PPARγ Agonism Modulates Synovial Macrophage and Cartilage Responses in an Equine Model of Synovial Inflammation—Implications for Joint Therapy
Abstract
1. Introduction
2. Materials and Methods
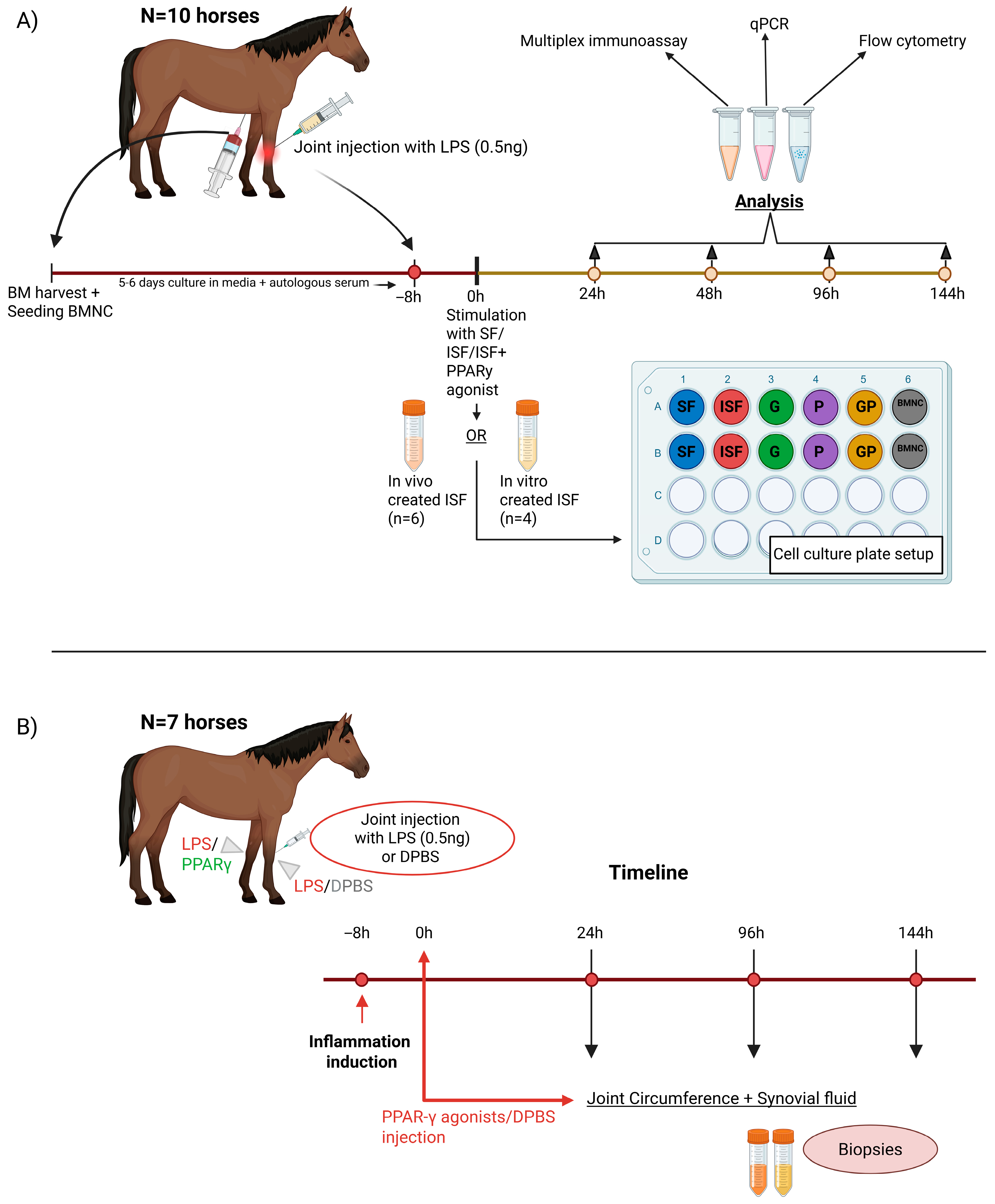
2.1. Study Design
2.1.1. In Vitro Study
2.1.2. In Vivo Study
2.2. BMNC Isolation and Macrophage Culture
2.3. Induction of Synovitis and Processing of Inflamed Synovial Fluid
2.4. Inflammatory Challenge and Treatment with PPARγ Agonists
2.5. Cell Harvesting and Processing
2.6. Gene Expression
2.7. Flow Cytometry
2.8. Joint Treatment and Synovial Fluid Sampling
2.9. Multiplex Immunoassay
2.10. Histology
2.11. Statistical Analysis
3. Results
3.1. Cell Culture Behavior
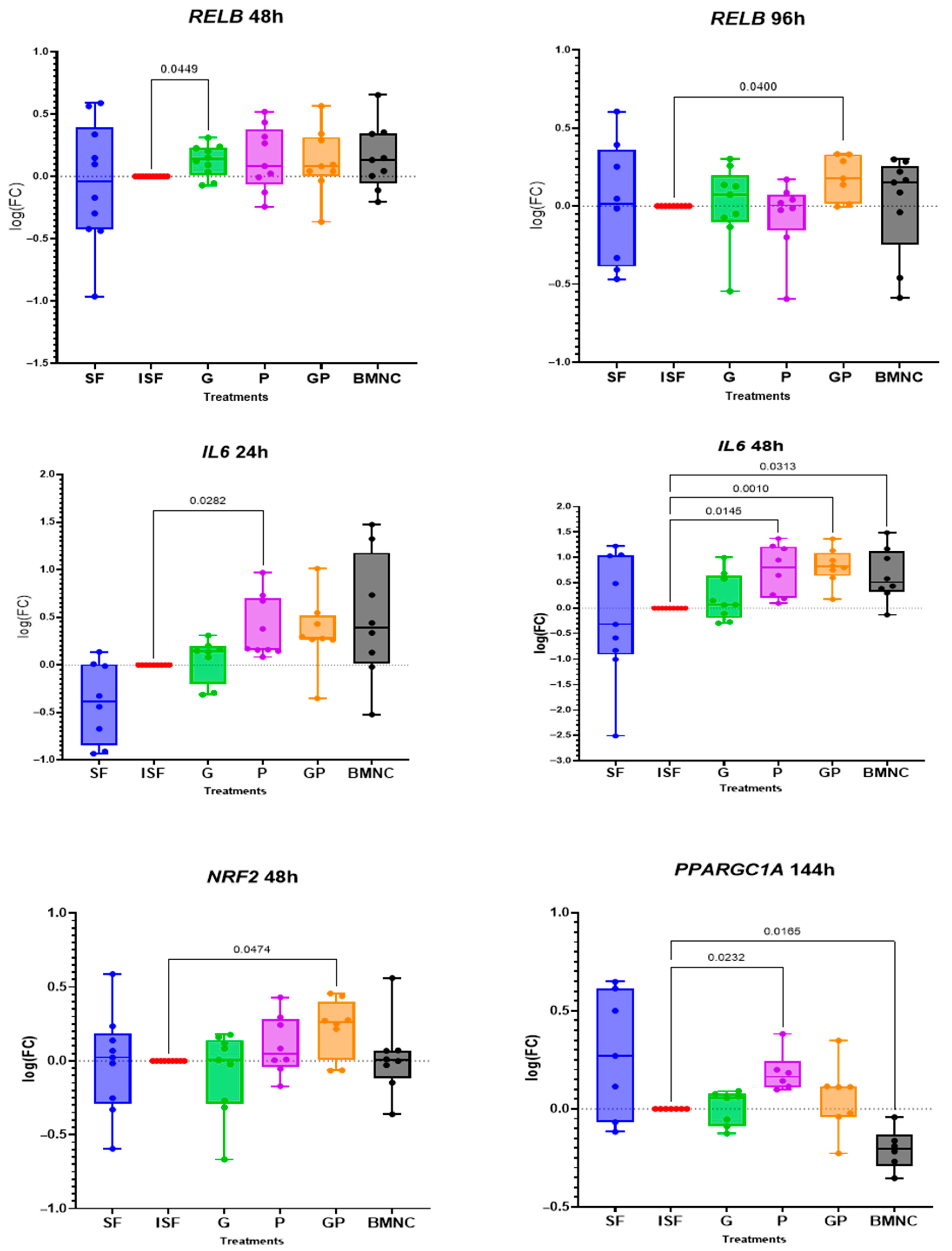
3.2. Gene Expression
3.3. Flow Cytometry
3.4. Cytokine/Chemokine Quantification
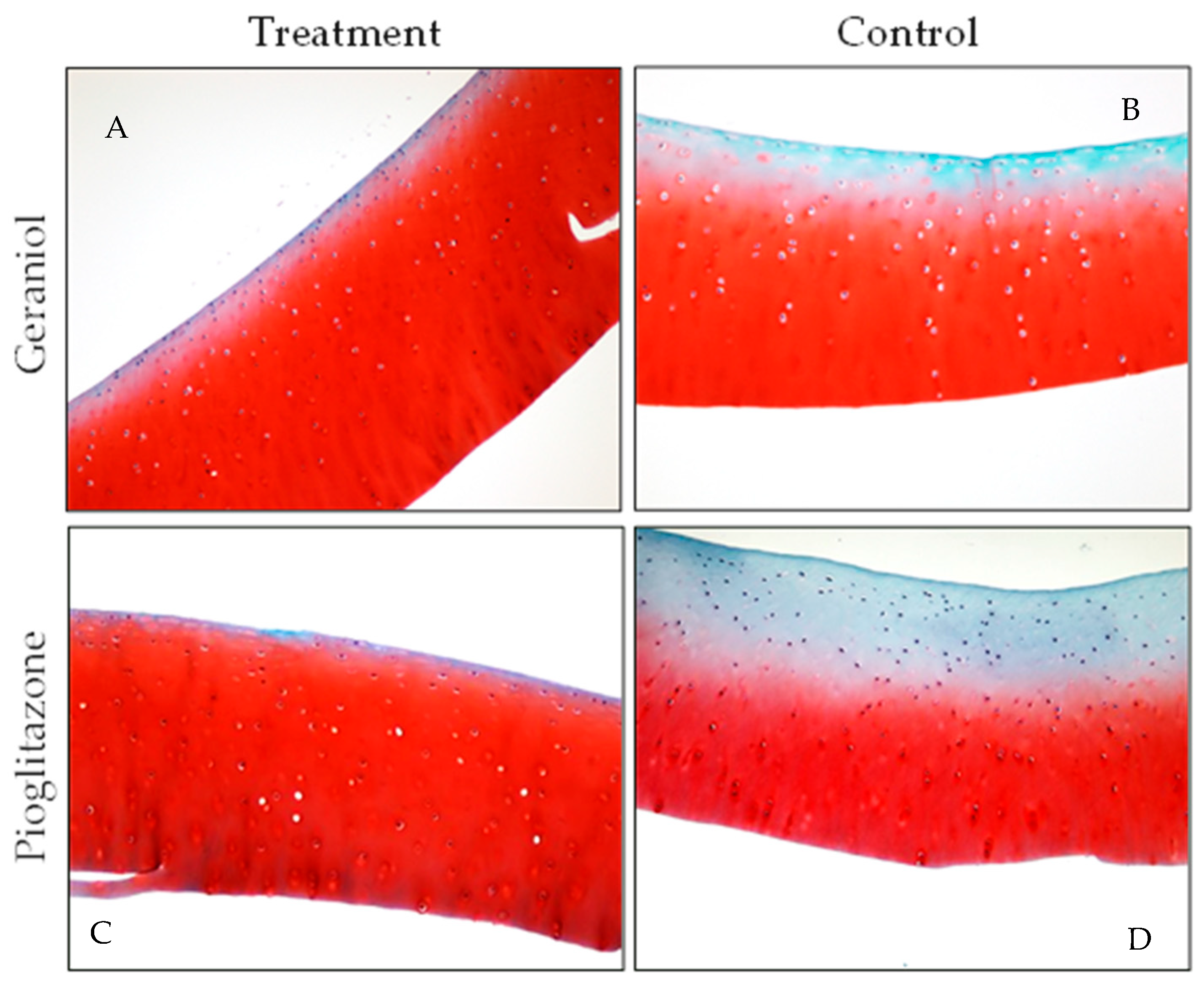
3.5. Gross Pathology and Histology
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PPARγ | peroxisome proliferator-activated receptor gamma |
| OA | osteoarthritis |
| NSAIDs | non-steroidal anti-inflammatory drugs |
| PPARGC1a | PPARγ coactivator 1A gene |
| RELB | transcription factor RELB gene |
| IL6 | interleukin 6 coding gene |
| IL6 | interleukin 6 protein |
| NRF2 | interleukin 6 protein |
| TNF-α | tumor necrosis factor alpha |
| PGE2 | prostaglandin E2 |
| NF-κB | nuclear factor kappa B |
| IL10 | interleukin-10 protein |
| IL1β | interleukin-1β protein |
| IL1α | interleukin-1α protein |
| IGF1 | insulin-like growth factor 1 |
| SDF1 | stromal cell-derived factor 1 |
| MCP1 | macrophage chemotactic protein 1 |
| BMNCs | bone marrow mononuclear cells |
| BMDMs | bone marrow-derived macrophages |
| G | geraniol |
| P | pioglitazone |
| GP | geraniol and pioglitazone combined |
| SF | synovial fluid |
| ISF | inflamed synovial fluid |
| PCR | polymerase chain reaction |
| ROS | reactive oxygen species |
| LPS | lipopolysaccharide |
| IU | international units |
| DPBS | Dulbecco’s phosphate-buffered saline |
| DMSO | dimethyl sulfoxide |
| FBS | fetal bovine serum |
| M-CSF | macrophage-colony stimulating factor |
| AVMA | American Veterinary Medical Association |
| mRNA | messenger ribonucleic acid |
| cDNA | complementary deoxyribonucleic acid |
| MdFI | median fluorescence intensity |
| MinDC | minimum detectable concentration |
| AZF | acetic zinc formalin |
| OARSI | Osteoarthritis Research Society International |
| LLOD | lower limit of detection |
| ANOVA | analysis of variance |
| H&E | hematoxylin and eosin |
| Saf-O | safranin O |
| RXR | retinoid X receptor |
References
- Boring, M.A.; Hootman, J.M.; Liu, Y.; Theis, K.A.; Murphy, L.B.; Barbour, K.E.; Helmick, C.G.; Brady, T.J.; Croft, J.B. Prevalence of Arthritis and Arthritis-Attributable Activity Limitation by Urban-Rural County Classification-United States, 2015. MMWR Morb. Mortal. Wkly. Rep. 2017, 66, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Cisternas, M.G.; Murphy, L.; Sacks, J.J.; Solomon, D.H.; Pasta, D.J.; Helmick, C.G. Alternative Methods for Defining Osteoarthritis and the Impact on Estimating Prevalence in a US Population-Based Survey. Arthritis Care Res. 2016, 68, 574–580. [Google Scholar] [CrossRef]
- Murphy, L.B.; Cisternas, M.G.; Pasta, D.J.; Helmick, C.G.; Yelin, E.H. Medical Expenditures and Earnings Losses Among US Adults with Arthritis in 2013. Arthritis Care Res. 2018, 70, 869–876. [Google Scholar] [CrossRef]
- Oke, S.; McIlwraith, C.W.; Moyer, W. Review of the Economic Impact of Osteoarthritis and Oral Joint-Health Supplements in Horses. Jt. AAEP Proc. 2010, 56, 12–16. [Google Scholar]
- Deloitte, L. The Economic Impact of the Horse Industry on the United States; Consulting, N., Deloitte, L.L., Eds.; American Horse Council: Washington, DC, USA, 2005. [Google Scholar]
- Wagner, B.; Hillegas, J.M.; Brinker, D.R.; Horohov, D.W.; Antczak, D.F. Characterization of monoclonal antibodies to equine interleukin-10 and detection of T regulatory 1 cells in horses. Vet. Immunol. Immunopathol. 2008, 122, 57–64. [Google Scholar] [CrossRef]
- Bondeson, J.; Blom, A.B.; Wainwright, S.; Hughes, C.; Caterson, B.; van den Berg, W.B. The role of synovial macrophages and macrophage-produced mediators in driving inflammatory and destructive responses in osteoarthritis. Arthritis Rheum. 2010, 62, 647–657. [Google Scholar] [CrossRef]
- Marder, W.; Khalatbari, S.; Myles, J.D.; Hench, R.; Lustig, S.; Yalavarthi, S.; Parameswaran, A.; Brook, R.D.; Kaplan, M.J. The peroxisome proliferator activated receptor-γ pioglitazone improves vascular function and decreases disease activity in patients with rheumatoid arthritis. J. Am. Heart Assoc. 2013, 2, e000441. [Google Scholar] [CrossRef] [PubMed]
- Ormseth, M.J.; Oeser, A.M.; Cunningham, A.; Bian, A.; Shintani, A.; Solus, J.; Tanner, S.; Stein, C.M. Peroxisome proliferator-activated receptor γ agonist effect on rheumatoid arthritis: A randomized controlled trial. Arthritis Res. Ther. 2013, 15, R110. [Google Scholar] [CrossRef]
- Souza, M.V.d. Osteoarthritis in horses—Part 2: A review of the intra-articular use of corticosteroids as a method of treatment. Braz. Arch. Biol. Technol. 2016, 59, 1–10. [Google Scholar] [CrossRef]
- McAlindon, T.E.; LaValley, M.P.; Harvey, W.F.; Price, L.L.; Driban, J.B.; Zhang, M.; Ward, R.J. Effect of Intra-articular Triamcinolone vs Saline on Knee Cartilage Volume and Pain in Patients with Knee Osteoarthritis: A Randomized Clinical Trial. JAMA 2017, 317, 1967–1975. [Google Scholar] [CrossRef] [PubMed]
- McAlindon, T.E.; Bannuru, R.R.; Sullivan, M.C.; Arden, N.K.; Berenbaum, F.; Bierma-Zeinstra, S.M.; Hawker, G.A.; Henrotin, Y.; Hunter, D.J.; Kawaguchi, H.; et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthr. Cartil. 2014, 22, 363–388. [Google Scholar] [CrossRef]
- Bellamy, N.; Campbell, J.; Robinson, V.; Gee, T.; Bourne, R.; Wells, G. Intraarticular corticosteroid for treatment of osteoarthritis of the knee. Cochrane Database Syst. Rev. 2006, Cd005328. [Google Scholar]
- Norling, L.V.; Headland, S.E.; Dalli, J.; Arnardottir, H.H.; Haworth, O.; Jones, H.R.; Irimia, D.; Serhan, C.N.; Perretti, M. Proresolving and cartilage-protective actions of resolvin D1 in inflammatory arthritis. JCI Insight 2016, 1, e85922. [Google Scholar] [CrossRef]
- Panigrahy, D.; Gilligan, M.M.; Serhan, C.N.; Kashfi, K. Resolution of inflammation: An organizing principle in biology and medicine. Pharmacol. Ther. 2021, 227, 107879. [Google Scholar] [CrossRef]
- Sugimoto, M.A.; Sousa, L.P.; Pinho, V.; Perretti, M.; Teixeira, M.M. Resolution of Inflammation: What Controls Its Onset? Front. Immunol. 2016, 7, 160. [Google Scholar] [CrossRef]
- Markworth, J.F.; Vella, L.; Lingard, B.S.; Tull, D.L.; Rupasinghe, T.W.; Sinclair, A.J.; Maddipati, K.R.; Cameron-Smith, D. Human inflammatory and resolving lipid mediator responses to resistance exercise and ibuprofen treatment. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, R1281–R1296. [Google Scholar] [CrossRef] [PubMed]
- Colbath, A.C.; Dow, S.W.; Hopkins, L.S.; Phillips, J.N.; McIlwraith, C.W.; Goodrich, L.R. Single and repeated intra-articular injections in the tarsocrural joint with allogeneic and autologous equine bone marrow-derived mesenchymal stem cells are safe, but did not reduce acute inflammation in an experimental interleukin-1β model of synovitis. Equine Vet. J. 2020, 52, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Broeckx, S.; Suls, M.; Beerts, C.; Vandenberghe, A.; Seys, B.; Wuertz-Kozak, K.; Duchateau, L.; Spaas, J.H. Allogenic mesenchymal stem cells as a treatment for equine degenerative joint disease: A pilot study. Curr. Stem Cell Res. Ther. 2014, 9, 497–503. [Google Scholar] [CrossRef]
- Campbell, K.A.; Saltzman, B.M.; Mascarenhas, R.; Khair, M.M.; Verma, N.N.; Bach, B.R., Jr.; Cole, B.J. Does Intra-articular Platelet-Rich Plasma Injection Provide Clinically Superior Outcomes Compared With Other Therapies in the Treatment of Knee Osteoarthritis? A Systematic Review of Overlapping Meta-analyses. Arthroscopy 2015, 31, 2213–2221. [Google Scholar] [CrossRef] [PubMed]
- Gobbi, A.; Lad, D.; Karnatzikos, G. The effects of repeated intra-articular PRP injections on clinical outcomes of early osteoarthritis of the knee. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 2170–2177. [Google Scholar] [CrossRef]
- Laudy, A.B.; Bakker, E.W.; Rekers, M.; Moen, M.H. Efficacy of platelet-rich plasma injections in osteoarthritis of the knee: A systematic review and meta-analysis. Br. J. Sports Med. 2015, 49, 657–672. [Google Scholar] [CrossRef]
- Textor, J.A.; Willits, N.H.; Tablin, F. Synovial fluid growth factor and cytokine concentrations after intra-articular injection of a platelet-rich product in horses. Vet. J. 2013, 198, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Manferdini, C.; Paolella, F.; Gabusi, E.; Silvestri, Y.; Gambari, L.; Cattini, L.; Filardo, G.; Fleury-Cappellesso, S.; Lisignoli, G. From osteoarthritic synovium to synovial-derived cells characterization: Synovial macrophages are key effector cells. Arthritis Res. Ther. 2016, 18, 83. [Google Scholar] [CrossRef]
- Barrera, P.; Blom, A.; van Lent, P.L.; van Bloois, L.; Beijnen, J.H.; van Rooijen, N.; de Waal Malefijt, M.C.; van de Putte, L.B.; Storm, G.; van den Berg, W.B. Synovial macrophage depletion with clodronate-containing liposomes in rheumatoid arthritis. Arthritis Rheum. 2000, 43, 1951–1959. [Google Scholar] [CrossRef]
- Haraden, C.A.; Huebner, J.L.; Hsueh, M.F.; Li, Y.J.; Kraus, V.B. Synovial fluid biomarkers associated with osteoarthritis severity reflect macrophage and neutrophil related inflammation. Arthritis Res. Ther. 2019, 21, 146. [Google Scholar] [CrossRef]
- Menarim, B.C.; Gillis, K.H.; Oliver, A.; Mason, C.; Ngo, Y.; Werre, S.R.; Barrett, S.H.; Luo, X.; Byron, C.R.; Dahlgren, L.A. Autologous bone marrow mononuclear cells modulate joint homeostasis in an equine in vivo model of synovitis. FASEB J. 2019, 33, 14337–14353. [Google Scholar] [CrossRef]
- Menarim, B.C.; Gillis, K.H.; Oliver, A.; Ngo, Y.; Werre, S.R.; Barrett, S.H.; Rodgerson, D.H.; Dahlgren, L.A. Macrophage Activation in the Synovium of Healthy and Osteoarthritic Equine Joints. Front. Vet. Sci. 2020, 7, 568756. [Google Scholar] [CrossRef]
- Everett, J.B.; Menarim, B.C.; Barrett, S.H.; Bogers, S.H.; Byron, C.R.; Pleasant, R.S.; Werre, S.R.; Dahlgren, L.A. Intra-articular bone marrow mononuclear cell therapy improves lameness from naturally occurring equine osteoarthritis. Front. Vet. Sci. 2023, 10. [Google Scholar] [CrossRef] [PubMed]
- Goncars, V.; Jakobsons, E.; Blums, K.; Briede, I.; Patetko, L.; Erglis, K.; Erglis, M.; Kalnberzs, K.; Muiznieks, I.; Erglis, A. The comparison of knee osteoarthritis treatment with single-dose bone marrow-derived mononuclear cells vs. hyaluronic acid injections. Medicina 2017, 53, 101–108. [Google Scholar] [CrossRef]
- Goncars, V.; Kalnberzs, K.; Jakobsons, E.; Enģele, I.; Briede, I.; Blums, K.; Erglis, K.; Erglis, M.; Patetko, L.; Muiznieks, I.; et al. Treatment of Knee Osteoarthritis with Bone Marrow–Derived Mononuclear Cell Injection: 12-Month Follow-up. Cartilage 2018, 10, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Menarim, B.C.; El-Sheikh Ali, H.; Loux, S.C.; Scoggin, K.E.; Kalbfleisch, T.S.; MacLeod, J.N.; Dahlgren, L.A. Transcriptional and Histochemical Signatures of Bone Marrow Mononuclear Cell-Mediated Resolution of Synovitis. Front. Immunol. 2021, 12, 734322. [Google Scholar] [CrossRef]
- Croasdell, A.; Duffney, P.F.; Kim, N.; Lacy, S.H.; Sime, P.J.; Phipps, R.P. PPARγ and the Innate Immune System Mediate the Resolution of Inflammation. PPAR Res. 2015, 2015, 549691. [Google Scholar] [CrossRef]
- Vasheghani, F.; Monemdjou, R.; Fahmi, H.; Zhang, Y.; Perez, G.; Blati, M.; St-Arnaud, R.; Pelletier, J.P.; Beier, F.; Martel-Pelletier, J.; et al. Adult cartilage-specific peroxisome proliferator-activated receptor gamma knockout mice exhibit the spontaneous osteoarthritis phenotype. Am. J. Pathol. 2013, 182, 1099–1106. [Google Scholar] [CrossRef]
- Boileau, C.; Martel-Pelletier, J.; Fahmi, H.; Mineau, F.; Boily, M.; Pelletier, J.-P. The peroxisome proliferator–activated receptor γ agonist pioglitazone reduces the development of cartilage lesions in an experimental dog model of osteoarthritis: In vivo protective effects mediated through the inhibition of key signaling and catabolic pathways. Arthritis Rheum. 2007, 56, 2288–2298. [Google Scholar] [CrossRef]
- Nebbaki, S.S.; El Mansouri, F.E.; Afif, H.; Kapoor, M.; Benderdour, M.; Pelletier, J.P.; Martel-Pelletier, J.; Fahmi, H. Expression of peroxisome proliferator-activated receptors α, β, γ, and H- and L-prostaglandin D synthase during osteoarthritis in the spontaneous hartley guinea pig and experimental dog models. J. Rheumatol. 2013, 40, 877–890. [Google Scholar] [CrossRef] [PubMed]
- Afif, H.; Benderdour, M.; Mfuna-Endam, L.; Martel-Pelletier, J.; Pelletier, J.P.; Duval, N.; Fahmi, H. Peroxisome proliferator-activated receptor gamma1 expression is diminished in human osteoarthritic cartilage and is downregulated by interleukin-1beta in articular chondrocytes. Arthritis Res. Ther. 2007, 9, R31. [Google Scholar] [CrossRef] [PubMed]
- Daniel, B.; Nagy, G.; Horvath, A.; Czimmerer, Z.; Cuaranta-Monroy, I.; Poliska, S.; Hays, T.T.; Sauer, S.; Francois-Deleuze, J.; Nagy, L. The IL-4/STAT6/PPARγ signaling axis is driving the expansion of the RXR heterodimer cistrome, providing complex ligand responsiveness in macrophages. Nucleic Acids Res. 2018, 46, 4425–4439. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Qian, H.; Wan, B.; Zhou, L.; Chen, C.; Lv, Y.; Chen, M.; Zhu, S.; Ye, L.; Wang, X.; et al. Geranylgeranyl diphosphate synthase deficiency hyperactivates macrophages and aggravates lipopolysaccharide-induced acute lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. 2021, 320, L1011–L1024. [Google Scholar] [CrossRef]
- Wei, L.; Zheng, Y.Y.; Sun, J.; Wang, P.; Tao, T.; Li, Y.; Chen, X.; Sang, Y.; Chong, D.; Zhao, W.; et al. GGPP depletion initiates metaflammation through disequilibrating CYB5R3-dependent eicosanoid metabolism. J. Biol. Chem. 2020, 295, 15988–16001. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.N.; Ye, J.; Hao, R.; Debose-Boyd, R.; Ye, J. Sufficient production of geranylgeraniol is required to maintain endotoxin tolerance in macrophages. J. Lipid Res. 2013, 54, 3430–3437. [Google Scholar] [CrossRef][Green Version]
- Matsubara, T.; Takakura, N.; Urata, M.; Muramatsu, Y.; Tsuboi, M.; Yasuda, K.; Addison, W.N.; Zhang, M.; Matsuo, K.; Nakatomi, C.; et al. Geranylgeraniol Induces PPARγ Expression and Enhances the Biological Effects of a PPARγ Agonist in Adipocyte Lineage Cells. In Vivo 2018, 32, 1339–1344. [Google Scholar] [CrossRef] [PubMed]
- Tricarico, P.M.; Kleiner, G.; Valencic, E.; Campisciano, G.; Girardelli, M.; Crovella, S.; Knowles, A.; Marcuzzi, A. Block of the mevalonate pathway triggers oxidative and inflammatory molecular mechanisms modulated by exogenous isoprenoid compounds. Int. J. Mol. Sci. 2014, 15, 6843–6856. [Google Scholar] [CrossRef] [PubMed]
- Marcuzzi, A.; Decorti, G.; Pontillo, A.; Ventura, A.; Tommasini, A. Decreased cholesterol levels reflect a consumption of anti-inflammatory isoprenoids associated with an impaired control of inflammation in a mouse model of mevalonate kinase deficiency. Inflamm. Res. 2010, 59, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, Z.; Fu, X.; Lin, Z.; Yu, K. Geraniol-mediated osteoarthritis improvement by down-regulating PI3K/Akt/NF-κB and MAPK signals: In vivo and in vitro studies. Int. Immunopharmacol. 2020, 86, 106713. [Google Scholar] [CrossRef]
- Barreira, A.P.B.; Alves, A.L.G.; Saito, M.E.; Amorim, R.L.; Kohayagawa, A.; Menarim, B.C.; Mota, L.S. Autologous Implant of Bone Mar-row Mononuclear Cells as Treatment of Induced Equine Tendinitis. Intern. J. Appl. Res. Vet. Med. 2008, 6, 46–54. [Google Scholar]
- Menarim, B.C.; Gillis, K.H.; Oliver, A.; Mason, C.; Werre, S.R.; Luo, X.; Byron, C.R.; Kalbfleisch, T.S.; MacLeod, J.N.; Dahlgren, L.A. Inflamed synovial fluid induces a homeostatic response in bone marrow mononuclear cells in vitro: Implications for joint therapy. FASEB J. 2020, 34, 4430–4444. [Google Scholar] [CrossRef]
- Corrêa, F.; Borlone, C.; Wittwer, F.; Bustamante, H.; Müller, A.; Ramirez, A.; Menarim, B.C. 121. Mononuclear cells concentrations in fractioned samples of bone marrow aspirate of horse sternum. In Proceedings of the 14th Annual Congress and International Society for Animal Clinical Pathology (ISACP), Ljubljana, Slovenia, 3–7 July 2012; pp. 1–47. [Google Scholar]
- Ma, C.; Zhang, Y.; Li, Y.-Q.; Chen, C.; Cai, W.; Zeng, Y.-L. The Role of PPARγ in Advanced Glycation End Products-Induced Inflammatory Response in Human Chondrocytes. PLoS ONE 2015, 10, e0125776. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Δ;ΔC(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Doherty, E.; Perl, A. Measurement of Mitochondrial Mass by Flow Cytometry during Oxidative Stress. React. Oxyg. Species (Apex) 2017, 4, 275–283. [Google Scholar] [CrossRef]
- Jayadev, C.; Rout, R.; Price, A.; Hulley, P.; Mahoney, D. Hyaluronidase treatment of synovial fluid to improve assay precision for biomarker research using multiplex immunoassay platforms. J. Immunol. Methods 2012, 386, 22–30. [Google Scholar] [CrossRef]
- McIlwraith, C.W.; Frisbie, D.D.; Kawcak, C.E.; Fuller, C.J.; Hurtig, M.; Cruz, A. The OARSI histopathology initiative-recommendations for histological assessments of osteoarthritis in the horse. Osteoarthr. Cartil. 2010, 18 (Suppl. S3), S93–S105. [Google Scholar] [CrossRef] [PubMed]
- de Waal Malefyt, R.; Abrams, J.; Bennett, B.; Figdor, C.G.; de Vries, J.E. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: An autoregulatory role of IL-10 produced by monocytes. J. Exp. Med. 1991, 174, 1209–1220. [Google Scholar] [CrossRef]
- Struzik, J.; Szulc-Dąbrowska, L. Manipulation of Non-canonical NF-κB Signaling by Non-oncogenic Viruses. Arch. Immunol. Ther. Exp. 2019, 67, 41–48. [Google Scholar] [CrossRef]
- Yu, H.; Lin, L.; Zhang, Z.; Zhang, H.; Hu, H. Targeting NF-κB pathway for the therapy of diseases: Mechanism and clinical study. Sig Transduc Target. Ther. 2020, 5, 209. [Google Scholar] [CrossRef]
- Fuster, J.J.; Walsh, K. The good, the bad, and the ugly of interleukin-6 signaling. EMBO J. 2014, 33, 1425–1427. [Google Scholar] [CrossRef]
- Scheller, J.; Chalaris, A.; Schmidt-Arras, D.; Rose-John, S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta Mol. Cell Res. 2011, 1813, 878–888. [Google Scholar] [CrossRef]
- Czimmerer, Z.; Nagy, L. Epigenomic regulation of macrophage polarization: Where do the nuclear receptors belong? Immunol. Rev. 2023, 317, 152–165. [Google Scholar] [CrossRef]
- Gureev, A.P.; Shaforostova, E.A.; Popov, V.N. Regulation of Mitochondrial Biogenesis as a Way for Active Longevity: Interaction Between the Nrf2 and PGC-1α Signaling Pathways. Front. Genet. 2019, 10, 435. [Google Scholar] [CrossRef]
- Wang, J.; Su, B.; Zhu, H.; Chen, C.; Zhao, G. Protective effect of geraniol inhibits inflammatory response, oxidative stress and apoptosis in traumatic injury of the spinal cord through modulation of NF-κB and p38 MAPK. Exp. Ther. Med. 2016, 12, 3607–3613. [Google Scholar] [CrossRef] [PubMed]
- Rekha, K.R.; Inmozhi Sivakamasundari, R. Geraniol Protects Against the Protein and Oxidative Stress Induced by Rotenone in an In Vitro Model of Parkinson’s Disease. Neurochem. Res. 2018, 43, 1947–1962. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, X.; Lotz, M.; Terkeltaub, R.; Liu-Bryan, R. Mitochondrial biogenesis is impaired in osteoarthritis chondrocytes but reversible via peroxisome proliferator-activated receptor γ coactivator 1α. Arthritis Rheum. 2015, 67, 2141–2153. [Google Scholar] [CrossRef]
- Seewald, L.A.; Sabino, I.G.; Montney, K.L.; Delco, M.L. Synovial fluid mitochondrial DNA concentration reflects the degree of cartilage damage after naturally occurring articular injury. Osteoarthr. Cartil. 2023, 31, 1056–1065. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Pham, T.; Wang, J.; O-Sullivan, I.; DiCamillo, A.; Du, S.; Mwale, F.; Farooqui, Z.; Votta-Velis, G.; Bruce, B.; et al. Nanoparticle-based inhibition of vascular endothelial growth factor receptors alleviates osteoarthritis pain and cartilage damage. Sci. Adv. 2024, 10, eadi5501. [Google Scholar] [CrossRef] [PubMed]
- Morris, E.A.; Treadwell, B.V. Effect of interleukin 1 on articular cartilage from young and aged horses and comparison with metabolism of osteoarthritic cartilage. Am. J. Vet. Res. 1994, 55, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Menarim, B.C.; MacLeod, J.N.; Dahlgren, L.A. Bone marrow mononuclear cells for joint therapy: The role of macrophages in inflammation resolution and tissue repair. World J. Stem Cells 2021, 13, 825–840. [Google Scholar] [CrossRef]
- Knych, H.K.; Weiner, D.; Harrison, L.; McKemie, D.S. Pharmacokinetics and pharmacodynamics of intra-articular isoflupredone following administration to horses with lipopolysaccharide-induced synovitis. BMC Vet. Res. 2022, 18, 436. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaimbeul, S.F.; Rodrigues, N.N.P.; Thurston, D.D.; Scoggin, K.E.; Janes, J.; Jacobs, C.A.; MacLeod, J.N.; Stone, A.V.; Menarim, B.C. PPARγ Agonism Modulates Synovial Macrophage and Cartilage Responses in an Equine Model of Synovial Inflammation—Implications for Joint Therapy. Biomolecules 2025, 15, 1267. https://doi.org/10.3390/biom15091267
Chaimbeul SF, Rodrigues NNP, Thurston DD, Scoggin KE, Janes J, Jacobs CA, MacLeod JN, Stone AV, Menarim BC. PPARγ Agonism Modulates Synovial Macrophage and Cartilage Responses in an Equine Model of Synovial Inflammation—Implications for Joint Therapy. Biomolecules. 2025; 15(9):1267. https://doi.org/10.3390/biom15091267
Chicago/Turabian StyleChaimbeul, Slàine F., Nubia N. P. Rodrigues, Danny D. Thurston, Kirsten E. Scoggin, Jennifer Janes, Cale A. Jacobs, James N. MacLeod, Austin V. Stone, and Bruno C. Menarim. 2025. "PPARγ Agonism Modulates Synovial Macrophage and Cartilage Responses in an Equine Model of Synovial Inflammation—Implications for Joint Therapy" Biomolecules 15, no. 9: 1267. https://doi.org/10.3390/biom15091267
APA StyleChaimbeul, S. F., Rodrigues, N. N. P., Thurston, D. D., Scoggin, K. E., Janes, J., Jacobs, C. A., MacLeod, J. N., Stone, A. V., & Menarim, B. C. (2025). PPARγ Agonism Modulates Synovial Macrophage and Cartilage Responses in an Equine Model of Synovial Inflammation—Implications for Joint Therapy. Biomolecules, 15(9), 1267. https://doi.org/10.3390/biom15091267






