Plasminogen Activator Inhibitor-1 in Skin Malignancies: Therapeutic Implications of Its Inhibition
Abstract
1. Introduction
2. PAI-1 in Skin Cancers
2.1. Malignant Melanoma
2.2. Cutaneous Squamous Cell Carcinoma (cSCC)
2.3. Cutaneous Angiosarcoma (CAS)
2.4. Mycosis Fungoides (MF), Cutaneous T Cell Lymphoma (CTCL)
3. Diverse Tumor-Promoting Effects of PAI-1 in the TME
3.1. Immunomodulatory Role of PAI-1 in TAM Regulation Within the TME
3.2. Significant Effects of PAI-1 on Cancer-Associated Fibroblasts (CAFs)
4. Future Perspective
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Atopic dermatitis |
| CAFs | Cancer associated fibroblasts |
| CAS | Cutaneous angiosarcoma |
| cSCC | Cutaneous squamous cell carcinoma |
| ECs | Endothelial cells |
| ECM | Extracellular matrix |
| HA | Hyaluronic acid |
| HS | Hidradenitis suppurativa |
| ICIs | Immune checkpoints inhibitors |
| MMP | Matrix metalloproteinase |
| MF | Mycosis fungoides |
| PAI-1 | plasminogen activator inhibitor-1 |
| PD-L1 | programmed cell death ligand 1 |
| SASP | Senescence-associated secretory phenotype |
| TAMs | tumor-associated macrophage |
| TKI | Tyrosine kinase inhibitor |
| TME | Tumor microenvironment |
References
- Placencio, V.R.; DeClerck, Y.A. Plasminogen Activator Inhibitor-1 in Cancer: Rationale and Insight for Future Therapeutic Testing. Cancer Res. 2015, 75, 2969–2974. [Google Scholar] [CrossRef]
- Iwaki, T.; Urano, T.; Umemura, K. PAI-1, progress in understanding the clinical problem and its aetiology. Br. J. Haematol. 2012, 157, 291–298. [Google Scholar] [CrossRef]
- Ohuchi, K.; Kambayashi, Y.; Hidaka, T.; Fujimura, T. Plasminogen activating inhibitor-1 might be a predictive marker for the efficacy of anti-PD1 antibody in advanced melanoma patients. Front. Oncol. 2021, 11, 798385. [Google Scholar] [CrossRef] [PubMed]
- Hisada, Y.; Mackman, N. Cancer-associated pathways and biomarkers of venous thrombosis. Blood 2017, 130, 1499–1506. [Google Scholar] [CrossRef] [PubMed]
- Bajou, K.; Peng, H.; Laug, W.E.; Maillard, C.; Noel, A.; Foidart, J.M.; Martial, J.A.; DeClerck, Y.A. Plasminogen activator inhibitor-1 protects endothelial cells from FasL-mediated apoptosis. Cancer Cell 2008, 14, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Bajou, K.; Maillard, C.; Jost, M.; Lijnen, R.H.; Gils, A.; Declerck, P.; Carmeliet, P.; Foidart, J.M.; Noel, A. Host-derived plasminogen activator inhibitor-1 (PAI-1) concentration is critical for in vivo tumoral angiogenesis and growth. Oncogene 2004, 23, 6986–6990. [Google Scholar] [CrossRef]
- Thapa, B.; Koo, B.H.; Kim, Y.H.; Kwon, H.J.; Kim, D.S. Plasminogen activator inhibitor-1 regulates infiltration of macrophages into melanoma via phosphorylation of FAK-Tyr925. Biochem. Biophys. Res. Commun. 2014, 450, 1696–1701. [Google Scholar] [CrossRef]
- Tseng, Y.J.; Lee, C.H.; Chen, W.Y.; Kwon, H.J.; Kim, D.S. Inhibition of PAI-1 Blocks PD-L1 Endocytosis and Improves the Response of Melanoma Cells to Immune Checkpoint Blockade. J. Investig. Dermatol. 2021, 141, 2690–2698. [Google Scholar] [CrossRef]
- Ibrahim, A.; Fujimura, T.; Uno, T.; Terada, T.; Dan, T.; Ohta, A.; Miyata, T.; Ando, K.; Yahata, T. Plasminogen activator inhibitor-1 promotes immune evasion in tumors by facilitating the expression of programmed cell death-ligand 1. Front. Immunol. 2024, 15, 1365894. [Google Scholar] [CrossRef]
- Fujimura, T.; Yoshino, K.; Kato, H.; Fukushima, S.; Ishizuki, S.; Otsuka, A.; Matsushita, S.; Amagai, R.; Muto, Y.; Yamazaki, E.; et al. A phase II multicentre study of plasminogen activator inhibitor-1 inhibitor (TM5614) plus nivolumab for treating anti-programmed cell death 1 antibody-refractory malignant melanoma: TM5614-MM trial. Br. J. Dermatol. 2024, 191, 691–697. [Google Scholar] [CrossRef]
- Takahashi, N.; Kameoka, Y.; Onizuka, M.; Onishi, Y.; Takahashi, F.; Dan, T.; Miyata, T.; Ando, K.; Harigae, H. Deep molecular response in patients with chronic phase chronic myeloid leukemia treated with the plasminogen activator inhibitor-1 inhibitor TM5614 combined with a tyrosine kinase inhibitor. Cancer Med. 2023, 12, 4250–4258. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, T.; Yoshino, K.; Nakamura, M.; Kato, H.; Ito, T.; Maekawa, T.; Fujisawa, Y.; Matsushita, S.; Amagai, R.; Yamazaki, E.; et al. Efficacy and safety of TM5614 in combination with paclitaxel in the treatment of paclitaxel-resistant cutaneous angiosarcoma: Phase II study protocol. Exp. Dermatol. 2024, 33, e14976. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Hirata, T.; Sakamoto, T.; Tsubata, Y.; Ichihara, E.; Kozuki, T.; Shoda, H.; Motonaga, M.; Yoshida, T.; Fukutani, M.; et al. Treatment rationale and protocol design: An. investigator-initiated phase II study of combination treatment of nivolumab and TM5614, a PAI-1 inhibitor for previously treated patients with non-small cell lung cancer. J. Thorac. Dis. 2024, 16, 3381–3388. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Shoushtari, A.N.; Betof Warner, A.; Si, L.; Tang, B.; Cui, C.; Yang, X.; Wei, X.; Quach, H.T.; Cann, C.G.; et al. Benefit and toxicity of programmed death-1 blockade vary by ethnicity in patients with advanced melanoma: An international multicentre observational study. Br. J. Dermatol. 2022, 187, 401–410. [Google Scholar] [CrossRef]
- Nakamura, Y.; Namikawa, K.; Yoshino, K.; Yoshikawa, S.; Uchi, H.; Goto, K.; Nakamura, Y.; Fukushima, S.; Kiniwa, Y.; Takenouchi, T.; et al. Anti-PD1 checkpoint inhibitor therapy in acral melanoma: A multicenter study of 193 Japanese patients. Anti-PD1 checkpoint inhibitor therapy in acral melanoma: A multicenter study of 193 Japanese patients. Ann. Oncol. 2020, 31, 1198–1206. [Google Scholar] [CrossRef]
- Minowa, T.; Murata, K.; Mizue, Y.; Murai, A.; Nakatsugawa, M.; Sasaki, K.; Tokita, S.; Kubo, T.; Kanaseki, T.; Tsukahara, T.; et al. Single-cell profiling of acral melanoma infiltrating lymphocytes reveals a suppressive tumor microenvironment. Sci. Transl. Med. 2024, 16, eadk8832. [Google Scholar] [CrossRef]
- Fujisawa, Y.; Yoshikawa, S.; Minagawa, A.; Takenouchi, T.; Yokota, K.; Uchi, H.; Noma, N.; Nakamura, Y.; Asai, J.; Kato, J.; et al. Clinical and histopathological characteristics and survival analysis of 4594 Japanese patients with melanoma. Cancer Med. 2019, 8, 2146–2156. [Google Scholar] [CrossRef]
- Hayward, N.K.; Wilmott, J.S.; Waddell, N.; Johansson, P.A.; Field, M.A.; Nones, K.; Patch, A.M.; Kakavand, H.; Alexandrov, L.B.; Burke, H.; et al. Whole-genome landscapes of major melanoma subtypes. Nature 2017, 545, 175–180. [Google Scholar] [CrossRef]
- Blank, C.U.; Rozeman, E.A.; Fanchi, L.F.; Sikorska, K.; van de Wiel, B.; Kvistborg, P.; Krijgsman, O.; van den Braber, M.; Philips, D.; Broeks, A.; et al. Neoadjuvant versus adjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma. Nat. Med. 2018, 24, 1655–1661. [Google Scholar] [CrossRef]
- Tawbi, H.A.; Schadendorf, D.; Lipson, E.J.; Ascierto, P.A.; Matamala, L.; Castillo Gutiérrez, E.; Rutkowski, P.; Gogas, H.J.; Lao, C.D.; De Menezes, J.J.; et al. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N. Engl. J. Med. 2022, 386, 24–34. [Google Scholar] [CrossRef]
- Sadik, A.; Somarribas Patterson, L.F.; Öztürk, S.; Mohapatra, S.R.; Panitz, V.; Secker, P.F.; Pfänder, P.; Loth, S.; Salem, H.; Prentzell, M.T.; et al. IL4I1 Is a Metabolic Immune Checkpoint that Activates the AHR and Promotes Tumor Progression. Cell 2020, 182, 1252–1270. [Google Scholar] [CrossRef] [PubMed]
- Dummer, R.; Long, G.V.; Robert, C.; Tawbi, H.A.; Flaherty, K.T.; Ascierto, P.A.; Nathan, P.D.; Rutkowski, P.; Leonov, O.; Dutriaux, C.; et al. Randomized Phase III Trial Evaluating Spartalizumab Plus Dabrafenib and Trametinib for BRAF V600-Mutant Unresectable or Metastatic Melanoma. J. Clin. Oncol. 2022, 40, 1428–1438. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.M.; Bernstein, D.; Higgins, S.P.; Higgins, C.E.; Higgins, P.J. SERPINE1 expression discriminates site-specific metastasis in human melanoma. Exp. Dermatol. 2012, 21, 551–554. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Hattori, N.; Senoo, T.; Akita, S.; Ishikawa, N.; Fujitaka, K.; Haruta, Y.; Murai, H.; Kohno, N. SK-216, an inhibitor of plasminogen activator inhibitor-1, limits tumor progression and angiogenesis. Mol. Cancer Ther. 2013, 12, 2378–2388. [Google Scholar] [CrossRef]
- Pires da Silva, I.; Ahmed, T.; Reijers, I.L.M.; Weppler, A.M.; Betof Warner, A.; Patrinely, J.R.; Serra-Bellver, P.; Allayous, C.; Mangana, J.; Nguyen, K.; et al. Ipilimumab alone or ipilimumab plus anti-PD-1 therapy in patients with metastatic melanoma resistant to anti-PD-(L)1 monotherapy: A multicentre, retrospective, cohort study. Lancet Oncol. 2021, 22, 836–847. [Google Scholar] [CrossRef]
- VanderWalde, A.; Bellasea, S.L.; Kendra, K.L.; Khushalani, N.I.; Campbell, K.M.; Scumpia, P.O.; Kuklinski, L.F.; Collichio, F.; Sosman, J.A.; Ikeguchi, A.; et al. Ipilimumab with or without nivolumab in PD-1 or PD-L1 blockade refractory metastatic melanoma: A randomized phase 2 trial. Nat. Med. 2023, 29, 2278–2285. [Google Scholar] [CrossRef]
- Takahashi, A.; Namikawa, K.; Ogata, D.; Jinnai, S.; Nakano, E.; Yamazaki, N. Updated analysis of nivolumab and ipilimumab combination therapy in Japanese patients with advanced melanoma. J. Dermatol. 2023, 50, 525–535. [Google Scholar] [CrossRef]
- Yamazaki, E.; Fujimura, T.; Takahashi-Watanabe, M.; Tada, S.; Kitayama, C.; Amagai, R.; Kambayashi, Y.; Watanabe, M.; Maekawa, M.; Mano, N.; et al. Decreased serum levels of IL-4 correlate with the efficacy of the PAI-1 inhibitor TM5614 in patients with malignant melanoma refractory to anti-PD-1 antibodies: Post hoc study of TM5614-MM trial. Br. J. Dermatol. 2024, 192, 167–169. [Google Scholar] [CrossRef]
- Sappino, A.P.; Belin, D.; Huarte, J.; Hirschel-Scholz, S.; Saurat, J.H.; Vassalli, J.D. Differential protease expression by cutaneous squamous and basal cell carcinomas. J. Clin. Investig. 1991, 88, 1073–1079. [Google Scholar] [CrossRef]
- Wilkins-Port, C.E.; Higgins, C.E.; Freytag, J.; Higgins, S.P.; Carlson, J.A.; Higgins, P.J. PAI-1 is a Critical Upstream Regulator of the TGF-beta1/EGF-Induced Invasive Phenotype in Mutant p53 Human Cutaneous Squamous Cell Carcinoma. J. Biomed. Biotechnol. 2007, 2007, 85208. [Google Scholar] [CrossRef]
- Mizrahi, A.; Barzilai, A.; Gur-Wahnon, D.; Ben-Dov, I.Z.; Glassberg, S.; Meningher, T.; Elharar, E.; Masalha, M.; Jacob-Hirsch, J.; Tabibian-Keissar, H.; et al. Alterations of microRNAs throughout the malignant evolution of cutaneous squamous cell carcinoma: The role of miR-497 in epithelial to mesenchymal transition of keratinocytes. Oncogene 2018, 37, 218–230. [Google Scholar] [CrossRef]
- Shaikh, S.B.; Balaya, R.D.A.; Dagamajalu, S.; Bhandary, Y.P.; Unwalla, H.; Prasad, T.S.K.; Rahman, I. A signaling pathway map of plasminogen activator inhibitor-1 (PAI-1/SERPINE-1): A review of an innovative frontier in molecular aging and cellular senescence. Cell Commun. Signal 2024, 22, 544. [Google Scholar] [CrossRef]
- Salminen, A. Cooperation between inhibitory immune checkpoints of senescent cells with immunosuppressive network to promote immunosenescence and the aging process. Ageing Res. Rev. 2025, 106, 102694. [Google Scholar] [CrossRef]
- Giroud, J.; Delvaux, P.; Carlier, L.; De Schutter, C.; Martin, N.; Rouget, R.; Bolouki, A.; De Glas, V.; Bouriez, I.; Bourdoux, F.; et al. Targeting ATF6alpha Attenuates UVB-Induced Senescence and Improves Skin Homeostasis by Regulating IL8 Expression. Aging Cell 2025, 24, e70024. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, J.; Pei, Z.; Zhu, Y.; Zhang, X.; Zhou, Z.; Ye, C.; Song, M.; Hu, Y.; Xue, P.; et al. Endothelial senescence induced by PAI-1 promotes endometrial fibrosis. Cell Death Discov. 2025, 11, 89. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, E.; Fujimura, T.; Takahashi-Watanabe, M.; Amagai, R.; Tamabuchi, E.; Oka, K.; Kambayashi, Y.; Hashimoto, A.; Omori, R.; Takahashi, T.; et al. An Evaluation of Prognostic Factors in Cutaneous Squamous Cell Carcinoma: A Single-Center Study of 237 Japanese Cases. J. Clin. Med. 2025, 14, 1243. [Google Scholar] [CrossRef]
- Meier, C.; Brieger, A. The role of IL-8 in cancer development and its impact on immunotherapy resistance. Eur. J. Cancer 2025, 218, 115267. [Google Scholar] [CrossRef] [PubMed]
- Alfaro, C.; Teijeira, A.; Oñate, C.; Pérez, G.; Sanmamed, M.F.; Andueza, M.P.; Alignani, D.; Labiano, S.; Azpilikueta, A.; Rodriguez-Paulete, A.; et al. Tumor-Produced Interleukin-8 Attracts Human Myeloid-Derived Suppressor Cells and Elicits Extrusion of Neutrophil Extracellular Traps (NETs). Clin. Cancer Res. 2016, 22, 3924–3936. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Guo, Z.Q.; Cai, X.T.; Rong, Z.X.; Fang, Y.; Chen, J.Q.; Zhuang, K.M.; Ruan, M.J.; Ma, S.C.; Lin, L.Y.; et al. PAI-1-driven SFRP2(high) cancer-associated fibroblasts hijack the abscopal effect of radioimmunotherapy. Cancer Cell 2025, 43, 856–874. [Google Scholar] [CrossRef]
- Che, Y.; Wang, J.; Li, Y.; Lu, Z.; Huang, J.; Sun, S.; Mao, S.; Lei, Y.; Zang, R.; Sun, N.; et al. Cisplatin-activated PAI-1 secretion in the cancer-associated fibroblasts with paracrine effects promoting esophageal squamous cell carcinoma progression and causing chemoresistance. Cell Death Dis. 2018, 9, 759. [Google Scholar] [CrossRef]
- Foekens, J.A.; Peters, H.A.; Look, M.P.; Portengen, H.; Schmitt, M.; Kramer, M.D.; Brünner, N.; Jänicke, F.; Meijer-van Gelder, M.E.; Henzen-Logmans, S.C.; et al. The urokinase system of plasminogen activation and prognosis in 2780 breast cancer patients. Cancer Res. 2000, 60, 636–643. [Google Scholar] [PubMed]
- Bagaria, S.P.; Gatalica, Z.; Maney, T.; Serie, D.; Parasramka, M.; Attia, S.; Krishna, M.; Joseph, R.W. Association Between Programmed Death-Ligand 1 Expression and the Vascular Endothelial Growth Factor Pathway in Angiosarcoma. Front. Oncol. 2018, 8, 71. [Google Scholar] [CrossRef]
- Thiebaud, J.A.; Ravi, V.; Litwin, S.; Schuetze, S.M.; Movva, S.; Agulnik, M.; Kraft, A.S.; Tetzlaff, E.D.; Somaiah, N.; von Mehren, M. OER-073: A multicenter phase 2 study evaluating the role of pazopanib in angiosarcoma. Cancer 2022, 128, 3516–3522. [Google Scholar] [CrossRef]
- Jones, R.L.; Ravi, V.; Brohl, A.S.; Chawla, S.; Ganjoo, K.N.; Italiano, A.; Attia, S.; Burgess, M.A.; Thornton, K.; Cranmer, L.D.; et al. Efficacy and Safety of TRC105 Plus Pazopanib vs Pazopanib Alone for Treatment of Patients with Advanced Angiosarcoma: A Randomized Clinical Trial. JAMA Oncol. 2022, 8, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Ohuchi, K.; Amagai, R.; Ikawa, T.; Muto, Y.; Roh, Y.; Endo, J.; Maekawa, T.; Kambayashi, Y.; Asano, Y.; Fujimura, T. Plasminogen activating inhibitor-1 promotes angiogenesis in cutaneous angiosarcomas. Exp. Dermatol. 2023, 32, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Fjæstad, K.Y.; Rømer, A.M.A.; Goitea, V.; Johansen, A.Z.; Thorseth, M.L.; Carretta, M.; Engelholm, L.H.; Grøntved, L.; Junker, N.; Madsen, D.H. Blockade of beta-adrenergic receptors reduces cancer growth and enhances the response to anti-CTLA4 therapy by modulating the tumor microenvironment. Oncogene 2022, 41, 1364–1375. [Google Scholar] [CrossRef]
- Wagner, M.J.; Cranmer, L.D.; Loggers, E.T.; Pollack, S.M. Propranolol for the treatment of vascular sarcomas. J. Exp. Pharmacol. 2018, 10, 51–58. [Google Scholar] [CrossRef]
- Mehta-Shah, N.; Horwitz, S.M.; Ansell, S.; Ai, W.Z.; Barnes, J.; Barta, S.K.; Clemens, M.W.; Dogan, A.; Fisher, K.; Goodman, A.M.; et al. NCCN Guidelines Insights: Primary Cutaneous Lymphomas, Version 2.2020. J. Natl. Compr. Canc Netw. 2020, 18, 522–536. [Google Scholar] [CrossRef]
- Vonderheid, E.C.; Hamilton, R.G.; Kadin, M.E. Mycosis Fungoides and Its Relationship to Atopy, Serum Total IgE, and Eosinophil Counts. Clin. Lymphoma Myeloma Leuk. 2021, 21, 279–288. [Google Scholar] [CrossRef]
- Ha, Y.J.; Tak, K.H.; Lee, J.L.; Kim, C.W.; Ah, Y.C.; Kim, S.S.; Moon, I.J.; Yoon, Y.S. Polynucleotides Enhance Skin Barrier Function and Reduce Inflammation in a 2,4-Dinitrochlorobenzene-Induced Mouse Model of Atopic Dermatitis. Skin. Res. Technol. 2025, 31, e70189. [Google Scholar] [CrossRef]
- Patil, K.; Kuttikrishnan, S.; Khan, A.Q.; Ahmad, F.; Alam, M.; Buddenkotte, J.; Ahmad, A.; Steinhoff, M.; Uddin, S. Molecular pathogenesis of Cutaneous T cell Lymphoma: Role of chemokines, cytokines, and dysregulated signaling pathways. Semin. Cancer Biol. 2022, 86, 382–399. [Google Scholar] [CrossRef]
- Bogdanowicz, P.; Bensadoun, P.; Noizet, M.; Béganton, B.; Philippe, A.; Alvarez-Georges, S.; Doat, G.; Tourette, A.; Bessou-Touya, S.; Lemaitre, J.M.; et al. Senomorphic activity of a combination of niacinamide and hyaluronic acid: Correlation with clinical improvement of skin aging. Sci. Rep. 2024, 14, 16321. [Google Scholar] [CrossRef]
- Ikawa, T.; Yamazaki, E.; Amagai, R.; Kambayashi, Y.; Sekine, M.; Takahashi, T.; Asano, Y.; Fujimura, T. Impact of Hyaluronic Acid on the Cutaneous T-Cell Lymphoma Microenvironment: A Novel Anti-Tumor Mechanism of Bexarotene. Cancers 2025, 17, 324. [Google Scholar] [CrossRef]
- Zheng, B.W.; Wang, B.Y.; Xiao, W.L.; Sun, Y.J.; Yang, C.; Zhao, B.T. Different molecular weight hyaluronic acid alleviates inflammation response in DNFB-induced mice atopic dermatitis and LPS-induced RAW 264.7 cells. Life Sci. 2022, 301, 120591. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, T.; Ohuchi, K.; Ikawa, T.; Kambayashi, Y.; Amagai, R.; Furudate, S.; Asano, Y. Plasminogen activator inhibitor-1 promote angiogenesis through matrix metalloproteinase 9 in advanced mycosis fungoides. Hematol. Oncol. 2024, 42, e3244. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Huang, L.; Enkhjargal, B.; Xu, W.; Umut, O.; Travis, Z.D.; Zhang, G.; Tang, J.; Liu, F.; Zhang, J.H. Activation of retinoid X receptor by bexarotene attenuates neuroinflammation via PPARgamma/SIRT6/FoxO3a pathway after subarachnoid hemorrhage in rats. J. Neuroinflamm. 2019, 16, 47. [Google Scholar] [CrossRef] [PubMed]
- Adhikary, K.; Paul, A.; Madan, A.; Islam, A.; Ashique, S.; Ramzan, M. Personalized precision: Revolutionizing cancer treatment with mRNA-based vaccines in melanoma therapy. Adv. Immunol. 2025, 166, 137–167. [Google Scholar]
- Wolchok, J.D.; Chiarion-Sileni, V.; Rutkowski, P.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Queirolo, P.; Dummer, R.; Butler, M.O.; Hill, A.G.; et al. Final, 10-Year Outcomes with Nivolumab plus Ipilimumab in Advanced Melanoma. N. Eng. J. Med. 2025, 392, 11–22. [Google Scholar] [CrossRef]
- Bai, X.; Lawless, A.R.; Czapla, J.A.; Gerstberger, S.C.; Park, B.C.; Jung, S.; Johnson, R.; Yamazaki, N.; Ogata, D.; Umeda, Y.; et al. Benefit, recurrence pattern, and toxicity to adjuvant anti-PD-1 monotherapy varies by ethnicity and melanoma subtype: An international multicenter cohort study. JAAD Int. 2024, 15, 105–114. [Google Scholar] [CrossRef]
- Bai, X.; Attrill, G.H.; Gide, T.N.; Ferguson, P.M.; Nahar, K.J.; Shang, P.; Vergara, I.A.; Palendira, U.; da Silva, I.P.; Carlino, M.S.; et al. Stroma-infiltrating T cell spatiotypes define immunotherapy outcomes in adolescent and young adult patients with melanoma. Nat. Commun. 2024, 15, 3014. [Google Scholar] [CrossRef]
- He, Q.; Xiang, L.; Luo, Y.; Wang, R.; Zheng, C.; Gao, Y.; Yao, H. Tumor-associated macrophages in colon cancer immunotherapy: Mechanisms, natural product interventions, and microenvironment remodeling. Front. Immunol. 2025, 16, 1642091. [Google Scholar] [CrossRef]
- Ye, Z.; Yi, J.; Jiang, X.; Shi, W.; Xu, H.; Cao, H.; Qin, L.; Liu, L.; Wang, T.; Ma, Z.; et al. Gastric cancer-derived exosomal let-7 g-5p mediated by SERPINE1 promotes macrophage M2 polarization and gastric cancer progression. J. Exp. Clin. Cancer Res. 2025, 44, 2. [Google Scholar] [CrossRef] [PubMed]
- Sturniolo, I.; Váróczy, C.; Regdon, Z.; Mázló, A.; Muzsai, S.; Bácsi, A.; Intili, G.; Hegedűs, C.; Boothby, M.R.; Holechek, J.; et al. PARP14 Contributes to the Development of the Tumor-Associated Macrophage Phenotype. Int. J. Mol. Sci. 2024, 25, 3601. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shi, Y.; Zhang, X.; Li, P.; Ma, L.; Hu, P.; Xu, L.; Dai, Y.; Xia, S.; Qiu, H. FGFR2 upregulates PAI-1 via JAK2/STAT3 signaling to induce M2 polarization of macrophages in colorectal cancer. Biochim. Biophys. Acta Mol. Basis Dis. 2023, 1869, 166665. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Zhang, X.; Yang, P.; Zhang, X.; Peng, Y.; Li, D.; Yu, Y.; Wu, Y.; Wang, Y.; Zhang, J.; et al. RNA m6A methylation orchestrates cancer growth and metastasis via macrophage reprogramming. Nat. Commun. 2021, 12, 1394. [Google Scholar] [CrossRef]
- Noonepalle, S.K.R.; Gracia-Hernandez, M.; Aghdam, N.; Berrigan, M.; Coulibaly, H.; Li, X.; Zevallos-Delgado, C.; Pletcher, A.; Weselman, B.; Palmer, E.; et al. Cell therapy using ex vivo reprogrammed macrophages enhances antitumor immune responses in melanoma. J. Exp. Clin. Cancer Res. 2024, 43, 263. [Google Scholar] [CrossRef]
- Chen, S.; Wang, M.; Lu, T.; Liu, Y.; Hong, W.; He, X.; Cheng, Y.; Liu, J.; Wei, Y.; Wei, X. JMJD6 in tumor-associated macrophage regulates macrophage polarization and cancer progression via STAT3/IL-10 axis. Oncogene 2023, 42, 2737–2750. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, H.; Li, Z.; Shi, Y.; Zhao, J.; Bai, Y.; Chen, Q.; Li, W. Prognostic Significance and Therapeutic Potential of SERPINE1 in Head and Neck Squamous Cell Carcinoma. Cancer Med. 2025, 14, e70605. [Google Scholar] [CrossRef]
- Kubala, M.H.; Punj, V.; Placencio-Hickok, V.R.; Fang, H.; Fernandez, G.E.; Sposto, R.; DeClerck, Y.A. Plasminogen Activator Inhibitor-1 Promotes the Recruitment and Polarization of Macrophages in Cancer. Cell Rep. 2018, 25, 2177–2191. [Google Scholar] [CrossRef]
- Romano, V.; Belviso, I.; Venuta, A.; Ruocco, M.R.; Masone, S.; Aliotta, F.; Fiume, G.; Montagnani, S.; Avagliano, A.; Arcucci, A. Influence of Tumor Microenvironment and Fibroblast Population Plasticity on Melanoma Growth, Therapy Resistance and Immunoescape. Int. J. Mol. Sci. 2021, 22, 5283. [Google Scholar] [CrossRef]
- Omland, S.H.; Wettergren, E.E.; Mollerup, S.; Asplund, M.; Mourier, T.; Hansen, A.J.; Gniadecki, R. Cancer associated fibroblasts (CAFs) are activated in cutaneous basal cell carcinoma and in the peritumoural skin. BMC Cancer 2017, 17, 675. [Google Scholar] [CrossRef] [PubMed]
- Wongm, P.F.; Wei, W.; Gupta, S.; Smithy, J.W.; Zelterman, D.; Kluger, H.M.; Rimm, D.L. Multiplex quantitative analysis of cancer-associated fibroblasts and immunotherapy outcome in metastatic melanoma. J. Immunother. Cancer 2019, 7, 194. [Google Scholar] [CrossRef] [PubMed]
- Forsthuber, A.; Aschenbrenner, B.; Korosec, A.; Jacob, T.; Annusver, K.; Krajic, N.; Kholodniuk, D.; Frech, S.; Zhu, S.; Purkhauser, K.; et al. Cancer-associated fibroblast subtypes modulate the tumor-immune microenvironment and are associated with skin cancer malignancy. Nat. Commun. 2024, 15, 9678. [Google Scholar] [CrossRef]
- Papaccio, F.; Kovacs, D.; Bellei, B.; Caputo, S.; Migliano, E.; Cota, C.; Picardo, M. Profiling Cancer-Associated Fibroblasts in Melanoma. Int. J. Mol. Sci. 2021, 22, 7255. [Google Scholar] [CrossRef]
- Aronovich, A.; Moyal, L.; Gorovitz, B.; Amitay-Laish, I.; Naveh, H.P.; Forer, Y.; Maron, L.; Knaneh, J.; Ad-El, D.; Yaacobi, D.; et al. Cancer-Associated Fibroblasts in Mycosis Fungoides Promote Tumor Cell Migration and Drug Resistance through CXCL12/CXCR4. J. Investig. Dermatol. 2021, 141, 619–627. [Google Scholar] [CrossRef]
- Wei, W.F.; Zhou, H.L.; Chen, P.Y.; Huang, X.L.; Huang, L.; Liang, L.J.; Guo, C.H.; Zhou, C.F.; Yu, L.; Fan, L.S.; et al. Cancer-associated fibroblast-derived PAI-1 promotes lymphatic metastasis via the induction of EndoMT in lymphatic endothelial cells. J. Exp. Clin. Cancer Res. 2023, 42, 160. [Google Scholar] [CrossRef]
- Masuda, T.; Nakashima, T.; Namba, M.; Yamaguchi, K.; Sakamoto, S.; Horimasu, Y.; Miyamoto, S.; Iwamoto, H.; Fujitaka, K.; Miyata, Y.; et al. Inhibition of PAI-1 limits chemotherapy resistance in lung cancer through suppressing myofibroblast characteristics of cancer-associated fibroblasts. J. Cell Mol. Med. 2019, 23, 2984–2994. [Google Scholar] [CrossRef]
- Chen, S.; Morine, Y.; Tokuda, K.; Yamada, S.; Saito, Y.; Nishi, M.; Ikemoto, T.; Shimada, M. Cancer-associated fibroblast-induced M2-polarized macrophages promote hepatocellular carcinoma progression via the plasminogen activator inhibitor-1 pathway. Int. J. Oncol. 2021, 59, 59. [Google Scholar] [CrossRef] [PubMed]
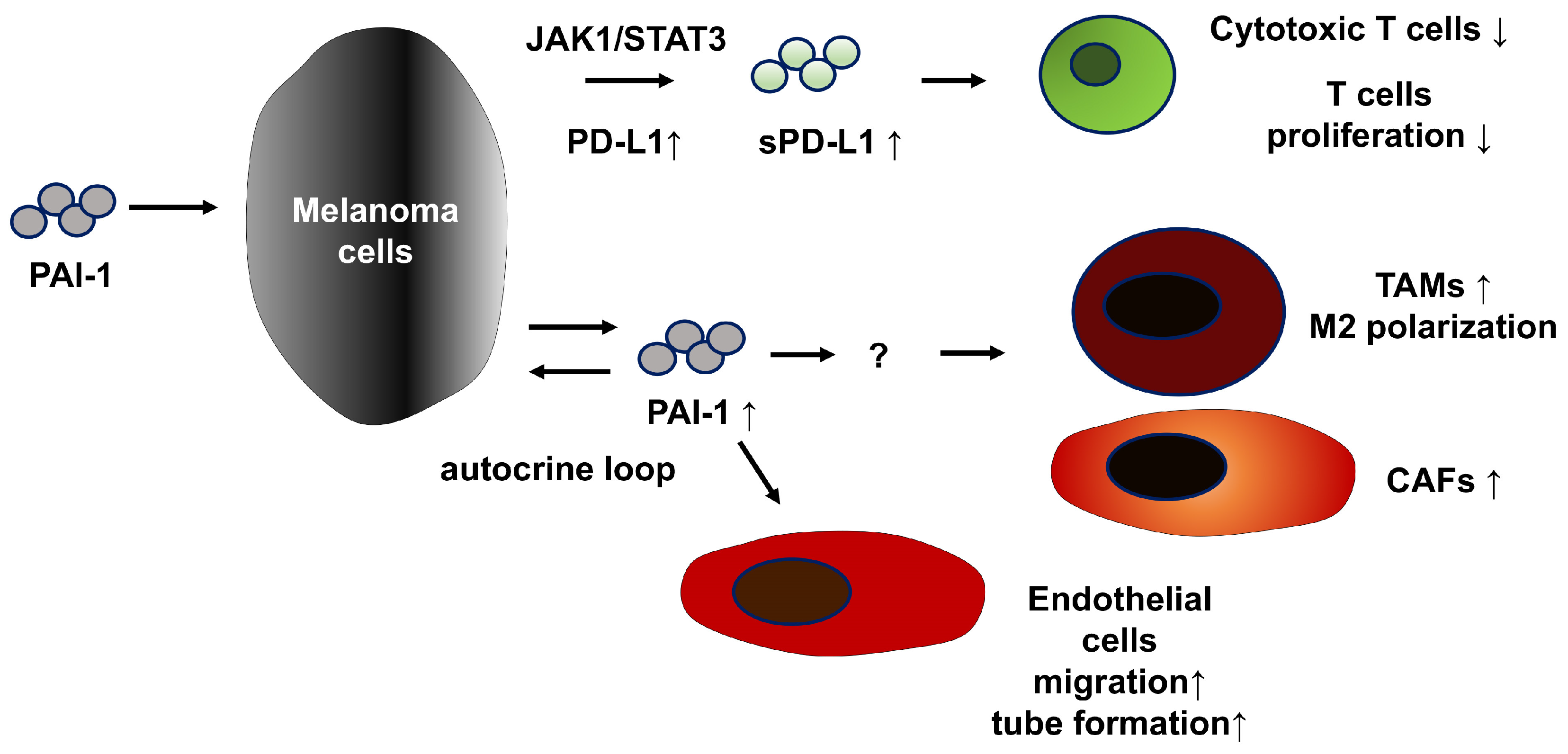
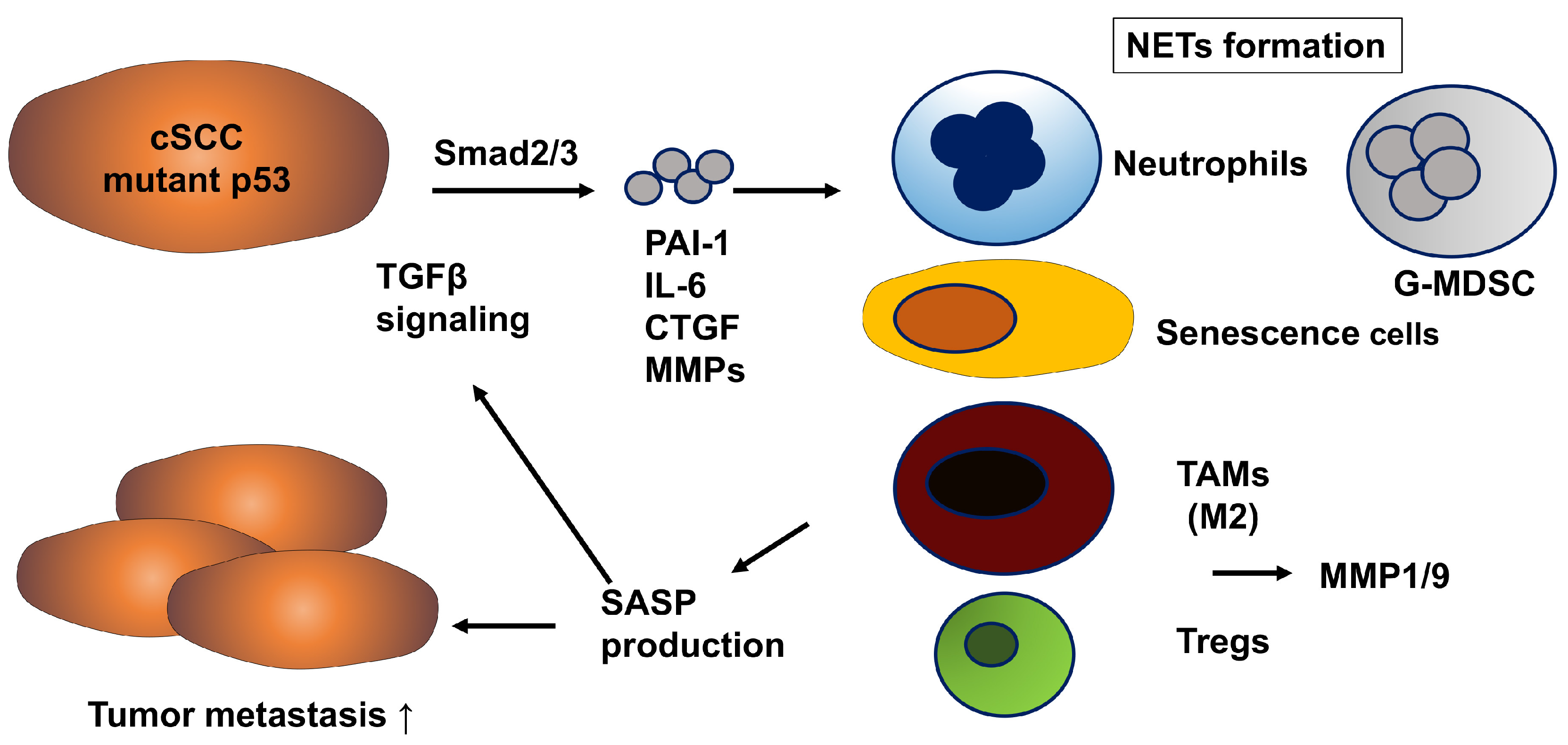
| Tumor Promoting Effects of PAI-1 | Possible Add-On Medication for TM5614 | ||
|---|---|---|---|
| Melanoma | increase | PD-L1 | nivolumab |
| sPD-L1 | |||
| ratio of Tregs | pembrolizumab | ||
| decrease | number of TAMs | ||
| number of CAFs | other anti-PD-1 Ab | ||
| cytotoxic function of T cells | |||
| cSCC | induced by | p53 mutant | cisplatin |
| Smad 2/3 signal | |||
| activate | p53/p21 pathway | oxaliplatin | |
| LRP1/p65 signal | |||
| increase | IL-6, IL-8, other SASP | radioimmunotherapy | |
| MMPs | |||
| CAS | increase | IL-23p19, VEGF-C, CXCL5 | paclitaxel |
| VEGF signal | |||
| activate | Fas-L mediated apoptosis | pazopanib | |
| p53/p21 signal | |||
| MF | induced by | hyalronic acid mediated SASP | bexarotene |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujimura, T.; Muto, Y.; Asano, Y. Plasminogen Activator Inhibitor-1 in Skin Malignancies: Therapeutic Implications of Its Inhibition. Biomolecules 2025, 15, 1317. https://doi.org/10.3390/biom15091317
Fujimura T, Muto Y, Asano Y. Plasminogen Activator Inhibitor-1 in Skin Malignancies: Therapeutic Implications of Its Inhibition. Biomolecules. 2025; 15(9):1317. https://doi.org/10.3390/biom15091317
Chicago/Turabian StyleFujimura, Taku, Yusuke Muto, and Yoshihide Asano. 2025. "Plasminogen Activator Inhibitor-1 in Skin Malignancies: Therapeutic Implications of Its Inhibition" Biomolecules 15, no. 9: 1317. https://doi.org/10.3390/biom15091317
APA StyleFujimura, T., Muto, Y., & Asano, Y. (2025). Plasminogen Activator Inhibitor-1 in Skin Malignancies: Therapeutic Implications of Its Inhibition. Biomolecules, 15(9), 1317. https://doi.org/10.3390/biom15091317






