EET-Based Therapeutics Mitigate Sorafenib-Associated Glomerular Cell Damage
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Methodology
2.3. Cell Viability Assay
2.4. Caspase 3/7 Activation for Apoptosis Analysis
2.5. Cell Confluence Analysis
2.6. Genomic Study
2.6.1. RNA-Seq Data Analysis of HRMCs
2.6.2. Bioinformatics Analysis
2.6.3. RT-qPCR
2.7. Statistical Analysis
3. Results
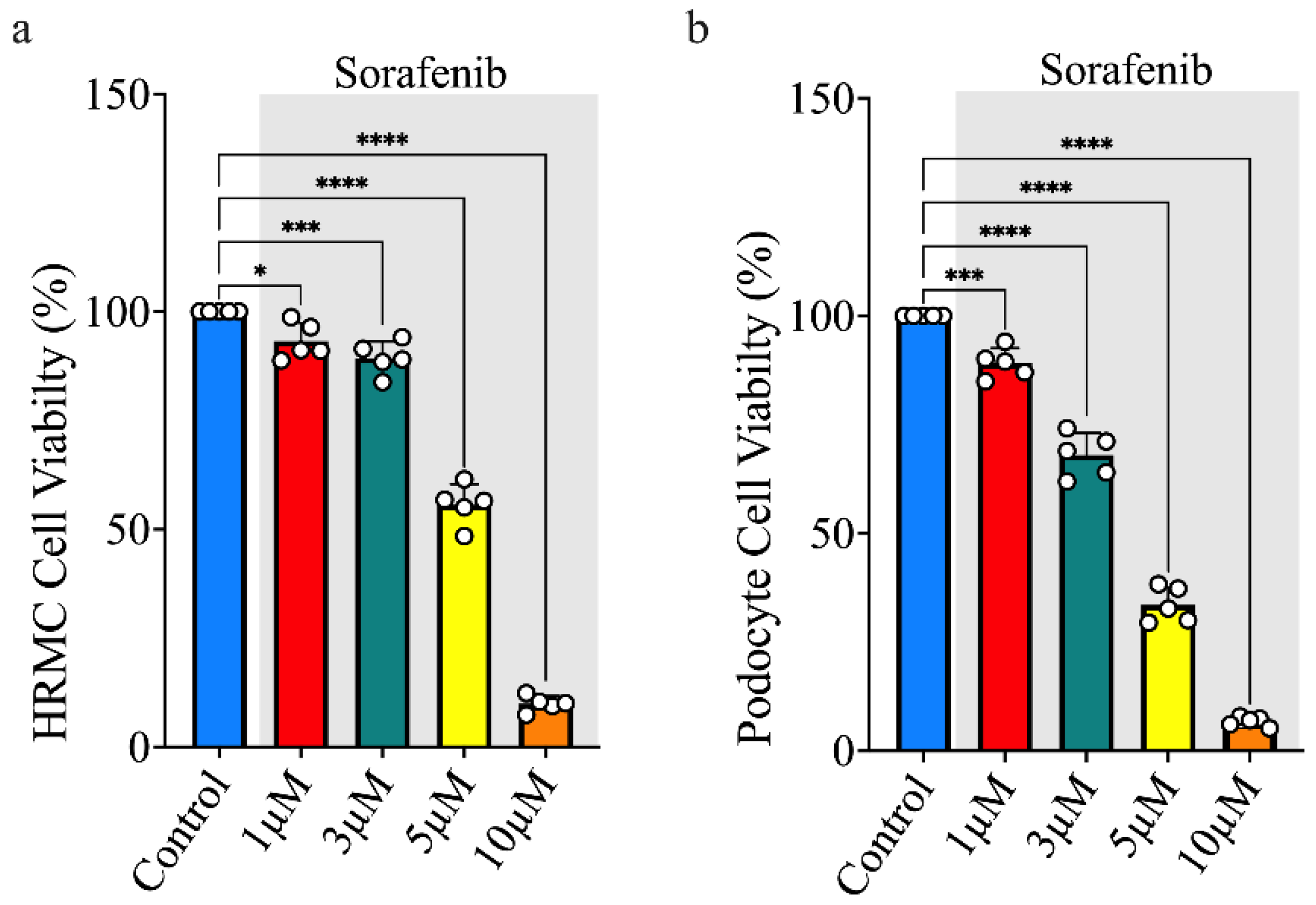
3.1. Sorafenib Treatment Decreases Cell Viability
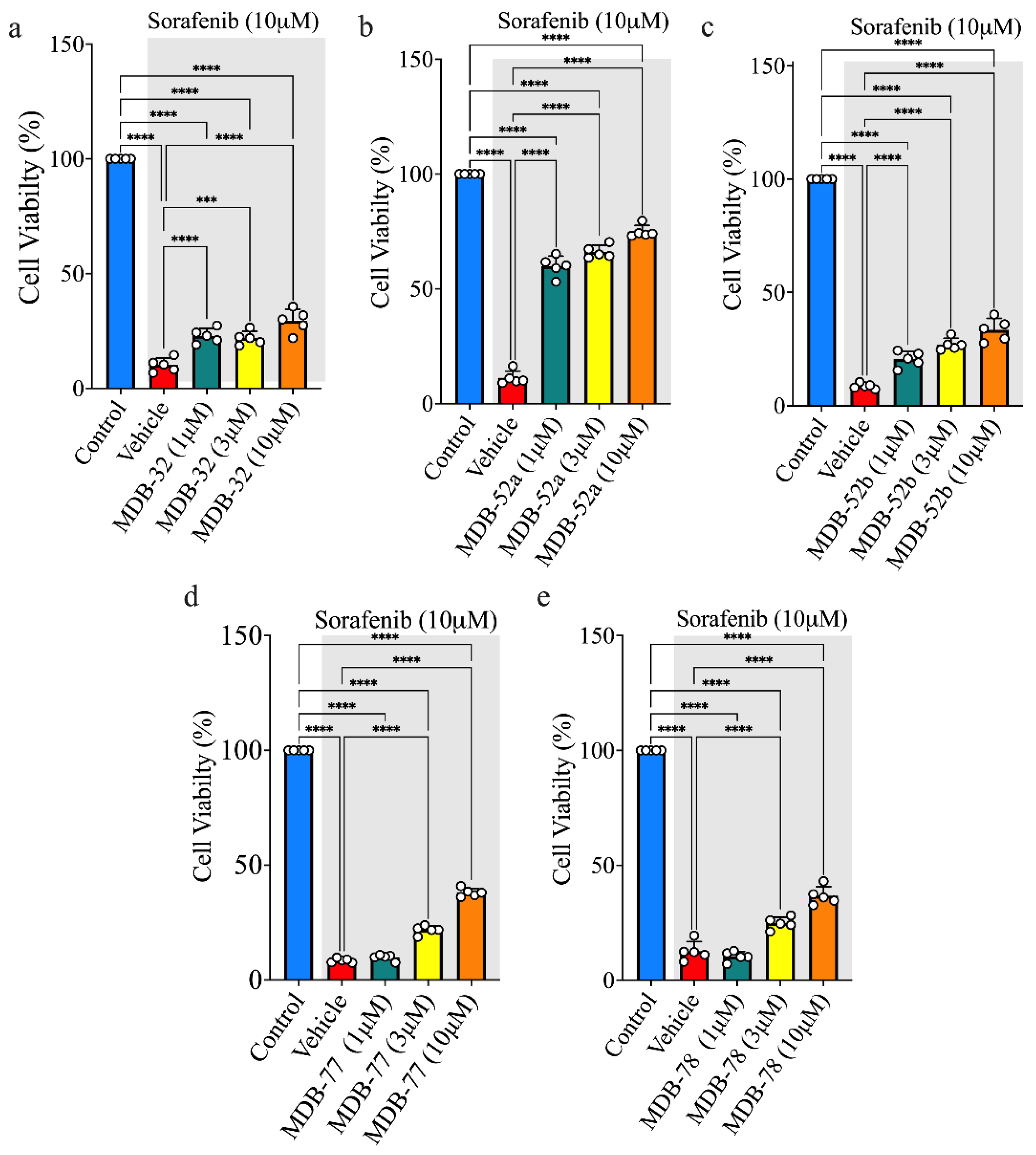
3.2. Synthetic Analogs of 8,9-EET Maintained the Viability of HRMCs Treated with Sorafenib
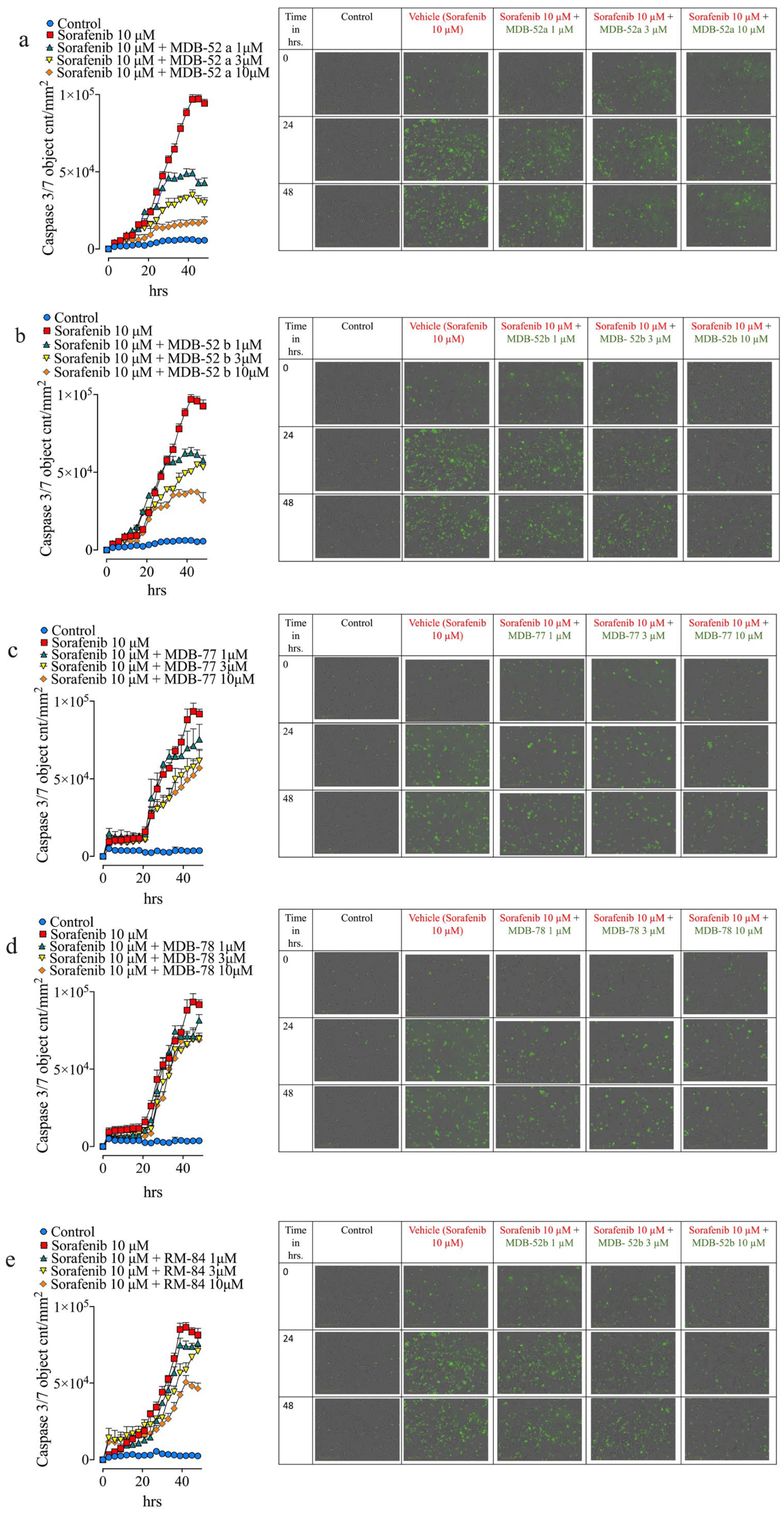
3.3. Synthetic Analogs of 8,9-EET Mitigate Sorafenib-Induced HRMCs Apoptosis
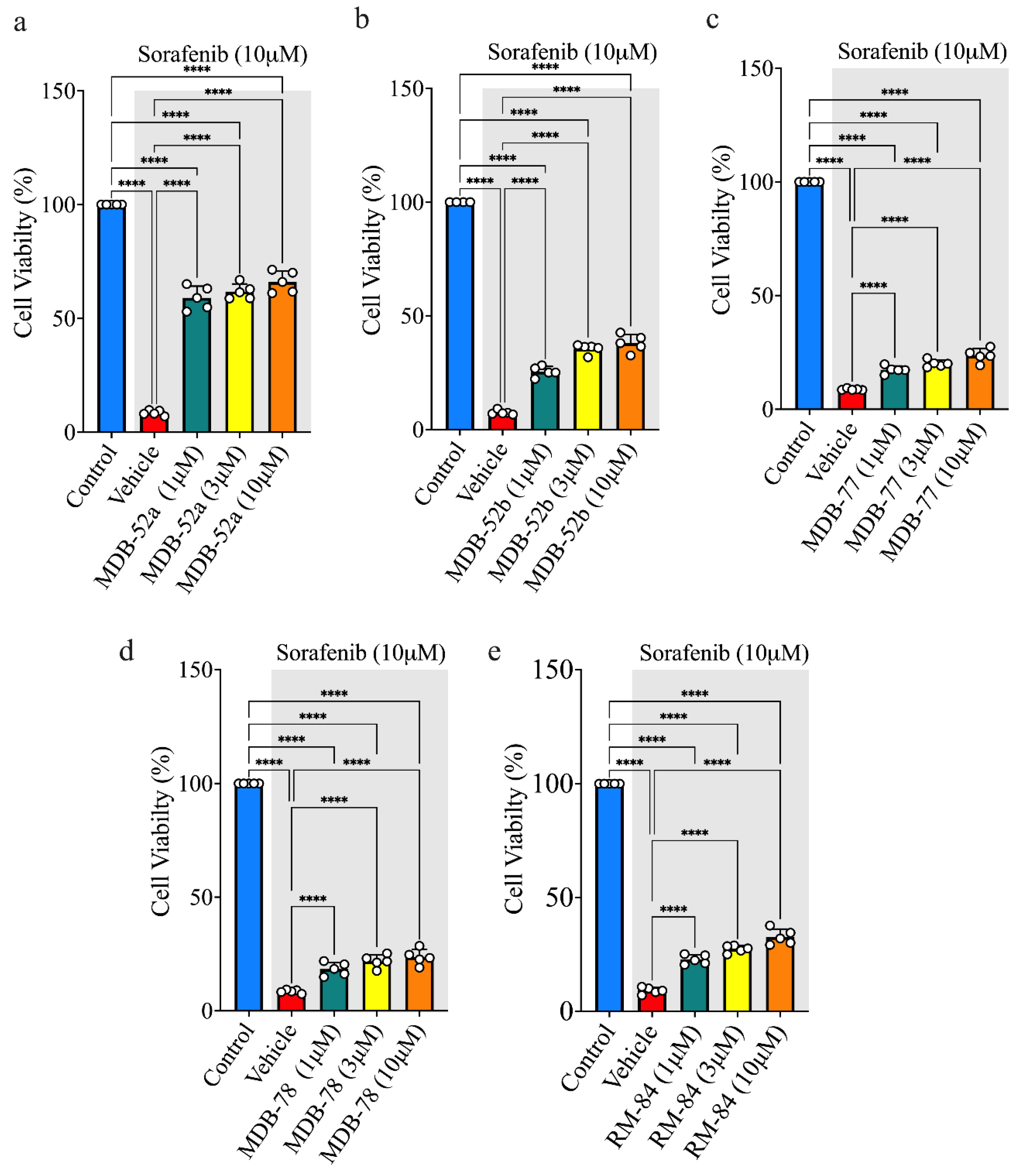
3.4. Synthetic Analogs of 8,9-EET Maintain Podocyte Cell Viability During Sorafenib Exposure
3.5. Synthetic 8,9-EET Analogs Reduce Apoptosis Caused by Sorafenib
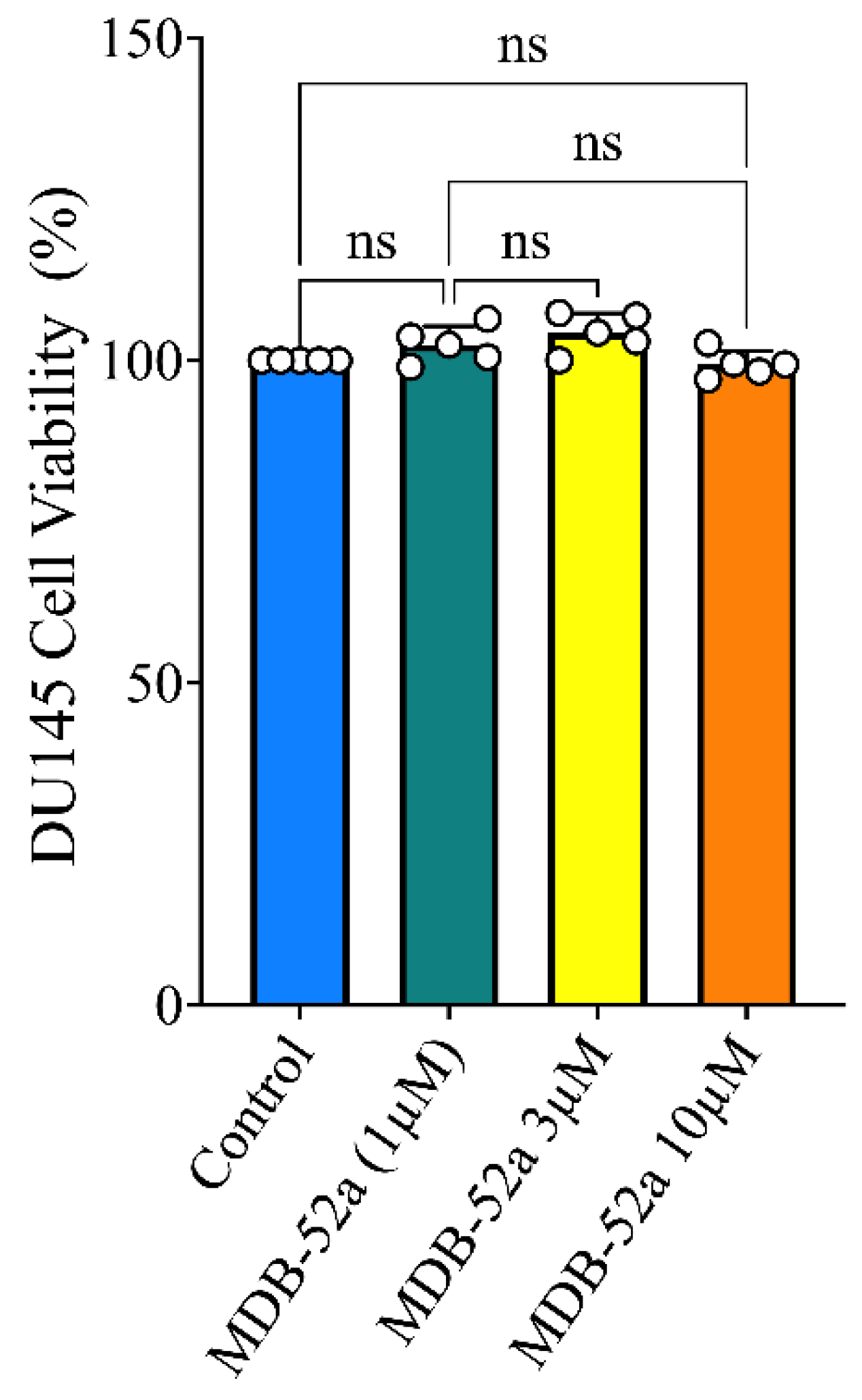
3.6. Synthetic Analog of 8,9-EET, MDB-52a, Does Not Interfere with Sorafenib’s Anticancer Activity
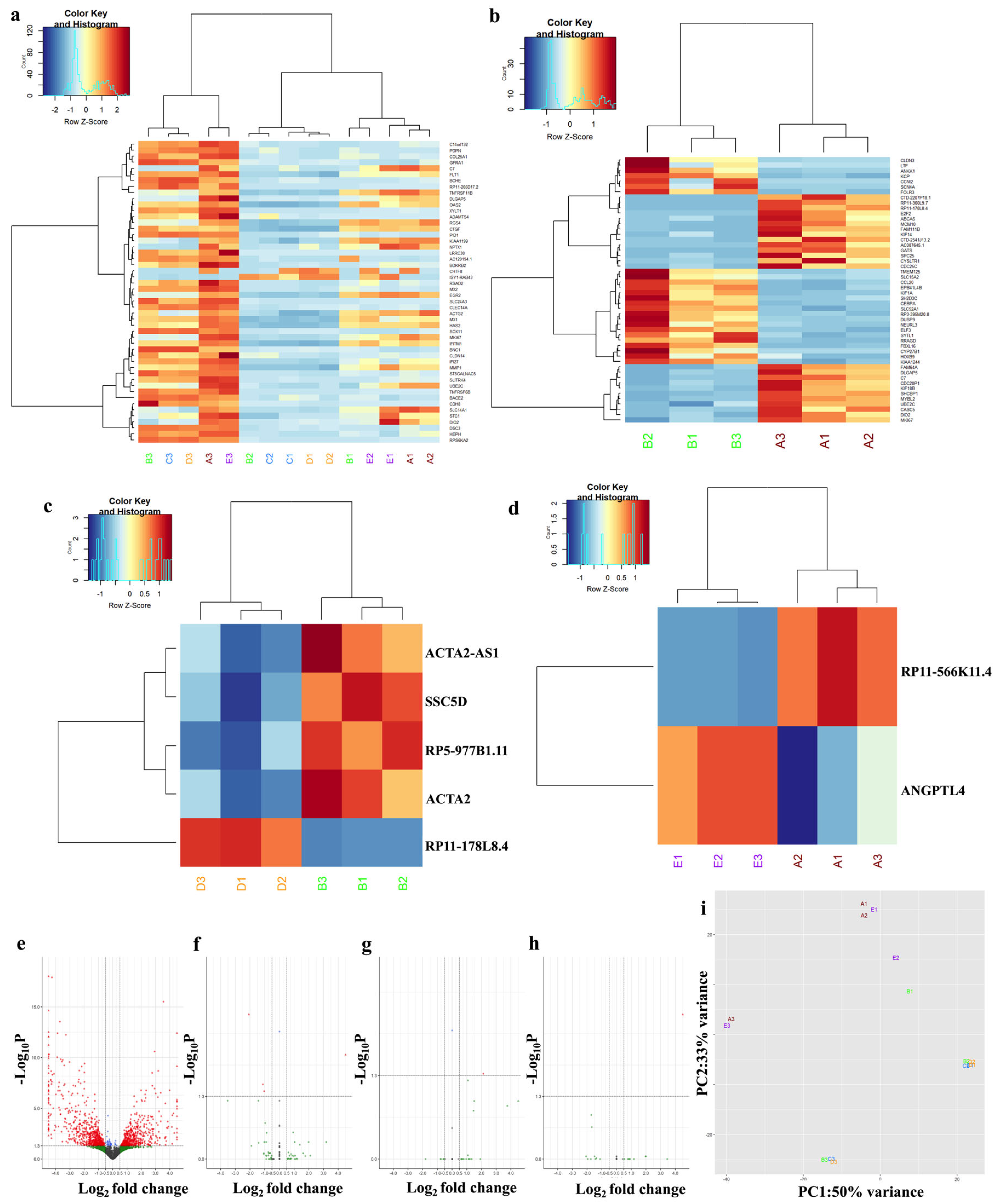
3.7. Sorafenib and Synthetic Analog MDB52a Affect Gene Expression in HRMC
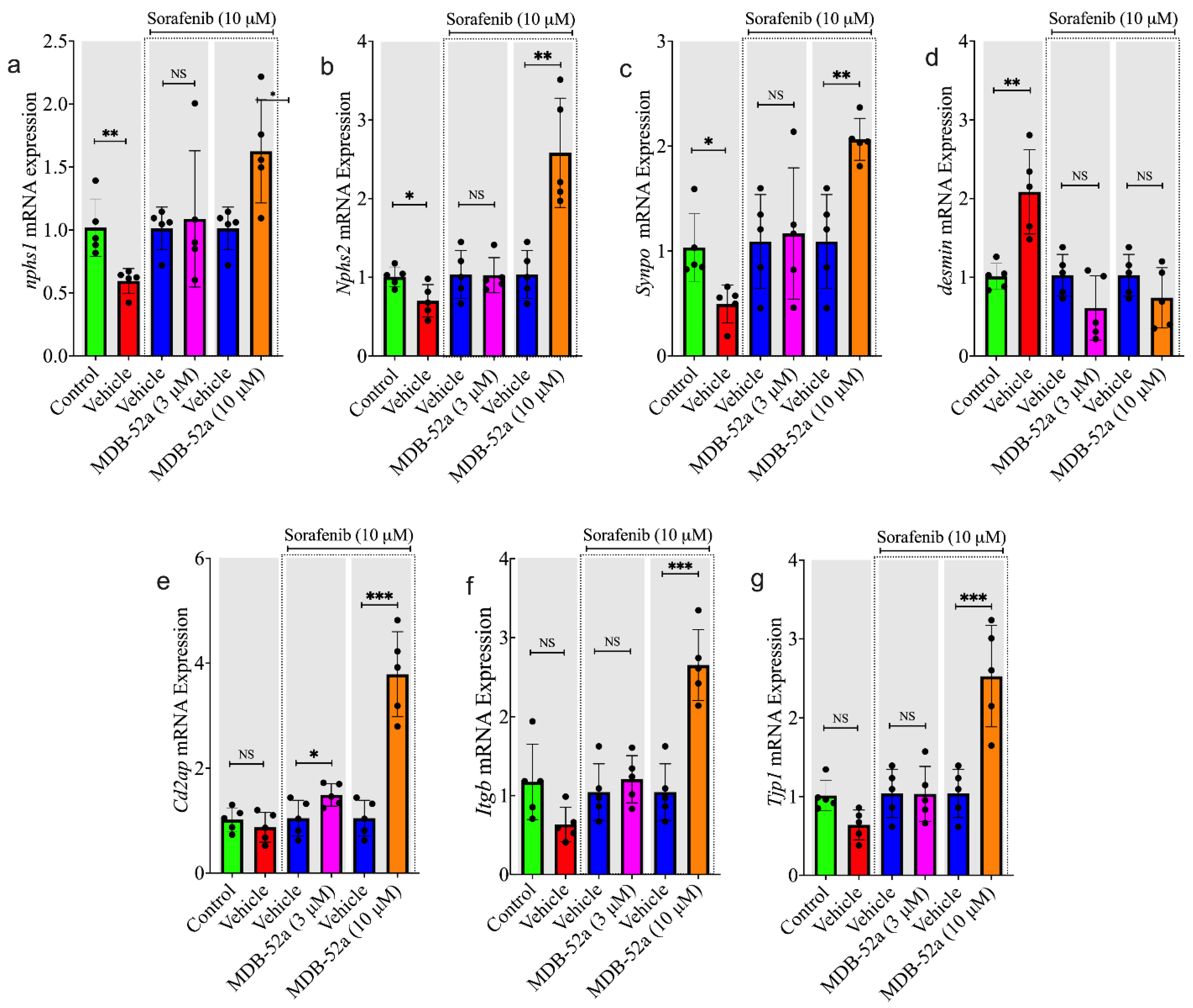
3.8. MDB-52a Influences Sorafenib-Induced Alterations in Podocyte-Specific Gene Expression
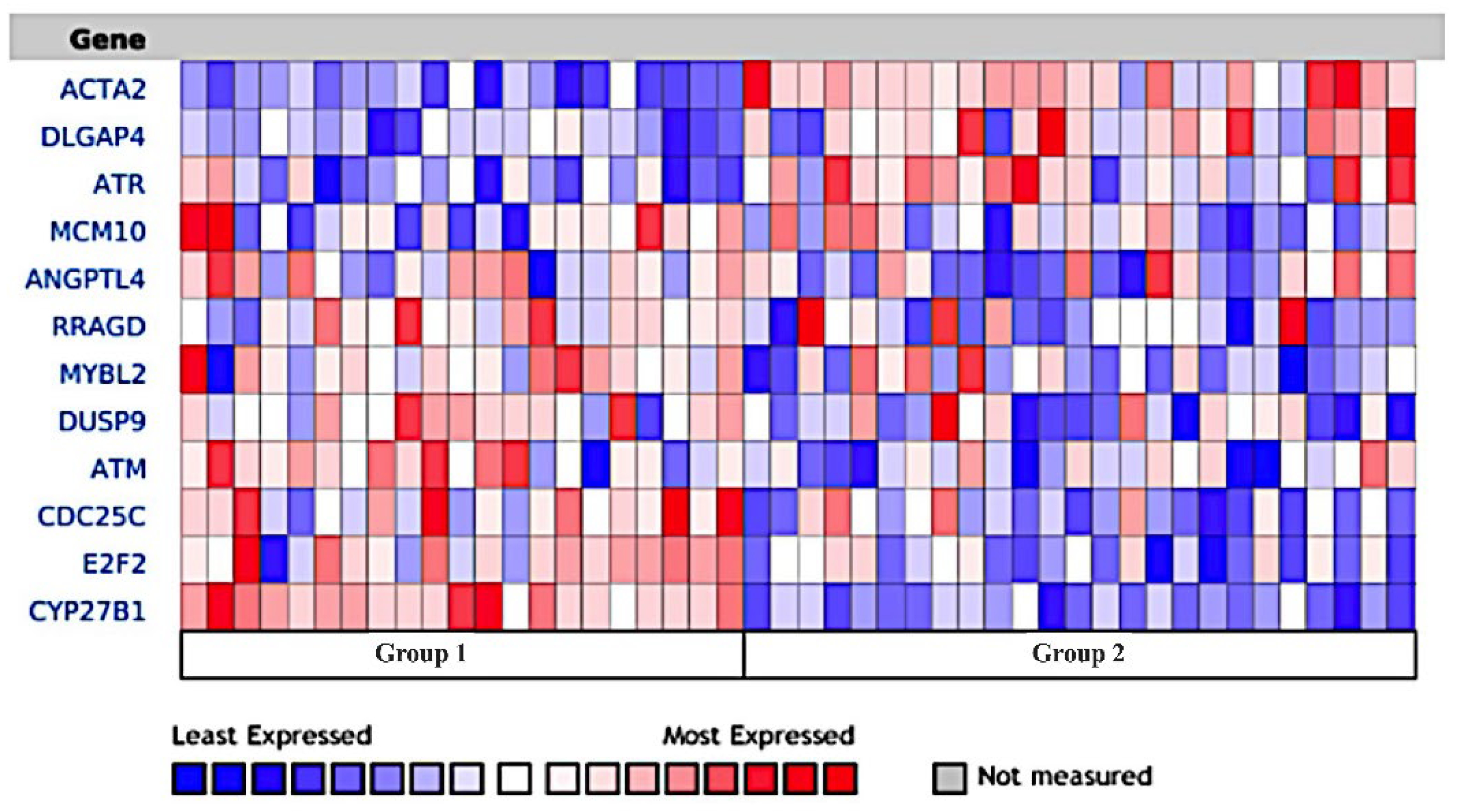
3.9. Naphroseq Analysis Identifies Genes Influenced by MDB-52a as Therapeutic Targets
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gawi Ermi, A.; Sarkar, D. Resistance to Tyrosine Kinase Inhibitors in Hepatocellular Carcinoma (HCC): Clinical Implications and Potential Strategies to Overcome the Resistance. Cancers 2024, 16, 3944. [Google Scholar] [CrossRef] [PubMed]
- Rashidi, A.; Wanchoo, R.; Izzedine, H. How I Manage Hypertension and Proteinuria Associated with VEGF Inhibitor. Clin. J. Am. Soc. Nephrol. 2023, 18, 121–123. [Google Scholar] [CrossRef] [PubMed]
- Rosner, M.H.; Jhaveri, K.D.; McMahon, B.A.; Perazella, M.A. Onconephrology: The Intersections between the Kidney and Cancer. CA Cancer J. Clin. 2021, 71, 47–77. [Google Scholar] [CrossRef] [PubMed]
- Avraham, S.; Korin, B.; Chung, J.-J.; Oxburgh, L.; Shaw, A.S. The Mesangial Cell—The Glomerular Stromal Cell. Nat. Rev. Nephrol. 2021, 17, 855–864. [Google Scholar] [CrossRef]
- Noh, M.R.; Jang, H.-S.; Salem, F.E.; Ferrer, F.A.; Kim, J.; Padanilam, B.J. Epoxyeicosatrienoic Acid Administration or Soluble Epoxide Hydrolase Inhibition Attenuates Renal Fibrogenesis in Obstructive Nephropathy. Am. J. Physiol.-Ren. Physiol. 2023, 324, F138–F151. [Google Scholar] [CrossRef]
- Elmarakby, A.A. Reno-Protective Mechanisms of Epoxyeicosatrienoic Acids in Cardiovascular Disease. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2012, 302, R321–R330. [Google Scholar] [CrossRef]
- Sporková, A.; Reddy, R.N.; Falck, J.R.; Imig, J.D.; Kopkan, L.; Sadowski, J.; Červenka, L. Interlobular Arteries From 2-Kidney, 1-Clip Goldblatt Hypertensive Rats’ Exhibit-Impaired Vasodilator Response to Epoxyeicosatrienoic Acids. Am. J. Med. Sci. 2016, 351, 513–519. [Google Scholar] [CrossRef]
- Campbell, W.B.; Imig, J.D.; Schmitz, J.M.; Falck, J.R. Orally Active Epoxyeicosatrienoic Acid Analogs. J. Cardiovasc. Pharmacol. 2017, 70, 211–224. [Google Scholar] [CrossRef]
- Imig, J.D. Prospective for Cytochrome P450 Epoxygenase Cardiovascular and Renal Therapeutics. Pharmacol. Ther. 2018, 192, 1–19. [Google Scholar] [CrossRef]
- Baranowska, I.; Walkowska, A.; Badzynska, B.; Vaneckova, I.; Gawrys, O.; Cervenka, L.; Kompanowska-Jezierska, E. 14,15-Epoxyeicosatrienoic Acid Analog Augments Hypotensive Effect of an Endothelin-A Receptor Blocker Antrasentan and Prevents Oedema and Organ Hypertrophy in Spontaneously Hypertensive Rats. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2025, 76. [Google Scholar] [CrossRef]
- Duflot, T.; Laurent, C.; Soudey, A.; Fonrose, X.; Hamzaoui, M.; Iacob, M.; Bertrand, D.; Favre, J.; Etienne, I.; Roche, C.; et al. Preservation of Epoxyeicosatrienoic Acid Bioavailability Prevents Renal Allograft Dysfunction and Cardiovascular Alterations in Kidney Transplant Recipients. Sci. Rep. 2021, 11, 3739. [Google Scholar] [CrossRef]
- Liu, J.-Y. Inhibition of Soluble Epoxide Hydrolase for Renal Health. Front. Pharmacol. 2019, 9, 1551. [Google Scholar] [CrossRef]
- Jíchová, Š.; Kopkan, L.; Husková, Z.; Doleželová, Š.; Neckář, J.; Kujal, P.; Vernerová, Z.; Kramer, H.J.; Sadowski, J.; Kompanowska-Jezierska, E.; et al. Epoxyeicosatrienoic Acid Analog Attenuates the Development of Malignant Hypertension, but Does Not Reverse It Once Established: A Study in Cyp1a1-Ren-2 Transgenic Rats. J. Hypertens. 2016, 34, 2008–2025. [Google Scholar] [CrossRef]
- Imig, J.D.; Hye Khan, M.A.; Burkhan, A.; Chen, G.; Adebesin, A.M.; Falck, J.R. Kidney-Targeted Epoxyeicosatrienoic Acid Analog, EET-F01, Reduces Inflammation, Oxidative Stress, and Cisplatin-Induced Nephrotoxicity. Int. J. Mol. Sci. 2021, 22, 2793. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.H.; Liu, J.; Kumar, G.; Skapek, S.X.; Falck, J.R.; Imig, J.D. Novel Orally Active Epoxyeicosatrienoic Acid (EET) Analogs Attenuate Cisplatin Nephrotoxicity. FASEB J. 2013, 27, 2946–2956. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; McCarthy, E.T.; Reddy, D.S.; Patel, P.K.; Savin, V.J.; Medhora, M.; Falck, J.R. 8,9-Epoxyeicosatrienoic Acid Protects the Glomerular Filtration Barrier. Prostaglandins Other Lipid Mediat. 2009, 89, 43–51. [Google Scholar] [CrossRef]
- De Bourg, M.; Mishra, A.; Mohammad, R.S.; Morisseau, C.; Hammock, B.D.; Imig, J.D.; Vik, A. Synthetic Epoxyeicosatrienoic Acid Mimics Protect Mesangial Cells from Sorafenib-Induced Cell Death. Molecules 2025, 30, 1445. [Google Scholar] [CrossRef]
- Chittiprol, S.; Chen, P.; Petrovic-Djergovic, D.; Eichler, T.; Ransom, R.F. Marker Expression, Behaviors, and Responses Vary in Different Lines of Conditionally Immortalized Cultured Podocytes. Am. J. Physiol.-Ren. Physiol. 2011, 301, F660–F671. [Google Scholar] [CrossRef]
- Ni, L.; Saleem, M.; Mathieson, P.W. Podocyte Culture: Tricks of the Trade: Podocyte Culture. Nephrology 2012, 17, 525–531. [Google Scholar] [CrossRef]
- Ewels, P.; Magnusson, M.; Lundin, S.; Käller, M. MultiQC: Summarize Analysis Results for Multiple Tools and Samples in a Single Report. Bioinformatics 2016, 32, 3047–3048. [Google Scholar] [CrossRef]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq—A Python Framework to Work with High-Throughput Sequencing Data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef]
- Law, C.W.; Chen, Y.; Shi, W.; Smyth, G.K. Voom: Precision Weights Unlock Linear Model Analysis Tools for RNA-Seq Read Counts. Genome Biol. 2014, 15, R29. [Google Scholar] [CrossRef]
- Robinson, M.D.; Oshlack, A. A Scaling Normalization Method for Differential Expression Analysis of RNA-Seq Data. Genome Biol. 2010, 11, R25. [Google Scholar] [CrossRef]
- Gurevich, F.; Perazella, M.A. Renal Effects of Anti-Angiogenesis Therapy: Update for the Internist. Am. J. Med. 2009, 122, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Nangaku, M. Angiogenesis and Hypoxia in the Kidney. Nat. Rev. Nephrol. 2013, 9, 211–222. [Google Scholar] [CrossRef]
- Uyl, T.J.J.; Ngo, A.; Pratt, D.; Cortez, I.; Mathijssen, R.H.J.; Versmissen, J.; Danser, A.H.J.; Mirabito Colafella, K.M. Mechanisms of Anti-VEGF Therapy-Induced Kidney Injury: Current Insights and Future Perspectives in Combination with Immune Checkpoint Inhibitors. Am. J. Physiol.-Ren. Physiol. 2025, 329, F284–F299. [Google Scholar] [CrossRef]
- Jankiewicz, W.K.; Barnett, S.D.; Stavniichuk, A.; Hwang, S.H.; Hammock, B.D.; Belayet, J.B.; Khan, A.H.; Imig, J.D. Dual sEH/COX-2 Inhibition Using PTUPB—A Promising Approach to Antiangiogenesis-Induced Nephrotoxicity. Front. Pharmacol. 2021, 12, 744776. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Teves, S.S.; Kemp, C.J.; Henikoff, S. Doxorubicin, DNA Torsion, and Chromatin Dynamics. Biochim. Biophys. Acta BBA—Rev. Cancer 2014, 1845, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Obeidat, M.; Obeidat, M.; Ballermann, B.J. Glomerular Endothelium: A Porous Sieve and Formidable Barrier. Exp. Cell Res. 2012, 318, 964–972. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Kowalkowski, K.; Ciurlionis, R.; Buck, W.R.; Glaser, K.B.; Albert, D.H.; Blomme, E.A.G. Identification of VEGF Signaling Inhibition-Induced Glomerular Injury in Rats through Site-Specific Urinary Biomarkers. Int. J. Mol. Sci. 2021, 22, 12629. [Google Scholar] [CrossRef]
- Li, J.; Zhang, L.; Ge, T.; Liu, J.; Wang, C.; Yu, Q. Understanding Sorafenib-Induced Cardiovascular Toxicity: Mechanisms and Treatment Implications. Drug Des. Devel. Ther. 2024, 18, 829–843. [Google Scholar] [CrossRef]
- Nagasawa, T.; Hye Khan, M.A.; Imig, J.D. Captopril Attenuates Hypertension and Renal Injury Induced by the Vascular Endothelial Growth Factor Inhibitor Sorafenib. Clin. Exp. Pharmacol. Physiol. 2012, 39, 454–461. [Google Scholar] [CrossRef]
- Shen, T.; Huang, S. The Role of Cdc25A in the Regulation of Cell Proliferation and Apoptosis. Anticancer Agents Med. Chem. 2012, 12, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Infante, A.; Laresgoiti, U.; Fernández-Rueda, J.; Fullaondo, A.; Galán, J.; Díaz-Uriarte, R.; Malumbres, M.; Field, S.J.; Zubiaga, A.M. E2F2 Represses Cell Cycle Regulators to Maintain Quiescence. Cell Cycle 2008, 7, 3915–3927. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Wang, Y.; Sun, L.; Zhang, Z.; Jiang, Z.; Qin, Z.; Han, H.; Liu, Z.; Li, X.; Tang, A.; et al. Decreased Expression of Dual-Specificity Phosphatase 9 Is Associated with Poor Prognosis in Clear Cell Renal Cell Carcinoma. BMC Cancer 2011, 11, 413. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Zhang, J.; Yang, R.; Bai, J.; Deng, B.; Cheng, L.; Gao, F.; Xie, J.; Zhang, B. The CaMKII Inhibitory Peptide AIP Alleviates Renal Fibrosis Through the TGF-β/Smad and RAF/ERK Pathways. J. Pharmacol. Exp. Ther. 2023, 386, 310–322. [Google Scholar] [CrossRef]
- Niu, C.; Hu, Y.; Xu, K.; Pan, X.; Wang, L.; Yu, G. The Role of the Cytoskeleton in Fibrotic Diseases. Front. Cell Dev. Biol. 2024, 12, 1490315. [Google Scholar] [CrossRef]
- Parrish, A.R. The Cytoskeleton as a Novel Target for Treatment of Renal Fibrosis. Pharmacol. Ther. 2016, 166, 1–8. [Google Scholar] [CrossRef]
- Durvasula, R.V.; Shankland, S.J. Podocyte Injury and Targeting Therapy: An Update. Curr. Opin. Nephrol. Hypertens. 2006, 15, 1–7. [Google Scholar] [CrossRef]
- Yu, S.M.-W.; Nissaisorakarn, P.; Husain, I.; Jim, B. Proteinuric Kidney Diseases: A Podocyte’s Slit Diaphragm and Cytoskeleton Approach. Front. Med. 2018, 5, 221. [Google Scholar] [CrossRef]

| Serial No. | Compound ID | Compound Structure | Molecular Weight |
|---|---|---|---|
| 1 | MDB-31 | 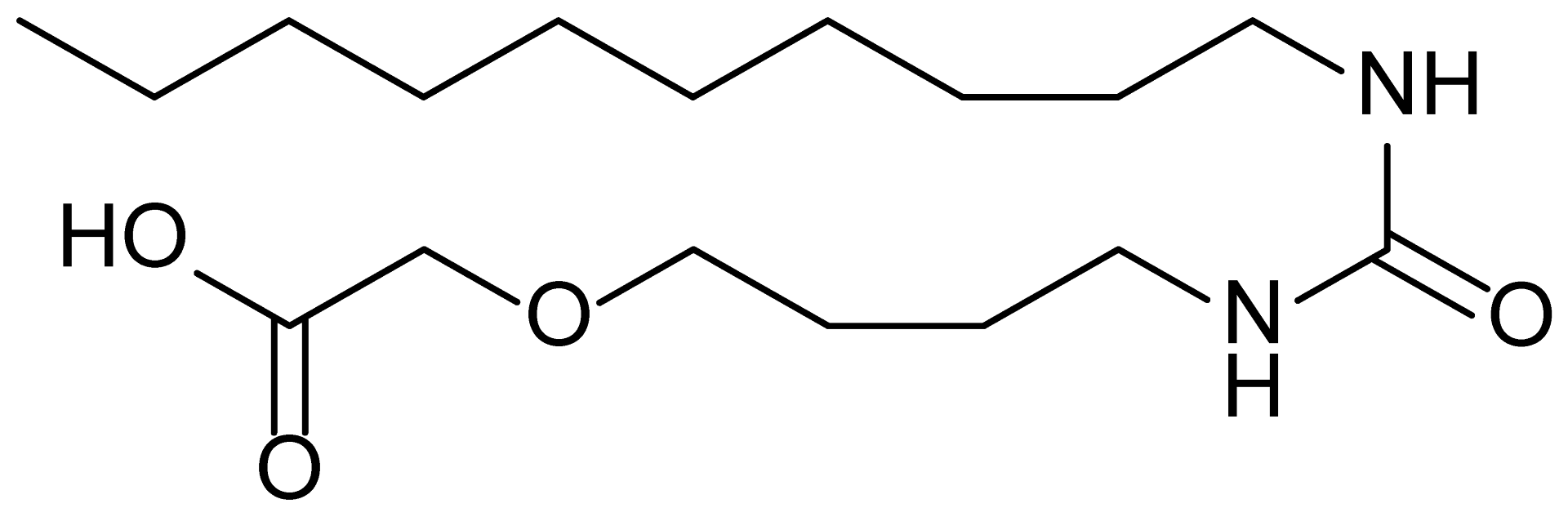 | 330.5 |
| 2 | MDB-32 | 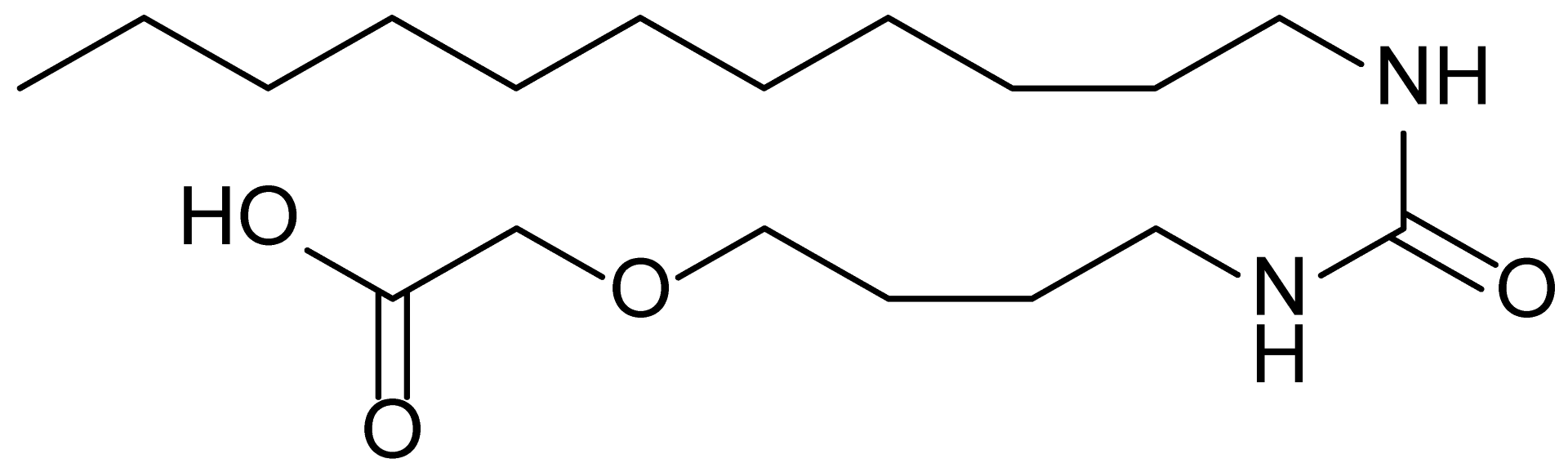 | 344.5 |
| 3 | MDB-33 |  | 328.5 |
| 4 | MDB-41 | 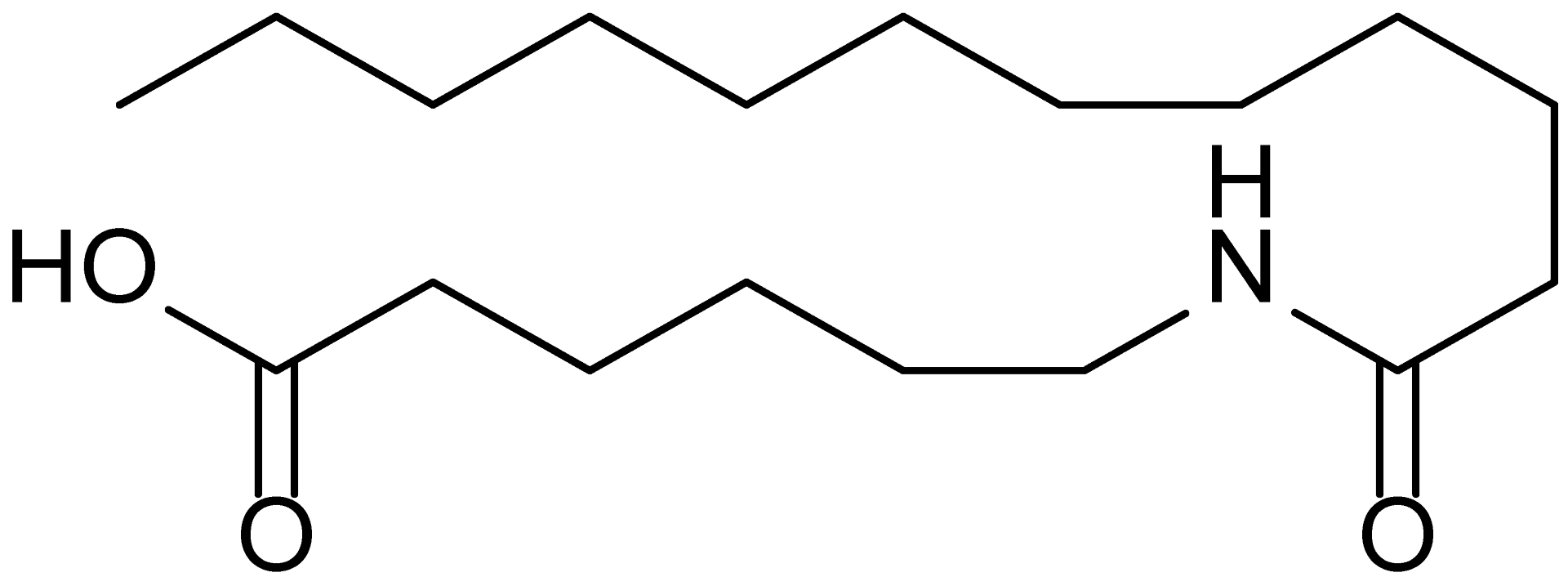 | 313.5 |
| 5 | MDB-44 |  | 340.5 |
| 6 | MDB-46 | 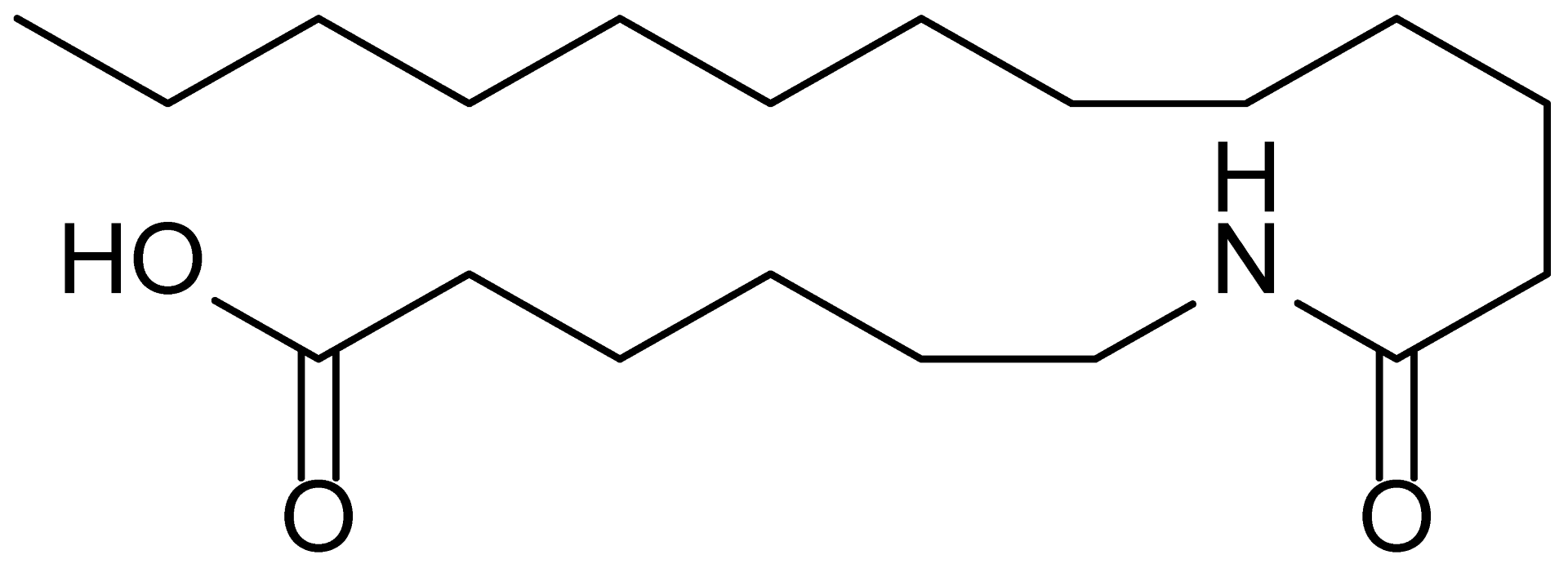 | 327.5 |
| 7 | MDB-52a | 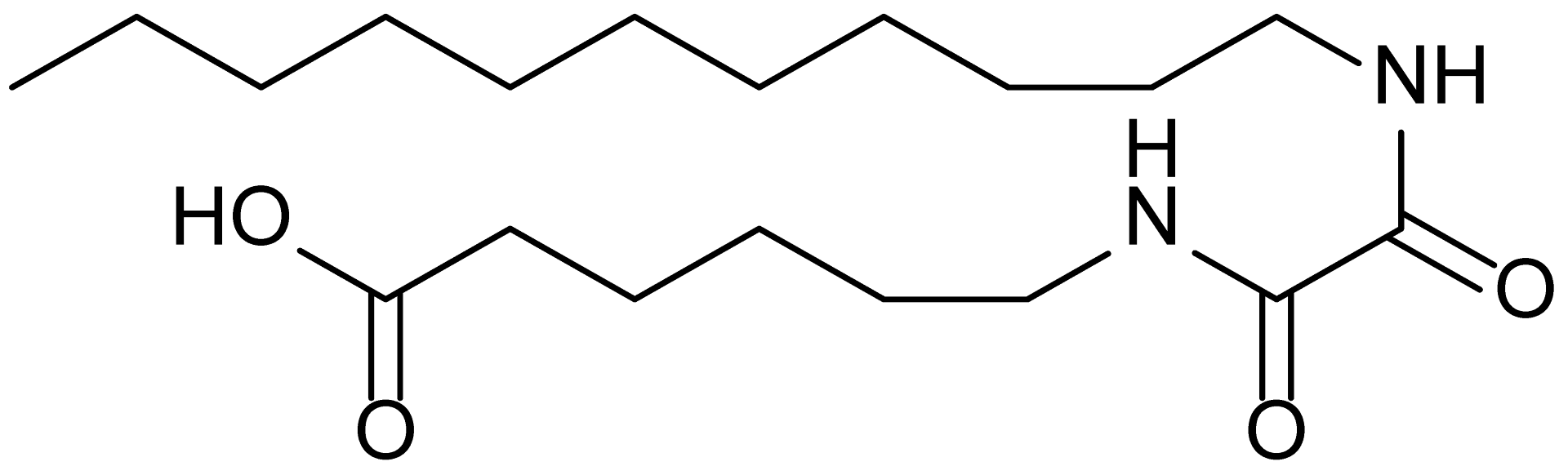 | 365.5 |
| 8 | MDB-52b | 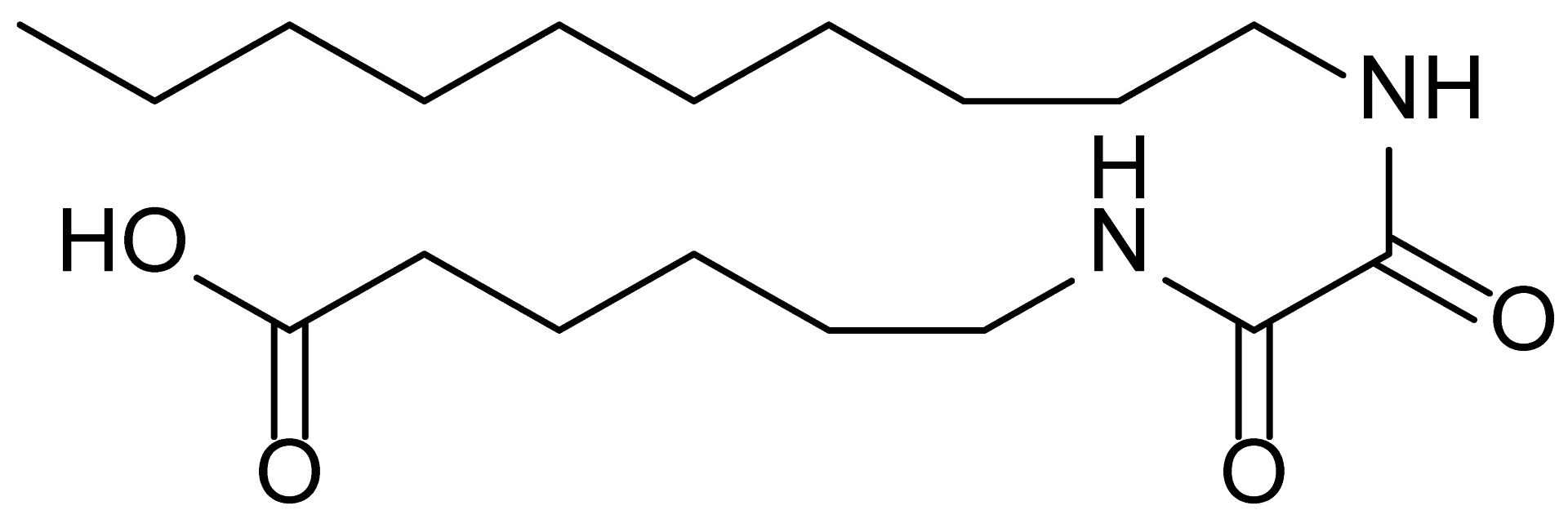 | 342.5 |
| 9 | MDB-58 | 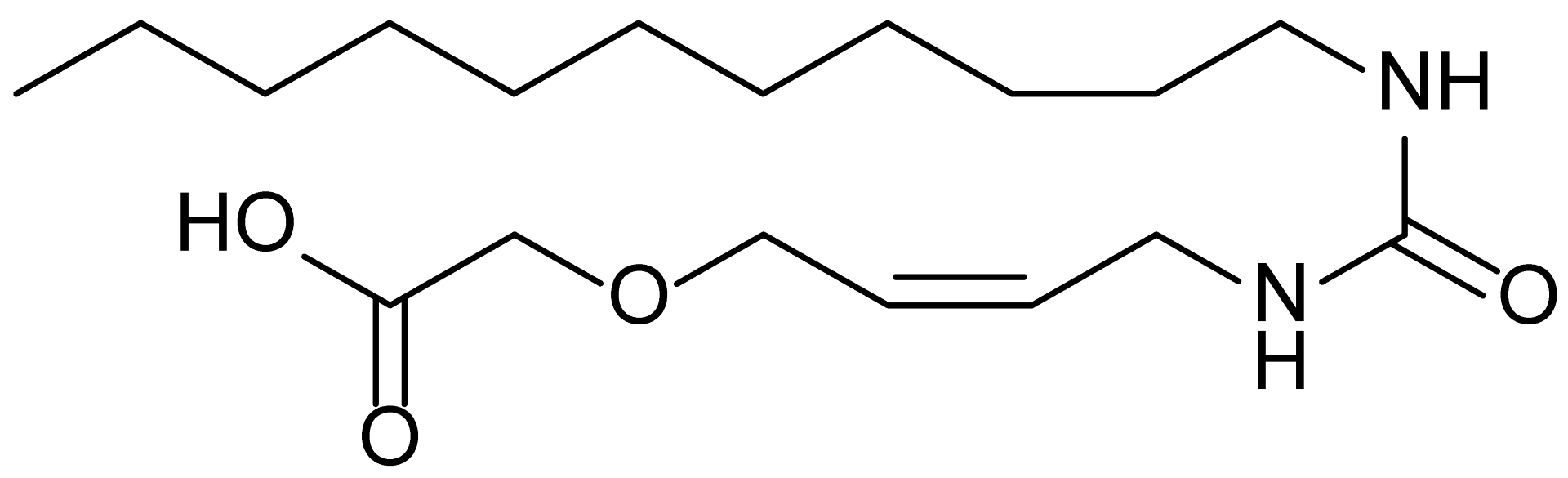 | 342.5 |
| 10 | MDB-76 |  | 327.5 |
| 11 | MDB-77 |  | 341.5 |
| 12 | MDB-78 | 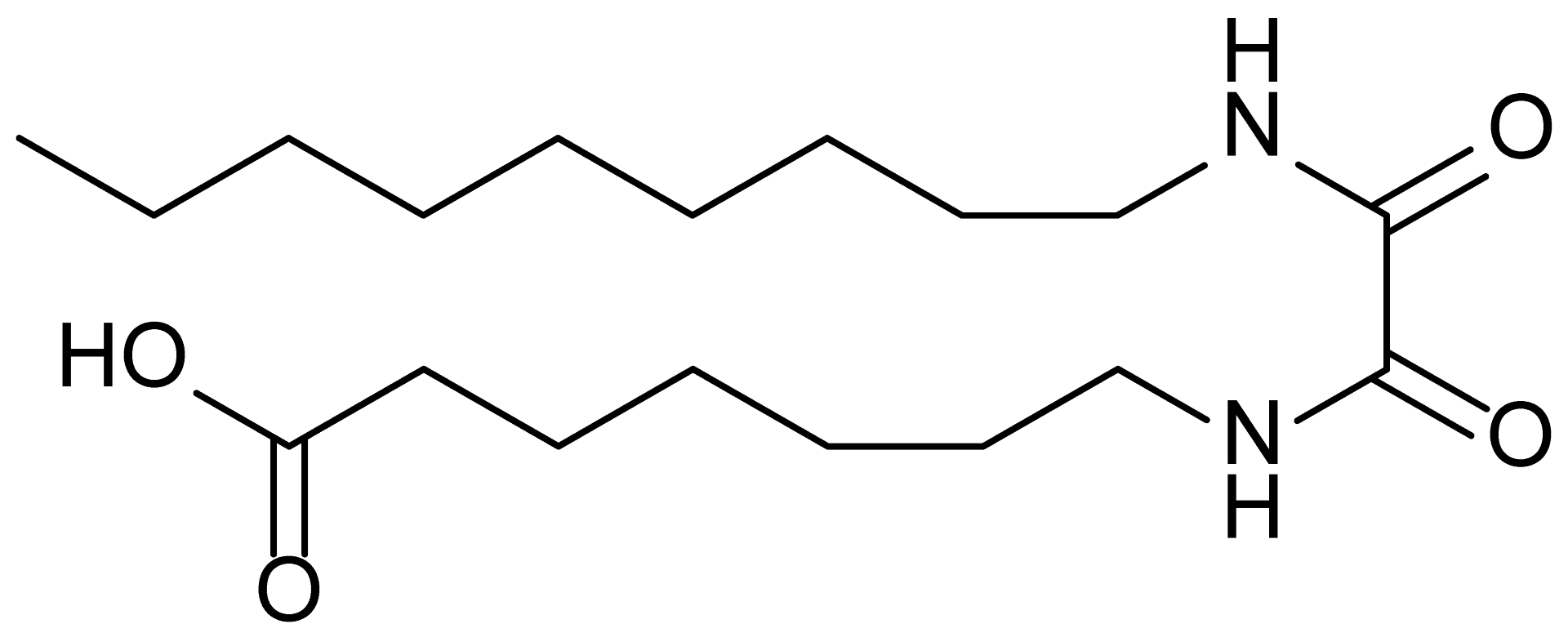 | 342.5 |
| 13 | MDB-79 | 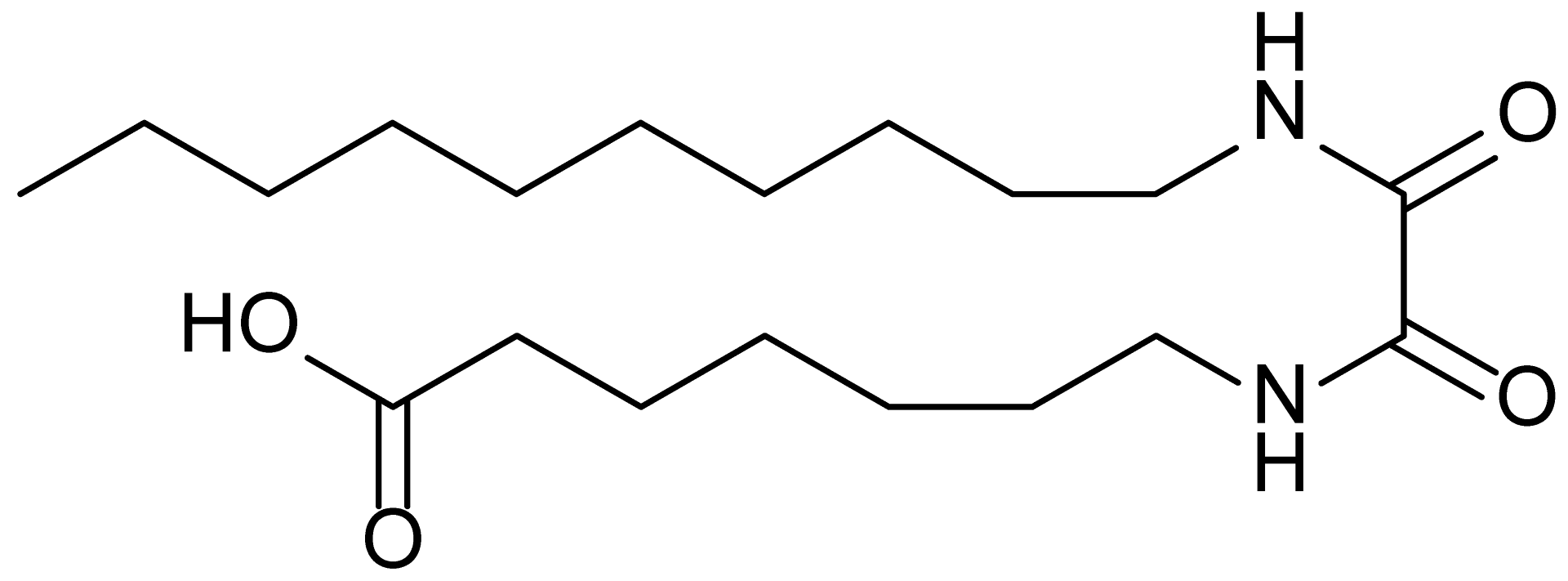 | 356.5 |
| 14 | MDB-80 |  | 327.5 |
| 15 | MDB-81 |  | 341.5 |
| 16 | MDB-18 | 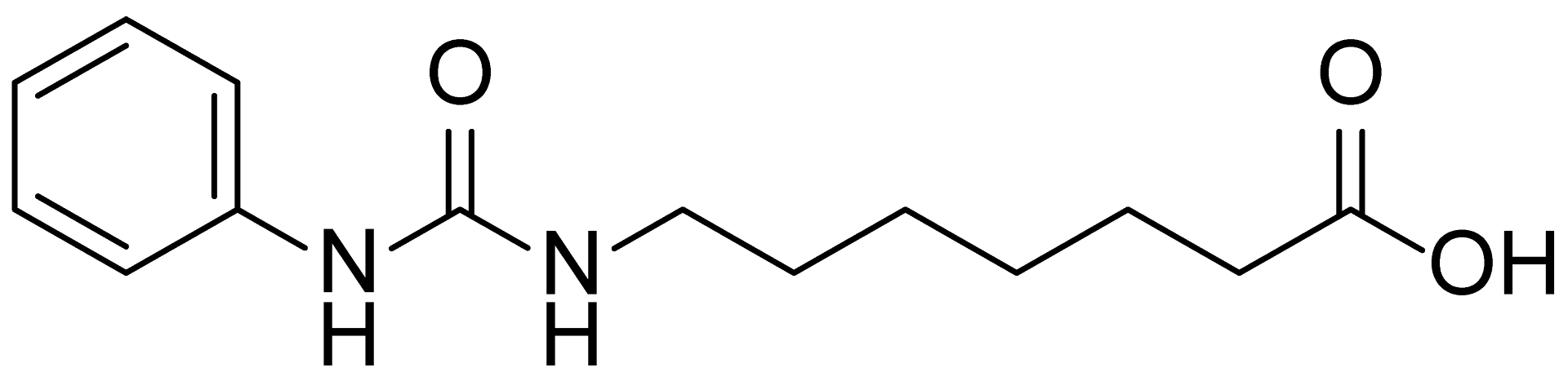 | 328.5 |
| 17 | RM-69 | 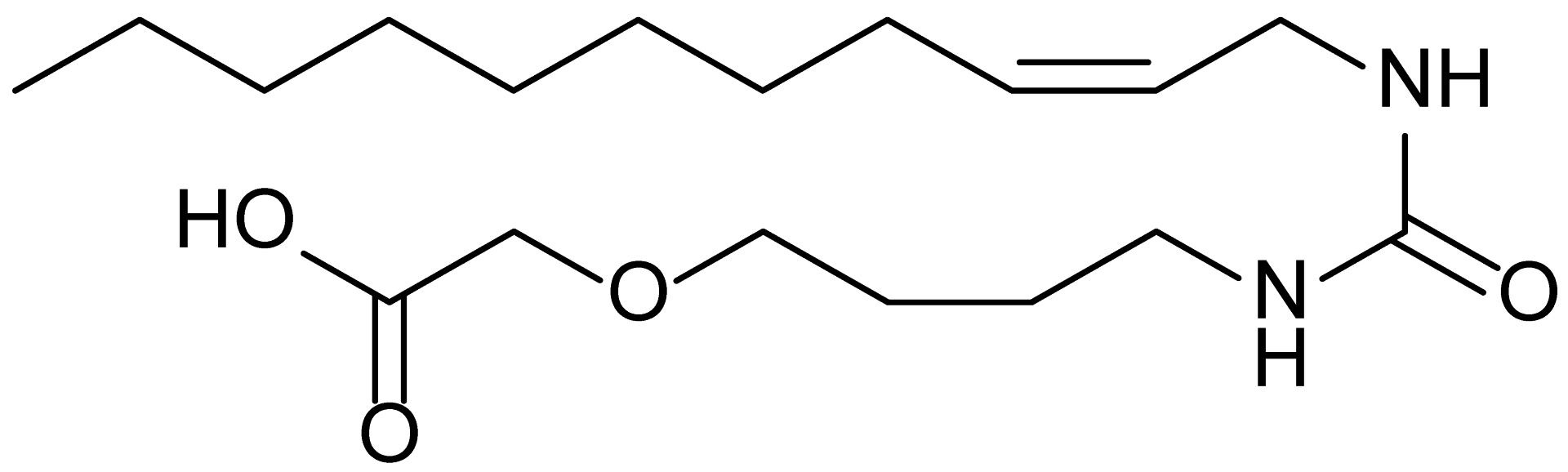 | 342.5 |
| 18 | RM-72 | 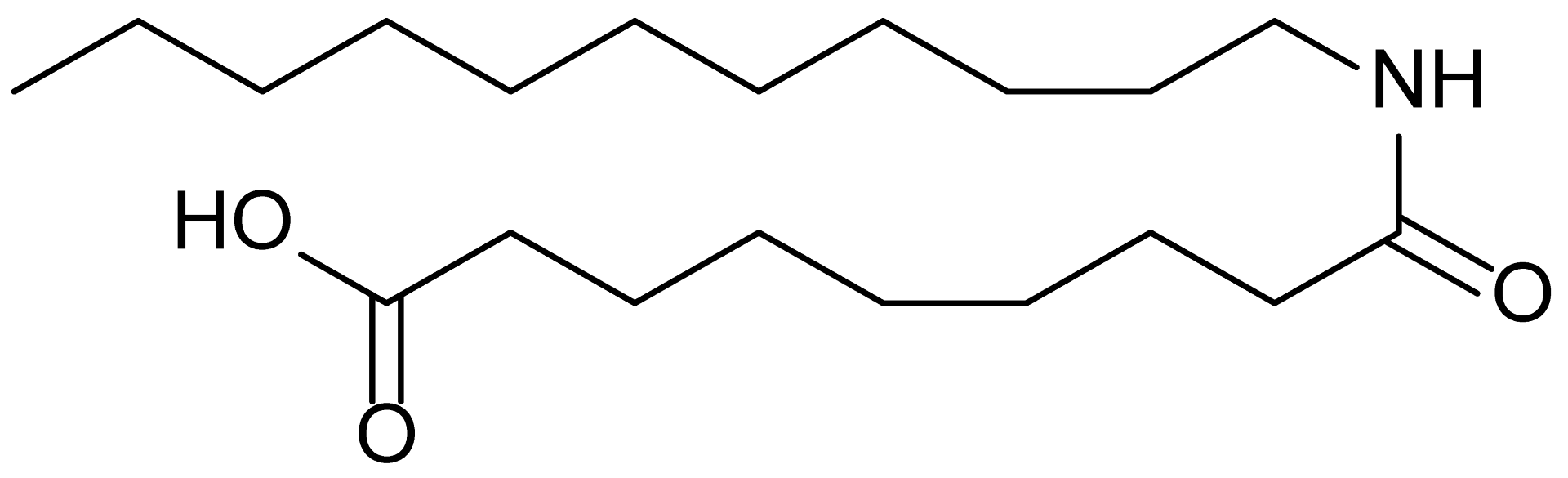 | 341.5 |
| 19 | RM-81 | 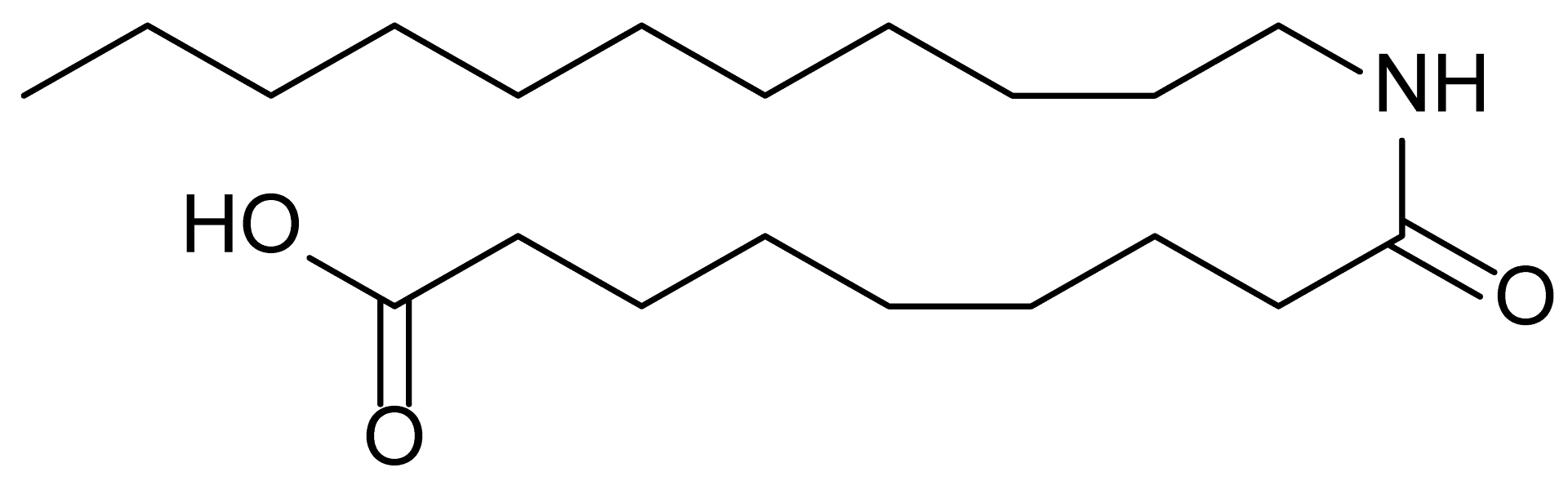 | 339.5 |
| 20 | RM-84 | 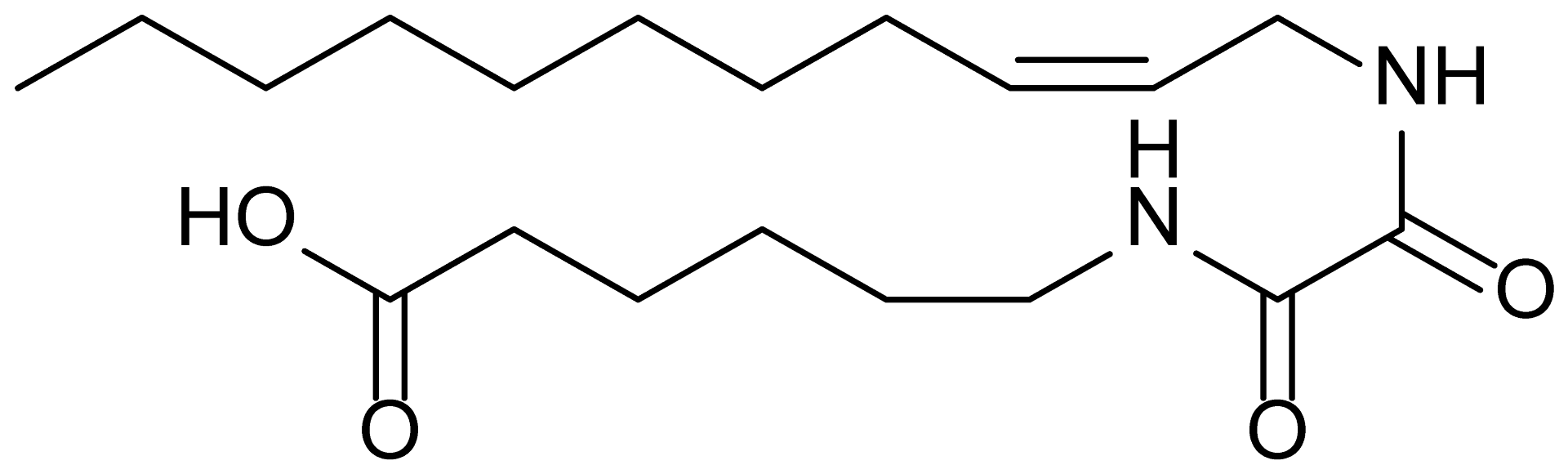 | 354.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mishra, A.; de Bourg, M.; Mohamed, R.S.; Hye Khan, M.A.; Weldemichael, T.; Johann, D.J., Jr.; Goorani, S.; Bommagani, S.; Jones, D.E.; Vik, A.; et al. EET-Based Therapeutics Mitigate Sorafenib-Associated Glomerular Cell Damage. Biomolecules 2025, 15, 1324. https://doi.org/10.3390/biom15091324
Mishra A, de Bourg M, Mohamed RS, Hye Khan MA, Weldemichael T, Johann DJ Jr., Goorani S, Bommagani S, Jones DE, Vik A, et al. EET-Based Therapeutics Mitigate Sorafenib-Associated Glomerular Cell Damage. Biomolecules. 2025; 15(9):1324. https://doi.org/10.3390/biom15091324
Chicago/Turabian StyleMishra, Abhishek, Marcus de Bourg, Rawand S. Mohamed, Md Abdul Hye Khan, Tsigereda Weldemichael, Donald J. Johann, Jr., Samaneh Goorani, Shobanbabu Bommagani, Darin E. Jones, Anders Vik, and et al. 2025. "EET-Based Therapeutics Mitigate Sorafenib-Associated Glomerular Cell Damage" Biomolecules 15, no. 9: 1324. https://doi.org/10.3390/biom15091324
APA StyleMishra, A., de Bourg, M., Mohamed, R. S., Hye Khan, M. A., Weldemichael, T., Johann, D. J., Jr., Goorani, S., Bommagani, S., Jones, D. E., Vik, A., & Imig, J. D. (2025). EET-Based Therapeutics Mitigate Sorafenib-Associated Glomerular Cell Damage. Biomolecules, 15(9), 1324. https://doi.org/10.3390/biom15091324









