Pinocembrin Downregulates Vascular Smooth Muscle Cells Proliferation and Migration Leading to Attenuate Neointima Formation in Balloon-Injured Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
2.3. Isolation of VSMCs
2.4. Balloon Injury Model
2.5. Cell Proliferation Assay
2.6. Cell Migration Assay
2.7. Zymography
2.8. Immunoblot Analysis
2.9. Cell Cycle Analysis
2.10. Immunocytochemistry
2.11. Morphometric Analysis
2.12. Immunohistochemistry
2.13. Statistical Analysis
3. Results
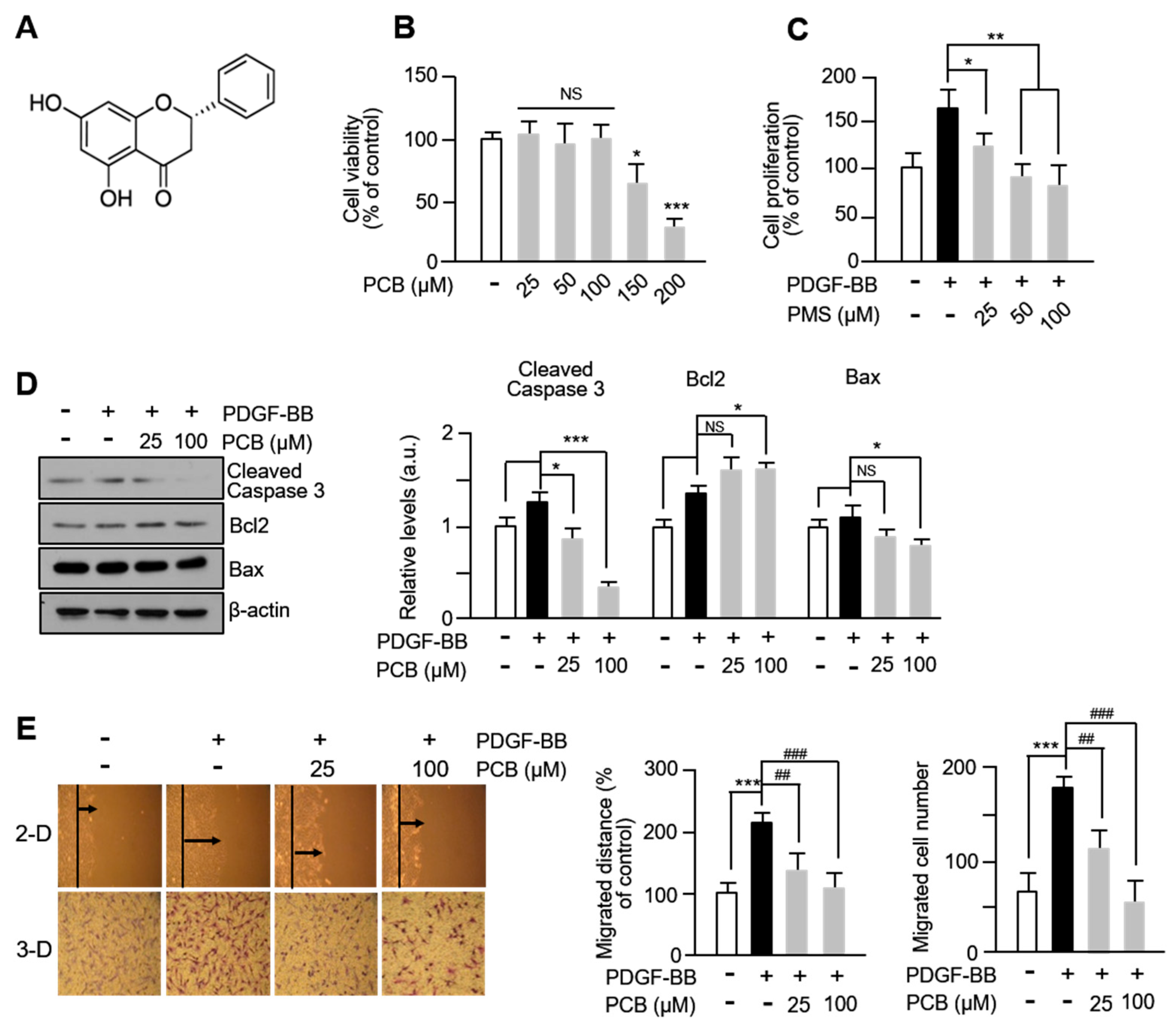
3.1. PCB Inhibits PDGF-BB-Induced VSMC Proliferation and Migration
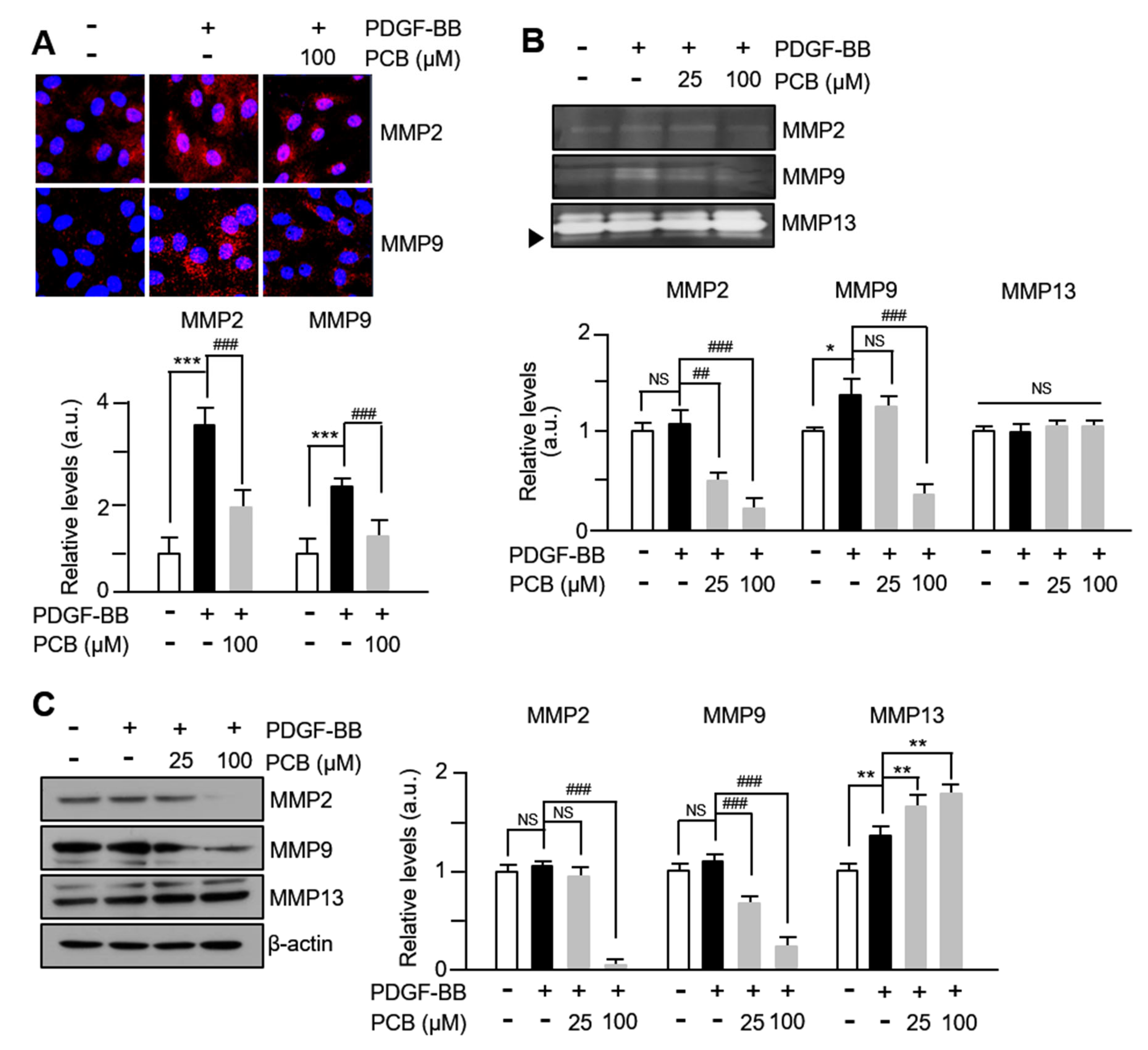
3.2. PCB Inhibits PDGF-BB Induced MMPs Expression and Activity
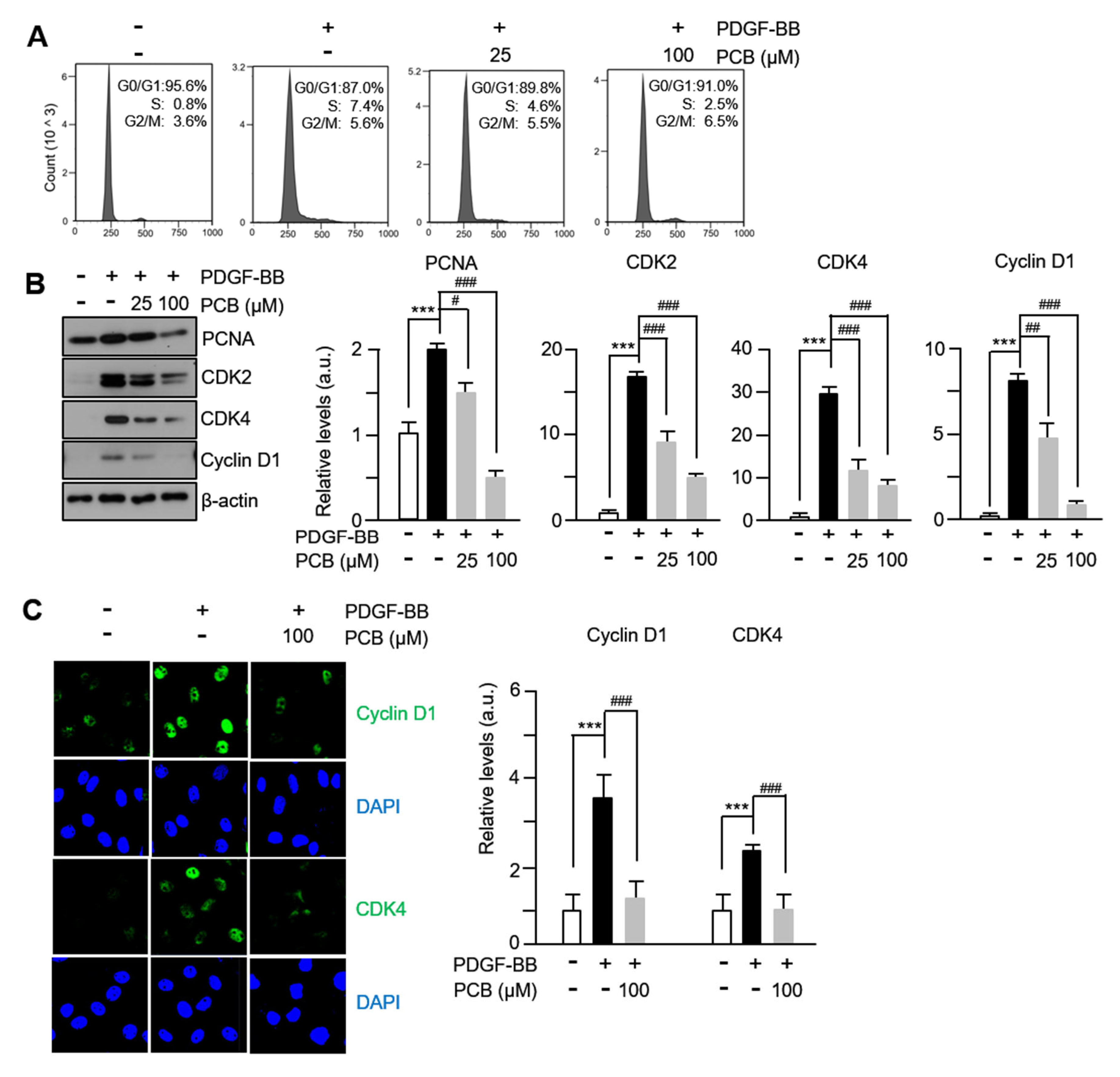
3.3. PCB Inhibits PDGF-BB Induced VSMC Proliferation
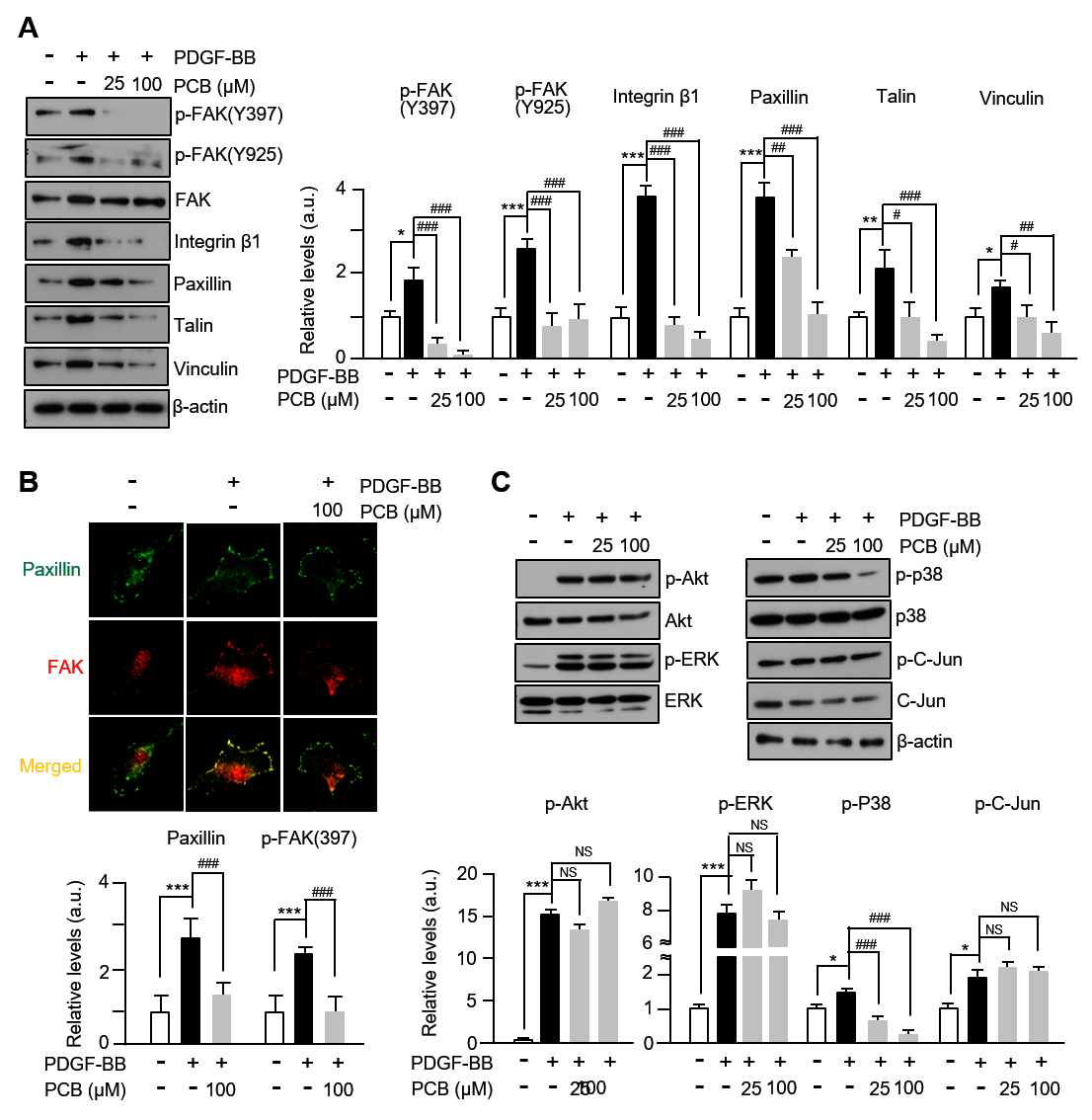
3.4. PCB Attenuates PDGF-BB-Induced the FAK-Related and p38 Signaling Pathways
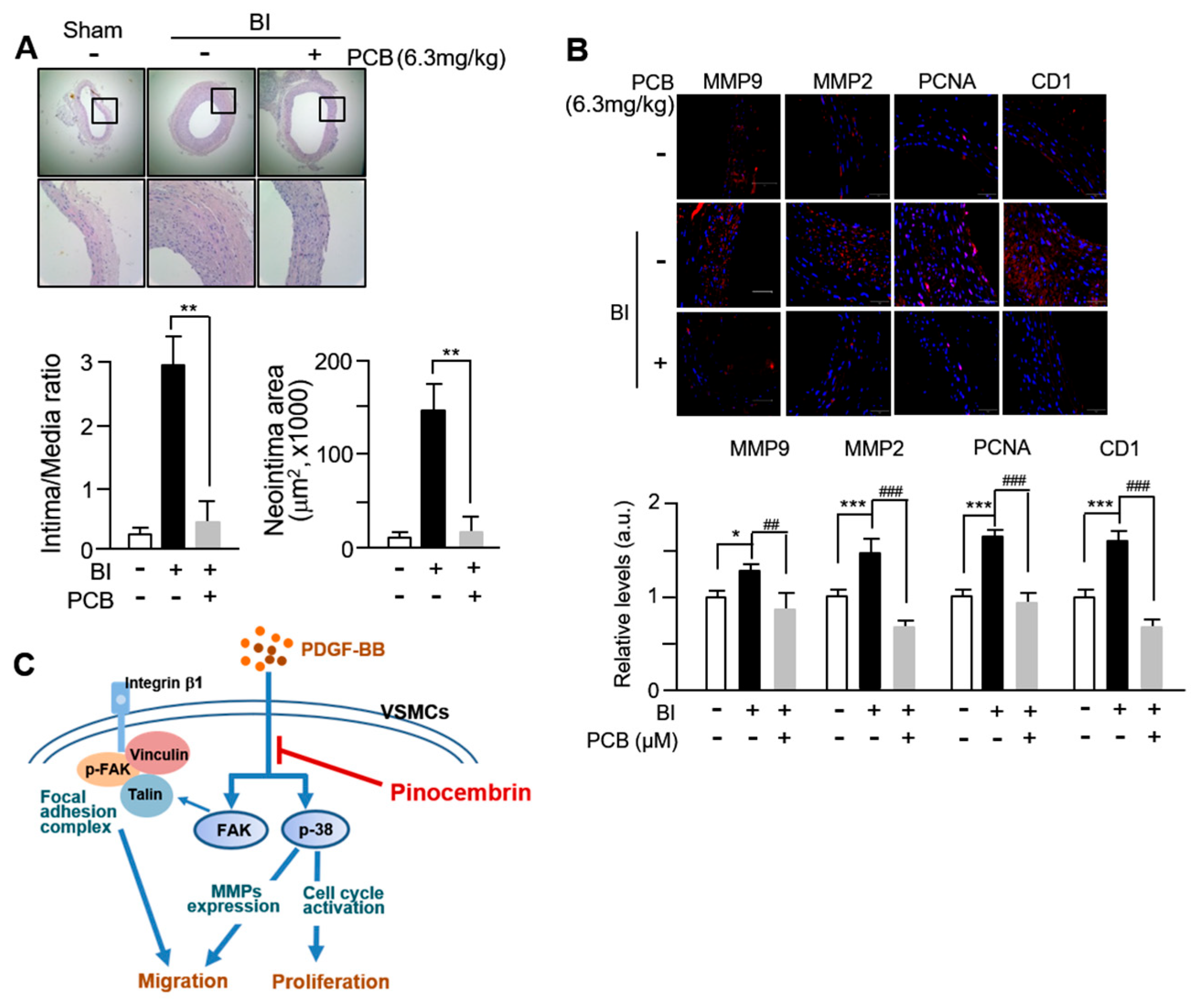
3.5. PCB Inhibits Neointima Formation Induced in BI Rat Carotid Arteries
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Frismantiene, A.; Philippova, M.; Erne, P.; Resink, T.J. Smooth muscle cell-driven vascular diseases and molecular mechanisms of VSMC plasticity. Cell. Signal. 2018, 52, 48–64. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Orekhov, A.N.; Bobryshev, Y.V. Vascular smooth muscle cell in atherosclerosis. Acta Physiol. 2015, 214, 33–50. [Google Scholar] [CrossRef]
- Ramadan, M.; Cooper, B.; Posnack, N.G. Bisphenols and phthalates: Plastic chemical exposures can contribute to adverse cardiovascular health outcomes. Birth Defects Res. 2020, 112, 1362–1385. [Google Scholar] [CrossRef]
- Lim, L.; Kim, H.; Jeong, J.; Han, S.; Yu, Y.; Song, H. Yohimbine inhibits PDGF-induced vascular smooth muscle cell proliferation and migration via FOXO3a factor. Int. J. Mol. Sci 2024, 25, 6899. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Park, H.; Hwang, B.; Kim, S.; Choi, Y.H.; Kim, W.J.; Moon, S.K. Bisphenol A exposure inhibits vascular smooth muscle cell responses: Involvement of proliferation, migration, and invasion. Environ. Toxicol. Pharmacol. 2023, 98, 104060. [Google Scholar] [CrossRef] [PubMed]
- Tanase, D.M.; Valasciuc, E.; Anton, I.B.; Gosav, E.M.; Dima, N.; Cucu, A.I.; Costea, C.F.; Floria, D.E.; Hurjui, L.L.; Tarniceriu, C.C.; et al. Matrix metalloproteinases: Pathophysiologic implications and potential therapeutic targets in cardiovascular disease. Biomolecules 2025, 15, 598. [Google Scholar] [CrossRef]
- Kim, J.; Ko, J. Human sLZIP promotes atherosclerosis via MMP-9 transcription and vascular smooth muscle cell migration. FASEB J. 2014, 28, 5010–5021. [Google Scholar] [CrossRef]
- Park, H.S.; Quan, K.T.; Han, J.H.; Jung, S.H.; Lee, D.H.; Jo, E.; Lim, T.W.; Heo, K.S.; Na, M.; Myung, C.S. Rubiarbonone C inhibits platelet-derived growth factor-induced proliferation and migration of vascular smooth muscle cells through the focal adhesion kinase, MAPK and STAT3 Tyr(705) signalling pathways. Br. J. Pharmacol. 2017, 174, 4140–4154. [Google Scholar] [CrossRef]
- Hsuan, C.F.; Kuo, Y.T.; Chang, T.H.; Chen, Y.L.; Houng, H.Y.; Chang, N.; Chang, S.; Chang, C.C.; Houng, J.Y. Abelmoschus manihot flower extract retards platelet-derived growth factor-BB-stimulated proliferation and migration in vascular smooth muscle cells by inhibiting the MAPK/NF-kB pathway and matrix metalloproteinase expressions. J. Med. Food 2025, 28, 921–933. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Zhang, T.; Zhang, W.; Zhao, Z.; Sun, J. Natural Drugs as a Treatment Strategy for Cardiovascular Disease through the Regulation of Oxidative Stress. Oxid. Med. Cell. Longev. 2020, 2020, 5430407. [Google Scholar] [CrossRef]
- Cheng, X.; Zhao, C.; Jin, Z.; Hu, J.; Zhang, Z.; Zhang, C. Natural products: Potential therapeutic agents for atherosclerosis. Chin. J. Nat. Med. 2022, 20, 830–845. [Google Scholar] [CrossRef] [PubMed]
- Rasul, A.; Millimouno, F.M.; Ali Eltayb, W.; Ali, M.; Li, J.; Li, X. Pinocembrin: A novel natural compound with versatile pharmacological and biological activities. Biomed Res Int 2013, 2013, 379850. [Google Scholar] [CrossRef]
- Elbatreek, M.H.; Mahdi, I.; Ouchari, W.; Mahmoud, M.F.; Sobeh, M. Current advances on the therapeutic potential of pinocembrin: An updated review. Biomed Pharmacother 2023, 157, 114032. [Google Scholar] [CrossRef]
- Pei, B.; Sun, J. Pinocembrin alleviates cognition deficits by inhibiting inflammation in diabetic mice. J. Neuroimmunol. 2018, 314, 42–49. [Google Scholar] [CrossRef]
- Said, M.M.; Azab, S.S.; Saeed, N.M.; El-Demerdash, E. Antifibrotic Mechanism of Pinocembrin: Impact on Oxidative Stress, Inflammation and TGF-β/Smad Inhibition in Rats. Ann. Hepatol. 2018, 17, 307–317. [Google Scholar] [CrossRef]
- Saad, M.A.; Abdel Salam, R.M.; Kenawy, S.A.; Attia, A.S. Pinocembrin attenuates hippocampal inflammation, oxidative perturbations and apoptosis in a rat model of global cerebral ischemia reperfusion. Pharmacol. Rep. 2015, 67, 115–122. [Google Scholar] [CrossRef]
- Shi, L.L.; Chen, B.N.; Gao, M.; Zhang, H.A.; Li, Y.J.; Wang, L.; Du, G.H. The characteristics of therapeutic effect of pinocembrin in transient global brain ischemia/reperfusion rats. Life Sci. 2011, 88, 521–528. [Google Scholar] [CrossRef]
- Tao, J.; Shen, C.; Sun, Y.; Chen, W.; Yan, G. Neuroprotective effects of pinocembrin on ischemia/reperfusion-induced brain injury by inhibiting autophagy. Biomed. Pharmacother. 2018, 106, 1003–1010. [Google Scholar] [CrossRef]
- Gao, J.; Lin, S.; Gao, Y.; Zou, X.; Zhu, J.; Chen, M.; Wan, H.; Zhu, H. Pinocembrin inhibits the proliferation and migration and promotes the apoptosis of ovarian cancer cells through down-regulating the mRNA levels of N-cadherin and GABAB receptor. Biomed. Pharmacother. 2019, 120, 109505. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Shao, Y.; Yuan, Y. Pinocembrin flavanone inhibits cell viability in PC-3 human prostate cancer by inducing cellular apoptosis, ROS production and cell cycle arrest. Acta Pharm. 2021, 71, 669–678. [Google Scholar] [CrossRef]
- Li, C.; Wan, W.; Ye, T.; Sun, Y.; Chen, X.; Liu, X.; Shi, S.; Zhang, Y.; Qu, C.; Yang, B.; et al. Pinocembrin alleviates lipopolysaccharide-induced myocardial injury and cardiac dysfunction in rats by inhibiting p38/JNK MAPK pathway. Life Sci. 2021, 277, 119418. [Google Scholar] [CrossRef] [PubMed]
- Lungkaphin, A.; Pongchaidecha, A.; Palee, S.; Arjinajarn, P.; Pompimon, W.; Chattipakorn, N. Pinocembrin reduces cardiac arrhythmia and infarct size in rats subjected to acute myocardial ischemia/reperfusion. Appl. Physiol. Nutr. Metab. 2015, 40, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Xu, J.; Hu, W.; Yu, D.; Bai, X. Effects of Pinocembrin Pretreatment on Connexin 43 (Cx43) Protein Expression After Rat Myocardial Ischemia-Reperfusion and Cardiac Arrhythmia. Med. Sci. Monit. 2018, 24, 5008–5014. [Google Scholar] [CrossRef]
- Ye, T.; Zhang, C.; Wu, G.; Wan, W.; Liang, J.; Liu, X.; Liu, D.; Yang, B. Pinocembrin attenuates autonomic dysfunction and atrial fibrillation susceptibility via inhibition of the NF-κB/TNF-α pathway in a rat model of myocardial infarction. Int. Immunopharmacol. 2019, 77, 105926. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.M.; Fang, L.H.; Li, Y.J.; Du, G.H. Endothelium-dependent and -independent relaxation induced by pinocembrin in rat aortic rings. Vascul. Pharmacol. 2007, 46, 160–165. [Google Scholar] [CrossRef]
- Sang, H.; Yuan, N.; Yao, S.; Li, F.; Wang, J.; Fang, Y.; Qin, S. Inhibitory effect of the combination therapy of simvastatin and pinocembrin on atherosclerosis in ApoE-deficient mice. Lipids Health Dis. 2012, 11, 166. [Google Scholar] [CrossRef]
- Chen, K.S.; Shi, M.D.; Chien, C.S.; Shih, Y.W. Pinocembrin suppresses TGF-β1-induced epithelial-mesenchymal transition and metastasis of human Y-79 retinoblastoma cells through inactivating αvβ3 integrin/FAK/p38α signaling pathway. Cell Biosci. 2014, 4, 41. [Google Scholar] [CrossRef]
- Zhu, X.; Li, R.; Wang, C.; Zhou, S.; Fan, Y.; Ma, S.; Gao, D.; Gai, N.; Yang, J. Pinocembrin Inhibits the Proliferation and Metastasis of Breast Cancer via Suppression of the PI3K/AKT Signaling Pathway. Front. Oncol. 2021, 11, 661184. [Google Scholar] [CrossRef]
- Lim, L.; Yun, J.J.; Jeong, J.E.; Wi, A.J.; Song, H. Inhibitory Effects of Nano-Extract from Dendropanax morbifera on Proliferation and Migration of Vascular Smooth Muscle Cells. J. Nanosci. Nanotechnol. 2015, 15, 116–119. [Google Scholar] [CrossRef]
- Lin, C.L.; Zhang, Z.X.; Tan, Z. Mechanisms of focal adhesion kinase in the proliferation of human pulmonary artery smooth cells under hypoxia. Zhonghua Yi Xue Za Zhi 2011, 91, 2274–2277. [Google Scholar] [PubMed]
- Miano, J.M.; Fisher, E.A.; Majesky, M.W. Fate and State of Vascular Smooth Muscle Cells in Atherosclerosis. Circulation 2021, 143, 2110–2116. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Qian, H.; Zhou, Q.; Lei, C. Hsa_circ_0007765 promotes Platelet-Derived Growth Factor-BB-induced proliferation and migration of human aortic vascular smooth muscle cells in atherosclerosis. Cardiovasc. Toxicol. 2024, 24, 1077–1089. [Google Scholar] [CrossRef]
- Koo, Y.E.; Song, J.; Bae, S. Use of Plant and Herb Derived Medicine for Therapeutic Usage in Cardiology. Medicines 2018, 5, 38. [Google Scholar] [CrossRef]
- He, W.; Zheng, Q.; Zou, T.; Yan, W.; Gao, X.; Wang, C.; Xiong, Y. Angiopoietin-like 4 facilitates human aortic smooth muscle cell phenotype switch and dysfunctions through the PI3K/Akt signaling in aortic dissection. Adv. Med. Sci. 2024, 69, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Uzui, H.; Lee, J.D.; Shimizu, H.; Tsutani, H.; Ueda, T. The role of protein-tyrosine phosphorylation and gelatinase production in the migration and proliferation of smooth muscle cells. Atherosclerosis 2000, 149, 51–59. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, W.Z.; Liu, T.; Feng, X.; Yang, N.; Zhou, H.F. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J. Recept. Signal Transduct. Res. 2015, 35, 600–604. [Google Scholar] [CrossRef]
- Johnson, C.; Galis, Z.S. Matrix metalloproteinase-2 and -9 differentially regulate smooth muscle cell migration and cell-mediated collagen organization. Arter. Thromb. Vasc. Biol. 2004, 24, 54–60. [Google Scholar] [CrossRef]
- Guan, X.; Liu, Y.; An, Y.; Wang, X.; Wei, L.; Qi, X. FAK family kinases: A potential therapeutic target for atherosclerosis. Diabetes Metab. Syndr. Obes. 2024, 17, 3151–3161. [Google Scholar] [CrossRef]
- Li, J.J.; Han, M.; Wen, J.K.; Li, A.Y. Osteopontin stimulates vascular smooth muscle cell migration by inducing FAK phosphorylation and ILK dephosphorylation. Biochem. Biophys. Res. Commun. 2007, 356, 13–19. [Google Scholar] [CrossRef]
- Bicknell, K.A.; Surry, E.L.; Brooks, G. Targeting the cell cycle machinery for the treatment of cardiovascular disease. J. Pharm. Pharmacol. 2003, 55, 571–591. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Q.; Li, Y.L.; Li, X.T.; Lv, J.Y.; Gao, Y.; Li, W.N.; Gong, Q.H.; Yang, D.L. Osthole Alleviates Neointimal Hyperplasia in Balloon-Induced Arterial Wall Injury by Suppressing Vascular Smooth Muscle Cell Proliferation and Downregulating Cyclin D1/CDK4 and Cyclin E1/CDK2 Expression. Front. Physiol. 2020, 11, 514494. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yu, J.; Xiao, X.; Li, W.; Zang, L.; Han, T.; Zhang, D.; Niu, X. The inhibitory effect of (-)-Epicatechin gallate on the proliferation and migration of vascular smooth muscle cells weakens and stabilizes atherosclerosis. Eur. J. Pharmacol. 2021, 891, 173761. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. FDA Guidance for Industry: Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/estimating-maximum-safe-starting-dose-initial-clinical-trials-therapeutics-adult-healthy-volunteers (accessed on 20 May 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Jung, J.; Yu, Y.-B.; Choi, D.-H.; Lim, L.; Song, H. Pinocembrin Downregulates Vascular Smooth Muscle Cells Proliferation and Migration Leading to Attenuate Neointima Formation in Balloon-Injured Rats. Biomolecules 2025, 15, 1325. https://doi.org/10.3390/biom15091325
Kim H, Jung J, Yu Y-B, Choi D-H, Lim L, Song H. Pinocembrin Downregulates Vascular Smooth Muscle Cells Proliferation and Migration Leading to Attenuate Neointima Formation in Balloon-Injured Rats. Biomolecules. 2025; 15(9):1325. https://doi.org/10.3390/biom15091325
Chicago/Turabian StyleKim, Hyeonhwa, Jihye Jung, Young-Bob Yu, Dong-Hyun Choi, Leejin Lim, and Heesang Song. 2025. "Pinocembrin Downregulates Vascular Smooth Muscle Cells Proliferation and Migration Leading to Attenuate Neointima Formation in Balloon-Injured Rats" Biomolecules 15, no. 9: 1325. https://doi.org/10.3390/biom15091325
APA StyleKim, H., Jung, J., Yu, Y.-B., Choi, D.-H., Lim, L., & Song, H. (2025). Pinocembrin Downregulates Vascular Smooth Muscle Cells Proliferation and Migration Leading to Attenuate Neointima Formation in Balloon-Injured Rats. Biomolecules, 15(9), 1325. https://doi.org/10.3390/biom15091325





