Coming Clean and Avoiding Bubble Trouble–Using Detergents Wisely in the Purification of Membrane Proteins for Cryo-EM Studies
Abstract
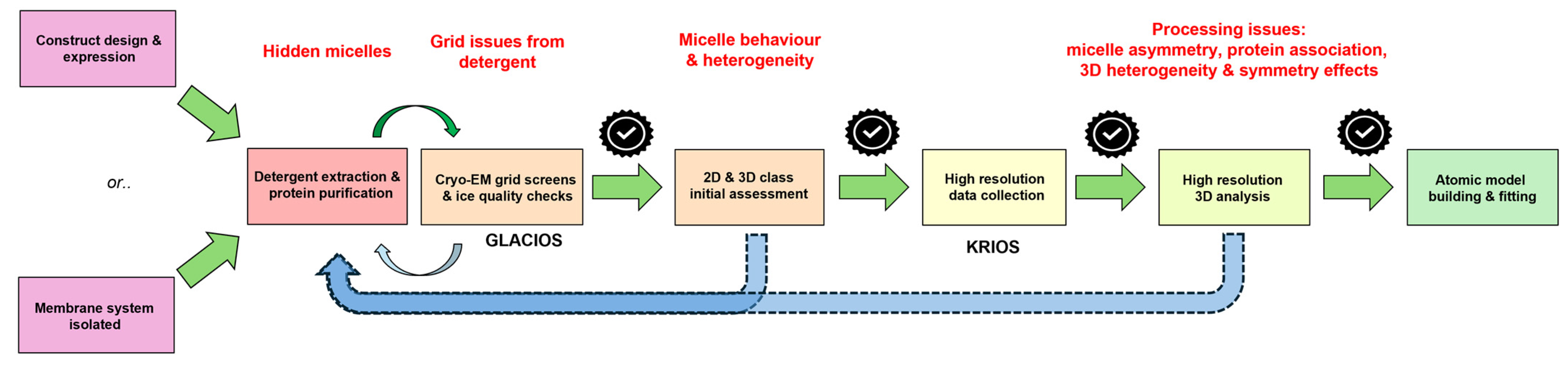
1. Introduction
2. Materials and Methods
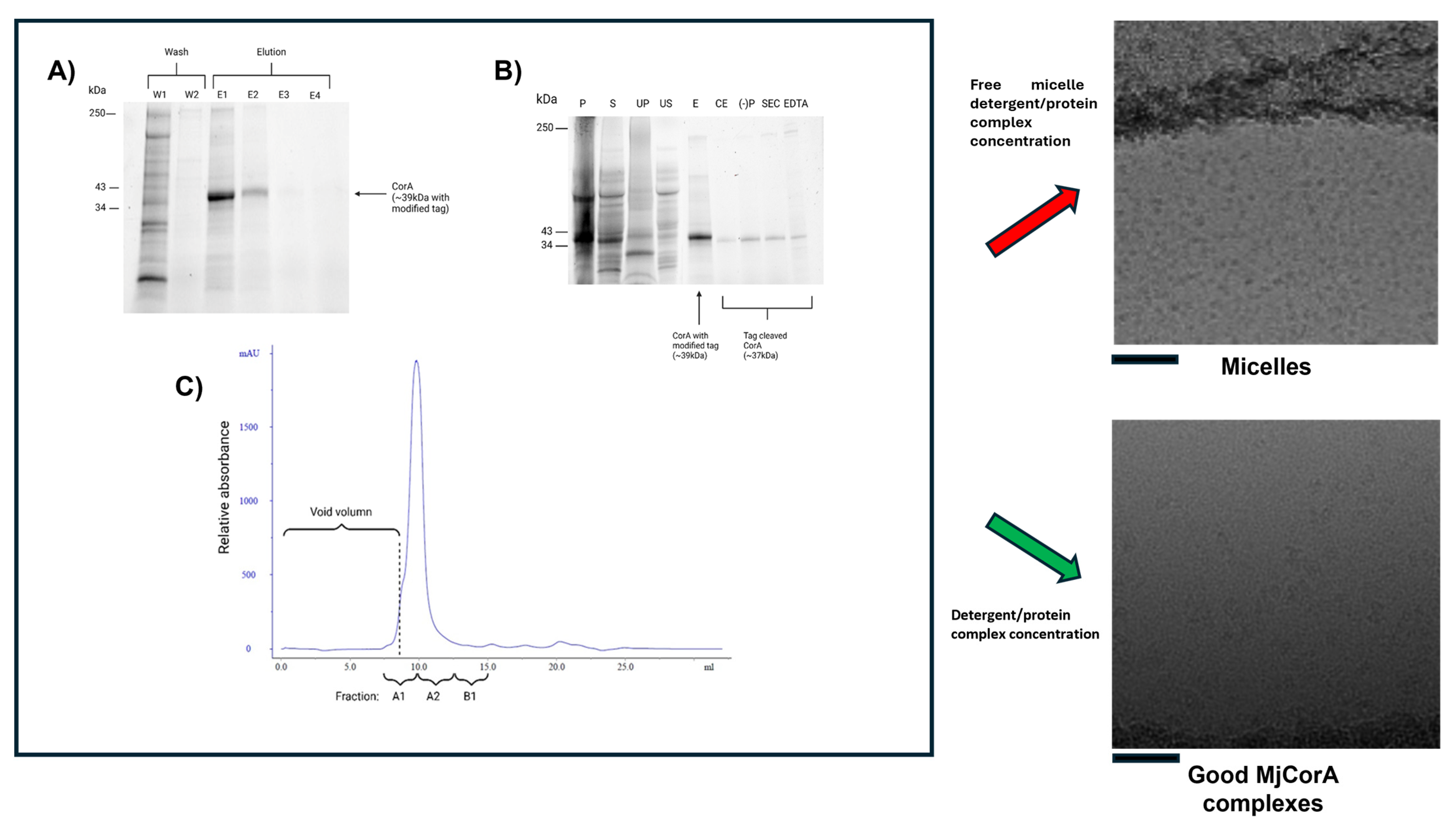
2.1. Cell Culture, Expression, and Purification of MjCorA
2.2. Cell Culture and Protein Purification of WzzBST
2.3. Sample Preparation for Cryo-EM for MjCorA & WzzBST
2.4. Image Processing and 3D Reconstruction for MjCorA & WzzBST
| Sample | MjCorA | WzzBST |
|---|---|---|
| Grid type | C-fFlat T40–200 or 300 Au mesh 1.3/1.2 | Quantifoil–300 Cu mesh 1.3/1.2 (chloroform- washed) |
| Glow dDischarge | Emitek K100X: 25 mv for 30 s glow discharge | Solarus II 955: 20 V/5 V with 1:1 H2:O2 20 s |
| Sample vVolume | 3 mL | |
| Grid blotting parameters | 4–6 s in Vitrobot IV: 5 s wait (100% humidity) at 22 °C | 2–4 s in Vitrobot IV (95% humidity) at 22 °C |
| Microscope | KRIOS G2 300 Kv | |
| Sampling | 1.06 or 1.072 Å/px | 1.06 Å/px |
| Total dose | 40 e/Å2 | 60 e/Å2 |
| Defocus (uM) | −0.75 to –2.25 | −1.0 to –2.25 |
| Images used | 25,000–36,000 total | ~5000 total |
| Camera | Gatan K3 or F4i/Selectris | Gatan K3 |
| MotionCorr | 512 or 1024 FFT box and 5 × 5 patches [68] | |
| CTF correction | CTFFIND 4.1 [69] | |
| CTF resolution | 30 Å, 2.5 Å (min, max) | |
| Processing | Topaz for picking [66] & Relion 5.0 with BLUSH [3] | |
| Data bBinning | x4 in process up to 3D refinement | |
| Symmetry | C1, C6, C12 | C1 and C5C1 & C5 |
2.5. Molecular Modelling and Refinement of WzzBST
2.6. Additionally Mentioned Protein Examples—Sample and Image Processing Information
- The membrane protein ion channel example (used in Figure 3) was purified into DDM/Tris buffer and concentrated to 2 mg/mL−1 before being loaded onto glow-discharged Quantifoil R1.2/1.3 300 mesh Cu grids. The sample was vitrified with a Leica GP2 with a blotting time of 3 s and the grid was screened and imaged with a ThermoScientific Glacios operated at 200 kV with a Falcon 4i detector at 1.192 Å/px.
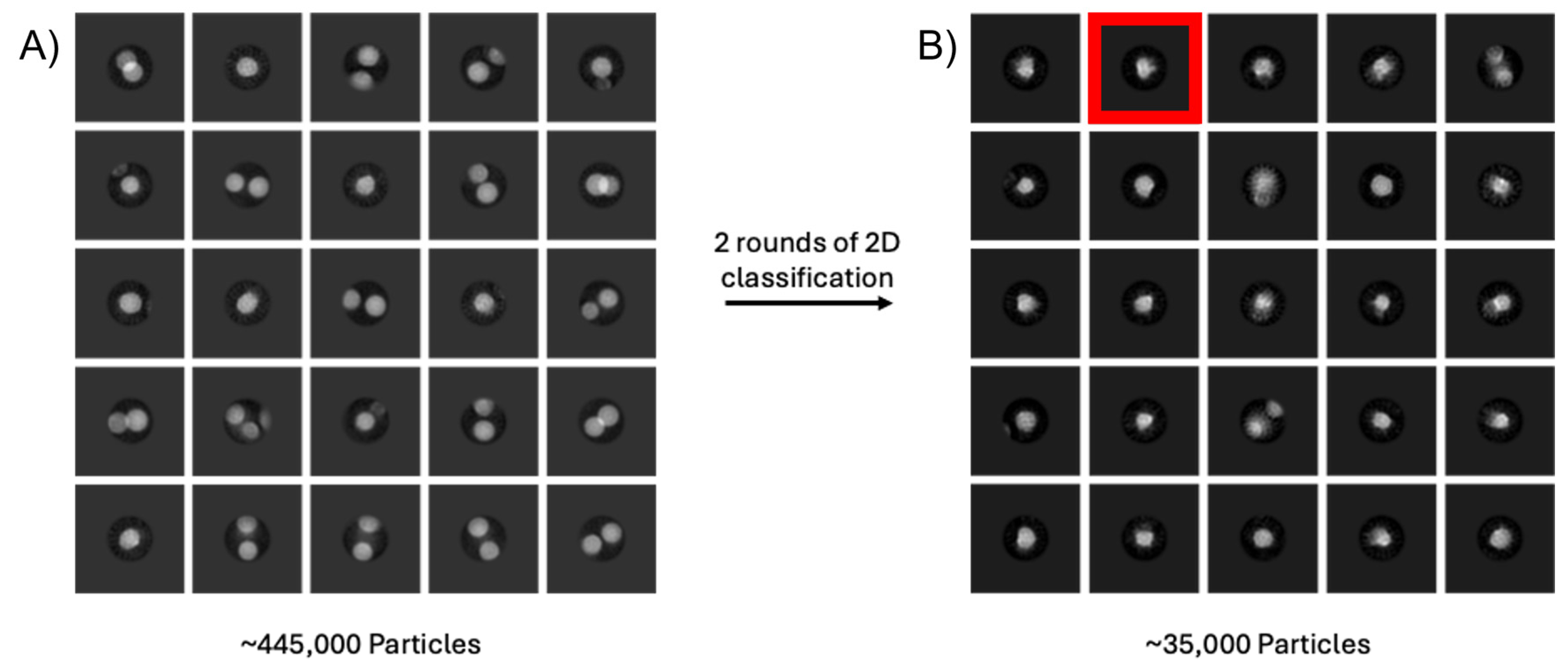
- The purified bacterial enzyme example (used in Figure 5) was purified from E. coli. For the experiment in Figure 5, the enzyme was solubilised into DDM with the added substrate also solubilised in DDM. A total of 3.5 μL of protein at 1.7 mg/mL−1 was applied to a glow-discharged Quantifoil Au 1.2/1.3 300 mesh grid (Harrick Plasma Cleaner, 60 s) and blotted for 2 s before vitrification in liquid ethane using a Vitrobot (Thermo Fisher). EM data were collected on a ThermoScientific Krios operated at 300 kV with a Gatan K3 camera and Bioquantum energy filter. A total of 1604 micrographs were collected at a magnification 130,000× with a physical pixel size of 0.651 Å/px. The total dose for each dataset was 60 e−/Å2 with a defocus range of −1.0 μm to −2.5 μm and a slit width of 20 eV. Movies were motion-corrected and ctf-corrected using Motion Corr [68] and Ctffind4 as part of the eBIC auto-processing pipeline (PATO) which uses Relion algorithms (https://ebic-pato.diamond.ac.uk/, accessed on 1 September 2025). Particle picking was performed using Blob Picker in CryoSPARC v 3.12 (https://cryosparc.com/, accessed on 1 September 2025), giving 445,289 picks in total. Two-dimensional classification was also performed in CryoSPARC, resulting in a final stack of 35,255 particles.
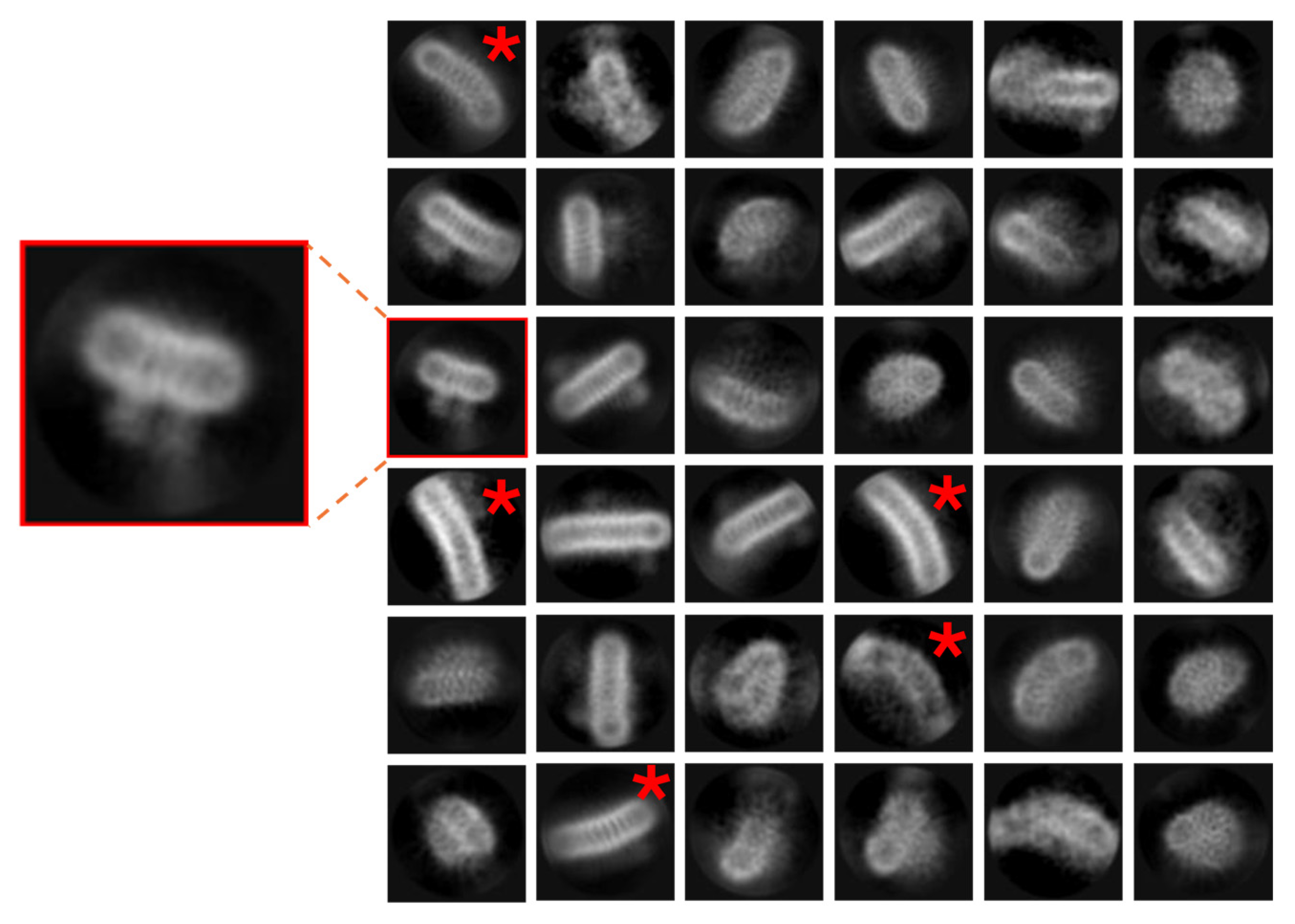
- For the experiment shown in Figure 6, the enzyme was purified from E. coli and solubilised into LMNG/Tris buffer. A total of 3.5 μL of protein at 2.2 mg/mL−1 was applied to a glow-discharged Quantifoil Au 1.2/1.3 300 mesh grid (Harrick Plasma Cleaner, 60 s) and blotted for 2 s before vitrification in liquid ethane using a Vitrobot (Thermo Fisher). Movies were collected on a ThermoScientific Krios operated at 300 kV with a Gatan K3 camera and Bioquantum energy filter. A total of 23,351 movies were collected at a magnification of 130,000× with a physical pixel size of 0.651 Å/px. The total dose was 60 e−/Å2 with a defocus range of −1.0 μm to −2.5 μm and a slit width of 20 eV. Movies were motion-corrected and CTF-corrected using Motion Corr 2 and Ctffind 4 as part of the eBIC auto-processing pipeline (PATO) using Relion (as above). A total of 1,748,933 particles were picked in the pipeline and subjected to multiple rounds of 2D classification to yield a final stack of 357,220 particles.
- The human major facilitator superfamily (MFS) (referred to in Figure 8) transporter was purified from the Baculovirus expression system. Protein was concentrated to 5.1 mg/mL−1 and grids were prepared using an SPT Labtech Chameleon using Quantifoil Active 1.2/0.8 grids, glow-discharged internally in the Chameleon. EM data were collected on a ThermoScientific Glacios operated at 200 kV with a Falcon 4 detector at 1.192 Å/px. A total of 1059 movies were collected at a magnification of 130,000× corresponding to a physical pixel size of 0.651 Å/px. The total dose for each dataset was 60 e−/A2 with a defocus range of −1.0 μm to −2.5 μm and a slit width of 20 eV. Movies were motion-corrected and CTF-corrected using Motion Corr 2 and Ctffind 4 as part of the eBIC auto-processing pipeline using Relion (as above). A total of 530,402 particles were picked and subjected to 2D classification.
3. Results and Discussion
3.1. Issue 1: Empty Micelle ‘Up-Concentration’
- His-tag purification on a Ni2+ affinity column;
- Tag cleavage and removal;
- A final size-exclusion chromatography (SEC) step to enhance purity and homogeneity but also reduce the DDM concentration from 0.04% to 0.015%.
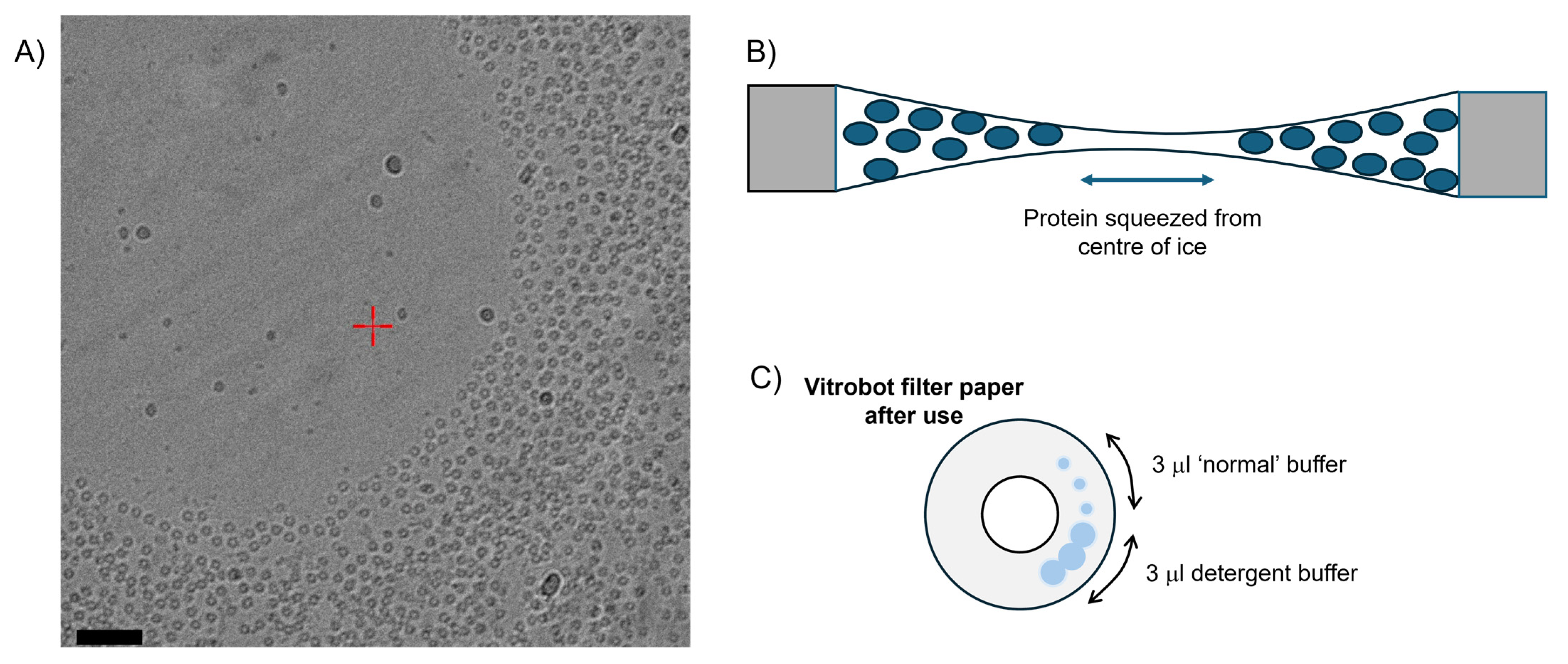
3.2. Issue 2: ‘Concave Lensing’ of Vitreous Ice in the Preparation of Cryo Grids
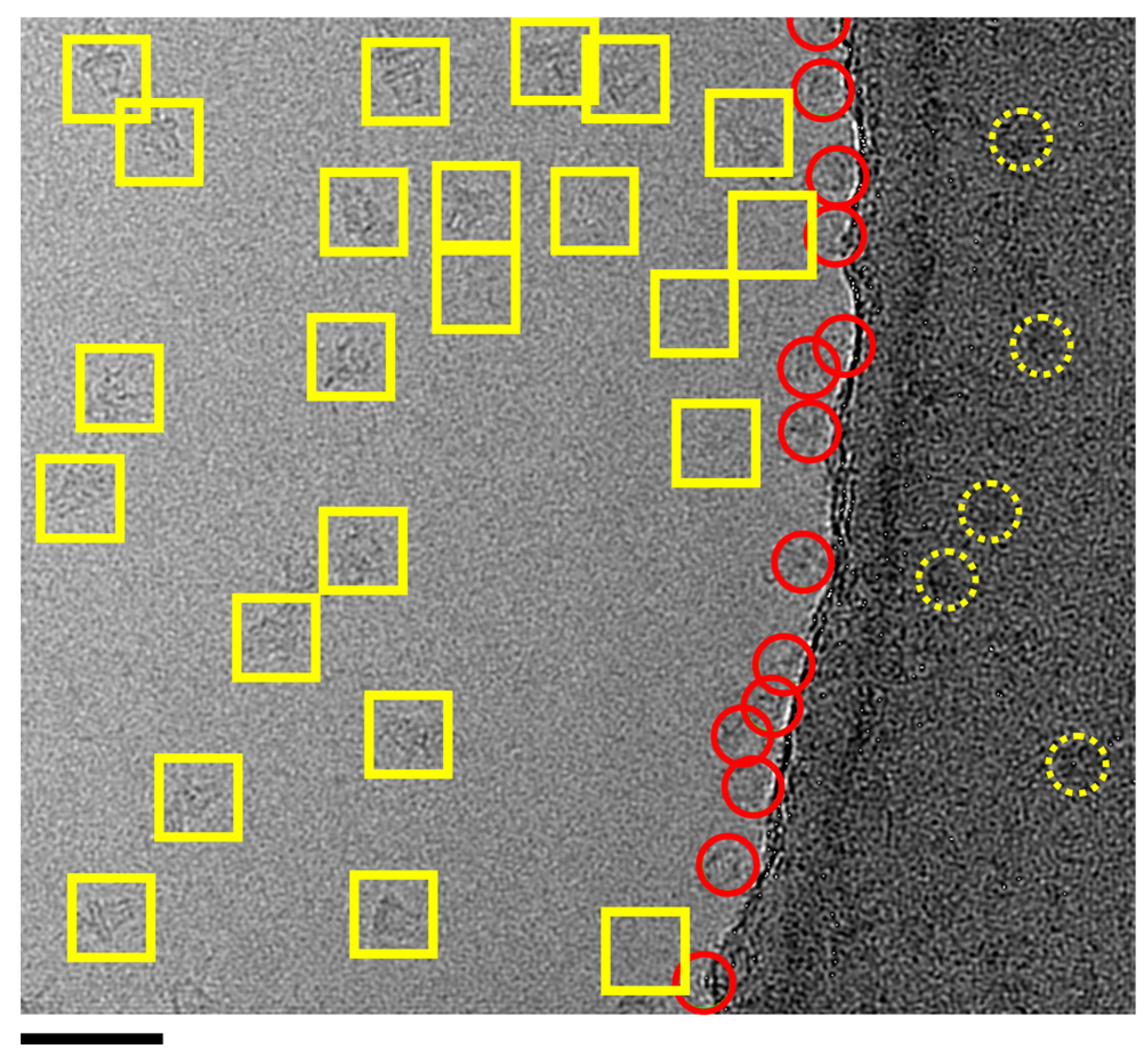
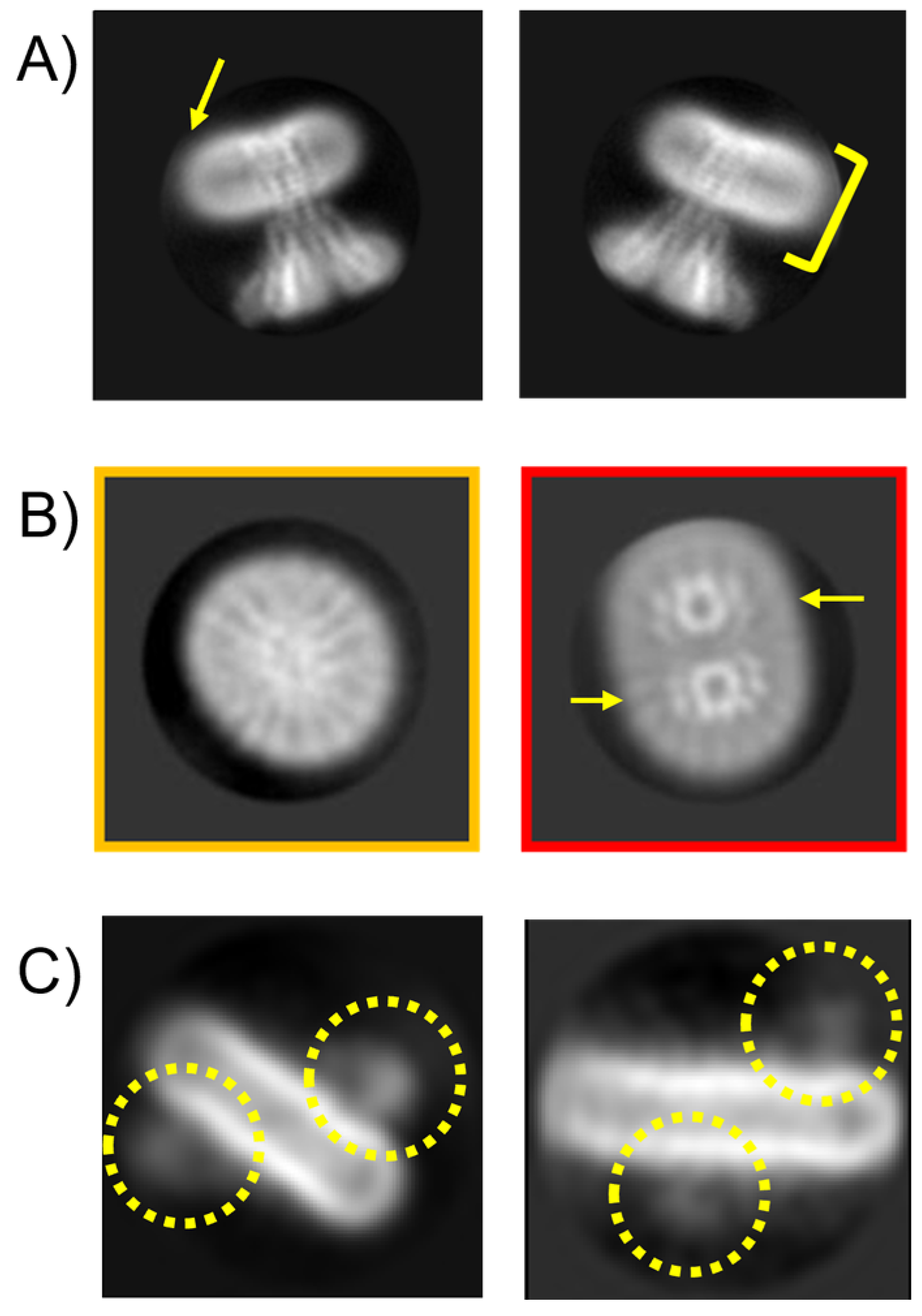
3.3. Issue 3: Empty Micelle ‘Swamping’ of 2D Classification and Unusual Micelle Structures
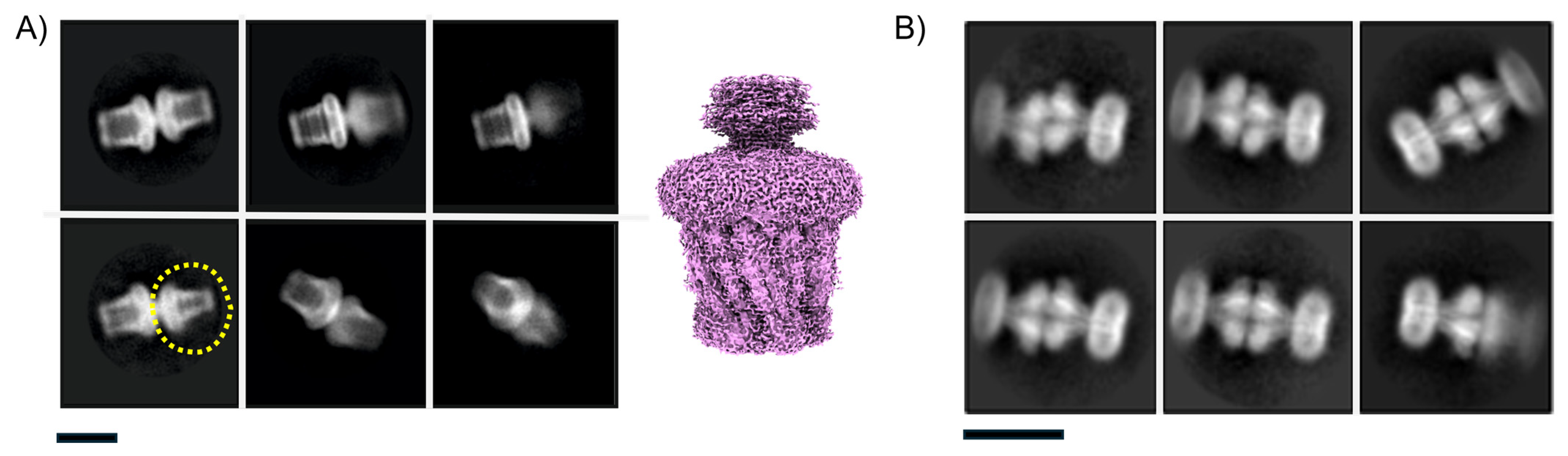
3.4. Issue 4: Projection Contamination from Detergent-Driven Oligomerisation
3.5. Issue 5: Irregular Micelle Insertions
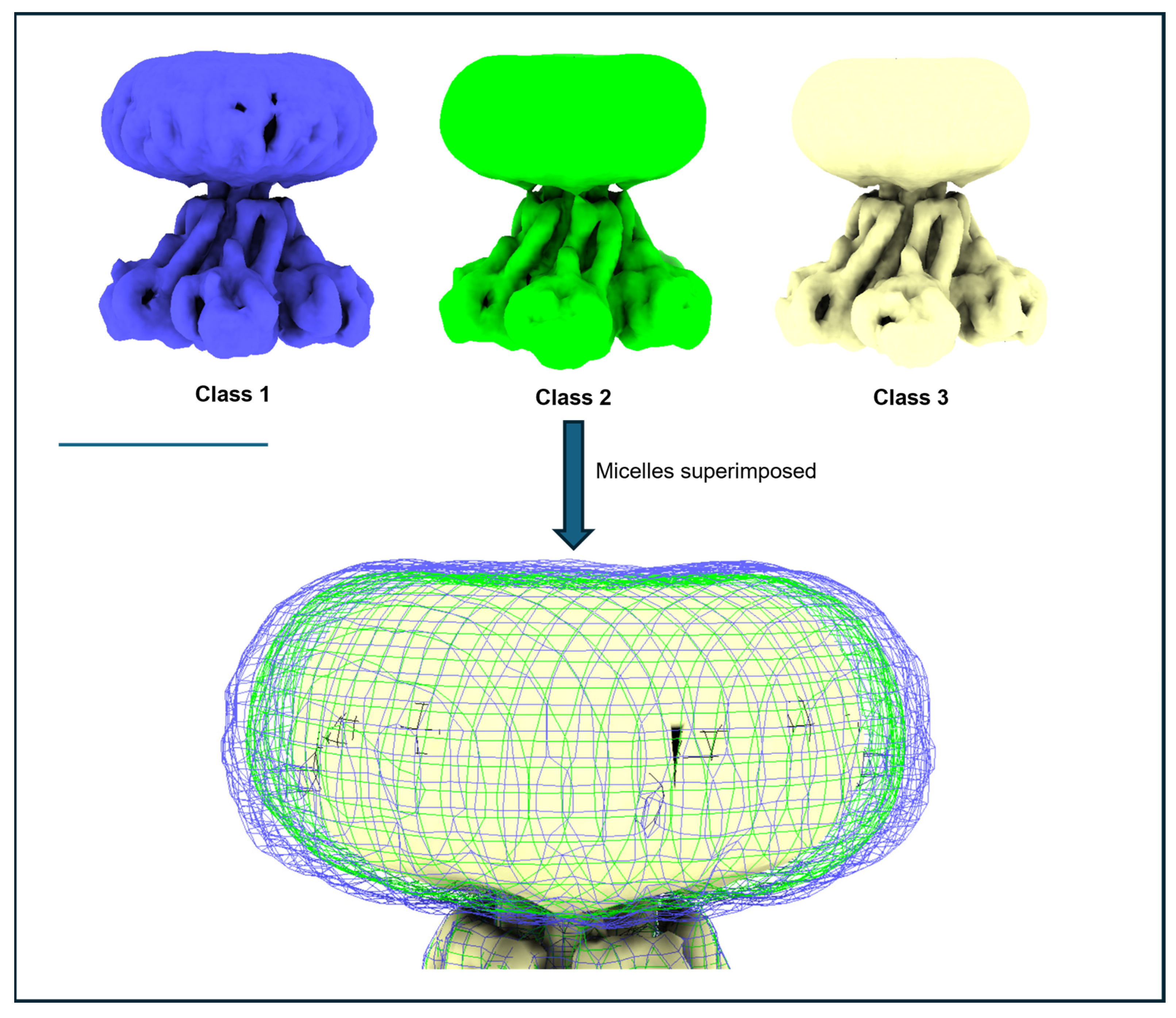
3.6. Issue 6: Variable Micelle Dimensions in 3D Structures
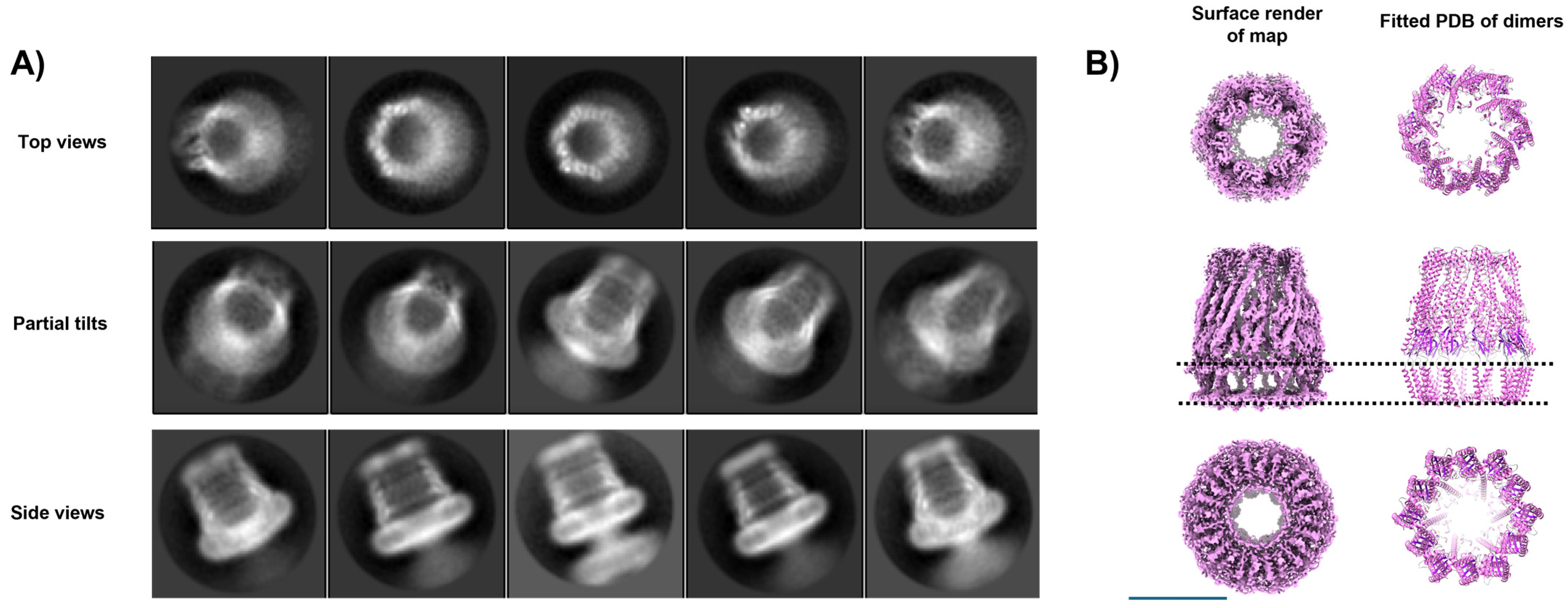
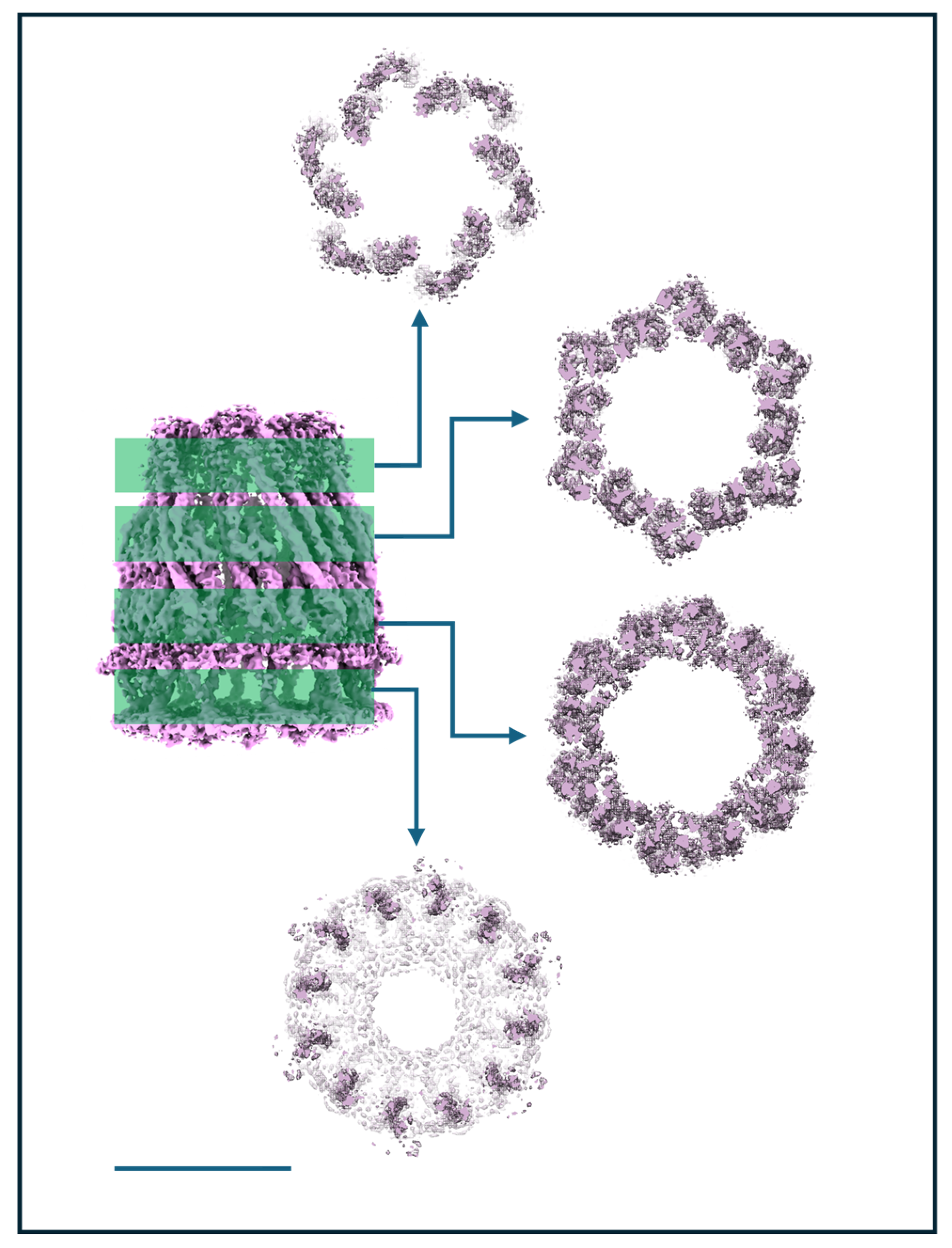
3.7. Issue 7: Unusual Symmetries
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, S.; Armache, J.P.; Cheng, Y. Single-particle cryo-EM data acquisition by using direct electron detection camera. Microscopy 2016, 65, 35–41. [Google Scholar] [CrossRef]
- Chari, A.; Stark, H. Prospects and Limitations of High-Resolution Single-Particle Cryo-Electron Microscopy. Annu. Rev. Biophys. 2023, 52, 391–411. [Google Scholar] [CrossRef]
- Burt, A.; Toader, B.; Warshamanage, R.; von Kügelgen, A.; Pyle, E.; Zivanov, J.; Kimanius, D.; Bharat, T.A.M.; Scheres, S.H.W. An image processing pipeline for electron cryo-tomography in RELION-5. FEBS Open Bio. 2024, 14, 1788–1804. [Google Scholar] [CrossRef]
- Grant, T.; Rohou, A.; Grigorieff, N. cisTEM, user-friendly software for single-particle image processing. Elife 2018, 7, e35383. [Google Scholar] [CrossRef] [PubMed]
- Kimanius, D.; Dong, L.; Sharov, G.; Nakane, T.; Scheres, S.H.W. New tools for automated cryo-EM single-particle analysis in RELION-4.0. Biochem. J. 2021, 478, 4169–4185. [Google Scholar] [CrossRef] [PubMed]
- Punjani, A.; Rubinstein, J.L.; Fleet, D.J.; Brubaker, M.A. cryoSPARC: Algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 2017, 14, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Střelák, D.; Marchán, D.; Carazo, J.M.; CO, S.S. Performance and Quality Comparison of Movie Alignment Software for Cryogenic Electron Microscopy. Micromachines 2023, 14, 1835. [Google Scholar] [CrossRef]
- Robajac, D.; Zámorová, M.; Katrlík, J.; Miković, Ž.; Nedić, O. Screening for the best detergent for the isolation of placental membrane proteins. Int. J. Biol. Macromol. 2017, 102, 431–437. [Google Scholar] [CrossRef]
- Dunstone, M.A.; de Marco, A. Cryo-electron tomography: An ideal method to study membrane-associated proteins. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017, 372, 20160210. [Google Scholar] [CrossRef]
- Neuhaus, A.; Selvaraj, M.; Salzer, R.; Langer, J.D.; Kruse, K.; Kirchner, L.; Sanders, K.; Daum, B.; Averhoff, B.; Gold, V.A.M. Cryo-electron microscopy reveals two distinct type IV pili assembled by the same bacterium. Nat. Commun. 2020, 11, 2231. [Google Scholar] [CrossRef]
- Li, M.; Ma, J.; Li, X.; Sui, S.F. In situ cryo-ET structure of phycobilisome-photosystem II supercomplex from red alga. Elife 2021, 10, e69635. [Google Scholar] [CrossRef]
- Anandan, A.; Vrielink, A. Detergents in Membrane Protein Purification and Crystallisation. Adv. Exp. Med. Biol. 2016, 922, 13–28. [Google Scholar]
- Lichtenberg, D.; Ahyayauch, H.; Goñi, F.M. The mechanism of detergent solubilization of lipid bilayers. Biophys. J. 2013, 105, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Le Maire, M.; Champeil, P.; Moller, J.V. Interaction of membrane proteins and lipids with solubilizing detergents. Biochim. Biophys. Acta 2000, 1508, 86–111. [Google Scholar] [CrossRef] [PubMed]
- Oliver, R.C.; Lipfert, J.; Fox, D.A.; Lo, R.H.; Doniach, S.; Columbus, L. Dependence of micelle size and shape on detergent alkyl chain length and head group. PLoS ONE 2013, 8, e62488. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Kalur, G.C.; Ziserman, L.; Danino, D.; Raghavan, S.R. Wormlike micelles of a C22-tailed zwitterionic betaine surfactant: From viscoelastic solutions to elastic gels. Langmuir 2007, 23, 12849–12856. [Google Scholar] [CrossRef]
- Arnold, T.; Linke, D. The use of detergents to purify membrane proteins. Curr. Protoc. Protein Sci. 2008, 53, 4.8.1–4.8.30. [Google Scholar] [CrossRef]
- Otzen, D. Protein-surfactant interactions: A tale of many states. Biochim. Biophys. Acta 2011, 1814, 562–591. [Google Scholar] [CrossRef]
- Cho, K.H.; Husri, M.; Amin, A.; Gotfryd, K.; Lee, H.J.; Go, J.; Kim, J.W.; Loland, C.J.; Guan, L.; Byrne, B.; et al. Maltose neopentyl glycol-3 (MNG-3) analogues for membrane protein study. Analyst 2015, 140, 3157–3163. [Google Scholar] [CrossRef][Green Version]
- Chae, P.S.; Rasmussen, S.G.; Rana, R.R.; Gotfryd, K.; Chandra, R.; Goren, M.A.; Kruse, A.C.; Nurva, S.; Loland, C.J.; Pierre, Y.; et al. Maltose-neopentyl glycol (MNG) amphiphiles for solubilization, stabilization and crystallization of membrane proteins. Nat. Methods 2010, 7, 1003–1008. [Google Scholar] [CrossRef]
- Doré, A.S.; Robertson, N.; Errey, J.C.; Ng, I.; Hollenstein, K.; Tehan, B.; Hurrell, E.; Bennett, K.; Congreve, M.; Magnani, F.; et al. Structure of the adenosine A(2A) receptor in complex with ZM241385 and the xanthines XAC and caffeine. Structure 2011, 19, 1283–1293. [Google Scholar] [CrossRef] [PubMed]
- Billesbølle, C.B.; de March, C.A.; van der Velden, W.J.C.; Ma, N.; Tewari, J.; Del Torrent, C.L.; Li, L.; Faust, B.; Vaidehi, N.; Matsunami, H.; et al. Structural basis of odorant recognition by a human odorant receptor. Nature 2023, 615, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Youn, T.; Kim, G.; Hariharan, P.; Li, X.; Ahmed, W.; Byrne, B.; Liu, X.; Guan, L.; Chae, P.S. Improved Pendant-Bearing Glucose-Neopentyl Glycols for Membrane Protein Stability. Bioconjug Chem. 2025, 36, 707–717. [Google Scholar] [CrossRef]
- Miyashita, Y.; Moriya, T.; Kato, T.; Kawasaki, M.; Yasuda, S.; Adachi, N.; Suzuki, K.; Ogasawara, S.; Saito, T.; Senda, T.; et al. Improved higher resolution cryo-EM structures reveal the binding modes of hERG channel inhibitors. Structure 2024, 32, 1926–1935.e3. [Google Scholar] [CrossRef]
- Choy, B.C.; Cater, R.J.; Mancia, F.; Pryor, E.E., Jr. A 10-year meta-analysis of membrane protein structural biology: Detergents, membrane mimetics, and structure determination techniques. Biochim. Biophys. Acta Biomembr. 2021, 1863, 183533. [Google Scholar] [CrossRef]
- Hutchison, J.M.; Lu, Z.; Li, G.C.; Travis, B.; Mittal, R.; Deatherage, C.L.; Sanders, C.R. Dodecyl-β-melibioside Detergent Micelles as a Medium for Membrane Proteins. Biochemistry 2017, 56, 5481–5484. [Google Scholar] [CrossRef]
- Bill, R.M.; Henderson, P.J.; Iwata, S.; Kunji, E.R.; Michel, H.; Neutze, R.; Newstead, S.; Poolman, B.; Tate, C.G.; Vogel, H. Overcoming barriers to membrane protein structure determination. Nat. Biotechnol. 2011, 29, 335–340. [Google Scholar] [CrossRef]
- Efremov, R.G.; Gatsogiannis, C.; Raunser, S. Lipid Nanodiscs as a Tool for High-Resolution Structure Determination of Membrane Proteins by Single-Particle Cryo-EM. Methods Enzymol. 2017, 594, 1–30. [Google Scholar]
- Hiotis, G.; Notti, R.Q.; Bao, H.; Walz, T. Nanodiscs remain indispensable for Cryo-EM studies of membrane proteins. Curr. Opin. Struct. Biol. 2025, 92, 103042. [Google Scholar] [CrossRef]
- Knowles, T.J.; Finka, R.; Smith, C.; Lin, Y.P.; Dafforn, T.; Overduin, M. Membrane proteins solubilized intact in lipid containing nanoparticles bounded by styrene maleic acid copolymer. J. Am. Chem. Soc. 2009, 131, 7484–7485. [Google Scholar] [CrossRef]
- Guo, Y. Detergent-free systems for structural studies of membrane proteins. Biochem. Soc. Trans. 2021, 49, 1361–1374. [Google Scholar] [CrossRef] [PubMed]
- Dimitrova, V.S.; Song, S.; Karagiaridi, A.; Marand, A.; Pinkett, H.W. Detergent Alternatives: Membrane Protein Purification Using Synthetic Nanodisc Polymers. Methods Mol. Biol. 2022, 2507, 375–387. [Google Scholar] [PubMed]
- Broadbent, L.; Depping, P.; Lodé, A.; Vaitsopoulou, A.; Hardy, D.; Ayub, H.; Mitchell-White, J.; Kerr, I.D.; Goddard, A.D.; Bill, R.M.; et al. Detergent-Free Membrane Protein Purification Using SMA Polymer. Methods Mol. Biol. 2022, 2507, 389–404. [Google Scholar] [PubMed]
- Bayburt, T.H.; Grinkova, Y.V.; Sligar, S.G. Assembly of single bacteriorhodopsin trimers in bilayer nanodiscs. Arch. Biochem. Biophys. 2006, 450, 215–222. [Google Scholar] [CrossRef]
- Denisov, I.G.; Sligar, S.G. Nanodiscs in Membrane Biochemistry and Biophysics. Chem. Rev. 2017, 117, 4669–4713. [Google Scholar] [CrossRef]
- Carlson, M.L.; Young, J.W.; Zhao, Z.; Fabre, L.; Jun, D.; Li, J.; Li, J.; Dhupar, H.S.; Wason, I.; Mills, A.T.; et al. The Peptidisc, a simple method for stabilizing membrane proteins in detergent-free solution. Elife 2018, 7, e34085. [Google Scholar] [CrossRef]
- Frauenfeld, J.; Löving, R.; Armache, J.P.; Sonnen, A.F.; Guettou, F.; Moberg, P.; Zhu, L.; Jegerschöld, C.; Flayhan, A.; Briggs, J.A.; et al. A saposin-lipoprotein nanoparticle system for membrane proteins. Nat. Methods 2016, 13, 345–351. [Google Scholar] [CrossRef]
- Mostafavi, S.; Custódio, T.F.; Jungnickel, K.E.J.; Löw, C. Salipro technology in membrane protein research. Curr. Opin. Struct. Biol. 2025, 93, 103050. [Google Scholar] [CrossRef]
- Esmaili, M.; Overduin, M. Membrane biology visualized in nanometer-sized discs formed by styrene maleic acid polymers. Biochim. Biophys. Acta Biomembr. 2018, 1860, 257–263. [Google Scholar] [CrossRef]
- Lee, S.C.; Knowles, T.J.; Postis, V.L.; Jamshad, M.; Parslow, R.A.; Lin, Y.P.; Goldman, A.; Sridhar, P.; Overduin, M.; Muench, S.P.; et al. A method for detergent-free isolation of membrane proteins in their local lipid environment. Nat. Protoc. 2016, 11, 1149–1162. [Google Scholar] [CrossRef]
- Dörr, J.M.; Koorengevel, M.C.; Schäfer, M.; Prokofyev, A.V.; Scheidelaar, S.; van der Cruijsen, E.A.; Dafforn, T.R.; Baldus, M.; Killian, J.A. Detergent-free isolation, characterization, and functional reconstitution of a tetrameric K+ channel: The power of native nanodiscs. Proc. Natl. Acad. Sci. USA 2014, 111, 18607–18612. [Google Scholar] [CrossRef]
- Jamshad, M.; Charlton, J.; Lin, Y.P.; Routledge, S.J.; Bawa, Z.; Knowles, T.J.; Overduin, M.; Dekker, N.; Dafforn, T.R.; Bill, R.M.; et al. G-protein coupled receptor solubilization and purification for biophysical analysis and functional studies, in the total absence of detergent. Biosci. Rep. 2015, 35, e00188. [Google Scholar] [CrossRef] [PubMed]
- Gulati, S.; Jamshad, M.; Knowles, T.J.; Morrison, K.A.; Downing, R.; Cant, N.; Collins, R.; Koenderink, J.B.; Ford, R.C.; Overduin, M.; et al. Detergent-free purification of ABC (ATP-binding-cassette) transporters. Biochem. J. 2014, 461, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Swainsbury, D.J.K.; Hawkings, F.R.; Martin, E.C.; Musiał, S.; Salisbury, J.H.; Jackson, P.J.; Farmer, D.A.; Johnson, M.P.; Siebert, C.A.; Hitchcock, A.; et al. Cryo-EM structure of the four-subunit Rhodobacter sphaeroides cytochrome bc(1) complex in styrene maleic acid nanodiscs. Proc. Natl. Acad. Sci. USA 2023, 120, e2217922120. [Google Scholar] [CrossRef] [PubMed]
- Orwick-Rydmark, M.; Lovett, J.E.; Graziadei, A.; Lindholm, L.; Hicks, M.R.; Watts, A. Detergent-free incorporation of a seven-transmembrane receptor protein into nanosized bilayer Lipodisq particles for functional and biophysical studies. Nano Lett. 2012, 12, 4687–4692. [Google Scholar] [CrossRef]
- Glukhov, G.; Karlova, M.; Kravchuk, E.; Glukhova, A.; Trifonova, E.; Sokolova, O.S. Purification of Potassium Ion Channels Using Styrene-Maleic Acid Copolymers. Methods Mol. Biol. 2024, 2796, 73–86. [Google Scholar]
- Oluwole, A.O.; Danielczak, B.; Meister, A.; Babalola, J.O.; Vargas, C.; Keller, S. Solubilization of Membrane Proteins into Functional Lipid-Bilayer Nanodiscs Using a Diisobutylene/Maleic Acid Copolymer. Angew. Chem. Int. Ed. Engl. 2017, 56, 1919–1924. [Google Scholar] [CrossRef]
- Gulamhussein, A.A.; Uddin, R.; Tighe, B.J.; Poyner, D.R.; Rothnie, A.J. A comparison of SMA (styrene maleic acid) and DIBMA (di-isobutylene maleic acid) for membrane protein purification. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183281. [Google Scholar] [CrossRef]
- Parmar, M.; Rawson, S.; Scarff, C.A.; Goldman, A.; Dafforn, T.R.; Muench, S.P.; Postis, V.L.G. Using a SMALP platform to determine a sub-nm single particle cryo-EM membrane protein structure. Biochim. Biophys. Acta Biomembr. 2018, 1860, 378–383. [Google Scholar]
- Sun, C.; Gennis, R.B. Single-particle cryo-EM studies of transmembrane proteins in SMA copolymer nanodiscs. Chem. Phys. Lipids 2019, 221, 114–119. [Google Scholar] [CrossRef]
- Hesketh, S.J.; Klebl, D.P.; Higgins, A.J.; Thomsen, M.; Pickles, I.B.; Sobott, F.; Sivaprasadarao, A.; Postis, V.L.G.; Muench, S.P. Styrene maleic-acid lipid particles (SMALPs) into detergent or amphipols: An exchange protocol for membrane protein characterisation. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183192. [Google Scholar] [CrossRef] [PubMed]
- Kuyler, G.C.; Barnard, E.; Sridhar, P.; Murray, R.J.; Pollock, N.L.; Wheatley, M.; Dafforn, T.R.; Klumperman, B. Tunable Terpolymer Series for the Systematic Investigation of Membrane Proteins. Biomacromolecules 2025, 26, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Akram, A.; Hadasha, W.; Kuyler, G.C.; Smith, M.P.; Bailey-Dallaway, S.; Preedy, A.; Browne, C.; Broadbent, L.; Hill, A.; Javaid, T.; et al. Solubilisation & purification of membrane proteins using benzylamine-modified SMA polymers. Biophys. Chem. 2025, 316, 107343. [Google Scholar]
- Danielczak, B.; Rasche, M.; Lenz, J.; Pérez Patallo, E.; Weyrauch, S.; Mahler, F.; Agbadaola, M.T.; Meister, A.; Babalola, J.O.; Vargas, C.; et al. A bioinspired glycopolymer for capturing membrane proteins in native-like lipid-bilayer nanodiscs. Nanoscale 2022, 14, 1855–1867. [Google Scholar] [CrossRef]
- Kuyler, G.C.; Barnard, E.; Cunningham, R.D.; Sibariboyi, S.; White, L.; Wessels, I.; Smith, M.P.; Motloung, B.; Klumperman, B. Amphiphilic Copolymers and Their Role in the Study of Membrane Proteins. J. Phys. Chem. Lett. 2025, 16, 5784–5799. [Google Scholar] [CrossRef]
- Yasuhara, K.; Arakida, J.; Ravula, T.; Ramadugu, S.K.; Sahoo, B.; Kikuchi, J.I.; Ramamoorthy, A. Spontaneous Lipid Nanodisc Fomation by Amphiphilic Polymethacrylate Copolymers. J. Am. Chem. Soc. 2017, 139, 18657–18663. [Google Scholar] [CrossRef]
- Krishnarjuna, B.; Ramamoorthy, A. Detergent-Free Isolation of Membrane Proteins and Strategies to Study Them in a Near-Native Membrane Environment. Biomolecules 2022, 12, 1076. [Google Scholar] [CrossRef]
- Simon, K.S.; Pollock, N.L.; Lee, S.C. Membrane protein nanoparticles: The shape of things to come. Biochem. Soc. Trans. 2018, 46, 1495–1504. [Google Scholar] [CrossRef]
- Zoonens, M.; Popot, J.L. Amphipols for each season. J. Membr. Biol. 2014, 247, 759–796. [Google Scholar] [CrossRef]
- Tribet, C.; Audebert, R.; Popot, J.L. Amphipols: Polymers that keep membrane proteins soluble in aqueous solutions. Proc. Natl. Acad. Sci. USA 1996, 93, 15047–15050. [Google Scholar] [CrossRef]
- Le Bon, C.; Marconnet, A.; Masscheleyn, S.; Popot, J.L.; Zoonens, M. Folding and stabilizing membrane proteins in amphipol A8-35. Methods 2018, 147, 95–105. [Google Scholar] [CrossRef]
- Higgins, A.J.; Flynn, A.J.; Marconnet, A.; Musgrove, L.J.; Postis, V.L.G.; Lippiat, J.D.; Chung, C.W.; Ceska, T.; Zoonens, M.; Sobott, F.; et al. Cycloalkane-modified amphiphilic polymers provide direct extraction of membrane proteins for CryoEM analysis. Commun. Biol. 2021, 4, 1337. [Google Scholar]
- Michon, B.; López-Sánchez, U.; Degrouard, J.; Nury, H.; Leforestier, A.; Rio, E.; Salonen, A.; Zoonens, M. Role of surfactants in electron cryo-microscopy film preparation. Biophys. J. 2023, 122, 1846–1857. [Google Scholar] [CrossRef] [PubMed]
- Kean, J.; Cleverley, R.M.; O’Ryan, L.; Ford, R.C.; Prince, S.M.; Derrick, J.P. Characterization of a CorA Mg2+ transport channel from Methanococcus jannaschii using a Thermofluor-based stability assay. Mol. Membr. Biol. 2008, 25, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Collins, R.F.; Kargas, V.; Clarke, B.R.; Siebert, C.A.; Clare, D.K.; Bond, P.J.; Whitfield, C.; Ford, R.C. Full-length, Oligomeric Structure of Wzz Determined by Cryoelectron Microscopy Reveals Insights into Membrane-Bound States. Structure 2017, 25, 806–815.e3. [Google Scholar] [CrossRef] [PubMed]
- Ripstein, Z.A.; Rubinstein, J.L. Processing of Cryo-EM Movie Data. Methods Enzymol. 2016, 579, 103–124. [Google Scholar]
- Rohou, A.; Grigorieff, N. CTFFIND4: Fast and accurate defocus estimation from electron micrographs. J. Struct. Biol. 2015, 192, 216–221. [Google Scholar] [CrossRef]
- Bepler, T.; Kelley, K.; Noble, A.J.; Berger, B. Topaz-Denoise: General deep denoising models for cryoEM and cryoET. Nat. Commun. 2020, 11, 5208. [Google Scholar]
- Kucukelbir, A.; Sigworth, F.J.; Tagare, H.D. Quantifying the local resolution of cryo-EM density maps. Nat. Methods 2014, 11, 63–65. [Google Scholar]
- Adams, P.D.; Afonine, P.V.; Bunkóczi, G.; Chen, V.B.; Davis, I.W.; Echols, N.; Headd, J.J.; Hung, L.W.; Kapral, G.J.; Grosse-Kunstleve, R.W.; et al. PHENIX: A comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 2010, 66, 213–221. [Google Scholar]
- Niebling, S.; Burastero, O.; García-Alai, M. Biophysical Characterization of Membrane Proteins. Methods Mol. Biol. 2023, 2652, 215–230. [Google Scholar] [PubMed]
- Newstead, S.; Ferrandon, S.; Iwata, S. Rationalizing alpha-helical membrane protein crystallization. Protein Sci. 2008, 17, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Olerinyova, A.; Sonn-Segev, A.; Gault, J.; Eichmann, C.; Schimpf, J.; Kopf, A.H.; Rudden, L.S.P.; Ashkinadze, D.; Bomba, R.; Frey, L.; et al. Mass Photometry of Membrane Proteins. Chem 2021, 7, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Timmins, P.; Pebay-Peyroula, E.; Welte, W. Detergent organisation in solutions and in crystals of membrane proteins. Biophys. Chem. 1994, 53, 27–36. [Google Scholar] [CrossRef]
- Passmore, L.A.; Russo, C.J. Specimen Preparation for High-Resolution Cryo-EM. Methods Enzymol. 2016, 579, 51–86. [Google Scholar]
- Quispe, J.; Damiano, J.; Mick, S.E.; Nackashi, D.P.; Fellmann, D.; Ajero, T.G.; Carragher, B.; Potter, C.S. An improved holey carbon film for cryo-electron microscopy. Microsc. Microanal. 2007, 13, 365–371. [Google Scholar] [CrossRef]
- Helenius, A.; Simons, K. Solubilization of membranes by detergents. Biochim. Biophys. Acta 1975, 415, 29–79. [Google Scholar] [CrossRef]
- Bangham, J.A.; Lea, E.J. The interaction of detergents with bilayer lipid membranes. Biochim. Biophys. Acta 1978, 511, 388–396. [Google Scholar] [CrossRef]
- Sgro, G.G.; Costa, T.R.D. Cryo-EM Grid Preparation of Membrane Protein Samples for Single Particle Analysis. Front. Mol. Biosci. 2018, 5, 74. [Google Scholar] [CrossRef]
- Bai, X.C.; Fernandez, I.S.; McMullan, G.; Scheres, S.H. Ribosome structures to near-atomic resolution from thirty thousand cryo-EM particles. Elife 2013, 2, e00461. [Google Scholar] [CrossRef]
- Kampjut, D.; Steiner, J.; Sazanov, L.A. Cryo-EM grid optimization for membrane proteins. iScience 2021, 24, 102139. [Google Scholar] [CrossRef]
- Iancu, C.V.; Tivol, W.F.; Schooler, J.B.; Dias, D.P.; Henderson, G.P.; Murphy, G.E.; Wright, E.R.; Li, Z.; Yu, Z.; Briegel, A.; et al. Electron cryotomography sample preparation using the Vitrobot. Nat. Protoc. 2006, 1, 2813–2819. [Google Scholar] [CrossRef]
- Naydenova, K.; Russo, C.J. Integrated wafer-scale manufacturing of electron cryomicroscopy specimen supports. Ultramicroscopy 2022, 232, 113396. [Google Scholar] [CrossRef]
- Levitz, T.S.; Weckener, M.; Fong, I.; Naismith, J.H.; Drennan, C.L.; Brignole, E.J.; Clare, D.K.; Darrow, M.C. Approaches to Using the Chameleon: Robust, Automated, Fast-Plunge cryoEM Specimen Preparation. Front. Mol. Biosci. 2022, 9, 903148. [Google Scholar] [CrossRef]
- Koning, R.I.; Vader, H.; van Nugteren, M.; Grocutt, P.A.; Yang, W.; Renault, L.L.R.; Koster, A.J.; Kamp, A.C.F.; Schwertner, M. Automated vitrification of cryo-EM samples with controllable sample thickness using suction and real-time optical inspection. Nat. Commun. 2022, 13, 2985. [Google Scholar] [CrossRef]
- Jain, T.; Sheehan, P.; Crum, J.; Carragher, B.; Potter, C.S. Spotiton: A prototype for an integrated inkjet dispense and vitrification system for cryo-TEM. J. Struct. Biol. 2012, 179, 68–75. [Google Scholar] [CrossRef]
- Gewering, T.; Januliene, D.; Ries, A.B.; Moeller, A. Know your detergents: A case study on detergent background in negative stain electron microscopy. J. Struct. Biol. 2018, 203, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Arachea, B.T.; Sun, Z.; Potente, N.; Malik, R.; Isailovic, D.; Viola, R.E. Detergent selection for enhanced extraction of membrane proteins. Protein Expr. Purif. 2012, 86, 12–20. [Google Scholar] [CrossRef]
- Hauer, F.; Gerle, C.; Fischer, N.; Oshima, A.; Shinzawa-Itoh, K.; Shimada, S.; Yokoyama, K.; Fujiyoshi, Y.; Stark, H. GraDeR: Membrane Protein Complex Preparation for Single-Particle Cryo-EM. Structure 2015, 23, 1769–1775. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Zhang, X.; Snee, M.; Lockhart-Cairns, M.P.; Levy, C.W.; Jowitt, T.A.; Birchenough, H.L.; Dean, L.; Collins, R.; Dodd, R.J.; et al. The structural organisation of pentraxin-3 and its interactions with heavy chains of inter-α-inhibitor regulate crosslinking of the hyaluronan matrix. Matrix Biol. 2025, 136, 52–68. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Menon, A.K. Transbilayer lipid asymmetry. Curr. Biol. 2018, 28, R386–R391. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, Z.; Csanády, L.; Gadsby, D.C.; Chen, J. Molecular Structure of the Human CFTR Ion Channel. Cell 2017, 169, 85–95.e8. [Google Scholar] [CrossRef] [PubMed]
- Jafurulla, M.; Chattopadhyay, A. Structural Stringency of Cholesterol for Membrane Protein Function Utilizing Stereoisomers as Novel Tools: A Review. Methods Mol. Biol. 2017, 1583, 21–39. [Google Scholar] [PubMed]
- Poitevin, F.; Kushner, A.; Li, X.; Dao Duc, K. Structural Heterogeneities of the Ribosome: New Frontiers and Opportunities for Cryo-EM. Molecules 2020, 25, 4262. [Google Scholar] [CrossRef]
- Whitfield, C.; Williams, D.M.; Kelly, S.D. Lipopolysaccharide O-antigens-bacterial glycans made to measure. J. Biol. Chem. 2020, 295, 10593–10609. [Google Scholar] [CrossRef]
- Weckener, M.; Woodward, L.S.; Clarke, B.R.; Liu, H.; Ward, P.N.; Le Bas, A.; Bhella, D.; Whitfield, C.; Naismith, J.H. The lipid linked oligosaccharide polymerase Wzy and its regulating co-polymerase, Wzz, from enterobacterial common antigen biosynthesis form a complex. Open Biol. 2023, 13, 220373. [Google Scholar] [CrossRef]
- Wiseman, B.; Nitharwal, R.G.; Widmalm, G.; Högbom, M. Structure of a full-length bacterial polysaccharide co-polymerase. Nat. Commun. 2021, 12, 369. [Google Scholar] [CrossRef]
- Collins, R.F.; Beis, K.; Dong, C.; Botting, C.H.; McDonnell, C.; Ford, R.C.; Clarke, B.R.; Whitfield, C.; Naismith, J.H. The 3D structure of a periplasm-spanning platform required for assembly of group 1 capsular polysaccharides in Escherichia coli. Proc. Natl. Acad. Sci. USA 2007, 104, 2390–2395. [Google Scholar] [CrossRef]
- Felix, J.; De Munck, S.; Bazan, J.F.; Savvides, S.N. Overcoming cryo-EM map anisotropy reveals ALK-cytokine assemblies with distinct stoichiometries. bioRxiv 2025. [Google Scholar] [CrossRef]
- Youn, T.; Yoon, S.; Byrne, B.; Chae, P.S. Foldable Detergents for Membrane Protein Stability. Chembiochem 2022, 23, e202200276. [Google Scholar] [CrossRef]
- Yoon, S.; Bae, H.E.; Hariharan, P.; Nygaard, A.; Lan, B.; Woubshete, M.; Sadaf, A.; Liu, X.; Loland, C.J.; Byrne, B.; et al. Rational Approach to Improve Detergent Efficacy for Membrane Protein Stabilization. Bioconjug Chem. 2024, 35, 223–231. [Google Scholar] [CrossRef]
- Lee, H.S.; Das, M.; Mahler, F.; Ahmed, W.; Wang, H.; Mortensen, J.S.; Hariharan, P.; Ghani, L.; Byrne, B.; Guan, L.; et al. 3,4-Bis(hydroxymethyl)hexane-1,6-diol-based Maltosides (HDMs) for Membrane-Protein Study: Importance of Detergent Rigidity-Flexibility Balance in Protein Stability. Chem. Asian J. 2022, 17, e202200941. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, B.; Harrison, P.; Kargas, V.; Pollock, N.; Ford, R.C.; Prince, S.M.; Collins, R.F. Coming Clean and Avoiding Bubble Trouble–Using Detergents Wisely in the Purification of Membrane Proteins for Cryo-EM Studies. Biomolecules 2025, 15, 1315. https://doi.org/10.3390/biom15091315
Chen B, Harrison P, Kargas V, Pollock N, Ford RC, Prince SM, Collins RF. Coming Clean and Avoiding Bubble Trouble–Using Detergents Wisely in the Purification of Membrane Proteins for Cryo-EM Studies. Biomolecules. 2025; 15(9):1315. https://doi.org/10.3390/biom15091315
Chicago/Turabian StyleChen, Bowen, Peter Harrison, Vasileios Kargas, Naomi Pollock, Robert C. Ford, Stephen M. Prince, and Richard F. Collins. 2025. "Coming Clean and Avoiding Bubble Trouble–Using Detergents Wisely in the Purification of Membrane Proteins for Cryo-EM Studies" Biomolecules 15, no. 9: 1315. https://doi.org/10.3390/biom15091315
APA StyleChen, B., Harrison, P., Kargas, V., Pollock, N., Ford, R. C., Prince, S. M., & Collins, R. F. (2025). Coming Clean and Avoiding Bubble Trouble–Using Detergents Wisely in the Purification of Membrane Proteins for Cryo-EM Studies. Biomolecules, 15(9), 1315. https://doi.org/10.3390/biom15091315






