Abstract
Diabetic cardiomyopathy (DCM) is a common and clinically relevant complication of diabetes mellitus, defined by myocardial dysfunction in the absence of overt coronary artery disease or systemic hypertension. Recent studies have identified proprotein convertase subtilisin/kexin type 9 (PCSK9) as a pivotal mediator in the pathogenesis of DCM. PCSK9 contributes not only to dyslipidemia via degradation of LDLR and consequent elevation of circulating LDL-C, but also to metabolic derangements and inflammation through interactions with receptors such as CD36 and Toll-like receptor 4 (TLR4). In DCM, PCSK9 has been shown to exacerbate inflammation and pyroptosis and is closely linked to impaired autophagic function. Elevated circulating PCSK9 has emerged as a potential biomarker for cardiovascular events in patients with type 2 diabetes mellitus (T2DM). At the same time, long-term administration of PCSK9 inhibitors (PCSK9i) has not been associated with a significant increase in incident diabetes. Furthermore, PCSK9 loss-of-function mutations have been linked to a modestly heightened risk of T2DM, underscoring its complex involvement in cardiometabolic regulation and disease. This review synthesizes current insights into the mechanistic and therapeutic roles of PCSK9 in DCM, aiming to inform precision cardiovascular risk management strategies in T2DM populations.
1. Introduction
Diabetes mellitus is a chronic metabolic disease that poses a serious threat to human health. Currently, type 2 diabetes mellitus (T2DM) affects approximately 540 million individuals worldwide, including 6.1 million in Europe. Projections estimate that by 2045, the global prevalence of diabetes will rise to 783 million, representing 12.2% of the worldwide population [1,2]. T2DM is the most prevalent form of diabetes, with cardiovascular complications constituting one of the leading causes of mortality [3]. Diabetic cardiomyopathy (DCM) is defined as myocardial systolic and/or diastolic dysfunction in the presence of diabetes mellitus. It is not dependent on coronary artery disease, hypertension, or other cardiovascular risk factors [2].
The pathogenesis of DCM is complex and involves multiple pathological processes, such as inflammatory response, oxidative stress, cardiomyocyte death, autophagy, metabolic disorders, insulin resistance, mitochondrial damage, endoplasmic reticulum stress, and accumulation of advanced glycation end products (AGEs) [4,5,6,7]. In recent years, proprotein convertase subtilisin/kexin type 9 (PCSK9), a key regulator of lipid metabolism, has been increasingly recognised for its non-canonical roles in glucose metabolism, inflammation, and cell death. Notably, PCSK9 expression is markedly elevated in T2DM and has been shown to correlate positively with major adverse cardiovascular events (MACEs), suggesting its potential pathogenic involvement in DCM warrants further investigation.
PCSK9 plays a pivotal role in lipid metabolism by regulating low-density lipoprotein receptor (LDLR) degradation. Beyond this canonical function, PCSK9 influences fatty acid uptake, inflammatory pathways, and mitochondrial function through its interactions with key signalling mediators such as CD36 and Toll-like receptor 4 (TLR4), exerting profound effects on myocardial homeostasis [8]. In addition, PCSK9 is involved in the pathogenesis of various diseases such as atherosclerotic cardiovascular disease, ischemia/reperfusion injury, cancer, and HIV [9,10].
Although Mendelian randomisation studies [11], meta-analyses [12], and clinical studies [13,14] suggest that PCSK9 inhibitors (PCSK9i) may slightly affect glucose metabolism, large-scale clinical studies have not found a significant increase in the risk of new-onset diabetes mellitus (NODM) [15,16] (Table 1). This suggests an apparent cardiovascular protective effect of PCSK9i. Although the conclusions of related studies have shown significant discrepancies, the role of PCSK9 and its inhibitors in diabetes and the effects of glucose-lowering drugs on PCSK9 have attracted much attention in recent years. In recent years, the interaction between PCSK9 and glucose-lowering drugs, such as the modulation of PCSK9 expression by SGLT2 inhibitors and GLP-1 receptor agonists, has also triggered research interest.
This review aims to systematically summarise the pathological mechanisms and recent advances concerning PCSK9 in DCM, focusing particularly on its cell-type-specific functions within the heart. It further explores the cross-talk between PCSK9 and key regulatory pathways, including lipid and glucose metabolism, inflammation, autophagy, and ferroptosis, while highlighting its potential clinical value as a therapeutic target.
2. Mechanisms of Diabetic Cardiomyopathy
DCM is characterised by myocardial hypertrophy and progressive impairment of cardiac function, which may ultimately culminate in heart failure [17]. Individuals with T2DM have approximately a 2.4-fold higher risk of developing heart failure compared to non-diabetic individuals [18]. More than 25% of patients with T2DM exhibit subclinical structural or functional cardiac abnormalities, with left ventricular diastolic dysfunction being the most prevalent. As the disease progresses, diabetic patients may develop heart failure with preserved ejection fraction (HFpEF) or reduced ejection fraction (HFrEF), with a particularly significant risk in female patients [2,19].
Metabolic dysregulation is a central pathological hallmark of DCM. Recent studies have identified the TGR5 signalling pathway as a critical regulator of lipid metabolism and myocardial function. Loss of TGR5 promotes aberrant membrane trafficking of CD36 through DHHC4-mediated palmitoylation, leading to intracellular lipid accumulation and compromised cardiac performance. Conversely, TGR5 activation significantly limits abnormal fatty acid influx into cardiomyocytes, exerting cardioprotective effects [20,21]. This novel mechanism expands the understanding of myocardial lipotoxicity and highlights the “TGR5–CD36” axis as a promising therapeutic target for DCM.
Diabetes is increasingly recognised as a chronic, low-grade inflammatory disease. Experimental studies have demonstrated that inhibition of reactive oxygen species (ROS) production significantly attenuates myocardial pro-inflammatory signalling, underscoring the ROS–NLRP3 inflammasome axis as a key molecular bridge linking metabolic dysregulation to cardiac injury [22,23]. In diabetic conditions, high-fat-induced mitochondrial ROS generation leads to mitochondrial damage and mitochondrial DNA (mtDNA) leakage, which activates the cytosolic DNA sensor cyclic GMP–AMP synthase (cGAS). It promotes stimulator of interferon genes (STING) translocation to the Golgi apparatus, subsequently activating IRF3, NF-κB, and thioredoxin-interacting protein (TXNIP), culminating in inflammation, apoptosis, and myocardial fibrosis [24,25,26]. Concurrently, increased ROS in the diabetic atrial myocardium stimulates the NF-κB pathway, which directly binds the Atrogin-1 promoter and enhances FBXO32 gene transcription, thereby accelerating SK2 protein degradation in an Atrogin-1-dependent manner [27].
AGEs further exacerbate inflammation by forming a ternary complex with TLR4 and MD2, activating pro-inflammatory cascades in the diabetic milieu [28]. Artesunate has been shown to target MD2 and disrupt this pathway, thereby reducing fibroblast proliferation, migration, and collagen deposition, ultimately improving cardiac function [29]. AGEs can also promote NLRP3 inflammasome activation via ROS, triggering inflammation and pyroptosis and impairing tissue repair in diabetic settings [30].
Chronic low-grade inflammation is a key player in driving insulin resistance (IR) in the context of obesity and T2DM. Inflammatory cascades begin with IgG deposition in adipose tissue, which triggers macrophage infiltration and disrupts insulin signalling [31]. Hyperactivation of CREBZF exacerbates adipose tissue inflammation and IR by competitively binding NF-κB p65 with inhibitor of NF-κB alpha (IκBα) [32]. Additionally, hypoxia-inducible factor 2α (HIF-2α) in macrophages maintains immunometabolic homeostasis through the H3K27me3–Cpt1a axis and suppresses activation of the NLRP3 inflammasome [33]. JJMJD8 enhances LPS-induced inflammation and worsens IR by interacting with interferon regulatory factor 3 [34] (Figure 1).
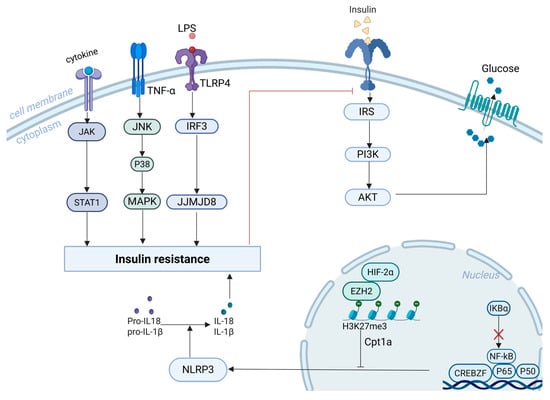
Figure 1.
Mechanistic Pathways Linking Inflammation and Insulin Resistance. Legend: the red lines represent inhibitory effects.
Branched-chain amino acids promote polarisation of macrophages toward the pro-inflammatory M1 phenotype via activation of the Janus kinase 1/signal transducer and activator of transcription 1 (JAK1/STAT1) pathway, thereby impairing insulin sensitivity [35]. Activation of c-Jun N-terminal kinase (JNK) and p38 mitogen-activated protein kinases is also strongly linked to IR [36] (Figure 1). Lactate metabolism also modulates immune responses. MCT1 facilitates lactate uptake into mitochondria, supporting M2 anti-inflammatory polarisation, while MCT4 promotes lactate export and M1 polarisation. In pancreatic β-cells, lactate suppresses insulin release by activating GPR81 and inhibiting the cAMP–PKA pathway [37].
SOSTDC1, secreted by CD4+ T cells, is elevated in obese individuals and mice on a high-fat diet. It promotes fat accumulation and worsens IR by boosting adipogenesis, blocking lipolysis, and activating the lipoprotein receptor-related protein 5 (LRP5)/6 β-catenin pathway [38]. Furthermore, endothelial loss or pharmacologic blockade of the adrenomedullin receptor ameliorates obesity-induced IR [39]. Statin therapy has also been implicated in worsening IR by reducing circulating glucagon-like peptide-1 (GLP-1) levels in a gut microbiota-dependent manner [40].
3. Mechanisms of PCSK9
3.1. PCSK9 Biology
PCSK9, a member of the proprotein convertase family, plays a pivotal role in the proteolytic activation, modification, and degradation of secreted proteins. The protein comprises 692 amino acids, with a molecular mass of approximately 74.3 kDa, and contains three characteristic functional domains arranged from the N-terminus: a prodomain, a catalytic domain, and a cysteine–histidine-rich C-terminal domain (CRD) [41,42,43]. PCSK9 is expressed primarily in the liver, kidneys, and intestines, but is also detectable in other tissues, including the lungs, pancreas, skin, and cerebrospinal fluid [44]. Notably, PCSK9 expression has also been identified in cardiomyocytes, endothelial cells (ECs) [45], macrophages [46], monocytes, and vascular smooth muscle cells (VSMCs) [47,48]
PCSK9 modulates several membrane-bound receptors on pancreatic β-cells, including very low-density lipoprotein receptor (VLDLR), CD36, and fatty acid transport proteins, thereby influencing insulin secretion and fatty acid uptake, and ultimately contributing to glucose homeostasis [49]. Circulating PCSK9 targets monoclonal antibodies that spatially site-block PCSK9 binding to the EGF-A structural domain of LDLR in hepatocytes and other tissues by recognising its catalytic subunit [50]. Interestingly, PCSK9-mediated regulation of VLDLR is independent of LDLR, as it forms an internalisation complex that facilitates lysosomal degradation through selective EGF-A domain binding [51]. Emerging evidence suggests that PCSK9 may further accelerate LDLR degradation by interfering with SNX17-dependent recycling mechanisms [52]. Beyond its receptor-level effects, PCSK9i has shown significant clinical efficacy in lowering LDL-C levels (p < 0.01), improving atherogenic lipid indices, including TC, non-HDL-C, TG, Lp(a), and ApoB (all p < 0.05), while simultaneously elevating HDL-C concentrations (p < 0.05) [53,54].
3.2. PCSK9 Demographics
PCSK9i, such as evolocumab, are suitable for patients across all age groups. Notably, initiating long-term evolocumab therapy in older adults with atherosclerotic cardiovascular disease yields cardiovascular benefits comparable to, if not exceeding, those seen in younger individuals [55]. Recent analyses further confirm that the efficacy and safety profile of evolocumab remains consistent in patients aged ≥75 years compared to those under 75 [56].
Emerging genomic data suggest that the metabolic effects of PCSK9 inhibition may vary by ancestry. Rosoff et al. [57] reported that prolonged pharmacologic or genetic inhibition of PCSK9 exerts a broadly neutral impact on glycemic control and T2DM risk among most non-European populations. However, a slightly increased risk was noted in African ancestry cohorts. Conversely, inhibition of HMGCR, the molecular target of statins, was linked to a higher incidence of T2DM in individuals of South Asian, East Asian, and European descent. Importantly, PCSK9-related genetic modulation did not significantly alter hepatic enzyme levels—including ALT, AST, GGT, and ALP—across all ethnic groups examined, supporting a reassuring hepatic safety profile. Moreover, variants associated with reduced PCSK9 expression and lower circulating PCSK9 protein were correlated with increased direct bilirubin levels, a finding in line with observational evidence indicating an inverse relationship between PCSK9 and bilirubin concentrations [58].
Sex has been identified as a biological determinant of plasma PCSK9 concentrations in patients with diabetes, with variations observed across different age strata. PCSK9i significantly reduces LDL-C and MACE risk in both sexes, with no significant sex differences in MACE reduction. However, LDL-C reduction is greater in males [59]. Women tend to initiate monoclonal antibody therapy targeting PCSK9 at a later age than men. In females, circulating PCSK9 concentrations increase with age, particularly following menopause, which may reflect enhanced therapeutic responsiveness in older versus younger women. Furthermore, sex—rather than age—was a stronger determinant of baseline LDL-C levels and the likelihood of achieving treatment goals [60].
3.3. PCSK9 and Lipid Metabolism
3.3.1. HNF1α
HNF1α serves as a central transcriptional activator in hepatic metabolic regulation by binding its conserved recognition sequence (5′-GTTAATNATTAAC-3′) via the N-terminal POU domain, thereby directly promoting PCSK9 transcription [61]. Conversely, FOXO3 functions as a negative regulator by competitively occupying the PCSK9 promoter, thereby attenuating HNF1α transcriptional activity [62]. Insulin negatively regulates HNF1α via the mTORC1–PKCδ signalling axis, decreasing PCSK9 expression and elevated LDLR levels [63]. The small-molecule compound DC371739 also downregulates PCSK9 by specifically inhibiting HNF1α-mediated transcriptional activity [64]. In terms of genetic regulation, Liu et al. [65] demonstrated that the MODY3-associated HNF1A IVS7-6G>A mutation induces aberrant mRNA splicing and yields truncated proteins lacking the transactivation domain, with the 282–501 region shown to be critical for HNF1A function. In addition, Jorge et al. [66] found that the long non-coding RNA HASTER modulates hepatic and pancreatic β-cell metabolic function by regulating HNF1A expression. Recent mechanistic evidence suggests that the HNF1α–PCSK9–LDLR signalling cascade may be involved in the lipid-modulatory effects of dapagliflozin [67] (Figure S1).
3.3.2. SREBP2
Sterol regulatory element-binding protein 2 (SREBP2) plays a pivotal role in regulating intracellular cholesterol homeostasis by modulating genes involved in lipid metabolism. Through its basic helix-loop-helix structural motif, SREBP2 selectively binds sterol regulatory elements (SREs) within the promoter regions of target genes, including PCSK9, LDLR, and HMGCR, thereby initiating their transcription [68] (Figure S1). In addition, Austin et al. [69] reported that caffeine inhibits SREBP2 transcriptional activity by elevating endoplasmic reticulum calcium levels in hepatocytes, which leads to increased LDLR expression and improved LDL-C clearance.
SREBP2 activation is also modulated by inflammatory signalling. Proinflammatory cytokines such as TNF-α and IFN-γ upregulate PCSK9 expression in hepatocytes through synergistic activation of SREBP2 [70]. Additionally, IL-1β enhances mTORC1 activity via the NF-κB pathway, impacting SREBP2 maturation and downstream LDLR regulation [71].
3.3.3. CD36
CD36 is a pivotal fatty acid translocase that regulates transmembrane fatty acid transport via a dynamic membrane recycling mechanism. Aberrant intracellular localisation of CD36 has been implicated in multiple metabolic disorders [72,73,74]. In diabetes, persistent surface localisation of CD36 promotes excessive fatty acid uptake, contributing to lipid imbalance. This process is modulated through a stable, non-degradable complex formed with PCSK9, positioning CD36 as a regulatory node within the gut–adipose tissue lipid axis [75,76]. Furthermore, exogenous PCSK9 has been shown to reduce basal fatty acid uptake in cardiomyocytes independently of CD36 expression, suggesting alternative regulatory pathways [77]. In peripheral nerves, PCSK9 deficiency results in CD36 upregulation, mitochondrial structural damage, lipid accumulation, and elevated acylcarnitine levels, ultimately leading to peripheral neuropathic symptoms [78]. CD36 modulates SREBP1 processing by interacting with insulin-induced gene 2 (INSIG2), thereby maintaining hepatic lipid homeostasis [79] (Figure S1).
3.4. PCSK9 and Glucose Metabolism
3.4.1. PCSK9 and T2DM
The functional role of PCSK9 within pancreatic islet cells remains controversial. Although circulating and liver-derived PCSK9 appear to exert minimal influence on pancreatic β-cell physiology, targeted PCSK9 deficiency within β-cells has been associated with intracellular cholesterol accumulation and impaired insulin secretion, suggesting a context-specific requirement for endogenous PCSK9 in maintaining islet homeostasis [80]. Conversely, other preclinical studies have shown that extensive inactivation of PCSK9 in β-cells does not alter glycemic control or insulin output, raising questions about its functional necessity in this compartment [8,15]. Emerging evidence indicates that δ-cell-secreted PCSK9 may play a paracrine protective role by limiting cholesterol uptake in β-cells, thereby attenuating lipotoxicity under metabolic stress conditions [81]. Insulin itself induces PCSK9 expression via the ERK signalling cascade in a dose- and time-dependent manner, forming a potential autocrine feedback loop [44].
From a clinical perspective, elevated serum PCSK9 concentrations have been documented in individuals with impaired glucose metabolism, including those with impaired glucose tolerance and T2DM [82]. In pregnant women, PCSK9 levels were significantly higher in the gestational diabetes mellitus (GDM) group than those with standard glucose tolerance (NGT), suggesting a possible pathophysiological role for PCSK9 in GDM-related dyslipidemia and glycemic dysregulation. Furthermore, Wu et al. [83] reported that serum PCSK9 levels were positively correlated with LDL-C, fasting plasma glucose, and HbA1c in GDM patients, reinforcing its potential role as a metabolic biomarker. These findings suggest that PCSK9’s role in islet biology may depend highly on cellular context and metabolic milieu. Further, mechanistic and translational studies are needed to elucidate its contribution to β-cell homeostasis.
Current evidence suggests that prolonged use of PCSK9i does not confer a heightened risk of developing NODM [84,85]. Among prediabetic individuals, no significant difference in NODM incidence was observed between treatment and control arms, and glycemic indices in patients with established T2DM remained unaffected by PCSK9i exposure [14,80]. Despite these findings, the long-term influence of PCSK9i on glucose metabolism remains an area of active investigation, particularly in light of the need to evaluate subgroup-specific susceptibility and the durability of glycemic neutrality. Given these uncertainties, further studies are warranted to examine the metabolic interplay between PCSK9 signalling and hypoglycemic therapies.
PCSK9i has consistently demonstrated substantial cardiovascular benefits in patients with diabetes mellitus. Meta-analyses and large-scale randomised controlled trials report an approximate 18% relative risk reduction in MACEs among diabetic patients receiving PCSK9i therapy, alongside significant improvements in lipid indices [86,87]. The FOURIER and FOURIER-OLE studies provided evidence of evolocumab’s efficacy, showing significant long-term MACE reduction in both multivessel disease (MVD) and non-MVD subgroups. While the MVD cohort derived earlier and more robust benefits, patients without MVD also exhibited a sustained 25% risk reduction over time, underscoring the broad clinical utility of PCSK9i across varying cardiovascular risk strata [88].

Table 1.
The Relationship Between PCSK9i, Lipid Profiles, and NODM (The arrows in the table indicate the changes: downward arrows indicate a decrease, while hyphens (–) represent no significant effect observed).
Table 1.
The Relationship Between PCSK9i, Lipid Profiles, and NODM (The arrows in the table indicate the changes: downward arrows indicate a decrease, while hyphens (–) represent no significant effect observed).
| Author | Dose of the Intervention | Year | Number of Participants | Follow-Up (Mean or Median) Years | Inclusion Criteria | Type Randomization | Outcome Change |
|---|---|---|---|---|---|---|---|
| Imbalzano et al. [89] | Alirocumab/ Evolocumab Varied (see individual trials) | 2023 | 20651(aggregated) | 51 weeks | DM + hypercholesterolemia, RCTs comparing PCSK9i vs. placebo | Meta-analysis of 8 RCTs | MACE 18% ↓; LDL-C, HDL-C, TG, Lp(a), and ApoB ↓. |
| Ray et al. [90] | Alirocumab 75 mg every 2 weeks (Q2W), increased to 150 mg if required | 2019 | 413 | 24 weeks | Type 2 Diabetes Mellitus (T2DM), mixed dyslipidemia, LDL-C ≥ 100 mg/dL, ASCVD, on stable maximally tolerated statin therapy | RCT | LDL-C, non-HDL-C, ApoB, and LDL particle number (LDL-PN) ↓ |
| Fischer et al. [91] | Alirocumab (75 mg or 150 mg) and Evolocumab (140 mg) | 2021 | 237 | Median 18 months | Age > 18 years; PCSK9 inhibitors (alirocumab or evolocumab) for secondary prevention; Available LDL-C data at baseline and during follow-up for at least 3 months | Observational study | MACE ↓; LDL-C, non-HDL-C, and ApoB ↓ |
| Rosenson et al. [92] | Evolocumab 420 mg subcutaneously once a month | 2019 | 421 | 12 weeks | Type 2 diabetes, hyperlipidemia or mixed dyslipidemia, background atorvastatin 20 mg/d | RCT | LDL-C 54.3% ↓ (12 weeks); non-HDL-C, and ApoB ↓. |
| Chen et al. [93] | Evolocumab 140 mg every 2 weeks/420 mg monthly | 2019 | 453 | 12 weeks | T2DM with hyperlipidemia, LDL-C ≥ 2.6 mmol/L on statin or ≥3.4 mmol/L without statin | RCT | LDL-C ↓ Non-HDL-C, ApoB100, triglycerides, and Lp(a) ↓; HbA1c and FSG(–) |
| Schwartz et al. [16] | Alirocumab (75 mg or 150 mg every 2 weeks) | 2025 | 8107 | Median follow-up of 2.4 years | Patients with recent acute coronary syndrome, elevated lipoproteins, and no diabetes at baseline | RCT | NODM(–) |
| Sabatine et al. [94] | Evolocumab 140 mg every 2 weeks or 420 mg monthly | 2017 | 27,564 | 104 weeks | Atherosclerotic cardiovascular disease, LDL-C ≥ 1.8 mmol/L, on statin therapy | RCT | NODM(–) |
| Moura et al. [95] | Evolocumab 140 mg every 2 weeks vs. placebo | 2025 | 9388 (No diabetes at baseline) | Median follow-up of 2.3 years | Age 40–85, stable cardiovascular disease, LDL ≥ 70 mg/dL, on statin therapy, no T2D at baseline | RCT | NODM(–) |
| González-Lleó et al. [14] | Alirocumab 75 or 150 mg or evolocumab 140 mg every 2 weeks | 2024 | 218 | Mean follow-up of 3.2 years | Patients over 18 years with hypercholesterolemia, including familial hypercholesterolemia (FH), undergoing treatment with PCSK9 inhibitors | Observational study | NODM (2.6%/year); Glycemic parameters(–) |
3.4.2. PCSK9 and Antihyperglycemic Drug
Emerging evidence indicates a complex and potentially synergistic relationship between PCSK9 signalling and antidiabetic pharmacotherapies. The current therapeutic landscape for DCM is dominated by SGLT-2 inhibitors, GLP-1 receptor agonists, and their combined use. While efficacious in glycemic control, these agents often induce gastrointestinal adverse effects that compromise long-term adherence. Combination regimens have shown enhanced clinical outcomes, including reductions in HbA1c, fasting plasma glucose, and body weight compared to monotherapy [96]. On a mechanistic level, liraglutide was found to suppress hepatic PCSK9 and LDLR expression through an HNF1α-dependent pathway in both HepG2 cells and db/db mice [97], and also downregulate PCSK9 transcription in adipose and hepatic tissues while promoting lipoprotein lipase expression in high-fat diet–induced mouse models [98].
Dapagliflozin similarly modulates hepatic lipid metabolism through the PCSK9/LDLR pathway [67]. A study showed no potentially deleterious effects on circulating PCSK9 levels in 78 patients with T2DM treated with 25 mg/d of empagliflozin for 4 weeks [99]. In animal models, dapagliflozin suppressed PCSK9 expression by inhibiting carbohydrate-responsive element-binding protein activity, resulting in reduced hepatic and serum PCSK9 levels in both diabetic and nondiabetic dyslipidemic mice [100]. Furthermore, PCSK9i and atorvastatin reduce cardiac impairment in ovariectomized prediabetic rats via improved mitochondrial function and Ca2+ regulation [101]. However, not all outcomes were favorable: alirocumab worsened renal dysfunction in obese ZSF1 rats, and empagliflozin failed to prevent kidney disease progression in the same model, despite its renoprotective effects in human diabetic kidney disease [102].
Insulin exerts a pivotal influence on both systemic and hepatic expression of PCSK9. Miao et al. [103] reported that insulin receptor knockdown in murine hepatocytes led to significant reductions in both PCSK9 mRNA and protein levels, highlighting the dependence of PCSK9 expression on insulin signalling. In parallel, glucagon was shown to suppress the PCSK9 gene and protein expression by approximately 50% in primary rat hepatocytes. Niesen et al. [104] consistently observed a marked decrease in hepatic PCSK9 protein levels in streptozotocin-induced insulin-deficient Sprague-Dawley rats. These hormonal effects appear to be mediated through post-transcriptional pathways, with PCSK9 acting as a downstream effector in the glucagon- and estrogen-induced regulation of LDL receptor availability [105].
3.4.3. PCSK9 and Atherogenic Dyslipidemia
Circulating PCSK9 has emerged as a potential prognostic biomarker for cardiovascular (CV) events in patients with T2DM [45,106,107]. While glucose metabolism alone does not appear to influence plasma PCSK9 concentrations directly, T2DM may modulate the relationship between PCSK9 and atherogenic lipid markers such as non-high-density lipoprotein cholesterol (non-HDL-C) and apolipoprotein B (ApoB) [108]. Several studies have demonstrated a significant positive correlation between circulating PCSK9 and LDL-C levels [109,110]. One cohort study found an inverse association between genetically determined LDL-C levels and T2DM risk, suggesting that PCSK9-mediated lipid regulation may have complex metabolic implications [111].
In a cohort of 2984 participants, higher serum PCSK9 levels were significantly associated with multivessel coronary heart disease (CHD) and elevated Gensini scores. They were identified as an independent predictor of CHD and MACEs in individuals with T2DM [107]. Furthermore, PCSK9 levels were found to predict carotid–femoral pulse wave velocity, accounting for 16.9% of its variance, thereby supporting its utility in cardiovascular risk stratification for diabetic patients [112]. Importantly, PCSK9i appear to reduce MACE risk and improve lipid profiles in patients with both diabetes and dyslipidemia, irrespective of baseline glycemic status [89] (Table 1).
3.4.4. PCSK9 Mutations and Gene Silencing
Genetic polymorphisms affecting PCSK9 function are increasingly recognised for their metabolic consequences. Several PCSK9 loss-of-function (LOF) variants, including rs11583680, rs11591147, rs2479409, and rs11206510, have been associated with modest elevations in fasting glucose, increased body weight, and a higher risk for T2DM development [113]. In contrast, gain-of-function (GOF) mutations such as S127R and R496W reduce PCSK9’s affinity for LDL particles, augmenting LDLR degradation and exacerbating hypercholesterolemia [114]. Paradoxically, LOF mutations in LDLR observed in patients with familial hypercholesterolemia appear to reduce T2DM risk, suggesting gene-specific metabolic compensation. Mechanistic studies in Pcsk9 knockout mice have demonstrated impaired mitochondrial bioenergetics in cardiac tissue, with diminished electron transport chain (ETC) activity and a shift toward glycolytic reliance that fails to sustain adequate energy output under physiological stress [115].
Therapeutically, gene silencing of PCSK9 is being explored as a novel strategy for mitigating DCM. Sustained suppression of PCSK9 expression has been achieved through the transient delivery of ethyl transfer RNA (ETR), leading to durable reductions in LDL-C [49]. In vitro studies using human microvascular endothelial cells (HMEC-1) have shown that PCSK9 silencing attenuates hyperglycemia-induced inflammatory signalling, oxidative stress, and lipid metabolic dysfunction, primarily via inhibition of the lectin-like oxidised LDL receptor-1 (LOX-1) pathway [45]. Recent advances in gene editing have further enabled epigenetic suppression of PCSK9 through targeted methylation. It is achieved by fusing catalytically inactive dCas9 to either DNA methyltransferase or demethylase domains, allowing for site-specific epigenetic modulation of the PCSK9 promoter guided by single-guide RNAs, resulting in efficient downregulation of PCSK9 and reduced LDL-C levels [116].
3.5. PCSK9 and Inflammation
3.5.1. PCSK9 Activates the TLR4/NF-κB Pathway
PCSK9 directly interacts with TLR4 via its cysteine-rich domain (CRD), activating the TLR4/MyD88/NF-κB signalling pathway and promoting the expression of key pro-inflammatory mediators, including TNF-α, IL-1β, and tissue factor [117,118,119,120]. Gene silencing of PCSK9 has been shown to inhibit NF-κB nuclear translocation and dampen inflammatory responses [51,121]. In addition, PCSK9 enhances the activity of NLRP3 Inflammasomes by activating this pathway, creating a positive feedback loop that exacerbates myocardial fibrosis and sepsis-associated endothelial dysfunction [122,123,124]. Interestingly, the TLR4 agonist lipopolysaccharide (LPS) has been found to reverse the upregulation of PCSK9, reinforcing an inflammation-metabolism loop. Furthermore, deletion of low-density LRP5—a known transporter of PCSK9—markedly reduces PCSK9 release and downstream TLR4 activation [125].
PCSK9 activates the NLRP3 inflammasome signalling pathway by inducing damage to mtDNA [126]. It regulates Smac translocation from mitochondria to cytosol, mediates hypoxia-induced apoptosis, and inhibits neovascularisation [127]. Clinical myocardial samples have demonstrated pronounced co-localisation of PCSK9 with N-terminal gasdermin D (GSDMD-NT) in the peri-infarct zone, implicating PCSK9 in pyroptotic injury during acute myocardial infarction [128]. Mechanistic studies have demonstrated that PCSK9 activates the NLRP3 inflammasome signalling pathway by inducing mtDNA damage, ultimately triggering caspase-1-dependent pyroptosis. In PCSK9 knockout (PCSK9-/-) mice, NLRP3 signalling was inhibited, with significant reductions in GSDMD-NT expression and lactate dehydrogenase release, supporting PCSK9’s role in the regulation of inflammatory cell death [126].
3.5.2. NLRP3 Inflammasome Via IL-1β Regulates PCSK9 Secretion
NLRP3 inflammasome also inversely regulates PCSK9 expression [44]. In vitro experiments had shown that ATP or Nigericin stimulation following LPS priming had led to increased PCSK9 expression in macrophages, with the effect being strictly dependent on apoptosis-associated speck-like protein containing a CARD (ASC), caspase-1, and IL-1β [44,109]. IL-1β further induces PCSK9 secretion through the MAPK signalling axis, revealing its role as a key molecular hub linking immuno-inflammation and lipid metabolism [44,126,129]. The CANTOS clinical trial validated the clinical translational value of this mechanism: the IL-1β monoclonal antibody canakinumab significantly reduced the recurrence of MACE and HF hospitalisation rates in patients with myocardial infarction, while lowering hs-CRP levels [130,131]. Furthermore, dapagliflozin suppresses PCSK9 expression through the NLRP3/IL-1β axis in a cardiotoxicity context, reinforcing the importance of this feedback network in cardiometabolic modulation [132].
3.6. Pyroptosis
Oxidative stress, endoplasmic reticulum stress, and immune activation induced by hyperglycemia are primary drivers of focal cellular death in diabetes. Elevated glucose concentrations stimulate the expression of multiple classes of endogenous non-coding RNAs—including miRNAs, circRNAs, and lncRNAs—which modulate transcriptional and post-transcriptional pathways involved in inflammation and programmed cell death [133].
Mechanistically, persistent hyperglycemia activates the CaMK2α/O-GlcNAcylation positive feedback loop in endothelial cells, which remains active even after restoring normoglycemia. It promotes phosphorylation of Stat1, enhances transcription of miR-15/16 precursors, increases secretion of small extracellular vesicle-associated miR-15/16, and sustains a state of chronic low-grade inflammation [134]. circGlis3 expression is significantly upregulated in both genetically and diet-induced obese mice. circGlis3 appears to exert a protective role by promoting insulin synthesis and secretion and binding to the pro-apoptotic protein SCOTIN in a caspase-3–dependent manner, thereby inhibiting β-cell apoptosis [135]. Furthermore, PCSK9 has been shown to influence mitochondrial function and apoptosis-related signalling in VSMCs, suggesting a possible contribution to diabetic vascular remodelling [126,136].
Activation of the NLRP3 inflammasome appears to be a critical pathogenic node linking programmed cell death to inflammatory amplification in diabetic vasculature. Through caspase-1-dependent release of proinflammatory mediators, NLRP3 drives localised immune activation within vascular tissues, contributing to endothelial dysfunction and structural remodelling of the vascular wall. This mechanistic axis provides a key inflammatory framework underpinning the progression of diabetic vascular complications [137].
3.7. Autophagy
Under low-grade inflammation and reduced PCSK9 expression, VSMCs mitigate cellular injury by activating autophagy or entering senescence via limited proliferation, allowing adaptation to external stressors. While these processes are considered protective, experimental studies show that VSMCs undergoing autophagy or senescence exhibit distinct morphological remodelling and metabolic reprogramming [138].
Emerging evidence suggests that PCSK9i may modulate autophagy-related mechanisms during vascular inflammation. For instance, PCSK9i suppresses IL-6–induced autophagy activation in endothelial cells [139]. Additionally, the lysosomal inhibitor NH4Cl reverses the downregulation of PCSK9 induced by the small molecule OY3, supporting a model in which OY3 promotes autophagy-dependent PCSK9 degradation. In contrast, the proteasome inhibitor carfilzomib exerts no significant effect, reinforcing that the degradation process bypasses the ubiquitin–proteasome system and is primarily autophagy-mediated [140]. Although current data support the involvement of PCSK9 in cardiovascular regulation via autophagic pathways, further studies are required to clarify whether it promotes or inhibits autophagy to confer cardioprotection.
3.8. Ferroptosis
Ferroptosis is a distinct form of programmed cell death characterized by intracellular iron accumulation, reactive oxygen species (ROS) generation via the Fenton reaction, and lipid peroxidation, ultimately leading to membrane disruption and cell death [141]. In DCM, hyperglycemia has been reported to trigger a DNA damage response that activates the DNA-dependent protein kinase complex, which may promote ferroptosis in endothelial cells [142].
Preclinical studies suggest that PCSK9 could be involved in regulating ferroptosis in cardiomyocytes, potentially through effects on mitochondrial dynamics and oxidative stress, thereby influencing disease progression. However, these findings are derived primarily from experimental models, and the clinical significance remains to be established.
Furthermore, it may represent a mechanistic link between inflammation and ferroptosis. Experimental silencing of TLR4 by siRNA has been shown to attenuate ferroptosis and ameliorate cardiac pathology in DCM models. In vitro and in vivo evidence indicates that PCSK9 expression correlates positively with TLR4 levels; overexpression of PCSK9 increases TLR4 expression in macrophages, whereas pharmacological or genetic inhibition of PCSK9 downregulates TLR4 in vascular tissues [143]. Concerning potential therapeutic strategies, agents such as Nicorandil have been reported to induce Parkin-dependent mitophagy through mitochondrial AMPKα1 activation, while concurrently inhibiting mitochondrial translocation of ACSL4, thereby attenuating the ferroptotic cascade. These findings offer a promising therapeutic strategy for targeting ferroptosis in DCM [144].
4. PCSK9 Involvement in the Pathological Processes of DCM
4.1. Cardiomyocytes
Despite its relatively low expression in cardiomyocytes, PCSK9 plays an essential role in cardiac metabolic regulation. Cardiomyocytes endogenously synthesise and secrete PCSK9, contributing to functional impairment via autocrine mechanisms [145]. PCSK9 deficiency is strongly linked to the development of cardiomyopathy, characterised by impaired mitochondrial oxidative capacity, altered substrate utilisation, and deteriorated contractile function [115]. In particular, disruption of PCSK9 affects cardiac lipid homeostasis through LDLR-independent pathways, facilitating the onset of HFpEF [146].
Experimental deletion of Pcsk9 specifically in cardiomyocytes induces a progressive, dilated cardiomyopathy-like phenotype in mice, marked by myocardial hypertrophy, fibrotic remodelling, and loss of systolic function, ultimately progressing to end-stage heart failure and early mortality [115]. Mechanistically, therapeutic silencing of the mitochondrial cholesterol transporter TSPO in CM-Pcsk9−/− mice prevents cholesterol accumulation within mitochondria and restores cardiac performance, suggesting a metabolic rescue strategy in PCSK9-deficient cardiomyopathy [147].
PCSK9 contributes to regulating glucose metabolism reprogramming in cardiomyocytes under pathological stress. In DCM, elevated β-hydroxybutyric acid (βOHB) levels promote epigenetic activation of the lipocalin-2 (Lcn2) promoter via H3K9bhb modification, leading to nuclear translocation of the NF-κB/RPS3 complex and initiating proinflammatory and profibrotic gene expression programs [148]. In a murine myocardial infarction model, increased expression of Foxk1 and Foxk2 has been shown to enhance cardiomyocyte proliferation, reduce scar formation, and improve cardiac function [149]. Furthermore, CTRP9-induced upregulation of CD36 occurs independently of LRP1 and LDLR pathways, indicating a distinct regulatory mechanism. Nevertheless, PCSK9 modulates receptor-mediated signalling in cardiomyocytes, particularly its influence on LRP1-dependent pathways [77].
Evidence indicates that PCSK9i mitigates cardiac fibrosis following myocardial infarction through multiple converging mechanisms. Evolocumab improves myocardial remodelling and cardiac function in metabolic syndrome models by suppressing PCSK9-mediated activation of the NLRP3 inflammasome and its downstream Caspase-1/IL-1β axis [150]. Independently, PCSK9 modulates post-infarction fibroblast phenotypic transition via the JAK2/STAT3 pathway, further contributing to fibrotic remodelling [151].
In reperfusion injury models, PCSK9i attenuate myocardial fibrosis by inhibiting the TGF-β1/Smad3 signalling cascade and dampening local inflammatory responses [152]. Additionally, under hypoxic conditions, PCSK9 inhibition reduces collagen synthesis, fibroblast migration, and the transdifferentiation of CFs into activated myofibroblasts, with these effects mediated via Notch1/Hes1 signalling, leading to improved cardiac function [153]. Moreover, GSK-3β–dependent activation of the NLRP3 inflammasome in CFs, followed by IL-1β secretion, promotes apoptosis and pyroptosis in both CFs and cardiomyocytes (CMs), reinforcing the role of inflammasome signalling in fibrotic and inflammatory myocardial injury [154].
4.2. Endothelial Cells
Endothelial cells constitute a dynamic interface for vascular immune responses. Deletion of endothelial epsins delays dysfunction by inhibiting FGFR1/TGF-β–driven EndMT, thereby preserving barrier integrity under stress conditions [155,156]. Loss of the transcription factor Gata6 downregulates Cmpk2, an upstream regulator of mitochondrial DNA signalling, ultimately suppressing NLRP3 inflammasome assembly, monocyte chemotaxis, and M1-type macrophage polarisation [157]. Furthermore, S1PR2 activation induces mitochondrial fission through Rho/ROCK1-mediated phosphorylation of DRP1, which initiates NLRP3-mediated pyroptosis and exacerbates reperfusion-associated endothelial injury [136].
PCSK9 emerges as a key modulator of endothelial integrity in diabetes. Hyperglycemic and hypoxic stimuli upregulate PCSK9 expression in endothelial cells, triggering focal cell death and functional decline [127]. In diabetic conditions, PCSK9 facilitates VEGFR2 ubiquitination by enhancing its interaction with the E3 ligase NEDD4, thus suppressing VEGFR2/AKT/eNOS-ERK signalling, a crucial pathway for angiogenic repair [158]. Additionally, PCSK9 accelerates ox-LDL–induced cytotoxicity via the UQCRC1/mitochondrial ROS axis, aggravating oxidative endothelial injury [159]. Genomic analyses also identify endothelial gene variants that predict individual responsiveness to lipid-lowering strategies, offering potential for precision medicine in cardiovascular risk reduction [160].
4.3. Monocyte
Monocytes are key cellular mediators in the inflammatory and immunological disturbances associated with diabetes. Under hyperglycemic stress, monocytes secrete pro-cathepsin D, which facilitates blood–brain barrier transcytosis and promotes neurovascular injury [161]. Additionally, CCL2 signalling within monocytes may increase susceptibility to asthma in diabetic individuals [162]. Hyperglycemia also fosters an immunosuppressive microenvironment, notably in colorectal cancer liver metastases, by recruiting circulating monocytes via the CCL3–CCR1 axis [163].
PCSK9 exerts immunoregulatory effects on monocytes, extending beyond lipid metabolism. Neutralising PCSK9 with monoclonal antibodies attenuates CCR2-mediated chemotaxis and dampens pro-inflammatory monocyte activation in familial hypercholesterolemia [164]. In patients with stable coronary artery disease (CAD), circulating PCSK9 levels exhibit subtype-specific correlations: classical monocytes (CMs) show a positive association (R = 0.29; p = 0.04), while non-classical monocytes (NCMs) are inversely related (R = −0.33; p = 0.02) [165]. Furthermore, PCSK9–Lp(a) complex concentrations and body mass index correlate with total monocyte counts, reinforcing the lipid–immune interface [166].
Evolocumab therapy has downregulated monocyte activation markers in high-risk ASCVD populations [167]. Interestingly, PCSK9 levels correlate with HMGB1, TLR4, and NCM proportion in CAD patients not on statin therapy. However, these associations are reversed in statin-treated patients, underscoring drug-specific immunometabolic shifts. Multivariate regression confirms HMGB1, NCM%, and IM% as independent positive predictors of PCSK9 in statin-naïve individuals, and as negative predictors under statin exposure [168].
4.4. Macrophage
Macrophages are increasingly recognised as central mediators of inflammatory remodelling in diabetic left ventricular fibrosis and myocardial dysfunction [169]. In the context of diabetic wound pathology, lysosomal destabilisation appears to suppress NLRP3 inflammasome activation, thereby facilitating reparative responses and accelerating tissue repair [170]. Preclinical data highlight a paracrine inflammatory axis wherein keratinocytes upregulate NLRP3 expression in infiltrating macrophages via IL-1R–dependent activation of the histone demethylase JMJD3 [171].
Phenylpyruvic acid, a microbial-derived metabolite, enters macrophages via CD36-dependent uptake, binds to palmitoyl-protein thioesterase 1 (PPT1), and inhibits its depalmitoylase activity. It enhances NLRP3 palmitoylation and activates the inflammasome, leading to proinflammatory cytokine secretion and M1-like macrophage polarisation [172]. In contrast, natural compounds such as bridging sterols can attenuate inflammasome activation and exert protective anti-inflammatory effects [173]. Additionally, macrophage-specific epsin deficiency suppresses foam cell formation through altered interactions with LRP1, suggesting an anti-atherogenic role [155].
PCSK9 emerges as a potent regulator of macrophage polarisation and function. By downregulating CD36 and inhibiting lipid uptake, PCSK9 reinforces an inflammatory macrophage phenotype characterised by increased IFN-γ production and T cell stimulation [70]. LRP5–PCSK9 interaction governs intracellular lipid processing, and LRP5 facilitates PCSK9 release [125]. PCSK9 depletion significantly impairs macrophage infiltration and reduces cytokine gene expression in vascular grafts [174].
Functionally, PCSK9 operates both intracellularly and as a circulating mediator to regulate macrophage antiviral immunity and vascular inflammation [175]. Circulating PCSK9 induces macrophage activation and vein graft lesion development via LDLR-independent mechanisms [176]. Notably, PCSK9-induced NF-κB activation in macrophages augments H/R-triggered cardiomyocyte injury through heightened secretion of IL-1β and TNF-α [177].
4.5. Vascular Smooth Muscle Cells
PCSK9 maintains mitochondrial homeostasis in VSMCs. Its upregulation leads to enhanced phosphorylation of p38 MAPK, which subsequently activates the mitochondrial fission mediator dynamin-related protein 1 (DRP1), resulting in marked changes in mitochondrial morphology and function. It is accompanied by increased intracellular reactive oxygen species (ROS) levels, ultimately impairing cellular function and viability. DRP1, as a central regulator of mitochondrial dynamics, appears to be a critical downstream effector of PCSK9, suggesting the presence of a feedback regulatory loop between PCSK9 and DRP1 [178]. Additionally, under diabetic conditions, oxidative stress activates the AMPK–Cx43–NLRP3 axis, contributing to extracellular matrix remodelling in gastric smooth muscle, as observed in diabetic gastroparesis models [179].
PCSK9 also promotes vascular ageing through multiple mechanisms. Its overexpression downregulates apolipoprotein E receptor 2, promotes polyploidization of VSMCs, and accelerates senescence-associated vascular changes [138]. Conversely, PCSK9 deficiency alleviates atherosclerosis-associated ageing phenotypes by significantly reducing neointimal thickening, vascular stenosis, and collagen deposition [174]. In diabetes, the metabolite palmitic acid also induces VSMCs’ senescence by activating the Dll4 signalling pathway in macrophages. This process depends on activating the TLR4/ERK/FOXC2 pathway, which increases plaque instability and vulnerability [180].
5. Research Progress on PCSK9 in Other Fields
5.1. PCSK9 and Ischemia/Reperfusion Injury
PCSK9 is increasingly recognised as a pathophysiological mediator and therapeutic target in ischemia–reperfusion (I/R) injury following ST-segment elevation myocardial infarction (STEMI). Experimental models indicate that PCSK9 inhibition via evolocumab protects cardiomyocytes by suppressing LIAS-dependent cuproptosis, a copper-induced cell death pathway implicated in I/R-associated myocardial damage [10].
Clinically, serial measurements in STEMI patients reveal a delayed but sustained rise in circulating PCSK9 levels within 48 h post-percutaneous coronary intervention. While early elevations (at 24 h) do not correlate with myocardial or microvascular injury, higher PCSK9 levels at 48 h are strongly associated with intramyocardial haemorrhage, microvascular obstruction, infarct expansion, and adverse clinical prognosis [9]. These findings highlight the temporal relevance of PCSK9 dynamics and reinforce its potential as a biomarker and therapeutic target in acute myocardial infarction management.
5.2. PCSK9 and Cancer
PCSK9 has been proposed as a multifunctional regulator of tumour progression and metastasis through its ability to reprogram the tumour microenvironment and intracellular signalling networks. It modulates metastatic competency by downregulating low-density lipoprotein receptor–related protein 1 (LRP1), thereby derepressing transcriptional programs associated with invasion and dissemination [181]. Beyond LRP1 suppression, experimental data indicate that PCSK9 could contribute to cancer progression by promoting epithelial–mesenchymal transition (EMT), activating the PI3K/AKT signalling pathway, and influencing macrophage polarisation toward pro-inflammatory and pro-metastatic phenotypes [182].
Mechanistically, PCSK9 overexpression has been associated with enhanced phosphorylation of mitogen-activated protein kinases (MAPKs), including p38MAPK, ERK1/2, and JNK, which may facilitate tumour cell migration and invasion. Gene silencing of PCSK9 in model systems attenuates this phosphorylation cascade and impairs tumour cell motility [183]. In anaplastic thyroid carcinoma, PCSK9 drives the lysosome-dependent degradation of E-cadherin, thereby facilitating EMT and tumour spread. Loss-of-function mutations in p53—especially the R248Q variant—further upregulate PCSK9 transcription by disrupting its repression, amplifying tumour malignancy. Targeted inhibition using the small molecule PF-846 suppresses PCSK9 expression and significantly reduces tumour proliferation and metastasis in vitro and in vivo [184].
Moreover, PCSK9 may also influence the metabolic adaptation of cancer cells in distinct microenvironments. In pancreatic cancer, low PCSK9 expression promotes extracellular cholesterol uptake, leveraging hepatic cholesterol abundance. Conversely, elevated PCSK9 levels shift cancer cells toward intrinsic cholesterol biosynthesis and antioxidant production, enhancing their survival in oxidative niches such as the lungs [185]. These observations highlight PCSK9 as a potential modulator of tumour biology. However, current evidence is derived mainly from in vitro and animal studies, and the translational and clinical relevance remains to be determined.
6. Conclusions and Perspectives
PCSK9 has garnered recognition beyond its canonical role in lipid metabolism, emerging as a pleiotropic effector in DCM. In this review, we delineate the multifaceted roles of PCSK9 in metabolic dysregulation, immune activation, and programmed cell death, and examine its cell-specific effects in cardiomyocytes, endothelial cells, macrophages, monocytes, and vascular smooth muscle cells. Mechanistically, PCSK9 contributes to chronic lipotoxic and inflammatory signalling via CD36- and TLR4-dependent pathways, fostering maladaptive cardiac remodelling in the diabetic milieu.
Pharmacologically, PCSK9 inhibition offers dual benefits—practical LDL-C reduction and favourable metabolic neutrality. Evidence from clinical trials and Mendelian randomisation studies suggests that PCSK9i do not increase the incidence of NODM, and that associated T2DM risk from partial loss-of-function mutations is minimal. Beyond lipid-lowering, PCSK9i demonstrate anti-inflammatory, anti-fibrotic, and cardio-metabolic effects that support their use in diabetic populations, especially among statin-intolerant patients or those with severe dysmetabolism.
Notable therapeutic candidates include Tafolecimab, which induces significant and sustained LDL-C reductions with favourable safety in Chinese HeFH cohorts at biweekly and monthly dosing intervals [186], and AZD0780, an orally administered PCSK9 modulator, provides potent dose-dependent LDL-C reductions and favourable pharmacokinetics, supporting further clinical development [187].
Critical knowledge gaps remain. Future studies must clarify the intracellular pathways modulated by PCSK9 in DCM, including mitochondrial bioenergetics, ferroptosis, and autophagy. Additionally, the pathophysiologic relevance of PCSK9 across various diabetic subtypes (T1DM, T2DM, GDM) must be elucidated through multicenter, phenotype-specific cohorts. Finally, combinatorial regimens with GLP-1 receptor agonists or SGLT2 inhibitors require rigorous investigation to determine additive or antagonistic effects. Of note, targeted epigenetic editing of the PCSK9 promoter has emerged as a promising strategy for personalised intervention.
In summary, targeting PCSK9 represents a novel and promising strategy for DCM management, potentially improving both survival outcomes and quality of life in patients with diabetes-related heart disease.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biom15091240/s1, Figure S1: Recent advances in PCSK9-mediated regulation of lipid metabolism.
Author Contributions
H.W., Z.Z., P.W., J.Z., Y.W. and S.D. performed the literature searches. H.W., Z.Z., P.W. and J.Z. wrote the first draft of the manuscript. Y.W. and S.D. wrote sections of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Clinical Cooperative Pilot Project of Traditional Chinese and Western Medicine for Major Diseases (no. Administration of State Administration of Traditional Chinese Medicine (2018), no. 3), National Key R&D Program of China (no. 2018YFC1311505), Gansu Provincial Clinical Research Center for Cardiovascular Diseases (no. 18JR2FA005), National Natural Science Foundation of China (no. 820608071003910).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, Regional and Country-Level Diabetes Prevalence Estimates for 2021 and Projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119, Erratum in Diabetes Res. Clin. Pract. 2023, 204, 110945. https://doi.org/10.1016/j.diabres.2023.110945. [Google Scholar] [CrossRef] [PubMed]
- Seferović, P.M.; Paulus, W.J.; Rosano, G.; Polovina, M.; Petrie, M.C.; Jhund, P.S.; Tschöpe, C.; Sattar, N.; Piepoli, M.; Papp, Z.; et al. Diabetic Myocardial Disorder. A Clinical Consensus Statement of the Heart Failure Association of the ESC and the ESC Working Group on Myocardial & Pericardial Diseases. Eur. J. Heart Fail. 2024, 26, 1893–1903. [Google Scholar] [CrossRef]
- Cavallari, I.; Bhatt, D.L.; Steg, P.G.; Leiter, L.A.; McGuire, D.K.; Mosenzon, O.; Im, K.; Raz, I.; Braunwald, E.; Scirica, B.M. Causes and Risk Factors for Death in Diabetes. J. Am. Coll. Cardiol. 2021, 77, 1837–1840. [Google Scholar] [CrossRef]
- Wu, Q.; Zeng, Y.; Geng, K.; Guo, M.; Teng, F.; Yan, P.; Lei, Y.; Long, Y.; Jiang, Z.; Law, B.Y.-K.; et al. The Role of IL-1 Family Cytokines in Diabetic Cardiomyopathy. Metabolism 2025, 163, 156083. [Google Scholar] [CrossRef]
- Akhtar, M.S.; Alavudeen, S.S.; Raza, A.; Imam, M.T.; Almalki, Z.S.; Tabassum, F.; Iqbal, M.J. Current Understanding of Structural and Molecular Changes in Diabetic Cardiomyopathy. Life Sci. 2023, 332, 122087. [Google Scholar] [CrossRef]
- Congur, I.; Mingrone, G.; Guan, K. Targeting Endoplasmic Reticulum Stress as a Potential Therapeutic Strategy for Diabetic Cardiomyopathy. Metabolism 2025, 162, 156062. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, Z.; Chen, H.; Chen, Q. Pyroptosis: Mechanisms and Links with Diabetic Cardiomyopathy. Ageing Res. Rev. 2024, 94, 102182. [Google Scholar] [CrossRef]
- Peyot, M.-L.; Roubtsova, A.; Lussier, R.; Chamberland, A.; Essalmani, R.; Murthy Madiraju, S.R.; Seidah, N.G.; Prentki, M.; Prat, A. Substantial PCSK9 Inactivation in β-Cells Does Not Modify Glucose Homeostasis or Insulin Secretion in Mice. Biochim. Biophys. Acta BBA-Mol. Cell Biol. Lipids 2021, 1866, 158968. [Google Scholar] [CrossRef] [PubMed]
- Tiller, C.; Holzknecht, M.; Lechner, I.; Oberhollenzer, F.; Von Der Emde, S.; Kremser, T.; Gollmann-Tepeköylü, C.; Mayr, A.; Bauer, A.; Metzler, B.; et al. Association of Circulating PCSK9 With Ischemia-Reperfusion Injury in Acute ST-Elevation Myocardial Infarction. Circ. Cardiovasc. Imaging 2024, 17, e016482. [Google Scholar] [CrossRef]
- Li, Z.-Z.; Guo, L.; An, Y.-L.; Yu, W.-J.; Shi, D.-Y.; Lin, Q.-Y.; Zhang, B. Evolocumab Attenuates Myocardial Ischemia/Reperfusion Injury by Blocking PCSK9/LIAS-Mediated Cuproptosis of Cardiomyocytes. Basic Res. Cardiol. 2025, 120, 301–320. [Google Scholar] [CrossRef] [PubMed]
- Ference, B.A.; Robinson, J.G.; Brook, R.D.; Catapano, A.L.; Chapman, M.J.; Neff, D.R.; Voros, S.; Giugliano, R.P.; Davey Smith, G.; Fazio, S.; et al. Variation in PCSK9 and HMGCR and Risk of Cardiovascular Disease and Diabetes. N. Engl. J. Med. 2016, 375, 2144–2153. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wu, G.; Li, C.; Qin, X.; Liu, R.; Zhang, M. Safety of Proprotein Convertase Subtilisin/Kexin Type 9 Monoclonal Antibodies in Regard to Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Am. J. Cardiovasc. Drugs 2020, 20, 343–353. [Google Scholar] [CrossRef]
- Rojo-Martínez, G.; Valdés, S.; Soriguer, F.; Vendrell, J.; Urrutia, I.; Pérez, V.; Ortega, E.; Ocón, P.; Montanya, E.; Menéndez, E.; et al. Incidence of Diabetes Mellitus in Spain as Results of the Nation-Wide Cohort Diabet.es Study. Sci. Rep. 2020, 10, 2765. [Google Scholar] [CrossRef]
- González-Lleó, A.M.; Sánchez-Hernández, R.M.; Plana, N.; Ibarretxe, D.; Rehues, P.; Ribalta, J.; Llop, D.; Wägner, A.M.; Masana, L.; Boronat, M. Impact of PCSK9 Inhibitors in Glycaemic Control and New-Onset Diabetes. Cardiovasc. Diabetol. 2024, 23, 4. [Google Scholar] [CrossRef]
- Ramin-Mangata, S.; Thedrez, A.; Nativel, B.; Diotel, N.; Blanchard, V.; Wargny, M.; Aguesse, A.; Billon-Crossouard, S.; Vindis, C.; Le May, C.; et al. Effects of Proprotein Convertase Subtilisin Kexin Type 9 Modulation in Human Pancreatic Beta Cells Function. Atherosclerosis 2021, 326, 47–55. [Google Scholar] [CrossRef]
- Schwartz, G.G.; Szarek, M.; Jukema, J.W.; Cobbaert, C.M.; Reijnders, E.; Bittner, V.A.; Schwertfeger, M.; Bhatt, D.L.; Fazio, S.; Garon, G.; et al. Risk of Incident Diabetes Related to Lipoprotein(a), LDL Cholesterol, and Their Changes with Alirocumab: Post Hoc Analyses of the ODYSSEY OUTCOMES Randomized Trial. Diabetes Care 2025, 48, 596–604. [Google Scholar] [CrossRef]
- Chen, W.; Zhao, M.; Zhao, S.; Lu, Q.; Ni, L.; Zou, C.; Lu, L.; Xu, X.; Guan, H.; Zheng, Z.; et al. Activation of the TXNIP/NLRP3 Inflammasome Pathway Contributes to Inflammation in Diabetic Retinopathy: A Novel Inhibitory Effect of Minocycline. Inflamm. Res. 2017, 66, 157–166. [Google Scholar] [CrossRef]
- Weissman, D.; Maack, C. Bile Acids for Diabetic Cardiomyopathy. Nat. Metab. 2024, 6, 993–995. [Google Scholar] [CrossRef] [PubMed]
- Winell, K.; Pietilä, A.; Salomaa, V. Incidence and Prognosis of Heart Failure in Persons with Type 2 Diabetes Compared with Individuals without Diabetes—A Nation-Wide Study from Finland in 1996–2012. Ann. Med. 2019, 51, 174–181. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Z.; Yin, Z.; Zhang, Y.; Xue, X.; Han, J.; Zhu, Y.; Zhang, J.; Emmert, M.Y.; Wang, H. Gender Differences in Fibrosis Remodeling in Patients with Long-Standing Persistent Atrial Fibrillation. Oncotarget 2017, 8, 53714–53729. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, J.; Cui, H.; Fan, C.; Xue, Y.; Liu, H.; Li, H.; Li, J.; Li, H.; Sun, Y.; et al. Inhibition of Fatty Acid Uptake by TGR5 Prevents Diabetic Cardiomyopathy. Nat. Metab. 2024, 6, 1161–1177. [Google Scholar] [CrossRef]
- Huynh, K.; Kiriazis, H.; Du, X.-J.; Love, J.E.; Gray, S.P.; Jandeleit-Dahm, K.A.; McMullen, J.R.; Ritchie, R.H. Targeting the Upregulation of Reactive Oxygen Species Subsequent to Hyperglycemia Prevents Type 1 Diabetic Cardiomyopathy in Mice. Free Radic. Biol. Med. 2013, 60, 307–317. [Google Scholar] [CrossRef]
- Mariappan, N.; Elks, C.M.; Sriramula, S.; Guggilam, A.; Liu, Z.; Borkhsenious, O.; Francis, J. NF-κB-Induced Oxidative Stress Contributes to Mitochondrial and Cardiac Dysfunction in Type II Diabetes. Cardiovasc. Res. 2010, 85, 473–483. [Google Scholar] [CrossRef]
- Meng, L.; Lu, Y.; Wang, X.; Cheng, C.; Xue, F.; Xie, L.; Zhang, Y.; Sui, W.; Zhang, M.; Zhang, Y.; et al. NPRC Deletion Attenuates Cardiac Fibrosis in Diabetic Mice by Activating PKA/PKG and Inhibiting TGF-Β1/Smad Pathways. Sci. Adv. 2023, 9, eadd4222. [Google Scholar] [CrossRef]
- Ralston, J.C.; Lyons, C.L.; Kennedy, E.B.; Kirwan, A.M.; Roche, H.M. Fatty Acids and NLRP3 Inflammasome–Mediated Inflammation in Metabolic Tissues. Annu. Rev. Nutr. 2017, 37, 77–102. [Google Scholar] [CrossRef]
- Zhang, Z.-Y.; Dang, S.-P.; Li, S.-S.; Liu, Y.; Qi, M.-M.; Wang, N.; Miao, L.-F.; Wu, Y.; Li, X.-Y.; Wang, C.-X.; et al. Glucose Fluctuations Aggravate Myocardial Fibrosis via the Nuclear Factor-κB-Mediated Nucleotide-Binding Oligomerization Domain-Like Receptor Protein 3 Inflammasome Activation. Front. Cardiovasc. Med. 2022, 9, 748183. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, D.; Ma, Y.; Du, H.; Wang, Y.; Luo, W.; Wang, R.; Yi, F. ROS in Diabetic Atria Regulate SK2 Degradation by Atrogin-1 through the NF-κB Signaling Pathway. J. Biol. Chem. 2024, 300, 105735. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Luo, W.; Han, J.; Khan, Z.A.; Fang, Q.; Jin, Y.; Chen, X.; Zhang, Y.; Wang, M.; Qian, J.; et al. MD2 Activation by Direct AGE Interaction Drives Inflammatory Diabetic Cardiomyopathy. Nat. Commun. 2020, 11, 2148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Thai, P.N.; Shivnaraine, R.V.; Ren, L.; Wu, X.; Siepe, D.H.; Liu, Y.; Tu, C.; Shin, H.S.; Caudal, A.; et al. Multiscale Drug Screening for Cardiac Fibrosis Identifies MD2 as a Therapeutic Target. Cell 2024, 187, 7143–7163.e22. [Google Scholar] [CrossRef]
- Wan, L.; Bai, X.; Zhou, Q.; Chen, C.; Wang, H.; Liu, T.; Xue, J.; Wei, C.; Xie, L. The Advanced Glycation End-Products (AGEs)/ROS/NLRP3 Inflammasome Axis Contributes to Delayed Diabetic Corneal Wound Healing and Nerve Regeneration. Int. J. Biol. Sci. 2022, 18, 809–825. [Google Scholar] [CrossRef]
- Yu, L.; Yang, Y.X.; Gong, Z.; Wan, Q.; Du, Y.; Zhou, Q.; Xiao, Y.; Zahr, T.; Wang, Z.; Yu, Z.; et al. FcRn-Dependent IgG Accumulation in Adipose Tissue Unmasks Obesity Pathophysiology. Cell Metab. 2025, 37, 656–672.e7. [Google Scholar] [CrossRef]
- Liu, Y.; Su, W.; Liu, Z.; Hu, Z.; Shen, J.; Zheng, Z.; Ding, D.; Huang, W.; Li, W.; Cai, G.; et al. Macrophage CREBZF Orchestrates Inflammatory Response to Potentiate Insulin Resistance and Type 2 Diabetes. Adv. Sci. 2024, 11, e2306685. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.; Xia, J.; Zhang, L.; Chen, B.; Lian, G.; Yun, C.; Yang, J.; Yan, Y.; Wang, P.; et al. Macrophage HIF-2α Suppresses NLRP3 Inflammasome Activation and Alleviates Insulin Resistance. Cell Rep. 2021, 36, 109607. [Google Scholar] [CrossRef]
- You, D.; Jung, B.C.; Villivalam, S.D.; Lim, H.-W.; Kang, S. JMJD8 Is a Novel Molecular Nexus Between Adipocyte-Intrinsic Inflammation and Insulin Resistance. Diabetes 2022, 71, 43–59. [Google Scholar] [CrossRef]
- Huang, H.; Chen, H.; Yao, Y.; Lou, X. Branched-Chain Amino Acids Supplementation Induces Insulin Resistance and pro-Inflammatory Macrophage Polarization via INFGR1/JAK1/STAT1 Signal Pathway. Mol. Med. 2024, 30, 149. [Google Scholar] [CrossRef]
- Larsen, J.K.; Stocks, B.; Henderson, J.; Andersson, D.; Bäckdahl, J.; Eriksson-Hogling, D.; Stidsen, J.V.; Sakamoto, K.; Højlund, K.; Rydén, M.; et al. Personalized Molecular Signatures of Insulin Resistance and Type 2 Diabetes 2024. Cell 2025, 188, 4106–4122.e16. [Google Scholar]
- Chen, L.; Lin, Y.; Zhu, X.; Zhuo, S.; Li, Z.; Guo, C.; Ye, X.; Chen, J.; Wang, S.; Chen, Y. MCT1-Mediated Lactate Shuttle to Mitochondria Governs Macrophage Polarization and Modulates Glucose Homeostasis by Affecting β Cells. Adv. Sci. 2025, e14760. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhu, J.; Zhang, M.; Shi, Q.; Guo, R.; Zhang, D.; Zheng, P.; Zhang, H.; Li, G.; Wu, J.; et al. SOSTDC1 Downregulation in CD4+ T Cells Confers Protection against Obesity-Induced Insulin Resistance. Cell Rep. 2025, 44, 115496. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Lai, C.-C.; Bonnavion, R.; Alnouri, M.W.; Wang, S.; Roquid, K.A.; Kawase, H.; Campos, D.; Chen, M.; Weinstein, L.S.; et al. Endothelial Insulin Resistance Induced by Adrenomedullin Mediates Obesity-Associated Diabetes. Science 2025, 387, 674–682. [Google Scholar] [CrossRef]
- She, J.; Tuerhongjiang, G.; Guo, M.; Liu, J.; Hao, X.; Guo, L.; Liu, N.; Xi, W.; Zheng, T.; Du, B.; et al. Statins Aggravate Insulin Resistance through Reduced Blood Glucagon-like Peptide-1 Levels in a Microbiota-Dependent Manner. Cell Metab. 2024, 36, 408–421.e5. [Google Scholar] [CrossRef] [PubMed]
- Seidah, N.G.; Prat, A. The Multifaceted Biology of PCSK9. Endocr. Rev. 2022, 43, 558–582. [Google Scholar] [CrossRef]
- Walley, K.R.; Thain, K.R.; Russell, J.A.; Reilly, M.P.; Meyer, N.J.; Ferguson, J.F.; Christie, J.D.; Nakada, T.; Fjell, C.D.; Thair, S.A.; et al. PCSK9 Is a Critical Regulator of the Innate Immune Response and Septic Shock Outcome. Sci. Transl. Med. 2014, 6, 258ra143. [Google Scholar] [CrossRef]
- Luna Saavedra, Y.G.; Zhang, J.; Seidah, N.G. PCSK9 Prosegment Chimera as Novel Inhibitors of LDLR Degradation. PLoS ONE 2013, 8, e72113. [Google Scholar] [CrossRef]
- Ding, Z.; Wang, X.; Liu, S.; Zhou, S.; Kore, R.A.; Mu, S.; Deng, X.; Fan, Y.; Mehta, J.L. NLRP3 Inflammasome via IL-1β Regulates PCSK9 Secretion. Theranostics 2020, 10, 7100–7110. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Liu, H. PCSK9/LOX-1 Is Associated with T2DM and Regulates High Glucose-Induced Lipid Metabolism Dysfunction in Human Microvascular Endothelial Cells. Endokrynol. Pol. 2025, 76, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Adorni, M.P.; Cipollari, E.; Favari, E.; Zanotti, I.; Zimetti, F.; Corsini, A.; Ricci, C.; Bernini, F.; Ferri, N. Inhibitory Effect of PCSK9 on Abca1 Protein Expression and Cholesterol Efflux in Macrophages. Atherosclerosis 2017, 256, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Liu, S.; Wang, X.; Deng, X.; Fan, Y.; Sun, C.; Wang, Y.; Mehta, J.L. Hemodynamic Shear Stress via ROS Modulates PCSK9 Expression in Human Vascular Endothelial and Smooth Muscle Cells and Along the Mouse Aorta. Antioxid. Redox Signal. 2015, 22, 760–771. [Google Scholar] [CrossRef]
- Ding, Z.; Liu, S.; Wang, X.; Deng, X.; Fan, Y.; Shahanawaz, J.; Shmookler Reis, R.J.; Varughese, K.I.; Sawamura, T.; Mehta, J.L. Cross-Talk between LOX-1 and PCSK9 in Vascular Tissues. Cardiovasc. Res. 2015, 107, 556–567. [Google Scholar] [CrossRef]
- Cappelluti, M.A.; Mollica Poeta, V.; Valsoni, S.; Quarato, P.; Merlin, S.; Merelli, I.; Lombardo, A. Durable and Efficient Gene Silencing in Vivo by Hit-and-Run Epigenome Editing. Nature 2024, 627, 416–423. [Google Scholar] [CrossRef]
- Reduction of Low-Density Lipoprotein Cholesterol by Monoclonal Antibody Inhibition of PCSK9 | Annual Reviews. Available online: https://www.annualreviews.org/content/journals/10.1146/annurev-med-022613-090402 (accessed on 7 December 2024).
- Tang, Y.; Li, S.-L.; Hu, J.-H.; Sun, K.-J.; Liu, L.-L.; Xu, D.-Y. Research Progress on Alternative Non-Classical Mechanisms of PCSK9 in Atherosclerosis in Patients with and without Diabetes. Cardiovasc. Diabetol. 2020, 19, 33. [Google Scholar] [CrossRef]
- Guan, Y.; Liu, X.; Yang, Z.; Zhu, X.; Liu, M.; Du, M.; Pan, X.; Wang, Y. PCSK9 Promotes LDLR Degradation by Preventing SNX17-Mediated LDLR Recycling. Circulation 2025, 151, 1512–1526. [Google Scholar] [CrossRef]
- Ding, Z.; Wang, X.; Liu, S.; Shahanawaz, J.; Theus, S.; Fan, Y.; Deng, X.; Zhou, S.; Mehta, J.L. PCSK9 Expression in the Ischaemic Heart and Its Relationship to Infarct Size, Cardiac Function, and Development of Autophagy. Cardiovasc. Res. 2018, 114, 1738–1751. [Google Scholar] [CrossRef]
- Seidah, N.G.; Awan, Z.; Chrétien, M.; Mbikay, M. PCSK9: A Key Modulator of Cardiovascular Health. Circ. Res. 2014, 114, 1022–1036. [Google Scholar] [CrossRef]
- Al Said, S.; O’Donoghue, M.L.; Ran, X.; Murphy, S.A.; Atar, D.; Keech, A.; Flores-Arredondo, J.H.; Wang, B.; Sabatine, M.S.; Giugliano, R.P. Long-Term Lipid Lowering with Evolocumab in Older Individuals. J. Am. Coll. Cardiol. 2025, 85, 504–512. [Google Scholar] [CrossRef]
- Dhar, K.; Berger, J.; Newman, J.; Schwartzbard, A.; Pernia, A.C.J.; Weintraub, H.S. Evolocumab in Older Individuals. J. Am. Coll. Cardiol. 2025, 85, 513–514. [Google Scholar] [CrossRef]
- Rosoff, D.B.; Wagner, J.; Jung, J.; Pacher, P.; Christodoulides, C.; Davey Smith, G.; Ray, D.; Lohoff, F.W. Multiomic Mendelian Randomization Study Investigating the Impact of PCSK9 and HMGCR Inhibition on Type 2 Diabetes Across Five Populations. Diabetes 2025, 74, 120–130. [Google Scholar] [CrossRef]
- Rosoff, D.B.; Bell, A.S.; Wagner, J.; Mavromatis, L.A.; Hamandi, A.; Park, L.; Jung, J.; Lohoff, F.W. Assessing the Impact of PCSK9 and HMGCR Inhibition on Liver Function: Drug-Target Mendelian Randomization Analyses in Four Ancestries. Cell. Mol. Gastroenterol. Hepatol. 2024, 17, 29–40. [Google Scholar] [CrossRef]
- Rivera, F.B.; Cha, S.W.; Aparece, J.P.; Rocimo, A.; Ong, B.A.; Golbin, J.M.; Alfonso, P.G.; Enkhmaa, B.; Khan, S.U.; Cainzos-Achirica, M.; et al. Sex Differences in Cardiovascular Outcomes and Cholesterol-Lowering Efficacy of PCSK9 Inhibitors. JACC Adv. 2023, 2, 100669. [Google Scholar] [CrossRef] [PubMed]
- Hodeda, L.; Eisen, A.; Rotholz, A.; Kornowski, R.; Gurevitz, C. Sex Differences in the Treatment with PCSK9 Monoclonal Antibodies- Real World Experience from a Dedicated Preventive Cardiology Clinic. Am. J. Prev. Cardiol. 2025, 23, 101022. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Dong, B.; Park, S.W.; Lee, H.-S.; Chen, W.; Liu, J. Hepatocyte Nuclear Factor 1α Plays a Critical Role in PCSK9 Gene Transcription and Regulation by the Natural Hypocholesterolemic Compound Berberine. J. Biol. Chem. 2009, 284, 28885–28895. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Yan, B.; Gui, Y.; Tang, Z.; Tai, S.; Zhou, S.; Zheng, X. Physiology and Role of PCSK9 in Vascular Disease: Potential Impact of Localized PCSK9 in Vascular Wall. J. Cell. Physiol. 2021, 236, 2333–2351. [Google Scholar] [CrossRef] [PubMed]
- Ai, D.; Chen, C.; Han, S.; Ganda, A.; Murphy, A.J.; Haeusler, R.; Thorp, E.; Accili, D.; Horton, J.D.; Tall, A.R. Regulation of Hepatic LDL Receptors by mTORC1 and PCSK9 in Mice. J. Clin. Invest. 2012, 122, 1262–1270. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, J.; Yan, C.; Xi, C.; Wu, C.; Zhao, J.; Li, F.; Ding, Y.; Zhang, R.; Qi, S.; et al. Identification and Evaluation of a Lipid-Lowering Small Compound in Preclinical Models and in a Phase I Trial. Cell Metab. 2022, 34, 667–680.e6. [Google Scholar] [CrossRef]
- Wang, M.; Shu, H.; Xie, J.; Huang, Y.; Wang, K.; Feng, R.; Yu, X.; Guan, J.; Feng, W.; Liu, M. An Intron Mutation of HNF1A Causes Abnormal Splicing and Impairs Its Activity as a Transcription Factor. Mol. Cell. Endocrinol. 2022, 545, 111575. [Google Scholar] [CrossRef]
- Beucher, A.; Miguel-Escalada, I.; Balboa, D.; De Vas, M.G.; Maestro, M.A.; Garcia-Hurtado, J.; Bernal, A.; Gonzalez-Franco, R.; Vargiu, P.; Heyn, H.; et al. The HASTER lncRNA Promoter Is a Cis-Acting Transcriptional Stabilizer of HNF1A. Nat. Cell Biol. 2022, 24, 1528–1540. [Google Scholar] [CrossRef]
- Lu, F.; Li, E.; Gao, Y.; Zhang, Y.; Kong, L.; Yang, X. Dapagliflozin Modulates Hepatic Lipid Metabolism through the Proprotein Convertase Subtilisin/Kexin Type 9/Low Density Lipoprotein Receptor Pathway. Diabetes Obes. Metab. 2025, 27, 2096–2109. [Google Scholar] [CrossRef]
- Luo, J.; Yang, H.; Song, B.-L. Mechanisms and Regulation of Cholesterol Homeostasis. Nat. Rev. Mol. Cell Biol. 2020, 21, 225–245. [Google Scholar] [CrossRef]
- Lebeau, P.F.; Byun, J.H.; Platko, K.; Saliba, P.; Sguazzin, M.; MacDonald, M.E.; Paré, G.; Steinberg, G.R.; Janssen, L.J.; Igdoura, S.A.; et al. Caffeine Blocks SREBP2-Induced Hepatic PCSK9 Expression to Enhance LDLR-Mediated Cholesterol Clearance. Nat. Commun. 2022, 13, 770. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xu, H.; Yu, J.; Cui, J.; Chen, Z.; Li, Y.; Niu, Y.; Wang, S.; Ran, S.; Zou, Y.; et al. Immune Regulation of the Liver Through the PCSK9/CD36 Pathway During Heart Transplant Rejection. Circulation 2023, 148, 336–353. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ma, K.L.; Zhang, Y.; Wu, Y.; Hu, Z.B.; Lv, L.L.; Tang, R.N.; Liu, H.; Ruan, X.Z.; Liu, B.C. Activation of mTORC1 Disrupted LDL Receptor Pathway: A Potential New Mechanism for the Progression of Non-Alcoholic Fatty Liver Disease. Int. J. Biochem. Cell Biol. 2015, 61, 8–19. [Google Scholar] [CrossRef]
- Hao, J.-W.; Wang, J.; Guo, H.; Zhao, Y.-Y.; Sun, H.-H.; Li, Y.-F.; Lai, X.-Y.; Zhao, N.; Wang, X.; Xie, C.; et al. CD36 Facilitates Fatty Acid Uptake by Dynamic Palmitoylation-Regulated Endocytosis. Nat. Commun. 2020, 11, 4765. [Google Scholar] [CrossRef]
- Heather, L.C.; Gopal, K.; Srnic, N.; Ussher, J.R. Redefining Diabetic Cardiomyopathy: Perturbations in Substrate Metabolism at the Heart of Its Pathology. Diabetes 2024, 73, 659–670. [Google Scholar] [CrossRef]
- Tan, Y.; Zhang, Z.; Zheng, C.; Wintergerst, K.A.; Keller, B.B.; Cai, L. Mechanisms of Diabetic Cardiomyopathy and Potential Therapeutic Strategies: Preclinical and Clinical Evidence. Nat. Rev. Cardiol. 2020, 17, 585–607. [Google Scholar] [CrossRef]
- Roubtsova, A.; Munkonda, M.N.; Awan, Z.; Marcinkiewicz, J.; Chamberland, A.; Lazure, C.; Cianflone, K.; Seidah, N.G.; Prat, A. Circulating Proprotein Convertase Subtilisin/Kexin 9 (PCSK9) Regulates VLDLR Protein and Triglyceride Accumulation in Visceral Adipose Tissue. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 785–791. [Google Scholar] [CrossRef]
- Levy, E.; Ouadda, A.B.D.; Spahis, S.; Sane, A.T.; Garofalo, C.; Grenier, É.; Emonnot, L.; Yara, S.; Couture, P.; Beaulieu, J.-F.; et al. PCSK9 Plays a Significant Role in Cholesterol Homeostasis and Lipid Transport in Intestinal Epithelial Cells. Atherosclerosis 2013, 227, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Rohrbach, S.; Li, L.; Novoyatleva, T.; Niemann, B.; Knapp, F.; Molenda, N.; Schulz, R. Impact of PCSK9 on CTRP9-Induced Metabolic Effects in Adult Rat Cardiomyocytes. Front. Physiol. 2021, 12, 593862. [Google Scholar] [CrossRef] [PubMed]
- Jaafar, A.K.; Paulo-Ramos, A.; Rastoldo, G.; Veeren, B.; Planesse, C.; Bringart, M.; Rondeau, P.; Chemello, K.; Meilhac, O.; Lambert, G.C.; et al. PCSK9 Deficiency Promotes the Development of Peripheral Neuropathy. JCI Insight 2025, 10, e183786. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Qin, H.; Liao, M.; Zheng, E.; Luo, X.; Xiao, A.; Li, Y.; Chen, L.; Wei, L.; Zhao, L.; et al. CD36 Promotes de Novo Lipogenesis in Hepatocytes through INSIG2-Dependent SREBP1 Processing. Mol. Metab. 2022, 57, 101428. [Google Scholar] [CrossRef]
- Da Dalt, L.; Ruscica, M.; Bonacina, F.; Balzarotti, G.; Dhyani, A.; Di Cairano, E.; Baragetti, A.; Arnaboldi, L.; De Metrio, S.; Pellegatta, F.; et al. PCSK9 Deficiency Reduces Insulin Secretion and Promotes Glucose Intolerance: The Role of the Low-Density Lipoprotein Receptor. Eur. Heart J. 2019, 40, 357–368. [Google Scholar] [CrossRef]
- Zaid, A.; Roubtsova, A.; Essalmani, R.; Marcinkiewicz, J.; Chamberland, A.; Hamelin, J.; Tremblay, M.; Jacques, H.; Jin, W.; Davignon, J.; et al. Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9): Hepatocyte-Specific Low-Density Lipoprotein Receptor Degradation and Critical Role in Mouse Liver Regeneration. Hepatology 2008, 48, 646–654. [Google Scholar] [CrossRef]
- Guo, W.; Gong, Y.; Gu, Y.; Fu, Z.; Fan, H.; Gao, B.; Zhu, X.; Fu, J.; Zhao, Y.; Sun, M.; et al. Circulating PCSK9 Levels and 2-hPG Are Positively Correlated in Metabolic Diseases in a Chinese Han Population. Lipids Health Dis. 2018, 17. [Google Scholar] [CrossRef]
- Wu, Y.; Shi, J.; Su, Q.; Yang, Z.; Qin, L. Correlation Between Circulating PCSK9 Levels and Gestational Diabetes Mellitus in a Chinese Population. Front. Endocrinol. 2022, 13, 826757. [Google Scholar] [CrossRef]
- Nelson, C.P.; Lai, F.Y.; Nath, M.; Ye, S.; Webb, T.R.; Schunkert, H.; Samani, N.J. Genetic Assessment of Potential Long-Term On-Target Side Effects of PCSK9 (Proprotein Convertase Subtilisin/Kexin Type 9) Inhibitors. Circ. Genomic Precis. Med. 2019, 12, e002196. [Google Scholar] [CrossRef]
- Ray, K.K.; Colhoun, H.M.; Szarek, M.; Baccara-Dinet, M.; Bhatt, D.L.; Bittner, V.A.; Budaj, A.J.; Diaz, R.; Goodman, S.G.; Hanotin, C.; et al. Effects of Alirocumab on Cardiovascular and Metabolic Outcomes after Acute Coronary Syndrome in Patients with or without Diabetes: A Prespecified Analysis of the ODYSSEY OUTCOMES Randomised Controlled Trial. Lancet Diabetes Endocrinol. 2019, 7, 618–628, Erratum in Lancet Diabetes Endocrinol. 2019, 7, 618–628. [Google Scholar] [CrossRef]
- Nishikido, T.; Ray, K.K. Targeting the Peptidase PCSK9 to Reduce Cardiovascular Risk: Implications for Basic Science and Upcoming Challenges. Br. J. Pharmacol. 2021, 178, 2168–2185. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, S.J. PCSK9 Inhibitors and Reduction in Cardiovascular Events: Current Evidence and Future Perspectives. Pol. Heart J. Kardiologia Pol. 2023, 81, 115–122. [Google Scholar] [CrossRef]
- McClintick, D.J.; O’Donoghue, M.L.; De Ferrari, G.M.; Ferreira, J.; Ran, X.; Im, K.; López, J.A.G.; Elliott-Davey, M.; Wang, B.; Monsalvo, M.L.; et al. Long-Term Efficacy of Evolocumab in Patients With or Without Multivessel Coronary Disease. J. Am. Coll. Cardiol. 2024, 83, 652–664. [Google Scholar] [CrossRef] [PubMed]
- Imbalzano, E.; Ilardi, F.; Orlando, L.; Pintaudi, B.; Savarese, G.; Rosano, G. The Efficacy of PCSK9 Inhibitors on Major Cardiovascular Events and Lipid Profile in Patients with Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Eur. Heart J.-Cardiovasc. Pharmacother. 2023, 9, 318–327. [Google Scholar] [CrossRef]
- Ray, K.K.; Del Prato, S.; Müller-Wieland, D.; Cariou, B.; Colhoun, H.M.; Tinahones, F.J.; Domenger, C.; Letierce, A.; Mandel, J.; Samuel, R.; et al. Alirocumab Therapy in Individuals with Type 2 Diabetes Mellitus and Atherosclerotic Cardiovascular Disease: Analysis of the ODYSSEY DM-DYSLIPIDEMIA and DM-INSULIN Studies. Cardiovasc. Diabetol. 2019, 18, 149. [Google Scholar] [CrossRef] [PubMed]
- Fischer, L.T.; Hochfellner, D.A.; Knoll, L.; Pöttler, T.; Mader, J.K.; Aberer, F. Real-World Data on Metabolic Effects of PCSK9 Inhibitors in a Tertiary Care Center in Patients with and without Diabetes Mellitus. Cardiovasc. Diabetol. 2021, 20, 89. [Google Scholar] [CrossRef]
- Rosenson, R.S.; Daviglus, M.L.; Handelsman, Y.; Pozzilli, P.; Bays, H.; Monsalvo, M.L.; Elliott-Davey, M.; Somaratne, R.; Reaven, P. Efficacy and Safety of Evolocumab in Individuals with Type 2 Diabetes Mellitus: Primary Results of the Randomised Controlled BANTING Study. Diabetologia 2019, 62, 948–958. [Google Scholar] [CrossRef]
- Chen, Y.; Yuan, Z.; Lu, J.; Eliaschewitz, F.G.; Lorenzatti, A.J.; Monsalvo, M.L.; Wang, N.; Hamer, A.W.; Ge, J. Randomized Study of Evolocumab in Patients with Type 2 Diabetes and Dyslipidaemia on Background Statin: Pre-specified Analysis of the Chinese Population from the BERSON Clinical Trial. Diabetes Obes. Metab. 2019, 21, 1464–1473. [Google Scholar] [CrossRef]
- Sabatine, M.S.; Leiter, L.A.; Wiviott, S.D.; Giugliano, R.P.; Deedwania, P.; De Ferrari, G.M.; Murphy, S.A.; Kuder, J.F.; Gouni-Berthold, I.; Lewis, B.S.; et al. Cardiovascular Safety and Efficacy of the PCSK9 Inhibitor Evolocumab in Patients with and without Diabetes and the Effect of Evolocumab on Glycaemia and Risk of New-Onset Diabetes: A Prespecified Analysis of the FOURIER Randomised Controlled Trial. Lancet Diabetes Endocrinol. 2017, 5, 941–950. [Google Scholar] [CrossRef]
- Moura, F.A.; Kamanu, F.K.; Wiviott, S.D.; Giugliano, R.P.; Udler, M.S.; Florez, J.C.; Ellinor, P.T.; Sabatine, M.S.; Ruff, C.T.; Marston, N.A. Type 2 Diabetes Genetic Risk and Incident Diabetes across Diabetes Risk Enhancers. Diabetes Obes. Metab. 2025, 27, 1287–1295. [Google Scholar] [CrossRef]
- Mousavi, A.; Shojaei, S.; Soleimani, H.; Semirani-Nezhad, D.; Ebrahimi, P.; Zafari, A.; Ebrahimi, R.; Roozbehi, K.; Harrison, A.; Syed, M.A.; et al. Safety, Efficacy, and Cardiovascular Benefits of Combination Therapy with SGLT-2 Inhibitors and GLP-1 Receptor Agonists in Patients with Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Diabetol. Metab. Syndr. 2025, 17, 68. [Google Scholar] [CrossRef]
- Yang, S.-H.; Xu, R.-X.; Cui, C.-J.; Wang, Y.; Du, Y.; Chen, Z.-G.; Yao, Y.-H.; Ma, C.-Y.; Zhu, C.-G.; Guo, Y.-L.; et al. Liraglutide Downregulates Hepatic LDL Receptor and PCSK9 Expression in HepG2 Cells and Db/Db Mice through a HNF-1a Dependent Mechanism. Cardiovasc. Diabetol. 2018, 17, 48. [Google Scholar] [CrossRef] [PubMed]
- Vergès, B.; Duvillard, L.; de Barros, J.P.P.; Bouillet, B.; Baillot-Rudoni, S.; Rouland, A.; Petit, J.M.; Degrace, P.; Demizieux, L. Liraglutide Increases the Catabolism of Apolipoprotein B100–Containing Lipoproteins in Patients with Type 2 Diabetes and Reduces Proprotein Convertase Subtilisin/Kexin Type 9 Expression. Diabetes Care 2021, 44, 1027–1037. [Google Scholar] [CrossRef] [PubMed]
- Tricò, D.; Raggi, F.; Distaso, M.; Ferrannini, E.; Solini, A. Effect of Empagliflozin on Plasma Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) in Patients with Type 2 Diabetes. Diabetes Res. Clin. Pract. 2022, 190, 109983. [Google Scholar] [CrossRef]
- Hu, D.; Guo, Y.; Wu, R.; Shao, T.; Long, J.; Yu, B.; Wang, H.; Luo, Y.; Lu, H.; Zhang, J.; et al. New Insight into Metformin-Induced Cholesterol-Lowering Effect Crosstalk Between Glucose and Cholesterol Homeostasis via ChREBP (Carbohydrate-Responsive Element-Binding Protein)-Mediated PCSK9 (Proprotein Convertase Subtilisin/Kexin Type 9) Regulation. Arterioscler. Thromb. Vasc. Biol. 2021, 41, e208–e223. [Google Scholar] [CrossRef] [PubMed]
- Amput, P.; Palee, S.; Arunsak, B.; Pratchayasakul, W.; Thonusin, C.; Kerdphoo, S.; Jaiwongkam, T.; Chattipakorn, S.C.; Chattipakorn, N. PCSK9 Inhibitor and Atorvastatin Reduce Cardiac Impairment in Ovariectomized Prediabetic Rats via Improved Mitochondrial Function and Ca2+ Regulation. J. Cell. Mol. Med. 2020, 24, 9189–9203. [Google Scholar] [CrossRef]
- Hummelgaard, S.; Hvid, H.; Birn, H.; Glerup, S.; Tom, N.; Bilgin, M.; Kirchhoff, J.E.; Weyer, K. Lack of Renoprotective Effects by Long-Term PCSK9 and SGLT2 Inhibition Using Alirocumab and Empagliflozin in Obese ZSF1 Rats. Am. J. Physiol.-Ren. Physiol. 2025, 328, F48–F67. [Google Scholar] [CrossRef]
- Miao, J.; Manthena, P.V.; Haas, M.E.; Ling, A.V.; Shin, D.-J.; Graham, M.J.; Crooke, R.M.; Liu, J.; Biddinger, S.B. Role of Insulin in the Regulation of Proprotein Convertase Subtilisin/Kexin Type 9. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1589–1596. [Google Scholar] [CrossRef]
- Niesen, M.; Bedi, M.; Lopez, D. Diabetes Alters LDL Receptor and PCSK9 Expression in Rat Liver. Arch. Biochem. Biophys. 2008, 470, 111–115. [Google Scholar] [CrossRef]
- Persson, L.; Gälman, C.; Angelin, B.; Rudling, M. Importance of Proprotein Convertase Subtilisin/Kexin Type 9 in the Hormonal and Dietary Regulation of Rat Liver Low-Density Lipoprotein Receptors. Endocrinology 2009, 150, 1140–1146. [Google Scholar] [CrossRef]
- Ruscica, M.; Macchi, C.; Giuliani, A.; Rizzuto, A.S.; Ramini, D.; Sbriscia, M.; Carugo, S.; Bonfigli, A.R.; Corsini, A.; Olivieri, F.; et al. Circulating PCSK9 as a Prognostic Biomarker of Cardiovascular Events in Individuals with Type 2 Diabetes: Evidence from a 16.8-Year Follow-up Study. Cardiovasc. Diabetol. 2023, 22, 222. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Gu, J.-X.; Wang, K.; Zhang, A.-M.; Hong, T.-T.; Li, S.-S.; Yao, X.-Q.; Yang, M.; Yin, Y.; Zhang, N.; et al. Association between Serum PCSK9 and Coronary Heart Disease in Patients with Type 2 Diabetes Mellitus. Diabetol. Metab. Syndr. 2023, 15, 260. [Google Scholar] [CrossRef] [PubMed]
- Brouwers, M.C.G.J.; Troutt, J.S.; Van Greevenbroek, M.M.J.; Ferreira, I.; Feskens, E.J.; Van Der Kallen, C.J.H.; Schaper, N.C.; Schalkwijk, C.G.; Konrad, R.J.; Stehouwer, C.D.A. Plasma Proprotein Convertase Subtilisin Kexin Type 9 Is Not Altered in Subjects with Impaired Glucose Metabolism and Type 2 Diabetes Mellitus, but Its Relationship with Non-HDL Cholesterol and Apolipoprotein B May Be Modified by Type 2 Diabetes Mellitus: The CODAM Study. Atherosclerosis 2011, 217, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Liu, S.; Wang, X.; Theus, S.; Deng, X.; Fan, Y.; Zhou, S.; Mehta, J.L. PCSK9 Regulates Expression of Scavenger Receptors and Ox-LDL Uptake in Macrophages. Cardiovasc. Res. 2018, 114, 1145–1153, Erratum in Cardiovasc. Res. 2022, 118, 1849. https://doi.org/110.1093/cvr/cvab354. [Google Scholar] [CrossRef]
- Ridker, P.M.; Rifai, N.; Bradwin, G.; Rose, L. Plasma Proprotein Convertase Subtilisin/Kexin Type 9 Levels and the Risk of First Cardiovascular Events. Eur. Heart J. 2016, 37, 554–560. [Google Scholar] [CrossRef]
- Ravi, A.; Koyama, S.; Ellinor, P.T.; Natarajan, P. Genetic Predisposition to Low-Density Lipoprotein Cholesterol and Incident Type 2 Diabetes. JAMA Cardiol. 2025, 10, 379. [Google Scholar] [CrossRef]
- Armentaro, G.; Carbone, F.; Cassano, V.; Liberale, L.; Minetti, S.; Bertolotto, M.B.; Mannino, G.; Fiorentino, T.V.; Perticone, M.; Succurro, E.; et al. Serum Proprotein Convertase Subtilisin/Kexin Type 9 and Vascular Disease in Type 2 Diabetic Patients. Eur. J. Clin. Invest. 2023, 53, e13900. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.F.; Swerdlow, D.I.; Holmes, M.V.; Patel, R.S.; Fairhurst-Hunter, Z.; Lyall, D.M.; Hartwig, F.P.; Horta, B.L.; Hyppönen, E.; Power, C.; et al. PCSK9 Genetic Variants and Risk of Type 2 Diabetes: A Mendelian Randomisation Study. Lancet Diabetes Endocrinol. 2017, 5, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.K.; Matyas, A.; Asikhia, I.; Hu, Z.; Golder, M.; Beehler, K.; Kosenko, T.; Lagace, T.A. Pathogenic Gain-of-Function Mutations in the Prodomain and C-Terminal Domain of PCSK9 Inhibit LDL Binding. Front. Physiol. 2022, 13, 960272. [Google Scholar] [CrossRef] [PubMed]
- Laudette, M. Cardiomyocyte-Specific PCSK9 Deficiency Compromises Mitochondrial Bioenergetics and Heart Function. J. Mol. Cell. Cardiol. 2022, 173, S184. [Google Scholar] [CrossRef]
- Tremblay, F.; Xiong, Q.; Shah, S.S.; Ko, C.-W.; Kelly, K.; Morrison, M.S.; Giancarlo, C.; Ramirez, R.N.; Hildebrand, E.M.; Voytek, S.B.; et al. A Potent Epigenetic Editor Targeting Human PCSK9 for Durable Reduction of Low-Density Lipoprotein Cholesterol Levels. Nat. Med. 2025, 31, 1329–1338. [Google Scholar] [CrossRef]
- Scalise, V.; Sanguinetti, C.; Neri, T.; Cianchetti, S.; Lai, M.; Carnicelli, V.; Celi, A.; Pedrinelli, R. PCSK9 Induces Tissue Factor Expression by Activation of TLR4/NFkB Signaling. Int. J. Mol. Sci. 2021, 22, 12640. [Google Scholar] [CrossRef]
- Punch, E.; Klein, J.; Diaba-Nuhoho, P.; Morawietz, H.; Garelnabi, M. Effects of PCSK9 Targeting: Alleviating Oxidation, Inflammation, and Atherosclerosis. J. Am. Heart Assoc. Cardiovasc. Cerebrovasc. Dis. 2022, 11, e023328. [Google Scholar] [CrossRef]
- Jang, H.-D.; Lee, S.E.; Yang, J.; Lee, H.-C.; Shin, D.; Lee, H.; Lee, J.; Jin, S.; Kim, S.; Lee, S.J.; et al. Cyclase-Associated Protein 1 Is a Binding Partner of Proprotein Convertase Subtilisin/Kexin Type-9 and Is Required for the Degradation of Low-Density Lipoprotein Receptors by Proprotein Convertase Subtilisin/Kexin Type-9. Eur. Heart J. 2020, 41, 239–252. [Google Scholar] [CrossRef]
- Lee, S.; Lee, H.-C.; Kwon, Y.-W.; Lee, S.E.; Cho, Y.; Kim, J.; Lee, S.; Kim, J.-Y.; Lee, J.; Yang, H.-M.; et al. Adenylyl Cyclase-Associated Protein 1(CAP1) Is a Receptor for Human Resistin and Mediates Inflammatory Actions of Human Monocytes. Cell Metab. 2014, 19, 484–497. [Google Scholar] [CrossRef]
- Tuñón, J.; Badimón, L.; Bochaton-Piallat, M.-L.; Cariou, B.; Daemen, M.J.; Egido, J.; Evans, P.C.; Hoefer, I.E.; Ketelhuth, D.F.J.; Lutgens, E.; et al. Identifying the Anti-Inflammatory Response to Lipid Lowering Therapy: A Position Paper from the Working Group on Atherosclerosis and Vascular Biology of the European Society of Cardiology. Cardiovasc. Res. 2019, 115, 10–19. [Google Scholar] [CrossRef]
- Chung, C.-C.; Kao, Y.-H.; Chen, Y.-C.; Lin, Y.-K.; Higa, S.; Hsu, K.-C.; Chen, Y.-J. PCSK9 Enhances Cardiac Fibrogenesis via the Activation of Toll-like Receptor and NLRP3 Inflammasome Signaling. Int. J. Mol. Sci. 2025, 26, 1921. [Google Scholar] [CrossRef]
- Huang, L.; Li, Y.; Cheng, Z.; Lv, Z.; Luo, S.; Xia, Y. PCSK9 Promotes Endothelial Dysfunction During Sepsis Via the TLR4/MyD88/NF-κB and NLRP3 Pathways. Inflammation 2023, 46, 115–128. [Google Scholar] [CrossRef]
- Huang, L.; Li, Y.; Cheng, Z.; Lv, Z.; Luo, S.; Xia, Y. PCSK9 Promotes Endothelial Dysfunction in Sepsis via TLR4/MyD88/NF-κB and NLRP3 Pathways. Research Square 2022. [Google Scholar] [CrossRef]
- Badimon, L.; Luquero, A.; Crespo, J.; Peña, E.; Borrell-Pages, M. PCSK9 and LRP5 in Macrophage Lipid Internalization and Inflammation. Cardiovasc. Res. 2021, 117, 2054–2068. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Liu, S.; Brickell, A.N.; Zhang, J.; Wu, Z.; Zhou, S.; Ding, Z. PCSK9 Regulates Pyroptosis via mtDNA Damage in Chronic Myocardial Ischemia. Basic Res. Cardiol. 2020, 115, 66. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Chen, Y.; Qiu, Y.; Sun, J.; He, J.; Liu, Z.; Shi, J.; Wei, W.; Wu, G.; Liang, J. PCSK9 Promotes Hypoxia-Induced EC Pyroptosis by Regulating Smac Mitochondrion-Cytoplasm Translocation in Critical Limb Ischemia. JACC Basic Transl. Sci. 2023, 8, 1060–1077. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Tu, Z.; Chen, K.; Xu, Y.; Chen, F.; Xu, S.; Shi, T.; Qian, J.; Shen, L.; Hwa, J.; et al. Gasdermin D Inhibition Confers Antineutrophil-Mediated Cardioprotection in Acute Myocardial Infarction. J. Clin. Invest. 2022, 132, e151268. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Thompson, B.J.; Hietakangas, V.; Cohen, S.M. MAPK/ERK Signaling Regulates Insulin Sensitivity to Control Glucose Metabolism in Drosophila. PLoS Genet. 2011, 7, e1002429. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Everett, B.M.; Cornel, J.H.; Lainscak, M.; Anker, S.D.; Abbate, A.; Thuren, T.; Libby, P.; Glynn, R.J.; Ridker, P.M. Anti-Inflammatory Therapy With Canakinumab for the Prevention of Hospitalization for Heart Failure. Circulation 2019, 139, 1289–1299. [Google Scholar] [CrossRef]
- Maurea, N.; Bisceglia, I.; Paccone, A.; Buccolo, S.; Iovine, M.; Quagliariello, V. Dapagliflozin Reduces Systemic Pcsk9 Levels in Preclinical Models of Short-Term Doxorubicin Cardiotoxicity through Nlrp3 Inflammasome/Il-1β: A First Evidence of Sglt-2/Pcsk9 Cross-Talk in Cardioncology. J. Am. Coll. Cardiol. 2023, 81, 2269. [Google Scholar] [CrossRef]
- Higashikuni, Y.; Liu, W.; Numata, G.; Tanaka, K.; Fukuda, D.; Tanaka, Y.; Hirata, Y.; Imamura, T.; Takimoto, E.; Komuro, I.; et al. NLRP3 Inflammasome Activation Through Heart-Brain Interaction Initiates Cardiac Inflammation and Hypertrophy During Pressure Overload. Circulation 2023, 147, 338–355. [Google Scholar] [CrossRef]
- Ding, M.; Shi, R.; Du, Y.; Chang, P.; Gao, T.; De, D.; Chen, Y.; Li, M.; Li, J.; Li, K.; et al. O-GlcNAcylation-Mediated Endothelial Metabolic Memory Contributes to Cardiac Damage via Small Extracellular Vesicles. Cell Metab. 2025, 37, 1344–1363.e6. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Y.; Xu, C.; Liu, J.; Chen, J.; Li, G.; Huang, B.; Pan, Y.; Zhang, Y.; Wei, Q.; et al. Circular RNA circGlis3 Protects against Islet β-Cell Dysfunction and Apoptosis in Obesity. Nat. Commun. 2023, 14, 351. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Li, Q.; Wu, J.; Zhou, C.; Liu, X.; Yue, J.; Chen, X.; Liu, J.; Zhang, Q.; Zhang, Y.; et al. A Detrimental Role of Endothelial S1PR2 in Cardiac Ischemia-Reperfusion Injury via Modulating Mitochondrial Dysfunction, NLRP3 Inflammasome Activation, and Pyroptosis. Redox Biol. 2024, 75, 103244. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-P.; Qiu, S.; Tai, G.-J.; Liu, Y.-M.; Wei, W.; Fu, M.-M.; Fang, P.-Q.; Otieno, J.N.; Battulga, T.; Li, X.-X.; et al. NLRP3 Inflammasome-Modulated Angiogenic Function of EPC via PI3K/ Akt/mTOR Pathway in Diabetic Myocardial Infarction. Cardiovasc. Diabetol. 2025, 24, 6. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, L.; Zhai, L.; Palade, P.; Wang, X.; Mehta, J.L. Direct Impact of PCSK9 on SMC Senescence and Apoptosis: A New Focus in Cardiovascular Diseases. Arterioscler. Thromb. Vasc. Biol. 2024, 44, 1491–1496. [Google Scholar] [CrossRef]
- D’Onofrio, N.; Prattichizzo, F.; Marfella, R.; Sardu, C.; Martino, E.; Scisciola, L.; Marfella, L.; Grotta, R.L.; Frigé, C.; Paolisso, G.; et al. SIRT3 Mediates the Effects of PCSK9 Inhibitors on Inflammation, Autophagy, and Oxidative Stress in Endothelial Cells. Theranostics 2023, 13, 531–542. [Google Scholar] [CrossRef]
- Ouyang, Z.; Ma, M.; Zhang, Z.; Wu, H.; Xue, Y.; Jian, Y.; Yin, K.; Yu, S.; Zhao, C.; Guo, W.; et al. Targeted Degradation of PCSK9 In Vivo by Autophagy-Tethering Compounds. J. Med. Chem. 2024, 67, 433–449. [Google Scholar] [CrossRef]
- Xu, Q.; Zhao, Y.; He, N.; Gao, R.; Xu, W.; Zhuo, X.; Ren, Z.; Wu, C.; Liu, L. PCSK9: A Emerging Participant in Heart Failure. Biomed. Pharmacother. 2023, 158, 114106. [Google Scholar] [CrossRef]
- Luo, C.; Fang, C.; Zou, R.; Jiang, J.; Zhang, M.; Ge, T.; Zhou, H.; Fan, X.; Zheng, B.; Zeng, Z. Hyperglycemia-Induced DNA Damage Response Activates DNA-PK Complex to Promote Endothelial Ferroptosis in Type 2 Diabetic Cardiomyopathy. Theranostics 2025, 15, 4507–4525. [Google Scholar] [CrossRef]
- Tang, Z.-H.; Peng, J.; Ren, Z.; Yang, J.; Li, T.-T.; Li, T.-H.; Wang, Z.; Wei, D.-H.; Liu, L.-S.; Zheng, X.-L.; et al. New Role of PCSK9 in Atherosclerotic Inflammation Promotion Involving the TLR4/NF-κB Pathway. Atherosclerosis 2017, 262, 113–122. [Google Scholar] [CrossRef]
- Chen, Z.; Li, S.; Liu, M.; Yin, M.; Chen, J.; Li, Y.; Li, Q.; Zhou, Y.; Xia, Y.; Chen, A.; et al. Nicorandil Alleviates Cardiac Microvascular Ferroptosis in Diabetic Cardiomyopathy: Role of the Mitochondria-Localized AMPK-Parkin-ACSL4 Signaling Pathway. Pharmacol. Res. 2024, 200, 107057. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.; Kutsche, H.S.; Schreckenberg, R.; Weber, M.; Li, L.; Rohrbach, S.; Schulz, R.; Schlüter, K.-D. Autocrine Effects of PCSK9 on Cardiomyocytes. Basic Res. Cardiol. 2020, 115, 65. [Google Scholar] [CrossRef] [PubMed]
- Da Dalt, L.; Castiglioni, L.; Baragetti, A.; Audano, M.; Svecla, M.; Bonacina, F.; Pedretti, S.; Uboldi, P.; Benzoni, P.; Giannetti, F.; et al. PCSK9 Deficiency Rewires Heart Metabolism and Drives Heart Failure with Preserved Ejection Fraction. Eur. Heart J. 2021, 42, 3078–3090. [Google Scholar] [CrossRef]
- Laudette, M.; Lindbom, M.; Cinato, M.; Bergh, P.-O.; Skålén, K.; Muhammad, A.; Miljanovic, A.; Czuba, T.; Perkins, R.; Smith, J.G.; et al. PCSK9 Regulates Cardiac Mitochondrial Cholesterol by Promoting TSPO Degradation. Circ. Res. 2025, 136, 924–942. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Wan, S.; Wu, Q.; Xing, Y.; He, Y.; Huang, W.; Long, Y.; Zhang, C.; Xu, Y.; Jiang, Z. BDH1 Overexpression Alleviates Diabetic Cardiomyopathy through Inhibiting H3K9bhb-Mediated Transcriptional Activation of LCN2. Cardiovasc. Diabetol. 2025, 24, 101. [Google Scholar] [CrossRef]
- Cai, D.; Liu, C.; Li, H.; Wang, C.; Bai, L.; Feng, J.; Hu, M.; Wang, H.; Song, S.; Xie, Y.; et al. Foxk1 and Foxk2 Promote Cardiomyocyte Proliferation and Heart Regeneration. Nat. Commun. 2025, 16, 2877. [Google Scholar] [CrossRef]
- Ahmed, E.I.; Moneam Shamardl, H.A.; Elsayed, A.M.; Sadik, S.A. Evolocumab Ameliorates Myocardial Fibrosis and Improves Metabolic Syndrome–Induced Cardiac Dysfunction in Rats via Inhibiting PCSK9/NLRP3 Inflammasome and Caspase-1/IL-1β Pathways. Eur. J. Pharmacol. 2025, 998, 177499. [Google Scholar] [CrossRef]
- Bao, H.; Wang, X.; Zhou, H.; Zhou, W.; Liao, F.; Wei, F.; Yang, S.; Luo, Z.; Li, W. PCSK9 Regulates Myofibroblast Transformation through the JAK2/STAT3 Pathway to Regulate Fibrosis after Myocardial Infarction. Biochem. Pharmacol. 2024, 220, 115996. [Google Scholar] [CrossRef]
- Huang, Q.; Zhou, Z.; Xu, L.; Zhan, P.; Huang, G. PCSK9 Inhibitor Attenuates Cardiac Fibrosis in Reperfusion Injury Rat by Suppressing Inflammatory Response and TGF-Β1/Smad3 Pathway. Biochem. Pharmacol. 2024, 230, 116563. [Google Scholar] [CrossRef]
- Wu, C.; Lin, D.; Ji, J.; Jiang, Y.; Jiang, F.; Wang, Y. PCSK9 Inhibition Regulates Infarction-Induced Cardiac Myofibroblast Transdifferentiation via Notch1 Signaling. Cell Biochem. Biophys. 2023, 81, 359–369. [Google Scholar] [CrossRef]
- Wang, S.-H.; Cui, L.-G.; Su, X.-L.; Komal, S.; Ni, R.-C.; Zang, M.-X.; Zhang, L.-R.; Han, S.-N. GSK-3β-Mediated Activation of NLRP3 Inflammasome Leads to Pyroptosis and Apoptosis of Rat Cardiomyocytes and Fibroblasts. Eur. J. Pharmacol. 2022, 920, 174830. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Wang, B.; Du, M.; Zhu, B.; Cui, K.; Li, K.; Yuan, K.; Cowan, D.B.; Bhattacharjee, S.; Wong, S.; et al. Targeting Epsins to Inhibit Fibroblast Growth Factor Signaling While Potentiating Transforming Growth Factor-β Signaling Constrains Endothelial-to-Mesenchymal Transition in Atherosclerosis. Circulation 2023, 147, 669–685. [Google Scholar] [CrossRef] [PubMed]
- Tuleta, I.; Hanna, A.; Humeres, C.; Aguilan, J.T.; Sidoli, S.; Zhu, F.; Frangogiannis, N.G. Fibroblast-Specific TGF-β Signaling Mediates Cardiac Dysfunction, Fibrosis, and Hypertrophy in Obese Diabetic Mice. Cardiovasc. Res. 2024, 120, 2047–2063. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Bao, W.; Chen, X.; Lu, Y.; Fang, J.; Liu, J.; Peng, S.; Pi, J.; Tomlinson, B.; Chan, P.; et al. Endothelial Gata6 Deletion Reduces Monocyte Recruitment and Proinflammatory Macrophage Formation and Attenuates Atherosclerosis through Cmpk2-Nlrp3 Pathways. Redox Biol. 2023, 64, 102775, Erratum in Redox Biol. 2023, 66, 102854. https://doi.org/10.1016/j.redox.2023.102854. [Google Scholar] [CrossRef]
- Gao, J.-J.; Wu, F.-Y.; Liu, Y.-J.; Li, L.; Lin, Y.-J.; Kang, Y.-T.; Peng, Y.-M.; Liu, Y.-F.; Wang, C.; Ma, Z.-S.; et al. Increase of PCSK9 Expression in Diabetes Promotes VEGFR2 Ubiquitination to Inhibit Endothelial Function and Skin Wound Healing. Sci. China Life Sci. 2024, 67, 2635–2649. [Google Scholar] [CrossRef]
- Zeng, J.; Tao, J.; Xi, L.; Wang, Z.; Liu, L. PCSK9 Mediates the Oxidative Low-density Lipoprotein-induced Pyroptosis of Vascular Endothelial Cells via the UQCRC1/ROS Pathway. Int. J. Mol. Med. 2021, 47, 53. [Google Scholar] [CrossRef]
- Marston, N.A.; Kamanu, F.K.; Melloni, G.E.M.; Schnitzler, G.; Hakim, A.; Ma, R.X.; Kang, H.; Chasman, D.I.; Giugliano, R.P.; Ellinor, P.T.; et al. Endothelial Cell-Related Genetic Variants Identify LDL Cholesterol-Sensitive Individuals Who Derive Greater Benefit from Aggressive Lipid Lowering. Nat. Med. 2025, 31, 963–969. [Google Scholar] [CrossRef]
- Zhao, D.; Huang, Z.-K.; Liang, Y.; Li, Z.-J.; Zhang, X.-W.; Li, K.-H.; Wu, H.; Zhang, X.-D.; Li, C.-S.; An, D.; et al. Monocytes Release Pro-Cathepsin D to Drive Blood-to-Brain Transcytosis in Diabetes. Circ. Res. 2024, 134, e17–e33. [Google Scholar] [CrossRef]
- Luo, T.; Guo, W.; Ji, W.; Du, W.; Lv, Y.; Feng, Z. Monocyte CCL2 Signaling Possibly Contributes to Increased Asthma Susceptibility in Type 2 Diabetes. Sci. Rep. 2025, 15, 10768. [Google Scholar] [CrossRef]
- Peng, H.; Pan, Y.; Sun, Y.; Wang, X.; Fan, X.; Wang, Y.; Zhao, L.; Li, X.; Dong, Y.; Chen, J.; et al. Hyperglycemia Induces an Immunosuppressive Microenvironment in Colorectal Cancer Liver Metastases by Recruiting Peripheral Blood Monocytes through the CCL3-CCR1 Axis. J. Immunother. Cancer 2025, 13, e011310. [Google Scholar] [CrossRef]
- Moens, S.J.B.; Neele, A.E.; Kroon, J.; Van Der Valk, F.M.; Van Den Bossche, J.; Hoeksema, M.A.; Hoogeveen, R.M.; Schnitzler, J.G.; Baccara-Dinet, M.T.; Manvelian, G.; et al. PCSK9 Monoclonal Antibodies Reverse the Pro-Inflammatory Profile of Monocytes in Familial Hypercholesterolaemia. Eur. Heart J. 2017, 38, 1584–1593. [Google Scholar] [CrossRef] [PubMed]
- Krychtiuk, K.A.; Lenz, M.; Hohensinner, P.; Distelmaier, K.; Schrutka, L.; Kastl, S.P.; Huber, K.; Dostal, E.; Oravec, S.; Hengstenberg, C.; et al. Circulating Levels of Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) Are Associated with Monocyte Subsets in Patients with Stable Coronary Artery Disease. J. Clin. Lipidol. 2021, 15, 512–521. [Google Scholar] [CrossRef]
- Filatova, A.Y.; Afanasieva, O.I.; Arefieva, T.I.; Potekhina, A.V.; Tyurina, A.V.; Klesareva, E.A.; Razova, O.A.; Ezhov, M.V.; Pokrovsky, S.N. The Concentration of PCSK9-Lp(a) Complexes and the Level of Blood Monocytes in Males with Coronary Atherosclerosis. J. Pers. Med. 2023, 13, 1077. [Google Scholar] [CrossRef]
- Rosenson, R.S.; Tate, A.; Mar, P.; Grushko, O.; Chen, Q.; Goonewardena, S.N. Inhibition of PCSK9 with Evolocumab Modulates Lipoproteins and Monocyte Activation in High-Risk ASCVD Subjects. Atherosclerosis 2024, 392, 117529. [Google Scholar] [CrossRef] [PubMed]
- Desouky, D.A.; Nosair, N.A.; Salama, M.K.; El-Magd, M.A.; Desouky, M.A.; Sherif, D.E. PCSK9 and Its Relationship with HMGB1, TLR4, and TNFα in Non-Statin and Statin-Treated Coronary Artery Disease Patients. Mol. Cell. Biochem. 2025, 480, 2935–2949. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-Z.; Wu, Y.; Li, M.; Mijiti, A.; Cheng, L.-F. Identification of Macrophage Driver Genes in Fibrosis Caused by Different Heart Diseases Based on Omics Integration. J. Transl. Med. 2024, 22, 839. [Google Scholar] [CrossRef]
- Mao, J.; Xia, W.; Wu, Y.; Li, M.; Zhao, Y.; Zhai, P.; Zhang, Y.; Zan, T.; Cui, W.; Sun, X. Biosynthesis of Lysosomally Escaped Apoptotic Bodies Inhibits Inflammasome Synthesis in Macrophages. Research 2025, 8, 0581. [Google Scholar] [CrossRef]
- Wolf, S.J.; Audu, C.O.; Moon, J.Y.; Joshi, A.D.; Melvin, W.J.; Barrett, E.C.; Mangum, K.; De Jimenez, G.S.; Rocco, S.; Buckley, S.; et al. Diabetic Wound Keratinocytes Induce Macrophage JMJD3-Mediated Nlrp3 Expression via IL-1R Signaling. Diabetes 2024, 73, 1462–1472. [Google Scholar] [CrossRef]
- Lv, D.; Cao, X.; Zhong, L.; Dong, Y.; Xu, Z.; Rong, Y.; Xu, H.; Wang, Z.; Yang, H.; Yin, R.; et al. Targeting Phenylpyruvate Restrains Excessive NLRP3 Inflammasome Activation and Pathological Inflammation in Diabetic Wound Healing. Cell Rep. Med. 2023, 4, 101129. [Google Scholar] [CrossRef]
- Zhang, X.; McDonald, J.G.; Aryal, B.; Canfrán-Duque, A.; Goldberg, E.L.; Araldi, E.; Ding, W.; Fan, Y.; Thompson, B.M.; Singh, A.K.; et al. Desmosterol Suppresses Macrophage Inflammasome Activation and Protects against Vascular Inflammation and Atherosclerosis. Proc. Natl. Acad. Sci. USA 2021, 118, e2107682118. [Google Scholar] [CrossRef]
- Zou, Y.; Chen, Z.; Zhang, X.; Yu, J.; Xu, H.; Cui, J.; Li, Y.; Niu, Y.; Zhou, C.; Xia, J.; et al. Targeting PCSK9 Ameliorates Graft Vascular Disease in Mice by Inhibiting NLRP3 Inflammasome Activation in Vascular Smooth Muscle Cells. Front. Immunol. 2022, 13, 894789. [Google Scholar] [CrossRef]
- Fang, H.; Shi, M.; Wang, C.; Zhang, S.; Kong, N.; Ji, M.; Wang, Y.; Zhou, Y.; Zhu, Q.; Zhang, Y.; et al. PCSK9 Potentiates Innate Immune Response to RNA Viruses by Preventing AIP4-Mediated Polyubiquitination and Degradation of VISA/MAVS. Proc. Natl. Acad. Sci. USA 2025, 122, e2412206122. [Google Scholar] [CrossRef]
- Katsuki, S.; Jha, P.K.; Lupieri, A.; Nakano, T.; Passos, L.S.A.; Rogers, M.A.; Becker-Greene, D.; Le, T.-D.; Decano, J.L.; Ho Lee, L.; et al. Proprotein Convertase Subtilisin/Kexin 9 (PCSK9) Promotes Macrophage Activation via LDL Receptor-Independent Mechanisms. Circ. Res. 2022, 131, 873–889. [Google Scholar] [CrossRef]
- Yang, C.-L.; Zeng, Y.-D.; Hu, Z.-X.; Liang, H. PCSK9 Promotes the Secretion of Pro-Inflammatory Cytokines by Macrophages to Aggravate H/R-Induced Cardiomyocyte Injury via Activating NF-κB Signalling. Gen. Physiol. Biophys. 2020, 39, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Li, T.; Luo, J.; Zhang, X.; Wang, T.; Wang, Y.; Ma, Y.; Yang, B.; Jia, J.; Dmytriw, A.A.; et al. PCSK9 Increases Vulnerability of Carotid Plaque by Promoting Mitochondrial Dysfunction and Apoptosis of Vascular Smooth Muscle Cells. CNS Neurosci. Ther. 2024, 30, e14640. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Zhang, G.; Bao, Y.; Zhang, M. Involvement of Oxidative Stress-AMPK-Cx43-NLRP3 Pathway in Extracellular Matrix Remodeling of Gastric Smooth Muscle Cells in Rats with Diabetic Gastroparesis. Cell Stress Chaperones 2024, 29, 440–455. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, L.; Liu, J.; Ma, Y.; Qiu, C.; Liu, C.; Gong, Y.; Yuwen, Y.; Guan, G.; Zhang, Y.; et al. Palmitic Acid in Type 2 Diabetes Mellitus Promotes Atherosclerotic Plaque Vulnerability via Macrophage Dll4 Signaling. Nat. Commun. 2024, 15, 1281. [Google Scholar] [CrossRef]
- Mei, W.; Faraj Tabrizi, S.; Godina, C.; Lovisa, A.F.; Isaksson, K.; Jernström, H.; Tavazoie, S.F. A Commonly Inherited Human PCSK9 Germline Variant Drives Breast Cancer Metastasis via LRP1 Receptor. Cell 2025, 188, 371–389.e28. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, S.; Luo, H.; Lu, Q.; Yu, S. PCSK9 Promotes the Progression and Metastasis of Colon Cancer Cells through Regulation of EMT and PI3K/AKT Signaling in Tumor Cells and Phenotypic Polarization of Macrophages. J. Exp. Clin. Cancer Res. 2022, 41, 303. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Li, S.; Fang, Y.; Zou, Y.; Song, D.; Zhang, S.; Cai, Y. Proprotein Convertase Subtilisin/Kexin Type 9 Promotes Gastric Cancer Metastasis and Suppresses Apoptosis by Facilitating MAPK Signaling Pathway Through HSP70 Up-Regulation. Front. Oncol. 2021, 10, 609663. [Google Scholar] [CrossRef]
- Zhang, Y.; Su, W.; Ji, X.; Yang, Z.; Guan, Q.; Pang, Y.; Zhong, L.; Wang, Y.; Xiang, J. PCSK9 Promotes Progression of Anaplastic Thyroid Cancer through E-Cadherin Endocytosis. Cell Death Dis. 2025, 16, 362. [Google Scholar] [CrossRef]
- Rademaker, G.; Hernandez, G.A.; Seo, Y.; Dahal, S.; Miller-Phillips, L.; Li, A.L.; Peng, X.L.; Luan, C.; Qiu, L.; Liegeois, M.A.; et al. PCSK9 Drives Sterol-Dependent Metastatic Organ Choice in Pancreatic Cancer. Nature 2025, 643, 1381–1390. [Google Scholar] [CrossRef]
- Chai, M.; He, Y.; Zhao, W.; Han, X.; Zhao, G.; Ma, X.; Qiao, P.; Shi, D.; Liu, Y.; Han, W.; et al. Efficacy and Safety of Tafolecimab in Chinese Patients with Heterozygous Familial Hypercholesterolemia: A Randomized, Double-Blind, Placebo-Controlled Phase 3 Trial (CREDIT-2). BMC Med. 2023, 21, 77. [Google Scholar] [CrossRef] [PubMed]
- Koren, M.J.; Vega, R.B.; Agrawal, N.; Xu, Y.; Barbour, A.M.; Yu, H.; Wallerstedt, E.; Carter, D.; Middlemiss, J.; Twaddle, L.; et al. An Oral PCSK9 Inhibitor for Treatment of Hypercholesterolemia. J. Am. Coll. Cardiol. 2025, 85, 1996–2007. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).