Light of Pharmacovigilance. Vaccines 2024, 12, 1378. [Google Scholar] [CrossRef] [PubMed]
- Scholkmann, F.; May, C.-A. COVID-19, Post-Acute COVID-19 Syndrome (PACS, “Long COVID”) and Post-COVID-19 Vaccination Syndrome (PCVS, “Post-COVIDvac-Syndrome”): Similarities and Differences. Pathol. Res. Pract. 2023, 246, 154497. [Google Scholar] [CrossRef]
- Büchner, R.; Sander, C.; Schindler, S.; Walter, M.; Scheibenbogen, C.; Schomerus, G. “Have You Considered That It Could Be Burnout?”—Psychologization and Stigmatization of Self-Reported Long COVID or Post-COVID-19 Vaccination Syndrome. BMC Med. 2025, 23, 488. [Google Scholar] [CrossRef]
- Halma, M.; Varon, J. Assessing Barriers to Care for the Vaccine Injured: The Vaccination Injury Treatment and Access to Essential Care (VITAE) Survey. Preprint 2025. [Google Scholar] [CrossRef]
- Zhao, J.; Li, J.; Li, G.; Chen, M. The Role of Mitochondria-Associated Membranes Mediated ROS on NLRP3 Inflammasome in Cardiovascular Diseases. Front. Cardiovasc. Med. 2022, 9, 1059576. [Google Scholar] [CrossRef]
- Martemucci, G.; Costagliola, C.; Mariano, M.; D’andrea, L.; Napolitano, P.; D’Alessandro, A.G. Free Radical Properties, Source and Targets, Antioxidant Consumption and Health. Oxygen 2022, 2, 48–78. [Google Scholar] [CrossRef]
- Nathan, C.; Cunningham-Bussel, A. Beyond Oxidative Stress: An Immunologist’s Guide to Reactive Oxygen Species. Nat. Rev. Immunol. 2013, 13, 349–361. [Google Scholar] [CrossRef]
- Canton, M.; Sánchez-Rodríguez, R.; Spera, I.; Venegas, F.C.; Favia, M.; Viola, A.; Castegna, A. Reactive Oxygen Species in Macrophages: Sources and Targets. Front. Immunol. 2021, 12, 734229. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.; Herb, M.; Schramm, M.; Langmann, T. The TSPO-NOX1 Axis Controls Phagocyte-Triggered Pathological Angiogenesis in the Eye. Nat. Commun. 2020, 11, 2709. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.F.; Qiao, M.; Schröder, K.; Zhao, Q.; Asmis, R. Nox4 Is a Novel Inducible Source of Reactive Oxygen Species in Monocytes and Macrophages and Mediates Oxidized Low Density Lipoprotein-Induced Macrophage Death. Circ. Res. 2010, 106, 1489–1497. [Google Scholar] [CrossRef] [PubMed]
- Casey, A.M.; Ryan, D.G.; Prag, H.A.; Chowdhury, S.R.; Marques, E.; Turner, K.; Gruszczyk, A.V.; Yang, M.; Wolf, D.M.; Miljkovic, J.L.; et al. Pro-Inflammatory Macrophages Produce Mitochondria-Derived Superoxide by Reverse Electron Transport at Complex I That Regulates IL-1β Release during NLRP3 Inflammasome Activation. Nat. Metab. 2025, 7, 493–507. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Rodríguez, R.; Munari, F.; Angioni, R.; Venegas, F.; Agnellini, A.; Castro-Gil, M.P.; Castegna, A.; Luisetto, R.; Viola, A.; Canton, M. Targeting Monoamine Oxidase to Dampen NLRP3 Inflammasome Activation in Inflammation. Cell. Mol. Immunol. 2021, 18, 1311–1313. [Google Scholar] [CrossRef]
- Di Lisa, F.; Giorgio, M.; Ferdinandy, P.; Schulz, R. New Aspects of p66Shc in Ischaemia Reperfusion Injury and Other Cardiovascular Diseases. Br. J. Pharmacol. 2017, 174, 1690–1703. [Google Scholar] [CrossRef]
- Bedard, K.; Krause, K.-H. The NOX Family of ROS-Generating NADPH Oxidases: Physiology and Pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef]
- Vendrov, A.E.; Lozhkin, A.; Hayami, T.; Levin, J.; Silveira Fernandes Chamon, J.; Abdel-Latif, A.; Runge, M.S.; Madamanchi, N.R. Mitochondrial Dysfunction and Metabolic Reprogramming Induce Macrophage Pro-Inflammatory Phenotype Switch and Atherosclerosis Progression in Aging. Front. Immunol. 2024, 15, 1410832. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, M.; Huang, W.; Chen, W.; Zhao, Y.; Schulte, M.L.; Volberding, P.; Gerbec, Z.; Zimmermann, M.T.; Zeighami, A.; et al. Mitochondrial Metabolic Reprogramming by CD36 Signaling Drives Macrophage Inflammatory Responses. Circ. Res. 2019, 125, 1087–1102. [Google Scholar] [CrossRef]
- Elesela, S.; Lukacs, N.W. Role of Mitochondria in Viral Infections. Life 2021, 11, 232. [Google Scholar] [CrossRef]
- Sun, X.; Sun, L.; Zhao, Y.; Li, Y.; Lin, W.; Chen, D.; Sun, Q. MAVS Maintains Mitochondrial Homeostasis via Autophagy. Cell Discov. 2016, 2, 16024. [Google Scholar] [CrossRef]
- Sharma, A.; Kontodimas, K.; Bosmann, M. The MAVS Immune Recognition Pathway in Viral Infection and Sepsis. Antioxid. Redox Signal. 2021, 35, 1376–1392. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhou, Y.; Zhou, X. The Role of Mitophagy in Innate Immune Responses Triggered by Mitochondrial Stress. Cell Commun. Signal. 2020, 18, 186. [Google Scholar] [CrossRef] [PubMed]
- Buskiewicz, I.A.; Montgomery, T.; Yasewicz, E.C.; Huber, S.A.; Murphy, M.P.; Hartley, R.C.; Kelly, R.; Crow, M.K.; Perl, A.; Budd, R.C.; et al. Reactive Oxygen Species Induce Virus-Independent MAVS Oligomerization in Systemic Lupus Erythematosus. Sci. Signal. 2016, 9, ra115. [Google Scholar] [CrossRef]
- Fu, W.; Liu, Y.; Yin, H. Mitochondrial Dynamics: Biogenesis, Fission, Fusion, and Mitophagy in the Regulation of Stem Cell Behaviors. Stem Cells Int. 2019, 2019, 9757201. [Google Scholar] [CrossRef]
- Ni, H.-M.; Williams, J.A.; Ding, W.-X. Mitochondrial Dynamics and Mitochondrial Quality Control. Redox Biol. 2015, 4, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Youle, R.J.; van der Bliek, A.M. Mitochondrial Fission, Fusion, and Stress. Science 2012, 337, 1062–1065. [Google Scholar] [CrossRef]
- Zanfardino, P.; Amati, A.; Perrone, M.; Petruzzella, V. The Balance of MFN2 and OPA1 in Mitochondrial Dynamics, Cellular Homeostasis, and Disease. Biomolecules 2025, 15, 433. [Google Scholar] [CrossRef]
- Zerihun, M.; Sukumaran, S.; Qvit, N. The Drp1-Mediated Mitochondrial Fission Protein Interactome as an Emerging Core Player in Mitochondrial Dynamics and Cardiovascular Disease Therapy. Int. J. Mol. Sci. 2023, 24, 5785. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.M.; Youle, R.J. PINK1- and Parkin-Mediated Mitophagy at a Glance. J. Cell Sci. 2012, 125, 795–799. [Google Scholar] [CrossRef]
- Gurung, P.; Lukens, J.R.; Kanneganti, T.-D. Mitochondria: Diversity in the Regulation of the NLRP3 Inflammasome. Trends Mol. Med. 2015, 21, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-J.; Yoon, J.-H.; Ryu, J.-H. Mitophagy: A Balance Regulator of NLRP3 Inflammasome Activation. BMB Rep. 2016, 49, 529–535. [Google Scholar] [CrossRef]
- Mouton-Liger, F.; Rosazza, T.; Sepulveda-Diaz, J.; Ieang, A.; Hassoun, S.-M.; Claire, E.; Mangone, G.; Brice, A.; Michel, P.P.; Corvol, J.-C.; et al. Parkin Deficiency Modulates NLRP3 Inflammasome Activation by Attenuating an A20-Dependent Negative Feedback Loop. Glia 2018, 66, 1736–1751. [Google Scholar] [CrossRef]
- Li, X.; Wu, K.; Zeng, S.; Zhao, F.; Fan, J.; Li, Z.; Yi, L.; Ding, H.; Zhao, M.; Fan, S.; et al. Viral Infection Modulates Mitochondrial Function. Int. J. Mol. Sci. 2021, 22, 4260. [Google Scholar] [CrossRef]
- Singh, S.; Dirani, K.; Kumar, A. Intricacy of Mitochondrial Dynamics and Antiviral Response During RNA Virus Infection. Front. Virol. 2022, 2, 918806. [Google Scholar] [CrossRef]
- Madsen, H.B.; Durhuus, J.A.; Andersen, O.; Straten, P.T.; Rahbech, A.; Desler, C. Mitochondrial Dysfunction in Acute and Post-Acute Phases of COVID-19 and Risk of Non-Communicable Diseases. Npj Metab. Health Dis. 2024, 2, 36. [Google Scholar] [CrossRef]
- Li, X.; Hou, P.; Ma, W.; Wang, X.; Wang, H.; Yu, Z.; Chang, H.; Wang, T.; Jin, S.; Wang, X.; et al. SARS-CoV-2 ORF10 Suppresses the Antiviral Innate Immune Response by Degrading MAVS through Mitophagy. Cell. Mol. Immunol. 2022, 19, 67–78. [Google Scholar] [CrossRef]
- Rai, P.; Fessler, M.B. The MAVS and MAV-Nots: PINK1 Clears Prion-like MAVS Aggregates to Extinguish Mitochondrial Inflammatory Signaling. Am. J. Respir. Cell Mol. Biol. 2021, 64, 528–530. [Google Scholar] [CrossRef]
- Kim, S.-H.; Shin, H.J.; Yoon, C.M.; Lee, S.W.; Sharma, L.; Dela Cruz, C.S.; Kang, M.-J. PINK1 Inhibits Multimeric Aggregation and Signaling of MAVS and MAVS-Dependent Lung Pathology. Am. J. Respir. Cell Mol. Biol. 2021, 64, 592–603. [Google Scholar] [CrossRef] [PubMed]
- Frallonardo, L.; Segala, F.V.; Chhaganlal, K.D.; Yelshazly, M.; Novara, R.; Cotugno, S.; Guido, G.; Papagni, R.; Colpani, A.; De Vito, A.; et al. Incidence and Burden of Long COVID in Africa: A Systematic Review and Meta-Analysis. Sci. Rep. 2023, 13, 21482. [Google Scholar] [CrossRef]
- Chilunga, F.P.; Appelman, B.; Van Vugt, M.; Kalverda, K.; Smeele, P.; Van Es, J.; Wiersinga, W.J.; Rostila, M.; Prins, M.; Stronks, K.; et al. Differences in Incidence, Nature of Symptoms, and Duration of Long COVID among Hospitalised Migrant and Non-Migrant Patients in the Netherlands: A Retrospective Cohort Study. Lancet Reg. Health—Eur. 2023, 29, 100630. [Google Scholar] [CrossRef]
- Qi, C.; Osborne, T.; Bailey, R.; Cooper, A.; Hollinghurst, J.P.; Akbari, A.; Crowder, R.; Peters, H.; Law, R.-J.; Lewis, R.; et al. Impact of COVID-19 Pandemic on Incidence of Long-Term Conditions in Wales: A Population Data Linkage Study Using Primary and Secondary Care Health Records. Br. J. Gen. Pract. 2023, 73, e332–e339. [Google Scholar] [CrossRef]
- Sedgley, R.; Winer-Jones, J.; Bonafede, M. Long COVID Incidence in a Large US Ambulatory Electronic Health Record System. Am. J. Epidemiol. 2023, 192, 1350–1357. [Google Scholar] [CrossRef]
- Bhowal, C.; Ghosh, S.; Ghatak, D.; De, R. Pathophysiological Involvement of Host Mitochondria in SARS-CoV-2 Infection That Causes COVID-19: A Comprehensive Evidential Insight. Mol. Cell. Biochem. 2023, 478, 1325–1343. [Google Scholar] [CrossRef] [PubMed]
- Guarnieri, J.W.; Lie, T.; Albrecht, Y.E.S.; Hewin, P.; Jurado, K.A.; Widjaja, G.A.; Zhu, Y.; McManus, M.J.; Kilbaugh, T.J.; Keith, K.; et al. Mitochondrial Antioxidants Abate SARS-COV-2 Pathology in Mice. Proc. Natl. Acad. Sci. USA 2024, 121, e2321972121. [Google Scholar] [CrossRef]
- Kern, D.M.; Sorum, B.; Mali, S.S.; Hoel, C.M.; Sridharan, S.; Remis, J.P.; Toso, D.B.; Kotecha, A.; Bautista, D.M.; Brohawn, S.G. Cryo-EM Structure of SARS-CoV-2 ORF3a in Lipid Nanodiscs. Nat. Struct. Mol. Biol. 2021, 28, 573–582. [Google Scholar] [CrossRef]
- Jiao, S.; Miranda, P.; Li, Y.; Maric, D.; Holmgren, M. Some Aspects of the Life of SARS-CoV-2 ORF3a Protein in Mammalian Cells. Heliyon 2023, 9, e18754. [Google Scholar] [CrossRef] [PubMed]
- Prasada Kabekkodu, S.; Chakrabarty, S.; Jayaram, P.; Mallya, S.; Thangaraj, K.; Singh, K.K.; Satyamoorthy, K. Severe Acute Respiratory Syndrome Coronaviruses Contributing to Mitochondrial Dysfunction: Implications for Post-COVID Complications. Mitochondrion 2023, 69, 43–56. [Google Scholar] [CrossRef]
- Qudus, M.S.; Afaq, U.; Liu, S.; Wu, K.; Yu, C.; Tian, M.; Wu, J. SARS-CoV-2-ORF-3a Mediates Apoptosis Through Mitochondrial Dysfunction Modulated by the K+ Ion Channel. Int. J. Mol. Sci. 2025, 26, 1575. [Google Scholar] [CrossRef]
- Bignon, E.; Marazzi, M.; Monari, A. Hijacking of Cellular Functions by Severe Acute Respiratory Syndrome Coronavirus-2. Permeabilization and Polarization of the Host Lipid Membrane by Viroporins. J. Phys. Chem. Lett. 2022, 13, 4642–4649. [Google Scholar] [CrossRef] [PubMed]
- Poggio, E.; Vallese, F.; Hartel, A.J.W.; Morgenstern, T.J.; Kanner, S.A.; Rauh, O.; Giamogante, F.; Barazzuol, L.; Shepard, K.L.; Colecraft, H.M.; et al. Perturbation of the Host Cell Ca2+ Homeostasis and ER-Mitochondria Contact Sites by the SARS-CoV-2 Structural Proteins E and M. Cell Death Dis. 2023, 14, 297. [Google Scholar] [CrossRef]
- Sala, C.; Ninu, A.; Balducci, V.; Allegro, G.; Montalbano, A.; Lulli, M.; Boccitto, M.L.; Guzzolino, E.; Spinelli, V.; Arcangeli, A.; et al. Stable Expression of SARS-CoV-2 Envelope Viroporin Promotes Intracellular Calcium Depletion in Human Cells: Relevance for Endoplasmic Reticulum Stress, Cell Proliferation, Pluripotency and Lineage Differentiation. Cell Calcium 2025, 128, 103032. [Google Scholar] [CrossRef] [PubMed]
- Berta, B.; Tordai, H.; Lukács, G.L.; Papp, B.; Enyedi, Á.; Padányi, R.; Hegedűs, T. SARS-CoV-2 Envelope Protein Alters Calcium Signaling via SERCA Interactions. Sci. Rep. 2024, 14, 21200. [Google Scholar] [CrossRef]
- López-Ayllón, B.D.; Marin, S.; Fernández, M.F.; García-García, T.; Fernández-Rodríguez, R.; de Lucas-Rius, A.; Redondo, N.; Mendoza-García, L.; Foguet, C.; Grigas, J.; et al. Metabolic and Mitochondria Alterations Induced by SARS-CoV-2 Accessory Proteins ORF3a, ORF9b, ORF9c and ORF10. J. Med. Virol. 2024, 96, e29752. [Google Scholar] [CrossRef]
- De Angelis, M.; Anichini, G.; Palamara, A.T.; Nencioni, L.; Gori Savellini, G. Dysregulation of Intracellular Redox Homeostasis by the SARS-CoV-2 ORF6 Protein. Virol. J. 2023, 20, 239. [Google Scholar] [CrossRef]
- Yue, M.; Hu, B.; Li, J.; Chen, R.; Yuan, Z.; Xiao, H.; Chang, H.; Jiu, Y.; Cai, K.; Ding, B. Coronaviral ORF6 Protein Mediates Inter-Organelle Contacts and Modulates Host Cell Lipid Flux for Virus Production. EMBO J. 2023, 42, e112542. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.-H.; Jeng, T.-H.; Lee, M.-Y.; Wang, H.-C.; Tsai, K.-F.; Chou, C.-K. Viral Mitochondriopathy in COVID-19. Redox Biol. 2025, 85, 103766. [Google Scholar] [CrossRef]
- Zandi, M.; Shafaati, M.; Kalantar-Neyestanaki, D.; Pourghadamyari, H.; Fani, M.; Soltani, S.; Kaleji, H.; Abbasi, S. The Role of SARS-CoV-2 Accessory Proteins in Immune Evasion. Biomed. Pharmacother. Biomed. Pharmacother. 2022, 156, 113889. [Google Scholar] [CrossRef] [PubMed]
- Mozzi, A.; Oldani, M.; Forcella, M.E.; Vantaggiato, C.; Cappelletti, G.; Pontremoli, C.; Valenti, F.; Forni, D.; Saresella, M.; Biasin, M.; et al. SARS-CoV-2 ORF3c Impairs Mitochondrial Respiratory Metabolism, Oxidative Stress, and Autophagic Flux. iScience 2023, 26, 107118. [Google Scholar] [CrossRef]
- García-García, T.; Fernández-Rodríguez, R.; Redondo, N.; de Lucas-Rius, A.; Zaldívar-López, S.; López-Ayllón, B.D.; Suárez-Cárdenas, J.M.; Jiménez-Marín, Á.; Montoya, M.; Garrido, J.J. Impairment of Antiviral Immune Response and Disruption of Cellular Functions by SARS-CoV-2 ORF7a and ORF7b. iScience 2022, 25, 105444. [Google Scholar] [CrossRef]
- Xiao, X.; Fu, Y.; You, W.; Huang, C.; Zeng, F.; Gu, X.; Sun, X.; Li, J.; Zhang, Q.; Du, W.; et al. Inhibition of the RLR Signaling Pathway by SARS-CoV-2 ORF7b Is Mediated by MAVS and Abrogated by ORF7b-Homologous Interfering Peptide. J. Virol. 2024, 98, e0157323. [Google Scholar] [CrossRef]
- Stukalov, A.; Girault, V.; Grass, V.; Karayel, O.; Bergant, V.; Urban, C.; Haas, D.A.; Huang, Y.; Oubraham, L.; Wang, A.; et al. Multilevel Proteomics Reveals Host Perturbations by SARS-CoV-2 and SARS-CoV. Nature 2021, 594, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Zandi, M. ORF9c and ORF10 as Accessory Proteins of SARS-CoV-2 in Immune Evasion. Nat. Rev. Immunol. 2022, 22, 331. [Google Scholar] [CrossRef]
- Faizan, M.I.; Chaudhuri, R.; Sagar, S.; Albogami, S.; Chaudhary, N.; Azmi, I.; Akhtar, A.; Ali, S.M.; Kumar, R.; Iqbal, J.; et al. NSP4 and ORF9b of SARS-CoV-2 Induce Pro-Inflammatory Mitochondrial DNA Release in Inner Membrane-Derived Vesicles. Cells 2022, 11, 2969. [Google Scholar] [CrossRef]
- Zong, S.; Wu, Y.; Li, W.; You, Q.; Peng, Q.; Wang, C.; Wan, P.; Bai, T.; Ma, Y.; Sun, B.; et al. SARS-CoV-2 Nsp8 Induces Mitophagy by Damaging Mitochondria. Virol. Sin. 2023, 38, 520–530. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Z.; Pan, T.; Sun, Q.; Chen, Q.; Wang, P.-H.; Li, X.; Kuang, E. SARS-CoV-2 Nsp8 Suppresses MDA5 Antiviral Immune Responses by Impairing TRIM4-Mediated K63-Linked Polyubiquitination. PLOS Pathog. 2023, 19, e1011792. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhuang, M.-W.; Han, L.; Zhang, J.; Nan, M.-L.; Zhan, P.; Kang, D.; Liu, X.; Gao, C.; Wang, P.-H. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Membrane (M) Protein Inhibits Type I and III Interferon Production by Targeting RIG-I/MDA-5 Signaling. Signal Transduct. Target. Ther. 2020, 5, 299. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, Y.; Meng, X.; Wang, Z.; Younis, M.; Liu, Y.; Wang, P.; Huang, X. SARS-CoV-2 Membrane Protein Causes the Mitochondrial Apoptosis and Pulmonary Edema via Targeting BOK. Cell Death Differ. 2022, 29, 1395–1408. [Google Scholar] [CrossRef]
- Shin, H.J.; Lee, W.; Ku, K.B.; Yoon, G.Y.; Moon, H.-W.; Kim, C.; Kim, M.-H.; Yi, Y.-S.; Jun, S.; Kim, B.-T.; et al. SARS-CoV-2 Aberrantly Elevates Mitochondrial Bioenergetics to Induce Robust Virus Propagation. Signal Transduct. Target. Ther. 2024, 9, 125. [Google Scholar] [CrossRef]
- Yu, H.; Yang, L.; Han, Z.; Zhou, X.; Zhang, Z.; Sun, T.; Zheng, F.; Yang, J.; Guan, F.; Xie, J.; et al. SARS-CoV-2 Nucleocapsid Protein Enhances the Level of Mitochondrial Reactive Oxygen Species. J. Med. Virol. 2023, 95, e29270. [Google Scholar] [CrossRef]
- Jayaraman, P.; Rajagopal, M.; Paranjpe, I.; Suarez-Farinas, M.; Liharska, L.E.; Thompson, R.C.; Del Valle, D.M.; Beckmann, N.D.; Lund, A.N.; Gownivaripally, P.; et al. Peripheral Transcriptomics in Acute and Long-Term Kidney Dysfunction in SARS-CoV-2 Infection. Kidney360 2025, 6, 921–936. [Google Scholar] [CrossRef]
- Millette, K.; Cuala, J.; Wang, P.; Marks, C.; Woo, V.; Hayun, M.; Kang, H.; Martin, M.; Dhawan, S.; Chao, L.; et al. SARS-CoV2 Infects Pancreatic Beta Cells in Vivo and Induces Cellular and Subcellular Disruptions That Reflect Beta Cell Dysfunction. Res. Sq. 2021. [Google Scholar] [CrossRef]
- Lawal, A.; Oluyede, D.; Olumegbon, L.; Adebimpe, M.; Crown, O.; Salako, E. Endothelial Dysfunction, Oxidative Stress and Inflammation: Implications in Atherogenesis, Cardiovascular Diseases and Gene Targeted Therapeutic Approach. J. Pharmacol. Toxicol. 2023, 18, 42–52. [Google Scholar] [CrossRef]
- Chang, R.; Mamun, A.; Dominic, A.; Le, N.-T. SARS-CoV-2 Mediated Endothelial Dysfunction: The Potential Role of Chronic Oxidative Stress. Front. Physiol. 2021, 11, 605908. [Google Scholar] [CrossRef] [PubMed]
- Gultom, M.; Lin, L.; Brandt, C.B.; Milusev, A.; Despont, A.; Shaw, J.; Döring, Y.; Luo, Y.; Rieben, R. Sustained Vascular Inflammatory Effects of SARS-CoV-2 Spike Protein on Human Endothelial Cells. Inflammation 2024, 48, 2531–2547. [Google Scholar] [CrossRef] [PubMed]
- Huynh, T.V.; Rethi, L.; Lee, T.-W.; Higa, S.; Kao, Y.-H.; Chen, Y.-J. Spike Protein Impairs Mitochondrial Function in Human Cardiomyocytes: Mechanisms Underlying Cardiac Injury in COVID-19. Cells 2023, 12, 877. [Google Scholar] [CrossRef]
- Van Tin, H.; Rethi, L.; Higa, S.; Kao, Y.-H.; Chen, Y.-J. Spike Protein of SARS-CoV-2 Activates Cardiac Fibrogenesis through NLRP3 Inflammasomes and NF-κB Signaling. Cells 2024, 13, 1331. [Google Scholar] [CrossRef]
- Mercado-Gómez, M.; Prieto-Fernández, E.; Goikoetxea-Usandizaga, N.; Vila-Vecilla, L.; Azkargorta, M.; Bravo, M.; Serrano-Maciá, M.; Egia-Mendikute, L.; Rodríguez-Agudo, R.; Lachiondo-Ortega, S.; et al. The Spike of SARS-CoV-2 Promotes Metabolic Rewiring in Hepatocytes. Commun. Biol. 2022, 5, 827. [Google Scholar] [CrossRef]
- De Lima, I.; De Menezes, D.; Uesugi, J.; Bichara, C.; Da Costa Vasconcelos, P.; Quaresma, J.; Falcão, L. Liver Function in Patients with Long-Term Coronavirus Disease 2019 of up to 20 Months: A Cross-Sectional Study. Int. J. Environ. Res. Public. Health 2023, 20, 5281. [Google Scholar] [CrossRef]
- Stasi, C. Post-COVID-19 Pandemic Sequelae in Liver Diseases. Life 2025, 15, 403. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, J.; Du, J.; Chen, S.; Chen, S.; Li, J.; Shen, B. Changes in Serum Liver Function for Patients with COVID-19: A 1-Year Follow-Up Study. Infect. Drug Resist. 2022, 15, 1857–1870. [Google Scholar] [CrossRef] [PubMed]
- Pita-Juarez, Y.; Karagkouni, D.; Kalavros, N.; Melms, J.C.; Niezen, S.; Delorey, T.M.; Essene, A.L.; Brook, O.R.; Pant, D.; Skelton-Badlani, D.; et al. A Single-Nucleus and Spatial Transcriptomic Atlas of the COVID-19 Liver Reveals Topological, Functional, and Regenerative Organ Disruption in Patients. Genome Biol. 2025, 26, 56. [Google Scholar] [CrossRef]
- Kaundal, R.K.; Kalvala, A.K.; Kumar, A. Neurological Implications of COVID-19: Role of Redox Imbalance and Mitochondrial Dysfunction. Mol. Neurobiol. 2021, 58, 4575–4587. [Google Scholar] [CrossRef]
- Thakur, A.; Sharma, V.; Averbek, S.; Liang, L.; Pandya, N.; Kumar, G.; Cili, A.; Zhang, K. Immune Landscape and Redox Imbalance during Neurological Disorders in COVID-19. Cell Death Dis. 2023, 14, 593. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, J.; Li, Y.; Zhao, Y.; Kuermanbayi, S.; Zhuang, J.; Zhang, H.; Xu, F.; Li, F. Matrix Stiffness-Dependent Microglia Activation in Response to Inflammatory Cues: In Situ Investigation by Scanning Electrochemical Microscopy. Chem. Sci. 2024, 15, 171–184. [Google Scholar] [CrossRef]
- Simpson, D.S.A.; Oliver, P.L. ROS Generation in Microglia: Understanding Oxidative Stress and Inflammation in Neurodegenerative Disease. Antioxidants 2020, 9, 743. [Google Scholar] [CrossRef]
- Laforge, M.; Elbim, C.; Frère, C.; Hémadi, M.; Massaad, C.; Nuss, P.; Benoliel, J.-J.; Becker, C. Tissue Damage from Neutrophil-Induced Oxidative Stress in COVID-19. Nat. Rev. Immunol. 2020, 20, 515–516. [Google Scholar] [CrossRef]
- Griendling, K.K.; Touyz, R.M.; Zweier, J.L.; Dikalov, S.; Chilian, W.; Chen, Y.-R.; Harrison, D.G.; Bhatnagar, A. Measurement of Reactive Oxygen Species, Reactive Nitrogen Species, and Redox-Dependent Signaling in the Cardiovascular System: A Scientific Statement From the American Heart Association. Circ. Res. 2016, 119, E39–E75. [Google Scholar] [CrossRef]
- Stufano, A.; Isgrò, C.; Palese, L.L.; Caretta, P.; De Maria, L.; Lovreglio, P.; Sardanelli, A.M. Oxidative Damage and Post-COVID Syndrome: A Cross-Sectional Study in a Cohort of Italian Workers. Int. J. Mol. Sci. 2023, 24, 7445. [Google Scholar] [CrossRef]
- Shankar, V.; Wilhelmy, J.; Curtis, E.J.; Michael, B.; Cervantes, L.; Mallajosyula, V.A.; Davis, R.W.; Snyder, M.; Younis, S.; Robinson, W.H.; et al. Oxidative Stress Is a Shared Characteristic of ME/CFS and Long COVID. J. Immunol. 2024, 212, 0664_6496. [Google Scholar] [CrossRef]
- Semo, D.; Shomanova, Z.; Sindermann, J.; Mohr, M.; Evers, G.; Motloch, L.J.; Reinecke, H.; Godfrey, R.; Pistulli, R. Persistent Monocytic Bioenergetic Impairment and Mitochondrial DNA Damage in PASC Patients with Cardiovascular Complications. Int. J. Mol. Sci. 2025, 26, 4562. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Cai, Q.; Zheng, P. Mitochondrial Metabolic Rescue in Post-COVID-19 Syndrome: MR Spectroscopy Insights and Precision Nutritional Therapeutics. Front. Immunol. 2025, 16, 1597370. [Google Scholar] [CrossRef]
- Tsilingiris, D.; Vallianou, N.G.; Karampela, I.; Christodoulatos, G.S.; Papavasileiou, G.; Petropoulou, D.; Magkos, F.; Dalamaga, M. Laboratory Findings and Biomarkers in Long COVID: What Do We Know So Far? Insights into Epidemiology, Pathogenesis, Therapeutic Perspectives and Challenges. Int. J. Mol. Sci. 2023, 24, 10458. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.S.; Mogensen, T.H.; Agergaard, J.; Schiøttz-Christensen, B.; Østergaard, L.; Vibholm, L.K.; Leth, S. High-Dose Coenzyme Q10 Therapy versus Placebo in Patients with Post COVID-19 Condition: A Randomized, Phase 2, Crossover Trial. Lancet Reg. Health—Eur. 2023, 24, 100539. [Google Scholar] [CrossRef]
- Tenório, M.C.D.S.; Graciliano, N.G.; Moura, F.A.; Oliveira, A.C.M.D.; Goulart, M.O.F. N-Acetylcysteine (NAC): Impacts on Human Health. Antioxidants 2021, 10, 967. [Google Scholar] [CrossRef]
- Mokra, D.; Porvaznik, I.; Mokry, J. N-Acetylcysteine in the Treatment of Acute Lung Injury: Perspectives and Limitations. Int. J. Mol. Sci. 2025, 26, 2657. [Google Scholar] [CrossRef]
- Postler, T.S.; Peng, V.; Bhatt, D.M.; Ghosh, S. Metformin Selectively Dampens the Acute Inflammatory Response through an AMPK-Dependent Mechanism. Sci. Rep. 2021, 11, 18721. [Google Scholar] [CrossRef]
- Bharath, L.P.; Nikolajczyk, B.S. The Intersection of Metformin and Inflammation. Am. J. Physiol.-Cell Physiol. 2021, 320, C873–C879. [Google Scholar] [CrossRef] [PubMed]
- Nicolson, G.L. Mitochondrial Dysfunction and Chronic Disease: Treatment With Natural Supplements. Integr. Med. Encinitas Calif. 2014, 13, 35–43. [Google Scholar]
- Mantle, D.; Hargreaves, I.P.; Domingo, J.C.; Castro-Marrero, J. Mitochondrial Dysfunction and Coenzyme Q10 Supplementation in Post-Viral Fatigue Syndrome: An Overview. Int. J. Mol. Sci. 2024, 25, 574. [Google Scholar] [CrossRef] [PubMed]
- Vial, G.; Detaille, D.; Guigas, B. Role of Mitochondria in the Mechanism(s) of Action of Metformin. Front. Endocrinol. 2019, 10, 294. [Google Scholar] [CrossRef]
- Reczek, C.R.; Chakrabarty, R.P.; D’Alessandro, K.B.; Sebo, Z.L.; Grant, R.A.; Gao, P.; Budinger, G.R.; Chandel, N.S. Metformin Targets Mitochondrial Complex I to Lower Blood Glucose Levels. Sci. Adv. 2024, 10, eads5466. [Google Scholar] [CrossRef]
- Evans, V.A.; O’Neill, L.A.J. Lessons from Glucocorticoids, Metformin, and Dimethyl Fumarate: Could Targeting Immunometabolism Lead to Better Anti-Inflammatory Therapies? Annu. Rev. Pharmacol. Toxicol. 2025, 66. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Guo, H.; Qiu, L.; Zhang, C.; Deng, Q.; Leng, Q. Immunomodulatory and Antiviral Activity of Metformin and Its Potential Implications in Treating Coronavirus Disease 2019 and Lung Injury. Front. Immunol. 2020, 11, 2056. [Google Scholar] [CrossRef]
- Izadpanah, A.; Mudd, J.C.; Garcia, J.G.N.; Srivastav, S.; Abdel-Mohsen, M.; Palmer, C.; Goldman, A.R.; Kolls, J.K.; Qin, X.; Rappaport, J. SARS-CoV-2 Infection Dysregulates NAD Metabolism. Front. Immunol. 2023, 14, 1158455. [Google Scholar] [CrossRef]
- Laguarta-Val, S.; Varillas-Delgado, D.; Lizcano-Álvarez, Á.; Molero-Sánchez, A.; Melian-Ortiz, A.; Cano-de-la-Cuerda, R.; Jiménez-Antona, C. Effects of Aerobic Exercise Therapy through Nordic Walking Program in Lactate Concentrations, Fatigue and Quality-of-Life in Patients with Long-COVID Syndrome: A Non-Randomized Parallel Controlled Trial. J. Clin. Med. 2024, 13, 1035. [Google Scholar] [CrossRef] [PubMed]
- Barletta, M.A.; Marino, G.; Spagnolo, B.; Bianchi, F.P.; Falappone, P.C.F.; Spagnolo, L.; Gatti, P. Coenzyme Q10 + Alpha Lipoic Acid for Chronic COVID Syndrome. Clin. Exp. Med. 2022, 23, 667–678. [Google Scholar] [CrossRef]
- Izzo, R.; Trimarco, V.; Mone, P.; Aloè, T.; Capra Marzani, M.; Diana, A.; Fazio, G.; Mallardo, M.; Maniscalco, M.; Marazzi, G.; et al. Combining L-Arginine with Vitamin C Improves Long-COVID Symptoms: The LINCOLN Survey. Pharmacol. Res. 2022, 183, 106360. [Google Scholar] [CrossRef] [PubMed]
- Bramante, C.T.; Beckman, K.B.; Mehta, T.; Karger, A.B.; Odde, D.J.; Tignanelli, C.J.; Buse, J.B.; Johnson, D.M.; Watson, R.H.B.; Daniel, J.J.; et al. Favorable Antiviral Effect of Metformin on SARS-CoV-2 Viral Load in a Randomized, Placebo-Controlled Clinical Trial of COVID-19. Clin. Infect. Dis. 2024, 79, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Atieh, O.; Daher, J.; Durieux, J.; Abboud, M.; Labbato, D.; Baissary, J.; Koberssy, Z.; Ailstock, K.; Cummings, M.; Funderburg, N.; et al. Vitamins K2 and D3 Improve Long COVID, Fungal Translocation, and Inflammation: Randomized Controlled Trial. Nutrients 2025, 17, 304. [Google Scholar] [CrossRef]
- Gaylis, N.B.; Kreychman, I.; Sagliani, J.; Mograbi, J.; Gabet, Y. The Results of a Unique Dietary Supplement (Nutraceutical Formulation) Used to Treat the Symptoms of Long-Haul COVID. Front. Nutr. 2022, 9, 1034169. [Google Scholar] [CrossRef]
- Slankamenac, J.; Ranisavljev, M.; Todorovic, N.; Ostojic, J.; Stajer, V.; Candow, D.G.; Ratgeber, L.; Betlehem, J.; Acs, P.; Ostojic, S.M. Eight-Week Creatine-Glucose Supplementation Alleviates Clinical Features of Long COVID. J. Nutr. Sci. Vitaminol. 2024, 70, 174–178. [Google Scholar] [CrossRef]
- Noce, A.; Marrone, G.; Di Lauro, M.; Vita, C.; Montalto, G.; Giorgino, G.; Chiaramonte, C.; D’Agostini, C.; Bernardini, S.; Pieri, M. Potential Anti-Inflammatory and Anti-Fatigue Effects of an Oral Food Supplement in Long COVID Patients. Pharmaceuticals 2024, 17, 463. [Google Scholar] [CrossRef]
- Charoenporn, V.; Tungsukruthai, P.; Teacharushatakit, P.; Hanvivattanakul, S.; Sriyakul, K.; Sukprasert, S.; Kamalashiran, C.; Tungsukruthai, S.; Charernboon, T. Effects of an 8-week High-dose Vitamin D Supplementation on Fatigue and Neuropsychiatric Manifestations in Post-COVID Syndrome: A Randomized Controlled Trial. Psychiatry Clin. Neurosci. 2024, 78, 595–604. [Google Scholar] [CrossRef]
- Graham, E.L.; Clark, J.R.; Orban, Z.S.; Lim, P.H.; Szymanski, A.L.; Taylor, C.; DiBiase, R.M.; Jia, D.T.; Balabanov, R.; Ho, S.U.; et al. Persistent Neurologic Symptoms and Cognitive Dysfunction in Non-Hospitalized COVID-19 “Long Haulers”. Ann. Clin. Transl. Neurol. 2021, 8, 1073–1085. [Google Scholar] [CrossRef]
- Kagan, V.E.; Wipf, P.; Stoyanovsky, D.; Greenberger, J.S.; Borisenko, G.; Belikova, N.A.; Yanamala, N.; Samhan Arias, A.K.; Tungekar, M.A.; Jiang, J.; et al. Mitochondrial Targeting of Electron Scavenging Antioxidants: Regulation of Selective Oxidation vs Random Chain Reactions. Adv. Drug Deliv. Rev. 2009, 61, 1375–1385. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, K.; Cheng, L.; Zhu, H.; Xu, T. Progress in Understanding the Molecular Mechanisms Underlying the Antitumour Effects of Ivermectin. Drug Des. Devel. Ther. 2020, 14, 285–296. [Google Scholar] [CrossRef]
- Bouayed, J.; Bohn, T. Exogenous Antioxidants--Double-Edged Swords in Cellular Redox State: Health Beneficial Effects at Physiologic Doses versus Deleterious Effects at High Doses. Oxid. Med. Cell. Longev. 2010, 3, 228–237. [Google Scholar] [CrossRef]
- Sotler, R.; Poljšak, B.; Dahmane, R.; Jukić, T.; Pavan Jukić, D.; Rotim, C.; Trebše, P.; Starc, A. Prooxidant Activities of Antioxidants and Their Impact on Health. Acta Clin. Croat. 2019, 58, 726–736. [Google Scholar] [CrossRef]
- Liu, Y.; Fang, S.; Sun, Q.; Liu, B. Anthelmintic Drug Ivermectin Inhibits Angiogenesis, Growth and Survival of Glioblastoma through Inducing Mitochondrial Dysfunction and Oxidative Stress. Biochem. Biophys. Res. Commun. 2016, 480, 415–421. [Google Scholar] [CrossRef]
- Wang, J.; Xu, Y.; Wan, H.; Hu, J. Antibiotic Ivermectin Selectively Induces Apoptosis in Chronic Myeloid Leukemia through Inducing Mitochondrial Dysfunction and Oxidative Stress. Biochem. Biophys. Res. Commun. 2018, 497, 241–247. [Google Scholar] [CrossRef]
- Abd-Elmawla, M.A.; Ghaiad, H.R.; Gad, E.S.; Ahmed, K.A.; Abdelmonem, M. Suppression of NLRP3 Inflammasome by Ivermectin Ameliorates Bleomycin-Induced Pulmonary Fibrosis. J. Zhejiang Univ. Sci. B 2023, 24, 723–733. [Google Scholar] [CrossRef]
- Habibi Razi, F.; Mohammad Jafari, R.; Manavi, M.A.; Sheibani, M.; Rashidian, A.; Tavangar, S.M.; Beighmohammadi, M.T.; Dehpour, A.R. Ivermectin Ameliorates Bleomycin-Induced Lung Fibrosis in Male Rats by Inhibiting the Inflammation and Oxidative Stress. Immunopharmacol. Immunotoxicol. 2024, 46, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Dibekoğlu, C.; Kemertaş, K.; Aygun, H.; Erbaş, O. Ivermectin Attenuates Methotrexate-Induced Liver Fibrosis by Reducing TGF-β and Syndecan-1 Expression. Med. Kaunas Lith. 2025, 61, 1036. [Google Scholar] [CrossRef] [PubMed]
- Seyyedabadi, B.; Babataheri, S.; Laher, I.; Soraya, H. Neuroprotective Effects of Ivermectin against Transient Cerebral Ischemia-Reperfusion in Rats. Metab. Brain Dis. 2023, 38, 2807–2815. [Google Scholar] [CrossRef]
- de Melo, G.D.; Lazarini, F.; Larrous, F.; Feige, L.; Kornobis, E.; Levallois, S.; Marchio, A.; Kergoat, L.; Hardy, D.; Cokelaer, T.; et al. Attenuation of Clinical and Immunological Outcomes during SARS-CoV-2 Infection by Ivermectin. EMBO Mol. Med. 2021, 13, e14122. [Google Scholar] [CrossRef]
- Farajpour, N.; Soraya, H. Neuroprotective Effects of Ivermectin on Alzheimer’s Model Induced by Streptozotocin in Rats. Neurodegener. Dis. Manag. 2025, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Poudel, S.B.; Frikha-Benayed, D.; Ruff, R.R.; Yildirim, G.; Dixit, M.; Korstanje, R.; Robinson, L.; Miller, R.A.; Harrison, D.E.; Strong, J.R.; et al. Targeting Mitochondrial Dysfunction Using Methylene Blue or Mitoquinone to Improve Skeletal Aging. Aging 2024, 16, 4948–4964. [Google Scholar] [CrossRef] [PubMed]
- Klosowski, E.M.; de Souza, B.T.L.; Mito, M.S.; Constantin, R.P.; Mantovanelli, G.C.; Mewes, J.M.; Bizerra, P.F.V.; Menezes, P.V.M.d.C.; Gilglioni, E.H.; Utsunomiya, K.S.; et al. The Photodynamic and Direct Actions of Methylene Blue on Mitochondrial Energy Metabolism: A Balance of the Useful and Harmful Effects of This Photosensitizer. Free. Radic. Biol. Med. 2020, 153, 34–53. [Google Scholar] [CrossRef] [PubMed]
- Atamna, H.; Nguyen, A.; Schultz, C.; Boyle, K.; Newberry, J.; Kato, H.; Ames, B.N. Methylene Blue Delays Cellular Senescence and Enhances Key Mitochondrial Biochemical Pathways. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2008, 22, 703–712. [Google Scholar] [CrossRef]
- Cagno, V.; Medaglia, C.; Cerny, A.; Cerny, T.; Zwygart, A.C.-A.; Cerny, E.; Tapparel, C. Methylene Blue Has a Potent Antiviral Activity against SARS-CoV-2 and H1N1 Influenza Virus in the Absence of UV-Activation in Vitro. Sci. Rep. 2021, 11, 14295. [Google Scholar] [CrossRef]
- Dibekoğlu, C.; Kemertaş, K.; Aygun, H.; Erbas, O. Methylene Blue Alleviates Inflammatory and Oxidative Lung Injury in a Rat Model of Feces-Induced Peritonitis. Medicina 2025, 61, 1456. [Google Scholar] [CrossRef]
- Wen, Y.; Li, W.; Poteet, E.C.; Xie, L.; Tan, C.; Yan, L.-J.; Ju, X.; Liu, R.; Qian, H.; Marvin, M.A.; et al. Alternative Mitochondrial Electron Transfer as a Novel Strategy for Neuroprotection. J. Biol. Chem. 2011, 286, 16504–16515. [Google Scholar] [CrossRef]
- Yang, S.-H.; Li, W.; Sumien, N.; Forster, M.; Simpkins, J.W.; Liu, R. Alternative Mitochondrial Electron Transfer for the Treatment of Neurodegenerative Diseases and Cancers: Methylene Blue Connects the Dots. Prog. Neurobiol. 2017, 157, 273–291. [Google Scholar] [CrossRef]
- Oz, M.; Lorke, D.E.; Hasan, M.; Petroianu, G.A. Cellular and Molecular Actions of Methylene Blue in the Nervous System. Med. Res. Rev. 2011, 31, 93–117. [Google Scholar] [CrossRef]
- Bulama, I.; Nasiru, S.; Bello, A.; Abbas, A.Y.; Nasiru, J.I.; Saidu, Y.; Chiroma, M.S.; Mohd Moklas, M.A.; Mat Taib, C.N.; Waziri, A.; et al. Antioxidant-Based Neuroprotective Effect of Dimethylsulfoxide against Induced Traumatic Brain Injury in a Rats Model. Front. Pharmacol. 2022, 13, 998179. [Google Scholar] [CrossRef] [PubMed]
- Sanmartín-Suárez, C.; Soto-Otero, R.; Sánchez-Sellero, I.; Méndez-Álvarez, E. Antioxidant Properties of Dimethyl Sulfoxide and Its Viability as a Solvent in the Evaluation of Neuroprotective Antioxidants. J. Pharmacol. Toxicol. Methods 2011, 63, 209–215. [Google Scholar] [CrossRef]
- Ahn, H.; Kim, J.; Jeung, E.-B.; Lee, G.-S. Dimethyl Sulfoxide Inhibits NLRP3 Inflammasome Activation. Immunobiology 2014, 219, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, S.; Simon, R.P.; Graham, S.H. Dimethylsulfoxide (DMSO) Treatment Reduces Infarction Volume after Permanent Focal Cerebral Ischemia in Rats. Neurosci. Lett. 1997, 239, 125–127. [Google Scholar] [CrossRef] [PubMed]
- Krumholz, H.M.; Wu, Y.; Sawano, M.; Shah, R.; Zhou, T.; Arun, A.S.; Khosla, P.; Kaleem, S.; Vashist, A.; Bhattacharjee, B.; et al. Post-Vaccination Syndrome: A Descriptive Analysis of Reported Symptoms and Patient Experiences After Covid-19 Immunization. medRxiv 2023. [Google Scholar] [CrossRef]
- Chang, C.-C.; Wang, Y.-H.; Yen, J.-C.; Liaw, C.-C.; Tsai, K.-C.; Wei, W.-C.; Chiou, W.-F.; Chiou, C.-T.; Liou, K.-T.; Shen, Y.-C.; et al. NRICM101 in Combatting COVID-19 Induced Brain Fog: Neuroprotective Effects and Neurovascular Integrity Preservation in hACE2 Mice. J. Tradit. Complement. Med. 2025, 15, 36–50. [Google Scholar] [CrossRef]
- Filler, K.; Lyon, D.; Bennett, J.; McCain, N.; Elswick, R.; Lukkahatai, N.; Saligan, L.N. Association of Mitochondrial Dysfunction and Fatigue: A Review of the Literature. BBA Clin. 2014, 1, 12–23. [Google Scholar] [CrossRef]
- Gorman, G.S.; Elson, J.L.; Newman, J.; Payne, B.; McFarland, R.; Newton, J.L.; Turnbull, D.M. Perceived Fatigue Is Highly Prevalent and Debilitating in Patients with Mitochondrial Disease. Neuromuscul. Disord. 2015, 25, 563–566. [Google Scholar] [CrossRef]
- Van Der Feltz-Cornelis, C.; Turk, F.; Sweetman, J.; Khunti, K.; Gabbay, M.; Shepherd, J.; Montgomery, H.; Strain, W.D.; Lip, G.Y.H.; Wootton, D.; et al. Prevalence of Mental Health Conditions and Brain Fog in People with Long COVID: A Systematic Review and Meta-Analysis. Gen. Hosp. Psychiatry 2024, 88, 10–22. [Google Scholar] [CrossRef]
- Kelly, C.; Junker, A.; Englestad, K.; Hirano, M.; Trumpff, C.; Picard, M. Perceived Association of Mood and Symptom Severity in Adults with Mitochondrial Diseases. Mitochondrion 2025, 84, 102033. [Google Scholar] [CrossRef] [PubMed]
- John, J.; Kinra, M.; Mudgal, J.; Viswanatha, G.L.; Nandakumar, K. Animal Models of Chemotherapy-Induced Cognitive Decline in Preclinical Drug Development. Psychopharmacology 2021, 238, 3025–3053. [Google Scholar] [CrossRef]
- Neha; Sodhi, R.K.; Jaggi, A.S.; Singh, N. Animal Models of Dementia and Cognitive Dysfunction. Life Sci. 2014, 109, 73–86. [Google Scholar] [CrossRef]
- Appelman, B.; Charlton, B.T.; Goulding, R.P.; Kerkhoff, T.J.; Breedveld, E.A.; Noort, W.; Offringa, C.; Bloemers, F.W.; Van Weeghel, M.; Schomakers, B.V.; et al. Muscle Abnormalities Worsen after Post-Exertional Malaise in Long COVID. Nat. Commun. 2024, 15, 17. [Google Scholar] [CrossRef]
- Santos, A.F.; Póvoa, P.; Paixão, P.; Mendonça, A.; Taborda-Barata, L. Changes in Glycolytic Pathway in SARS-COV 2 Infection and Their Importance in Understanding the Severity of COVID-19. Front. Chem. 2021, 9, 685196. [Google Scholar] [CrossRef]
- Guarnieri, J.W.; Dybas, J.M.; Fazelinia, H.; Kim, M.S.; Frere, J.; Zhang, Y.; Soto Albrecht, Y.; Murdock, D.G.; Angelin, A.; Singh, L.N.; et al. Core Mitochondrial Genes Are Down-Regulated during SARS-CoV-2 Infection of Rodent and Human Hosts. Sci. Transl. Med. 2023, 15, eabq1533. [Google Scholar] [CrossRef]
- Palikaras, K.; Lionaki, E.; Tavernarakis, N. Mechanisms of Mitophagy in Cellular Homeostasis, Physiology and Pathology. Nat. Cell Biol. 2018, 20, 1013–1022. [Google Scholar] [CrossRef]
- Liang, S.; Bao, C.; Yang, Z.; Liu, S.; Sun, Y.; Cao, W.; Wang, T.; Schwantes-An, T.-H.; Choy, J.S.; Naidu, S.; et al. SARS-CoV-2 Spike Protein Induces IL-18-Mediated Cardiopulmonary Inflammation via Reduced Mitophagy. Signal Transduct. Target. Ther. 2023, 8, 108. [Google Scholar] [CrossRef] [PubMed]
- Halma, M.T.J.; Marik, P.E.; Saleeby, Y.M. Exploring Autophagy in Treating SARS-CoV-2 Spike Protein-Related Pathology. Endocr. Metab. Sci. 2024, 14, 100163. [Google Scholar] [CrossRef]
- Patterson, B.K.; Francisco, E.B.; Yogendra, R.; Long, E.; Pise, A.; Rodrigues, H.; Hall, E.; Herrera, M.; Parikh, P.; Guevara-Coto, J.; et al. Persistence of SARS CoV-2 S1 Protein in CD16+ Monocytes in Post-Acute Sequelae of COVID-19 (PASC) up to 15 Months Post-Infection. Front. Immunol. 2022, 12, 746021. [Google Scholar] [CrossRef]
- Craddock, V.; Mahajan, A.; Spikes, L.; Krishnamachary, B.; Ram, A.K.; Kumar, A.; Chen, L.; Chalise, P.; Dhillon, N.K. Persistent Circulation of Soluble and Extracellular Vesicle-linked Spike Protein in Individuals with Postacute Sequelae of COVID-19. J. Med. Virol. 2023, 95, e28568. [Google Scholar] [CrossRef]
- Swank, Z.; Senussi, Y.; Manickas-Hill, Z.; Yu, X.G.; Li, J.Z.; Alter, G.; Walt, D.R. Persistent Circulating Severe Acute Respiratory Syndrome Coronavirus 2 Spike Is Associated With Post-Acute Coronavirus Disease 2019 Sequelae. Clin. Infect. Dis. 2023, 76, e487–e490. [Google Scholar] [CrossRef]
- Swank, Z.; Borberg, E.; Chen, Y.; Senussi, Y.; Chalise, S.; Manickas-Hill, Z.; Yu, X.G.; Li, J.Z.; Alter, G.; Henrich, T.J.; et al. Measurement of Circulating Viral Antigens Post-SARS-CoV-2 Infection in a Multicohort Study. Clin. Microbiol. Infect. 2024, 30, 1599–1605. [Google Scholar] [CrossRef]
- Fehrer, A.; Sotzny, F.; Hoheisel, F.; Stein, E.; Kim, L.; Kedor, C.; Freitag, H.; Heindrich, C.; Bauer, S.; Rust, R.; et al. Long-Term Serum Spike Protein Persistence but No Correlation with Post-COVID Syndrome. medRxiv 2024. [Google Scholar] [CrossRef]
- Bhattacharjee, B.; Lu, P.; Monteiro, V.S.; Tabachnikova, A.; Wang, K.; Hooper, W.B.; Bastos, V.; Greene, K.; Sawano, M.; Guirgis, C.; et al. Immunological and Antigenic Signatures Associated with Chronic Illnesses after COVID-19 Vaccination. medRxiv 2025. [Google Scholar] [CrossRef]
- Fehrer, A.; Sotzny, F.; Kim, L.; Kedor, C.; Freitag, H.; Heindrich, C.; Grabowski, P.; Babel, N.; Scheibenbogen, C.; Wittke, K. Serum Spike Protein Persistence Post COVID Is Not Associated with ME/CFS. J. Clin. Med. 2025, 14, 1086. [Google Scholar] [CrossRef]
- McCullough, P.A.; Wynn, C.; Procter, B.C. Clinical Rationale for SARS-CoV-2 Base Spike Protein Detoxification in Post COVID-19 and Vaccine Injury Syndromes. J. Am. Physicians Surg. 2023, 28, 90–93. [Google Scholar] [CrossRef]
- Halma, M.T.J.; Plothe, C.; Marik, P.; Lawrie, T.A. Strategies for the Management of Spike Protein-Related Pathology. Microorganisms 2023, 11, 1308. [Google Scholar] [CrossRef] [PubMed]
- Hulscher, N.; McCullough, P.A.; Marotta, D.E. Strategic Deactivation of mRNA COVID-19 Vaccines: New Applications for siRNA Therapy and RIBOTACs. J. Gene Med. 2024, 26, e3733. [Google Scholar] [CrossRef]
- Kyriakopoulos, A.M.; Nigh, G.; McCullough, P.A.; Seneff, S. Clinical Rationale for Dietary Lutein Supplementation in Long COVID and mRNA Vaccine Injury Syndromes. F1000Research 2024, 13, 191. [Google Scholar] [CrossRef] [PubMed]
- Tanikawa, T.; Kiba, Y.; Yu, J.; Hsu, K.; Chen, S.; Ishii, A.; Yokogawa, T.; Suzuki, R.; Inoue, Y.; Kitamura, M. Degradative Effect of Nattokinase on Spike Protein of SARS-CoV-2. Molecules 2022, 27, 5405. [Google Scholar] [CrossRef] [PubMed]
- Grixti, J.M.; Theron, C.W.; Salcedo-Sora, J.E.; Pretorius, E.; Kell, D.B. Automated, Microscopic Measurement of Fibrinaloid Microclots and Their Degradation by Nattokinase, the Main Natto Protease. J. Exp. Clin. Appl. Chin. Med. 2024, 5, 30–55. [Google Scholar] [CrossRef]
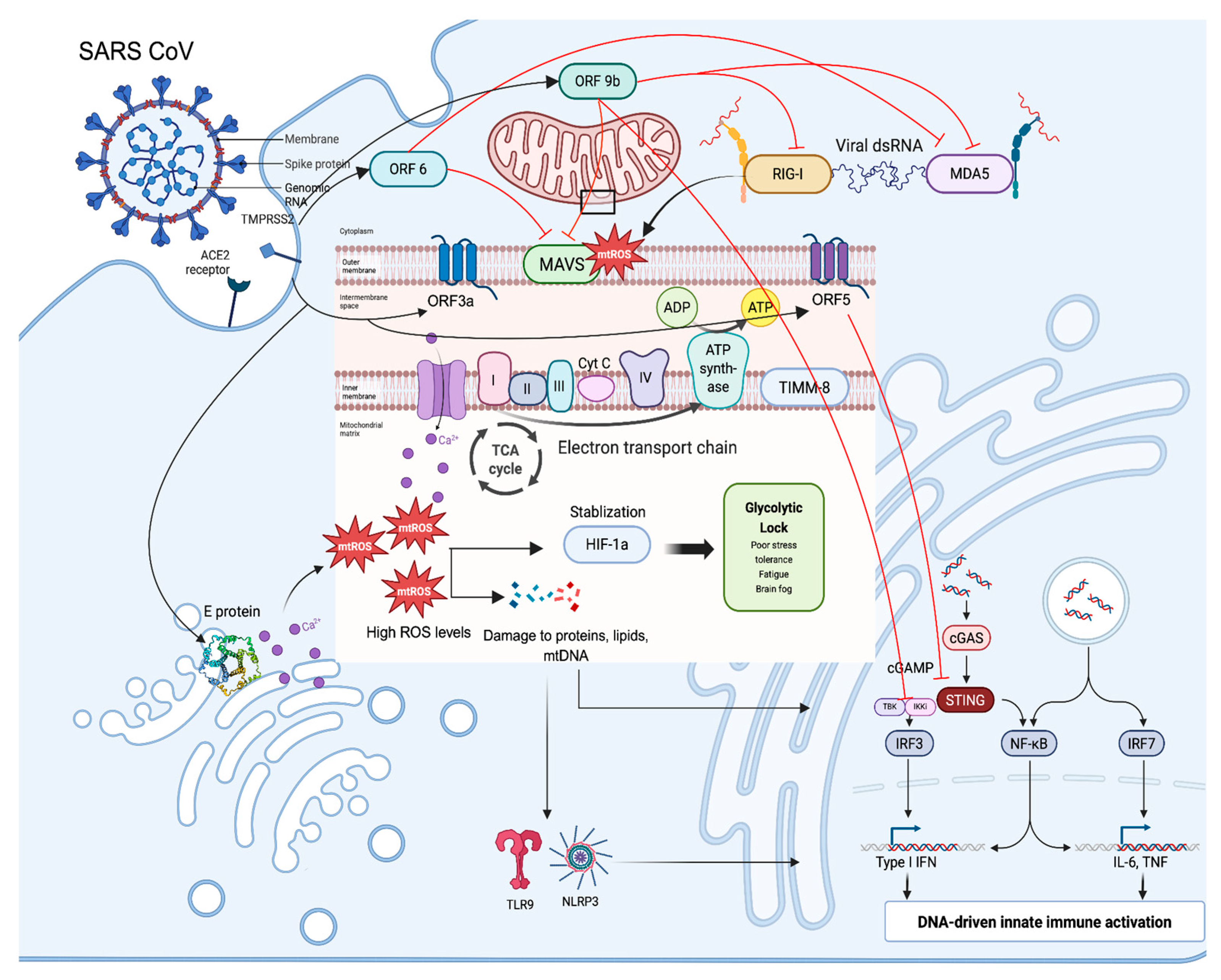
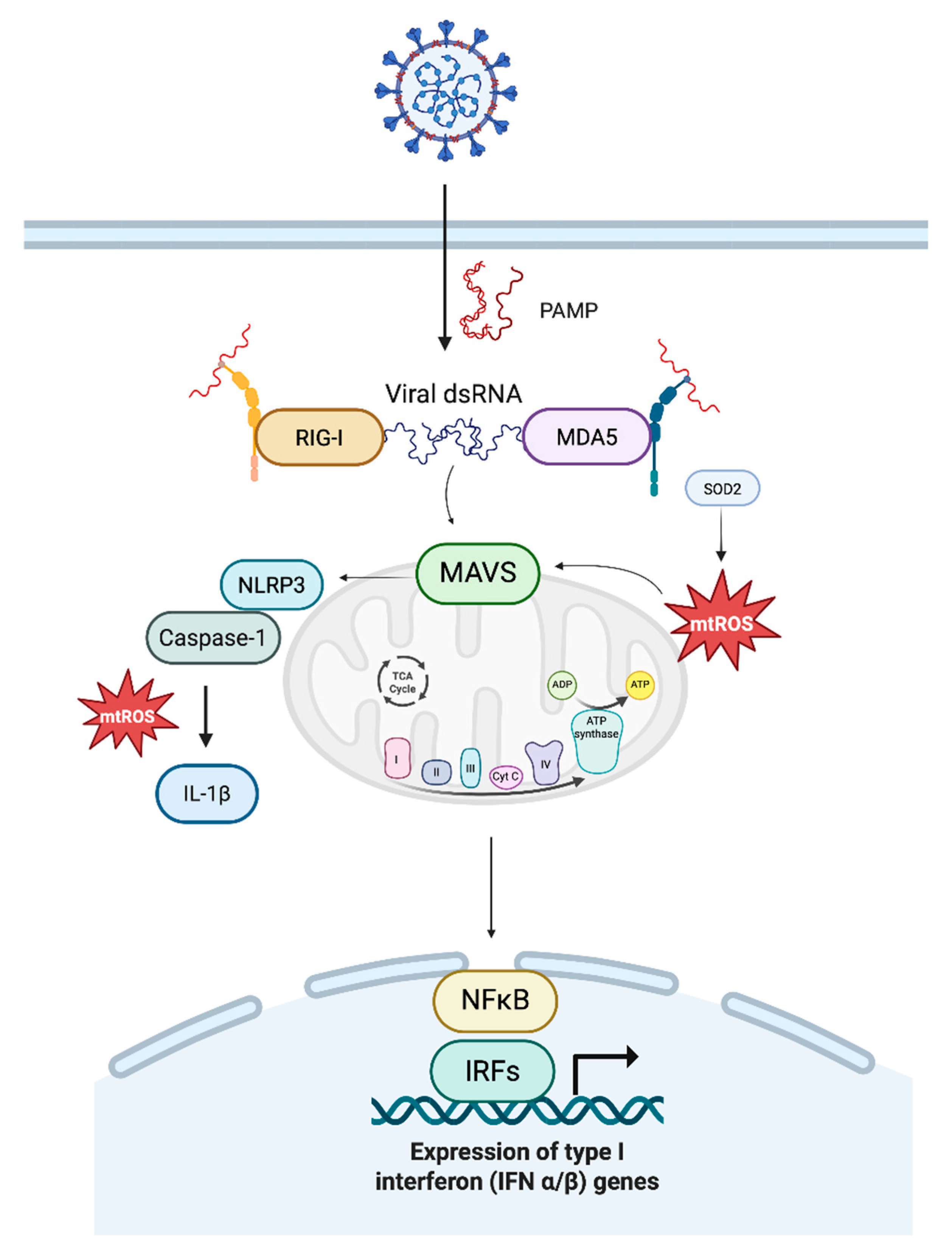
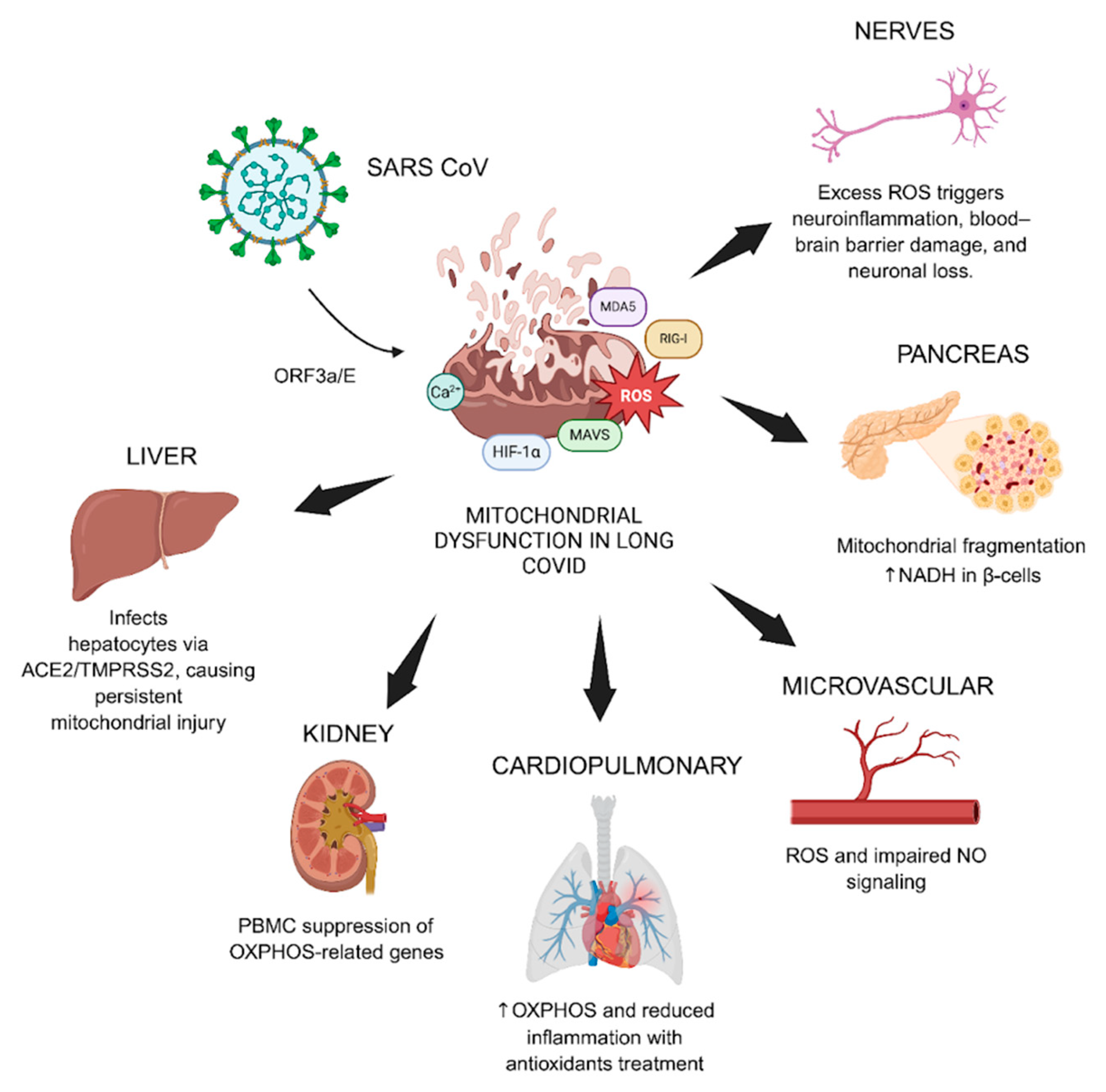
| Protein | Mitochondrial Target | Function/Key effects |
|---|---|---|
| ORF3a | Outer mitochondrial membrane pore-forming subunit ATP/potassium channel; ER–mitochondria contact site | Increase ROS, apoptosis, and decrease INF signaling; disrupts mitochondrial membrane [85,90] |
| ORF5 | MAVS signaling axis | Downregulates MAVS; limits TBK1/IRF3 recruitment; downregulates interferon response [73] |
| ORF6 | SAM complex, outer mitochondrial membrane | Metabolic reprogramming (lipolysis, fatty acid oxidation) attenuates MAVS. Immune suppression and altered mitochondrial proteome [91,92] |
| ORF9b | TOM70 (outer membrane), RIG-I/MDA5–MAVS | Immune evasion, inflammation, oxidative damage, and altered OXPHOS; inhibits MAVS; decreases interferon I/III signaling [93,94] |
| ORF9c | Complex I accessory complex (NDUFB9, NDUFAF1), Cristea | Impairs Complex I; increases ROS; induces mitochondrial fragmentation; decrease IFN production; immune evasion, inflammation, oxidative damage, and altered OXPHOS [90,94] |
| ORF3c | TOM70, TOM20, MAVS modulation (outer membrane) | Alters metabolism; increases ROS; blocks autophagy; increased ROS altered autophagy; Impairs INF and immunosuppression [93,95] |
| ORF7b | MAVS (outer membrane), MAM (endoplasmic reticulum) | Inhibits MAVS–TRAF6 interaction; increases ROS via interaction with MAM; decrease production of IFN-ß [96,97,98] |
| ORF10 | NIX (outer mitochondrial membrane) | Triggers mitophagy; disrupts MAVS, disrupts mitochondria, and decreases IFN signaling [73,99] |
| NSP4 | BAX (outer mitochondrial membrane) | Induces macropore formation; release of mtDNA, pro-apoptotic effects, and inflammation [100] |
| NSP8 | Colocalizes with the outer mitochondrial membrane | Induces incomplete mitophagy; causes mitochondrial damage; disrupts autophagy; reduces IFN signaling, and dampens innate immunity [101,102] |
| M protein | MAVS (outer mitochondrial membrane) | Triggers mitophagy; inhibits MAVS signaling, suppresses interferon I and III production, and causes irreversible loss of mitochondrial membrane potential, leakage of cytochrome C, and apoptosis [103,104] |
| Nucleocapsid | Localizes to the mitochondria and impairs mitochondrial transcription machinery | Increases ROS, decreases, and can also increase ATP production, and inhibits antioxidant enzymes; it increases oxidative stress and, indirectly, causes mitochondrial dysfunction [105,106] |
| E protein | ER/ERGIC/Golgi membranes; indirectly impacts mitochondria | Disrupts ER calcium stores; impairs ER–mitochondrial Ca2+ transfer; contributes to mitochondrial dysfunction [73] |
| Study Type | Study Name | Intervention Specifics | Outcome | Reference |
|---|---|---|---|---|
| Observational | Coenzyme Q10 + Alpha Lipoic Acid for Chronic COVID Syndrome | 500 mg/day CoQ10 + alpha lipoic acid (Requpero®) vs. no treatment | 53.5% achieved full fatigue response vs. 3.5% in control; significant symptom reduction | [143] |
| Randomized cross-over trial | High-Dose Coenzyme Q10 for Post-COVID Condition | 500 mg/day CoQ10 for 6 weeks vs. placebo (2×2 cross-over design) | No significant benefit over placebo in reducing post-COVID-19 condition symptoms; both groups improved similarly over time, suggesting natural recovery or placebo effect rather than a treatment effect | [130] |
| Observational | L-Arginine and Vitamin C for Long COVID | Includes a combination of L-arginine and Vitamin C supplements | L-arginine and Vitamin C group experienced less severe long COVID symptoms, with favorable effects on all symptoms | [144] |
| Double blind randomized controlled trial | Favorable Antiviral Effect of Metformin on SARS-CoV-2 Viral Load in a Randomized, Placebo-Controlled Clinical Trial of COVID-19 | Metformin, fluvoxamine, and ivermectin | Metformin significantly reduced SARS-CoV-2 viral load | [145] |
| Randomized controlled trial | Vitamins K2 and D3 Improve Long COVID, Fungal Translocation, and Inflammation: Randomized Controlled Trial | Vitamins K2 and D3 | Improved number of Long COVID symptoms, significantly lowered markers of inflammation (sTNF-RI, sCD163), oxidative stress (oxidized LDL), and fungal translocation (β-D-glucan) | [146] |
| One-arm open-label study | The results of a unique dietary supplement (nutraceutical formulation) used to treat the symptoms of long-haul COVID | β-caryophyllene and pregnenolone supplement | Statistically significant improvements in their overall symptoms after 2 and 4 weeks of treatment There were some symptoms, such as fatigue and brain fog, that appeared to respond more than others; however, no baseline presentations were able to predict individual symptomatic responses | [147] |
| Randomized controlled trial | Eight-Week Creatine-Glucose Supplementation Alleviates Clinical Features of Long COVID | Creatine, creatine, and glucose combined | Significantly elevated brain creatine levels and significantly reduced symptoms such as body aches, concentration difficulties, and headache compared with placebo | [148] |
| Double-blind, placebo-controlled randomized trial | Potential anti-inflammatory and anti-fatigue effects of an oral food supplement in long COVID patients | Echinacea angustifolia, rosehip, propolis, royal jelly, and zinc | Significant reduction in the inflammatory parameters during the OFS period, in comparison to the placebo. Statistically significant increase in serum values of vitamin D after the OFS | [149] |
| Randomized placebo-controlled trial | Effects of an 8-week high-dose vitamin D supplementation on fatigue and neuropsychiatric manifestations in post-COVID-19 syndrome: A randomized controlled trial | Vitamin D | Improved fatigue, reduced anxiety, and enhanced cognitive function (ACE score: +2.1, p = 0.012), with no meaningful changes in sleep quality, depression, or inflammatory markers (IL-6, CRP), and no serious adverse events | [150] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, E.; Ozigbo, A.A.; Varon, J.; Halma, M.; Laezzo, M.; Ang, S.P.; Iglesias, J. Mitochondrial Reactive Oxygen Species: A Unifying Mechanism in Long COVID and Spike Protein-Associated Injury: A Narrative Review. Biomolecules 2025, 15, 1339. https://doi.org/10.3390/biom15091339
Lee E, Ozigbo AA, Varon J, Halma M, Laezzo M, Ang SP, Iglesias J. Mitochondrial Reactive Oxygen Species: A Unifying Mechanism in Long COVID and Spike Protein-Associated Injury: A Narrative Review. Biomolecules. 2025; 15(9):1339. https://doi.org/10.3390/biom15091339
Chicago/Turabian StyleLee, Eunseuk, Adaobi Amelia Ozigbo, Joseph Varon, Mathew Halma, Madison Laezzo, Song Peng Ang, and Jose Iglesias. 2025. "Mitochondrial Reactive Oxygen Species: A Unifying Mechanism in Long COVID and Spike Protein-Associated Injury: A Narrative Review" Biomolecules 15, no. 9: 1339. https://doi.org/10.3390/biom15091339
APA StyleLee, E., Ozigbo, A. A., Varon, J., Halma, M., Laezzo, M., Ang, S. P., & Iglesias, J. (2025). Mitochondrial Reactive Oxygen Species: A Unifying Mechanism in Long COVID and Spike Protein-Associated Injury: A Narrative Review. Biomolecules, 15(9), 1339. https://doi.org/10.3390/biom15091339






