The Skin Microenvironment: A Dynamic Regulator of Hair Follicle Development, Cycling and Disease
Abstract
1. Introduction
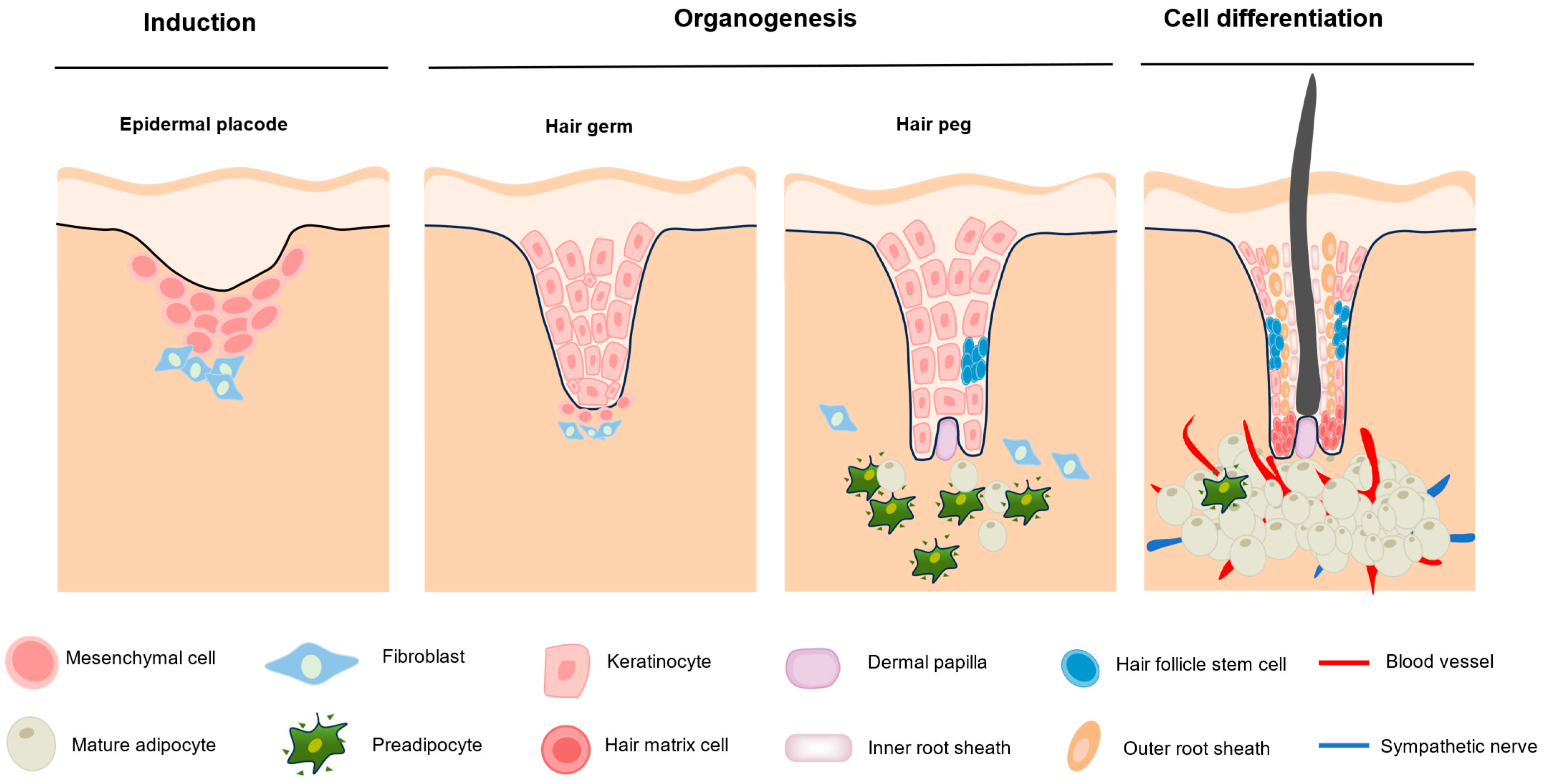
2. Hair Follicle Morphogenesis and Cycling
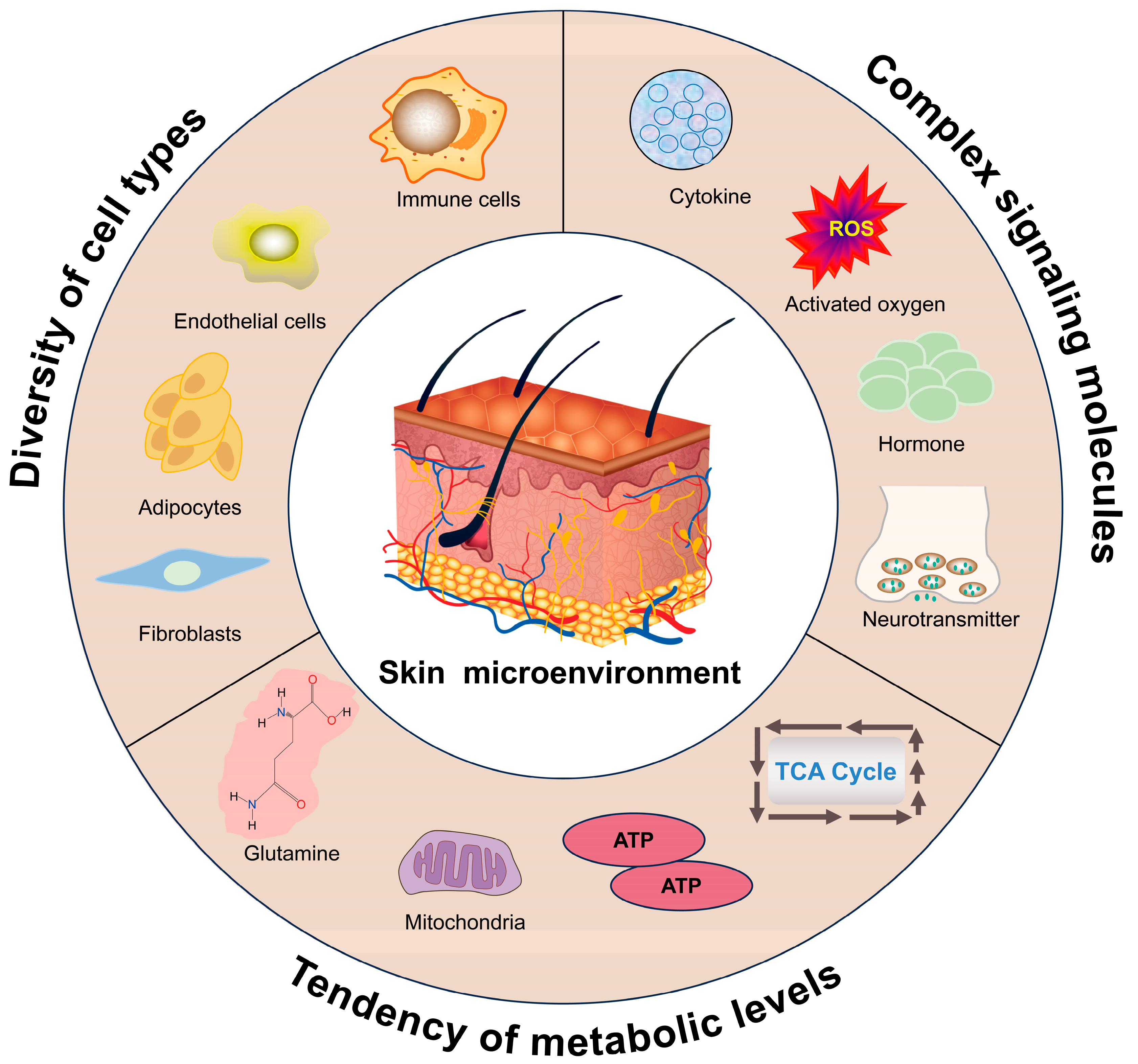
3. Key Factors Affecting the Hair Follicle Microenvironment
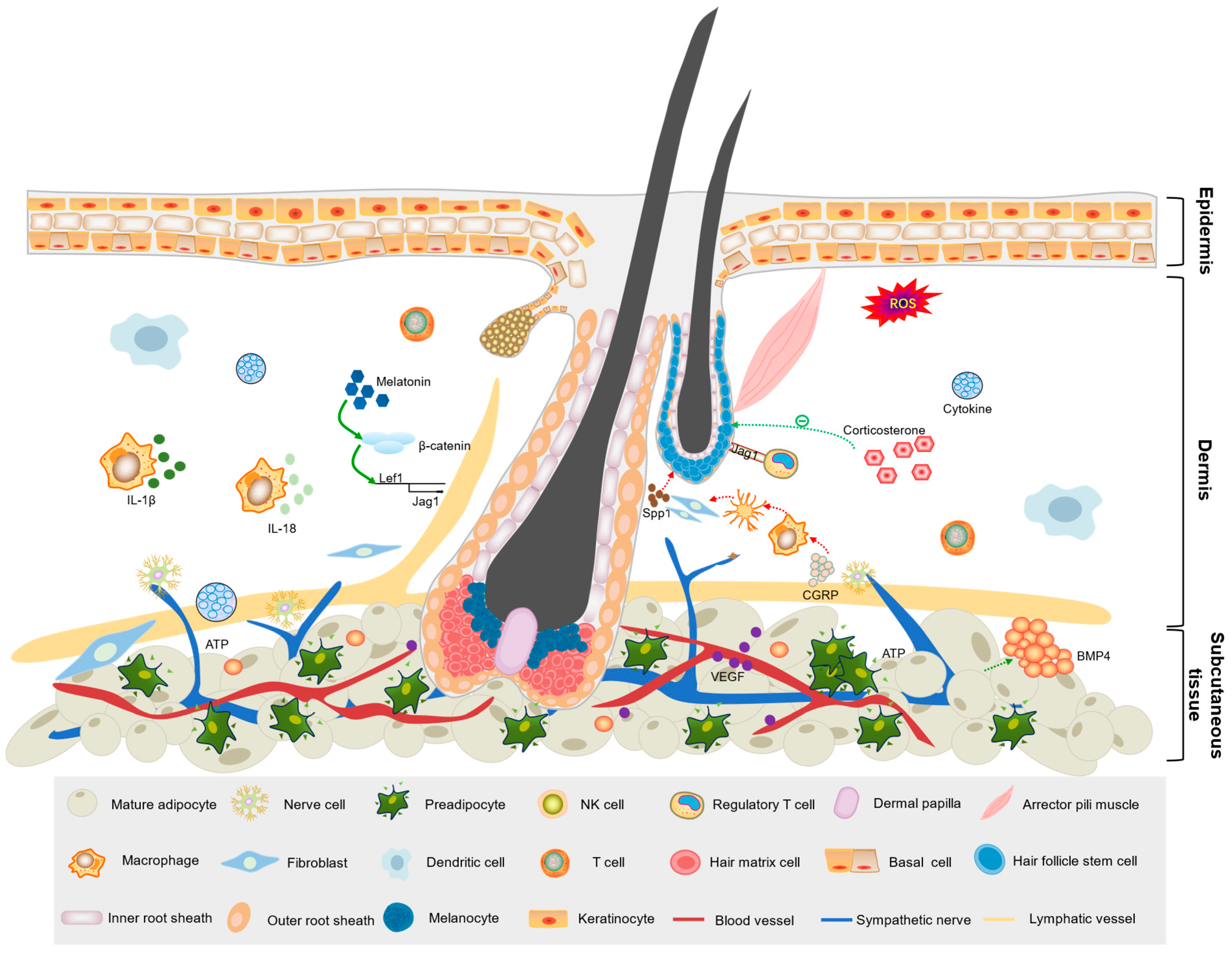
3.1. Influence of Cell Types in the Skin Microenvironment on Hair Follicle Development
3.1.1. Immune Cells
3.1.2. Adipocytes
3.1.3. Vascular Cells
3.2. Signaling Molecules
3.2.1. Hormones
3.2.2. Vitamins
3.2.3. Growth Factors
3.2.4. Other Signaling Molecules
| Signaling Molecule | Source | Mechanism | Reference |
|---|---|---|---|
| Interferon-γ | Immune cells | Enhances TGF-β 2 immunoreactivity and mRNA transcription levels | [114] |
| Tumor necrosis factor | Macrophages | Induces AKT/β-catenin signaling in HFSCs | [115] |
| Prolactin | Pituitary gland | Affects the proliferation and apoptosis of follicular keratinocytes | [116] |
| Hepatocyte growth factor | Dermal white adipose tissue | Upregulates WNT6 and WNT10B, inhibits SFRP1, and activates Wnt/β-catenin activity | [117] |
| Signal peptide CUB-EGF like domain-containing protein 3 | Dermal cells | Regulated by Hedgehog signaling, activates TGF-β signaling | [118] |
| Osteoblastin (SPP1) | Melanocytes | Signals to epithelial stem cells in adjacent hair follicles via CD44 receptors | [119] |
| Neutrophil elastase | Neutrophils | Specifically degrades collagen XVII (COL17A1) via hydrolysis | [120] |
| ANGPTL7 ANGPTL4 | Skin stem cells | ANGPTL7 promotes lymphatic drainage, resulting in capillary lymphatic vessels tightly surrounding the HFSC niche, helping maintain the stem cell resting state. When stem cells are activated, they shift from expressing ANGPTL7 to expressing ANGPTL4. This shift triggers the transient dissociation of lymphatic drainage, promoting stem cell activation and tissue regeneration | [121] |
| Platelet-derived growth factor | Platelets | Promotes HFSCs proliferation and self-renewal | [122] |
| Histone deacetylases 1 Histone deacetylases 2 | Papilla cells | During anagen, histone deacetylases 1 and 2 protect DPCs from apoptosis by inhibiting P53 activity and maintaining Wnt activity in DPCs to promote hair follicle growth | [123] |
| Major histocompatibility complex II and Aire | Thymic epithelial cells | Irreversibly transform the hair follicle pluripotent stem cells’ fates | [124] |
| Thyrotropin-releasing hormone | Hypothalamus | Stimulates keratinocyte proliferation and promotes hair shaft elongation | [125] |
| Galanin | Nervous tissues | Reduces stromal keratinocyte proliferation and shortens the growth period | [126] |
3.3. Metabolic Reprogramming
4. Interventions Targeting the Skin Microenvironment to Improve Hair Follicle Growth
5. Challenges and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grünwald, B.T.; Devisme, A.; Andrieux, G.; Vyas, F.; Aliar, K.; McCloskey, C.W.; Macklin, A.; Jang, G.H.; Denroche, R.; Romero, J.M. Spatially confined sub-tumor microenvironments in pancreatic cancer. Cell 2021, 184, 5577–5592. [Google Scholar] [CrossRef]
- Karimi, E.; Yu, M.W.; Maritan, S.M.; Perus, L.J.; Rezanejad, M.; Sorin, M.; Dankner, M.; Fallah, P.; Doré, S.; Zuo, D. Single-cell spatial immune landscapes of primary and metastatic brain tumours. Nature 2023, 614, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Du, W.L.; Chen, X.Y.; Zhang, Y.N. Organoid models of the tumor microenvironment and their applications. J. Cell. Mol. Med. 2021, 25, 5829–5841. [Google Scholar] [CrossRef]
- Ozan, V.B.; Wang, H.; Akshay, A.; Anand, D.; Hibaoui, Y.; Feki, A.; Gote-Schniering, J.; Gheinani, A.H.; Heller, M.; Uldry, A.-C. Influence of Microenvironmental Orchestration on Multicellular Lung Alveolar Organoid Development from Human Induced Pluripotent Stem Cells. Stem Cell Rev. Rep. 2025, 21, 254–275. [Google Scholar] [CrossRef]
- Dai, B.; Sha, R.N.; Yuan, J.L.; Liu, D.J. Multiple potential roles of thymosin β4 in the growth and development of hair follicles. J. Cell. Mol. Med. 2021, 25, 1350–1358. [Google Scholar] [CrossRef] [PubMed]
- Hawkshaw, N.; Hardman, J.; Alam, M.; Jimenez, F.; Paus, R. A study of a group of genes involved in the hair growth cycle. Br. J. Dermatol. 2020, 182, e169. [Google Scholar] [CrossRef]
- Ji, S.; Zhu, Z.; Sun, X.; Fu, X. Functional hair follicle regeneration: An updated review. Signal Transduct. Target. Ther. 2021, 6, 66. [Google Scholar] [CrossRef]
- Mou, C.; Jackson, B.; Schneider, P.; Overbeek, P.A.; Headon, D.J. Generation of the primary hair follicle pattern. Proc. Natl. Acad. Sci. USA 2006, 103, 9075–9080. [Google Scholar] [CrossRef]
- Vu, R.; Jin, S.; Sun, P.; Haensel, D.; Nguyen, Q.H.; Dragan, M.; Kessenbrock, K.; Nie, Q.; Dai, X. Wound healing in aged skin exhibits systems-level alterations in cellular composition and cell-cell communication. Cell Rep. 2022, 40, 111155. [Google Scholar] [CrossRef]
- Wang, E.C.; Dai, Z.; Ferrante, A.W.; Drake, C.G.; Christiano, A.M. A subset of TREM2+ dermal macrophages secretes oncostatin M to maintain hair follicle stem cell quiescence and inhibit hair growth. Cell Stem Cell 2019, 24, 654–669. [Google Scholar] [CrossRef]
- Castellana, D.; Paus, R.; Perez-Moreno, M. Macrophages contribute to the cyclic activation of adult hair follicle stem cells. PLoS Biol. 2014, 12, e1002002. [Google Scholar] [CrossRef]
- Paus, R.; Van Der Veen, C.; Eichmüller, S.; Kopp, T.; Hagen, E.; Müller-Röver, S.; Hofmann, U. Generation and cyclic remodeling of the hair follicle immune system in mice. J. Investig. Dermatol. 1998, 111, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Plikus, M.V.; Mayer, J.A.; de La Cruz, D.; Baker, R.E.; Maini, P.K.; Maxson, R.; Chuong, C.-M. Cyclic dermal BMP signalling regulates stem cell activation during hair regeneration. Nature 2008, 451, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Kwack, M.H.; Ahn, J.S.; Kim, M.K.; Kim, J.C.; Sung, Y.K. Dihydrotestosterone-inducible IL-6 inhibits elongation of human hair shafts by suppressing matrix cell proliferation and promotes regression of hair follicles in mice. J. Investig. Dermatol. 2012, 132, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Ananthan, A.S.; Kataria, S.; Pincha, N.; Dutta, A.; Athreya, S.; Bhosale, A.; Dey, R.; Jamora, C. Extracellular caspase-1 regulates hair follicle stem cell migration during wound-healing. bioRxiv 2019, 548529. [Google Scholar] [CrossRef]
- Shi, Y.; Cao, J.; Wang, Y. Rethinking regeneration: Empowerment of stem cells by inflammation. Cell Death Differ. 2015, 22, 1891–1892. [Google Scholar] [CrossRef]
- Morita, R.; Sanzen, N.; Sasaki, H.; Hayashi, T.; Umeda, M.; Yoshimura, M.; Yamamoto, T.; Shibata, T.; Abe, T.; Kiyonari, H. Tracing the origin of hair follicle stem cells. Nature 2021, 594, 547–552. [Google Scholar] [CrossRef]
- Gao, Y.; Song, W.; Hao, F.; Duo, L.; Zhe, X.; Gao, C.; Guo, X.; Liu, D. Effect of fibroblast growth factor 10 and an interacting non-coding RNA on Secondary Hair Follicle Dermal Papilla Cells in Cashmere goats’ follicle development assessed by whole-transcriptome sequencing technology. Animals 2023, 13, 2234. [Google Scholar] [CrossRef]
- Liu, Y.; Lyle, S.; Yang, Z.; Cotsarelis, G. Keratin 15 promoter targets putative epithelial stem cells in the hair follicle bulge. J. Investig. Dermatol. 2003, 121, 963–968. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, W.; Jiang, K.; Yu, Z.; Huang, H.; Wang, F.; Zhou, B.; Chen, T. Embryonic attenuated Embryonic attenuated Wnt/β-catenin signaling defines niche location and long-term stem cell fate in hair follicle. Elife 2015, 4, e10567. [Google Scholar] [CrossRef]
- Chai, M.; Jiang, M.; Vergnes, L.; Fu, X.; de Barros, S.C.; Doan, N.B.; Huang, W.; Chu, J.; Jiao, J.; Herschman, H. Stimulation of hair growth by small molecules that activate autophagy. Cell Rep. 2019, 27, 3413–3421. [Google Scholar] [CrossRef] [PubMed]
- Suen, W.-J.; Li, S.-T.; Yang, L.-T. Hes1 regulates anagen initiation and hair follicle regeneration through modulation of hedgehog signaling. Stem Cells 2020, 38, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Stenn, K.; Paus, R. Controls of hair follicle cycling. Physiol. Rev. 2001, 81, 449–494. [Google Scholar] [CrossRef]
- Müller-Röver, S.; Foitzik, K.; Paus, R.; Handjiski, B.; van der Veen, C.; Eichmüller, S.; McKay, I.A.; Stenn, K.S. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J. Investig. Dermatol. 2001, 117, 3–15. [Google Scholar] [CrossRef]
- Geyfman, M.; Plikus, M.V.; Treffeisen, E.; Andersen, B.; Paus, R. Resting no more: Re-defining telogen, the maintenance stage of the hair growth cycle. Biol. Rev. 2015, 90, 1179–1196. [Google Scholar] [CrossRef]
- Son, M.J.; Jeong, J.K.; Kwon, Y.; Ryu, J.-S.; Mun, S.J.; Kim, H.J.; Kim, S.-w.; Yoo, S.; Kook, J.; Lee, H. A novel and safe small molecule enhances hair follicle regeneration by facilitating metabolic reprogramming. Exp. Mol. Med. 2018, 50, 1–15. [Google Scholar] [CrossRef]
- Philpott, M.P.; Kealey, T. Metabolic studies on isolated hair follicles: Hair follicles engaged in aerobic glycolysis and do not demonstrate the glucose fatty acid cycle. J. Investig. Dermatol. 1990, 96, 875–879. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, T. Local and systemic mechanisms that control the hair follicle stem cell niche. Nat. Rev. Mol. Cell Biol. 2024, 25, 87–100. [Google Scholar] [CrossRef]
- Zhou, S.; Li, Z.; Li, X.; Ye, Y.; Wang, M.; Jiang, J.; Tao, L.; Wang, Y.; Tung, C.-T.; Chung, Y. Crosstalk between endothelial cells and dermal papilla entails hair regeneration and angiogenesis during aging. J. Adv. Res. 2025, 70, 339–353. [Google Scholar] [CrossRef]
- Sun, Q.; Lee, W.; Hu, H.; Ogawa, T.; De Leon, S.; Katehis, I.; Lim, C.H.; Takeo, M.; Cammer, M.; Taketo, M.M. Dedifferentiation maintains melanocyte stem cells in a dynamic niche. Nature 2023, 616, 774–782. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Miao, Y.; Gur-Cohen, S.; Gomez, N.; Yang, H.; Nikolova, M.; Polak, L.; Hu, Y.; Verma, A.; Elemento, O. The aging skin microenvironment dictates stem cell behavior. Proc. Natl. Acad. Sci. USA 2020, 117, 5339–5350. [Google Scholar] [CrossRef]
- Gribonika, I.; Band, V.I.; Chi, L.; Perez-Chaparro, P.J.; Link, V.M.; Ansaldo, E.; Oguz, C.; Bousbaine, D.; Fischbach, M.A.; Belkaid, Y. Skin autonomous antibody production regulates host–microbiota interactions. Nature 2024, 638, 1043–1053. [Google Scholar] [CrossRef]
- Duan, M.; Xu, Y.; Li, Y.; Feng, H.; Chen, Y. Targeting brain-peripheral immune responses for secondary brain injury after ischemic and hemorrhagic stroke. J. Neuroinflammation 2024, 21, 102. [Google Scholar] [CrossRef] [PubMed]
- Niederkorn, J.Y. Corneal nerves, CD11c+ dendritic cells and their impact on ocular immune privilege. Front. Immunol. 2021, 12, 701935. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Chéret, J.; Dinelli, F.S.; Rajabi-Estarabadi, A.; Akhundlu, A.; Demetrius, D.-L.; Gherardini, J.; Keren, A.; Harries, M.; Rodriguez-Feliz, J. Interleukin-15 is a hair follicle immune privilege guardian. J. Autoimmun. 2024, 145, 103217. [Google Scholar] [CrossRef]
- Ito, T.; Ito, N.; Saatoff, M.; Hashizume, H.; Fukamizu, H.; Nickoloff, B.J.; Takigawa, M.; Paus, R. Maintenance of hair follicle immune privilege is linked to prevention of NK cell attack. J. Investig. Dermatol. 2008, 128, 1196–1206. [Google Scholar] [CrossRef]
- Li, X.; An, T.; Yang, Y.; Xu, Z.; Chen, S.; Yi, Z.; Deng, C.; Zhou, F.; Man, Y.; Hu, C. TLR9 activation in large wound induces tissue repair and hair follicle regeneration via γδT cells. Cell Death Dis. 2024, 15, 598. [Google Scholar] [CrossRef]
- Dalessandri, T.; Kasper, M. TREMendous macrophages inhibit hair growth. Cell Stem Cell 2019, 24, 501–502. [Google Scholar] [CrossRef] [PubMed]
- Paus, R.; Christoph, T.; Müller-Röver, S. Immunology of the hair follicle: A short journey into terra incognita. J. Investig. Dermatol. Symp. Proc. 1999, 4, 226–234. [Google Scholar] [CrossRef]
- Ali, N.; Zirak, B.; Rodriguez, R.S.; Pauli, M.L.; Truong, H.-A.; Lai, K.; Ahn, R.; Corbin, K.; Lowe, M.M.; Scharschmidt, T.C. Regulatory T cells in skin facilitate epithelial stem cell differentiation. Cell 2017, 169, 1119–1129. [Google Scholar] [CrossRef]
- Xiao, X.; Gao, Y.; Yan, L.; Deng, C.; Wu, W.; Lu, X.; Lu, Q.; Zhong, W.; Xu, Y.; Zhang, C. M1 polarization of macrophages promotes stress-induced hair loss via interleukin-18 and interleukin-1β. J. Cell. Physiol. 2024, 239, e31181. [Google Scholar] [CrossRef]
- Gay, D.; Ghinatti, G.; Guerrero-Juarez, C.F.; Ferrer, R.A.; Ferri, F.; Lim, C.H.; Murakami, S.; Gault, N.; Barroca, V.; Rombeau, I. Phagocytosis of Wnt inhibitor SFRP4 by late wound macrophages drives chronic Wnt activity for fibrotic skin healing. Science advances 2020, 6, eaay3704. [Google Scholar] [CrossRef]
- Liu, Z.; Hu, X.; Liang, Y.; Yu, J.; Li, H.; Shokhirev, M.N.; Zheng, Y. Glucocorticoid signaling and regulatory T cells cooperate to maintain the hair-follicle stem-cell niche. Nat. Immunol. 2022, 23, 1086–1097. [Google Scholar] [CrossRef] [PubMed]
- Peña-Jimenez, D.; Fontenete, S.; Megias, D.; Fustero-Torre, C.; Graña-Castro, O.; Castellana, D.; Loewe, R.; Perez-Moreno, M. Lymphatic vessels interact dynamically with the hair follicle stem cell niche during skin regeneration in vivo. EMBO J. 2019, 38, e101688. [Google Scholar] [CrossRef] [PubMed]
- Nagao, K.; Kobayashi, T.; Moro, K.; Ohyama, M.; Adachi, T.; Kitashima, D.Y.; Ueha, S.; Horiuchi, K.; Tanizaki, H.; Kabashima, K. Stress-induced production of chemokines by hair follicles regulates the trafficking of dendritic cells in skin. Nat. Immunol. 2012, 13, 744–752. [Google Scholar] [CrossRef]
- Wang, Y.; Szretter, K.J.; Vermi, W.; Gilfillan, S.; Rossini, C.; Cella, M.; Barrow, A.D.; Diamond, M.S.; Colonna, M. IL-34 is a tissue-restricted ligand of CSF1R required for the development of Langerhans cells and microglia. Nat. Immunol. 2012, 13, 753–760. [Google Scholar] [CrossRef]
- Park, J.; Shin, S.; Liu, L.; Jahan, I.; Ong, S.-G.; Xu, P.; Berry, D.C.; Jiang, Y. Progenitor-like characteristics in a subgroup of UCP1+ cells within white adipose tissue. Dev. Cell 2021, 56, 985–999.e984. [Google Scholar] [PubMed]
- Zhang, L.-j.; Guerrero-Juarez, C.F.; Chen, S.X.; Zhang, X.; Yin, M.; Li, F.; Wu, S.; Chen, J.; Li, M.; Liu, Y. Diet-induced obesity promotes infection by impairment of the innate antimicrobial defense function of dermal adipocyte progenitors. Sci. Transl. Med. 2021, 13, eabb5280. [Google Scholar] [CrossRef]
- Kruglikov, I.L.; Zhang, Z.; Scherer, P.E. The role of immature and mature adipocytes in hair cycling. Trends Endocrinol. Metab. 2019, 30, 93–105. [Google Scholar] [CrossRef]
- Chase, H.B.; Montagna, W.; Malone, J.D. Changes in the skin in relation to the hair growth cycle. Anat. Rec. 1953, 116, 75–81. [Google Scholar] [CrossRef]
- Rivera-Gonzalez, G.; Shook, B.; Horsley, V. Adipocytes in skin health and disease. Cold Spring Harb. Perspect. Med. 2014, 4, a015271. [Google Scholar] [CrossRef]
- Connolly, K.D.; Guschina, I.A.; Yeung, V.; Clayton, A.; Draman, M.S.; Von Ruhland, C.; Ludgate, M.; James, P.E.; Rees, D.A. Characterisation of adipocyte-derived extracellular vesicles released pre-and post-adipogenesis. J. Extracell. Vesicles 2015, 4, 29159. [Google Scholar] [CrossRef]
- Guerrero-Juarez, C.F.; Plikus, M.V. Emerging nonmetabolic functions of skin fat. Nat. Rev. Endocrinol. 2018, 14, 163–173. [Google Scholar] [CrossRef]
- Festa, E.; Fretz, J.; Berry, R.; Schmidt, B.; Rodeheffer, M.; Horowitz, M.; Horsley, V. Adipocyte lineage cells contribute to the skin stem cell niche to drive hair cycling. Cell 2011, 146, 761–771. [Google Scholar] [CrossRef]
- Sardella, C.; Winkler, C.; Quignodon, L.; Hardman, J.A.; Toffoli, B.; Attianese, G.M.P.G.; Hundt, J.E.; Michalik, L.; Vinson, C.R.; Paus, R. Delayed hair follicle morphogenesis and hair follicle dystrophy in a lipoatrophy mouse model of Pparg total deletion. J. Investig. Dermatol. 2018, 138, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tang, W.; Tian, W.; Yu, M. Research progress in regulation of hair growth by dermal adipose tissue. Zhongguo xiu fu Chong Jian wai ke za zhi= Zhongguo Xiufu Chongjian Waike Zazhi. Chin. J. Reparative Reconstr. Surg. 2024, 38, 626–632. [Google Scholar]
- Chen, H.C.; Smith, S.J.; Tow, B.; Elias, P.M.; Farese, R.V. Leptin modulates the effects of acyl CoA: Diacylglycerol acyltransferase deficiency on murine fur and sebaceous glands. J. Clin. Investig. 2002, 109, 175–181. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, M.; Zhang, H.; Hou, Z.; Liu, D. Hypoxia treatment of adipose mesenchymal stem cells promotes the growth of dermal papilla cells via HIF-1α and ERK1/2 signaling pathways. Int. J. Mol. Sci. 2023, 24, 11198. [Google Scholar] [CrossRef]
- Kang, Y.; Yeo, M.; Derman, I.D.; Ravnic, D.J.; Singh, Y.P.; Alioglu, M.A.; Wu, Y.; Makkar, J.; Driskell, R.R.; Ozbolat, I.T. Intraoperative bioprinting of human adipose-derived stem cells and extra-cellular matrix induces hair follicle-like downgrowths and adipose tissue formation during full-thickness craniomaxillofacial skin reconstruction. Bioact. Mater. 2024, 33, 114–128. [Google Scholar] [CrossRef] [PubMed]
- Citrin, K.M.; Chaube, B.; Fernández-Hernando, C.; Suárez, Y. Intracellular endothelial cell metabolism in vascular function and dysfunction. Trends Endocrinol. Metab. 2025, 36, 744–755. [Google Scholar] [CrossRef] [PubMed]
- Mecklenburg, L.; Tobin, D.J.; Müller-Röver, S.; Handjiski, B.; Wendt, G.; Peters, E.M.; Pohl, S.; Moll, I.; Paus, R. Active hair growth (anagen) is associated with angiogenesis. J. Investig. Dermatol. 2000, 114, 909–916. [Google Scholar] [CrossRef]
- Gunawardena, T.N.A.; Rahman, M.T.; Abdullah, B.J.J.; Abu Kasim, N.H. Conditioned media derived from mesenchymal stem cell cultures: The next generation for regenerative medicine. J. Tissue Eng. Regen. Med. 2019, 13, 569–586. [Google Scholar] [CrossRef] [PubMed]
- Vizoso, F.J.; Eiro, N.; Cid, S.; Schneider, J.; Perez-Fernandez, R. Mesenchymal stem cell secretome: Toward cell-free therapeutic strategies in regenerative medicine. Int. J. Mol. Sci. 2017, 18, 1852. [Google Scholar] [CrossRef]
- Kageyama, T.; Chun, Y.-S.; Fukuda, J. Hair follicle germs containing vascular endothelial cells for hair regenerative medicine. Sci. Rep. 2021, 11, 624. [Google Scholar] [CrossRef]
- Li, K.N.; Chovatiya, G.; Ko, D.Y.; Sureshbabu, S.; Tumbar, T. Blood endothelial ALK1-BMP4 signaling axis regulates adult hair follicle stem cell activation. EMBO J. 2023, 42, e112196. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zheng, Y.; Zhao, D.; Zhao, L.; Geng, L.; Ma, S.; Cai, Y.; Liu, C.; Yan, Y.; Belmonte, J.C.I. Single-cell profiling reveals a potent role of quercetin in promoting hair regeneration. Protein Cell 2023, 14, 398–415. [Google Scholar] [CrossRef]
- Xie, B.; Chen, M.; Ding, P.; Lei, L.; Zhang, X.; Zhu, D.; Zou, Y.; Deng, Z.; Sun, G.; Li, J. Induction of dermal fibroblasts into dermal papilla cell-like cells in hydrogel microcapsules for enhanced hair follicle regeneration. Appl. Mater. Today 2020, 21, 100805. [Google Scholar] [CrossRef]
- Cerqueira, M.; Pirraco, R.P.; Santos, T.; Rodrigues, D.; Frias, A.; Martins, A.; Reis, R.; Marques, A. Human adipose stem cells cell sheet constructs impact epidermal morphogenesis in full-thickness excisional wounds. Biomacromolecules 2013, 14, 3997–4008. [Google Scholar] [CrossRef]
- Fujiwara, H.; Ferreira, M.; Donati, G.; Marciano, D.K.; Linton, J.M.; Sato, Y.; Hartner, A.; Sekiguchi, K.; Reichardt, L.F.; Watt, F.M. The basement membrane of hair follicle stem cells is a muscle cell niche. Cell 2011, 144, 577–589. [Google Scholar] [CrossRef]
- Hoffmann, J.P.; Liu, J.A.; Seddu, K.; Klein, S.L. Sex hormone signaling and regulation of immune function. Immunity 2023, 56, 2472–2491. [Google Scholar] [CrossRef]
- Chesnokova, V.; Melmed, S. Peptide hormone regulation of DNA damage responses. Endocr. Rev. 2020, 41, bnaa009. [Google Scholar] [CrossRef]
- Sato, T.; Matsumoto, T.; Yamada, T.; Watanabe, T.; Kawano, H.; Kato, S. Late onset of obesity in male androgen receptor-deficient (AR KO) mice. Biochem. Biophys. Res. Commun. 2003, 300, 167–171. [Google Scholar] [CrossRef]
- Dawson, H.L. On hair growth: A study of the effect of pregnancy on the activity of the follicle in the guinea-pig (Cavia cobaya). Am. J. Anat. 1933, 53, 89–115. [Google Scholar] [CrossRef]
- Williams, W.L.; Gardner, W.; DeVita, J. Local inhibition of hair growth in dogs by ercutaneous application of estrone1. Endocrinology 1946, 38, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Blume-Peytavi, U.; Vogt, A. Current standards in the diagnostics and therapy of hair diseases–hair consultation. JDDG J. Der Dtsch. Dermatol. Ges. 2011, 9, 394–412. [Google Scholar] [CrossRef] [PubMed]
- Fraser, A.; Nay, T. Growth of the mouse coat II. Effect of sex and pregnancy. Aust. J. Biol. Sci. 1953, 6, 645–656. [Google Scholar] [CrossRef]
- Bodó, E.; Kromminga, A.; Bíró, T.; Borbíró, I.; Gáspár, E.; Zmijewski, M.A.; Van Beek, N.; Langbein, L.; Slominski, A.T.; Paus, R. Human female hair follicles are a direct, nonclassical target for thyroid-stimulating hormone. J. Investig. Dermatol. 2009, 129, 1126–1139. [Google Scholar] [CrossRef] [PubMed]
- Knuever, J.; Poeggeler, B.; Gáspár, E.; Klinger, M.; Hellwig-Burgel, T.; Hardenbicker, C.; Tóth, B.I.; Bíró, T.; Paus, R. Thyrotropin-releasing hormone controls mitochondrial biology in human epidermis. J. Clin. Endocrinol. Metab. 2012, 97, 978–986. [Google Scholar] [CrossRef]
- Slominski, R.M.; Tuckey, R.C.; Manna, P.R.; Jetten, A.M.; Postlethwaite, A.; Raman, C.; Slominski, A.T. Extra-adrenal glucocorticoid biosynthesis: Implications for autoimmune and inflammatory disorders. Genes Immun. 2020, 21, 150–168. [Google Scholar] [CrossRef]
- Choi, S.; Zhang, B.; Ma, S.; Gonzalez-Celeiro, M.; Stein, D.; Jin, X.; Kim, S.T.; Kang, Y.-L.; Besnard, A.; Rezza, A. Corticosterone inhibits GAS6 to govern hair follicle stem-cell quiescence. Nature 2021, 592, 428–432. [Google Scholar] [CrossRef]
- Zhang, B.; Ma, S.; Rachmin, I.; He, M.; Baral, P.; Choi, S.; Gonçalves, W.A.; Shwartz, Y.; Fast, E.M.; Su, Y. Hyperactivation of sympathetic nerves drives depletion of melanocyte stem cells. Nature 2020, 577, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Fischer, T.; Naumann, V.; Bodo, E.; Paus, R. Towards new aspects of melatonin research in dermato-endocrinology. Exp. Dermatol. 2008, 17, 625. [Google Scholar] [CrossRef]
- Niu, Y.L.; Li, Y.K.; Gao, C.X.; Li, W.W.; Li, L.; Wang, H.; Shen, W.; Ge, W. Melatonin promotes hair regeneration by modulating the Wnt/β-catenin signalling pathway. Cell Prolif. 2024, 57, e13656. [Google Scholar] [CrossRef] [PubMed]
- Diao, X.; Duan, C.; Yao, L.; Qin, J.; He, L.; Zhang, W. Melatonin promotes the development of secondary hair follicles in adult cashmere goats by activating the Keap1-Nrf2 signaling pathway and inhibiting the inflammatory transcription factors NFκB and AP-1. Int. J. Mol. Sci. 2023, 24, 3403. [Google Scholar] [CrossRef]
- Jeon, S.-M.; Shin, E.-A. Exploring vitamin D metabolism and function in cancer. Exp. Mol. Med. 2018, 50, 1–14. [Google Scholar] [CrossRef]
- Traber, M.G. Vitamin E: Necessary nutrient for neural development and cognitive function. Proc. Nutr. Soc. 2021, 80, 319–326. [Google Scholar] [CrossRef]
- Tierney, M.T.; Polak, L.; Yang, Y.; Abdusselamoglu, M.D.; Baek, I.; Stewart, K.S.; Fuchs, E. Vitamin A resolves lineage plasticity to orchestrate stem cell lineage choices. Science 2024, 383, eadi7342. [Google Scholar] [CrossRef]
- Arnson, Y.; Amital, H.; Shoenfeld, Y. Vitamin D and autoimmunity: New aetiological and therapeutic considerations. Ann. Rheum. Dis. 2007, 66, 1137–1142. [Google Scholar] [CrossRef]
- Baeke, F.; van Etten, E.; Gysemans, C.; Overbergh, L.; Mathieu, C. Vitamin D signaling in immune-mediated disorders: Evolving insights and therapeutic opportunities. Mol. Asp. Med. 2008, 29, 376–387. [Google Scholar] [CrossRef]
- Bikle, D.D.; Oda, Y.; Tu, C.-L.; Jiang, Y. Novel mechanisms for the vitamin D receptor (VDR) in the skin and in skin cancer. J. Steroid Biochem. Mol. Biol. 2015, 148, 47–51. [Google Scholar] [CrossRef] [PubMed]
- van Etten, E.; Mathieu, C. Immunoregulation by 1, 25-dihydroxyvitamin D3: Basic concepts. J. Steroid Biochem. Mol. Biol. 2005, 97, 93–101. [Google Scholar] [CrossRef]
- Kim, D.H.; Lee, J.W.; Kim, I.S.; Choi, S.Y.; Lim, Y.Y.; Kim, H.M.; Kim, B.J.; Kim, M.N. Successful treatment of alopecia areata with topical calcipotriol. Ann. Dermatol. 2012, 24, 341–344. [Google Scholar] [CrossRef]
- Gerkowicz, A.; Chyl-Surdacka, K.; Krasowska, D.; Chodorowska, G. The role of vitamin D in non-scarring alopecia. Int. J. Mol. Sci. 2017, 18, 2653. [Google Scholar] [CrossRef]
- Almohanna, H.M.; Ahmed, A.A.; Tsatalis, J.P.; Tosti, A. The role of vitamins and minerals in hair loss: A review. Dermatol. Ther. 2019, 9, 51–70. [Google Scholar] [CrossRef]
- Zarafonetis, J.C. Darkening of gray hair during para-amino-benzoic acid therapy. J. Investig. Dermatol. 1950, 15, 399–401. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, L.J.; Lenzy, Y. Nutrition and hair. Clin. Dermatol. 2010, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Mindur, J.E.; Swirski, F.K. Growth factors as immunotherapeutic targets in cardiovascular disease. Arteriosclerosis. Thromb. Vasc. Biol. 2019, 39, 1275–1287. [Google Scholar] [CrossRef]
- Wang, F.; Tan, Y.-Q.; Zhang, J.; Zhou, G. Insulin-like growth factor 1 exhibits the pro-autophagic and anti-apoptotic activity on T cells of oral lichen planus. Int. J. Biol. Macromol. 2019, 133, 640–646. [Google Scholar] [CrossRef]
- Hu, X.; Hao, F.; Li, X.; Xun, Z.; Gao, Y.; Ren, B.; Cang, M.; Liang, H.; Liu, D. Generation of VEGF knock-in Cashmere goat via the CRISPR/Cas9 system. Int. J. Biol. Sci. 2021, 17, 1026. [Google Scholar] [CrossRef]
- Li, W.; Man, X.-Y.; Li, C.-M.; Chen, J.-Q.; Zhou, J.; Cai, S.-Q.; Lu, Z.-F.; Zheng, M. VEGF induces proliferation of human hair follicle dermal papilla cells through VEGFR-2-mediated activation of ERK. Exp. Cell Res. 2012, 318, 1633–1640. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhou, D.; Ma, T.; Liu, Q. Vascular endothelial growth factor protects CD200-rich and CD34-positive hair follicle stem cells against androgen-induced apoptosis through the phosphoinositide 3-kinase/Akt pathway in patients with androgenic alopecia. Dermatol. Surg. 2020, 46, 358–368. [Google Scholar] [CrossRef]
- Lin, Y.; Liu, C.; Zhan, X.; Wang, B.; Li, K.; Li, J. Jagged1 and epidermal growth factor promoted androgen-suppressed mouse hair growth in vitro and in vivo. Front. Pharmacol. 2020, 10, 1634. [Google Scholar] [CrossRef]
- Kawen, A.A. Effects of oral minoxidil on serum VEGF and hair regrowth in androgenetic alopecia. Acta Dermatoven. Alp. 2025, 34, 7–12. [Google Scholar]
- Bai, T.; Liu, F.; Zou, F.; Zhao, G.; Jiang, Y.; Liu, L.; Shi, J.; Hao, D.; Zhang, Q.; Zheng, T. Epidermal growth factor induces proliferation of hair follicle-derived mesenchymal stem cells through epidermal growth factor receptor-mediated activation of ERK and AKT signaling pathways associated with upregulation of cyclin D1 and downregulation of p16. Stem Cells Dev. 2017, 26, 113–122. [Google Scholar] [CrossRef]
- Sugawara, K.; Schneider, M.R.; Dahlhoff, M.; Kloepper, J.E.; Paus, R. Cutaneous consequences of inhibiting EGF receptor signaling in vivo: Normal hair follicle development, but retarded hair cycle induction and inhibition of adipocyte growth in EgfrWa5 mice. J. Dermatol. Sci. 2010, 57, 155–161. [Google Scholar] [CrossRef]
- DM, D. Keratinocyte growth factor is an important endogeneous mediator of hair follicle growth, development, and differentiation. Normalization of the nu/nu follicular differentiation defect and amelioration of chemotherapy-induced alopecia. Am. J. Pathol. 1995, 147, 145–154. [Google Scholar]
- Kawano, M.; Komi-Kuramochi, A.; Asada, M.; Suzuki, M.; Oki, J.; Jiang, J.; Imamura, T. Comprehensive analysis of FGF and FGFR expression in skin: FGF18 is highly expressed in hair follicles and capable of inducing anagen from telogen stage hair follicles. J. Investig. Dermatol. 2005, 124, 877–885. [Google Scholar] [CrossRef]
- Li, Y.; Song, S.; Zhang, Z.; Liu, X.; Zhang, Y.; E, G.; Ma, Y.; Jiang, L. A deletion variant within the FGF5 gene in goats is associated with gene expression levels and cashmere growth. Anim. Genet. 2022, 53, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Chai, B.; Zhang, K. Topical application of fibroblast growth factor 10-PLGA microsphere accelerates wound healing via inhibition of ER stress, Oxid. Med. Cell. Longev. 2020, 2020, 8586314. [Google Scholar]
- Xu, W.; Fan, W.; Yao, K. Cyclosporine A stimulated hair growth from mouse vibrissae follicles in an organ culture model. J. Biomed. Res. 2012, 26, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shaanan, T.L.; Knöpper, K.; Duan, L.; Liu, R.; Taglinao, H.; Xu, Y.; An, J.; Plikus, M.V.; Cyster, J.G. Dermal TRPV1 innervations engage a macrophage-and fibroblast-containing pathway to activate hair growth in mice. Dev. Cell 2024, 59, 2818–2833.e7. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, Y.; He, J.; Wang, J.; Chen, X.; Yang, R. Regulation of signaling pathways in hair follicle stem cells. Burns. Trauma 2022, 10, tkac022. [Google Scholar] [CrossRef]
- Hu, X.-M.; Li, Z.-X.; Zhang, D.-Y.; Yang, Y.-C.; Fu, S.-a.; Zhang, Z.-Q.; Yang, R.-H.; Xiong, K. A systematic summary of survival and death signalling during the life of hair follicle stem cells. Stem Cell Res. Ther. 2021, 12, 453. [Google Scholar] [CrossRef] [PubMed]
- Harel, S.; Higgins, C.A.; Cerise, J.E.; Dai, Z.; Chen, J.C.; Clynes, R.; Christiano, A.M. Pharmacologic inhibition of JAK-STAT signaling promotes hair growth. Sci. Adv. 2015, 1, e1500973. [Google Scholar] [CrossRef]
- Ito, T.; Ito, N.; Saathoff, M.; Bettermann, A.; Takigawa, M.; Paus, R. Interferon-γ is a potent inducer of catagen-like changes in cultured human anagen hair follicles. Br. J. Dermatol. 2005, 152, 623–631. [Google Scholar] [CrossRef]
- Wang, X.; Chen, H.; Tian, R.; Zhang, Y.; Drutskaya, M.S.; Wang, C.; Ge, J.; Fan, Z.; Kong, D.; Wang, X. Macrophages induce AKT/β-catenin-dependent Lgr5+ stem cell activation and hair follicle regeneration through TNF. Nat. Commun. 2017, 8, 14091. [Google Scholar] [CrossRef]
- Craven, A.; Nixon, A.; Ashby, M.; Ormandy, C.; Blazek, K.; Wilkins, R.J.; Pearson, A. Prolactin delays hair regrowth in mice. J. Endocrinol. 2006, 191, 415–425. [Google Scholar] [CrossRef]
- Nicu, C.; O’Sullivan, J.D.; Ramos, R.; Timperi, L.; Lai, T.; Farjo, N.; Farjo, B.; Pople, J.; Bhogal, R.; Hardman, J.A. Dermal adipose tissue secretes HGF to promote human hair growth and pigmentation. J. Investig. Dermatol. 2021, 141, 1633–1645.e13. [Google Scholar] [CrossRef]
- Liu, Y.; Guerrero-Juarez, C.F.; Xiao, F.; Shettigar, N.U.; Ramos, R.; Kuan, C.-H.; Lin, Y.-C.; Lomeli, L.d.J.M.; Park, J.M.; Oh, J.W. Hedgehog signaling reprograms hair follicle niche fibroblasts to a hyper-activated state. Dev. Cell 2022, 57, 1758–1775.e1757. [Google Scholar] [CrossRef]
- Wang, X.; Ramos, R.; Phan, A.Q.; Yamaga, K.; Flesher, J.L.; Jiang, S.; Oh, J.W.; Jin, S.; Jahid, S.; Kuan, C.-H. Signalling by senescent melanocytes hyperactivates hair growth. Nature 2023, 618, 808–817. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, H.; Mohri, Y.; Binh, N.T.; Morinaga, H.; Fukuda, M.; Ito, M.; Kurata, S.; Hoeijmakers, J.; Nishimura, E.K. Hair follicle aging is driven by transepidermal elimination of stem cells via COL17A1 proteolysis. Science 2016, 351, aad4395. [Google Scholar] [CrossRef]
- Gur-Cohen, S.; Yang, H.; Baksh, S.C.; Miao, Y.; Levorse, J.; Kataru, R.P.; Liu, X.; De La Cruz-Racelis, J.; Mehrara, B.J.; Fuchs, E. Stem cell–driven lymphatic remodeling coordinates tissue regeneration. Science 2019, 366, 1218–1225. [Google Scholar] [CrossRef]
- González, R.; Moffatt, G.; Hagner, A.; Sinha, S.; Shin, W.; Rahmani, W.; Chojnacki, A.; Biernaskie, J. Platelet-derived growth factor signaling modulates adult hair follicle dermal stem cell maintenance and self-renewal. NPJ Regen. Med. 2017, 2, 11. [Google Scholar] [CrossRef] [PubMed]
- Sibony-Benyamini, H.; Aamar, E.; Enshell-Seijffers, D. Hdac1 and Hdac2 regulate the quiescent state and survival of hair-follicle mesenchymal niche. Nat. Commun. 2023, 14, 4820. [Google Scholar] [CrossRef]
- Bonfanti, P.; Claudinot, S.; Amici, A.W.; Farley, A.; Blackburn, C.C.; Barrandon, Y. Microenvironmental reprogramming of thymic epithelial cells to skin multipotent stem cells. Nature 2010, 466, 978–982. [Google Scholar] [CrossRef] [PubMed]
- Gáspár, E.; Hardenbicker, C.; Bodó, E.; Wenzel, B.; Ramot, Y.; Funk, W.; Kromminga, A.; Paus, R. Thyrotropin releasing hormone (TRH): A new player in human hair-growth control. FASEB J. 2010, 24, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Holub, B.; Kloepper, J.E.; Tóth, B.I.; Bíró, T.; Kofler, B.; Paus, R. The neuropeptide galanin is a novel inhibitor of human hair growth. Br. J. Dermatol. 2012, 167, 10–16. [Google Scholar] [CrossRef]
- Yang, K.; Wang, X.; Song, C.; He, Z.; Wang, R.; Xu, Y.; Jiang, G.; Wan, Y.; Mei, J.; Mao, W. The role of lipid metabolic reprogramming in tumor microenvironment. Theranostics 2023, 13, 1774. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, D.; Yang, L.; Zhang, Y. Metabolic reprogramming in the immunosuppression of tumor-associated macrophages. Chin. Med. J. 2022, 135, 2405–2416. [Google Scholar] [CrossRef]
- Sun, P.; Wang, Z.; Li, S.; Yin, J.; Gan, Y.; Liu, S.; Lin, Z.; Wang, H.; Fan, Z.; Qu, Q. Autophagy induces hair follicle stem cell activation and hair follicle regeneration by regulating glycolysis. Cell Biosci. 2024, 14, 6. [Google Scholar] [CrossRef]
- Kim, C.S.; Ding, X.; Allmeroth, K.; Biggs, L.C.; Kolenc, O.I.; L’Hoest, N.; Chacón-Martínez, C.A.; Edlich-Muth, C.; Giavalisco, P.; Quinn, K.P. Glutamine metabolism controls stem cell fate reversibility and long-term maintenance in the hair follicle. Cell Metab. 2020, 32, 629–642.e8. [Google Scholar] [CrossRef]
- Wang, G.; Sweren, E.; Andrews, W.; Li, Y.; Chen, J.; Xue, Y.; Wier, E.; Alphonse, M.P.; Luo, L.; Miao, Y. Commensal microbiome promotes hair follicle regeneration by inducing keratinocyte HIF-1α signaling and glutamine metabolism. Sci. Adv. 2023, 9, eabo7555. [Google Scholar] [CrossRef]
- Morinaga, H.; Mohri, Y.; Grachtchouk, M.; Asakawa, K.; Matsumura, H.; Oshima, M.; Takayama, N.; Kato, T.; Nishimori, Y.; Sorimachi, Y. Obesity accelerates hair thinning by stem cell-centric converging mechanisms. Nature 2021, 595, 266–271. [Google Scholar] [CrossRef]
- Chen, H.; Liu, C.; Cui, S.; Xia, Y.; Zhang, K.; Cheng, H.; Peng, J.; Yu, X.; Li, L.; Yu, H. Intermittent fasting triggers interorgan communication to suppress hair follicle regeneration. Cell 2025, 188, 157–174.e22. [Google Scholar] [CrossRef]
- Ma, S.; Cao, W.; Ma, X.; Ye, X.; Qin, C.; Li, B.; Liu, W.; Lu, Q.; Wu, C.; Fu, X. Metabolomics reveals metabolites associated with hair follicle cycle in cashmere goats. BMC Vet. Res. 2024, 20, 208. [Google Scholar] [CrossRef]
- Feaster, B.; Onamusi, T.; Cooley, J.E.; McMichael, A.J. Oral minoxidil use in androgenetic alopecia and telogen effluvium. Arch. Dermatol. Res. 2023, 315, 201–205. [Google Scholar] [CrossRef]
- Bai, L.; Wang, Y.; Wang, K.; Chen, X.; Zhao, Y.; Liu, C.; Qu, X. Materiobiomodulated ROS Therapy for De Novo Hair Growth. Adv. Mater. 2024, 36, 2311459. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Xiong, J.; Guo, R.; Li, Y.; Jiang, H. A comprehensive review of microneedling as a potential treatment option for androgenetic alopecia. Aesthetic Plast. Surg. 2022, 46, 2979–2994. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.-W.; Li, Y.; Zhang, Z.-W.; Dao, J.-W.; Wei, D.-X. Hydrogel forming microneedles loaded with VEGF and Ritlecitinib/polyhydroxyalkanoates nanoparticles for mini-invasive androgenetic alopecia treatment. Bioact. Mater. 2024, 38, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Jeong, K.H.; Kim, J.E.; Woo, Y.J.; Kim, B.J.; Kang, H. Repeated Microneedle Stimulation Induces Enhanced Hair Growth in a Murine Model. Ann. Dermatol. 2016, 28, 586–592. [Google Scholar] [CrossRef]
- Zhang, Y.; Sheng, Y.Y.; Zeng, Y.B.; Hu, R.M.; Zhao, J.; Wang, W.Q.; Yang, Q.P. Randomized trial of microneedling combined with 2% minoxidil topical solution for the treatment of female pattern hair loss in a Chinese population. J. Cosmet. Dermatol. 2022, 21, 6985–6991. [Google Scholar] [CrossRef]
- Liebl, H.; Kloth, L.C. Skin cell proliferation stimulated by microneedles. J. Am. Coll. Clin. Wound Spec. 2012, 4, 2–6. [Google Scholar] [CrossRef]
- Kanti, V.; Messenger, A.; Dobos, G.; Reygagne, P.; Finner, A.; Blumeyer, A.; Trakatelli, M.; Tosti, A.; del Marmol, V.; Piraccini, B.M. Evidence-based (S3) guideline for the treatment of androgenetic alopecia in women and in men–short version. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 11–22. [Google Scholar] [CrossRef]
- Esmat, S.M.; Hegazy, R.A.; Gawdat, H.I.; Abdel Hay, R.M.; Allam, R.S.; El Naggar, R.; Moneib, H. Low level light-minoxidil 5% combination versus either therapeutic modality alone in management of female patterned hair loss: A randomized controlled study. Lasers Surg. Med. 2017, 49, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Adav, S.S.; Ng, K.W. Recent omics advances in hair aging biology and hair biomarkers analysis. Ageing Res. Rev. 2023, 91, 102041. [Google Scholar] [CrossRef]
- Wang, M.M.; Wang, M.Y.; Jiang, J.W.; Li, K.; Liang, H.; Wang, N.O.; Zou, Y.; Wang, D.H.; Zhou, S.Y.; Tang, Y.C.; et al. THSD4 promotes hair growth by facilitating dermal papilla and hair matrix interactions. Theranostics 2025, 15, 3571–3588. [Google Scholar] [CrossRef] [PubMed]
- Masone, M.C. Single-cell and spatial sequencing of kidney cancer. Nat. Rev. Urol. 2023, 20, 8. [Google Scholar] [CrossRef]
- Takahashi, R.; Grzenda, A.; Allison, T.F.; Rawnsley, J.; Balin, S.J.; Sabri, S.; Plath, K.; Lowry, W.E. Defining Transcriptional Signatures of Human Hair Follicle Cell States. J. Investig. Dermatol. 2020, 140, 764. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jiang, Y.; Ni, G.Y.; Li, S.J.; Balderson, B.; Zou, Q.; Liu, H.T.; Jiang, Y.F.; Sun, J.C.; Ding, X.D. Integrating Single-Cell and Spatial Transcriptomics Reveals Heterogeneity of Early Pig Skin Development and a Subpopulation with Hair Placode Formation. Adv. Sci. 2024, 11, 202306703. [Google Scholar] [CrossRef] [PubMed]
- Ganier, C.; Mazin, P.; Herrera-Oropeza, G.; Du-Harpur, X.; Blakeley, M.; Gabriel, J.; Predeus, A.V.; Cakir, B.; Prete, M.; Harun, N. Multiscale spatial mapping of cell populations across anatomical sites in healthy human skin and basal cell carcinoma. Proc. Natl. Acad. Sci. USA 2024, 121, e2313326120. [Google Scholar] [CrossRef]
- Takagi, R.; Ishimaru, J.; Sugawara, A.; Toyoshima, K.; Ishida, K.; Ogawa, M.; Sakakibara, K.; Asakawa, K.; Kashiwakura, A.; Oshima, M.; et al. Bioengineering a 3D integumentary organ system from iPS cells using an in vivo transplantation model. Sci. Adv. 2016, 2, e1500887. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Rabbani, C.C.; Gao, H.Y.; Steinhart, M.R.; Woodruff, B.M.; Pflum, Z.E.; Kim, A.; Heller, S.; Liu, Y.L.; Shipchandler, T.Z.; et al. Hair-bearing human skin generated entirely from pluripotent stem cells. Nature 2020, 582, 399–404. [Google Scholar] [CrossRef]
- Chu, X.; Zhou, Z.T.; Qian, X.F.; Shen, H.Y.; Cheng, H.X.; Zhang, J.F. Functional regeneration strategies of hair follicles: Advances and challenges. Stem Cell Res. Ther. 2025, 16, 77. [Google Scholar] [CrossRef]
- Kang, D.N.; Liu, Z.; Qian, C.M.; Huang, J.F.; Zhou, Y.; Mao, X.Y.; Qu, Q.; Liu, B.C.; Wang, J.; Hu, Z.Q.; et al. 3D bioprinting of a gelatin-alginate hydrogel for tissue-engineered hair follicle regeneration. Acta Biomater. 2023, 165, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Catarino, C.M.; Schuck, D.C.; Dechiario, L.; Karande, P. Incorporation of hair follicles in 3D bioprinted models of human skin. Sci. Adv. 2023, 9, eadg0297. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, W.; Peng, M.; Ma, Q.; Han, X.; Gao, C.; Zhang, W.; Liu, D. The Skin Microenvironment: A Dynamic Regulator of Hair Follicle Development, Cycling and Disease. Biomolecules 2025, 15, 1335. https://doi.org/10.3390/biom15091335
Song W, Peng M, Ma Q, Han X, Gao C, Zhang W, Liu D. The Skin Microenvironment: A Dynamic Regulator of Hair Follicle Development, Cycling and Disease. Biomolecules. 2025; 15(9):1335. https://doi.org/10.3390/biom15091335
Chicago/Turabian StyleSong, Weiguo, Mingli Peng, Qiqi Ma, Xiaoyu Han, Chunyan Gao, Wenqi Zhang, and Dongjun Liu. 2025. "The Skin Microenvironment: A Dynamic Regulator of Hair Follicle Development, Cycling and Disease" Biomolecules 15, no. 9: 1335. https://doi.org/10.3390/biom15091335
APA StyleSong, W., Peng, M., Ma, Q., Han, X., Gao, C., Zhang, W., & Liu, D. (2025). The Skin Microenvironment: A Dynamic Regulator of Hair Follicle Development, Cycling and Disease. Biomolecules, 15(9), 1335. https://doi.org/10.3390/biom15091335






