LncRNA TSPEAR-AS2 Maintains the Stemness of Gastric Cancer Stem Cells by Regulating the miR-15a-5p/CCND1 Axis
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Tissue Samples
2.2. Cell Culture
2.3. Isolation of CD54+ CD44+ and CD54− CD44− Populations
2.4. Quantitative Real-Time Polymerase Chain Reaction (RT-qPCR) Analysis
2.5. Construction and Transfection
2.6. Western Blotting Assay
2.7. Immunohistochemistry (IHC)
2.8. Dual-Luciferase Reporter Assay
2.9. RNA Immunoprecipitation (RIP) Assay
2.10. In Vivo Tumor Xenograft Model
2.11. Statistical Analysis
3. Results
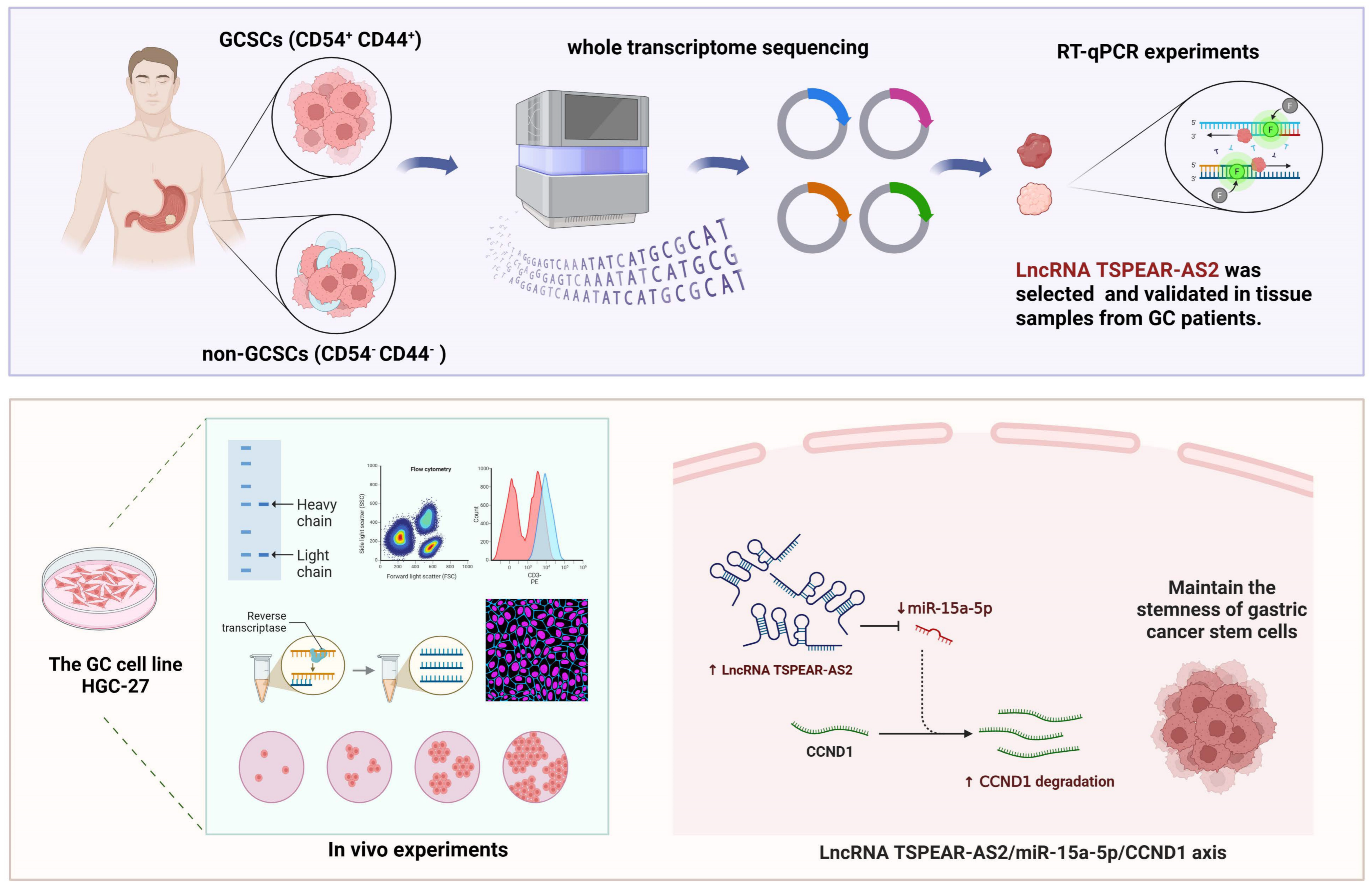
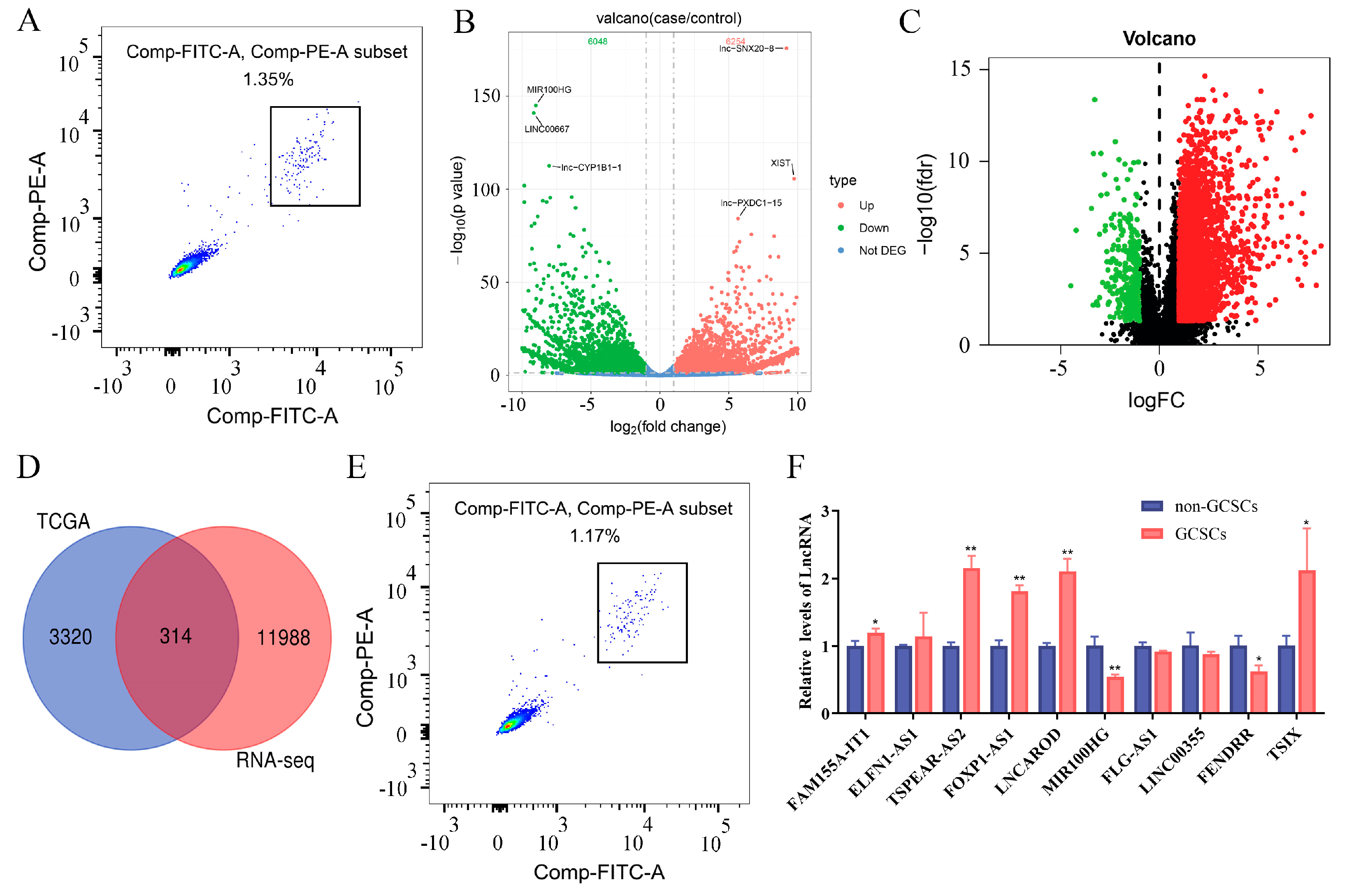
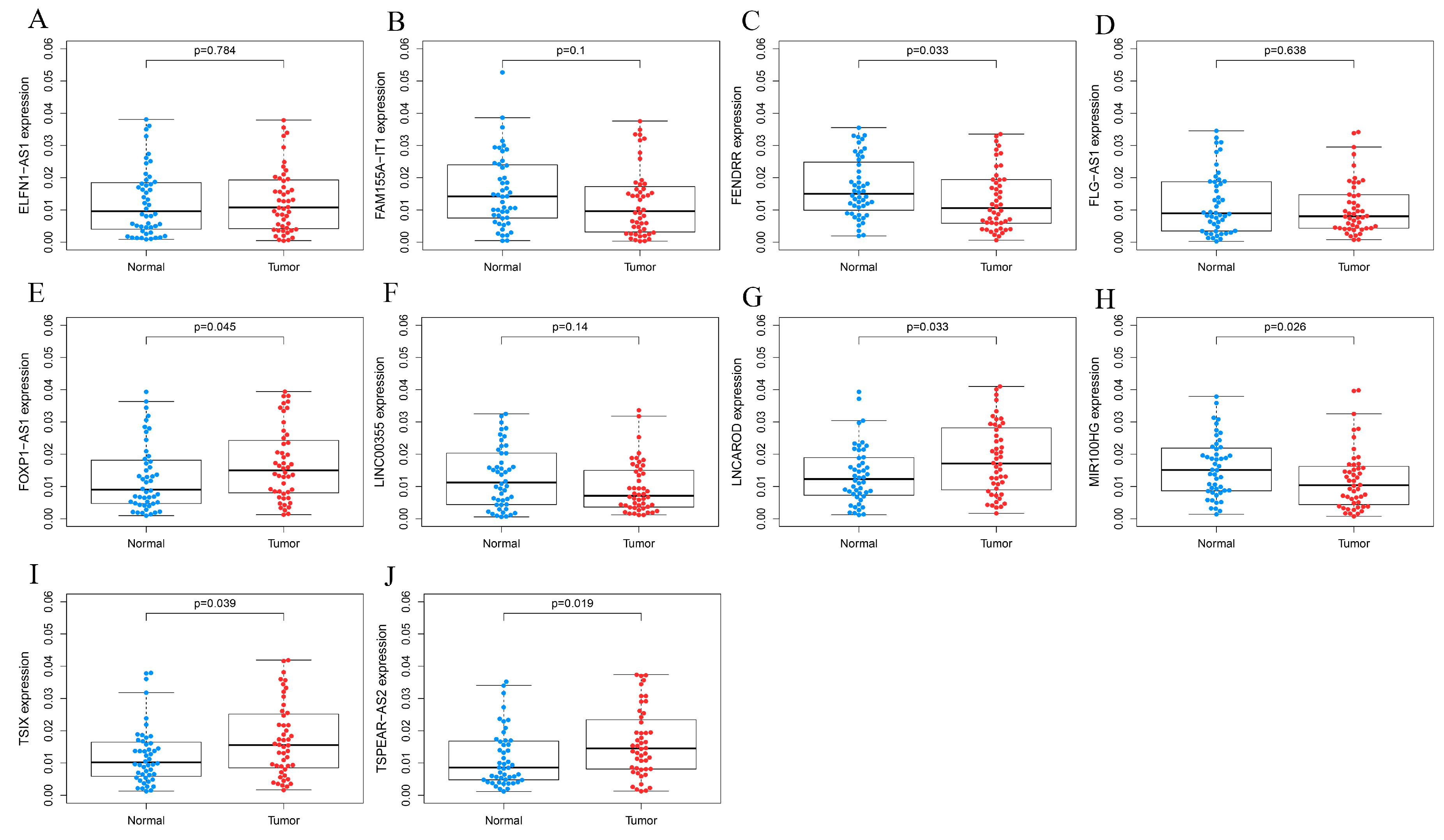
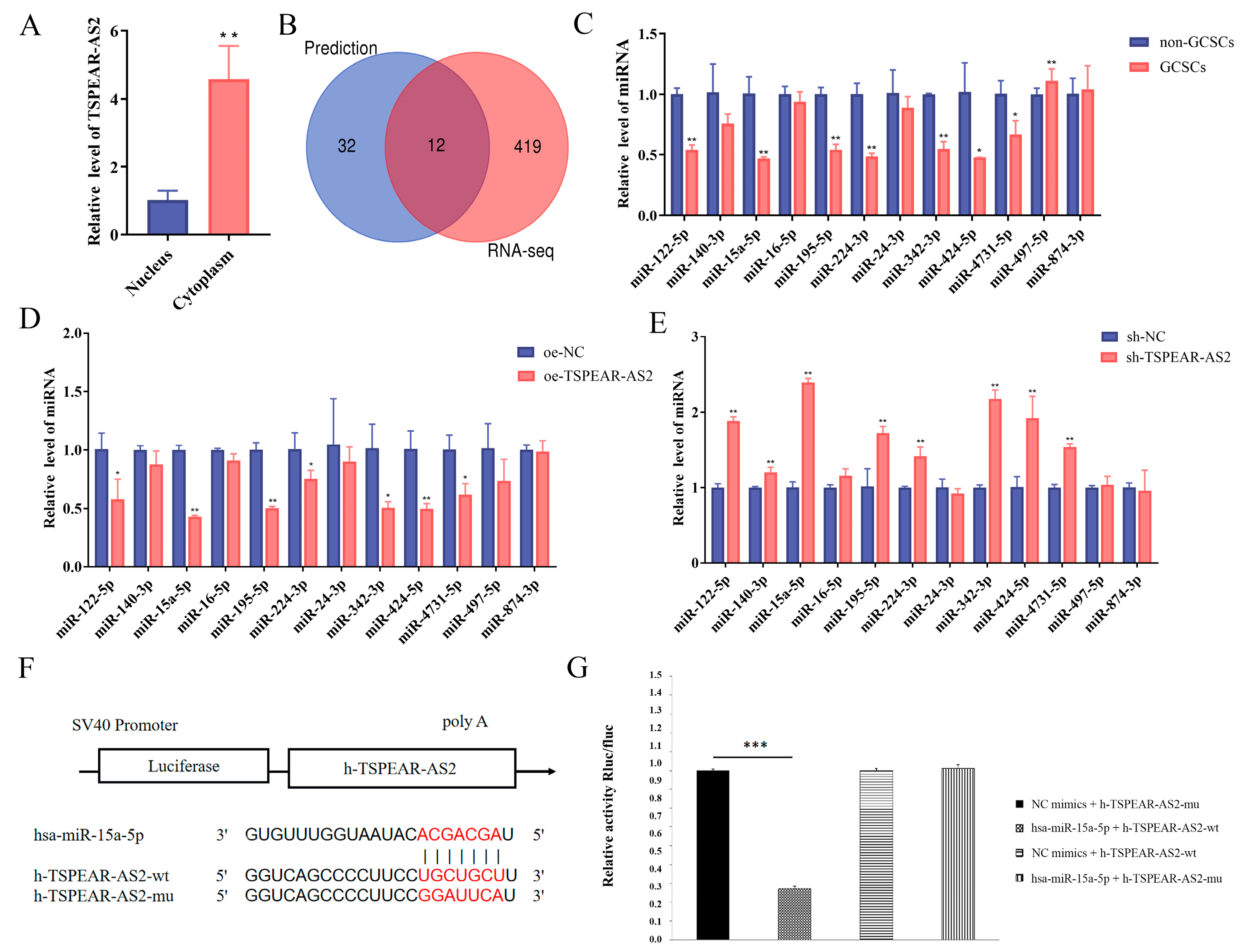
3.1. LncRNA TSPEAR-AS2 Is Overexpressed in GCSCs
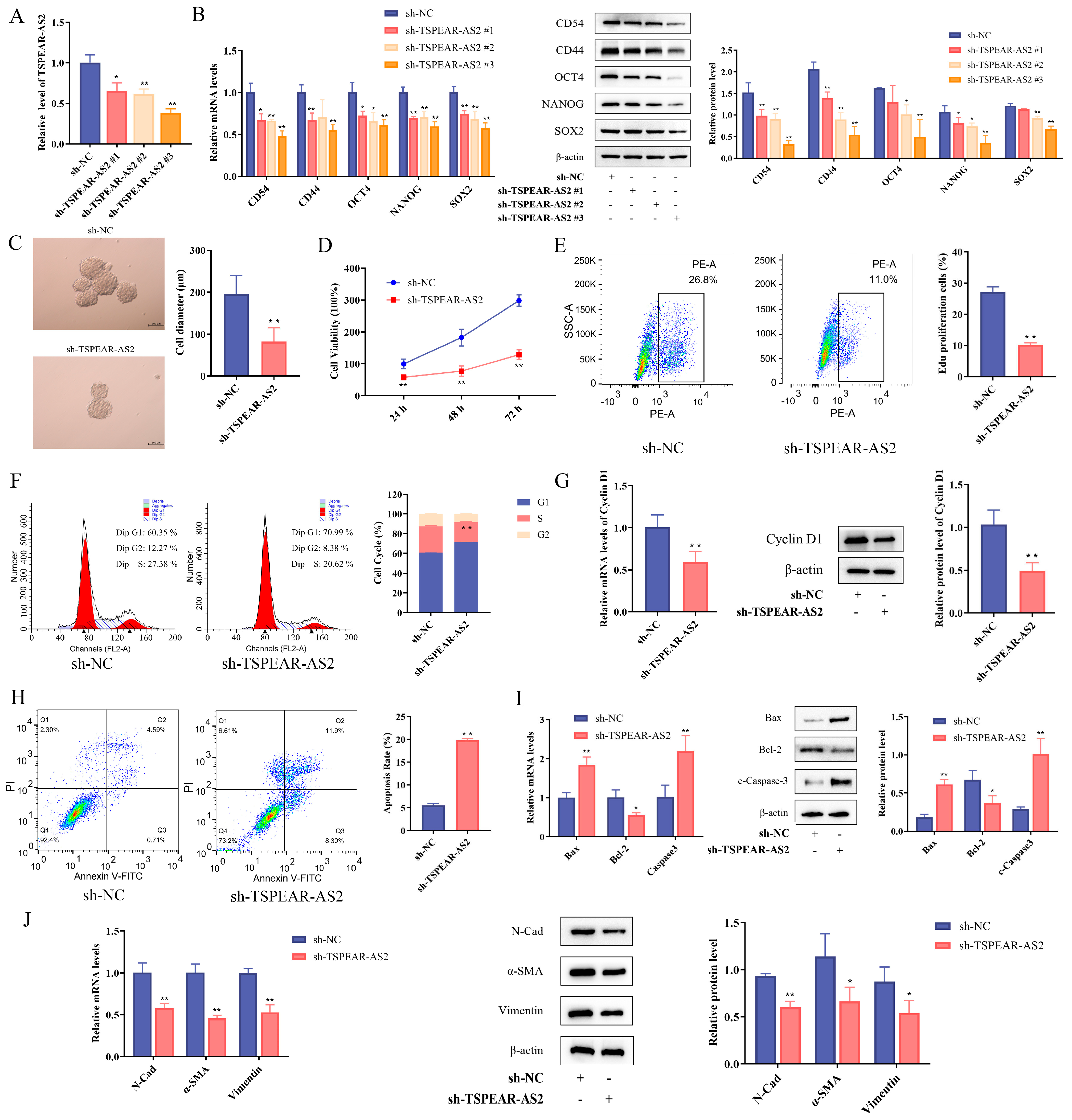
3.2. LncRNA TSPEAR-AS2 Maintains the Stemness of GCSCs
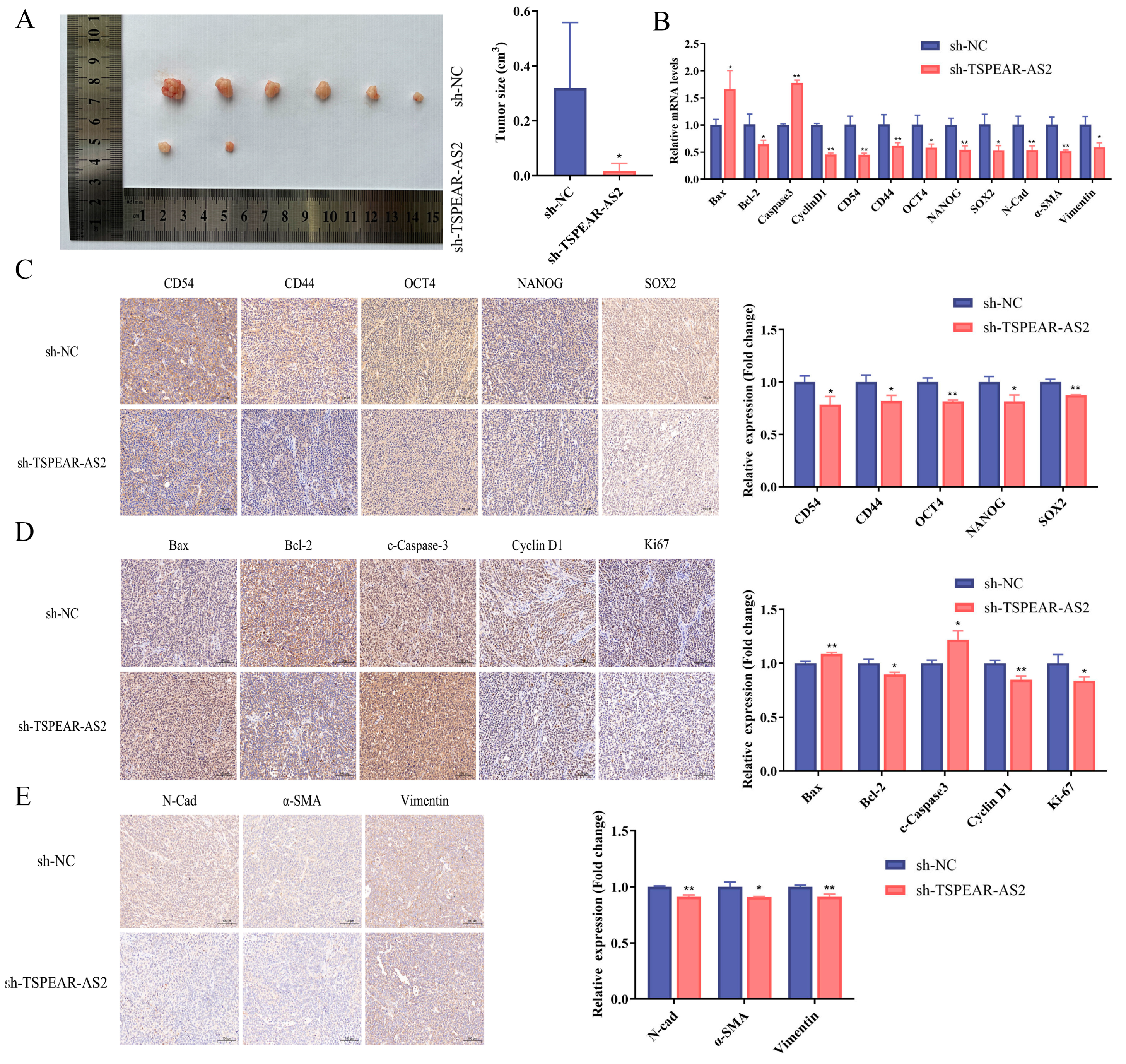
3.3. TSPEAR-AS2 Accelerated Cell Growth of GCSCs In Vivo
3.4. TSPEAR-AS2 Directly Targeted miR-15a-5p in GCSCs
3.5. miR-15a-5p Inhibited GCSC Stemness
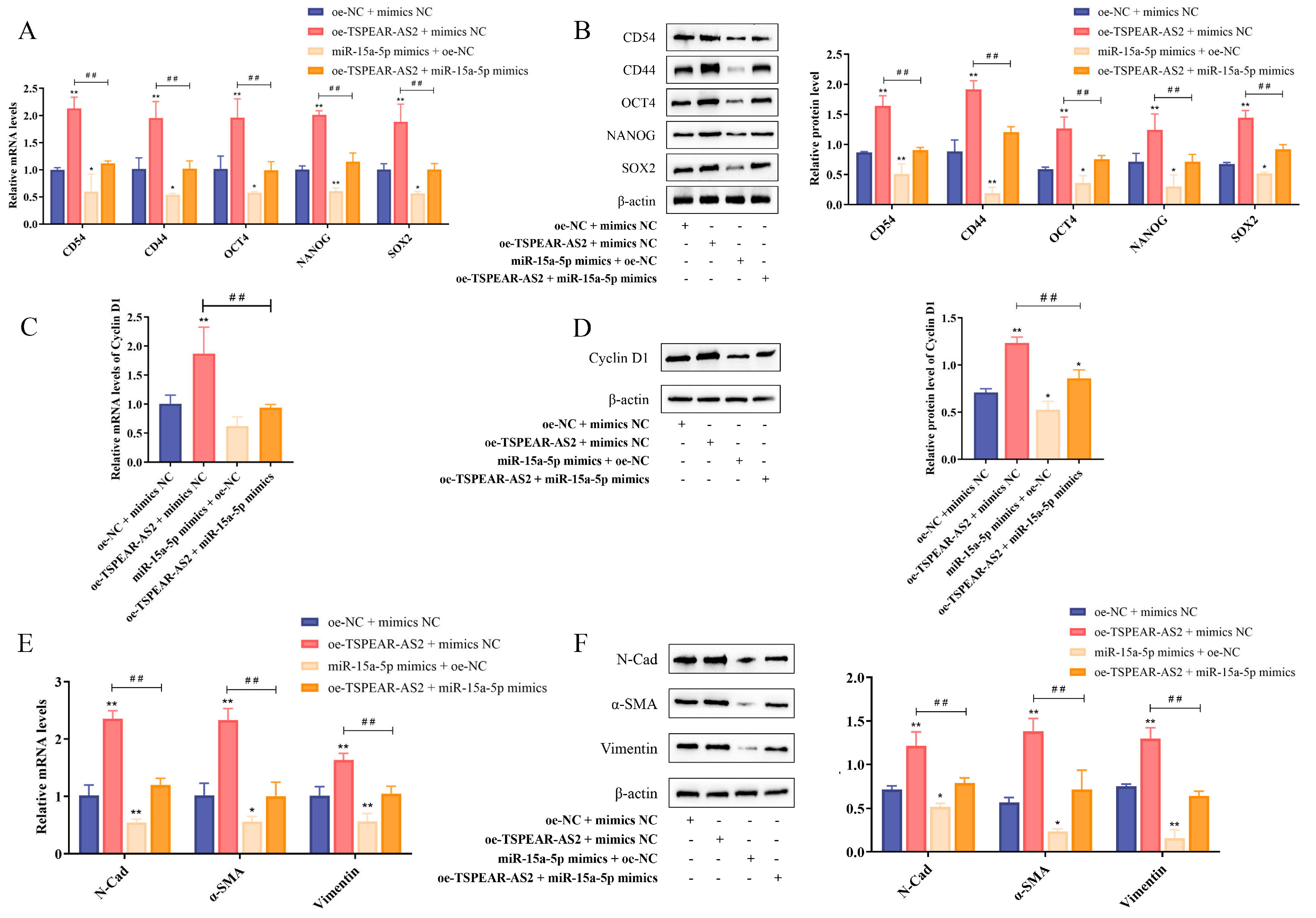
3.6. TSPEAR-AS2 Sponged miR-15a-5p to Regulate the Stemness of GCSCs
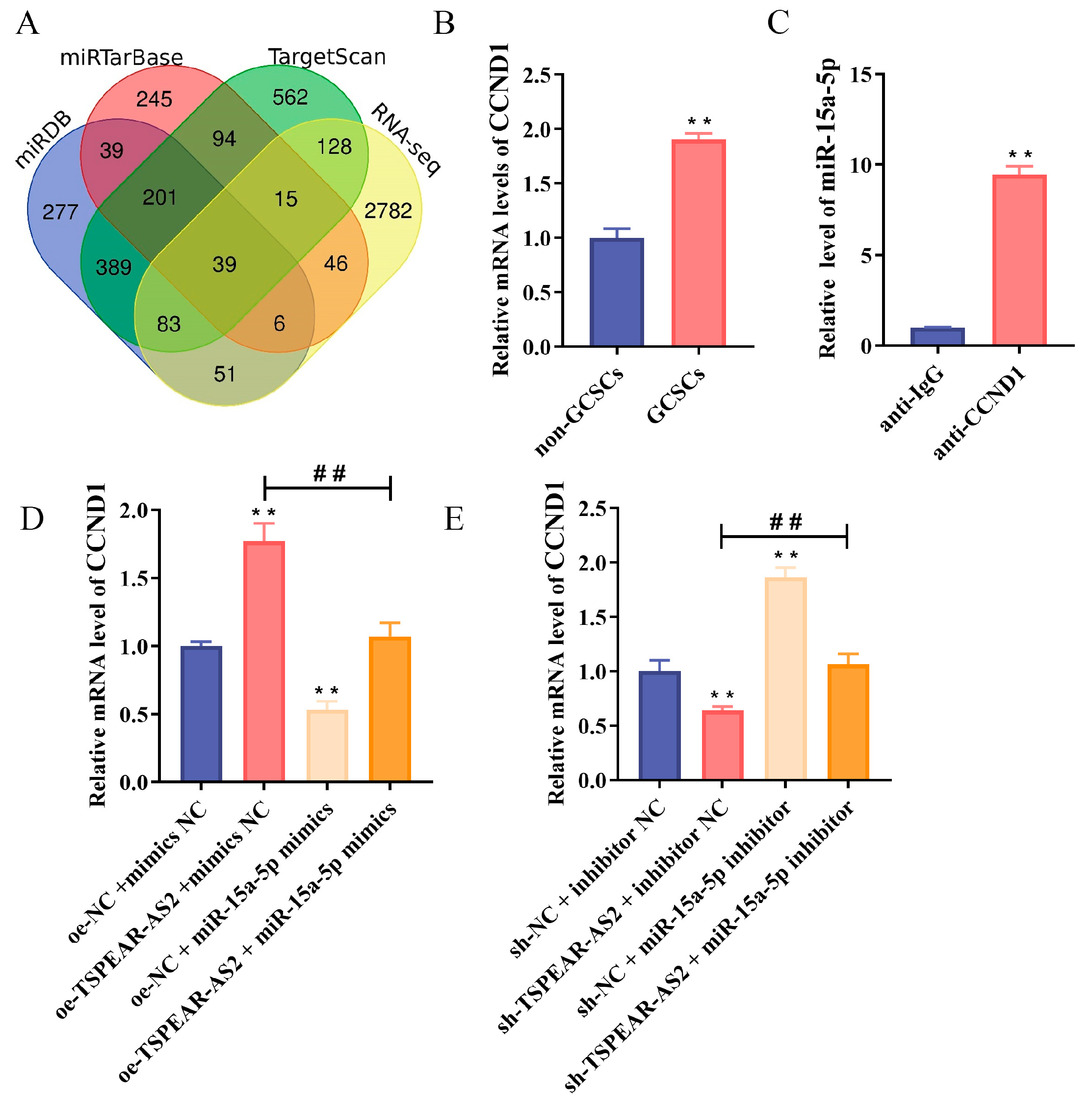
3.7. CCND1 Is a Direct Target of miR-15a-5p
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CSCs | cancer stem cells |
| ceRNA | competing endogenous RNA |
| EMT | epithelial–mesenchymal transition |
| GC | gastric cancer |
| GCSCs | gastric cancer stem cells |
| GEO | Gene Expression Omnibus |
| IARC | International Agency for Research on Cancer |
| IHC | immunohistochemistry |
| LncRNAs | long non-coding RNAs |
| RIP | RNA immunoprecipitation |
| RT-qPCR | quantitative real-time polymerase chain reaction |
| TCGA | The Cancer Genome Atlas |
References
- Smyth, E.C.; Nilsson, M.; Grabsch, H.I.; van Grieken, N.C.; Lordick, F. Gastric cancer. Lancet 2020, 396, 635–648. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Venerito, M.; Link, A.; Rokkas, T.; Malfertheiner, P. Gastric cancer–clinical and epidemiological aspects. Helicobacter 2016, 21, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Machlowska, J.; Baj, J.; Sitarz, M.; Maciejewski, R.; Sitarz, R. Gastric Cancer: Epidemiology, Risk Factors, Classification, Genomic Characteristics and Treatment Strategies. Int. J. Mol. Sci. 2020, 21, 4012. [Google Scholar] [CrossRef] [PubMed]
- Warsinggih; Syarifuddin, E.; Marhamah; Lusikooy, R.E.; Labeda, I.; Sampetoding, S.; Dani, M.I.; Kusuma, M.I.; Uwuratuw, J.A.; Prihantono; et al. Association of clinicopathological features and gastric cancer incidence in a single institution. Asian J. Surg. 2022, 45, 246–249. [Google Scholar] [CrossRef]
- Lin, Y.; Zheng, Y.; Wang, H.L.; Wu, J. Global Patterns and Trends in Gastric Cancer Incidence Rates (1988-2012) and Predictions to 2030. Gastroenterology 2021, 161, 116–127.e8. [Google Scholar] [CrossRef]
- Choi, I.J.; Kim, C.G.; Lee, J.Y.; Kim, Y.-I.; Kook, M.-C.; Park, B.; Joo, J. Family History of Gastric Cancer and Helicobacter pylori Treatment. N. Engl. J. Med. 2020, 382, 427–436. [Google Scholar] [CrossRef]
- Digklia, A.; Wagner, A.D. Advanced gastric cancer: Current treatment landscape and future perspectives. World J. Gastroenterol. 2016, 22, 2403–2414. [Google Scholar] [CrossRef]
- Piccioni, S.A.; Costantini, M.; Petrioli, R.; Bagnacci, G.; Ferrara, D.; Andreucci, E.; Carbone, L.; Ongaro, A.; Calomino, N.; Sandini, M. Impact of HER2 and microsatellite instability status on response to neoadjuvant/conversion therapy and survival in patients with gastric cancer. Eur. J. Cancer Prev. 2025, 10, 1097. [Google Scholar] [CrossRef]
- Visvader, J.E.; Lindeman, G.J. Cancer stem cells: Current status and evolving complexities. Cell Stem Cell 2012, 10, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Lapidot, T.; Sirard, C.; Vormoor, J.; Murdoch, B.; Hoang, T.; Caceres-Cortes, J.; Minden, M.; Paterson, B.; Caligiuri, M.A.; Dick, J.E. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature 1994, 367, 645–648. [Google Scholar] [CrossRef]
- Zeng, X.; Liu, C.; Yao, J.; Wan, H.; Wan, G.; Li, Y.; Chen, N. Breast cancer stem cells, heterogeneity, targeting therapies and therapeutic implications. Pharmacol. Res. 2021, 163, 105320. [Google Scholar] [CrossRef]
- Norollahi, S.E.; Mansour-Ghanaei, F.; Joukar, F.; Ghadarjani, S.; Mojtahedi, K.; Nejad, K.G.; Hemmati, H.; Gharibpoor, F.; Khaksar, R.; Samadani, A.A. Therapeutic approach of Cancer stem cells (CSCs) in gastric adenocarcinoma; DNA methyltransferases enzymes in cancer targeted therapy. Biomed. Pharmacother. 2019, 115, 108958. [Google Scholar] [CrossRef] [PubMed]
- Bapat, S.A.; Mali, A.M.; Koppikar, C.B.; Kurrey, N.K. Stem and progenitor-like cells contribute to the aggressive behavior of human epithelial ovarian cancer. Cancer Res. 2005, 65, 3025–3029. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.K.; Guan, X.Y.; Ma, S. Cancer stem cells in hepatocellular carcinoma—from origin to clinical implications. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 26–44. [Google Scholar] [CrossRef]
- Heng, W.S.; Gosens, R.; Kruyt, F.A.E. Lung cancer stem cells: Origin, features, maintenance mechanisms and therapeutic targeting. Biochem. Pharmacol. 2019, 160, 121–133. [Google Scholar] [CrossRef]
- Rao, X.; Zhang, C.; Luo, H.; Zhang, J.; Zhuang, Z.; Liang, Z.; Wu, X. Targeting Gastric Cancer Stem Cells to Enhance Treatment Response. Cells 2022, 11, 2828. [Google Scholar] [CrossRef]
- Huang, T.; Song, X.; Xu, D.; Tiek, D.; Goenka, A.; Wu, B.; Sastry, N.; Hu, B.; Cheng, S.-Y. Stem cell programs in cancer initiation, progression, and therapy resistance. Theranostics 2020, 10, 8721–8743. [Google Scholar] [CrossRef]
- Hsieh, H.L.; Yu, M.C.; Cheng, L.C.; Yeh, T.S.; Tsai, M.M. Molecular mechanism of therapeutic approaches for human gastric cancer stem cells. World J. Stem Cells 2022, 14, 76–91. [Google Scholar] [CrossRef]
- Li, K.; Dan, Z.; Nie, Y.Q. Gastric cancer stem cells in gastric carcinogenesis, progression, prevention and treatment. World J. Gastroenterol. 2014, 20, 5420–5426. [Google Scholar] [CrossRef]
- Stojnev, S.; Krstic, M.; Ristic-Petrovic, A.; Stefanovic, V.; Hattori, T. Gastric cancer stem cells: Therapeutic targets. Gastric Cancer 2014, 17, 13–25. [Google Scholar] [CrossRef]
- Otaegi-Ugartemendia, M.; Matheu, A.; Carrasco-Garcia, E. Impact of Cancer Stem Cells on Therapy Resistance in Gastric Cancer. Cancers 2022, 14, 1457. [Google Scholar] [CrossRef]
- Wang, K.C.; Chang, H.Y. Molecular mechanisms of long noncoding RNAs. Mol. Cell 2011, 43, 904–914. [Google Scholar] [CrossRef]
- Kopp, F.; Mendell, J.T. Functional Classification and Experimental Dissection of Long Noncoding RNAs. Cell 2018, 172, 393–407. [Google Scholar] [CrossRef]
- Herman, A.B.; Tsitsipatis, D.; Gorospe, M. Integrated lncRNA function upon genomic and epigenomic regulation. Mol. Cell 2022, 82, 2252–2266. [Google Scholar] [CrossRef]
- Jiang, M.C.; Ni, J.J.; Cui, W.Y.; Wang, B.Y.; Zhuo, W. Emerging roles of lncRNA in cancer and therapeutic opportunities. Am. J. Cancer Res. 2019, 9, 1354–1366. [Google Scholar]
- Yang, M.; Lu, H.; Liu, J.; Wu, S.; Kim, P.; Zhou, X. lncRNAfunc: A knowledgebase of lncRNA function in human cancer. Nucleic Acids Res. 2022, 50, D1295–D1306. [Google Scholar] [CrossRef]
- McCabe, E.M.; Rasmussen, T.P. lncRNA involvement in cancer stem cell function and epithelial-mesenchymal transitions. Semin. Cancer Biol. 2021, 75, 38–48. [Google Scholar] [CrossRef]
- Venkatesh, J.; Wasson, M.D.; Brown, J.M.; Fernando, W.; Marcato, P. LncRNA-miRNA axes in breast cancer: Novel points of interaction for strategic attack. Cancer Lett. 2021, 509, 81–88. [Google Scholar] [CrossRef]
- Liu, X.; Chen, X.; Yu, X.; Tao, Y.; Bode, A.M.; Dong, Z.; Cao, Y. Regulation of microRNAs by epigenetics and their interplay involved in cancer. J. Exp. Clin. Cancer Res. 2013, 32, 96. [Google Scholar] [CrossRef]
- Yan, H.; Bu, P. Non-coding RNAs in cancer stem cells. Cancer Lett. 2018, 421, 121–126. [Google Scholar] [CrossRef]
- Salmena, L.; Poliseno, L.; Tay, Y.; Kats, L.; Pandolfi, P.P. A ceRNA hypothesis: The Rosetta Stone of a hidden RNA language? Cell 2011, 146, 353–358. [Google Scholar] [CrossRef] [PubMed]
- López-Urrutia, E.; Bustamante Montes, L.P.; Ladrón de Guevara Cervantes, D.; Pérez-Plasencia, C.; Campos-Parra, A.D. Crosstalk Between Long Non-coding RNAs, Micro-RNAs and mRNAs: Deciphering Molecular Mechanisms of Master Regulators in Cancer. Front. Oncol. 2019, 9, 669. [Google Scholar] [CrossRef]
- Rawla, P.; Barsouk, A. Epidemiology of gastric cancer: Global trends, risk factors and prevention. Gastroenterol. Rev./Przegląd Gastroenterol. 2019, 14, 26–38. [Google Scholar] [CrossRef]
- Yang, W.-J.; Zhao, H.-P.; Yu, Y.; Wang, J.-H.; Guo, L.; Liu, J.-Y.; Pu, J.; Lv, J. Updates on global epidemiology, risk and prognostic factors of gastric cancer. World J. Gastroenterol. 2023, 29, 2452–2468. [Google Scholar] [CrossRef]
- Najafi, M.; Farhood, B.; Mortezaee, K. Cancer stem cells (CSCs) in cancer progression and therapy. J. Cell. Physiol. 2019, 234, 8381–8395. [Google Scholar] [CrossRef]
- Plaks, V.; Kong, N.; Werb, Z. The cancer stem cell niche: How essential is the niche in regulating stemness of tumor cells? Cell Stem Cell 2015, 16, 225–238. [Google Scholar] [CrossRef]
- Eun, K.; Ham, S.W.; Kim, H. Cancer stem cell heterogeneity: Origin and new perspectives on CSC targeting. BMB Rep. 2017, 50, 117–125. [Google Scholar] [CrossRef]
- Cui, M.; Chang, Y.; Fang, Q.-G.; Du, W.; Wu, J.-F.; Wang, J.-H.; Liu, S.-T.; Luo, S.-X. Non-Coding RNA Pvt1 Promotes Cancer Stem Cell-Like Traits in Nasopharyngeal Cancer via Inhibiting miR-1207. Pathol. Oncol. Res. 2019, 25, 1411–1422. [Google Scholar] [CrossRef]
- Fu, Z.; Chen, C.; Zhou, Q.; Wang, Y.; Zhao, Y.; Zhao, X.; Li, W.; Zheng, S.; Ye, H.; Wang, L.; et al. LncRNA HOTTIP modulates cancer stem cell properties in human pancreatic cancer by regulating HOXA9. Cancer Lett. 2017, 410, 68–81. [Google Scholar] [CrossRef]
- Li, W.; Zhang, L.; Guo, B.; Deng, J.; Wu, S.; Li, F.; Wang, Y.; Lu, J.; Zhou, Y. Exosomal FMR1-AS1 facilitates maintaining cancer stem-like cell dynamic equilibrium via TLR7/NFκB/c-Myc signaling in female esophageal carcinoma. Mol. Cancer 2019, 18, 22. [Google Scholar] [CrossRef]
- Peng, Y.; Xu, C.; Wen, J.; Zhang, Y.; Wang, M.; Liu, X.; Zhao, K.; Wang, Z.; Liu, Y.; Zhang, T. Fatty Acid Metabolism-Related lncRNAs Are Potential Biomarkers for Predicting the Overall Survival of Patients With Colorectal Cancer. Front. Oncol. 2021, 11, 704038. [Google Scholar] [CrossRef]
- Xia, Y.C.; Cao, J.; Yang, J.; Zhang, Y.; Li, Y.S. lncRNA TSPEAR-AS2, a Novel Prognostic Biomarker, Promotes Oral Squamous Cell Carcinoma Progression by Upregulating PPM1A via Sponging miR-487a-3p. Dis. Markers 2021, 2021, 2217663. [Google Scholar] [CrossRef]
- Ma, Z.-H.; Shuai, Y.; Gao, X.-Y.; Yan, Y.; Wang, K.-M.; Wen, X.-Z.; Ji, J.-F. BTEB2-Activated lncRNA TSPEAR-AS2 Drives GC Progression through Suppressing GJA1 Expression and Upregulating CLDN4 Expression. Mol. Ther. Nucleic Acids 2020, 22, 1129–1141. [Google Scholar] [CrossRef]
- Najafi, M.; Mortezaee, K.; Majidpoor, J. Cancer stem cell (CSC) resistance drivers. Life Sci. 2019, 234, 116781. [Google Scholar] [CrossRef]
- Ma, L.; Wang, F.; Du, C.; Zhang, Z.; Guo, H.; Xie, X.; Gao, H.; Zhuang, Y.; Kornmann, M.; Gao, H.; et al. Long non-coding RNA MEG3 functions as a tumour suppressor and has prognostic predictive value in human pancreatic cancer. Oncol. Rep. 2018, 39, 1132–1140. [Google Scholar] [CrossRef]
- Li, Y.; Hu, J.; Guo, D.; Ma, W.; Zhang, X.; Zhang, Z.; Lu, G.; He, S. LncRNA SNHG5 promotes the proliferation and cancer stem cell-like properties of HCC by regulating UPF1 and Wnt-signaling pathway. Cancer Gene Ther. 2022, 29, 1373–1383. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, T.; Xia, L.; Zhang, M. LncRNA WDFY3-AS2 promotes cisplatin resistance and the cancer stem cell in ovarian cancer by regulating hsa-miR-139-5p/SDC4 axis. Cancer Cell Int. 2021, 21, 284. [Google Scholar] [CrossRef]
- Sun, J.; Zhou, H.; Bao, X.; Wu, Y.; Jia, H.; Zhao, H.; Liu, G.; Papaccio, G. lncRNA TUG1 Facilitates Colorectal Cancer Stem Cell Characteristics and Chemoresistance by Enhancing GATA6 Protein Stability. Stem Cells Int. 2021, 2021, 1075481. [Google Scholar] [CrossRef]
- Fong, L.Y.; Nguyen, V.T.; Farber, J.L.; Huebner, K.; Magee, P.N. Early deregulation of the the p16ink4a-cyclin D1/cyclin-dependent kinase 4-retinoblastoma pathway in cell proliferation-driven esophageal tumorigenesis in zinc-deficient rats. Cancer Res. 2000, 60, 4589–4595. [Google Scholar]
- Wang, M.; Li, Y.; Xiao, G.; Zheng, X.; Wang, J.; Xu, C.; Qin, S.; Ren, H.; Tang, S.; Sun, X. H19 regulation of oestrogen induction of symmetric division is achieved by antagonizing Let-7c in breast cancer stem-like cells. Cell Prolif. 2019, 52, e12534. [Google Scholar] [CrossRef]
- Xu, R.; Zhu, X.; Chen, F.; Huang, C.; Ai, K.; Wu, H.; Zhang, L.; Zhao, X. LncRNA XIST/miR-200c regulates the stemness properties and tumourigenicity of human bladder cancer stem cell-like cells. Cancer Cell Int. 2018, 18, 41. [Google Scholar] [CrossRef]
- Wang, R.; Dong, H.X.; Zeng, J.; Pan, J.; Jin, X.Y. LncRNA DGCR5 contributes to CSC-like properties via modulating miR-330-5p/CD44 in NSCLC. J. Cell. Physiol. 2018, 233, 7447–7456. [Google Scholar] [CrossRef]
- He, B.; Peng, F.; Li, W.; Jiang, Y. Interaction of lncRNA-MALAT1 and miR-124 regulates HBx-induced cancer stem cell properties in HepG2 through PI3K/Akt signaling. J. Cell. Biochem. 2019, 120, 2908–2918. [Google Scholar] [CrossRef]
- Shen, C.; Wang, J.; Xu, Z.; Zhang, L.; Gu, W.; Zhou, X. ONECUT2 which is targeted by hsa-miR-15a-5p enhances stemness maintenance of gastric cancer stem cells. Exp. Biol. Med. 2021, 246, 2645–2659. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Wang, Y.; Chen, L.; Shen, Y.; Zhang, S.; Yue, D.; Chen, X. LncRNA TSPEAR-AS2 Maintains the Stemness of Gastric Cancer Stem Cells by Regulating the miR-15a-5p/CCND1 Axis. Biomolecules 2025, 15, 1227. https://doi.org/10.3390/biom15091227
Li Q, Wang Y, Chen L, Shen Y, Zhang S, Yue D, Chen X. LncRNA TSPEAR-AS2 Maintains the Stemness of Gastric Cancer Stem Cells by Regulating the miR-15a-5p/CCND1 Axis. Biomolecules. 2025; 15(9):1227. https://doi.org/10.3390/biom15091227
Chicago/Turabian StyleLi, Qiong, Yanan Wang, Liyang Chen, Yan Shen, Shijiao Zhang, Dengyuan Yue, and Xiaowei Chen. 2025. "LncRNA TSPEAR-AS2 Maintains the Stemness of Gastric Cancer Stem Cells by Regulating the miR-15a-5p/CCND1 Axis" Biomolecules 15, no. 9: 1227. https://doi.org/10.3390/biom15091227
APA StyleLi, Q., Wang, Y., Chen, L., Shen, Y., Zhang, S., Yue, D., & Chen, X. (2025). LncRNA TSPEAR-AS2 Maintains the Stemness of Gastric Cancer Stem Cells by Regulating the miR-15a-5p/CCND1 Axis. Biomolecules, 15(9), 1227. https://doi.org/10.3390/biom15091227





