Dual Perspectives on Peptide–Zinc Complexation: Highlighting Aquatic Sources While Contextualizing Other Natural Origins
Abstract
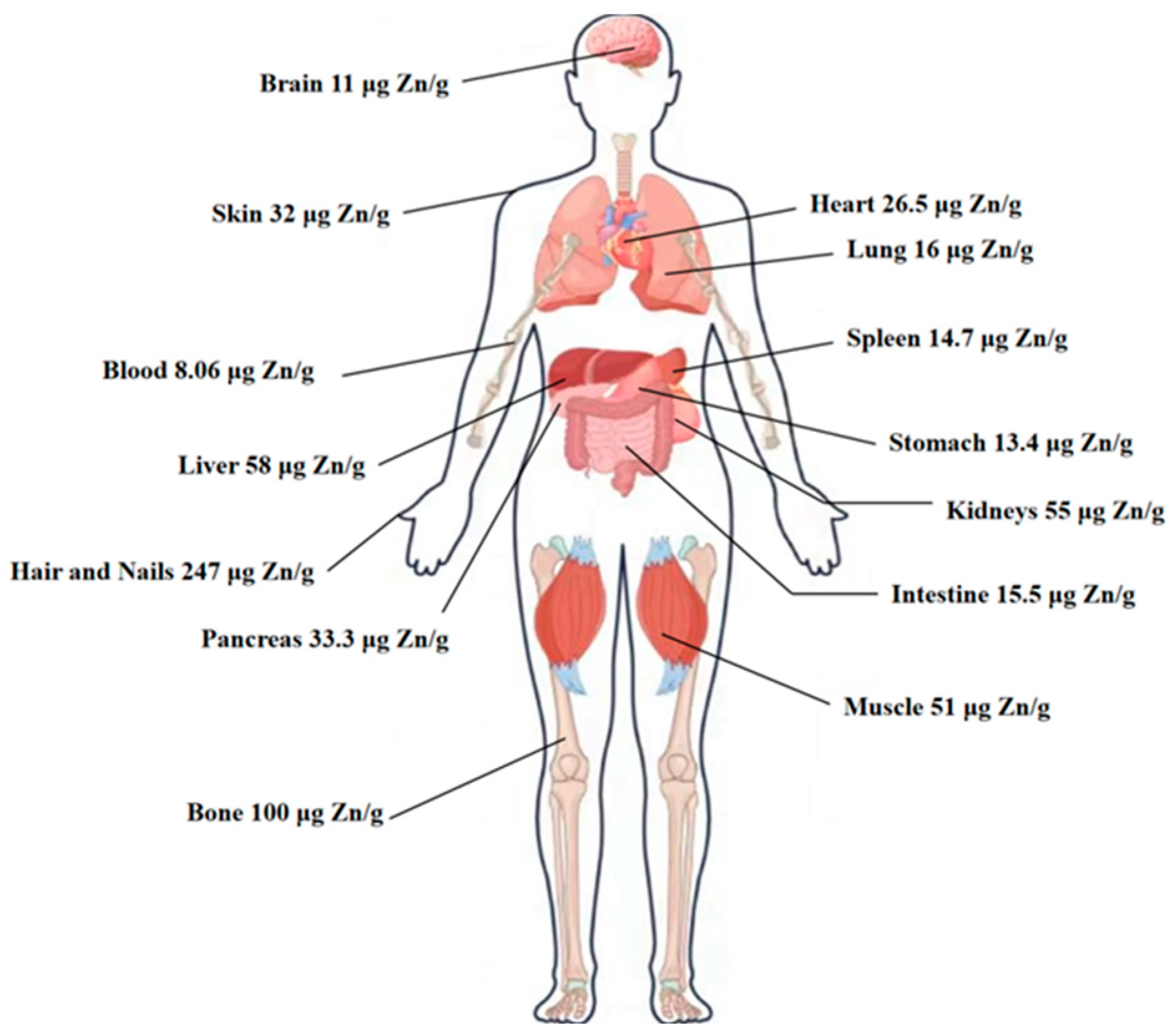
1. Introduction
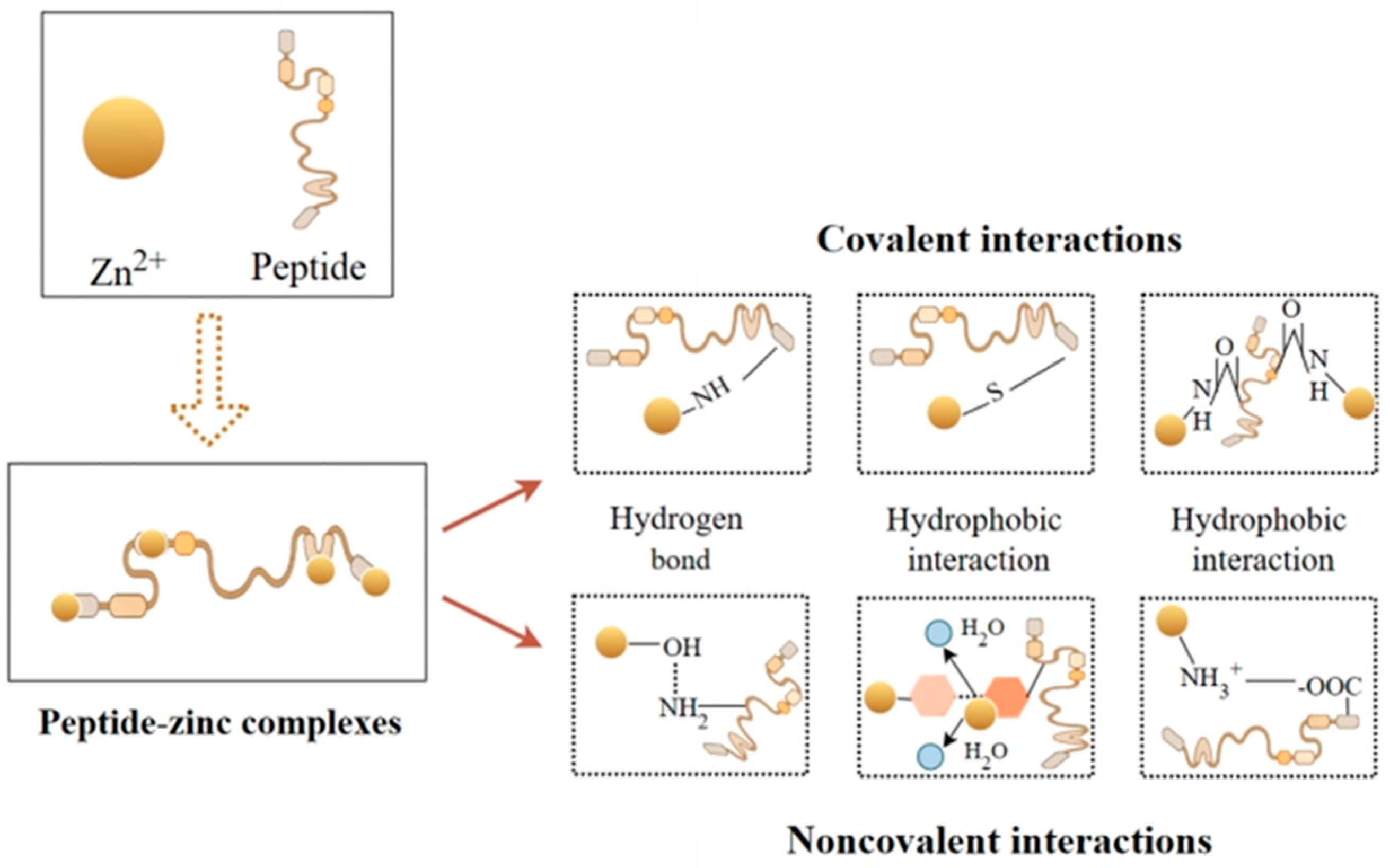
2. Peptide–Zinc Chelation Model
2.1. Theoretical Models of Peptide–Zinc Interactions
2.2. Phosphate Group–Zinc Chelation Pattern
2.3. Carboxyl–Zinc Chelation Method
2.4. The Key Role of Aromatic Amino Acids in Peptide–Zinc Coordination
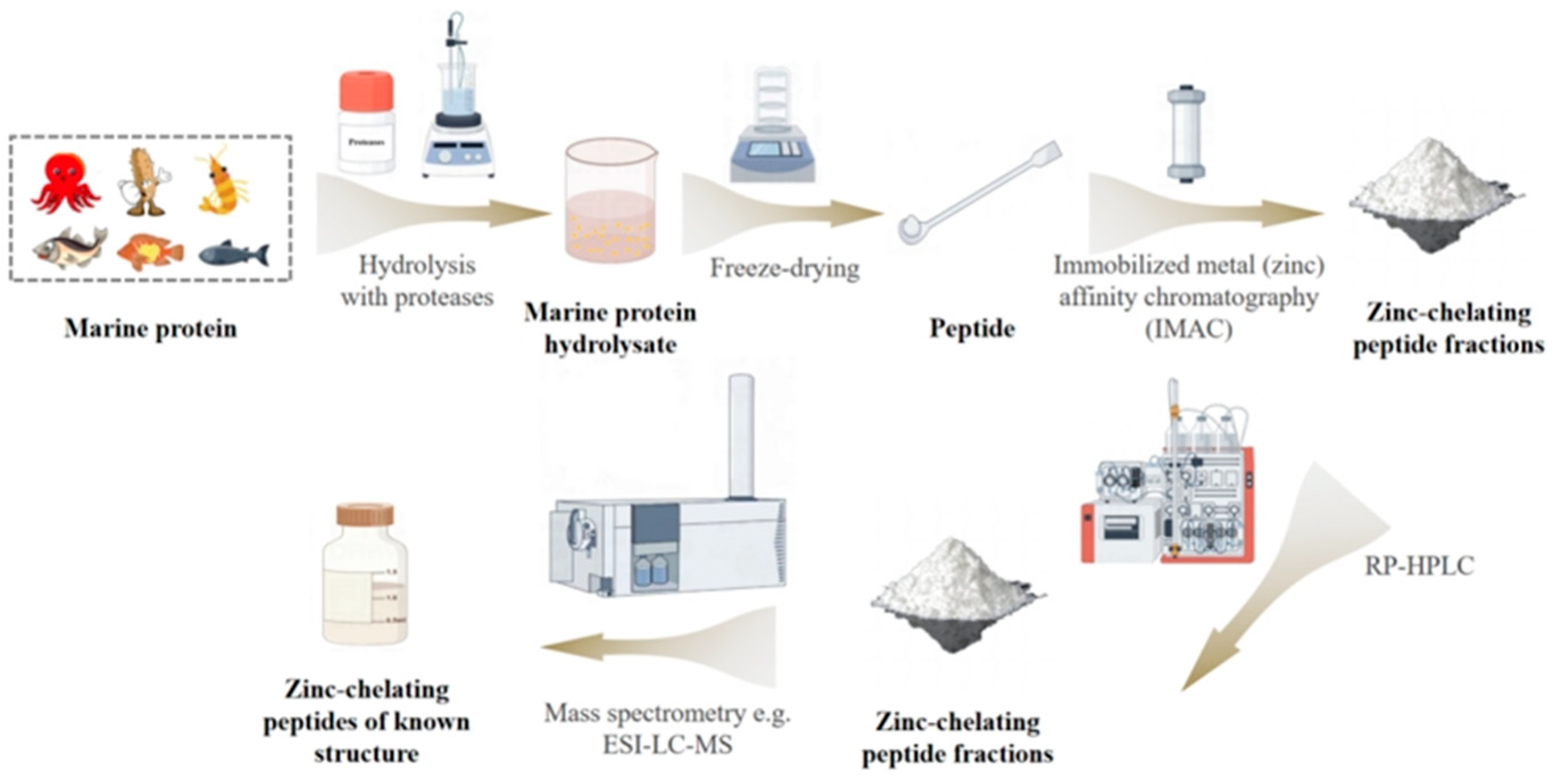
3. Production and Purification of Zinc Chelate Peptides from Proteins of Marine Origin
4. Zinc-Chelating Peptides of Marine Origin
4.1. Aquatic Sources
4.1.1. Tilapia
4.1.2. Oyster
4.1.3. Patinopecten Yessoensis
4.1.4. Alaska Pollock
4.1.5. Antarctic Krill
4.1.6. Octopus
4.1.7. Sea Cucumbers
4.1.8. Silver Carp
5. Current Insights into Peptide–Zinc Bioavailability
5.1. Role of Aquatic Proteins in Zinc Bioavailability
5.2. Recent Advances in Peptide–Zinc Interaction Studies
6. Clinical Implications of Peptide–Zinc Interactions
6.1. Peptide–Zinc Complexes in Nutritional Supplements
6.2. Therapeutic Potential of Peptide–Zinc Chelation
6.3. Clinical Trials on Peptide–Zinc Bioavailability
7. Technological Advances in Peptide–Zinc Research
7.1. Analytical Techniques for Studying Peptide–Zinc Interactions
7.2. Innovations in Peptide–Zinc Complex Synthesis
7.3. Computational Approaches to Peptide–Zinc Interaction Modeling
8. Effect of Peptides on Intestinal Zinc Absorption
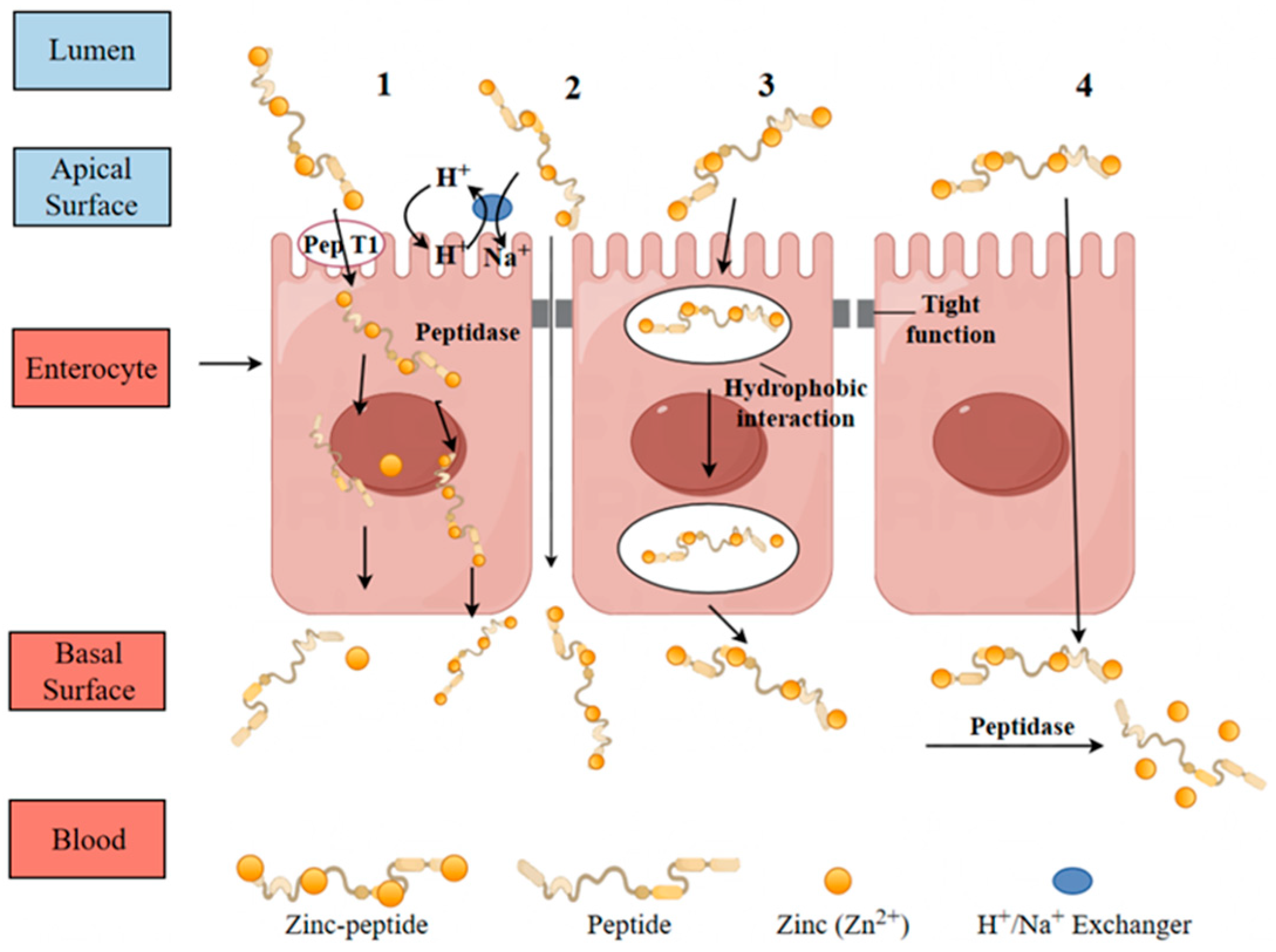
8.1. Intestinal Absorption Pathway
8.1.1. Transcellular Pathway
8.1.2. Paracellular Transport
8.2. Research Progress of Zinc Absorption Promoted by Peptides
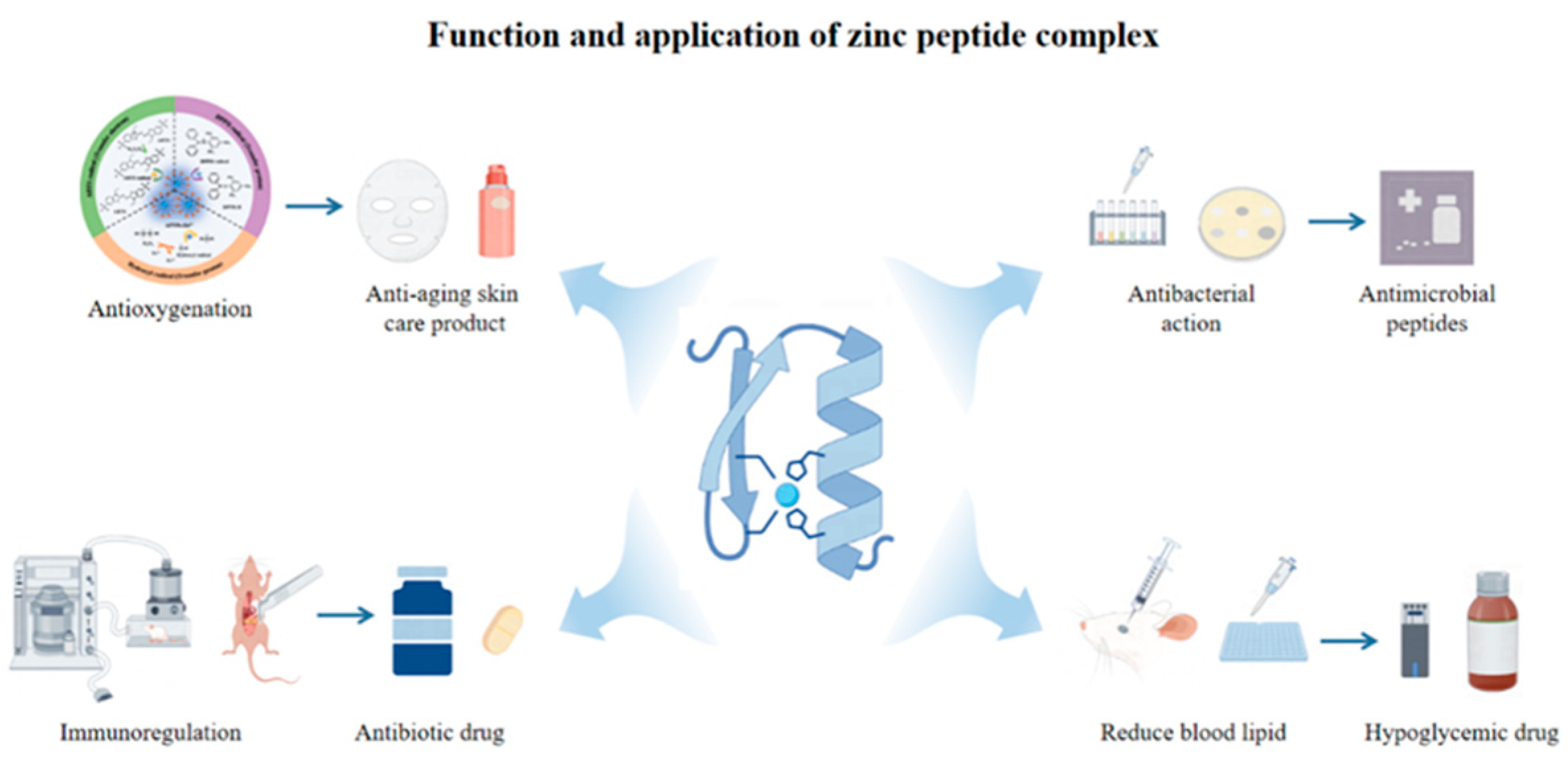
9. Function and Application of Zinc Peptide Complex
10. Conclusions and Future Trends
10.1. Emerging Trends in Peptide–Zinc Chelation Research
10.2. Prospective Applications of Peptide–Zinc Complexes
10.3. Challenges and Opportunities in Peptide–Zinc Bioavailability Research
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- King, J.C.; Brown, K.H.; Gibson, R.S.; Krebs, N.F.; Lowe, N.M.; Siekmann, J.H.; Raiten, D.J. Biomarkers of Nutrition for Development (BOND)—Zinc Review. J. Nutr. 2016, 146, 858S–885S. [Google Scholar] [CrossRef]
- Bozalioğlu, S.; Özkan, Y.; Turan, M.; Şimşek, B. Prevalence of zinc deficiency and immune response in short-term hemodialysis. J. Trace Elem. Med. Biol. 2005, 18, 243–249. [Google Scholar] [CrossRef]
- Udechukwu, M.C.; Collins, S.A.; Udenigwe, C.C. Prospects of enhancing dietary zinc bioavailability with food-derived zinc-chelating peptides. Food Funct. 2016, 7, 4137–4144. [Google Scholar] [CrossRef]
- Sun, R.; Liu, X.; Yu, Y.; Miao, J.; Leng, K.; Gao, H. Preparation process optimization, structural characterization and in vitro digestion stability analysis of Antarctic krill (Euphausia superba) peptides-zinc chelate. Food Chem. 2021, 340, 128056. [Google Scholar] [CrossRef]
- Zhang, S.Q.; Yu, X.F.; Zhang, H.B.; Peng, N.; Chen, Z.X.; Cheng, Q.; Zhang, X.L.; Cheng, S.H.; Zhang, Y. Comparison of the Oral Absorption, Distribution, Excretion, and Bioavailability of Zinc Sulfate, Zinc Gluconate, and Zinc-Enriched Yeast in Rats. Mol. Nutr. Food Res. 2018, 62, e1700981. [Google Scholar] [CrossRef]
- Rérat, A.; Nunes, C.S.; Mendy, F.; Roger, L. Amino acid absorption and production of pancreatic hormones in non-anaesthetized pigs after duodenal infusions of a milk enzymic hydrolysate or of free amino acids. Br. J. Nutr. 1988, 60, 121–136. [Google Scholar] [CrossRef]
- Meisel, H.; Frister, H. Chemical Characterization of a Caseinophosphopeptide Isolated from in vivo Digests of a Casein Diet. Biol. Chem. Hoppe-Seyler 1988, 369, 1275–1280. [Google Scholar] [CrossRef]
- Mehdi, A.; Ali, H.; Mehdi, A. Seaweed Proteins as a Source of Bioactive Peptides. Curr. Pharm. Des. 2021, 27, 1342–1352. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Zhou, Z.; Yao, X.; Fu, Y. Mineral-chelating peptides derived from fish collagen: Preparation, bioactivity and bioavailability. LWT 2020, 134, 110209. [Google Scholar] [CrossRef]
- Hu, S.; Fan, X.; Qi, P.; Zhang, X. Identification of anti-diabetes peptides from Spirulina platensis. J. Funct. Foods 2019, 56, 333–341. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, S.; Cao, R.; Hu, M. Review on bioactive peptides from Antarctic krill: From preparation to structure-activity relationship and tech-functionality. Curr. Res. Food Sci. 2025, 10, 101093. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, S.; Liu, H.; Zhang, T.; Cheng, S.; Du, M. A dual absorption pathway of novel oyster-derived peptide-zinc complex enhances zinc bioavailability and restores mitochondrial function. J. Adv. Res. 2025; in press. [Google Scholar]
- Reinhold, J.; Lahimgarzadeh, A.; Nasr, K.; Hedayati, H. Effects of Purified Phytate and Phytate-Rich Bread Upon Metabolism of Zinc, Calcium, Phosphorus, and Nitrogen in Man. Lancet 1973, 301, 283–288. [Google Scholar] [CrossRef]
- Mukhamedov, N.; Asrorov, A.; Yashinov, A.; Kayumov, M.; Wali, A.; Mirzaakhmedov, S.; Aisa, H.A.; Yili, A. Synthesis and Characterisation of Chickpea Peptides-Zinc Chelates Having ACE2 Inhibitory Activity. Protein J. 2023, 42, 547–562. [Google Scholar] [CrossRef]
- Timón, V.; Gálvez, Ó.; Maté, B.; Tanarro, I.; Herrero, V.J.; Escribano, R. Theoretical model of the interaction of glycine with hydrogenated amorphous carbon (HAC). Phys. Chem. Chem. Phys. 2015, 17, 28966–28976. [Google Scholar] [CrossRef]
- Miquel, E.; Gómez, J.Á.; Alegría, A.; Barberá, R.; Farré, R.; Recio, I. Identification of Casein Phosphopeptides Released after Simulated Digestion of Milk-Based Infant Formulas. J. Agric. Food Chem. 2005, 53, 3426–3433. [Google Scholar] [CrossRef] [PubMed]
- Prakash, D.; Lakshmi, A.J. Preparation of caseinophosphopeptides and assessing their efficacy in enhancing the bioaccessibility of iron and zinc. J. Food Sci. Technol. 2015, 52, 7493–7499. [Google Scholar] [CrossRef]
- Lönnerdal, B. Dietary Factors Influencing Zinc Absorption. J. Nutr. 2000, 130, 1378S–1383S. [Google Scholar] [CrossRef]
- Pérès, J.M.; Bouhallab, S.; Petit, C.; Bureau, F.; Maubois, J.L.; Arhan, P.; Bouglé, D. Improvement of zinc intestinal absorption and reduction of zinc/iron interaction using metal bound to the caseinophosphopeptide 1-25 of beta- casein. Reprod. Nutr. Dev. 1998, 38, 465–472. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Z.; Yin, F.; Liu, Y.; Qin, N.; Nakamura, Y.; Shahidi, F.; Yu, C.; Zhou, D.; Zhu, B. Zinc-Chelating Mechanism of Sea Cucumber (Stichopus japonicus)-Derived Synthetic Peptides. Mar. Drugs 2019, 17, 438. [Google Scholar] [CrossRef] [PubMed]
- Katimba, H.A.; Wang, R.; Cheng, C. Current findings support the potential use of bioactive peptides in enhancing zinc absorption in humans. Crit. Rev. Food Sci. Nutr. 2023, 63, 3959–3979. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, J.; Ma, X.; Liu, X.; Yin, F.; Li, D.; Nakamura, Y.; Yu, C.; Zhou, D. Characterization of a synthetic zinc-chelating peptide from sea cucumber (Stichopus japonicus) and its gastrointestinal digestion and absorption in vitro. J. Sci. Food Agric. 2022, 102, 4542–4550. [Google Scholar] [CrossRef]
- Chen, D.; Liu, Z.; Huang, W.; Zhao, Y.; Dong, S.; Zeng, M. Purification and characterisation of a zinc-binding peptide from oyster protein hydrolysate. J. Funct. Foods 2013, 5, 689–697. [Google Scholar] [CrossRef]
- Maji, M.S.; Paul, B.; Bhunia, S.; Purushotham, M.; Karan, G. Exploring Chemical Modifications of Aromatic Amino Acid Residues in Peptides. Synthesis 2023, 55, 3701–3724. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Sarkar, R. Site-selective cleavage of peptides and proteins targeting aromatic amino acid residues. RSC Adv. 2025, 15, 9159–9179. [Google Scholar] [CrossRef]
- Braun, C.; Ewert, J.; Stressler, T. Characterization of cross-linked enzyme aggregates (CLEAs) of the fusion protein FUS-PepN_PepX and their application for milk protein hydrolysis. Eur. Food Res. Technol. 2017, 243, 1815–1828. [Google Scholar] [CrossRef]
- Yu, J.; Mikiashvili, N.; Bonku, R.; Smith, I.N. Allergenicity, antioxidant activity and ACE-inhibitory activity of protease hydrolyzed peanut flour. Food Chem. 2021, 360, 129992. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Yin, R.; Howell, K.; Zhang, P. Activity and bioavailability of food protein-derived angiotensin-I-converting enzyme–inhibitory peptides. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1150–1187. [Google Scholar] [CrossRef] [PubMed]
- Pavlicevic, M.; Marmiroli, N.; Maestri, E. Immunomodulatory peptides—A promising source for novel functional food production and drug discovery. Peptides 2022, 148, 170696. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-Castilla, J.; Hernández-Álvarez, A.J.; Jiménez-Martínez, C.; Jacinto-Hernández, C.; Alaiz, M.; Girón-Calle, J.; Vioque, J.; Dávila-Ortiz, G. Antioxidant and metal chelating activities of Phaseolus vulgaris L. var. Jamapa protein isolates, phaseolin and lectin hydrolysates. Food Chem. 2012, 131, 1157–1164. [Google Scholar] [CrossRef]
- Luo, Q.; Chen, D.; Boom, R.M.; Janssen, A.E.M. Revisiting the enzymatic kinetics of pepsin using isothermal titration calorimetry. Food Chem. 2018, 268, 94–100. [Google Scholar] [CrossRef]
- Marcet, I.; Carpintero, M.; Rendueles, M.; Díaz, M. Antioxidant Activity of Egg Yolk Protein Hydrolysates Obtained by Enzymatic and Sub-Critical Water Hydrolysis. Molecule 2023, 28, 7836. [Google Scholar] [CrossRef]
- Abadía-García, L.; Castaño-Tostado, E.; Ozimek, L.; Romero-Gómez, S.; Ozuna, C.; Amaya-Llano, S.L. Impact of ultrasound pretreatment on whey protein hydrolysis by vegetable proteases. Innov. Food Sci. Emerg. Technol. 2016, 37, 84–90. [Google Scholar] [CrossRef]
- Zhao, D.; Li, H.; Huang, M.; Wang, T.; Hu, Y.; Wang, L.; Xu, D.; Mao, S.; Li, C.; Zhou, G. Influence of proteolytic enzyme treatment on the changes in volatile compounds and odors of beef longissimus dorsi. Food Chem. 2020, 333, 127549. [Google Scholar] [CrossRef]
- Moss, S.; Subramanian, V.; Acharya, K.R. Crystal structure of peptide-bound neprilysin reveals key binding interactions. FEBS Lett. 2020, 594, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Cheng, S.; Wu, D.; Xu, Z.; Xu, S.; Chen, H.; Du, M. Hydrophobic peptides from oyster protein hydrolysates show better zinc-chelating ability. Food Biosci. 2021, 41, 100985. [Google Scholar] [CrossRef]
- Chen, Q.; Guo, L.; Du, F.; Chen, T.; Hou, H.; Li, B. The chelating peptide (GPAGPHGPPG) derived from Alaska pollock skin enhances calcium, zinc and iron transport in Caco-2 cells. Int. J. Food Sci. Technol. 2017, 52, 1283–1290. [Google Scholar] [CrossRef]
- Lin, Y.; Tang, X.; Xu, L.; Wang, S. Antibacterial properties and possible action mechanism of chelating peptides-zinc nanocomposite against Escherichia coli. Food Control 2019, 106, 106675. [Google Scholar] [CrossRef]
- Guo, H.; Yu, Y.; Hong, Z.; Zhang, Y.; Xie, Q.; Chen, H. Effect of Collagen Peptide-Chelated Zinc Nanoparticles from Pufferfish Skin on Zinc Bioavailability in Rats. J. Med. Food 2021, 24, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Ruiz, K.L.; López-Cervantes, J.; Sánchez-Machado, D.I.; Campas-Baypoli, O.N.; Quintero-Guerrero, A.A.; de Lourdes Grijalva-Delgado, M.; Chávez-Almanza, A.F. Collagen peptide fractions from tilapia (Oreochromis aureus Steindachner, 1864) scales: Chemical characterization and biological activity. Food Biosci. 2023, 53, 102658. [Google Scholar] [CrossRef]
- Meng, K.; Chen, L.; Xia, G.; Shen, X. Effects of zinc sulfate and zinc lactate on the properties of tilapia (Oreochromis Niloticus) skin collagen peptide chelate zinc. Food Chem. 2021, 347, 129043. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sun, J.; Li, Y.; Yu, X.; Yin, F.; Li, D.; Jiang, P.; Nakamura, Y.; Zhou, D. Characterisation of zinc-chelating peptides derived from scallop (Patinopecten yessoensis) mantle and their intestinal transport in everted rat sacs. Int. J. Food Sci. Technol. 2023, 58, 2236–2246. [Google Scholar] [CrossRef]
- Sun, J.; Liu, X.; Wang, Z.; Yin, F.; Liu, H.; Nakamura, Y.; Yu, C.; Zhou, D. Gastrointestinal digestion and absorption characterization in vitro of zinc-chelating hydrolysate from scallop adductor (Patinopecten yessoensis). J. Sci. Food Agric. 2022, 102, 3277–3286. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Wang, B.; Li, B.; Wang, C.; Luo, Y. Preparation and identification of peptides and their zinc complexes with antimicrobial activities from silver carp (Hypophthalmichthys molitrix) protein hydrolysates. Food Res. Int. 2014, 64, 91–98. [Google Scholar] [CrossRef]
- Johnson, W.M.; Alexander, H.; Bier, R.L.; Miller, D.R.; Muscarella, M.E.; Pitz, K.J.; Smith, H. Auxotrophic interactions: A stabilizing attribute of aquatic microbial communities? FEMS Microbiol. Ecol. 2020, 96, fiaa115. [Google Scholar] [CrossRef]
- Ma, S.; Lin, D. The biophysicochemical interactions at the interfaces between nanoparticles and aquatic organisms: Adsorption and internalization. Environ. Sci. Process. Impacts 2013, 15, 145–160. [Google Scholar] [CrossRef]
- Kim, S.-K.; Wijesekara, I. Development and biological activities of marine-derived bioactive peptides: A review. J. Funct. Foods 2010, 2, 1–9. [Google Scholar] [CrossRef]
- Dewapriya, P.; Kim, S.-k. Marine microorganisms: An emerging avenue in modern nutraceuticals and functional foods. Food Res. Int. 2014, 56, 115–125. [Google Scholar] [CrossRef]
- Chen, L.; Shen, X.; Xia, G. Effect of Molecular Weight of Tilapia (Oreochromis Niloticus) Skin Collagen Peptide Fractions on Zinc-Chelating Capacity and Bioaccessibility of the Zinc-Peptide Fractions Complexes in Vitro Digestion. Appl. Sci. 2020, 10, 2041. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, F.; Liu, X.; Zhao, M. Particulate nanocomposite from oyster (Crassostrea rivularis) hydrolysates via zinc chelation improves zinc solubility and peptide activity. Food Chem. 2018, 258, 269–277. [Google Scholar] [CrossRef]
- Chu, F.; Wang, D.; Liu, T.; Han, H.; Yu, Y.; Yang, Q. An optimized cocktail of chitinolytic enzymes to produce N,N′-diacetylchitobiose and N-acetyl-d-glucosamine from defatted krill by-products. Int. J. Biol. Macromol. 2019, 133, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Viciano, E.; Iglesias, J.; Lago, M.J.; Sánchez, F.J.; Otero, J.J.; Navarro, J.C. Fatty acid composition of polar and neutral lipid fractions of Octopus vulgaris Cuvier, 1797 paralarvae reared with enriched on-grown Artemia. Aquac. Res. 2011, 42, 704–709. [Google Scholar] [CrossRef]
- Nurdiani, R.; Dissanayake, M.; Street, W.E.; Donkor, O.N.; Singh, T.K.; Vasiljevic, T. Sustainable use of marine resources—Turning waste into food ingredients. Int. J. Food Sci. Technol. 2015, 50, 2329–2339. [Google Scholar] [CrossRef]
- Fang, Z.; Xu, L.; Lin, Y.; Cai, X.; Wang, S. The preservative potential of Octopus scraps peptides−Zinc chelate against Staphylococcus aureus: Its fabrication, antibacterial activity and action mode. Food Control 2019, 98, 24–33. [Google Scholar] [CrossRef]
- Bordbar, S.; Anwar, F.; Saari, N. High-Value Components and Bioactives from Sea Cucumbers for Functional Foods—A Review. Mar. Drugs 2011, 9, 1761–1805. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Kunisaki, N.; Urano, N.; Kimura, S. Collagen as the Major Edible Component of Sea Cucumber (Stichopus japonicus). J. Food Sci. 2002, 67, 1319–1322. [Google Scholar] [CrossRef]
- Rafiuddin Ahmed, M.; Venkateshwarlu, U.; Jayakumar, R. Multilayered peptide incorporated collagen tubules for peripheral nerve repair. Biomaterials 2004, 25, 2585–2594. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Z.; Zhang, J.; Song, L.; Li, D.; Wu, Z.; Zhu, B.; Nakamura, Y.; Shahidi, F.; Yu, C.; et al. Isolation and identification of zinc-chelating peptides from sea cucumber (Stichopus japonicus) protein hydrolysate. J. Sci. Food Agric. 2019, 99, 6400–6407. [Google Scholar] [CrossRef]
- Tokur, B.; Ozkütük, S.; Atici, E.; Ozyurt, G.; Ozyurt, C.E. Chemical and sensory quality changes of fish fingers, made from mirror carp (Cyprinus carpio L., 1758), during frozen storage (−18 °C). Food Chem. 2006, 99, 335–341. [Google Scholar] [CrossRef]
- Dong, S.; Zeng, M.; Wang, D.; Liu, Z.; Zhao, Y.; Yang, H. Antioxidant and biochemical properties of protein hydrolysates prepared from Silver carp (Hypophthalmichthys molitrix). Food Chem. 2008, 107, 1485–1493. [Google Scholar] [CrossRef]
- Feng, Y.; Zhu, S.; Yang, Y.; Li, S.; Zhao, Z.; Wu, H. Caseinophosphopeptides Overcome Calcium Phytate Inhibition on Zinc Bioavailability by Retaining Zinc from Coprecipitation as Zinc/Calcium Phytate Nanocomplexes. J. Agric. Food Chem. 2024, 72, 4757–4764. [Google Scholar] [CrossRef]
- Qian, R.; Sun, C.; Bai, T.; Yan, J.; Cheng, J.; Zhang, J. Recent advances and challenges in the interaction between myofibrillar proteins and flavor substances. Front. Nutr. 2024, 11, 1378884. [Google Scholar] [CrossRef]
- Okamoto, K.; Sako, Y. Recent advances in FRET for the study of protein interactions and dynamics. Curr. Opin. Struct. Biol. 2017, 46, 16–23. [Google Scholar] [CrossRef]
- Lakatos, A.; Gyurcsik, B.; Nagy, N.V.; Csendes, Z.; Wéber, E.; Fülöp, L.; Kiss, T. Histidine-rich branched peptides as Cu(II) and Zn(II) chelators with potential therapeutic application in Alzheimer’s disease. Dalton Trans. 2012, 41, 1713–1726. [Google Scholar] [CrossRef]
- Bizup, B.; Brutsaert, S.; Cunningham, C.L.; Thathiah, A.; Tzounopoulos, T. Cochlear zinc signaling dysregulation is associated with noise-induced hearing loss, and zinc chelation enhances cochlear recovery. Proc. Natl. Acad. Sci. USA 2024, 121, e2310561121. [Google Scholar] [CrossRef] [PubMed]
- Zivković, J.; Kumar, K.A.; Rushendran, R.; Ilango, K.; Fahmy, N.M.; El-Nashar, H.A.S.; El-Shazly, M.; Ezzat, S.M.; Melgar-Lalanne, G.; Romero-Montero, A.; et al. Pharmacological properties of mangiferin: Bioavailability, mechanisms of action and clinical perspectives. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2024, 397, 763–781. [Google Scholar] [CrossRef] [PubMed]
- Thilakarathna, S.H.; Rupasinghe, H.P.V. Flavonoid Bioavailability and Attempts for Bioavailability Enhancement. Nutrients 2013, 5, 3367–3387. [Google Scholar] [CrossRef] [PubMed]
- Carlton, D.D.; Schug, K.A. A review on the interrogation of peptide–metal interactions using electrospray ionization-mass spectrometry. Anal. Chim. Acta 2011, 686, 19–39. [Google Scholar] [CrossRef]
- Liao, W.; Lai, T.; Chen, L.; Fu, J.; Sreenivasan, S.T.; Yu, Z.; Ren, J. Synthesis and Characterization of a Walnut Peptides–Zinc Complex and Its Antiproliferative Activity against Human Breast Carcinoma Cells through the Induction of Apoptosis. J. Agric. Food Chem. 2016, 64, 1509–1519. [Google Scholar] [CrossRef]
- Molga, K.; Beker, W.; Roszak, R.; Czerwiński, A.; Grzybowski, B.A. Hierarchical Reaction Logic Enables Computational Design of Complex Peptide Syntheses. J. Am. Chem. Soc. 2025, 147, 7644–7662. [Google Scholar] [CrossRef]
- Zhou, Y.; Ni, Z.; Chen, K.; Liu, H.; Chen, L.; Lian, C.; Yan, L. Modeling Protein–Peptide Recognition Based on Classical Quantitative Structure–Affinity Relationship Approach: Implication for Proteome-Wide Inference of Peptide-Mediated Interactions. Protein J. 2013, 32, 568–578. [Google Scholar] [CrossRef]
- Xuan-Yu, M.; Mihaly, M.; Meng, C. Computational Approaches for Modeling GPCR Dimerization. Curr. Pharm. Biotechnol. 2014, 15, 996–1006. [Google Scholar] [CrossRef]
- Maares, M.; Haase, H. A Guide to Human Zinc Absorption: General Overview and Recent Advances of In Vitro Intestinal Models. Nutrients 2020, 12, 762. [Google Scholar] [CrossRef]
- Wang, B.; Xiao, S.; Zhou, G.; Wang, J. Novel Casein-Derived Peptide-Zinc Chelate: Zinc Chelation and Transepithelial Transport Characteristics. J. Agric. Food Chem. 2023, 71, 6978–6986. [Google Scholar] [CrossRef]
- Lee, H.H.; Prasad, A.S.; Brewer, G.J.; Owyang, C. Zinc absorption in human small intestine. Am. J. Physiol.-Gastrointest. Liver Physiol. 1989, 256, G87–G91. [Google Scholar] [CrossRef]
- Sandström, B. Dose dependence of zinc and manganese absorption in man. Proc. Nutr. Soc. 1992, 51, 211–218. [Google Scholar] [CrossRef]
- Davies, N.T. Studies on the absorption of zinc by rat intestine. Br. J. Nutr. 1980, 43, 189–203. [Google Scholar] [CrossRef]
- Seal, C.J.; Heaton, F.W. Chemical factors affecting the intestinal absorption of zinc in vitro and in vivo. Br. J. Nutr. 1983, 50, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Seal, C.J.; Mathers, J.C. Intestinal zinc transfer by everted gut sacs from rats given diets containing different amounts and types of dietary fibre. Br. J. Nutr. 1989, 62, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Fan, H.; Yu, W.; Hong, H.; Wu, J. Transport Study of Egg-Derived Antihypertensive Peptides (LKP and IQW) Using Caco-2 and HT29 Coculture Monolayers. J. Agric. Food Chem. 2017, 65, 7406–7414. [Google Scholar] [CrossRef]
- Wang, S.-C.; Chen, Y.-S.; Chen, S.-M.; Young, T.-K. Possible Site of Decreased Intestinal Zinc Absorption in Chronic Uremic Rats. Nephron 2001, 89, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Wu, Z.; Dai, Z.; Wang, G.; Wu, G. Protein hydrolysates in animal nutrition: Industrial production, bioactive peptides, and functional significance. J. Anim. Sci. Biotechnol. 2017, 8, 24. [Google Scholar] [CrossRef]
- Ulluwishewa, D.; Anderson, R.C.; McNabb, W.C.; Moughan, P.J.; Wells, J.M.; Roy, N.C. Regulation of Tight Junction Permeability by Intestinal Bacteria and Dietary Components. J. Nutr. 2011, 141, 769–776. [Google Scholar] [CrossRef]
- Linnankoski, J.; Mäkelä, J.; Palmgren, J.; Mauriala, T.; Vedin, C.; Ungell, A.L.; Lazorova, L.; Artursson, P.; Urtti, A.; Yliperttula, M. Paracellular Porosity and Pore Size of the Human Intestinal Epithelium in Tissue and Cell Culture Models. J. Pharm. Sci. 2010, 99, 2166–2175. [Google Scholar] [CrossRef]
- Lin, Q.; Xu, Q.; Bai, J.; Wu, W.; Hong, H.; Wu, J. Transport of soybean protein-derived antihypertensive peptide LSW across Caco-2 monolayers. J. Funct. Foods 2017, 39, 96–102. [Google Scholar] [CrossRef]
- Bejjani, S.; Wu, J. Transport of IRW, an Ovotransferrin-Derived Antihypertensive Peptide, in Human Intestinal Epithelial Caco-2 Cells. J. Agric. Food Chem. 2013, 61, 1487–1492. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Musoles, R.; Salom, J.B.; Castelló-Ruiz, M.; Contreras, M.d.M.; Recio, I.; Manzanares, P. Bioavailability of antihypertensive lactoferricin B-derived peptides: Transepithelial transport and resistance to intestinal and plasma peptidases. Int. Dairy J. 2013, 32, 169–174. [Google Scholar] [CrossRef]
- Xu, Q.; Yan, X.; Zhang, Y.; Wu, J. Current understanding of transport and bioavailability of bioactive peptides derived from dairy proteins: A review. Int. J. Food Sci. Technol. 2019, 54, 1930–1941. [Google Scholar] [CrossRef]
- Guo, L.; Harnedy, P.A.; Li, B.; Hou, H.; Zhang, Z.; Zhao, X.; FitzGerald, R.J. Food protein-derived chelating peptides: Biofunctional ingredients for dietary mineral bioavailability enhancement. Trends Food Sci. Technol. 2014, 37, 92–105. [Google Scholar] [CrossRef]
- Davies, S.L.; Gibbons, C.E.; Steward, M.C.; Ward, D.T. Extracellular calcium- and magnesium-mediated regulation of passive calcium transport across Caco-2 monolayers. Biochim. Biophys. Acta-Biomembr. 2008, 1778, 2318–2324. [Google Scholar] [CrossRef] [PubMed]
- Cámara, F.; Amaro, M.A. Nutrional aspect of zinc availability. Int. J. Food Sci. Nutr. 2003, 54, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Gobbetti, M.; Stepaniak, L.; De Angelis, M.; Corsetti, A.; Di Cagno, R. Latent Bioactive Peptides in Milk Proteins: Proteolytic Activation and Significance in Dairy Processing. Crit. Rev. Food Sci. Nutr. 2002, 42, 223–239. [Google Scholar] [CrossRef] [PubMed]
- Grogan, J.; McKnight, C.J.; Troxler, R.F.; Oppenheim, F.G. Zinc and copper bind to unique sites of histatin 5. FEBS Lett. 2001, 491, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Wang, Y.; Sun, L.; Liu, H.; Li, J. Inhibitory activity of a novel antibacterial peptide AMPNT-6 from Bacillus subtilis against Vibrio parahaemolyticus in shrimp. Food Control 2013, 30, 58–61. [Google Scholar] [CrossRef]
- Dashper, S.G.; Liu, S.W.; Reynolds, E.C. Antimicrobial Peptides and their Potential as Oral Therapeutic Agents. Int. J. Pept. Res. Ther. 2007, 13, 505–516. [Google Scholar] [CrossRef]
- Qi, Z.-D.; Lin, Y.; Zhou, B.; Ren, X.-D.; Pang, D.-W.; Liu, Y. Characterization of the Mechanism of the Staphylococcus aureus Cell Envelope by Bacitracin and Bacitracin-Metal Ions. J. Membr. Biol. 2008, 225, 27–37. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, L.; Dong, N.; Yang, J.; Hu, B. Dual Perspectives on Peptide–Zinc Complexation: Highlighting Aquatic Sources While Contextualizing Other Natural Origins. Biomolecules 2025, 15, 1311. https://doi.org/10.3390/biom15091311
Han L, Dong N, Yang J, Hu B. Dual Perspectives on Peptide–Zinc Complexation: Highlighting Aquatic Sources While Contextualizing Other Natural Origins. Biomolecules. 2025; 15(9):1311. https://doi.org/10.3390/biom15091311
Chicago/Turabian StyleHan, Lingyu, Nuo Dong, Jixin Yang, and Bing Hu. 2025. "Dual Perspectives on Peptide–Zinc Complexation: Highlighting Aquatic Sources While Contextualizing Other Natural Origins" Biomolecules 15, no. 9: 1311. https://doi.org/10.3390/biom15091311
APA StyleHan, L., Dong, N., Yang, J., & Hu, B. (2025). Dual Perspectives on Peptide–Zinc Complexation: Highlighting Aquatic Sources While Contextualizing Other Natural Origins. Biomolecules, 15(9), 1311. https://doi.org/10.3390/biom15091311






