Therapeutic Effect of Selenium Nanoparticles, Sorafenib, and Selenium–Sorafenib Nanocomplex in the Lungs and Kidneys of Mice with TAA-Induced HCC
Abstract
1. Introduction
2. Materials & Methods
2.1. Ethical Statement
2.2. Animal Injection Protocols
2.3. Isolation of Total RNA from Lungs and Kidneys, Reverse Transcription
2.4. Synthesis and Characterization of SeNPs, So, and SeSo
2.5. Real-Time PCR
2.6. Western Blotting
2.7. Quantitative Assessment of Se Contents in the Lungs, Kidneys, and Liver by the Colorimetric Method
2.8. Quantitative Assessment of Se Contents in Lungs, Kidneys, and Liver by Hydride Generation Atomic Absorption Spectrometry (HG-AAS)
2.9. Statistical Data Processing
3. Results and Discussion
3.1. SeNP Injections Promote the Most Effective Accumulation of Se in the Liver, Lungs, and Kidneys Compared to SeSo
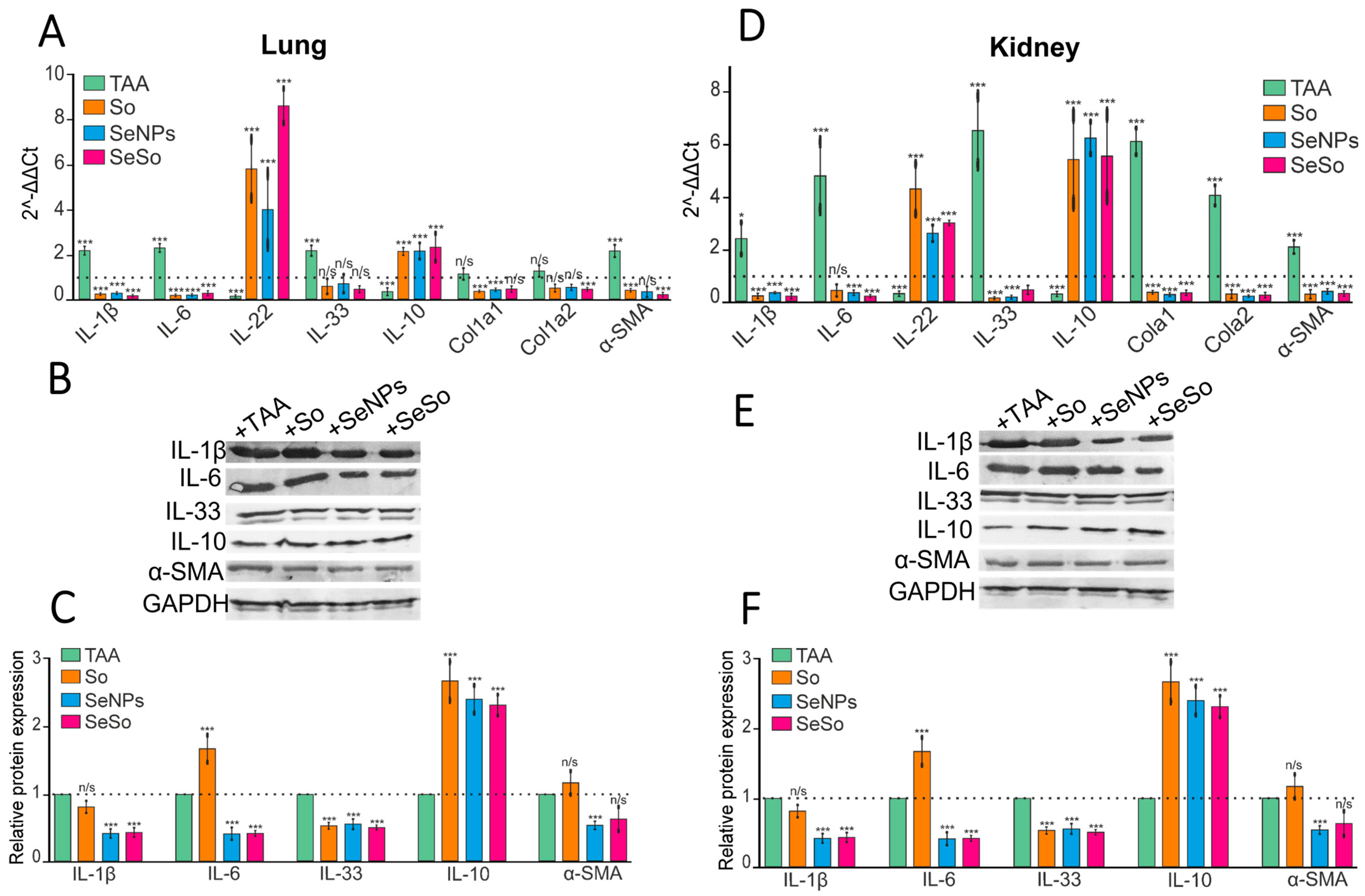
3.2. Injections of SeNPs, So, and SeSo Effectively Reduce the Inflammatory Status and Fibrotic Changes in the Liver and Lungs in Mice with TAA-Induced HCC
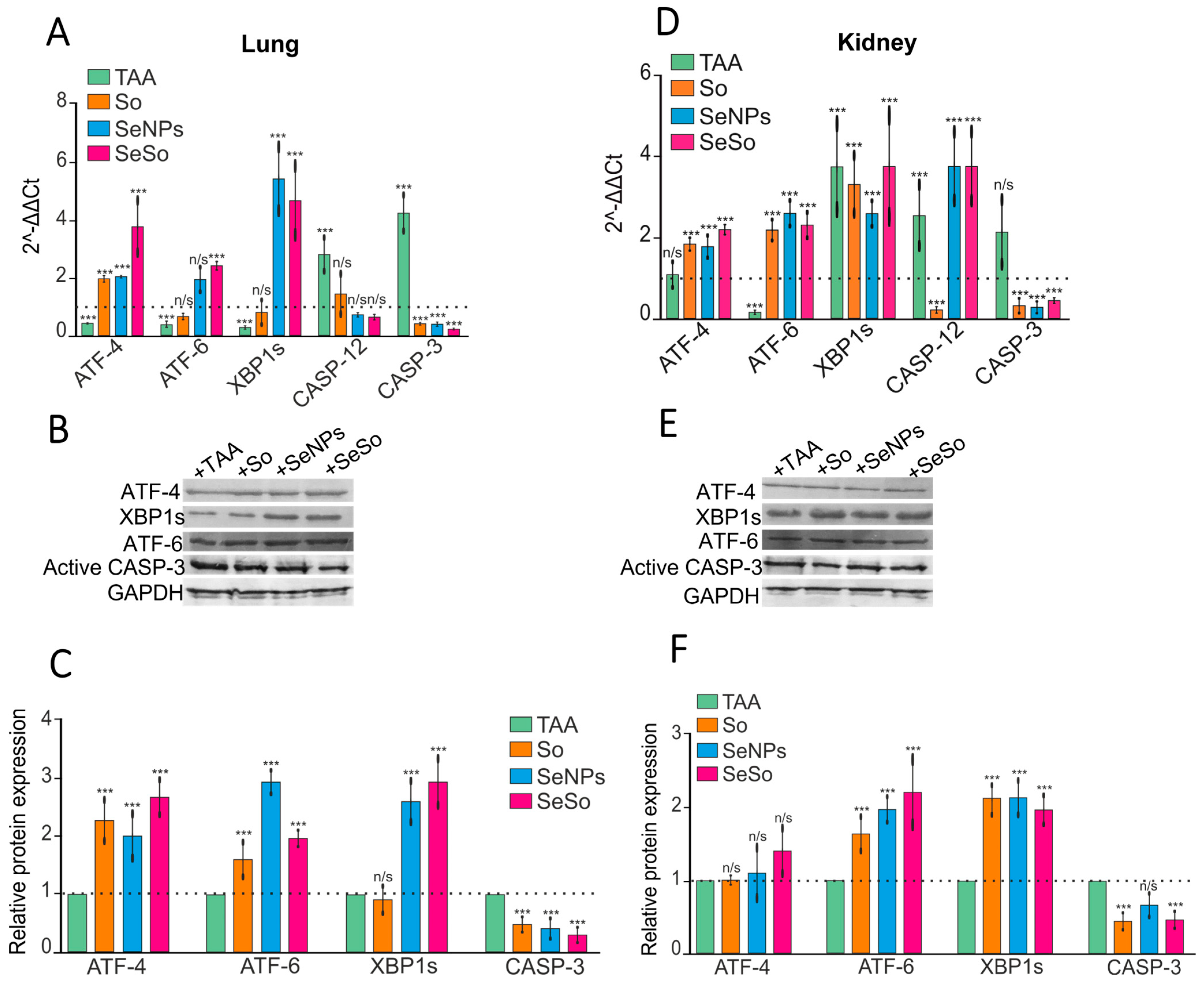
3.3. Injections of SeNPs, So, and SeSo Effectively Reduce Apoptotic Cell Death in the Lungs and Kidneys of Mice with TAA-Induced HCC
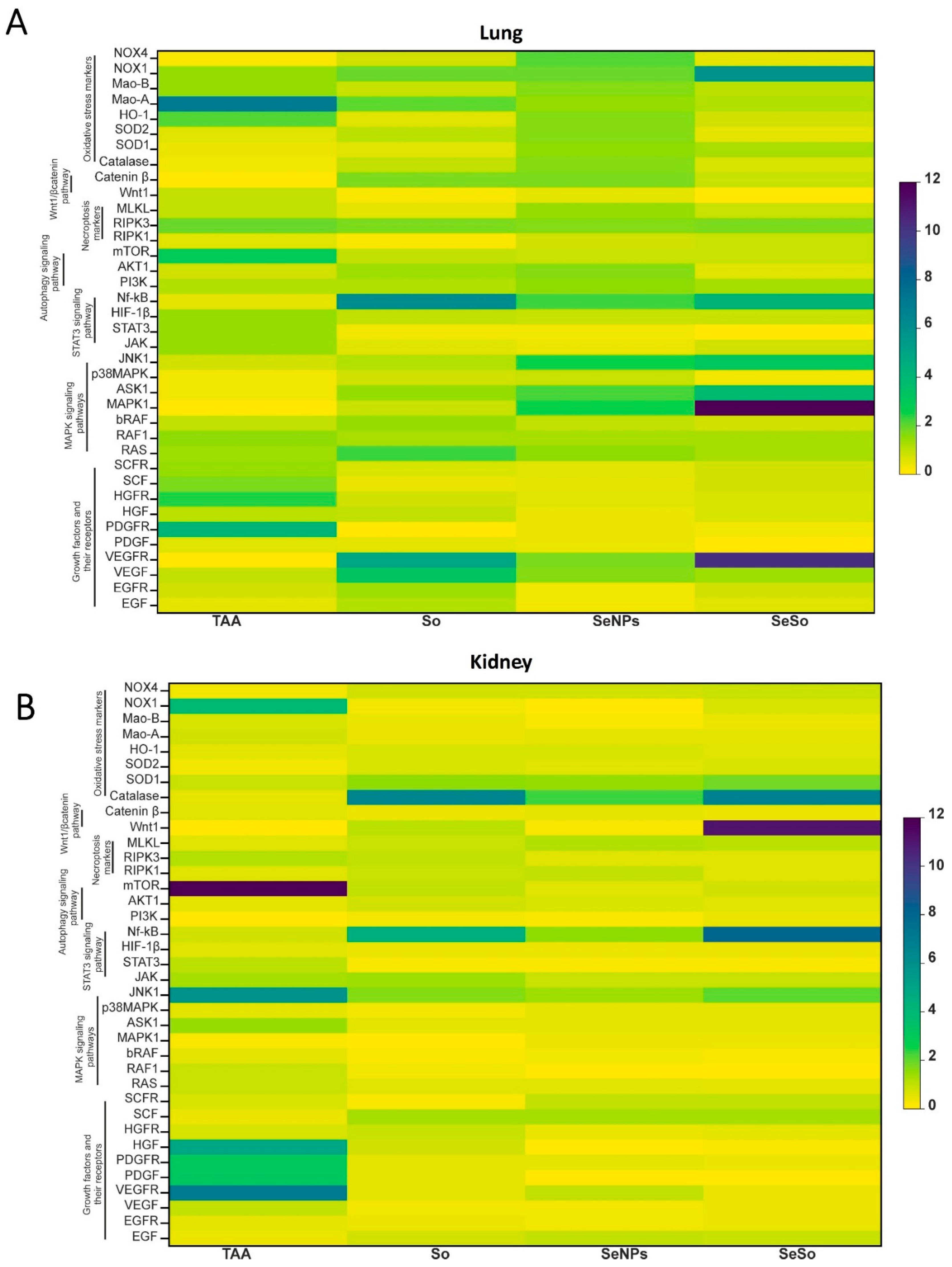
3.4. Analysis of the Screening of mRNA Expression Patterns of Markers of Various Signaling Pathways in the Kidneys and Lungs of Mice with HCC Induced by TAA and After Injections of So, SeNPs, and SeSo
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wagle, D.G.; Moore, R.H.; Murphy, G.P. Secondary carcinomas of the kidney. J. Urol. 1975, 114, 30–32. [Google Scholar] [CrossRef]
- Sawabe, M.; Nakamura, T.; Kanno, J.; Kasuga, T. Analysis of morphological factors of hepatocellular carcinoma in 98 autopsy cases with respect to pulmonary metastasis. Acta Pathol. Jpn. 1987, 37, 1389–1404. [Google Scholar] [CrossRef]
- Elhameed, A.G.A.; Helal, M.G.; Said, E.; Salem, H.A. Saxagliptin defers thioacetamide-induced hepatocarcinogenesis in rats: A novel suppressive impact on Wnt/Hedgehog/Notch1 signaling. Env. Toxicol. Pharmacol. 2021, 86, 103668. [Google Scholar] [CrossRef]
- Galvão, F.H.F.; Traldi, M.C.C.; Araújo, R.S.S.; Stefano, J.T.; D’Albuquerque, L.A.C.; Oliveira, C.P. Preclinical models of liver câncer. Arq. Gastroenterol. 2023, 60, 383–392. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Zaghloul, R.A.; Abdelghany, A.M.; El Gayar, A.M. Selenium nanoparticles and quercetin suppress thioacetamide-induced hepatocellular carcinoma in rats: Attenuation of inflammation involvement. J. Biochem. Mol. Toxicol. 2022, 36, e22989. [Google Scholar] [CrossRef]
- Nalkurthi, C.; Schroder, W.A.; Melino, M.; Irvine, K.M.; Nyuydzefe, M.; Chen, W.; Liu, J.; Teng, M.W.L.; Hill, G.R.; Bertolino, P.; et al. ROCK2 inhibition attenuates profibrogenic immune cell function to reverse thioacetamide-induced liver fibrosis. JHEP Rep. 2021, 4, 100386. [Google Scholar] [CrossRef]
- Varlamova, E.G.; Goltyaev, M.V.; Rogachev, V.V.; Gudkov, S.V.; Karaduleva, E.V.; Turovsky, E.A. Antifibrotic Effect of Selenium-Containing Nanoparticles on a Model of TAA-Induced Liver Fibrosis. Cells 2023, 12, 2723. [Google Scholar] [CrossRef]
- Atorrasagasti, C.; Piccioni, F.; Borowski, S.; Tirado-González, I.; Freitag, N.; Cantero, M.J.; Bayo, J.; Mazzolini, G.; Alaniz, L.D.; Blois, S.M.; et al. Acceleration of TAA-Induced Liver Fibrosis by Stress Exposure Is Associated with Upregulation of Nerve Growth Factor and Glycopattern Deviations. Int. J. Mol. Sci. 2021, 22, 5055. [Google Scholar] [CrossRef]
- Varlamova, E.G.; Khabatova, V.V.; Gudkov, S.V.; Turovsky, E.A. Ca2+-Dependent Effects of the Selenium-Sorafenib Nanocomplex on Glioblastoma Cells and Astrocytes of the Cerebral Cortex: Anticancer Agent and Cytoprotector. Int. J. Mol. Sci. 2023, 24, 2411. [Google Scholar] [CrossRef]
- Varlamova, E.G. Molecular Mechanisms of the Therapeutic Effect of Selenium Nanoparticles in Hepatocellular Carcinoma. Cells 2024, 13, 1102. [Google Scholar] [CrossRef]
- Maiyo, F.; Singh, M. Selenium nanoparticles: Potential in cancer gene and drug delivery. Nanomedicine 2017, 12, 1075–1089. [Google Scholar] [CrossRef]
- Varlamova, E.G.; Goltyaev, M.V.; Simakin, A.V.; Gudkov, S.V.; Turovsky, E.A. Comparative Analysis of the Cytotoxic Effect of a Complex of Selenium Nanoparticles Doped with Sorafenib, “Naked” Selenium Nanoparticles, and Sorafenib on Human Hepatocyte Carcinoma HepG2 Cells. Int. J. Mol. Sci. 2022, 23, 6641. [Google Scholar] [CrossRef]
- Varlamova, E.G.; Burmistrov, D.E.; Gudkov, S.V.; Rogachev, V.V.; Turovsky, E.A. Comparative analysis of anticancer properties of So and selenium nanoparticles in a thioacetamide-induced HCC model. Biochem. Biophys. Res. Commun. 2025, 778, 152364. [Google Scholar] [CrossRef]
- Marciel, M.P.; Hoffmann, P.R. Molecular Mechanisms by Which Selenoprotein K Regulates Immunity and Cancer. Biol. Trace Elem. Res. 2019, 192, 60–68. [Google Scholar] [CrossRef]
- Varlamova, E.G. Selenium-containing compounds, selenium nanoparticles and selenoproteins in the prevention and treatment of lung cancer. J. Trace Elem. Med. Biol. 2025, 88, 127620. [Google Scholar] [CrossRef]
- Varlamova, E.G. Roles of selenium-containing glutathione peroxidases and thioredoxin reductases in the regulation of processes associated with glioblastoma progression. Arch. Biochem. Biophys. 2025, 766, 110344. [Google Scholar] [CrossRef]
- da Costa, N.S.; Lima, L.S.; Oliveira, F.A.M.; Galiciolli, M.E.A.; Manzano, M.I.; Garlet, Q.I.; Irioda, A.C.; Oliveira, C.S. Antiproliferative Effect of Inorganic and Organic Selenium Compounds in Breast Cell Lines. Biomedicines 2023, 11, 1346. [Google Scholar] [CrossRef]
- Zhang, F.; Li, X.; Wei, Y. Selenium and Selenoproteins in Health. Biomolecules 2023, 13, 799. [Google Scholar] [CrossRef]
- Medina, D.; Thompson, H.; Ganther, H.; Ip, C. Se-methylselenocysteine: A new compound for chemoprevention of breast cancer. Nutr. Cancer 2001, 40, 12–17. [Google Scholar] [CrossRef]
- Varlamova, E.G.; Goltyaev, M.V.; Fesenko, E.E. Expression of human selenoprotein genes selh, selk, selm, sels, selv, and gpx-6 in various tumor cell lines. Dokl. Biochem. Biophys. 2016, 468, 203–205. [Google Scholar] [CrossRef]
- Varlamova, E.G.; Goltyaev, M.V.; Fesenko, E.E. Protein Partners of Selenoprotein SELM and the Role of Selenium Compounds in Regulation of Its Expression in Human Cancer Cells. Dokl. Biochem. Biophys. 2019, 488, 300–303. [Google Scholar] [CrossRef]
- Ali, W.; Chen, Y.; Gandahi, J.A.; Qazi, I.H.; Sun, J.; Wang, T.; Liu, Z.; Zou, H. Cross-Talk Between Selenium Nanoparticles and Cancer Treatment Through Autophagy. Biol. Trace Elem. Res. 2024, 202, 2931–2940. [Google Scholar] [CrossRef]
- Anjum, S.; Hashim, M.; Imran, M.; Babur, S.; Adnan, S.; Hano, C.; Ibrahim, W.N. Selenium Nanoparticles in Cancer Therapy: Unveiling Cytotoxic Mechanisms and Therapeutic Potential. Cancer Rep. 2025, 8, e70210. [Google Scholar] [CrossRef]
- Varlamova, E.G.; Gudkov, S.V.; Blinova, E.V.; Blinov, D.S.; Turovsky, E.A. Anticancer signal transduction pathways of selenium nanoparticles in mouse colorectal cancer model. Biochem. Biophys. Res. Commun. 2025, 769, 151962. [Google Scholar] [CrossRef]
- Martínez-Esquivias, F.; Gutiérrez-Angulo, M.; Pérez-Larios, A.; Sánchez-Burgos, J.A.; Becerra-Ruiz, J.S.; Guzmán-Flores, J.M. Anticancer Activity of Selenium Nanoparticles In Vitro Studies. Anticancer Agents Med. Chem. 2022, 22, 1658–1673. [Google Scholar] [CrossRef]
- Shehata, N.S.; Elwakil, B.H.; Elshewemi, S.S.; Ghareeb, D.A.; Olama, Z.A. Selenium nanoparticles coated bacterial polysaccharide with potent antimicrobial and anti-lung cancer activities. Sci. Rep. 2023, 13, 21871. [Google Scholar] [CrossRef]
- Menon, S.; Jayakodi, S.; Yadav, K.K.; Somu, P.; Isaq, M.; Shanmugam, V.K.; Chaitanyakumar, A.; Basavegowda, N. Preparation of Paclitaxel-Encapsulated Bio-Functionalized Selenium Nanoparticles and Evaluation of Their Efficacy against Cervical Cancer. Molecules 2022, 27, 7290. [Google Scholar] [CrossRef]
- Ahmed, M.E.; Alzahrani, K.K.; Fahmy, N.M.; Almutairi, H.H.; Almansour, Z.H.; Alam, M.W. Colistin-Conjugated Selenium Nanoparticles: A Dual-Action Strategy Against Drug-Resistant Infections and Cancer. Pharmaceutics 2025, 17, 556. [Google Scholar] [CrossRef]
- Li, S.; Liu, T.; Li, C.; Zhang, Z.; Zhang, J.; Sun, D. Overcoming immunotherapy resistance in colorectal cancer through nano-selenium probiotic complexes and IL-32 modulation. Biomaterials 2025, 320, 123233. [Google Scholar] [CrossRef]
- Zhou, M.; Niu, H.; Huang, G.; Zhou, M.; Cui, D.; Li, H.; Wen, H.; Zhang, H.; Liang, F.; Chen, R. Biomimetic Nano-delivery of Small-Molecule Piceatannol Modulates Tumor Stemness and Suppresses Colorectal Cancer Metastasis via Hippo/YAP1/SOX9 Signaling. Small 2025, 21, e2407191. [Google Scholar] [CrossRef]
- Guo, K.; Yang, X.; Wang, J.; Chang, W.; Liu, S.; Zhang, S.; Zhang, T.; Yan, H.; Yan, Y.; Wang, J.; et al. Synthesis and Bioactivity of Selenium Nanoparticles From Tussilago farfara L. Polysaccharides: Antioxidant Properties and MCF-7 Cell Inhibition. Chem. Biodivers. 2025, 22, e202402677. [Google Scholar] [CrossRef]
- Asl, F.D.; Arvejeh, P.M.; Rezaee, M.; Saffari-Chaleshtori, J.; Deris, F.; Satari, A.; Asgharzadeh, S.; Khosravian, P. Folic Acid Targeted Selenium-Curcumin Nanoparticles to Enhance Apoptosis in Breast Cancer Cells. Chem. Biodivers. 2025, 16, e00183. [Google Scholar] [CrossRef]
- He, L.; Zhang, L.; Peng, Y.; He, Z. Selenium in cancer management: Exploring the therapeutic potential. Front. Oncol. 2025, 14, 1490740. [Google Scholar] [CrossRef]
- Ranjbar, M.H.; Einafshar, E.; Javid, H.; Jafari, N.; Sajjadi, S.S.; Darban, R.A.; Hashemy, S.I. Enhancing the anticancer effects of rosmarinic acid in PC3 and LNCaP prostate cancer cells using titanium oxide and selenium-doped graphene oxide nanoparticles. Sci. Rep. 2025, 15, 11568. [Google Scholar] [CrossRef]
- Irannejad, F.; Shahbazi, S.; Reiisi, S.; Heidari, R. Study of the effect of zinc oxide, selenium, and silver nanoparticles on the expression level of oxidative stress-associated genes in ovarian cancer. Med. Oncol. 2025, 42, 39. [Google Scholar] [CrossRef]
- Cole, J.T.; Holland, N.B. Multifunctional nanoparticles for use in theranostic applications. Drug Deliv. Transl. Res. 2015, 5, 295–309. [Google Scholar] [CrossRef]
- Liu, Y.; Tan, J.; Thomas, A.; Ou-Yang, D.; Muzykantov, V.R. The shape of things to come: Importance of design in nanotechnology for drug delivery. Ther. Deliv. 2012, 3, 181–194. [Google Scholar] [CrossRef]
- Zambonino, M.C.; Quizhpe, E.M.; Mouheb, L.; Rahman, A.; Agathos, S.N.; Dahoumane, S.A. Biogenic Selenium Nanoparticles in Biomedical Sciences: Properties, Current Trends, Novel Opportunities and Emerging Challenges in Theranostic Nanomedicine. Nanomaterials 2023, 13, 424. [Google Scholar] [CrossRef]
- Verma, A.; Stellacci, F. Effect of surface properties on nanoparticle-cell interactions. Small 2010, 6, 12–21. [Google Scholar] [CrossRef]
- Turovsky, E.A.; Varlamova, E.G.; Rogachev, V.V.; Gudkov, S.V. Tellurium nanoparticles produced by laser ablation induce selective anticancer effects via ROS-mediated apoptosis and calcium signaling pathways: In vitro screening. Biochem. Biophys. Res. Commun. 2025, 782, 152555. [Google Scholar] [CrossRef]
- Varlamova, E.G.; Gudkov, S.V.; Turovsky, E.A. Opposite Effects of Small and Large Diameter Selenium Nanoparticles on the Redox-Status and Survival of Cortical Cells in Toxic Models In Vitro. Biol. Trace Elem. Res. 2025, 1–22. [Google Scholar] [CrossRef]
- Wilhelm, S.M.; Carter, C.; Tang, L.; Wilkie, D.; McNabola, A.; Rong, H.; Chen, C.; Zhang, X.; Vincent, P.; McHugh, M.; et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004, 64, 7099–7109. [Google Scholar] [CrossRef]
- Li, M.; Su, Y.; Zhang, F.; Chen, K.; Xu, X.; Xu, L.; Zhou, J.; Wang, W. A dual-targeting reconstituted high density lipoprotein leveraging the synergy of sorafenib and antimiRNA21 for enhanced hepatocellular carcinoma therapy. Acta Biomater. 2018, 75, 413–426. [Google Scholar] [CrossRef]
- Mancuso, A.; Airoldi, A.; Vigano, R.; Pinzello, G. Fatal gastric bleeding during sorafenib treatment for hepatocellular carcinoma recurrence after liver transplantation. Dig. Liver Dis. 2011, 43, 754. [Google Scholar] [CrossRef]
- Hart, W.E.; Marczak, S.P.; Kneller, A.R.; French, R.A.; Morris, D.L. The abilities of selenium dioxide and selenite ion to coordinate DNA-bound metal ions and decrease oxidative DNA damage. J. Inorg. Biochem. 2013, 125, 1–8. [Google Scholar] [CrossRef]
- Kiełczykowska, M.; Kocot, J.; Paździor, M.; Musik, I. Selenium—A fascinating antioxidant of protective properties. Adv. Clin. Exp. Med. 2018, 27, 245–255. [Google Scholar] [CrossRef]
- Khurana, A.; Tekula, S.; Saifi, M.A.; Venkatesh, P.; Godugu, C. Therapeutic applications of selenium nanoparticles. Biomed. Pharmacother. 2019, 111, 802–812. [Google Scholar] [CrossRef]
- Savory, L.A.; Kerr, C.J.; Whiting, P.; Finer, N.; McEneny, J.; Ashton, T. Selenium supplementation and exercise: Effect on oxidant stress in overweight adults. Obesity 2012, 20, 794–801. [Google Scholar] [CrossRef]
- Ganther, H.E. Selenium metabolism, selenoproteins and mechanisms of cancer prevention: Complexities with thioredoxin reductase. Carcinogenesis 1999, 20, 1657–1666. [Google Scholar] [CrossRef]
- Varlamova, E.G.; Turovsky, E.A. The Nature of Changes in mRNA Expression and the Relative Content of Selenoproteins in a Mouse Model with TAA-Induced Fibrosis and Hepatocellular Carcinoma (HCC). OM&P 2024, 11, 136–151. [Google Scholar] [CrossRef]
- Rua, R.M.; Nogales, F.; Carreras, O.; Ojeda, M.L. Selenium, selenoproteins and cancer of the thyroid. J. Trace Elem. Med. Biol. 2023, 76, 127115. [Google Scholar] [CrossRef]
- Wu, Y.; Min, J.; Ge, C.; Shu, J.; Tian, D.; Yuan, Y.; Zhou, D. Interleukin 22 in Liver Injury, Inflammation and Cancer. Int. J. Biol. Sci. 2020, 16, 2405–2413. [Google Scholar] [CrossRef]
- Budhu, A.; Wang, X.W. The role of cytokines in hepatocellular carcinoma. J. Leukoc. Biol. 2006, 80, 1197–1213. [Google Scholar] [CrossRef]
- Chan, S.L.; Mo, F.K.F.; Wong, C.S.C.; Chan, C.M.L.; Leung, L.K.S.; Hui, E.P.; Ma, B.B.; Chan, A.T.C.; Mok, T.S.K.; Yeo, W. A study of circulating interleukin 10 in prognostication of unresectable hepatocellular carcinoma. Cancer 2012, 118, 3984–3992. [Google Scholar] [CrossRef]
- Pan, X.; Chen, X.; Ren, Q.; Yue, L.; Niu, S.; Li, Z.; Zhu, R.; Chen, X.; Jia, Z.; Zhen, R.; et al. Single-cell transcriptomics identifies Col1a1 and Col1a2 as hub genes in obesity-induced cardiac fibrosis. Biochem. Biophys. Res. Commun. 2022, 61, 830–837. [Google Scholar] [CrossRef]
- Zhou, D.-J.; Mu, D.; Jiang, M.-D.; Zheng, S.-M.; Zhang, Y.; He, S.; Weng, M.; Zeng, W.-Z. Hepatoprotective effect of juglone on dimethylnitrosamine-induced liver fibrosis and its effect on hepatic antioxidant defence and the expression levels of α-SMA and collagen III. Mol. Med. Rep. 2015, 12, 4095–4102. [Google Scholar] [CrossRef]
- Scorrano, L.; Oakes, S.A.; Opferman, J.T.; Cheng, E.H.; Sorcinelli, M.D.; Pozzan, T.; Korsmeyer, S.J. BAX and BAK regulation of endoplasmic reticulum Ca2+: A control point for apoptosis. Science 2003, 300, 135–139. [Google Scholar] [CrossRef]
- Nakagawa, T.; Yuan, J. Cross-talk between two cysteine protease families. Activation of caspase-12 by calpain in apoptosis. J. Cell Biol. 2000, 150, 887–894. [Google Scholar] [CrossRef]
- Orrenius, S.; Zhivotovsky, B.; Nicotera, P. Regulation of cell death: The calcium-apoptosis link. Nat. Rev. Mol. Cell Biol. 2003, 4, 552–565. [Google Scholar] [CrossRef]
- Goltyaev, M.V.; Varlamova, E.G.; Novoselov, S.V.; Fesenko, E.E. Activation of Signal Pathways of Apoptosis under Conditions of Prolonged ER-Stress Caused by Exposure of Mouse Testicular Teratoma Cells to Selenium-Containing Compounds. Dokl. Biochem. Biophys. 2020, 490, 9–11. [Google Scholar] [CrossRef]
- Shigemi, Z.; Manabe, K.; Hara, N.; Baba, Y.; Hosokawa, K.; Kagawa, H.; Watanabe, T.; Fujimuro, M. Methylseleninic acid and sodium selenite induce severe ER stress and subsequent apoptosis through UPR activation in PEL cells. Chem. Biol. Interact. 2017, 266, 28–37. [Google Scholar] [CrossRef]
- Zachariah, M.; Maamoun, H.; Milano, L.; Rayman, M.P.; Meira, L.B.; Agouni, A. Endoplasmic reticulum stress and oxidative stress drive endothelial dysfunction induced by high selenium. J. Cell Physiol. 2021, 236, 4348–4359. [Google Scholar] [CrossRef]
- Marinko, J.T.; Huang, H.; Penn, W.D.; Capra, J.A.; Schlebach, J.P.; Sanders, C.R. Folding and Misfolding of Human Membrane Proteins in Health and Disease: From Single Molecules to Cellular Proteostasis. Chem. Rev. 2019, 119, 5537–5606. [Google Scholar] [CrossRef]
- Su, X.; Chen, H.; Xiang, H.; Ke, H.; Dong, C.; Song, Q.; Zhou, J.; Jiang, Q.; Wang, Y.; Chen, L.; et al. Selenium participates in the formation of kidney stones by alleviating endoplasmic reticulum stress and apoptosis of renal tubular epithelial cells. Redox Rep. 2024, 29, 2416825. [Google Scholar] [CrossRef]
- An, K.J.; Hanato, A.N.; Hui, K.W.; Pitts, M.W.; Seale, L.A.; Nicholson, J.L.; Toh, P.; Kim, J.K.; Berry, M.J.; Torres, D.J. Selenium Protects Mouse Hypothalamic Cells from Glucocorticoid-Induced Endoplasmic Reticulum Stress Vulnerability and Insulin Signaling Impairment. Antioxidants 2023, 12, 526. [Google Scholar] [CrossRef]
- Yao, L.; Du, Q.; Yao, H.; Chen, X.; Zhang, Z.; Xu, S. Roles of oxidative stress and endoplasmic reticulum stress in selenium deficiency-induced apoptosis in chicken liver. Biometals 2015, 28, 255–265. [Google Scholar] [CrossRef]
- Varias, D.C.; Moon, S.-H.; Shin, S.H.; Ryu, B.-Y. Selenium protects mouse spermatogonia against ivermectin-induced apoptosis by alleviating endoplasmic reticulum stress in vitro. Ecotoxicol. Environ. Saf. 2024, 287, 117307. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, L.; Li, Y.; Luo, X.; He, J. Excessive Selenium Supplementation Induced Oxidative Stress and Endoplasmic Reticulum Stress in Chicken Spleen. Biol. Trace Elem. Res. 2016, 172, 481–487. [Google Scholar] [CrossRef]
- Wang, F.; Sun, N.; Zeng, H.; Gao, Y.; Zhang, N.; Zhang, W. Selenium Deficiency Leads to Inflammation, Autophagy, Endoplasmic Reticulum Stress, Apoptosis and Contraction Abnormalities via Affecting Intestinal Flora in Intestinal Smooth Muscle of Mice. Front. Immunol. 2022, 13, 947655. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, B.; Guan, H.; Jiao, X.; Yang, J.; Cai, J.; Liu, Q.; Zhang, Z. Selenium deficiency causes apoptosis through endoplasmic reticulum stress in swine small intestine. Biofactors 2021, 47, 788–800. [Google Scholar] [CrossRef]
- Okada, T.; Yoshida, H.; Akazawa, R.; Negishi, M.; Mori, K. Distinct roles of activating transcription factor 6 (ATF6) and double-stranded RNA-activated protein kinase-like endoplasmic reticulum kinase (PERK) in transcription during the mammalian unfolded protein response. Biochem. J. 2002, 366, 585–594. [Google Scholar] [CrossRef]
- Harding, H.P.; Zhang, Y.; Ron, D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 1999, 397, 271–274. [Google Scholar] [CrossRef]
- Yoshida, H.; Matsui, T.; Yamamoto, A.; Okada, T.; Mori, K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 2001, 107, 881–891. [Google Scholar] [CrossRef]
- Ezzoukhry, Z.; Louandre, C.; Trécherel, E.; Godin, C.; Chauffert, B.; Dupont, S.; Diouf, M.; Barbare, J.; Mazière, J.; Galmiche, A. EGFR activation is a potential determinant of primary resistance of hepatocellular carcinoma cells to sorafenib. Int. J. Cancer 2012, 131, 2961–2969. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, C.; Wu, J.; Nan, Y. ADAMTS8 targets ERK to suppress cell proliferation, invasion, and metastasis of hepatocellular carcinoma. Onco Targets Ther. 2018, 11, 7569–7578. [Google Scholar] [CrossRef]
- Li, D.; Fan, X.; Zuo, L.; Wu, X.; Wu, Y.; Zhang, Y.; Zou, F.; Sun, Z.; Zhang, W. Prognostic analysis of RAS-related lncRNAs in liver hepatocellular carcinoma. Ann. Transl. Med. 2022, 10, 1356. [Google Scholar] [CrossRef]
- Yang, S.Y.; Miah, A.; Pabari, A.; Winslet, M. Growth Factors and their receptors in cancer metastases. Front. Biosci. 2011, 16, 531–538. [Google Scholar] [CrossRef]
- Di Domenico, M.; Giordano, A. Signal transduction growth factors: The effective governance of transcription and cellular adhesion in cancer invasion. Oncotarget 2017, 8, 36869–36884. [Google Scholar] [CrossRef][Green Version]
- Markezana, A.; Paldor, M.; Liao, H.; Ahmed, M.; Zorde-Khvalevsky, E.; Rozenblum, N.; Stechele, M.; Salvermoser, L.; Laville, F.; Goldmann, S.; et al. Fibroblast growth factors induce hepatic tumorigenesis post radiofrequency ablation. Sci. Rep. 2023, 13, 16341. [Google Scholar] [CrossRef]
- Ruff, S.M.; Pawlik, T.M. Emerging therapies targeting growth factors in hepatocellular carcinoma. Expert Opin. Pharmacother. 2024, 25, 255–262. [Google Scholar] [CrossRef]
- Furuse, J. Growth factors as therapeutic targets in HCC. Crit. Rev. Oncol. Hematol. 2008, 67, 8–15. [Google Scholar] [CrossRef]
- Iida, G.; Asano, K.; Seki, M.; Sakai, M.; Kutara, K.; Ishigaki, K.; Kagawa, Y.; Yoshida, O.; Teshima, K.; Edamura, K.; et al. Gene expression of growth factors and growth factor receptors for potential targeted therapy of canine hepatocellular carcinoma. J. Vet. Med. Sci. 2014, 76, 301–306. [Google Scholar] [CrossRef][Green Version]
- Nalesnik, M.A.; Michalopoulos, G.K. Growth factor pathways in development and progression of hepatocellular carcinoma. Front. Biosci. 2012, 4, 1487–1515. [Google Scholar] [CrossRef]
- Zheng, J.; Wang, S.; Xia, L.; Sun, Z.; Chan, K.M.; Bernards, R.; Qin, W.; Chen, J.; Xia, Q.; Jin, H. Hepatocellular carcinoma: Signaling pathways and therapeutic advances. Signal. Transduct. Target Ther. 2025, 10, 35. [Google Scholar] [CrossRef]
- Chen, J.; Gingold, J.A.; Su, X. Immunomodulatory TGF-β Signaling in Hepatocellular Carcinoma. Trends Mol. Med. 2019, 25, 1010–1023. [Google Scholar] [CrossRef]
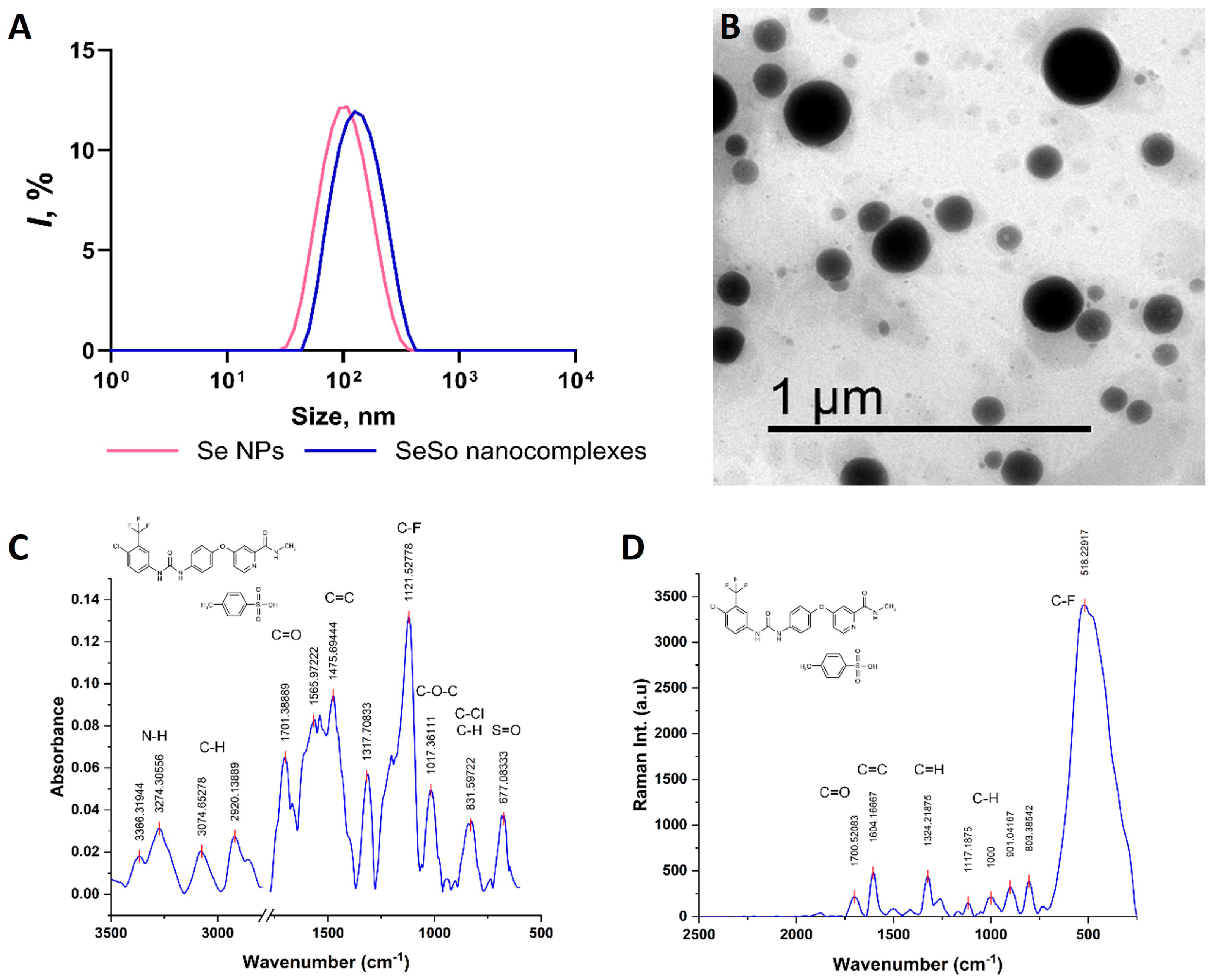

| Gene | Forward Primer 5′-> 3′ | Reverse Primer 5′-> 3′ |
|---|---|---|
| α-SMA | AGGAAGGATCTCTATGCTAACAAC | ACTTAGAAGCATTTGCGGTGG |
| COL1a1 | CATCACCTATCACTGCAAGAAC | AGGTCTTGGTGGTTTTGTTATTC |
| COL1a2 | TCTCAGAACATCACCTACCAC | CACGGAATTCTTGGTCAGCAC |
| IL-1β | CGTGCTGTCGGACCCATATG | GCTCTTGACTTCTATCTTGTTG |
| IL-6 | TCCAGAGATACAAAGAAATGATG | TTGGAAATTGGGGTAGGAAGG |
| IL-22 | GCTCCCCCAGTCAGACAGG | TAGAAGGCAGGAAGGAGCAG |
| IL-33 | TTTTGGAGAATGGATGTTATGTG | TTTGTGAAGGACGAAGAAGGC |
| IL-10 | AGCATGGCCCAGAAATCAAGG | AGACTCAATACACACTGCAGG |
| CASP-1 | AGAGAAATGAAGTTGCTGCTGG | ATCACCTTGGGCTTGTCTTTC |
| BAK | CACAGCCGGGAATGCCTAC | TCAGGATGGGGTCTCTACGC |
| BAX | TTCAACTGGGGCCGCGTGG | TTCCAGATGGTGAGCGAGGC |
| PUMA | TGAAGATCTGCGCCGGGAG | GAGAGGGACATGACGCGTG |
| BIM | AATGGCCGGCTATGGATGATG | GCCAATTGGGTTCACTGTCTG |
| CHOP | CAGCTGGGAGCTGGAAGCCTG | GACCACTCTGTTTCCGTTTCC |
| CASP-3 | CTCTTCATCATTCAGGCCTGC | GACCCGTCCTTTGAATTTCTC |
| BCL-2 | AAGTCAACACAAACCCCAAGTCCTC | GCAGATCTTCAGGTTCCTCCTGAGA |
| ATF4 | TCGGGTTTGGGGGCTGAAG | AAACAGAGCATCGAAGTCAAAC |
| ATF6 | AGGAGGGGAGATACGTTTTAC | CGAGGAGCTTTTGATGTGGAG |
| XBPs1 | AGTCCGCAGCACAGCAGGT | AGAGAAAGGGAGGCTGGTAAG |
| CASP-12 | TGTTGGTGTTATCATTTGGAGG | TTTTCTTTTCTTCTCAGCTACAG |
| PI3K | TGGCTGGGGAATGAAAATACC | AGGGAGCTGTACAGGTTGTAG |
| AKT1 | ATGTGTATGAGAAGAAGCTGAG | GCGGGGCTTCTGGACTCGG |
| mTOR | TGGCCAGTCAGTCGAAATTTTG | AGTTACCAGAAAGGGCACCAG |
| RIPK1 | TTGGAACTACAGGTACAGGAG | GGGTTCAGGTGTTCATCAGTC |
| RIPK3 | TTCGATGGCCCAACCTCCC | TGCCCGAAGGTGCCAAGCC |
| MLKL | AAGATCCCATTTGAAGGCTGTG | GGCTCATGGGCACGACACTC |
| CAT | TGCGGACATTCTACACAAAGG | CGGAGTTACAGGTTAGCTTTTC |
| SOD1 | TGGGGACAATACACAAGGCTG | ATCTTGTTTCTCATGGACCACC |
| SOD2 | GGAGAGTTGCTGGAGGCTATC | GAAGGTAGTAAGCGTGCTCC |
| HO-1 | AGGGTGACAGAAGAGGCTAAG | AATTCCCACTGCCACTGTTGC |
| MAO-A | TGAATGCTCTAGGAAAAGTTGC | AATTCATCCTCACTTTCCTTTAC |
| MAO-B | GGCTGCTACACAACCTACTTC | GGTAATGGGTCGTGCAGGGA |
| NOX1 | ACAAGAGATGGAGGAATTAGG | TTCCTAGGATCCAGACTCGAG |
| NOX4 | TACCTCAGTCAAACAGATGGG | TGTCCCATATGAGTTGTTCCG |
| JAK | CGAGAAGAGTAAAAGTCCACC | GAGCTTTGTTCTGGTTCTGGA |
| STAT3 | CCCCGTACCTGAAGACCAAG | ATGGGGTTCGGCTGCTTAGG |
| HIF1α | GGCGACTGTGCACCTACTATG | TGATCCAAAGCTCTGAGTAATTC |
| NFkB | TTAAAGAAACACTCAACAGCCAG | TTCAGCACTCGCACGGACAC |
| RAS | AACAAGTGTGACCTGGCTGC | TCCGGCACCTCCATGTCCTG |
| RAF1 | CCCACATCAACAACCGAGAC | CGATGCAGGGAAGGCTCAG |
| bRAF | CTGCTGGCCCGCTCATTGC | TAAGAAATAAAGAGTAGATGCTGC |
| MAPK1 | GGAGCAGTATTATGACCCAAG | GCTGAGACGGGCTGAAGAC |
| ASK1 | CCTAACAAGAACAGACACCCC | GGGCAGGGGATTGGAGTGG |
| p38MAPK | CTGTCGACCTACTGGAGAAG | TAGACAGAACAGAAACCAGGTG |
| JNK1 | AAGCCCCACCACCAAAGATC | TCTGTATCCGAGGCCAAAGTC |
| WNT | GTTCATCTTCGCAATCACCTC | GGCATTTGCACTCTTGGCGC |
| CATENINβ | GGGGTCCTCTGTGAACTTGC | GCACCAATGTCCAGTCCAAG |
| EGF | CCTTGGTTTGTGGTCCTAGAG | CTGGGGTCCTCTGTCACTTG |
| EGFR | ATGGAGGATGTAGTTGATGCTG | ACATATTCAGGTACAGGGAGG |
| VEGF | CCCGGTTTAAATCCTGGAGC | CTTTCCGGTGAGAGGTCTGG |
| VEGFR | TGCAGATCCACATTTTCATTCC | CTTGCTTTTACTCGCTATTCTC |
| PDGF | ATTGAGATTGTGCGAAAGAAGC | GGGGGCAATACAGCAAATACC |
| PDGFR | GCCGTGCAGCCCAATGAGAG | GGCTCTGCTTGCTGTGGCTC |
| HGF | TACAATTGATTTACCTAGTTATGG | ACCATTCTCATTTTGTGTTGTTC |
| HGFR | CTCTTATCCCGACGTGAACAC | ATCATGTGTTCCCCTCGCCA |
| SCF | CCAAAATGCTGCGGAGTAATG | GTGTTATTTTTGTCCAAGCTGTG |
| SCFR | GGAAGCAGCCCCTACCCAG | CACGGGGTTCTCTGGGTTG |
| GAPDH | GTAAAGACCTCTATGCCAACAC | GGTGCACGATGGAGGGGC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turovsky, E.A.; Gudkov, S.V.; Varlamova, E.G. Therapeutic Effect of Selenium Nanoparticles, Sorafenib, and Selenium–Sorafenib Nanocomplex in the Lungs and Kidneys of Mice with TAA-Induced HCC. Biomolecules 2025, 15, 1336. https://doi.org/10.3390/biom15091336
Turovsky EA, Gudkov SV, Varlamova EG. Therapeutic Effect of Selenium Nanoparticles, Sorafenib, and Selenium–Sorafenib Nanocomplex in the Lungs and Kidneys of Mice with TAA-Induced HCC. Biomolecules. 2025; 15(9):1336. https://doi.org/10.3390/biom15091336
Chicago/Turabian StyleTurovsky, Egor A., Sergey V. Gudkov, and Elena G. Varlamova. 2025. "Therapeutic Effect of Selenium Nanoparticles, Sorafenib, and Selenium–Sorafenib Nanocomplex in the Lungs and Kidneys of Mice with TAA-Induced HCC" Biomolecules 15, no. 9: 1336. https://doi.org/10.3390/biom15091336
APA StyleTurovsky, E. A., Gudkov, S. V., & Varlamova, E. G. (2025). Therapeutic Effect of Selenium Nanoparticles, Sorafenib, and Selenium–Sorafenib Nanocomplex in the Lungs and Kidneys of Mice with TAA-Induced HCC. Biomolecules, 15(9), 1336. https://doi.org/10.3390/biom15091336









