Anatomical, Physiological, and Transcriptome Analyses Revealing Pod Shattering of Medicago ruthenica Associated with Pericarp Lignin Biosynthesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Sampling Preparation
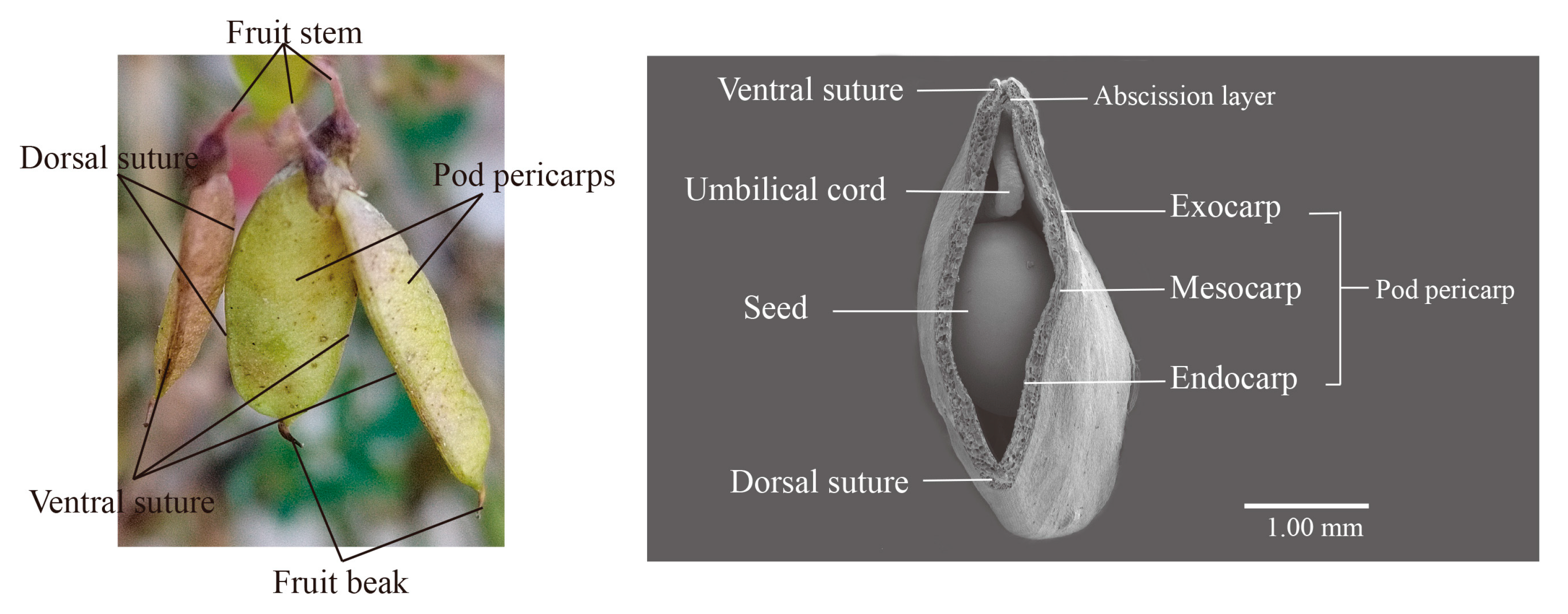
2.3. Anatomical Profile of Pod Pericarps
2.4. Component Analysis and Related Synthetase Activities
2.5. RNA Extraction and Transcriptomic Analysis
2.6. Quantitative Real Time-Polymerase Chain Reaction (RT-qPCR) Analysis
2.7. Promoter Motif Analysis
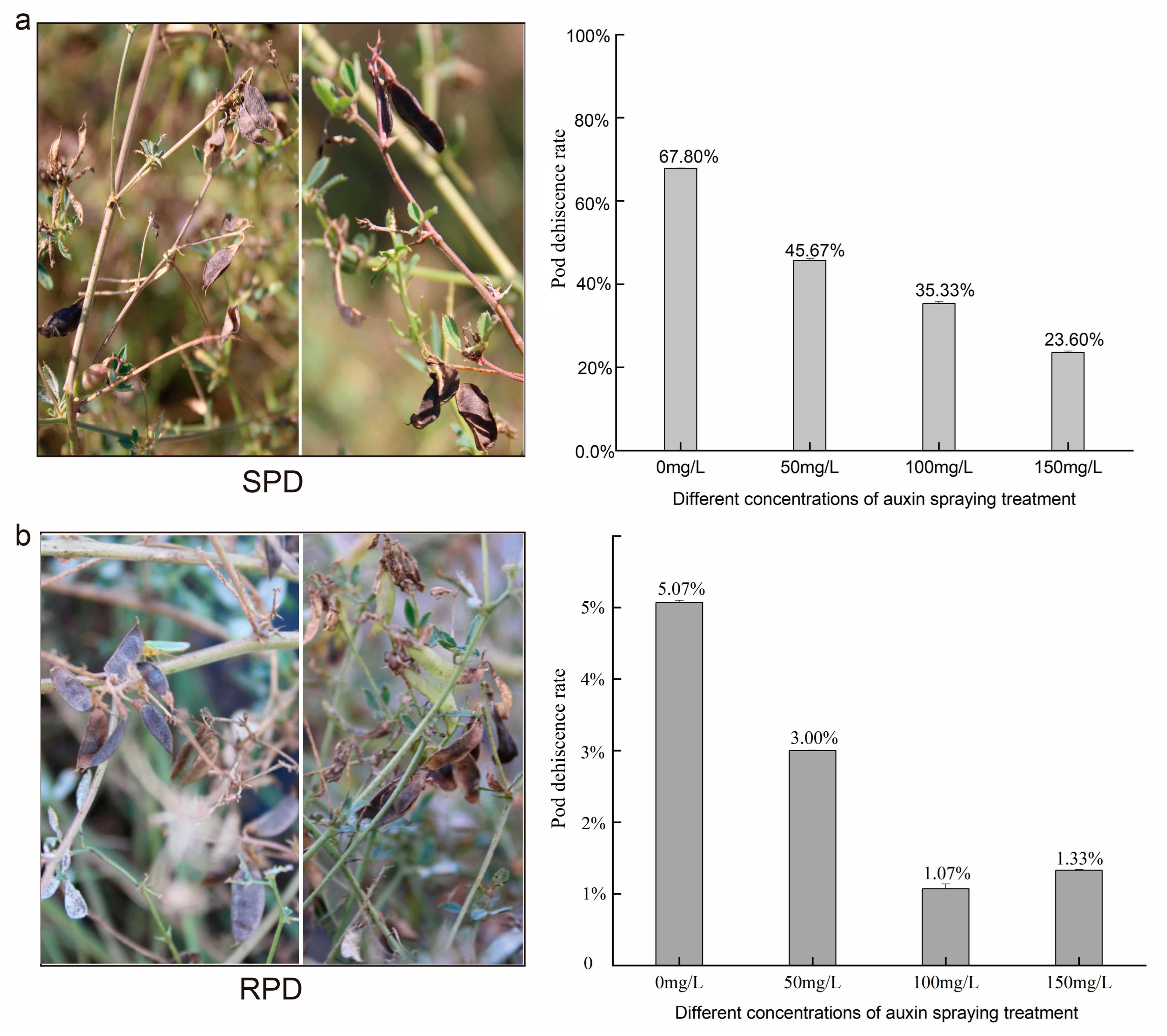
2.8. Verification of Exogenous Auxin Spraying
2.9. Statistical Analysis
3. Results
3.1. Anatomical Characteristics Associated with Pod Shattering
3.2. Analysis of Physiological Characteristics Associated with Pod Shattering
3.3. Analysis of Differentially Expressed Genes (DEGs) and Annotated Pathways Associated with Pod Shattering
3.4. Possible Upstream Regulators of POD Gene Expression
4. Discussion
4.1. Increased Lignin Content Altered the Properties of the Pericarp Material and Promoted Complete Pod Shattering
4.2. Auxin Negatively Regulates Phenylpropanoid Biosynthesis and the Explosive Opening of M. ruthenica Pods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ernest, S. Alfalfa and Relatives: Evolution and Classification of Medicago: The Other Species (Indicates a Species of Uncertain Status) 84; NRC Research Press: Ottawa, ON, Canada, 2011; pp. 565–570. ISBN 9780660199795. [Google Scholar]
- Small, E.; Jomphe, M. A synopsis of the genus Medicago (Leguminosae). Can. J. Bot. 1989, 67, 3260–3294. [Google Scholar] [CrossRef]
- Campbell, T.A.; Bao, G.; Xia, Z.L. Agronomic evaluation of Medicago ruthenica collected in Inner Mongolia. Crop Sci. 1997, 37, 599–604. [Google Scholar] [CrossRef]
- Campbell, T.A.; Bao, G.; Xia, Z.L. Completion of the agronomic evaluations of Medicago ruthenica (L.) Ledebour germplasm collected in Inner Mongolia. Genet. Resour. Crop Evol. 1999, 46, 477–484. [Google Scholar] [CrossRef]
- Li, H.Y.; Li, Z.Y.; Cai, L.Y.; Shi, W.G.; Mi, F.G.; Shi, F.L. Analysis of genetic diversity of Ruthenia Medic (Medicago ruthenica (L.) Trautv.) in Inner Mongolia using ISSR and SSR markers. Genet. Resour. Crop Evol. 2013, 60, 1687–1694. [Google Scholar] [CrossRef]
- Wang, T.; Ren, L.; Li, C.; Zhang, D.; Zhang, X.; Zhou, G.; Gao, D.; Chen, R.; Chen, Y.; Wang, Z.; et al. The genome of a wild Medicago species provides insights into the tolerant mechanisms of legume forage to environmental stress. BMC Biol. 2021, 19, 96. [Google Scholar] [CrossRef] [PubMed]
- Østergaard, L.; Kempin, S.A.; Bies, D.; Klee, H.J.; Yanofsky, M.F. Pod shatter-resistant Brassica fruit produced by ectopic expression of the FRUITFULL gene. Plant Biotechnol. J. 2006, 4, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.W.; Zhu, L.; Li, H.Y.; Liu, W.P.; Wu, Z.N.; Wang, C.H.; Liu, L.; Li, Z.Y.; Li, J. Mechanism of pod shattering in the forage legume Medicago ruthenica. Plant Physiol. Biochem. 2022, 185, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Yang, X.; Liu, J.; Wang, B.H.; Liu, B.L.; Wang, Y.Z. Pod shattering resistance associated with domestication is mediated by a NAC gene in soybean. Nat. Commun. 2014, 5, 3352. [Google Scholar] [CrossRef]
- Funatsuki, H.; Suzuki, M.; Hirose, A.; Inaba, H.; Yamada, T.; Hajika, M.; Komatsu, K.; Katayama, T.; Sayama, T.; Ishimoto, M.; et al. Molecular basis of a shattering resistance boosting global dissemination of soybean. Proc. Natl. Acad. Sci. USA 2014, 111, 17797–17802. [Google Scholar] [CrossRef]
- Liljegren, S.J.; Ditta, G.S.; Eshed, Y.; Savidge, B.; Bowman, J.L.; Yanofsky, M.F. SHATTERPROOF MADS-box genes control seed dispersal in Arabidopsis. Nature 2000, 404, 766–770. [Google Scholar] [CrossRef]
- Ferrándiz, C.; Liljegren, S.J.; Yanofsky, M.F. Negative regulation of the SHATTERPROOF genes by FRUITFULL during Arabidopsis fruit development. Science 2000, 289, 436–438. [Google Scholar] [CrossRef]
- Liljegren, S.J.; Roeder, A.H.K.; Kempin, S.A.; Gremski, K.; Østergaard, L.; Guimil, S.; Reyes, D.K.; Yanofsky, M.F. Control of fruit patterning in Arabidopsis by INDEHISCENT. Cell 2004, 116, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Romkaew, J.; Nagaya, Y.; Goto, M.; Suzuki, K.; Umezaki, T. Pod dehiscence in relation to chemical components of pod shell in soybean. Plant Prod. Sci. 2008, 11, 278–282. [Google Scholar] [CrossRef]
- Wu, J.Y.; Li, J.; Li, Z.Y.; Wang, S.Y. The study on pod dehiscence in Medicago ruthenica. Chin. J. Grassl. 2021, 43, 115–120. [Google Scholar] [CrossRef]
- Aguilar-Benitez, D.; Rubio, J.; Millan, T.; Gil, J.; Die, J.V.; Castro, P. Genetic analysis reveals PDH1 as a candidate gene for control of pod dehiscence in chickpea. Mol. Breed. 2020, 40, 40. [Google Scholar] [CrossRef]
- Parker, T.A.; de Sousa, L.L.; de Oliveira Floriani, T.; Palkovic, A.; Gepts, P. Toward the introgression of PvPdh1 for increased resistance to pod shattering in common bean. Theoretical and Applied Genetics. Theor. Und Angew. Genet. 2021, 134, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Parker, T.A.; Lo, S.; Gepts, P. Pod shattering in grain legumes: Emerging genetic and environment-related patterns. Plant Cell 2021, 33, 179–199. [Google Scholar] [CrossRef]
- Bin, Y.; Weiwei, Z.; Siming, W.; Bingbing, L.; Yan, W.; Nan, X.; Jiangjie, Q.C.; Chaoying, H. Parallel selection of loss-of-function alleles of Pdh1 orthologous genes in warm-season legumes for pod indehiscence and plasticity is related to precipitation. New Phytol. 2023, 240, 863–879. [Google Scholar] [CrossRef]
- Fourquin, C.; del Cerro, C.; Victoria, F.C.; Vialette-Guiraud, A.; de Oliveira, A.C.; Ferrándiz, C. A change in SHATTERPROOF protein lies at the origin of a fruit morphological novelty and a new strategy for seed dispersal in Medicago Genus. Plant Physiol. 2013, 162, 907–917. [Google Scholar] [CrossRef]
- Lo, S.; Parker, T.; Muñoz-Amatriaín, M.; Berny-Mier YTeran, J.C.; Jernstedt, J.; Close, T.J.; Gepts, P. Genetic, anatomical, and environmental patterns related to pod shattering resistance in domesticated cowpea [Vigna unguiculata (L.) Walp]. J. Exp. Bot. 2021, 72, 6219–6229. [Google Scholar] [CrossRef]
- Sperber, K.; Steinbrecher, T.; Graeber, K.; Scherer, G.; Clausing, S.; Wiegand, N.; Hourston, J.E.; Kurre, R.; Leubner-Metzger, G.; Mummenhoff, K. Fruit fracture biomechanics and the release of Lepidium didymum pericarp-imposed mechanical dormancy by fungi. Nat. Commun. 2017, 8, 1868. [Google Scholar] [CrossRef]
- Xue, L.; Sun, M.; Wu, Z.; Yu, L.; Yu, Q.; Tang, Y.; Jiang, F. LncRNA regulates tomato fruit cracking by coordinating gene expression via a hormone-redox-cell wall network. BMC Plant Biol. 2020, 20, 162. [Google Scholar] [CrossRef] [PubMed]
- Van Soest, P.; Wine, R. Use of detergents in the analysis of fibrous feeds. IV. Determination of plant cell-wall constituents. J. Assoc. Off. Anal. Chem. 1967, 50, 50–55. [Google Scholar] [CrossRef]
- Li, M.; Wang, L.; Zhang, J.; Zhan, R.; An, N.; Sun, Y.; Wu, F.; Yang, J.; Su, H. Single-walled carbon nanotubes promotes wood formation in Populus davidiana × P. bolleana. Plant Physiol. Biochem. 2022, 184, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.W.; Su, X.G.; You, Y.L.; Qu, H.X.; Li, Y.B.; Jiang, Y.M. Effect of nitric oxide on pericarp browning of harvested longan fruit in relation to phenolic metabolism. Food Chem. 2007, 104, 571–576. [Google Scholar] [CrossRef]
- Yin, M.; Zhang, S.; Du, X.; Mateo, R.G.; Guo, W.; Li, A.; Wang, Z.; Wu, S.; Chen, J.; Liu, J.; et al. Genomic analysis of Medicago ruthenica provides insights into its tolerance to abiotic stress and demographic history. Mol. Ecol. Resour. 2021, 21, 1641–1657. [Google Scholar] [CrossRef]
- Schmittgen, T.; Livak, K. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; et al. TBtools-II: A "one for all, all for one" bioinformatics platform for biological big-data mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef]
- Liu, D.; Li, J.; Li, Z.; Pei, Y. Hydrogen sulfide inhibits ethylene-induced petiole abscission in tomato (Solanum lycopersicum L.). Hortic. Res. 2000, 7, 14. [Google Scholar] [CrossRef]
- Christiansen, L.C.; Dal Degan, F.; Ulvskov, P.; Borkhardt, B. Examination of the dehiscence zone in soybean pods and isolation of a dehiscence-related endopolygalacturonase gene. Plant Cell Environ. 2010, 25, 479–490. [Google Scholar] [CrossRef]
- Petersen, M.; Sander, L.; Child, R.; van Onckelen, H.; Ulvskov, P.; Borkhardt, B. Isolation and characterisation of a pod dehiscence zone-specific polygalacturonase from Brassica napus. Plant Mol. Biol. 1996, 31, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.A.; Whitelaw, C.A.; Gonzalez-Carranza, Z.H.; McManus, M.T. Cell separation processes in plants—Models, mechanisms and manipulation. Ann. Bot. 2000, 86, 223–235. [Google Scholar] [CrossRef]
- Cullen, E.; Hay, A. Creating an explosion: Form and function in explosive fruit. Curr. Opin. Plant Biol. 2024, 79, 102543. [Google Scholar] [CrossRef]
- Timothy, M.S.; Marco, L.H.G.; Rong, Z.; Shahab, S.; Suzanne, R.A.; Adrian, J.C. Dehydration and dehiscence in siliques of Brassica napus and Brassica rapa. Can. J. Bot.-Rev. Can. Bot. 2003, 81, 248–254. [Google Scholar] [CrossRef]
- Fu, Z.; Chen, J.; Zhang, Y.; Xie, F.; Lu, Y. Review on wood deformation and cracking during moisture loss. Polymers 2023, 15, 3295. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Shakher, C. Experimental characterization of the hygroscopic properties of wood during convective drying using digital holographic interferometry. Appl. Opt. 2016, 55, 960–968. [Google Scholar] [CrossRef]
- Fu, Z.; Wang, H.; Li, J.; Lu, Y. Determination of Moisture Content and Shrinkage Strain during Wood Water Loss with Electrochemical Method. Polymers 2022, 14, 778. [Google Scholar] [CrossRef]
- Hu, W.J.; Harding, S.A.; Lung, J.; Popko, J.L.; Ralph, J.; Stokke, D.D.; Tsai, C.J.; Chiang, V.L. Repression of lignin biosynthesis promotes cellulose accumulation and growth in transgenic trees. Nat. Biotechnol. 1999, 17, 808–812. [Google Scholar] [CrossRef]
- Hofhuis, H.; Moulton, D.; Lessinnes, T.; Routier-Kierzkowska, A.L.; Bomphrey, R.J.; Mosca, G.; Reinhardt, H.; Sarchet, P.; Gan, X.; Tsiantis, M.; et al. Morphomechanical innovation drives explosive seed dispersal. Cell 2016, 166, 222–233. [Google Scholar] [CrossRef]
- Wang, L.; He, C.; Li, X.; Yang, X. Performance analysis of ternary composites with lignin, Eucalyptus Fiber, and polyvinyl chloride. BioResources 2018, 13, 6510–6523. [Google Scholar] [CrossRef]
- Dong, X.M.; Chen, J.W.; Zhou, Q.; Luo, D.; Fang, L.F.; Liu, W.X.; Liu, Z.P. Pod-shattering characteristic differences between shattering-resistant and shattering-susceptible common vetch accessions are associated with lignin biosynthesis. J. Integr. Agric. 2024, 3, 1–31. [Google Scholar] [CrossRef]
- Cousins, W. Elastic modulus of lignin as related to moisture content. Wood Sci. Technol. 1976, 10, 9–17. [Google Scholar] [CrossRef]
- Pejić, B.; Kostić, M.; Škundrić, P.; Praskalo, J. The effects of hemicelluloses and lignin removal on water uptake behavior of hemp fibers. Bioresour. Technol. 2008, 99, 7152–7159. [Google Scholar] [CrossRef] [PubMed]
- Meier, K.K.; Jones, S.M.; Kaper, T.; Hansson, H.; Koetsier, M.J.; Karkehabadi, S.; Solomon, E.I.; Sandgren, M.; Kelemen, B. Oxygen activation by Cu LPMOs in recalcitrant carbohydrate polysaccharide conversion to monomer sugars. Chem. Rev. 2018, 118, 2593–2635. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Chen, Q.; Fang, C.; Zhang, S.; Liu, X.; Fei, B. Effect of chemical composition of bamboo fibers on water sorption. Cellulose 2021, 28, 7273–7282. [Google Scholar] [CrossRef]
- Murgia, M.L.; Attene, G.; Rodriguez, M.; Bitocchi, E.; Bellucci, E.; Fois, D.; Nanni, L.; Gioia, T.; Albani, D.M.; Papa, R.; et al. A comprehensive phenotypic investigation of the “pod-shattering syndrome” in common bean. Front. Plant Sci. 2017, 8, 251. [Google Scholar] [CrossRef]
- Jinmi, Y.; LaeHyeon, C.; Htet, W.A.; HeeJong, K.; Gynheung, A. KNOX protein OSH15 induces grain shattering by repressing lignin biosynthesis genes. Plant Physiol. 2017, 174, 312–325. [Google Scholar] [CrossRef]
- Zhao, X.; Xie, W.; Zhang, J.; Zhang, Z.; Wang, Y. Histological characteristics, cell wall hydrolytic enzymes activity and candidate genes expression associated with seed shattering of Elymus sibiricus accessions. Front. Plant Sci. 2017, 8, 606. [Google Scholar] [CrossRef]
- Ralph, J.; Lundquist, K.; Brunow, G.; Lu, F.; Kim, H.; Schatz, P.F.; Marita, J.M.; Hatfield, R.D.; Sally, A.; Ralph, S.A.; et al. Lignins: Natural polymers from oxidative coupling of 4-hydroxyphenyl-propanoids. Phytochem. Rev. 2004, 3, 29–60. [Google Scholar] [CrossRef]
- Ong, S.S.; Wickneswari, R. Expression profile of small RNAs in Acacia mangium secondary xylem tissue with contrasting lignin content—Potential regulatory sequences in monolignol biosynthetic pathway. BMC Genom. 2011, 12, S13. [Google Scholar] [CrossRef] [PubMed]
- Cosio, C.; Dunand, C. Transcriptome analysis of various flower and silique development stages indicates a set of class III peroxidase genes potentially involved in pod shattering in Arabidopsis thaliana. BMC Genom. 2010, 11, 528. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.P.; Guo, M.W.; Zhu, L. Effects of endogenous hormones on pod cracking of Medicago ruthenica. Chin. J. Grassl. 2024, 46, 27–34. (In Chinese) [Google Scholar] [CrossRef]
- Sorefan, K.; Girin, T.; Liljegren, S.J.; Ljung, K.; Robles, P.; Galván-Ampudia, C.S.; Offringa, R.; Friml, J.; Yanofsky, M.F.; Østergaard, L. A regulated auxin minimum is required for seed dispersal in Arabidopsis. Nature 2009, 459, 583–586. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Du, Q.; Zhuo, C.; Qi, L.; Wang, H. LBD29-involved auxin signaling represses NAC master regulators and fiber wall biosynthesis. Plant Physiol. 2019, 181, 595–608. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Plant Physiology, 5th ed.; Science Press: Beijing, China, 2015; pp. 297–460. ISBN 978-7-03-044040-2. [Google Scholar]
- Pencík, A.; Casanova-Sáez, R.; Pilarová, V.; Žukauskaite, A.; Pinto, R.; Micol, J.L.; Ljung, K.; Novák, O. Ultra-rapid auxin metabolite profiling for high-throughput mutant screening in Arabidopsis. J. Exp. Bot. 2018, 69, 2569–2579. [Google Scholar] [CrossRef]




| Principal Component | Eigenvalue | Percentage of Variance (%) | Cumulative (%) | Eigen Vectors | |||
|---|---|---|---|---|---|---|---|
| Pec | Cel | Hec | Lig | ||||
| PC1 | 1.819 | 45.477 | 45.477 | −0.623 | 0.358 | 0.688 | −0.106 |
| PC2 | 1.305 | 32.622 | 78.099 | 0.316 | 0.603 | 0.085 | 0.727 |
| PC3 | 0.642 | 16.061 | 94.160 | 0.329 | 0.652 | −0.144 | −0.668 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, L.; Guo, M.; Li, Z.; Li, J.; Li, H.; Wu, Z.; Tian, Y.; Zhao, C. Anatomical, Physiological, and Transcriptome Analyses Revealing Pod Shattering of Medicago ruthenica Associated with Pericarp Lignin Biosynthesis. Biomolecules 2025, 15, 1269. https://doi.org/10.3390/biom15091269
Zhu L, Guo M, Li Z, Li J, Li H, Wu Z, Tian Y, Zhao C. Anatomical, Physiological, and Transcriptome Analyses Revealing Pod Shattering of Medicago ruthenica Associated with Pericarp Lignin Biosynthesis. Biomolecules. 2025; 15(9):1269. https://doi.org/10.3390/biom15091269
Chicago/Turabian StyleZhu, Lin, Maowei Guo, Zhiyong Li, Jun Li, Hongyan Li, Zinian Wu, Yonglei Tian, and Chenggui Zhao. 2025. "Anatomical, Physiological, and Transcriptome Analyses Revealing Pod Shattering of Medicago ruthenica Associated with Pericarp Lignin Biosynthesis" Biomolecules 15, no. 9: 1269. https://doi.org/10.3390/biom15091269
APA StyleZhu, L., Guo, M., Li, Z., Li, J., Li, H., Wu, Z., Tian, Y., & Zhao, C. (2025). Anatomical, Physiological, and Transcriptome Analyses Revealing Pod Shattering of Medicago ruthenica Associated with Pericarp Lignin Biosynthesis. Biomolecules, 15(9), 1269. https://doi.org/10.3390/biom15091269







