In Vivo Anti-Inflammatory Activity of Lipids Extracted from the Most Abundant Cyanobacterial Strains of the Therapeutic Euganean Thermal Muds
Abstract
1. Introduction
2. Materials and Methods
2.1. Cyanobacterial Strains and Maintenance
2.2. Cyanobacterial Strains Cultivation at Optimal Growth Conditions
2.3. Growth and Biomass Assessment
2.4. Chlorophyll a Extraction and Quantification
2.5. Lipid Extraction and Quantification
2.6. Total Lipid Extracts Chemical Characterization
2.7. In Vitro Cell Viability Assay
2.8. Zebrafish Maintenance
2.9. Fish Embryo Acute Toxicity (FET) Test
2.10. Chemical Treatment with Copper Sulphate and Anti-Inflammatory Tests
2.10.1. Analysis of Morphological Traits and Image Processing
2.10.2. Locomotor Activity Test
2.11. Statistical Analysis
3. Results and Discussion
3.1. Cyanobacteria Culture Features
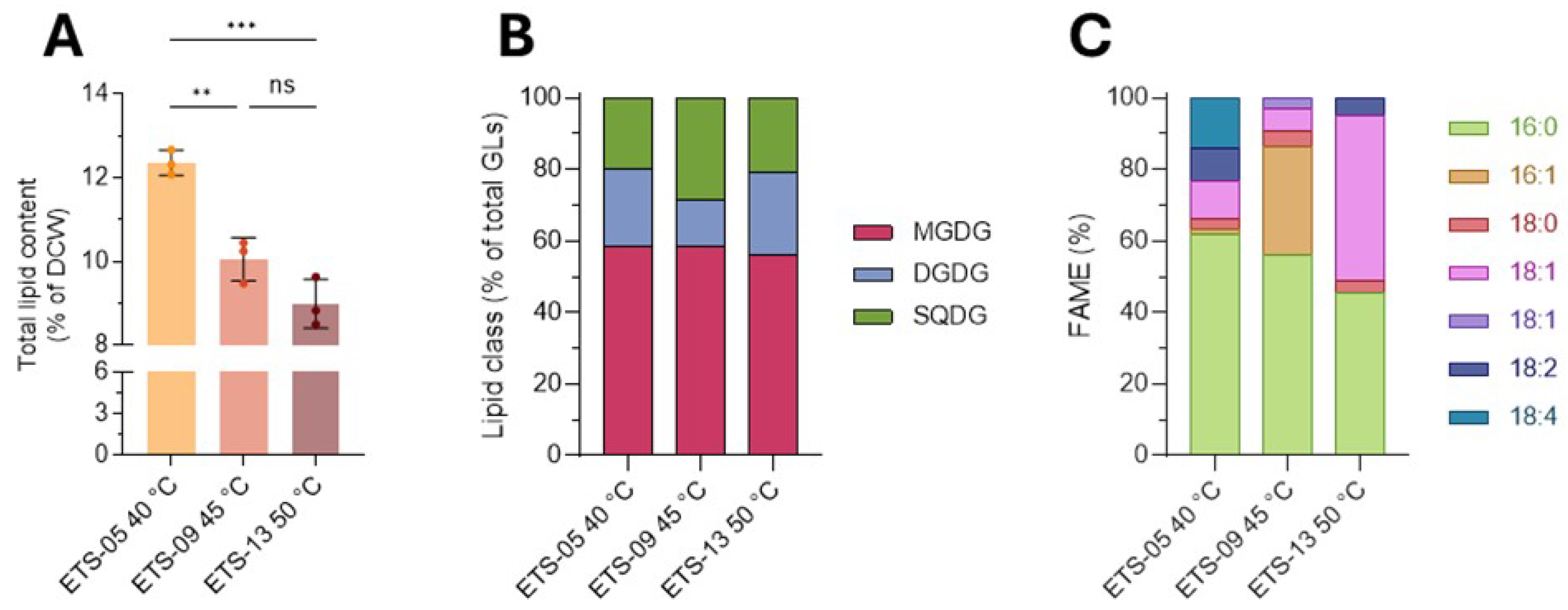
3.2. Lipid Extracts Characterization
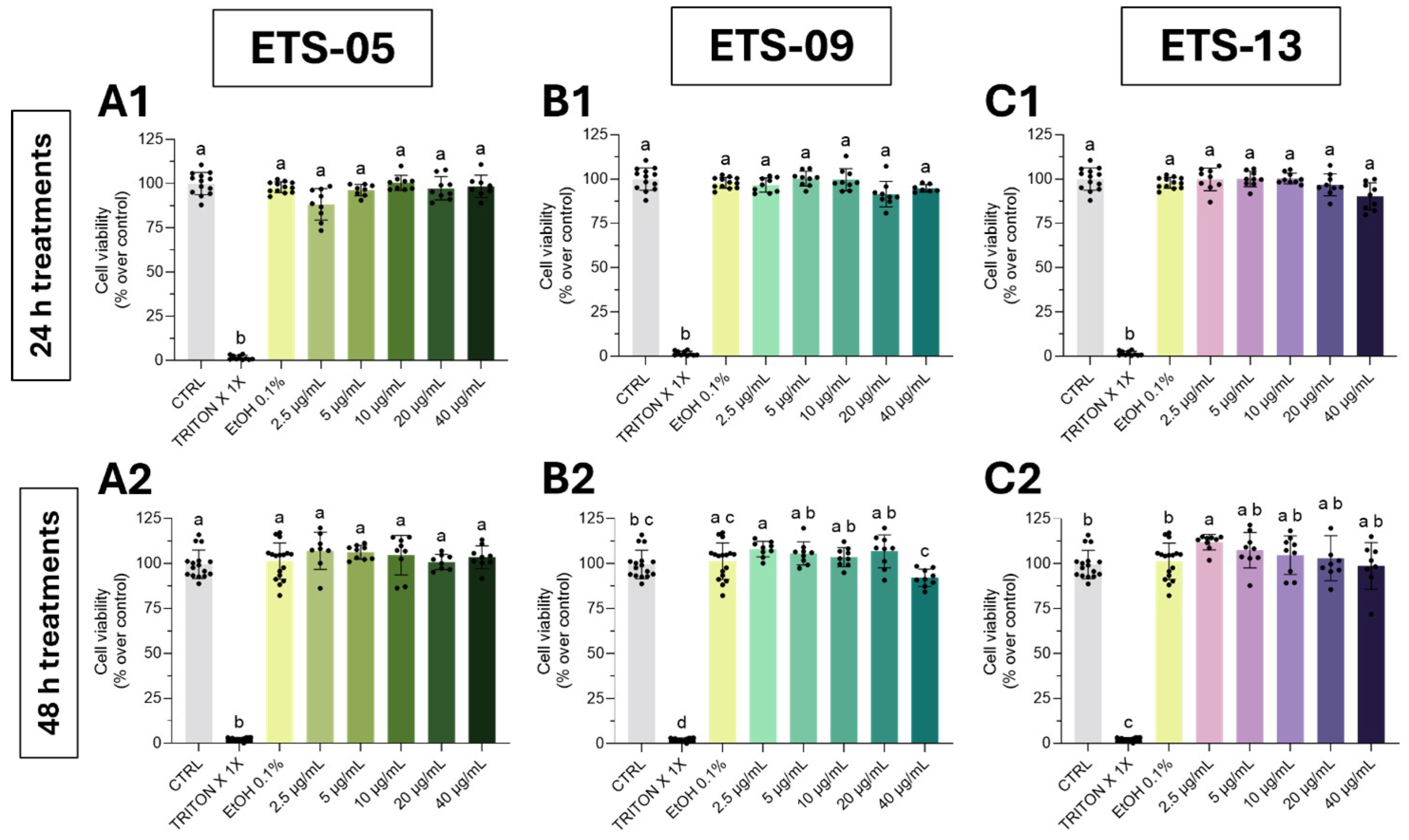
3.3. Human Skin Fibroblasts Viability Assay
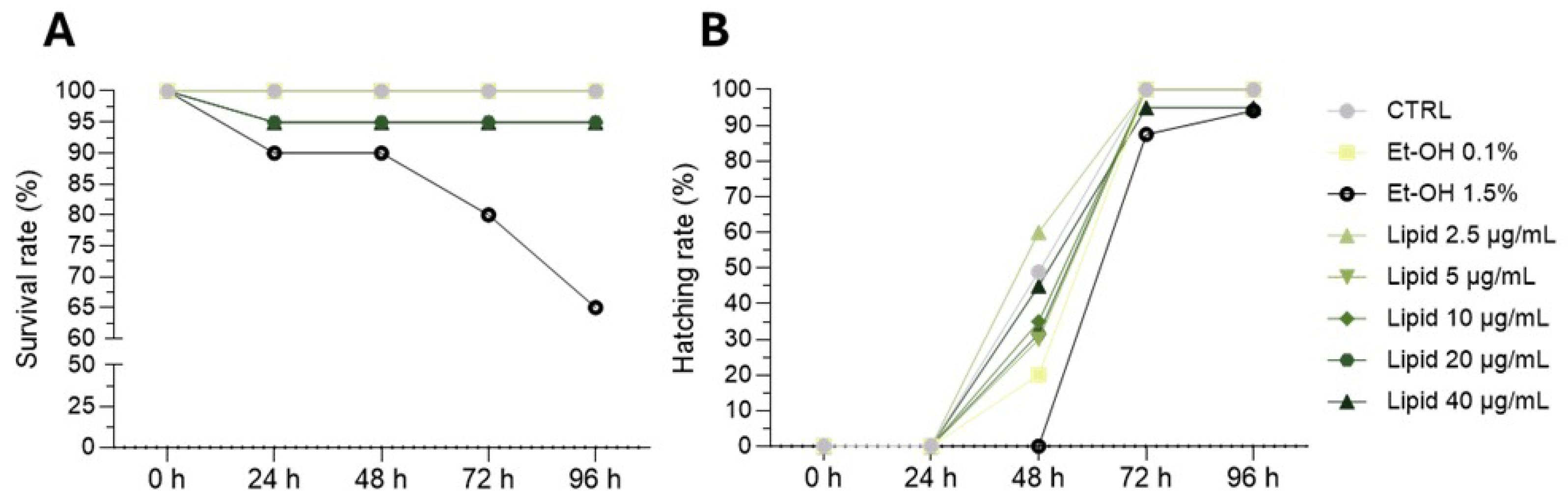
3.4. Zebrafish Embryo/Larvae Developmental Toxicity Assay
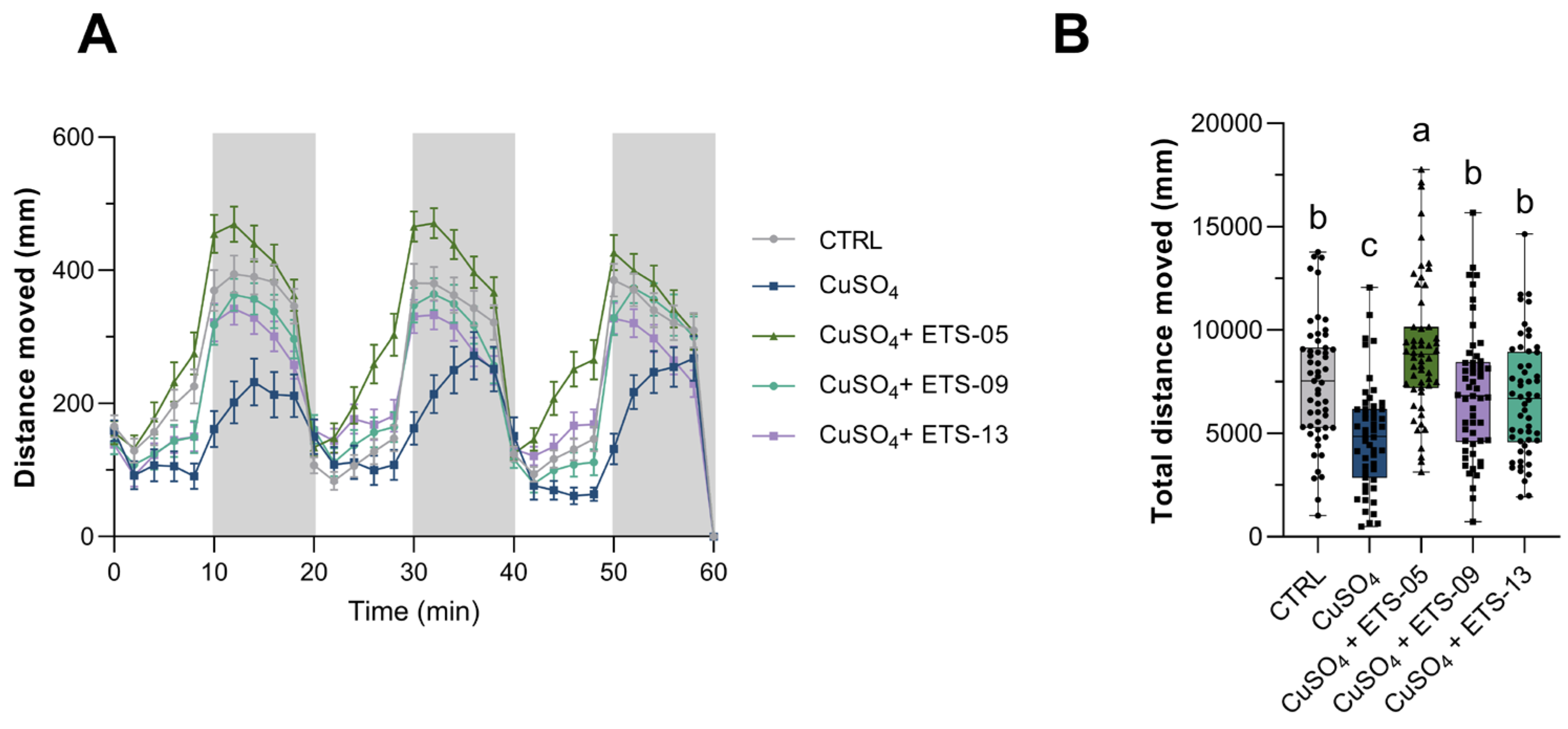
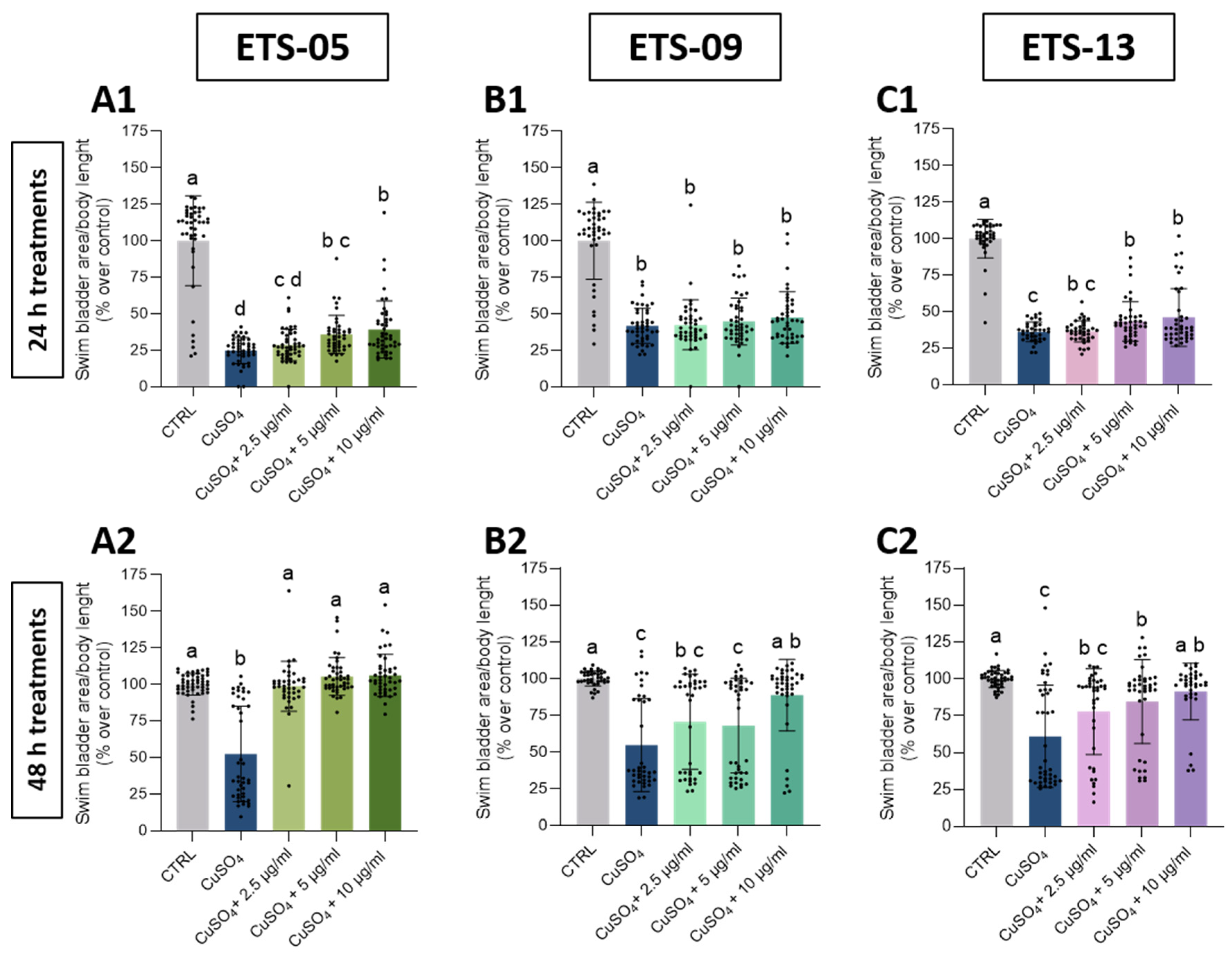
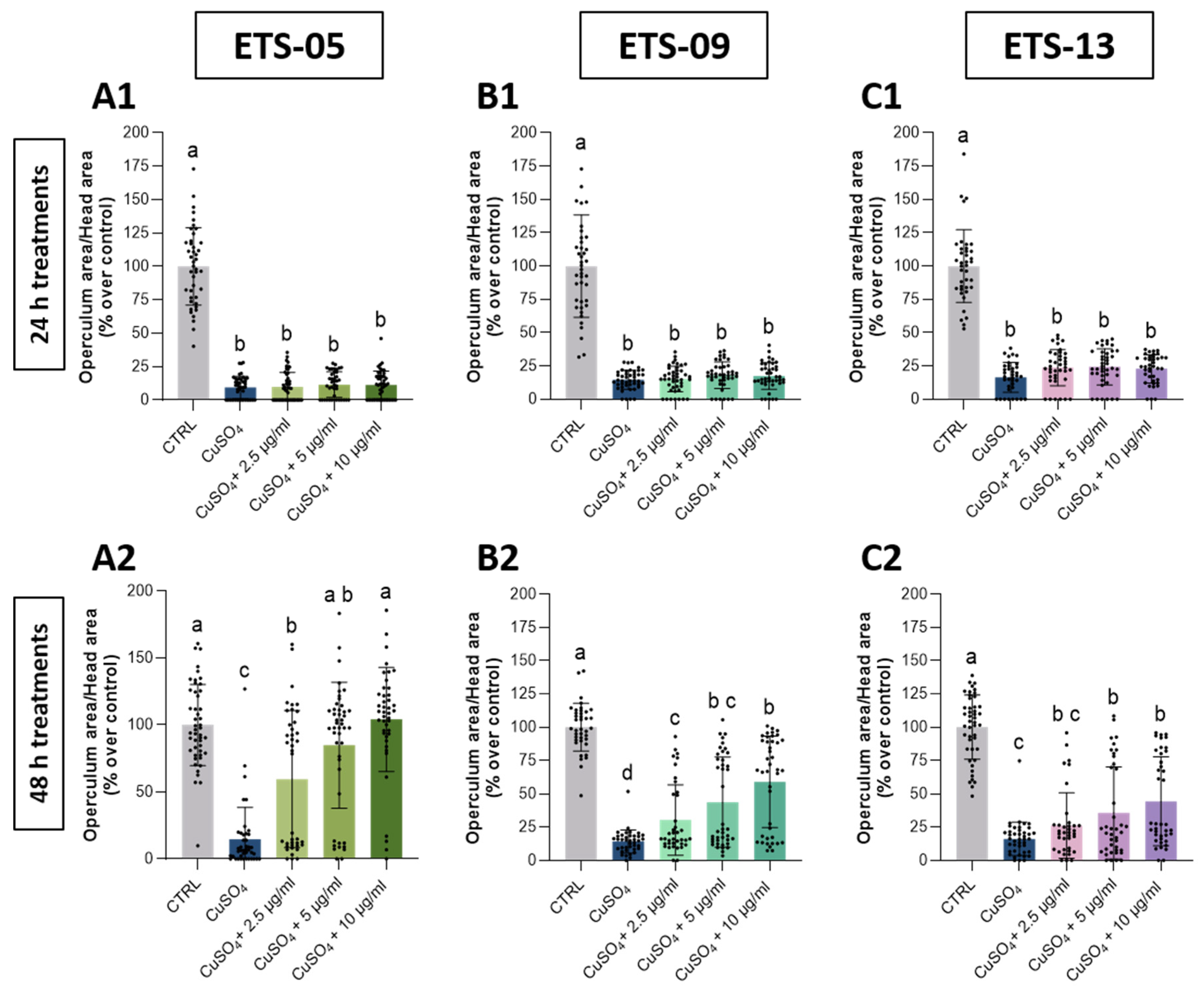
3.5. Analysis of Anti-Inflammatory Capabilities on Zebrafish Larvae
3.6. Swimming Behavior of Zebrafish Larvae After Lipid Treatments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Kini, S.; Divyashree, M.; Mani, M.K.; Mamatha, B.S. Algae and Cyanobacteria as a Source of Novel Bioactive Compounds for Biomedical Applications. In Advances in Cyanobacterial Biology; Academic Press: Cambridge, MA, USA, 2020; pp. 173–194. [Google Scholar] [CrossRef]
- Mourelle, M.L.; Gómez, C.P.; Legido, J.L. Microalgal Peloids for Cosmetic and Wellness Uses. Mar. Drugs 2021, 19, 666. [Google Scholar] [CrossRef]
- Demay, J.; Halary, S.; Knittel-Obrecht, A.; Villa, P.; Duval, C.; Hamlaoui, S.; Roussel, T.; Yéprémian, C.; Reinhardt, A.; Bernard, C.; et al. Anti-Inflammatory, Antioxidant, and Wound-Healing Properties of Cyanobacteria from Thermal Mud of Balaruc-Les-Bains, France: A Multi-Approach Study. Biomolecules 2021, 11, 28. [Google Scholar] [CrossRef] [PubMed]
- Calderan, A.; Carraro, A.; Honisch, C.; Lalli, A.; Ruzza, P.; Tateo, F. Euganean Therapeutic Mud (NE Italy): Chlorophyll a Variations over Two Years and Relationships with Mineralogy and Geochemistry. Appl. Clay. Sci. 2020, 185, 105361. [Google Scholar] [CrossRef]
- Caldara, F.; Chiappetta, A.; Scimemi, P. Thermal Scientific Research and Therapies in the Euganean Area. La Rivista di Engramma; Associazione Culturale Engramma, University ofVenice: Iuav, Italy, 2024.
- Bollettino Ufficiale Regione Del Veneto: Venezia. Regolamento d’uso Del Marchio Collettivo d’Origine Fango D.O.C. Thermae Abano Montegrotto—Regione Veneto. 2015. Available online: https://bur.regione.veneto.it/BurvServices/pubblica/Download.aspx?name=293_AllegatoA0_294348.pdf&type=9&storico=False (accessed on 7 September 2025).
- Gris, B.; Treu, L.; Zampieri, R.M.; Caldara, F.; Romualdi, C.; Campanaro, S.; La Rocca, N. Microbiota of the Therapeutic Euganean Thermal Muds with a Focus on the Main Cyanobacteria Species. Microorganisms 2020, 8, 1590. [Google Scholar] [CrossRef] [PubMed]
- Caichiolo, M.; Zampieri, R.M.; Adessi, A.; Ciani, M.; Caldara, F.; Dalla Valle, L.; La Rocca, N. Microbial Polysaccharides Extracted from Different Mature Muds of the Euganean Thermal District Show Similar Anti-Inflammatory Activity In Vivo. Int. J. Mol. Sci. 2024, 25, 4999. [Google Scholar] [CrossRef]
- Bruno, A.; Rossi, C.; Marcolongo, G.; Di Lena, A.; Venzo, A.; Berrie, C.P.; Corda, D. Selective in Vivo Anti-Inflammatory Action of the Galactolipid Monogalactosyldiacylglycerol. Eur. J. Pharmacol. 2005, 524, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Lenti, M.; Gentili, C.; Pianezzi, A.; Marcolongo, G.; Lalli, A.; Cancedda, R.; Cancedda, F.D. Monogalactosyldiacylglycerol Anti-Inflammatory Activity on Adult Articular Cartilage. Nat. Prod. Res. 2009, 23, 754–762. [Google Scholar] [CrossRef]
- Ulivi, V.; Lenti, M.; Gentili, C.; Marcolongo, G.; Cancedda, R.; Descalzi Cancedda, F. Anti-Inflammatory Activity of Monogalactosyldiacylglycerol in Human Articular Cartilage in Vitro: Activation of an Anti-Inflammatory Cyclooxygenase-2 (COX-2) Pathway. Arthritis Res. Ther. 2011, 13, R92. [Google Scholar] [CrossRef]
- Zampieri, R.M.; Adessi, A.; Caldara, F.; Codato, A.; Furlan, M.; Rampazzo, C.; De Philippis, R.; La Rocca, N.; Valle, L.D. Anti-Inflammatory Activity of Exopolysaccharides from Phormidium sp. ETS05, the Most Abundant Cyanobacterium of the Therapeutic Euganean Thermal Muds, Using the Zebrafish Model. Biomolecules 2020, 10, 582. [Google Scholar] [CrossRef]
- Zhang, J.; Li, C.; Yu, G.; Guan, H. Total Synthesis and Structure-Activity Relationship of Glycoglycerolipids from Marine Organisms. Mar. Drugs 2014, 12, 3634–3659. [Google Scholar] [CrossRef]
- Boudière, L.; Michaud, M.; Petroutsos, D.; Rébeillé, F.; Falconet, D.; Bastien, O.; Roy, S.; Finazzi, G.; Rolland, N.; Jouhet, J.; et al. Glycerolipids in Photosynthesis: Composition, Synthesis and Trafficking. Biochim. Biophys. Acta Bioenerg. 2014, 1837, 470–480. [Google Scholar] [CrossRef]
- Marcolongo, G.; De Appolonia, F.; Venzo, A.; Berrie, C.P.; Carofiglio, T.; Ceschi Berrini, C. Diacylglycerolipids Isolated from a Thermophile Cyanobacterium from the Euganean Hot Springs. Nat. Prod. Res. 2006, 20, 766–774. [Google Scholar] [CrossRef]
- Lalli, A.; Andreoli, C.; Ceschi Berrini, C.; De Appolonia, F.; Marcolongo, G. Anti-Inflammatory Active Principles in Euganean Thermal Mud. European Patent 1571203 (B1), 7 July 2013. [Google Scholar]
- Moro, I.; Fuiano, M.A.; Rascio, N.; De Philippis, R.; La Rocca, N. Phylogenetic, Morphological and Biochemical Studies on Thermospirulina andreolii Gen. & Sp. Nov. (Cyanophyta) from the Euganean Thermal District (Italy). Phycologia 2021, 60, 487–496. [Google Scholar] [CrossRef]
- Zampieri, R.M.; Bizzotto, E.; Campanaro, S.; Caldara, F.; Bellucci, M.; La Rocca, N. Kovacikia euganea Sp. Nov. (Leptolyngbyaceae, Cyanobacteria), a New Chlorophyll f Producing Cyanobacterium from the Euganean Thermal District (Italy). Front. Microbiol. 2025, 16, 1545008. [Google Scholar] [CrossRef]
- Russell, N.J.; Fukunaga, N. A Comparison of Thermal Adaptation of Membrane Lipids in Psychrophilic and Thermophilic Bacteria. FEMS Microbiol. Lett. 1990, 75, 171–182. [Google Scholar] [CrossRef]
- Kiseleva, L.L.; Horvàth, I.; Vigh, L.; Los, D.A. Temperature-Induced Specific Lipid Desaturation in the Thermophilic Cyanobacterium Synechococcus vulcanus. FEMS Microbiol. Lett. 1999, 175, 179–183. [Google Scholar] [CrossRef]
- Zampieri, R.M.; Caldara, F.; La Rocca, N. Assessment of Optimal Growth Conditions for Biomass and Exopolysaccharides Production in the Thermotolerant Cyanobacterium Phormidium sp. ETS-05. J. Appl. Phycol. 2023, 35, 1575–1587. [Google Scholar] [CrossRef]
- Crawford, A.D.; Esguerra, C.V.; De Witte, P.A.M. Fishing for Drugs from Nature: Zebrafish as a Technology Platform for Natural Product Discovery. Planta. Med. 2008, 74, 624–632. [Google Scholar] [CrossRef]
- MacRae, C.A.; Peterson, R.T. Zebrafish as Tools for Drug Discovery. Nat. Rev. Drug. Discov. 2015, 14, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Dash, S.N.; Patnaik, L. Flight for Fish in Drug Discovery: A Review of Zebrafish-Based Screening of Molecules. Biol. Lett. 2023, 19, 20220541. [Google Scholar] [CrossRef]
- Berrini, C.C.; De Appolonia, F.; Valle, L.; Dalla; Komárek, J.; Andreoli, C. Morphological and Molecular Characterization of a Thermophilic Cyanobacterium (Oscillatoriales) from the Euganean Thermal Springs (Padua, Italy). Algol. Stud./Arch. Für Hydrobiol. Suppl. Vol. 2004, 113, 73–85. [Google Scholar] [CrossRef]
- Rippka, R.; Deruelles, J.; Waterbury, J.B. Generic Assignments, Strain Histories and Properties of Pure Cultures of Cyanobacteria. J. Gen. Microbiol. 1979, 111, 1–61. [Google Scholar] [CrossRef]
- Moran, R. Formulae for Determination of Chlorophyllous Pigments Extracted with N,N-Dimethylformamide. Plant Physiol. 1982, 69, 1376–1381. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A Rapid Method of Total Lipid Extraction and Purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Cupo, A.; Landi, S.; Morra, S.; Nuzzo, G.; Gallo, C.; Manzo, E.; Fontana, A.; d’Ippolito, G. Autotrophic vs. Heterotrophic Cultivation of the Marine Diatom Cyclotella cryptica for EPA Production. Mar. Drugs 2021, 19, 355. [Google Scholar] [CrossRef]
- Nuzzo, G.; Gallo, C.; D’Ippolito, G.; Cutignano, A.; Sardo, A.; Fontana, A. Composition and Quantitation of Microalgal Lipids by ERETIC 1H-NMR Method. Mar. Drugs 2013, 11, 3742–3753. [Google Scholar] [CrossRef]
- Morra, S.; Lanzilli, M.; Grazioso, A.; Cupo, A.; Landi, S.; Nuzzo, G.; Castiglia, D.; Gallo, C.; Manzo, E.; Fontana, A.; et al. Potential of Lipid Biosynthesis under Heterotrophy in the Marine Diatom Cyclotella Cryptica. ACS Sustain. Chem. Eng. 2023, 11, 17607–17615. [Google Scholar] [CrossRef]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of Embryonic Development of the Zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef]
- OECD Test No. 236: Fish Embryo Acute Toxicity (FET) Test. In OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 2013; pp. 1–22.
- Tarasco, M.; Laizé, V.; Cardeira, J.; Cancela, M.L.; Gavaia, P.J. The Zebrafish Operculum: A Powerful System to Assess Osteogenic Bioactivities of Molecules with Pharmacological and Toxicological Relevance. Comp. Biochem. Physiol. Part C: Toxicol. Pharmacol. 2017, 197, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, O.; Kishida, T. Temperature-Induced Changes in the Lipid Molecular Species of a Thermophilic Cyanobacterium, Mastigocladus laminosm. Agric. Biol. Chem. 1991, 55, 781–785. [Google Scholar] [CrossRef]
- Sheng, J.; Vannela, R.; Rittmann, B.E. Evaluation of Methods to Extract and Quantify Lipids from Synechocystis PCC 6803. Bioresour. Technol. 2011, 102, 1697–1703. [Google Scholar] [CrossRef] [PubMed]
- Passos, L.S.; de Freitas, P.N.N.; Menezes, R.B.; de Souza, A.O.; da Silva, M.F.; Converti, A.; Pinto, E. Content of Lipids, Fatty Acids, Carbohydrates, and Proteins in Continental Cyanobacteria: A Systematic Analysis and Database Application. Appl. Sci. 2023, 13, 3162. [Google Scholar] [CrossRef]
- Maslova, I.P.; Mouradyan, E.A.; Lapina, S.S.; Klyachko-Gurvich, G.L.; Los, D.A. Lipid Fatty Acid Composition and Thermophilicity of Cyanobacteria. Russ. J. Plant Physiol. 2004, 51, 353–360. [Google Scholar] [CrossRef]
- Gara-Ali, M.; Zili, F.; Hosni, K.; Ben Ouada, H.; Ben-Mahrez, K. Lipophilic Extracts of the Thermophilic Cyanobacterium Leptolyngbya sp. and Chlorophyte Graesiella Sp. and Their Potential Use as Food and Anticancer Agents. Algal. Res. 2021, 60, 102511. [Google Scholar] [CrossRef]
- Pereira, T.C.B.; Campos, M.M.; Bogo, M.R. Copper Toxicology, Oxidative Stress and Inflammation Using Zebrafish as Experimental Model. J. Appl. Toxicol. 2016, 36, 876–885. [Google Scholar] [CrossRef]
- Zampieri, R.M.; Adessi, A.; Caldara, F.; De Philippis, R.; Dalla Valle, L.; La Rocca, N. In Vivo Anti-Inflammatory and Antioxidant Effects of Microbial Polysaccharides Extracted from Euganean Therapeutic Muds. Int. J. Biol. Macromol. 2022, 209 Pt B, 1710–1719. [Google Scholar] [CrossRef]
- Haverroth, G.M.B.; Welang, C.; Mocelin, R.N.; Postay, D.; Bertoncello, K.T.; Franscescon, F.; Rosemberg, D.B.; Dal Magro, J.; Dalla Corte, C.L. Copper Acutely Impairs Behavioral Function and Muscle Acetylcholinesterase Activity in Zebrafish (Danio Rerio). Ecotoxicol. Environ. Saf. 2015, 122, 440–447. [Google Scholar] [CrossRef]
- Sonnack, L.; Kampe, S.; Muth-Köhne, E.; Erdinger, L.; Henny, N.; Hollert, H.; Schäfers, C.; Fenske, M. Effects of Metal Exposure on Motor Neuron Development, Neuromasts and the Escape Response of Zebrafish Embryos. Neurotoxicol. Teratol. 2015, 50, 33–42. [Google Scholar] [CrossRef] [PubMed]
- MacPhail, R.C.; Brooks, J.; Hunter, D.L.; Padnos, B.; Irons, T.D.; Padilla, S. Locomotion in Larval Zebrafish: Influence of Time of Day, Lighting and Ethanol. Neurotoxicology 2009, 30, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Tabarzad, M.; Atabaki, V.; Hosseinabadi, T. Anti-Inflammatory Activity of Bioactive Compounds from Microalgae and Cyanobacteria by Focusing on the Mechanisms of Action. Mol. Biol. Rep. 2020, 47, 6193–6205. [Google Scholar] [CrossRef]
- Oliveira, F.; Conde, T.; Pinho, M.; Melo, T.; Hentschke, G.S.; Urbatzka, R.; Pereira, H.; Costa, M.; Vasconcelos, V.; Domingues, M.R. Unveiling the Biotechnological Potential of Cyanobacteria from the Portuguese LEGE-CC Collection Through Lipidomics and Antioxidant and Lipid-Lowering Properties. Molecules 2025, 30, 2504. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caichiolo, M.; d’Ippolito, G.; Grazioso, A.; Rampazzo, C.; Marchetto, A.; Caldara, F.; Dalla Valle, L.; La Rocca, N. In Vivo Anti-Inflammatory Activity of Lipids Extracted from the Most Abundant Cyanobacterial Strains of the Therapeutic Euganean Thermal Muds. Biomolecules 2025, 15, 1301. https://doi.org/10.3390/biom15091301
Caichiolo M, d’Ippolito G, Grazioso A, Rampazzo C, Marchetto A, Caldara F, Dalla Valle L, La Rocca N. In Vivo Anti-Inflammatory Activity of Lipids Extracted from the Most Abundant Cyanobacterial Strains of the Therapeutic Euganean Thermal Muds. Biomolecules. 2025; 15(9):1301. https://doi.org/10.3390/biom15091301
Chicago/Turabian StyleCaichiolo, Micol, Giuliana d’Ippolito, Angela Grazioso, Chiara Rampazzo, Angelica Marchetto, Fabrizio Caldara, Luisa Dalla Valle, and Nicoletta La Rocca. 2025. "In Vivo Anti-Inflammatory Activity of Lipids Extracted from the Most Abundant Cyanobacterial Strains of the Therapeutic Euganean Thermal Muds" Biomolecules 15, no. 9: 1301. https://doi.org/10.3390/biom15091301
APA StyleCaichiolo, M., d’Ippolito, G., Grazioso, A., Rampazzo, C., Marchetto, A., Caldara, F., Dalla Valle, L., & La Rocca, N. (2025). In Vivo Anti-Inflammatory Activity of Lipids Extracted from the Most Abundant Cyanobacterial Strains of the Therapeutic Euganean Thermal Muds. Biomolecules, 15(9), 1301. https://doi.org/10.3390/biom15091301







