The Role of Reactive Oxygen Species in Lung Cancer Development: Nanomedicine as a Therapeutic Strategy
Abstract
1. Introduction
2. The Role of ROS in Cancer: Focus on Lung Malignancies
2.1. The Origin of ROS in the Cell
2.2. Pivotal Role of ROS in Lung Cancer

2.2.1. Role of NRF2 in Lung Cancer
2.2.2. Role of ROS in Promoting Lung Cancer
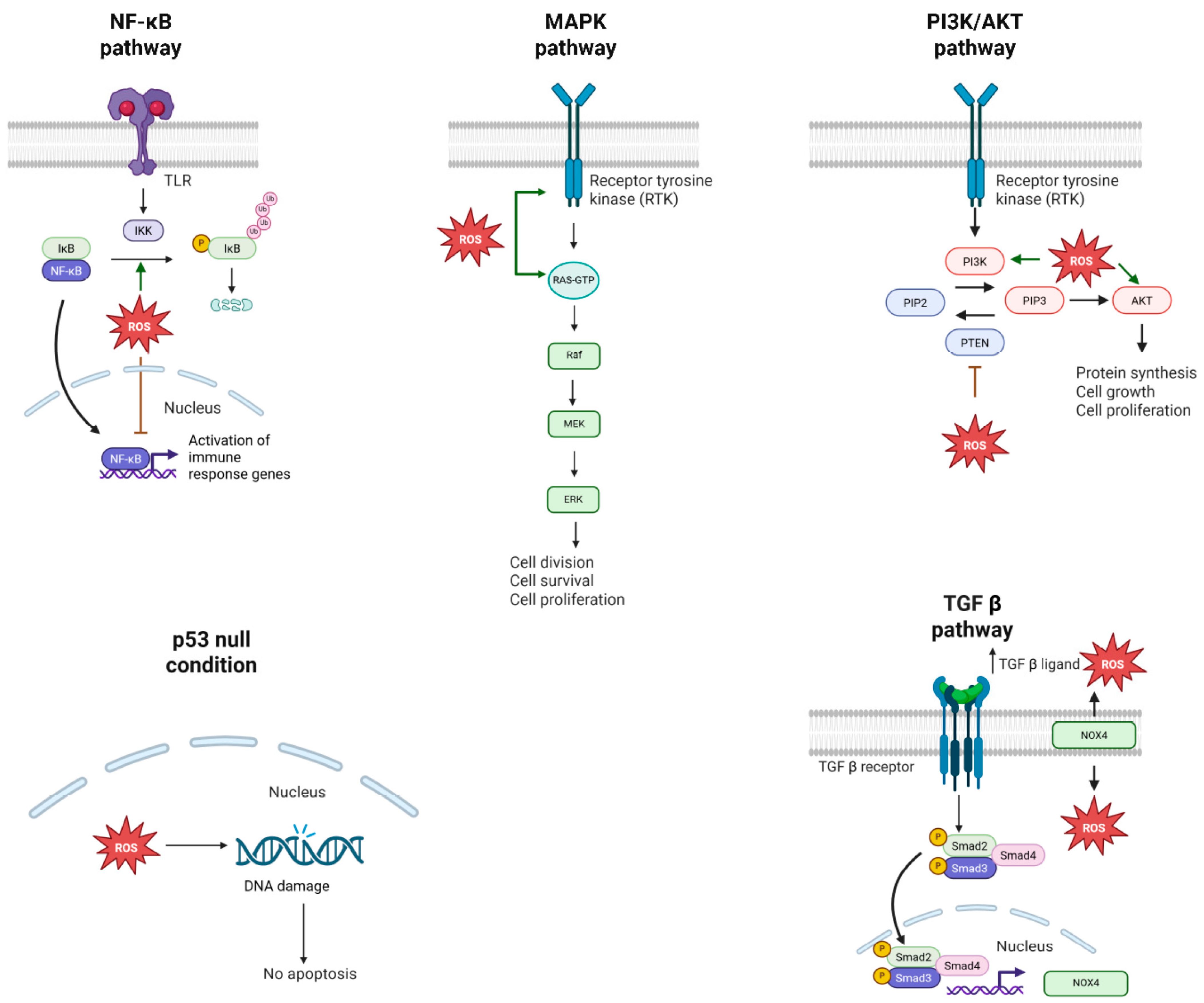
2.2.3. Role of ROS in Protecting from Lung Cancer
3. Targeted Therapy for Lung Cancer
3.1. The Origin of Targeted Therapy: Introduction to Small Molecule Inhibitors
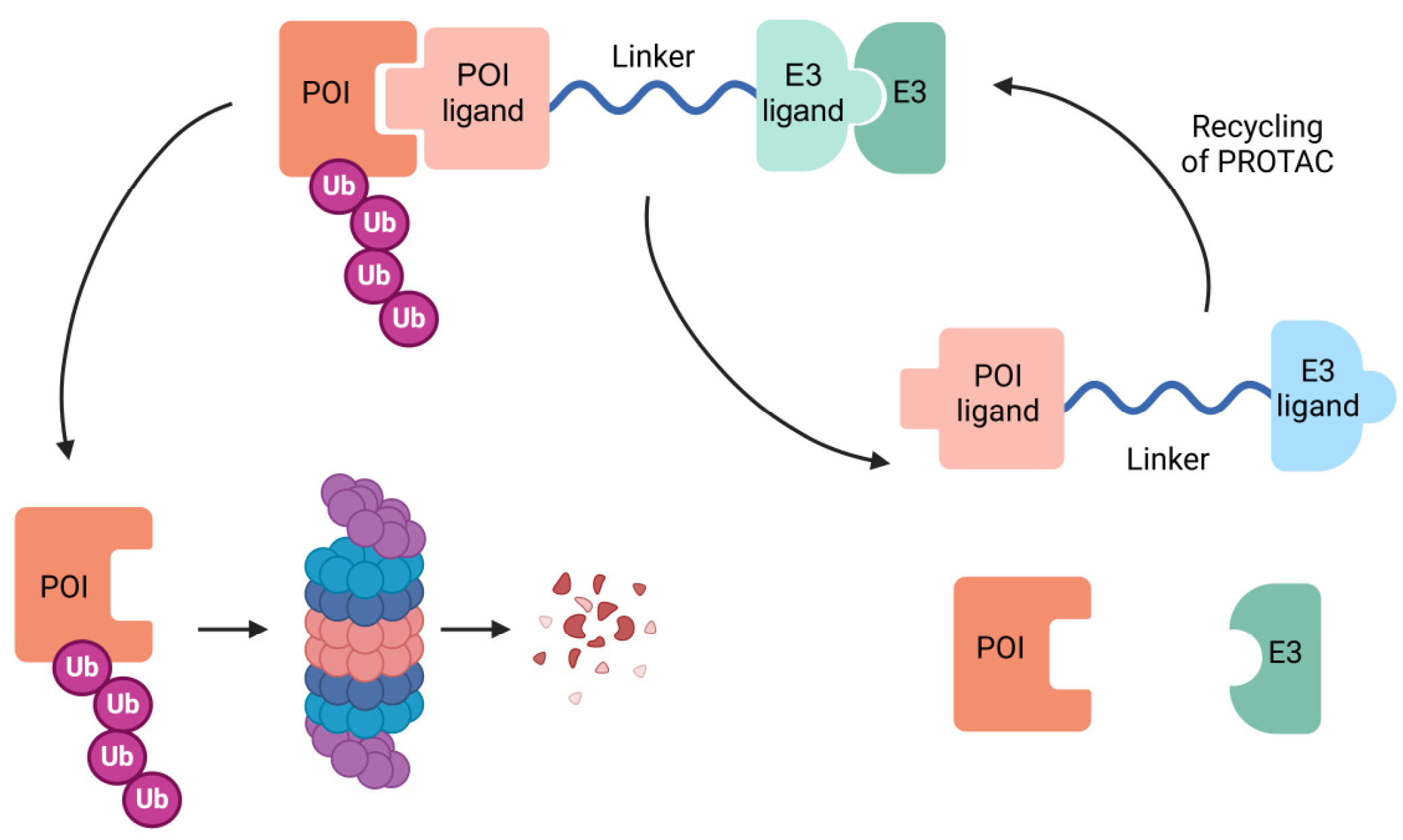
3.2. The Revolution of Targeted Therapy: PROTAC Technology
| E3 Ligase | Protein of Interest (POI) | Reference |
|---|---|---|
| KEAP1 | FAK Tau BRD4 CDK9 | [135,136,137,138] |
| DCAF1 | BRD4 FKBP12 | [139] |
| DCAF11 | FKBP12 AR | [140] |
| DCAF15 | BRD4 BRD7 BRD9 | [141,142] |
| DCAF16 | PARP2 FKBP12 | [143,144] |
| LM3BTL3 | BRD2 FKBP12 | [145,146] |
| KLH20 | BRD2 BRD3 | [147] |
| AhR | BRD2 BRD3 BRD4 | [148] |
| FEM1B | BCR-ABL BRD4 | [149] |
| RNF4 | BRD4 | [149] |
| RNF114 | BCR-ABL BRD4 | [150] |
3.3. Nanomedicine Avenues: Transforming Cancer Drug Delivery
3.4. ROS Inductor Nano PROTAC as a Potential Therapy for Lung Cancer
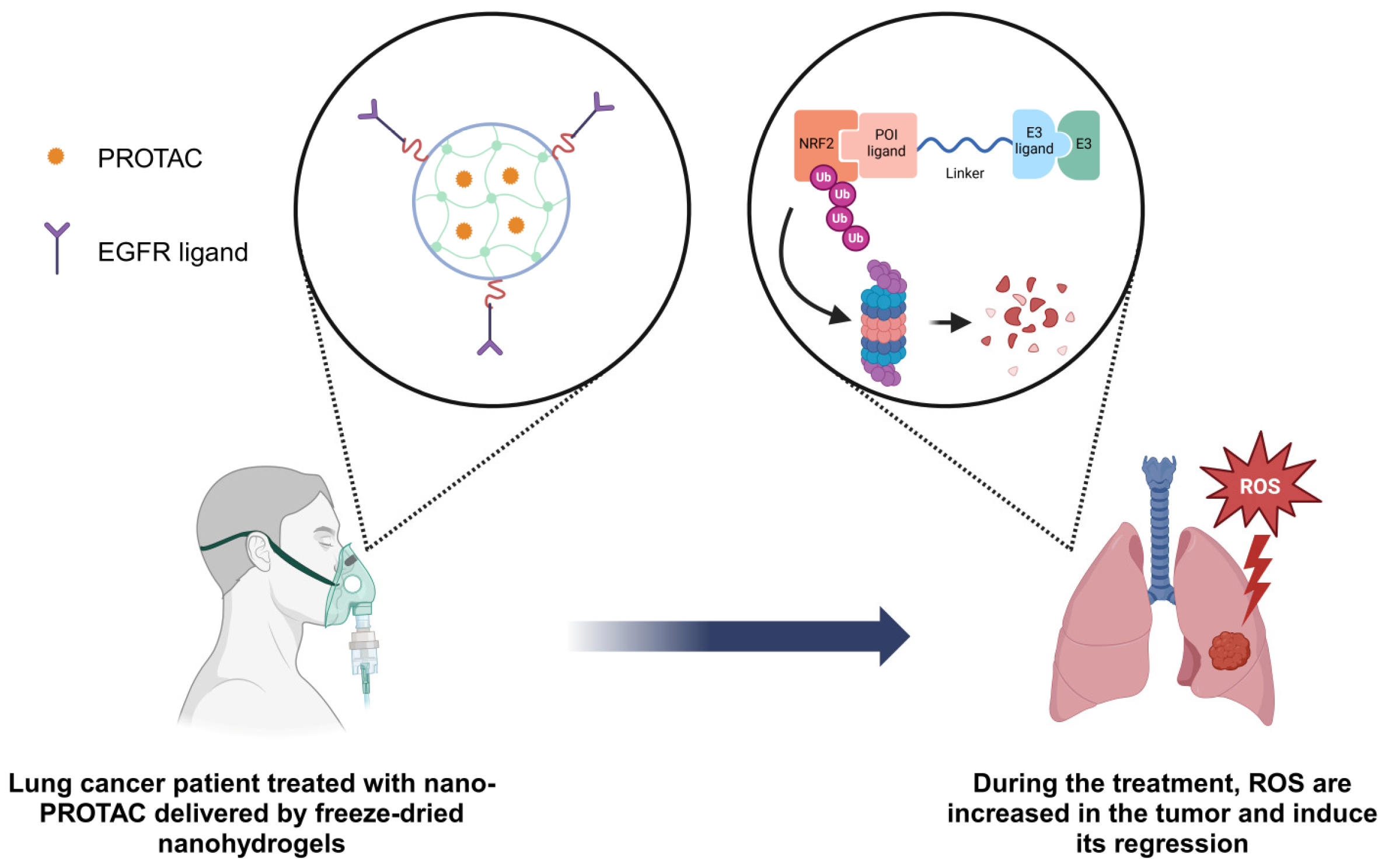
4. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Cardona, A.F.; Ricaurte, L.; Zatarain-Barrón, Z.L.; Arrieta, O. Squamous Cell Lung Cancer: Genomic Evolution and Personalized Therapy. Salud Publica Mex. 2019, 61, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Howlader, N.; Forjaz, G.; Mooradian, M.J.; Meza, R.; Kong, C.Y.; Cronin, K.A.; Mariotto, A.B.; Lowy, D.R.; Feuer, E.J. The Effect of Advances in Lung-Cancer Treatment on Population Mortality. N. Engl. J. Med. 2020, 383, 640–649. [Google Scholar] [CrossRef]
- Barta, J.A.; Powell, C.A.; Wisnivesky, J.P. Global Epidemiology of Lung Cancer. Ann. Glob. Health 2019, 85, 8. [Google Scholar] [CrossRef]
- Inamura, K. Lung Cancer: Understanding Its Molecular Pathology and the 2015 WHO Classification. Front. Oncol. 2017, 7, 193. [Google Scholar] [CrossRef]
- Oser, M.G.; Niederst, M.J.; Sequist, L.V.; Engelman, J.A. Transformation from Non-Small-Cell Lung Cancer to Small-Cell Lung Cancer: Molecular Drivers and Cells of Origin. Lancet Oncol. 2015, 16, e165–e172. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Ortega, M.; Carrera, A.C.; Garrido, A. Role of NRF2 in Lung Cancer. Cells 2021, 10, 1879. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Wang, Z.Y.; Li, Y.K.; Ye, D.M.; Zeng, J.; Hu, J.L.; Chen, P.F.; Xiao, J.; Zou, J.; Li, Z. Nuclear Factor Erythroid 2 (NF-E2)-Related Factor 2 (Nrf2) in Non-Small Cell Lung Cancer. Life Sci. 2020, 254, 117325. [Google Scholar] [CrossRef]
- Taniguchi, H.; Sen, T.; Rudin, C.M. Targeted Therapies and Biomarkers in Small Cell Lung Cancer. Front. Oncol. 2020, 10, 741. [Google Scholar] [CrossRef]
- Noronha, V.; Pinninti, R.; Patil, V.M.; Joshi, A.; Prabhash, K. Lung Cancer in the Indian Subcontinent. South Asian J. Cancer 2016, 5, 95. [Google Scholar] [CrossRef]
- Dakal, T.C.; Dhakar, R.; Beura, A.; Moar, K.; Maurya, P.K.; Sharma, N.K.; Ranga, V.; Kumar, A. Emerging Methods and Techniques for Cancer Biomarker Discovery. Pathol. Res. Pract. 2024, 262, 155567. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, M. Targeted Therapies for Cancer. BMC Med. 2022, 20, 90. [Google Scholar] [CrossRef]
- Lin, P.C.; Tsai, Y.S.; Yeh, Y.M.; Shen, M.R. Cutting-Edge AI Technologies Meet Precision Medicine to Improve Cancer Care. Biomolecules 2022, 12, 1133. [Google Scholar] [CrossRef]
- Mery, B.; Vallard, A.; Rowinski, E.; Magne, N. High-Throughput Sequencing in Clinical Oncology: From Past to Present. Swiss Med. Wkly. 2019, 149, w20057. [Google Scholar] [CrossRef]
- Hammerman, P.S.; Voet, D.; Lawrence, M.S.; Voet, D.; Jing, R.; Cibulskis, K.; Sivachenko, A.; Stojanov, P.; McKenna, A.; Lander, E.S.; et al. Comprehensive Genomic Characterization of Squamous Cell Lung Cancers. Nature 2012, 489, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.D.; Alexandrov, A.; Kim, J.; Wala, J.; Berger, A.H.; Pedamallu, C.S.; Shukla, S.A.; Guo, G.; Brooks, A.N.; Murray, B.A.; et al. Distinct Patterns of Somatic Genome Alterations in Lung Adenocarcinomas and Squamous Cell Carcinomas. Nat. Genet. 2016, 48, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Ortega, M.; Garrido, A.; Cirauqui, C.; Sanz-Gonzalez, L.; Hernández, M.C.; González-García, A.; Obregon, K.; Ferrer, I.; Paz-Ares, L.; Carrera, A.C. A Potential Therapeutic Strategy Based on Acute Oxidative Stress Induction for Wild-Type NRF2/KEAP1 Lung Squamous Cell Carcinoma. Redox Biol. 2024, 75, 103305. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q. Role of Nrf2 in Oxidative Stress and Toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- Luchkova, A.; Mata, A.; Cadenas, S. Nrf2 as a Regulator of Energy Metabolism and Mitochondrial Function. FEBS Lett. 2024, 598, 2092–2105. [Google Scholar] [CrossRef]
- Duan, Y.; Guan, Y.; Qin, W.; Zhai, X.; Yu, B.; Liu, H. Targeting Brd4 for Cancer Therapy: Inhibitors and Degraders. Medchemcomm 2018, 9, 1779. [Google Scholar] [CrossRef]
- An, S.; Fu, L. Small-Molecule PROTACs: An Emerging and Promising Approach for the Development of Targeted Therapy Drugs. EBioMedicine 2018, 36, 553–562. [Google Scholar] [CrossRef]
- Chen, L.; Liu, Z.P.; Li, X. Recent Advances in Dual BRD4-Kinase Inhibitors Based on Polypharmacology. ChemMedChem 2022, 17, e202100731. [Google Scholar] [CrossRef]
- Pu, C.; Wang, S.; Liu, L.; Feng, Z.; Zhang, H.; Gong, Q.; Sun, Y.; Guo, Y.; Li, R. Current Strategies for Improving Limitations of Proteolysis Targeting Chimeras. Chin. Chem. Lett. 2023, 34, 107927. [Google Scholar] [CrossRef]
- Song, Y.; Dong, Q.Q.; Ni, Y.K.; Xu, X.L.; Chen, C.X.; Chen, W. Nano-Proteolysis Targeting Chimeras (Nano-PROTACs) in Cancer Therapy. Int. J. Nanomed. 2024, 19, 5739–5761. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.Y.; Hou, D.Y.; Hu, X.J.; Liang, J.X.; Wang, M.D.; Song, Z.Z.; Yi, L.; Wang, Z.J.; An, H.W.; Xu, W.; et al. Nano Proteolysis Targeting Chimeras (PROTACs) with Anti-Hook Effect for Tumor Therapy. Angew. Chem. Int. Ed. 2023, 62, e202308049. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Zhao, Y.; Zhang, C.; Pu, K. Advancing Proteolysis Targeting Chimera (PROTAC) Nanotechnology in Protein Homeostasis Reprograming for Disease Treatment. ACS Nano 2024, 18, 28502–28530. [Google Scholar] [CrossRef] [PubMed]
- Winterbourn, C.C. Hydrogen Peroxide Reactivity and Specificity in Thiol-Based Cell Signalling. Biochem. Soc. Trans. 2020, 48, 745–754. [Google Scholar] [CrossRef]
- Kong, H.; Chandel, N.S. Regulation of Redox Balance in Cancer and T Cells. J. Biol. Chem. 2018, 293, 7499–7507. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H. Targeting Oxidative Stress in Disease: Promise and Limitations of Antioxidant Therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef]
- Chávez, V.; Mohri-Shiomi, A.; Garsin, D.A. Ce-Duox1/BLI-3 Generates Reactive Oxygen Species as a Protective Innate Immune Mechanism in Caenorhabditis Elegans. Infect. Immun. 2009, 77, 4983–4989. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide Dismutases: Dual Roles in Controlling ROS Damage and Regulating ROS Signaling. J. Cell Biol. 2018, 217, 1915. [Google Scholar] [CrossRef]
- Nandi, A.; Yan, L.J.; Jana, C.K.; Das, N. Role of Catalase in Oxidative Stress—And Age-Associated Degenerative Diseases. Oxid. Med. Cell Longev. 2019, 2019, 9613090. [Google Scholar] [CrossRef]
- Li, X.; Fang, P.; Mai, J.; Choi, E.T.; Wang, H.; Yang, X.F. Targeting Mitochondrial Reactive Oxygen Species as Novel Therapy for Inflammatory Diseases and Cancers. J. Hematol. Oncol. 2013, 6, 19. [Google Scholar] [CrossRef]
- Hadrava Vanova, K.; Kraus, M.; Neuzil, J.; Rohlena, J. Mitochondrial Complex II and Reactive Oxygen Species in Disease and Therapy. Redox Rep. 2020, 25, 26. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.Z.; Jiang, S.; Zhang, L.; Yu, Z. Bin Mitochondrial Electron Transport Chain, ROS Generation and Uncoupling (Review). Int. J. Mol. Med. 2019, 44, 3–15. [Google Scholar] [CrossRef]
- Bezawork-Geleta, A.; Rohlena, J.; Dong, L.; Pacak, K.; Neuzil, J. Mitochondrial Complex II: At the Crossroads. Trends Biochem. Sci. 2017, 42, 312–325. [Google Scholar] [CrossRef] [PubMed]
- Fransen, M.; Lismont, C.; Walton, P. The Peroxisome-Mitochondria Connection: How and Why? Int. J. Mol. Sci. 2017, 18, 1126. [Google Scholar] [CrossRef]
- Baumgart, E.; Vanhorebeek, I.; Grabenbauer, M.; Borgers, M.; Declercq, P.E.; Fahimi, H.D.; Baes, M. Mitochondrial Alterations Caused by Defective Peroxisomal Biogenesis in a Mouse Model for Zellweger Syndrome (PEX5 Knockout Mouse). Am. J. Pathol. 2001, 159, 1477–1494. [Google Scholar] [CrossRef]
- Fourcade, S.; Ferrer, I.; Pujol, A. Oxidative Stress, Mitochondrial and Proteostasis Malfunction in Adrenoleukodystrophy: A Paradigm for Axonal Degeneration. Free Radic. Biol. Med. 2015, 88, 18–29. [Google Scholar] [CrossRef]
- Van Veldhoven, P.P. Biochemistry and Genetics of Inherited Disorders of Peroxisomal Fatty Acid Metabolism. J. Lipid Res. 2010, 51, 2863–2895. [Google Scholar] [CrossRef]
- Nisimoto, Y.; Diebold, B.A.; Constentino-Gomes, D.; Lambeth, J.D. Nox4: A Hydrogen Peroxide-Generating Oxygen Sensor. Biochemistry 2014, 53, 5111–5120. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative Stress: A Concept in Redox Biology and Medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef]
- Bayo Jimenez, M.T.; Frenis, K.; Hahad, O.; Steven, S.; Cohen, G.; Cuadrado, A.; Münzel, T.; Daiber, A. Protective Actions of Nuclear Factor Erythroid 2-Related Factor 2 (NRF2) and Downstream Pathways against Environmental Stressors. Free Radic. Biol. Med. 2022, 187, 72–91. [Google Scholar] [CrossRef]
- Mudway, I.S.; Kelly, F.J.; Holgate, S.T. Oxidative Stress in Air Pollution Research. Free Radic. Biol. Med. 2020, 151, 2–6. [Google Scholar] [CrossRef] [PubMed]
- ArulJothi, K.N.; Kumaran, K.; Senthil, S.; Nidhu, A.B.; Munaff, N.; Janitri, V.B.; Kirubakaran, R.; Singh, S.K.; Gupt, G.; Dua, K.; et al. Implications of Reactive Oxygen Species in Lung Cancer and Exploiting It for Therapeutic Interventions. Med. Oncol. 2022, 40, 43. [Google Scholar] [CrossRef]
- Szatrowski, T.P.; Nathan, C.F. Production of Large Amounts of Hydrogen Peroxide by Human Tumor Cells. Cancer Res. 1991, 51, 794–798. [Google Scholar] [PubMed]
- Finkel, T. Signal Transduction by Reactive Oxygen Species. J. Cell Biol. 2011, 194, 7–15. [Google Scholar] [CrossRef]
- Bauer, A.K.; Hill, T.; Alexander, C.M. The Involvement of NRF2 in Lung Cancer. Oxid. Med. Cell Longev. 2013, 2013, 746432. [Google Scholar] [CrossRef]
- Kobayashi, A.; Kang, M.-I.; Okawa, H.; Ohtsuji, M.; Zenke, Y.; Chiba, T.; Igarashi, K.; Yamamoto, M. Oxidative Stress Sensor Keap1 Functions as an Adaptor for Cul3-Based E3 Ligase to Regulate Proteasomal Degradation of Nrf2. Mol. Cell Biol. 2004, 24, 7130–7139. [Google Scholar] [CrossRef]
- McMahon, M.; Lamont, D.J.; Beattie, K.A.; Hayes, J.D. Keap1 Perceives Stress via Three Sensors for the Endogenous Signaling Molecules Nitric Oxide, Zinc, and Alkenals. Proc. Natl. Acad. Sci. USA 2010, 107, 18838–18843. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Holtzclaw, W.D.; Cole, R.N.; Itoh, K.; Wakabayashi, N.; Katoh, Y.; Yamamoto, M.; Talalay, P. Direct Evidence That Sulfhydryl Groups of Keap1 Are the Sensors Regulating Induction of Phase 2 Enzymes That Protect against Carcinogens and Oxidants. Proc. Natl. Acad. Sci. USA 2002, 99, 11908–11913. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Eggler, A.L.; Liu, D.; Liu, G.; Mesecar, A.D.; van Breemen, R.B. Sites of Alkylation of Human Keap1 by Natural Chemoprevention Agents. J. Am. Soc. Mass Spectrom. 2007, 18, 2226–2232. [Google Scholar] [CrossRef] [PubMed]
- Smolková, K.; Mikó, E.; Kovács, T.; Leguina-Ruzzi, A.; Sipos, A.; Bai, P. Nuclear Factor Erythroid 2-Related Factor 2 in Regulating Cancer Metabolism. Antioxid. Redox Signal. 2020, 33, 966. [Google Scholar] [CrossRef]
- Jaramillo, M.C.; Zhang, D.D. The Emerging Role of the Nrf2-Keap1 Signaling Pathway in Cancer. Genes Dev. 2013, 27, 2179–2191. [Google Scholar] [CrossRef]
- Menegon, S.; Columbano, A.; Giordano, S. The Dual Roles of NRF2 in Cancer. Trends Mol. Med. 2016, 22, 578–593. [Google Scholar] [CrossRef]
- Kandoth, C.; McLellan, M.D.; Vandin, F.; Ye, K.; Niu, B.; Lu, C.; Xie, M.; Zhang, Q.; McMichael, J.F.; Wyczalkowski, M.A.; et al. Mutational Landscape and Significance across 12 Major Cancer Types. Nature 2013, 502, 333–339. [Google Scholar] [CrossRef]
- Sporn, M.B.; Liby, K.T. NRF2 and Cancer: The Good, the Bad and the Importance of Context. Nat. Rev. Cancer 2012, 12, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; de la Vega, M.R.; Chapman, E.; Ooi, A.; Zhang, D.D. The Effects of NRF2 Modulation on the Initiation and Progression of Chemically and Genetically Induced Lung Cancer. Mol. Carcinog. 2018, 57, 182–192. [Google Scholar] [CrossRef]
- Satoh, H.; Moriguchi, T.; Takai, J.; Ebina, M.; Yamamoto, M. Nrf2 Prevents Initiation but Accelerates Progression through the Kras Signaling Pathway during Lung Carcinogenesis. Cancer Res. 2013, 73, 4158–4168. [Google Scholar] [CrossRef]
- Satoh, H.; Moriguchi, T.; Saigusa, D.; Baird, L.; Yu, L.; Rokutan, H.; Igarashi, K.; Ebina, M.; Shibata, T.; Yamamoto, M. NRF2 Intensifies Host Defense Systems to Prevent Lung Carcinogenesis, but after Tumor Initiation Accelerates Malignant Cell Growth. Cancer Res. 2016, 76, 3088–3096. [Google Scholar] [CrossRef]
- Ray, P.D.; Huang, B.W.; Tsuji, Y. Reactive Oxygen Species (ROS) Homeostasis and Redox Regulation in Cellular Signaling. Cell. Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Houée-Lévin, C.; Bobrowski, K.; Horakova, L.; Karademir, B.; Schöneich, C.; Davies, M.J.; Spickett, C.M. Exploring Oxidative Modifications of Tyrosine: An Update on Mechanisms of Formation, Advances in Analysis and Biological Consequences. Free Radic. Res. 2015, 49, 347–373. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Jin, Y.; Chen, X.; Ye, X.; Shen, X.; Lin, M.; Zeng, C.; Zhou, T.; Zhang, J. NF-ΚB in Biology and Targeted Therapy: New Insights and Translational Implications. Signal Transduct. Target. Ther. 2024, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Liu, D.; Shishodia, S.; Ozburn, N.; Behrens, C.; Lee, J.J.; Waun, K.H.; Aggarwal, B.B.; Wistuba, I.I. Nuclear Factor-KappaB (NF-KappaB) Is Frequently Expressed in Lung Cancer and Preneoplastic Lesions. Cancer 2006, 107, 2637–2646. [Google Scholar] [CrossRef] [PubMed]
- El-Nikhely, N.; Karger, A.; Sarode, P.; Singh, I.; Weigert, A.; Wietelmann, A.; Stiewe, T.; Dammann, R.; Fink, L.; Grimminger, F.; et al. Metastasis-Associated Protein 2 Represses NF-ΚB to Reduce Lung Tumor Growth and Inflammation. Cancer Res. 2020, 80, 4199–4211. [Google Scholar] [CrossRef]
- Dimitrakopoulos, F.I.D.; Kottorou, A.E.; Kalofonou, M.; Kalofonos, H.P. The Fire Within: NF-ΚB Involvement in Non-Small Cell Lung Cancer. Cancer Res. 2020, 80, 4025–4036. [Google Scholar] [CrossRef]
- Jung, Y.; Kim, H.; Sun, H.M.; Sue, G.R.; Jeong, W. Dynein Light Chain LC8 Negatively Regulates NF-ΚB through the Redox-Dependent Interaction with IκBα. J. Biol. Chem. 2008, 283, 23863. [Google Scholar] [CrossRef]
- Kretz-Remy, C.; Mirault, M.E.; Arrigo, A.P. Inhibition of I kappa B-alpha phosphorylation and degradation and subsequent NF-kappa B activation by glutathione peroxidase overexpression. J. Cell Biol. 1996, 133, 1083–1093. [Google Scholar] [CrossRef]
- Wu, M.; Bian, Q.; Liu, Y.; Fernandes, A.F.; Taylor, A.; Pereira, P.; Shang, F. Sustained Oxidative Stress Inhibits NF-KappaB Activation Partially via Inactivating the Proteasome. Free Radic. Biol. Med. 2009, 46, 62–69. [Google Scholar] [CrossRef]
- Chen, P.M.; Wu, T.C.; Wang, Y.C.; Cheng, Y.W.; Sheu, G.T.; Chen, C.Y.; Lee, H. Activation of NF-ΚB by SOD2 Promotes the Aggressiveness of Lung Adenocarcinoma by Modulating NKX2-1-Mediated IKKβ Expression. Carcinogenesis 2013, 34, 2655–2663. [Google Scholar] [CrossRef]
- Bahar, M.E.; Kim, H.J.; Kim, D.R. Targeting the RAS/RAF/MAPK Pathway for Cancer Therapy: From Mechanism to Clinical Studies. Signal Transduct. Target. Ther. 2023, 8, 1–38. [Google Scholar] [CrossRef]
- Herbst, R.S.; Morgensztern, D.; Boshoff, C. The Biology and Management of Non-Small Cell Lung Cancer. Nature 2018, 553, 446–454. [Google Scholar] [CrossRef]
- Blair, H.A. Sotorasib: First Approval. Drugs 2021, 81, 1573–1579. [Google Scholar] [CrossRef] [PubMed]
- Messina, S.; De Simone, G.; Ascenzi, P. Cysteine-Based Regulation of Redox-Sensitive Ras Small GTPases. Redox Biol. 2019, 26, 101282. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, F.; Hamanaka, R.; Wheaton, W.W.; Weinberg, S.; Joseph, J.; Lopez, M.; Kalyanaraman, B.; Mutlu, G.M.; Budinger, G.R.S.; Chandel, N.S. Mitochondrial Metabolism and ROS Generation Are Essential for Kras-Mediated Tumorigenicity. Proc. Natl. Acad. Sci. USA 2010, 107, 8788–8793. [Google Scholar] [CrossRef]
- Liou, G.Y.; Döppler, H.; DelGiorno, K.E.; Zhang, L.; Leitges, M.; Crawford, H.C.; Murphy, M.P.; Storz, P. Mutant KRas-Induced Mitochondrial Oxidative Stress in Acinar Cells Upregulates EGFR Signaling to Drive Formation of Pancreatic Precancerous Lesions. Cell Rep. 2016, 14, 2325–2336. [Google Scholar] [CrossRef]
- Glaviano, A.; Foo, A.S.C.; Lam, H.Y.; Yap, K.C.H.; Jacot, W.; Jones, R.H.; Eng, H.; Nair, M.G.; Makvandi, P.; Geoerger, B.; et al. PI3K/AKT/MTOR Signaling Transduction Pathway and Targeted Therapies in Cancer. Mol. Cancer 2023, 22, 138. [Google Scholar] [CrossRef]
- Sanaei, M.J.; Razi, S.; Pourbagheri-Sigaroodi, A.; Bashash, D. The PI3K/Akt/MTOR Pathway in Lung Cancer; Oncogenic Alterations, Therapeutic Opportunities, Challenges, and a Glance at the Application of Nanoparticles. Transl. Oncol. 2022, 18, 101364. [Google Scholar] [CrossRef] [PubMed]
- Le, X.; Puri, S.; Negrao, M.V.; Nilsson, M.B.; Robichaux, J.; Boyle, T.; Kevin Hicks, J.; Lovinger, K.L.; Roarty, E.; Rinsurongkawong, W.; et al. Landscape of EGFR-Dependent and -Independent Resistance Mechanisms to Osimertinib and Continuation Therapy Beyond Progression in EGFR-Mutant NSCLC. Clin. Cancer Res. 2018, 24, 6195–6203. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Li, J.; Li, J.; Che, G. Clinical Significance of PIK3CA Gene in Non-Small-Cell Lung Cancer: A Systematic Review and Meta-Analysis. Biomed. Res. Int. 2020, 2020, 3608241. [Google Scholar] [CrossRef] [PubMed]
- Lastwika, K.J.; Wilson, W.; Li, Q.K.; Norris, J.; Xu, H.; Ghazarian, S.R.; Kitagawa, H.; Kawabata, S.; Taube, J.M.; Yao, S.; et al. Control of PD-L1 Expression by Oncogenic Activation of the AKT-MTOR Pathway in Non-Small Cell Lung Cancer. Cancer Res. 2016, 76, 227–238. [Google Scholar] [CrossRef]
- Kma, L.; Baruah, T.J. The interplay of ROS and the PI3K/Akt pathway in autophagy regulation. Biotechnol. Appl. Biochem. 2022, 69, 248–264. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Burchfield, J.G.; Yang, P.; Humphrey, S.J.; Yang, G.; Francis, D.; Yasmin, S.; Shin, S.Y.; Norris, D.M.; Kearney, A.L.; et al. Global Redox Proteome and Phosphoproteome Analysis Reveals Redox Switch in Akt. Nat. Commun. 2019, 10, 1–18. [Google Scholar] [CrossRef]
- Leslie, N.R.; Bennett, D.; Lindsay, Y.E.; Stewart, H.; Gray, A.; Downes, C.P. Redox Regulation of PI 3-Kinase Signalling via Inactivation of PTEN. EMBO J. 2003, 22, 5501–5510. [Google Scholar] [CrossRef] [PubMed]
- Peuget, S.; Zhou, X.; Selivanova, G. Translating P53-Based Therapies for Cancer into the Clinic. Nat. Rev. Cancer 2024, 24, 192–215. [Google Scholar] [CrossRef]
- Vaddavalli, P.L.; Schumacher, B. The P53 Network: Cellular and Systemic DNA Damage Responses in Cancer and Aging. Trends Genet. 2022, 38, 598–612. [Google Scholar] [CrossRef]
- Collisson, E.A.; Campbell, J.D.; Brooks, A.N.; Berger, A.H.; Lee, W.; Chmielecki, J.; Beer, D.G.; Cope, L.; Creighton, C.J.; Danilova, L.; et al. Comprehensive Molecular Profiling of Lung Adenocarcinoma. Nature 2014, 511, 543–550. [Google Scholar] [CrossRef]
- Tan, M.; Li, S.; Swaroop, M.; Guan, K.; Oberley, L.W.; Sun, Y. Transcriptional Activation of the Human Glutathione Peroxidase Promoter by P53. J. Biol. Chem. 1999, 274, 12061–12066. [Google Scholar] [CrossRef]
- Cano, C.E.; Gommeaux, J.; Pietri, S.; Culcasi, M.; Garcia, S.; Seux, M.; Barelier, S.; Vasseur, S.; Spoto, R.P.; Pébusque, M.J.; et al. Tumor Protein 53-Induced Nuclear Protein 1 Is a Major Mediator of P53 Antioxidant Function. Cancer Res. 2009, 69, 219–226. [Google Scholar] [CrossRef]
- Yoon, K.A.; Nakamura, Y.; Arakawa, H. Identification of ALDH4 as a P53-Inducible Gene and Its Protective Role in Cellular Stresses. J. Hum. Genet. 2004, 49, 134–140. [Google Scholar] [CrossRef]
- Faraonio, R.; Vergara, P.; Di Marzo, D.; Pierantoni, M.G.; Napolitano, M.; Russo, T.; Cimino, F. P53 Suppresses the Nrf2-Dependent Transcription of Antioxidant Response Genes. J. Biol. Chem. 2006, 281, 39776–39784. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, C.Y.; Duan, F.G.; Fan, X.X.; Yao, X.J.; Parks, R.J.; Tang, Y.J.; Wang, M.F.; Liu, L.; Tsang, B.K.; et al. P53 Sensitizes Chemoresistant Non-Small Cell Lung Cancer via Elevation of Reactive Oxygen Species and Suppression of EGFR/PI3K/AKT Signaling. Cancer Cell Int. 2019, 19, 188. [Google Scholar] [CrossRef]
- Chougoni, K.K.; Neely, V.; Ding, B.; Oduah, E.; Lam, V.T.; Hu, B.; Koblinski, J.E.; Windle, B.E.; Palit Deb, S.; Deb, S.; et al. Oncogenic Mutant P53 Sensitizes Non–Small Cell Lung Cancer Cells to Proteasome Inhibition via Oxidative Stress—Dependent Induction of Mitochondrial Apoptosis. Cancer Res. Commun. 2024, 4, 2685. [Google Scholar] [CrossRef]
- Liu, B.; Chen, Y.; St Clair, D.K. ROS and P53: A Versatile Partnership. Free Radic. Biol. Med. 2008, 44, 1529–1535. [Google Scholar] [CrossRef]
- Yu, H.; Gao, H.; Guo, H.; Wang, G.; Yang, Y.; Hu, Q.; Liang, L.; Zhao, Q.; Xie, D.; Rao, Y.; et al. Upregulation of Wild-Type P53 by Small Molecule-Induced Elevation of NQO1 in Non-Small Cell Lung Cancer Cells. Acta Pharmacol. Sin. 2021, 43, 692. [Google Scholar] [CrossRef] [PubMed]
- Asher, G.; Lotem, J.; Cohen, B.; Sachs, L.; Shaul, Y. Regulation of P53 Stability and P53-Dependent Apoptosis by NADH Quinone Oxidoreductase 1. Proc. Natl. Acad. Sci. USA 2001, 98, 1188–1193. [Google Scholar] [CrossRef]
- Deng, Z.; Fan, T.; Xiao, C.; Tian, H.; Zheng, Y.; Li, C.; He, J. TGF-β Signaling in Health, Disease, and Therapeutics. Signal Transduct. Target. Ther. 2024, 9, 61. [Google Scholar] [CrossRef]
- Donatelli, S.S.; Zhou, J.M.; Gilvary, D.L.; Eksioglu, E.A.; Chen, X.; Cress, W.D.; Haura, E.B.; Schabath, M.B.; Coppola, D.; Wei, S.; et al. TGF-β-Inducible MicroRNA-183 Silences Tumor-Associated Natural Killer Cells. Proc. Natl. Acad. Sci. USA 2014, 111, 4203–4208. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tong, X.; Zhou, Z.; Wang, S.; Lei, Z.; Zhang, T.; Liu, Z.; Zeng, Y.; Li, C.; Zhao, J.; et al. Circular RNA Hsa_circ_0008305 (CircPTK2) Inhibits TGF-β-Induced Epithelial-Mesenchymal Transition and Metastasis by Controlling TIF1γ in Non-Small Cell Lung Cancer. Mol. Cancer 2018, 17, 140. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Shi, F.; Zhai, R.; Wang, H.; Li, K.; Xu, C.; Yao, W.; Zhou, F. TGF-β Promote Epithelial-Mesenchymal Transition via NF-ΚB/NOX4/ROS Signal Pathway in Lung Cancer Cells. Mol. Biol. Rep. 2021, 48, 2365–2375. [Google Scholar] [CrossRef] [PubMed]
- Jobling, M.F.; Mott, J.D.; Finnegan, M.T.; Jurukovski, V.; Erickson, A.C.; Walian, P.J.; Taylor, S.E.; Ledbetter, S.; Lawrence, C.M.; Rifkin, D.B.; et al. Isoform-Specific Activation of Latent Transforming Growth Factor Beta (LTGF-Beta) by Reactive Oxygen Species. Radiat. Res. 2006, 166, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Gorowiec, M.R.; Borthwick, L.A.; Parker, S.M.; Kirby, J.A.; Saretzki, G.C.; Fisher, A.J. Free Radical Generation Induces Epithelial-to-Mesenchymal Transition in Lung Epithelium via a TGF-Β1-Dependent Mechanism. Free Radic. Biol. Med. 2012, 52, 1024–1032. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Letai, A.; Sarosiek, K. Regulation of Apoptosis in Health and Disease: The Balancing Act of BCL-2 Family Proteins. Nat. Rev. Mol. Cell Biol. 2019, 20, 175–193. [Google Scholar] [CrossRef]
- Wilkie-Grantham, R.P.; Matsuzawa, S.I.; Reed, J.C. Novel Phosphorylation and Ubiquitination Sites Regulate Reactive Oxygen Species-Dependent Degradation of Anti-Apoptotic c-FLIP Protein. J. Biol. Chem. 2013, 288, 12777–12790. [Google Scholar] [CrossRef]
- Villalpando-Rodriguez, G.E.; Gibson, S.B. Reactive Oxygen Species (ROS) Regulates Different Types of Cell Death by Acting as a Rheostat. Oxid. Med. Cell Longev. 2021, 2021, 9912436. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Yang, W.S.; Stockwell, B.R. Ferroptosis: Death by Lipid Peroxidation. Trends Cell Biol. 2016, 26, 165–176. [Google Scholar] [CrossRef]
- Hassannia, B.; Vandenabeele, P.; Vanden Berghe, T. Targeting Ferroptosis to Iron out Cancer. Cancer Cell 2019, 35, 830–849. [Google Scholar] [CrossRef]
- Scherz-Shouval, R.; Shvets, E.; Fass, E.; Shorer, H.; Gil, L.; Elazar, Z. Reactive Oxygen Species Are Essential for Autophagy and Specifically Regulate the Activity of Atg4. EMBO J. 2007, 26, 1749–1760. [Google Scholar] [CrossRef]
- Alexander, A.; Cai, S.L.; Kim, J.; Nanez, A.; Sahin, M.; MacLean, K.H.; Inoki, K.; Guan, K.L.; Shen, J.; Person, M.D.; et al. ATM Signals to TSC2 in the Cytoplasm to Regulate MTORC1 in Response to ROS. Proc. Natl. Acad. Sci. USA 2010, 107, 4153–4158. [Google Scholar] [CrossRef]
- De Raedt, T.; Walton, Z.; Yecies, J.L.; Li, D.; Chen, Y.; Malone, C.F.; Maertens, O.; Jeong, S.M.; Bronson, R.T.; Lebleu, V.; et al. Exploiting Cancer Cell Vulnerabilities to Develop a Combination Therapy for Ras-Driven Tumors. Cancer Cell 2011, 20, 400–413. [Google Scholar] [CrossRef]
- Vandenabeele, P.; Galluzzi, L.; Vanden Berghe, T.; Kroemer, G. Molecular Mechanisms of Necroptosis: An Ordered Cellular Explosion. Nat. Rev. Mol. Cell Biol. 2010, 11, 700–714. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.W.; Shao, J.; Lin, J.; Zhang, N.; Lu, B.J.; Lin, S.C.; Dong, M.Q.; Han, J. RIP3, an Energy Metabolism Regulator That Switches TNF-Induced Cell Death from Apoptosis to Necrosis. Science 2009, 325, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Bedard, P.L.; Hyman, D.M.; Davids, M.S.; Siu, L.L. Small Molecules, Big Impact: 20 Years of Targeted Therapy in Oncology. Lancet 2020, 395, 1078–1088. [Google Scholar] [CrossRef]
- Zhong, L.; Li, Y.; Xiong, L.; Wang, W.; Wu, M.; Yuan, T.; Yang, W.; Tian, C.; Miao, Z.; Wang, T.; et al. Small Molecules in Targeted Cancer Therapy: Advances, Challenges, and Future Perspectives. Signal Transduct. Target. Ther. 2021, 6, 201. [Google Scholar] [CrossRef] [PubMed]
- Klein, E.A.; Thompson, I.M.; Tangen, C.M.; Crowley, J.J.; Lucia, S.; Goodman, P.J.; Minasian, L.M.; Ford, L.G.; Parnes, H.L.; Gaziano, J.M.; et al. Vitamin E and the Risk of Prostate Cancer: The Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2011, 306, 1549–1556. [Google Scholar] [CrossRef]
- Omenn, G.S.; Goodman, G.E.; Thornquist, M.D.; Balmes, J.; Cullen, M.R.; Glass, A.; Keogh, J.P.; Meyskens, F.L.; Valanis, B.; Williams, J.H.; et al. Effects of a Combination of Beta Carotene and Vitamin A on Lung Cancer and Cardiovascular Disease. N. Engl. J. Med. 1996, 334, 1150–1155. [Google Scholar] [CrossRef]
- Wiel, C.; Le Gal, K.; Ibrahim, M.X.; Jahangir, C.A.; Kashif, M.; Yao, H.; Ziegler, D.V.; Xu, X.; Ghosh, T.; Mondal, T.; et al. BACH1 Stabilization by Antioxidants Stimulates Lung Cancer Metastasis. Cell 2019, 178, 330–345.e22. [Google Scholar] [CrossRef]
- Panieri, E.; Santoro, M.M. ROS Homeostasis and Metabolism: A Dangerous Liason in Cancer Cells. Cell Death Dis. 2016, 7, e2253. [Google Scholar] [CrossRef]
- Pottier, C.; Fresnais, M.; Gilon, M.; Jérusalem, G.; Longuespée, R.; Sounni, N.E. Tyrosine Kinase Inhibitors in Cancer: Breakthrough and Challenges of Targeted Therapy. Cancers 2020, 12, 731. [Google Scholar] [CrossRef]
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of Multidrug Resistance in Cancer Chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233. [Google Scholar] [CrossRef] [PubMed]
- Turke, A.B.; Zejnullahu, K.; Wu, Y.L.; Song, Y.; Dias-Santagata, D.; Lifshits, E.; Toschi, L.; Rogers, A.; Mok, T.; Sequist, L.; et al. Preexistence and Clonal Selection of MET Amplification in EGFR Mutant NSCLC. Cancer Cell 2010, 17, 77–88. [Google Scholar] [CrossRef]
- Najafi, M.; Mortezaee, K.; Majidpoor, J. Cancer Stem Cell (CSC) Resistance Drivers. Life Sci. 2019, 234, 116781. [Google Scholar] [CrossRef]
- Mesci, S.; Marakli, S.; Yazgan, B.; Yıldırım, T. The Effect of ATP-Binding Cassette (ABC) Transporters in Human Cancers. Int. J. Sci. Lett. 2019, 1, 14–19. [Google Scholar] [CrossRef]
- Mele, L.; del Vecchio, V.; Liccardo, D.; Prisco, C.; Schwerdtfeger, M.; Robinson, N.; Desiderio, V.; Tirino, V.; Papaccio, G.; La Noce, M. The Role of Autophagy in Resistance to Targeted Therapies. Cancer Treat. Rev. 2020, 88. [Google Scholar] [CrossRef]
- Cox, A.D.; Fesik, S.W.; Kimmelman, A.C.; Luo, J.; Der, C.J. Drugging the Undruggable RAS: Mission Possible? Nat. Rev. Drug Discov. 2014, 13, 828–851. [Google Scholar] [CrossRef] [PubMed]
- Amm, I.; Sommer, T.; Wolf, D.H. Protein Quality Control and Elimination of Protein Waste: The Role of the Ubiquitin–Proteasome System. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2014, 1843, 182–196. [Google Scholar] [CrossRef]
- Komander, D.; Rape, M. The Ubiquitin Code. Annu. Rev. Biochem. 2012, 81, 203–229. [Google Scholar] [CrossRef]
- Grice, G.L.; Nathan, J.A. The Recognition of Ubiquitinated Proteins by the Proteasome. Cell Mol. Life Sci. 2016, 73, 3497–3506. [Google Scholar] [CrossRef]
- Harrigan, J.A.; Jacq, X.; Martin, N.M.; Jackson, S.P. Deubiquitylating Enzymes and Drug Discovery: Emerging Opportunities. Nat. Rev. Drug Discov. 2018, 17, 57–77. [Google Scholar] [CrossRef]
- Sakamoto, K.M.; Kim, K.B.; Kumagai, A.; Mercurio, F.; Crews, C.M.; Deshaies, R.J. Protacs: Chimeric Molecules That Target Proteins to the Skp1-Cullin-F Box Complex for Ubiquitination and Degradation. Proc. Natl. Acad. Sci. USA 2001, 98, 8554–8559. [Google Scholar] [CrossRef]
- Mi, D.; Li, Y.; Gu, H.; Li, Y.; Chen, Y. Current Advances of Small Molecule E3 Ligands for Proteolysis-Targeting Chimeras Design. Eur. J. Med. Chem. 2023, 256, 115444. [Google Scholar] [CrossRef]
- Poongavanam, V.; Atilaw, Y.; Siegel, S.; Giese, A.; Lehmann, L.; Meibom, D.; Erdelyi, M.; Kihlberg, J. Linker-Dependent Folding Rationalizes PROTAC Cell Permeability. J. Med. Chem. 2022, 65, 13029–13040. [Google Scholar] [CrossRef] [PubMed]
- Du, G.; Jiang, J.; Henning, N.J.; Safaee, N.; Koide, E.; Nowak, R.P.; Donovan, K.A.; Yoon, H.; You, I.; Yue, H.; et al. Exploring the Target Scope of KEAP1 E3 Ligase-Based PROTACs. Cell Chem. Biol. 2022, 29, 1470–1481.e31. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Liu, T.; Jiao, Q.; Ji, J.; Tao, M.; Liu, Y.; You, Q.; Jiang, Z. Discovery of a Keap1-Dependent Peptide PROTAC to Knockdown Tau by Ubiquitination-Proteasome Degradation Pathway. Eur. J. Med. Chem. 2018, 146, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Tong, B.; Luo, M.; Xie, Y.; Spradlin, J.N.; Tallarico, J.A.; McKenna, J.M.; Schirle, M.; Maimone, T.J.; Nomura, D.K. Bardoxolone Conjugation Enables Targeted Protein Degradation of BRD4. Sci. Rep. 2020, 10, 15543. [Google Scholar] [CrossRef]
- Pei, J.; Xiao, Y.; Liu, X.; Hu, W.; Sobh, A.; Yuan, Y.; Zhou, S.; Hua, N.; Mackintosh, S.G.; Zhang, X.; et al. Piperlongumine Conjugates Induce Targeted Protein Degradation. Cell Chem. Biol. 2023, 30, 203–213.e17. [Google Scholar] [CrossRef]
- Tao, Y.; Remillard, D.; Vinogradova, E.V.; Yokoyama, M.; Banchenko, S.; Schwefel, D.; Melillo, B.; Schreiber, S.L.; Zhang, X.; Cravatt, B.F. Targeted Protein Degradation by Electrophilic PROTACs That Stereoselectively and Site-Specifically Engage DCAF1. J. Am. Chem. Soc. 2022, 144, 18688–18699. [Google Scholar] [CrossRef]
- Zhang, X.; Luukkonen, L.M.; Eissler, C.L.; Crowley, V.M.; Yamashita, Y.; Schafroth, M.A.; Kikuchi, S.; Weinstein, D.S.; Symons, K.T.; Nordin, B.E.; et al. DCAF11 Supports Targeted Protein Degradation by Electrophilic Proteolysis-Targeting Chimeras. J. Am. Chem. Soc. 2021, 143, 5141–5149. [Google Scholar] [CrossRef]
- Zoppi, V.; Hughes, S.J.; Maniaci, C.; Testa, A.; Gmaschitz, T.; Wieshofer, C.; Koegl, M.; Riching, K.M.; Daniels, D.L.; Spallarossa, A.; et al. Iterative Design and Optimization of Initially Inactive Proteolysis Targeting Chimeras (PROTACs) Identify VZ185 as a Potent, Fast, and Selective von Hippel-Lindau (VHL) Based Dual Degrader Probe of BRD9 and BRD7. J. Med. Chem. 2019, 62, 699–726. [Google Scholar] [CrossRef]
- Li, L.; Mi, D.; Pei, H.; Duan, Q.; Wang, X.; Zhou, W.; Jin, J.; Li, D.; Liu, M.; Chen, Y. In Vivo Target Protein Degradation Induced by PROTACs Based on E3 Ligase DCAF15. Signal Transduct. Target. Ther. 2020, 5, 129. [Google Scholar] [CrossRef]
- Pu, C.; Tong, Y.; Liu, Y.; Lan, S.; Wang, S.; Yan, G.; Zhang, H.; Luo, D.; Ma, X.; Yu, S.; et al. Selective Degradation of PARP2 by PROTACs via Recruiting DCAF16 for Triple-Negative Breast Cancer. Eur. J. Med. Chem. 2022, 236, 114321. [Google Scholar] [CrossRef]
- Zhang, X.; Crowley, V.M.; Wucherpfennig, T.G.; Dix, M.M.; Cravatt, B.F. Electrophilic PROTACs That Degrade Nuclear Proteins by Engaging DCAF16. Nat. Chem. Biol. 2019, 15, 737–746. [Google Scholar] [CrossRef]
- Nalawansha, D.A.; Li, K.; Hines, J.; Crews, C.M. Hijacking Methyl Reader Proteins for Nuclear-Specific Protein Degradation. J. Am. Chem. Soc. 2022, 144, 5594–5605. [Google Scholar] [CrossRef] [PubMed]
- Leng, F.; Yu, J.; Zhang, C.; Alejo, S.; Hoang, N.; Sun, H.; Lu, F.; Zhang, H. Methylated DNMT1 and E2F1 are targeted for proteolysis by L3MBTL3 and CRL4(DCAF5) ubiquitin ligase. Nat. Commun. 2018, 9, 1641–1657. [Google Scholar]
- Ohoka, N.; Tsuji, G.; Shoda, T.; Fujisato, T.; Kurihara, M.; Demizu, Y.; Naito, M. Development of Small Molecule Chimeras That Recruit AhR E3 Ligase to Target Proteins. ACS Chem. Biol. 2019, 14, 2822–2832. [Google Scholar] [CrossRef] [PubMed]
- Henning, N.J.; Manford, A.G.; Spradlin, J.N.; Brittain, S.M.; Zhang, E.; McKenna, J.M.; Tallarico, J.A.; Schirle, M.; Rape, M.; Nomura, D.K. Discovery of a Covalent FEM1B Recruiter for Targeted Protein Degradation Applications. J. Am. Chem. Soc. 2022, 144, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Ward, C.C.; Kleinman, J.I.; Brittain, S.M.; Lee, P.S.; Chung, C.Y.S.; Kim, K.; Petri, Y.; Thomas, J.R.; Tallarico, J.A.; McKenna, J.M.; et al. Covalent Ligand Screening Uncovers a RNF4 E3 Ligase Recruiter for Targeted Protein Degradation Applications. ACS Chem. Biol. 2019, 14, 2430–2440. [Google Scholar] [CrossRef]
- Luo, M.; Spradlin, J.N.; Boike, L.; Tong, B.; Brittain, S.M.; McKenna, J.M.; Tallarico, J.A.; Schirle, M.; Maimone, T.J.; Nomura, D.K. Chemoproteomics-Enabled Discovery of Covalent RNF114-Based Degraders That Mimic Natural Product Function. Cell Chem. Biol. 2021, 28, 559–566.e15. [Google Scholar] [CrossRef]
- Zhong, G.; Chang, X.; Xie, W.; Zhou, X. Targeted Protein Degradation: Advances in Drug Discovery and Clinical Practice. Signal Transduct. Target. Ther. 2024, 9, 308. [Google Scholar] [CrossRef]
- Herbst, R.S.; Fukuoka, M.; Baselga, J. Gefitinib—A Novel Targeted Approach to Treating Cancer. Nat. Rev. Cancer 2004, 4, 956–965. [Google Scholar] [CrossRef]
- Cappuzzo, F.; Ciuleanu, T.; Stelmakh, L.; Cicenas, S.; Szczésna, A.; Juhász, E.; Esteban, E.; Molinier, O.; Brugger, W.; Melezínek, I.; et al. Erlotinib as Maintenance Treatment in Advanced Non-Small-Cell Lung Cancer: A Multicentre, Randomised, Placebo-Controlled Phase 3 Study. Lancet Oncol. 2010, 11, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Cross, D.A.E.; Ashton, S.E.; Ghiorghiu, S.; Eberlein, C.; Nebhan, C.A.; Spitzler, P.J.; Orme, J.P.; Finlay, M.R.V.; Ward, R.A.; Mellor, M.J.; et al. AZD9291, an Irreversible EGFR TKI, Overcomes T790M-Mediated Resistance to EGFR Inhibitors in Lung Cancer. Cancer Discov. 2014, 4, 1046–1061. [Google Scholar] [CrossRef]
- Dong, R.F.; Zhu, M.L.; Liu, M.M.; Xu, Y.T.; Yuan, L.L.; Bian, J.; Xia, Y.Z.; Kong, L.Y. EGFR Mutation Mediates Resistance to EGFR Tyrosine Kinase Inhibitors in NSCLC: From Molecular Mechanisms to Clinical Research. Pharmacol. Res. 2021, 167, 105583. [Google Scholar] [CrossRef]
- Zhao, H.Y.; Wang, H.P.; Mao, Y.Z.; Zhang, H.; Xin, M.; Xi, X.X.; Lei, H.; Mao, S.; Li, D.H.; Zhang, S.Q. Discovery of Potent PROTACs Targeting EGFR Mutants through the Optimization of Covalent EGFR Ligands. J. Med. Chem. 2022, 65, 4709–4726. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhou, H. Proteolysis Targeting Chimera (PROTAC) for Epidermal Growth Factor Receptor Enhances Anti-Tumor Immunity in Non-Small Cell Lung Cancer. Drug Dev. Res. 2021, 82, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; To, C.; De Clercq, D.J.H.; Park, E.; Ponthier, C.M.; Shin, B.H.; Mushajiang, M.; Nowak, R.P.; Fischer, E.S.; Eck, M.J.; et al. Mutant-Selective Allosteric EGFR Degraders Are Effective Against a Broad Range of Drug-Resistant Mutations. Angew. Chem. Int. Ed. 2020, 59, 14481–14489. [Google Scholar] [CrossRef]
- Du, Y.; Chen, Y.; Wang, Y.; Chen, J.; Lu, X.; Zhang, L.; Li, Y.; Wang, Z.; Ye, G.; Zhang, G. HJM-561, a Potent, Selective, and Orally Bioavailable EGFR PROTAC That Overcomes Osimertinib-Resistant EGFR Triple Mutations. Mol. Cancer Ther. 2022, 21, 1060–1066. [Google Scholar] [CrossRef]
- Haisco Pharmaceutical Group Co., Ltd. Phase I Study of HSK40118 in NSCLC Patients with EGFR Mutation; NCT06050980; Haisco Pharmaceutical Group Co., Ltd.: Tibet, China, 2023. [Google Scholar]
- Haisco Pharmaceutical Group Co., Ltd. Phase I Study of HSK42360 in Solid Tumors with BRAF V600 Mutation; NCT06536400; Haisco Pharmaceutical Group Co., Ltd.: Tibet, China, 2024. [Google Scholar]
- Li, J.W.; Zheng, G.; Kaye, F.J.; Wu, L. PROTAC Therapy as a New Targeted Therapy for Lung Cancer. Mol. Ther. 2023, 31, 647–656. [Google Scholar] [CrossRef]
- García Jiménez, D.; Rossi Sebastiano, M.; Vallaro, M.; Mileo, V.; Pizzirani, D.; Moretti, E.; Ermondi, G.; Caron, G. Designing Soluble PROTACs: Strategies and Preliminary Guidelines. J. Med. Chem. 2022, 65, 12639–12649. [Google Scholar] [CrossRef]
- Guenette, R.G.; Yang, S.W.; Min, J.; Pei, B.; Potts, P.R. Target and Tissue Selectivity of PROTAC Degraders. Chem. Soc. Rev. 2022, 51, 5740–5756. [Google Scholar] [CrossRef]
- Pike, A.; Williamson, B.; Harlfinger, S.; Martin, S.; McGinnity, D.F. Optimising Proteolysis-Targeting Chimeras (PROTACs) for Oral Drug Delivery: A Drug Metabolism and Pharmacokinetics Perspective. Drug Discov. Today 2020, 25, 1793–1800. [Google Scholar] [CrossRef]
- Wu, K.; Yu, B.; Li, D.; Tian, Y.; Liu, Y.; Jiang, J. Recent Advances in Nanoplatforms for the Treatment of Osteosarcoma. Front. Oncol. 2022, 12, 805978. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Huang, D.; Peppas, N.A. Advanced Engineered Nanoparticulate Platforms to Address Key Biological Barriers for Delivering Chemotherapeutic Agents to Target Sites. Adv. Drug Deliv. Rev. 2020, 167, 170–188. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Li, D.; Wu, Z.; Wang, Q.; Ma, Z.; Zhang, C. Research Progress and Prospect of Nanoplatforms for Treatment of Oral Cancer. Front. Pharmacol. 2020, 11, 616101. [Google Scholar] [CrossRef]
- Mauro, N.; Utzeri, M.A.; Varvarà, P.; Cavallaro, G. Functionalization of Metal and Carbon Nanoparticles with Potential in Cancer Theranostics. Molecules 2021, 26, 3085. [Google Scholar] [CrossRef]
- Zhong, J.; Zhao, R.; Wang, Y.; Su, Y.X.; Lan, X. Nano-PROTACs: State of the Art and Perspectives. Nanoscale 2024, 16, 4378–4391. [Google Scholar] [CrossRef]
- Andra, V.V.S.N.L.; Pammi, S.V.N.; Bhatraju, L.V.K.P.; Ruddaraju, L.K. A Comprehensive Review on Novel Liposomal Methodologies, Commercial Formulations, Clinical Trials and Patents. Bionanoscience 2022, 12, 274–291. [Google Scholar] [CrossRef] [PubMed]
- Ickenstein, L.M.; Garidel, P. Lipid-Based Nanoparticle Formulations for Small Molecules and RNA Drugs. Expert Opin. Drug Deliv. 2019, 16, 1205–1226. [Google Scholar] [CrossRef]
- Yao, L.; Yang, N.; Zhou, W.; Akhtar, M.H.; Zhou, W.; Liu, C.; Song, S.; Li, Y.; Han, W.; Yu, C. Exploiting Cancer Vulnerabilities by Blocking of the DHODH and GPX4 Pathways: A Multifunctional Bodipy/PROTAC Nanoplatform for the Efficient Synergistic Ferroptosis Therapy. Adv. Healthc. Mater. 2023, 12, e2300871. [Google Scholar] [CrossRef]
- Fu, Y.; Rathod, D.; Patel, K. Protein Kinase C Inhibitor Anchored BRD4 PROTAC PEGylated Nanoliposomes for the Treatment of Vemurafenib-Resistant Melanoma. Exp. Cell Res. 2020, 396, 112275. [Google Scholar] [CrossRef]
- Chen, J.; Qiu, M.; Ma, F.; Yang, L.; Glass, Z.; Xu, Q. Enhanced Protein Degradation by Intracellular Delivery of Pre-Fused PROTACs Using Lipid-like Nanoparticles. J. Control. Release 2021, 330, 1244–1249. [Google Scholar] [CrossRef]
- Ma, B.; Fan, Y.; Zhang, D.; Wei, Y.; Jian, Y.; Liu, D.; Wang, Z.; Gao, Y.; Ma, J.; Chen, Y.; et al. De Novo Design of an Androgen Receptor DNA Binding Domain-Targeted Peptide PROTAC for Prostate Cancer Therapy. Adv. Sci. 2022, 9, e2201859. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Zhou, L.; Yu, D.; Zhu, R.; Chen, Y.; Song, M.; Liu, X.; Liao, Y.; Ding, T.; Fan, W.; et al. Near-Infrared-Activatable PROTAC Nanocages for Controllable Target Protein Degradation and On-Demand Antitumor Therapy. J. Med. Chem. 2023, 66, 10458–10472. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.F.; He, Z.D. Design and Fabrication of Hydrogel-Based Nanoparticulate Systems for in Vivo Drug Delivery. J. Control. Release 2016, 243, 269–282. [Google Scholar] [CrossRef]
- Beach, M.A.; Nayanathara, U.; Gao, Y.; Zhang, C.; Xiong, Y.; Wang, Y.; Such, G.K. Polymeric Nanoparticles for Drug Delivery. Chem. Rev. 2024, 124, 5505–5616. [Google Scholar] [CrossRef]
- Liu, H.J.; Chen, W.; Wu, G.; Zhou, J.; Liu, C.; Tang, Z.; Huang, X.; Gao, J.; Xiao, Y.; Kong, N.; et al. Glutathione-Scavenging Nanoparticle-Mediated PROTACs Delivery for Targeted Protein Degradation and Amplified Antitumor Effects. Adv. Sci. 2023, 10, e2207439. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zan, X.; Miao, J.; Wang, B.; Wu, Y.; Shen, Y.; Chen, X.; Gou, H.; Zheng, S.; Huang, N.; et al. Enhanced Anti-Glioma Efficacy of Doxorubicin with BRD4 PROTAC Degrader Using Targeted Nanoparticles. Mater. Today Bio 2022, 16, 100423. [Google Scholar] [CrossRef]
- Guan, X.; Xu, X.; Tao, Y.; Deng, X.; He, L.; Lin, Z.; Chang, J.; Huang, J.; Zhou, D.; Yu, X.; et al. Dual Targeting and Bioresponsive Nano-PROTAC Induced Precise and Effective Lung Cancer Therapy. J. Nanobiotechnol. 2024, 22, 692. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, C.; Chen, X.; Liao, K.; Li, F.; Song, X.; Liu, C.; Su, M.Y.; Sun, H.; Hou, T.; et al. Linker-Free PROTACs Efficiently Induce the Degradation of Oncoproteins. Nat. Commun. 2025, 16, 4794. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, Z.; Chen, C.; Yang, F.; Liu, P.; Fang, S.; Wang, B.; Chen, S.; Li, X. A Small-Molecule Degrader Selectively Inhibits the Growth of ALK-Rearranged Lung Cancer with Ceritinib Resistance. iScience 2024, 27, 109015. [Google Scholar] [CrossRef]
- Khan, S.; Cao, L.; Wiegand, J.; Zhang, P.; Zajac-Kaye, M.; Kaye, F.J.; Zheng, G.; Zhou, D. PROTAC-Mediated Dual Degradation of BCL-XL and BCL-2 Is a Highly Effective Therapeutic Strategy in Small-Cell Lung Cancer. Cells 2024, 13, 528. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.X.; Ding, X.; Tang, L.; Cao, W.J.; Su, B.; Zhang, J. PROTAC EZH2 Degrader-1 Overcomes the Resistance of Podophyllotoxin Derivatives in Refractory Small Cell Lung Cancer with Leptomeningeal Metastasis. BMC Cancer 2024, 24, 504. [Google Scholar] [CrossRef]
- Zhang, D.; Ma, B.; Dragovich, P.S.; Ma, L.; Chen, S.; Chen, E.C.; Ye, X.; Liu, J.; Pizzano, J.; Bortolon, E.; et al. Tissue Distribution and Retention Drives Efficacy of Rapidly Clearing VHL-Based PROTACs. Commun. Med. 2024, 4, 87. [Google Scholar] [CrossRef]
- Kotagiri, S.; Wang, Y.; Han, Y.; Liang, X.; Blazanin, N.; Nguyen, P.K.; Jiang, Y.; Lissanu, Y. Discovery of Novel, Potent and Orally Bioavailable SMARCA2 PROTACs with Synergistic Anti-Tumor Activity in Combination with KRAS G12C Inhibitors. bioRxiv 2024. [Google Scholar] [CrossRef]
- Wang, D.; Liu, Y.; Chen, Y.; Dai, C.; Hu, W.; Han, J.; Li, Z.; Yin, F.; Zhang, Y.; Shi, C. EGFR Targeted Liposomal PROTAC Assisted with Epigenetic Regulation as an Efficient Strategy for Osimertinib-Resistant Lung Cancer Therapy. Adv. Sci. 2025, e10197. [Google Scholar] [CrossRef]
- Vartak, R.; Patel, K. Targeted Nanoliposomes of Oncogenic Protein Degraders: Significant Inhibition of Tumor in Lung-Cancer Bearing Mice. J. Control. Release 2024, 376, 502–517. [Google Scholar] [CrossRef]
- Lim, S.M.; Hong, M.H.; Kim, H.R. Immunotherapy for Non-Small Cell Lung Cancer: Current Landscape and Future Perspectives. Immune Netw. 2020, 20, e10. [Google Scholar] [CrossRef]
- Zhu, H.F.; Li, Y. Small-Molecule Targets in Tumor Immunotherapy. Nat. Prod. Bioprospect. 2018, 8, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, R.; Jiang, T.; Gao, Y.; Zhong, K.; Cheng, H.; Chen, X.; Li, S. Inhalable Nanomedicine for Lung Cancer Treatment. Smart Mater. Med. 2024, 5, 261–280. [Google Scholar] [CrossRef]
- Kumar, M.; Jha, A.; DR, M.; Mishra, B. Targeted Drug Nanocrystals for Pulmonary Delivery: A Potential Strategy for Lung Cancer Therapy. Expert Opin. Drug Deliv. 2020, 17, 1459–1472. [Google Scholar] [CrossRef]
- Sakamoto, K.M. Protacs for Treatment of Cancer. Pediatr. Res. 2010, 67, 505–508. [Google Scholar] [CrossRef]
- Pérez, E.; Fernández-Olleros, A.M.; Olmo, R.; Teijón, J.M.; Blanco, M.D. PH and Glutathion-Responsive Hydrogel for Localized Delivery of Paclitaxel. Colloids Surf. B Biointerfaces 2014, 116, 247–256. [Google Scholar] [CrossRef]
- Ji, J.; Ma, S.; Zhu, Y.; Zhao, J.; Tong, Y.; You, Q.; Jiang, Z. ARE-PROTACs Enable Co-Degradation of an Nrf2-MafG Heterodimer. J. Med. Chem. 2023, 66, 6070–6081. [Google Scholar] [CrossRef]
- Barba, A.A.; Syahputra, E.W.; Lee, H.; Cho, H.; Park, H.J.; Park, K.-S.; Hwang, D. PROTAC Delivery Strategies for Overcoming Physicochemical Properties and Physiological Barriers in Targeted Protein Degradation. Pharmaceutics 2025, 17, 501. [Google Scholar] [CrossRef]
- Eaton, M.A.W.; Levy, L.; Fontaine, O.M.A. Delivering Nanomedicines to Patients: A Practical Guide. Nanomedicine 2015, 11, 983–992. [Google Scholar] [CrossRef] [PubMed]
- Đorđević, S.; Gonzalez, M.M.; Conejos-Sánchez, I.; Carreira, B.; Pozzi, S.; Acúrcio, R.C.; Satchi-Fainaro, R.; Florindo, H.F.; Vicent, M.J. Current Hurdles to the Translation of Nanomedicines from Bench to the Clinic. Drug Deliv. Transl. Res. 2022, 12, 500–525. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olazábal-Morán, M.; Pérez, E.; Esteban-Arranz, A.; Garrido, A. The Role of Reactive Oxygen Species in Lung Cancer Development: Nanomedicine as a Therapeutic Strategy. Biomolecules 2025, 15, 1316. https://doi.org/10.3390/biom15091316
Olazábal-Morán M, Pérez E, Esteban-Arranz A, Garrido A. The Role of Reactive Oxygen Species in Lung Cancer Development: Nanomedicine as a Therapeutic Strategy. Biomolecules. 2025; 15(9):1316. https://doi.org/10.3390/biom15091316
Chicago/Turabian StyleOlazábal-Morán, Manuel, Elena Pérez, Adrián Esteban-Arranz, and Antonio Garrido. 2025. "The Role of Reactive Oxygen Species in Lung Cancer Development: Nanomedicine as a Therapeutic Strategy" Biomolecules 15, no. 9: 1316. https://doi.org/10.3390/biom15091316
APA StyleOlazábal-Morán, M., Pérez, E., Esteban-Arranz, A., & Garrido, A. (2025). The Role of Reactive Oxygen Species in Lung Cancer Development: Nanomedicine as a Therapeutic Strategy. Biomolecules, 15(9), 1316. https://doi.org/10.3390/biom15091316







