Metabolic Adaptations Determine the Evolutionary Trajectory of TOR Signaling in Diverse Eukaryotes
Abstract
1. Introduction
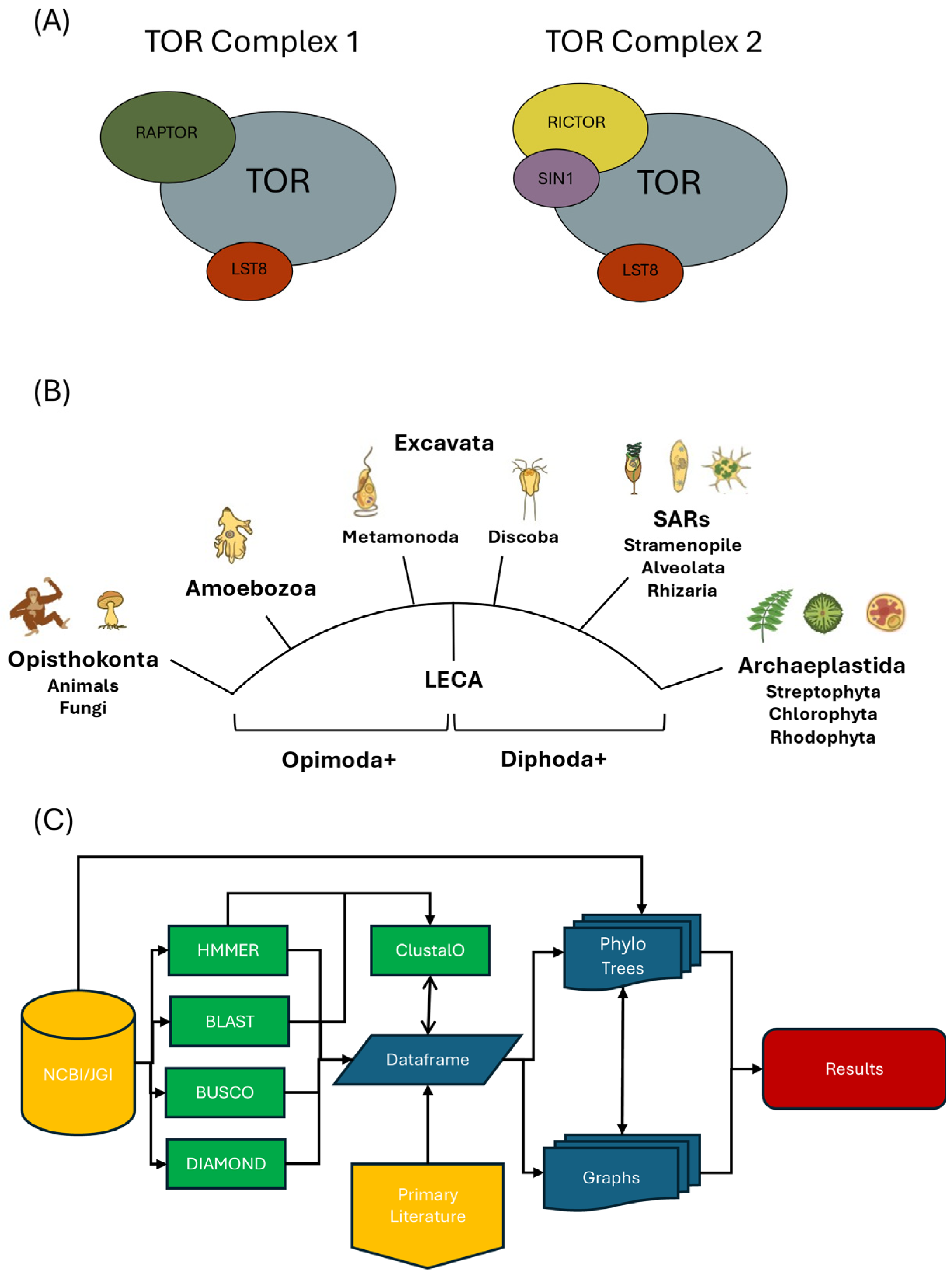
2. Materials and Methods
2.1. Data Acquisition
2.2. Establishing a Scoring Rubric for Strength of Homology
2.3. Sequence Read Archive (SRA) Data Analysis
2.4. Generating Phylogenetic Trees
3. Results
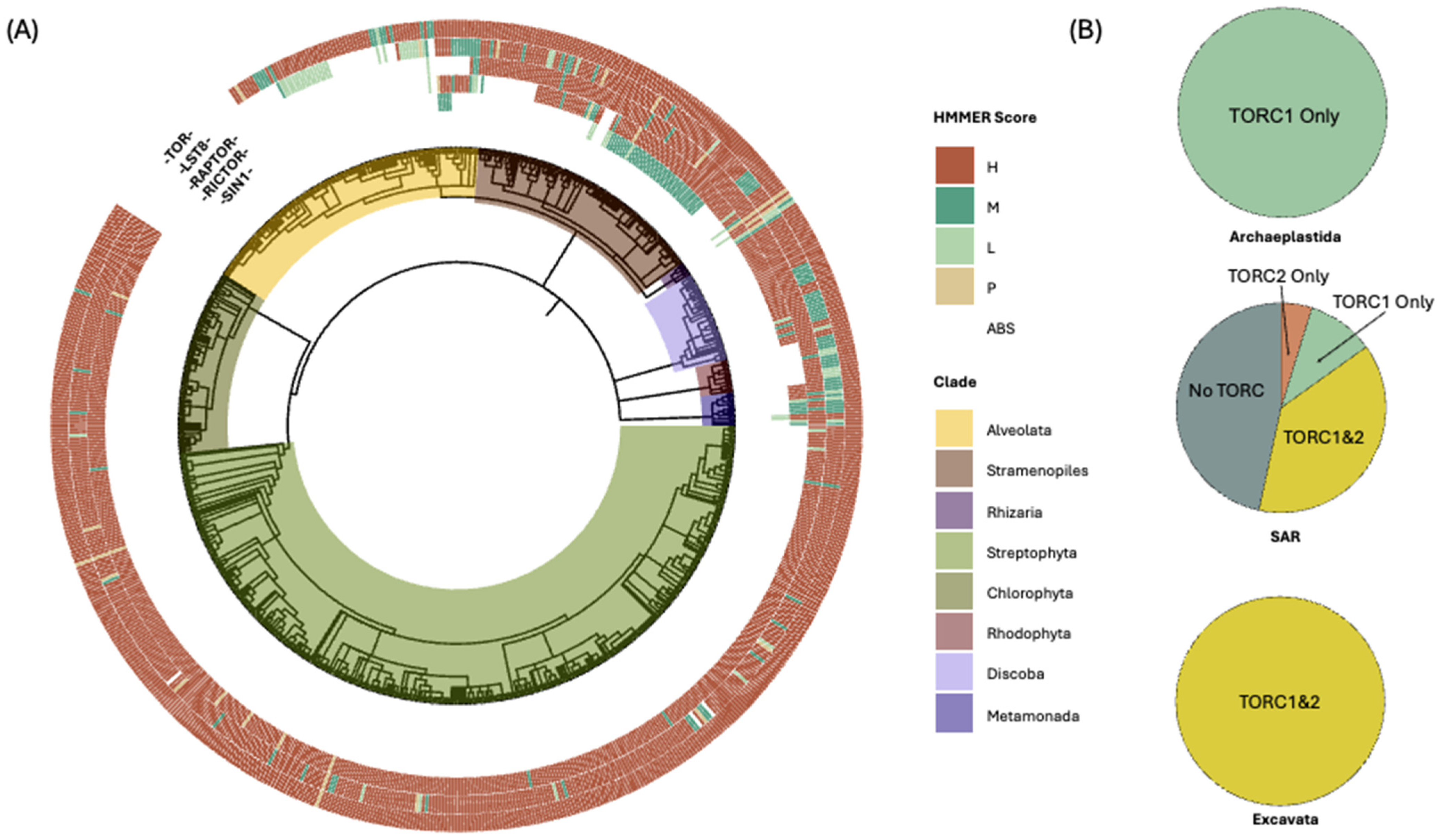
3.1. Phylogenetically Informed Detection of TOR Complex Components
3.2. Lineage-Specific Variation in the Distribution of TOR Complexes
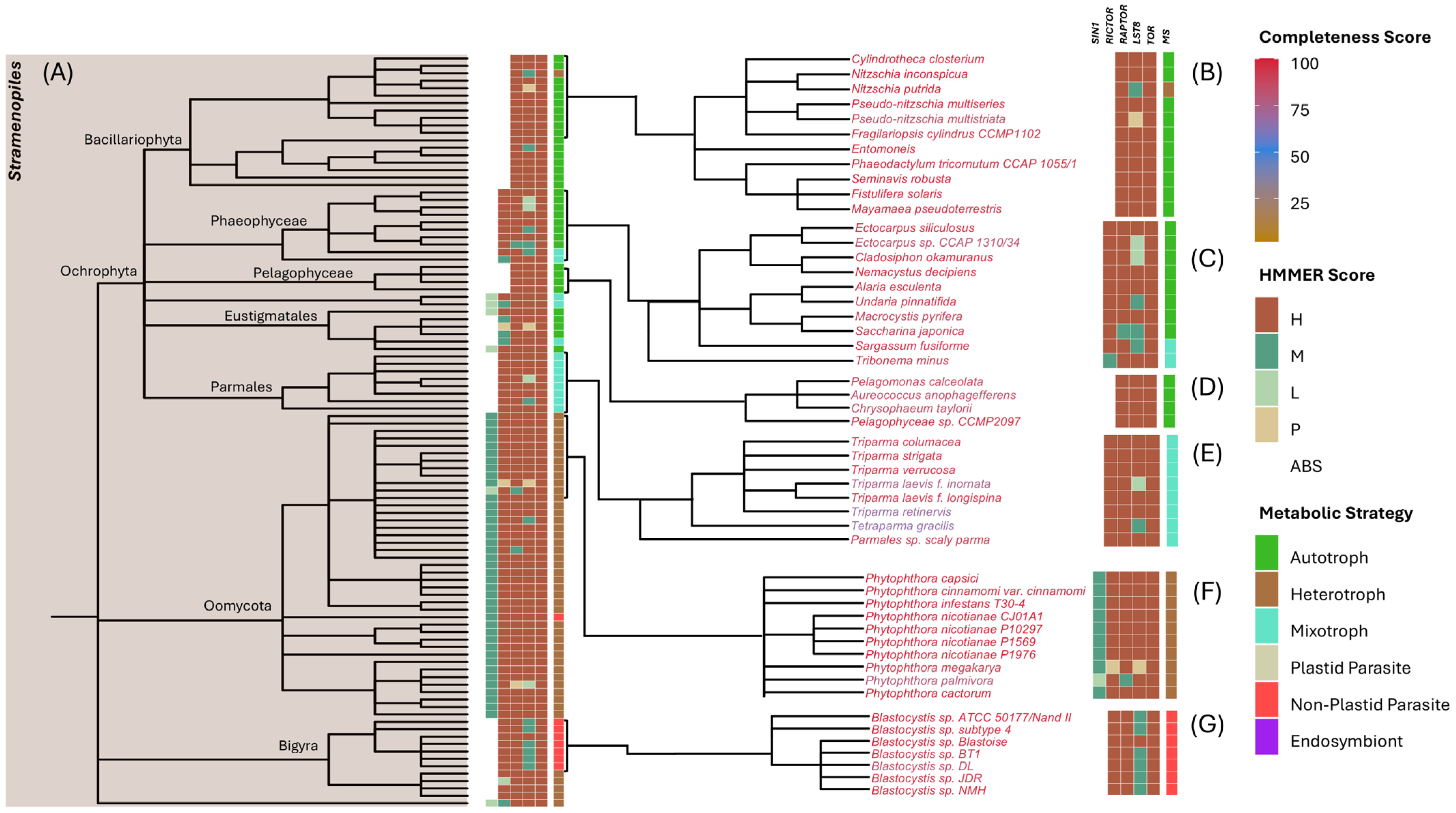
3.3. Tracing TOR Complex Differences in SAR Lineages
3.3.1. Stramenopiles
3.3.2. Alveolata
3.3.3. Rhizaria
3.4. Symbiotic Species and TOR Complex Composition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BUSCO | Benchmarking Universal Single-Copy Orthologs |
| HMMER | profile hidden Markov model search suite |
| JGI | Joint Genome Institute |
| LECA | last eukaryotic common ancestor |
| LST8 | Lethal with SEC Thirteen 8 |
| MSA | multiple sequence alignment |
| NCBI | National Center for Biotechnology Information |
| RAPTOR | Regulatory-Associated Protein of TOR (TORC1 subunit) |
| RICTOR | Rapamycin-Insensitive Companion of TOR (TORC2 scaffold) |
| ROS | reactive oxygen species |
| SAR | Stramenopiles, Alveolata, Rhizaria |
| SIN1 | Stress-Activated MAPK–Interacting Protein 1 (TORC2 subunit) |
| TOR | Target of Rapamycin |
| TORC1/TORC2 | TOR Complex 1/TOR Complex 2 |
References
- Selosse, M.-A.; Charpin, M.; Not, F. Mixotrophy Everywhere on Land and in Water: The Grand Écart Hypothesis. Ecol. Lett. 2017, 20, 246–263. [Google Scholar] [CrossRef]
- Keeling, P.J. The Endosymbiotic Origin, Diversification and Fate of Plastids. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010, 365, 729–748. [Google Scholar] [CrossRef]
- Judge, A.; Dodd, M.S. Metabolism. Essays Biochem. 2020, 64, 607–647. [Google Scholar] [CrossRef] [PubMed]
- Perlin, M.H.; Poulin, R.; de Bekker, C. Invasion of the Four Kingdoms: The Parasite Journey across Plant and Non-Plant Hosts. Biol. Rev. Camb. Philos. Soc. 2025, 100, 936–968. [Google Scholar] [CrossRef]
- López-García, P.; Eme, L.; Moreira, D. Symbiosis in Eukaryotic Evolution. J. Theor. Biol. 2017, 434, 20–33. [Google Scholar] [CrossRef] [PubMed]
- Efeyan, A.; Comb, W.C.; Sabatini, D.M. Nutrient-Sensing Mechanisms and Pathways. Nature 2015, 517, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Loewith, R.; Hall, M.N. Target of Rapamycin (TOR) in Nutrient Signaling and Growth Control. Genetics 2011, 189, 1177–1201. [Google Scholar] [CrossRef]
- Zoncu, R.; Efeyan, A.; Sabatini, D.M. mTOR: From Growth Signal Integration to Cancer, Diabetes and Ageing. Nat. Rev. Mol. Cell Biol. 2011, 12, 21–35. [Google Scholar] [CrossRef]
- Mossmann, D.; Park, S.; Hall, M.N. mTOR Signalling and Cellular Metabolism Are Mutual Determinants in Cancer. Nat. Rev. Cancer 2018, 18, 744–757. [Google Scholar] [CrossRef]
- Eltschinger, S.; Loewith, R. TOR Complexes and the Maintenance of Cellular Homeostasis. Trends Cell Biol. 2016, 26, 148–159. [Google Scholar] [CrossRef]
- Barbet, N.C.; Schneider, U.; Helliwell, S.B.; Stansfield, I.; Tuite, M.F.; Hall, M.N. TOR Controls Translation Initiation and Early G1 Progression in Yeast. Mol. Biol. Cell 1996, 7, 25–42. [Google Scholar] [CrossRef]
- Powers, T.; Walter, P. Regulation of Ribosome Biogenesis by the Rapamycin-Sensitive TOR-Signaling Pathway in Saccharomyces Cerevisiae. Mol. Biol. Cell 1999, 10, 987–1000. [Google Scholar] [CrossRef]
- Loewith, R.; Jacinto, E.; Wullschleger, S.; Lorberg, A.; Crespo, J.L.; Bonenfant, D.; Oppliger, W.; Jenoe, P.; Hall, M.N. Two TOR Complexes, Only One of Which Is Rapamycin Sensitive, Have Distinct Roles in Cell Growth Control. Mol. Cell 2002, 10, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Helliwell, S.B.; Howald, I.; Barbet, N.; Hall, M.N. TOR2 Is Part of Two Related Signaling Pathways Coordinating Cell Growth in Saccharomyces Cerevisiae. Genetics 1998, 148, 99–112. [Google Scholar] [CrossRef]
- Wedaman, K.P.; Reinke, A.; Anderson, S.; Yates, J.; McCaffery, J.M.; Powers, T. Tor Kinases Are in Distinct Membrane-Associated Protein Complexes in Saccharomyces Cerevisiae. Mol. Biol. Cell 2003, 14, 1204–1220. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; Kunz, J.; Hall, M.N. TOR2 Is Required for Organization of the Actin Cytoskeleton in Yeast. Proc. Natl. Acad. Sci. USA 1996, 93, 13780–13785. [Google Scholar] [CrossRef] [PubMed]
- Emmerstorfer-Augustin, A.; Thorner, J. Regulation of TORC2 Function and Localization in Yeast. Annu. Rev. Cell Dev. Biol. 2023, 39, 363–389. [Google Scholar] [CrossRef]
- Riggi, M.; Kusmider, B.; Loewith, R. The Flipside of the TOR Coin–TORC2 and Plasma Membrane Homeostasis at a Glance. J Cell Sci 2020, 133, jcs242040. [Google Scholar] [CrossRef]
- Aronova, S.; Wedaman, K.; Aronov, P.A.; Fontes, K.; Ramos, K.; Hammock, B.D.; Powers, T. Regulation of Ceramide Biosynthesis by TOR Complex 2. Cell Metab. 2008, 7, 148–158. [Google Scholar] [CrossRef]
- Fu, W.; Hall, M.N. Regulation of mTORC2 Signaling. Genes 2020, 11, 1045. [Google Scholar] [CrossRef]
- Jacinto, E.; Loewith, R.; Schmidt, A.; Lin, S.; Rüegg, M.A.; Hall, A.; Hall, M.N. Mammalian TOR Complex 2 Controls the Actin Cytoskeleton and Is Rapamycin Insensitive. Nat. Cell Biol. 2004, 6, 1122–1128. [Google Scholar] [CrossRef]
- Sarbassov, D.D.; Guertin, D.A.; Ali, S.M.; Sabatini, D.M. Phosphorylation and Regulation of Akt/PKB by the Rictor-mTOR Complex. Science 2005, 307, 1098–1101. [Google Scholar] [CrossRef] [PubMed]
- Guri, Y.; Colombi, M.; Dazert, E.; Hindupur, S.K.; Roszik, J.; Moes, S.; Jenoe, P.; Heim, M.H.; Riezman, I.; Riezman, H.; et al. mTORC2 Promotes Tumorigenesis via Lipid Synthesis. Cancer Cell 2017, 32, 807–823.e12. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.; Kupiec, M.; Weisman, R. Glucose Activates TORC2-Gad8 Protein via Positive Regulation of the cAMP/cAMP-Dependent Protein Kinase A (PKA) Pathway and Negative Regulation of the Pmk1 Protein-Mitogen-Activated Protein Kinase Pathway. J. Biol. Chem. 2014, 289, 21727–21737. [Google Scholar] [CrossRef] [PubMed]
- Hatano, T.; Morigasaki, S.; Tatebe, H.; Ikeda, K.; Shiozaki, K. Fission Yeast Ryh1 GTPase Activates TOR Complex 2 in Response to Glucose. Cell Cycle 2015, 14, 848–856. [Google Scholar] [CrossRef]
- Kim, D.-H.; Sarbassov, D.D.; Ali, S.M.; King, J.E.; Latek, R.R.; Erdjument-Bromage, H.; Tempst, P.; Sabatini, D.M. mTOR Interacts with Raptor to Form a Nutrient-Sensitive Complex That Signals to the Cell Growth Machinery. Cell 2002, 110, 163–175. [Google Scholar] [CrossRef]
- Kim, D.-H.; Sarbassov, D.D.; Ali, S.M.; Latek, R.R.; Guntur, K.V.P.; Erdjument-Bromage, H.; Tempst, P.; Sabatini, D.M. GbetaL, a Positive Regulator of the Rapamycin-Sensitive Pathway Required for the Nutrient-Sensitive Interaction between Raptor and mTOR. Mol. Cell 2003, 11, 895–904. [Google Scholar] [CrossRef]
- Aylett, C.H.S.; Sauer, E.; Imseng, S.; Boehringer, D.; Hall, M.N.; Ban, N.; Maier, T. Architecture of Human mTOR Complex 1. Science 2016, 351, 48–52. [Google Scholar] [CrossRef]
- Sarbassov, D.D.; Ali, S.M.; Kim, D.-H.; Guertin, D.A.; Latek, R.R.; Erdjument-Bromage, H.; Tempst, P.; Sabatini, D.M. Rictor, a Novel Binding Partner of mTOR, Defines a Rapamycin-Insensitive and Raptor-Independent Pathway That Regulates the Cytoskeleton. Curr. Biol. 2004, 14, 1296–1302. [Google Scholar] [CrossRef]
- Jacinto, E.; Facchinetti, V.; Liu, D.; Soto, N.; Wei, S.; Jung, S.Y.; Huang, Q.; Qin, J.; Su, B. SIN1/MIP1 Maintains Rictor-mTOR Complex Integrity and Regulates Akt Phosphorylation and Substrate Specificity. Cell 2006, 127, 125–137. [Google Scholar] [CrossRef]
- Scaiola, A.; Mangia, F.; Imseng, S.; Boehringer, D.; Berneiser, K.; Shimobayashi, M.; Stuttfeld, E.; Hall, M.N.; Ban, N.; Maier, T. The 3.2-Å Resolution Structure of Human mTORC2. Sci. Adv. 2020, 6, eabc1251. [Google Scholar] [CrossRef] [PubMed]
- Tsverov, J.; Yegorov, K.; Powers, T. Identification of Defined Structural Elements within TOR2 Kinase Required for TOR Complex 2 Assembly and Function in Saccharomyces Cerevisiae. Mol. Biol. Cell 2022, 33, ar44. [Google Scholar] [CrossRef] [PubMed]
- Tatebe, H.; Shiozaki, K. Evolutionary Conservation of the Components in the TOR Signaling Pathways. Biomolecules 2017, 7, 77. [Google Scholar] [CrossRef] [PubMed]
- van Dam, T.J.P.; Zwartkruis, F.J.T.; Bos, J.L.; Snel, B. Evolution of the TOR Pathway. J. Mol. Evol. 2011, 73, 209–220. [Google Scholar] [CrossRef]
- Ingargiola, C.; Turqueto Duarte, G.; Robaglia, C.; Leprince, A.-S.; Meyer, C. The Plant Target of Rapamycin: A Conduc TOR of Nutrition and Metabolism in Photosynthetic Organisms. Genes 2020, 11, 1285. [Google Scholar] [CrossRef]
- Mallén-Ponce, M.J.; Pérez-Pérez, M.E.; Crespo, J.L. Deciphering the Function and Evolution of the Target of Rapamycin Signaling Pathway in Microalgae. J. Exp. Bot. 2022, 73, 6993–7005. [Google Scholar] [CrossRef]
- Brunkard, J.O. Exaptive Evolution of Target of Rapamycin Signaling in Multicellular Eukaryotes. Dev. Cell 2020, 54, 142–155. [Google Scholar] [CrossRef]
- Shertz, C.A.; Bastidas, R.J.; Li, W.; Heitman, J.; Cardenas, M.E. Conservation, Duplication, and Loss of the Tor Signaling Pathway in the Fungal Kingdom. BMC Genom. 2010, 11, 510. [Google Scholar] [CrossRef]
- Keeling, P.J.; Burki, F. Progress towards the Tree of Eukaryotes. Curr. Biol. 2019, 29, R808–R817. [Google Scholar] [CrossRef]
- Williamson, K.; Eme, L.; Baños, H.; McCarthy, C.G.P.; Susko, E.; Kamikawa, R.; Orr, R.J.S.; Muñoz-Gómez, S.A.; Minh, B.Q.; Simpson, A.G.B.; et al. A Robustly Rooted Tree of Eukaryotes Reveals Their Excavate Ancestry. Nature 2025, 640, 974–981. [Google Scholar] [CrossRef]
- Keeling, P.J.; Eglit, Y. Openly Available Illustrations as Tools to Describe Eukaryotic Microbial Diversity. PLoS Biol. 2023, 21, e3002395. [Google Scholar] [CrossRef]
- Sayers, E.W.; Beck, J.; Bolton, E.E.; Brister, J.R.; Chan, J.; Connor, R.; Feldgarden, M.; Fine, A.M.; Funk, K.; Hoffman, J.; et al. Database Resources of the National Center for Biotechnology Information in 2025. Nucleic Acids Res. 2024, 53, D20–D29. [Google Scholar] [CrossRef]
- Grigoriev, I.V.; Hayes, R.D.; Calhoun, S.; Kamel, B.; Wang, A.; Ahrendt, S.; Dusheyko, S.; Nikitin, R.; Mondo, S.J.; Salamov, A.; et al. PhycoCosm, a Comparative Algal Genomics Resource. Nucleic Acids Res. 2021, 49, D1004–D1011. [Google Scholar] [CrossRef] [PubMed]
- Schoch, C.L.; Ciufo, S.; Domrachev, M.; Hotton, C.L.; Kannan, S.; Khovanskaya, R.; Leipe, D.; Mcveigh, R.; O’Neill, K.; Robbertse, B.; et al. NCBI Taxonomy: A Comprehensive Update on Curation, Resources and Tools. Database 2020, 2020, baaa062. [Google Scholar] [CrossRef]
- Sayers, E.W.; Cavanaugh, M.; Clark, K.; Ostell, J.; Pruitt, K.D.; Karsch-Mizrachi, I. GenBank. Nucleic Acids Res. 2019, 47, D94–D99. [Google Scholar] [CrossRef] [PubMed]
- Burki, F.; Roger, A.J.; Brown, M.W.; Simpson, A.G.B. The New Tree of Eukaryotes. Trends Ecol. Evol. 2020, 35, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Blum, M.; Andreeva, A.; Florentino, L.C.; Chuguransky, S.R.; Grego, T.; Hobbs, E.; Pinto, B.L.; Orr, A.; Paysan-Lafosse, T.; Ponamareva, I.; et al. InterPro: The Protein Sequence Classification Resource in 2025. Nucleic Acids Res. 2025, 53, D444–D456. [Google Scholar] [CrossRef]
- Mi, H.; Ebert, D.; Muruganujan, A.; Mills, C.; Albou, L.-P.; Mushayamaha, T.; Thomas, P.D. PANTHER Version 16: A Revised Family Classification, Tree-Based Classification Tool, Enhancer Regions and Extensive API. Nucleic Acids Res. 2021, 49, D394–D403. [Google Scholar] [CrossRef]
- Thomas, P.D.; Ebert, D.; Muruganujan, A.; Mushayahama, T.; Albou, L.-P.; Mi, H. PANTHER: Making Genome-Scale Phylogenetics Accessible to All. Protein Sci. 2022, 31, 8–22. [Google Scholar] [CrossRef]
- Eddy, S.R. Accelerated Profile HMM Searches. PLoS Comput. Biol. 2011, 7, e1002195. [Google Scholar] [CrossRef]
- Manni, M.; Berkeley, M.R.; Seppey, M.; Zdobnov, E.M. BUSCO: Assessing Genomic Data Quality and Beyond. Curr. Protoc. 2021, 1, e323. [Google Scholar] [CrossRef]
- Pearson, W.R. An Introduction to Sequence Similarity (“Homology”) Searching. Curr. Protoc. Bioinform. 2013, 42, 3.1.1–3.1.8. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Urrea-Castellanos, R.; Calderan-Rodrigues, M.J.; Artins, A.; Musialak-Lange, M.; Macharanda-Ganesh, A.; Fernie, A.R.; Wahl, V.; Caldana, C. The Regulatory-Associated Protein of Target of Rapamycin 1B (RAPTOR 1B) Interconnects with the Photoperiod Pathway to Promote Flowering in Arabidopsis. Proc. Natl. Acad. Sci. USA 2025, 122, e2405536122. [Google Scholar] [CrossRef] [PubMed]
- Sievers, F.; Wilm, A.; Dineen, D.; Gibson, T.J.; Karplus, K.; Li, W.; Lopez, R.; McWilliam, H.; Remmert, M.; Söding, J.; et al. Fast, Scalable Generation of High-Quality Protein Multiple Sequence Alignments Using Clustal Omega. Mol. Syst. Biol. 2011, 7, 539. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.M.; Procter, J.B.; Martin, D.M.A.; Clamp, M.; Barton, G.J. Jalview Version 2—A Multiple Sequence Alignment Editor and Analysis Workbench. Bioinformatics 2009, 25, 1189–1191. [Google Scholar] [CrossRef]
- Buchfink, B.; Xie, C.; Huson, D.H. Fast and Sensitive Protein Alignment Using DIAMOND. Nat. Methods 2015, 12, 59–60. [Google Scholar] [CrossRef]
- Yu, G.; Smith, D.K.; Zhu, H.; Guan, Y.; Lam, T.T.-Y. Ggtree: An r Package for Visualization and Annotation of Phylogenetic Trees with Their Covariates and Other Associated Data. Methods Ecol. Evol. 2017, 8, 28–36. [Google Scholar] [CrossRef]
- Torruella, G.; Galindo, L.J.; Moreira, D.; López-García, P. Phylogenomics of Neglected Flagellated Protists Supports a Revised Eukaryotic Tree of Life. Curr. Biol. 2025, 35, 198–207.e4. [Google Scholar] [CrossRef]
- Bowles, A.M.C.; Williamson, C.J.; Williams, T.A.; Lenton, T.M.; Donoghue, P.C.J. The Origin and Early Evolution of Plants. Trends Plant Sci. 2023, 28, 312–329. [Google Scholar] [CrossRef]
- Ševčíková, T.; Horák, A.; Klimeš, V.; Zbránková, V.; Demir-Hilton, E.; Sudek, S.; Jenkins, J.; Schmutz, J.; Přibyl, P.; Fousek, J.; et al. Updating Algal Evolutionary Relationships through Plastid Genome Sequencing: Did Alveolate Plastids Emerge Through Endosymbiosis of an Ochrophyte? Sci. Rep. 2015, 5, 10134. [Google Scholar] [CrossRef]
- Ban, H.; Sato, S.; Yoshikawa, S.; Yamada, K.; Nakamura, Y.; Ichinomiya, M.; Sato, N.; Blanc-Mathieu, R.; Endo, H.; Kuwata, A.; et al. Genome Analysis of Parmales, the Sister Group of Diatoms, Reveals the Evolutionary Specialization of Diatoms from Phago-Mixotrophs to Photoautotrophs. Commun. Biol. 2023, 6, 697. [Google Scholar] [CrossRef]
- Keeling, P.J. Chromalveolates and the Evolution of Plastids by Secondary Endosymbiosis. J. Eukaryot. Microbiol. 2009, 56, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sibbald, S.J.; Archibald, J.M. Genomic Insights into Plastid Evolution. Genome Biol. Evol. 2020, 12, 978–990. [Google Scholar] [CrossRef] [PubMed]
- McFadden, G.I.; Yeh, E. The Apicoplast: Now You See It, Now You Don’t. Int. J. Parasitol. 2017, 47, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Woo, Y.H.; Ansari, H.; Otto, T.D.; Klinger, C.M.; Kolisko, M.; Michálek, J.; Saxena, A.; Shanmugam, D.; Tayyrov, A.; Veluchamy, A.; et al. Chromerid Genomes Reveal the Evolutionary Path from Photosynthetic Algae to Obligate Intracellular Parasites. eLife 2015, 4, e06974. [Google Scholar] [CrossRef]
- Macorano, L.; Nowack, E.C.M. Paulinella Chromatophora. Curr. Biol. 2021, 31, R1024–R1026. [Google Scholar] [CrossRef]
- Roelants, F.M.; Leskoske, K.L.; Martinez Marshall, M.N.; Locke, M.N.; Thorner, J. The TORC2-Dependent Signaling Network in the Yeast Saccharomyces Cerevisiae. Biomolecules 2017, 7, 66. [Google Scholar] [CrossRef]
- Waszczak, C.; Carmody, M.; Kangasjärvi, J. Reactive Oxygen Species in Plant Signaling. Annu. Rev. Plant Biol. 2018, 69, 209–236. [Google Scholar] [CrossRef]
- Denoeud, F.; Godfroy, O.; Cruaud, C.; Heesch, S.; Nehr, Z.; Tadrent, N.; Couloux, A.; Brillet-Guéguen, L.; Delage, L.; Mckeown, D.; et al. Evolutionary Genomics of the Emergence of Brown Algae as Key Components of Coastal Ecosystems. Cell 2024, 187, 6943–6965.e39. [Google Scholar] [CrossRef]
- Strassert, J.F.H.; Irisarri, I.; Williams, T.A.; Burki, F. A Molecular Timescale for Eukaryote Evolution with Implications for the Origin of Red Algal-Derived Plastids. Nat. Commun. 2021, 12, 1879. [Google Scholar] [CrossRef] [PubMed]
- Bailleul, B.; Berne, N.; Murik, O.; Petroutsos, D.; Prihoda, J.; Tanaka, A.; Villanova, V.; Bligny, R.; Flori, S.; Falconet, D.; et al. Energetic Coupling between Plastids and Mitochondria Drives CO2 Assimilation in Diatoms. Nature 2015, 524, 366–369. [Google Scholar] [CrossRef] [PubMed]
- Yadavalli, R.; Umeda, K.; Fernández Robledo, J.A. Perkinsus Marinus. Trends Parasitol. 2020, 36, 1013–1014. [Google Scholar] [CrossRef]
- Kamikawa, R.; Mochizuki, T.; Sakamoto, M.; Tanizawa, Y.; Nakayama, T.; Onuma, R.; Cenci, U.; Moog, D.; Speak, S.; Sarkozi, K.; et al. Genome Evolution of a Nonparasitic Secondary Heterotroph, the Diatom Nitzschia Putrida. Sci. Adv. 2022, 8, eabi5075. [Google Scholar] [CrossRef]
- Selosse, M.-A.; Alaux, P.-L.; Deloche, L.; Delannoy, E.; Minasiewicz, J.; Tsiftsis, S.; Figura, T.; Martos, F. Mixotrophy in Orchids: Facts, Questions, and Perspectives. New Phytol. 2025, 246, 1912–1921. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Endoh, R.; Manabe, R.; Ohkuma, M.; Hirakawa, Y. Multiple Losses of Photosynthesis and Convergent Reductive Genome Evolution in the Colourless Green Algae Prototheca. Sci. Rep. 2018, 8, 940. [Google Scholar] [CrossRef]
- Yan, D.; Wang, Y.; Murakami, T.; Shen, Y.; Gong, J.; Jiang, H.; Smith, D.R.; Pombert, J.-F.; Dai, J.; Wu, Q. Auxenochlorella Protothecoides and Prototheca Wickerhamii Plastid Genome Sequences Give Insight into the Origins of Non-Photosynthetic Algae. Sci. Rep. 2015, 5, 14465. [Google Scholar] [CrossRef]
- Pringsheim, E.G.; Hovasse, R. The Loss of Chromatophores in Euglena Gracilis. New Phytol. 1948, 47, 52–87. [Google Scholar] [CrossRef]
- Gain, G.; Vega de Luna, F.; Cordoba, J.; Perez, E.; Degand, H.; Morsomme, P.; Thiry, M.; Baurain, D.; Pierangelini, M.; Cardol, P. Trophic State Alters the Mechanism Whereby Energetic Coupling between Photosynthesis and Respiration Occurs in Euglena Gracilis. New Phytol. 2021, 232, 1603–1617. [Google Scholar] [CrossRef]
- Barua, D.; Płecha, M.; Muszewska, A. Non-Dikarya Fungi Share the TORC1 Pathway with Animals, Not with Saccharomyces Cerevisiae. Sci. Rep. 2025, 15, 5926. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnson, K.; Pourkeramati, D.; Korf, I.; Powers, T. Metabolic Adaptations Determine the Evolutionary Trajectory of TOR Signaling in Diverse Eukaryotes. Biomolecules 2025, 15, 1295. https://doi.org/10.3390/biom15091295
Johnson K, Pourkeramati D, Korf I, Powers T. Metabolic Adaptations Determine the Evolutionary Trajectory of TOR Signaling in Diverse Eukaryotes. Biomolecules. 2025; 15(9):1295. https://doi.org/10.3390/biom15091295
Chicago/Turabian StyleJohnson, Kyle, Dellaraam Pourkeramati, Ian Korf, and Ted Powers. 2025. "Metabolic Adaptations Determine the Evolutionary Trajectory of TOR Signaling in Diverse Eukaryotes" Biomolecules 15, no. 9: 1295. https://doi.org/10.3390/biom15091295
APA StyleJohnson, K., Pourkeramati, D., Korf, I., & Powers, T. (2025). Metabolic Adaptations Determine the Evolutionary Trajectory of TOR Signaling in Diverse Eukaryotes. Biomolecules, 15(9), 1295. https://doi.org/10.3390/biom15091295







