Improvement of Soluble Expression, Stability, and Activity of Acetaldehyde Lyase by Elastin-like Polypeptides Fusion for Acetoin Production from Acetaldehyde
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Construction and Expression of Fusion Protein
2.3. Purification of Recombinant Protein
- (1) Purification by Ni-NTA affinity chromatography (His-ALS (I28V/L482E))
- (2) Purification by ITC (ELP-ALS (I28V/L482E))
2.4. ALS Enzyme Activity Assay
2.5. Determination of Optimal pH and Temperature
2.6. Enzyme Stability and Substrate Tolerance
2.7. Kinetic Parameter Activity Assay
2.8. Fed-Batch Strategy for Acetoin Production
3. Results
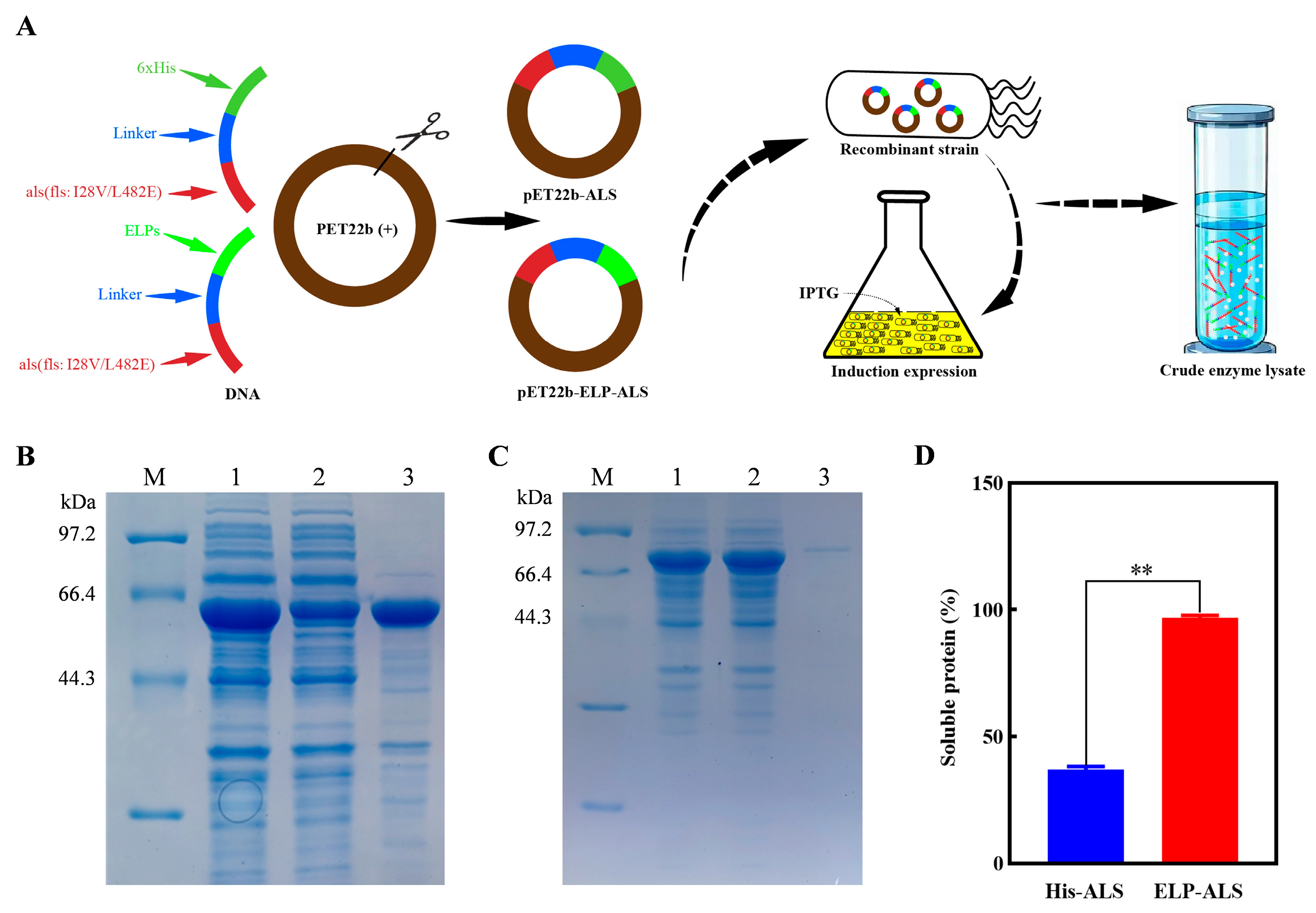
3.1. Expression of His-ALS and ELP-ALS
3.2. Purification of His-ALS and ELP-ALS
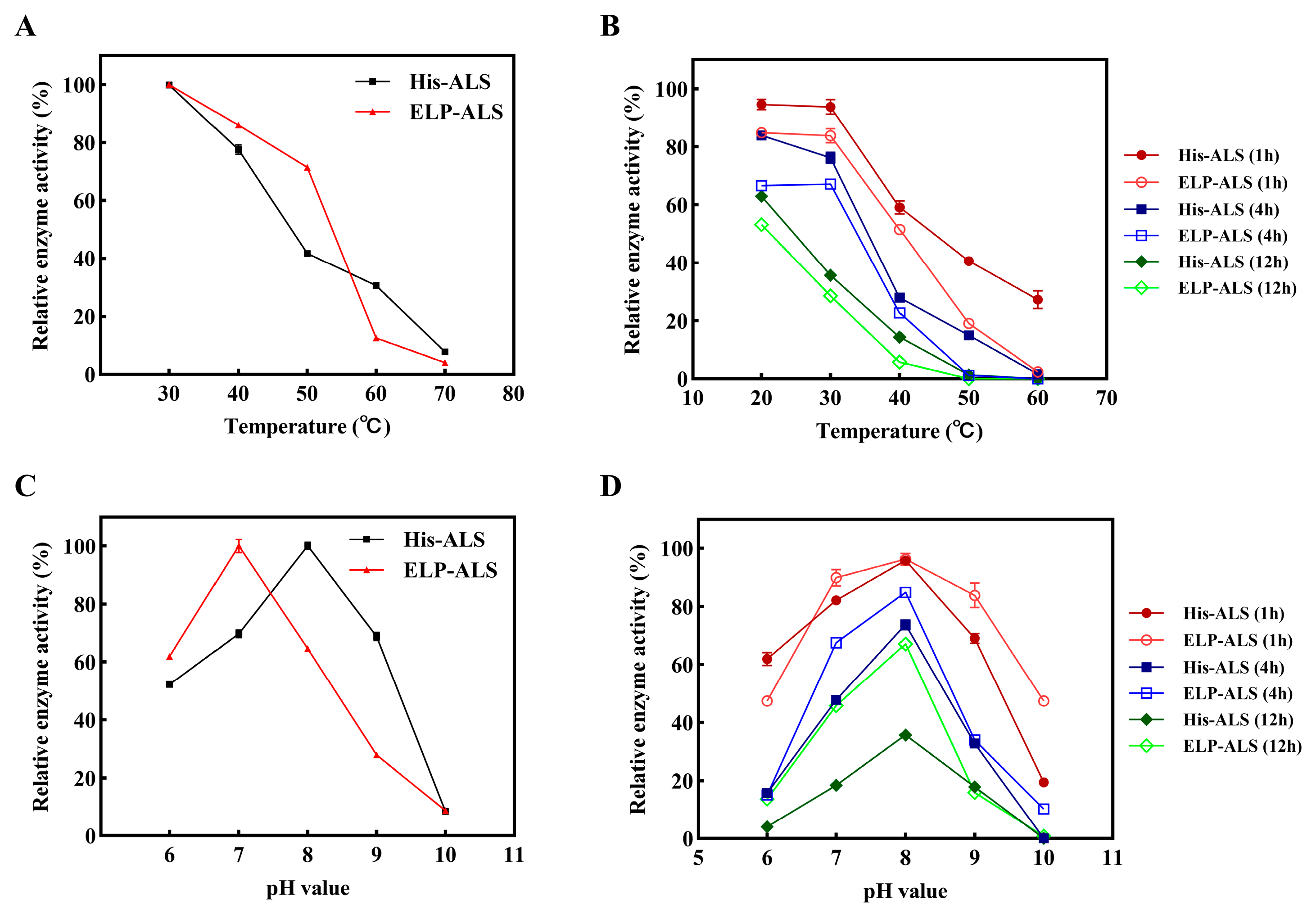
3.3. Evaluation of the Effect of pH and Temperature
3.4. Storage Stability and Substrate Tolerance
3.5. Kinetic Parameters
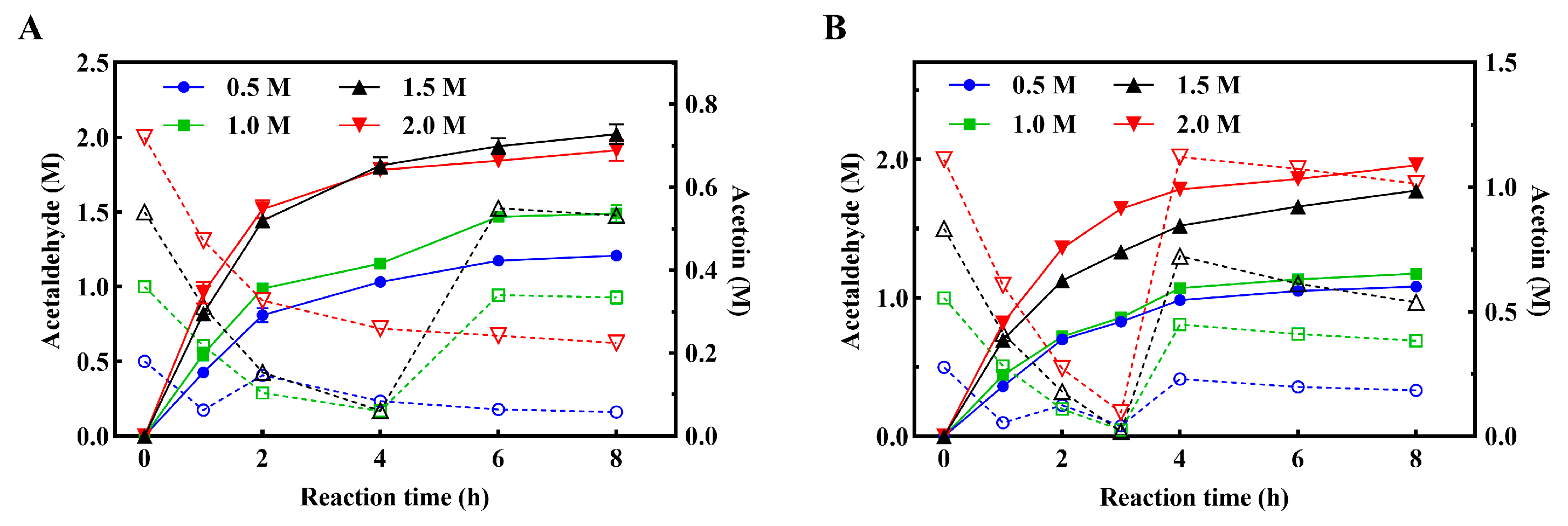
3.6. Fed-Batch Reaction
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALS | Acetaldehyde lyase |
| ELPs | Elastin-like polypeptides |
| ITC | Inverse transition cycling |
| FLS | Formolase |
| Ni-NTA | Nickel-nitrilotriacetic acid |
| SDGs | Sustainable Development Goals |
| IPTG | Isopropyl-β-d-thiogalactoside |
| TEMED | Tetramethylethylenediamine |
| TPP | Thiamine pyrophosphate |
| SDS | Sodium dodecyl sulfate |
| Tris | Tris(hydroxymethyl)aminomethane |
| Xyl | Xylanase |
| LB | Luria–bertani |
| His | Histone |
| MBP | Maltose-Binding Protein |
| TrxA | Thioredoxin A |
| CLP | Collagen-like polypeptide |
| SOD | Superoxide dismutase |
| DAAO | D-amino acid oxidase |
References
- Jiang, Y.; Woortman, A.J.; van Ekenstein, G.O.; Loos, K. Enzyme-catalyzed synthesis of unsaturated aliphatic polyesters based on green monomers from renewable resources. Biomolecules 2013, 3, 461–480. [Google Scholar] [CrossRef]
- Becker, M.; Nikel, P.; Andexer, J.N.; Lütz, S.; Rosenthal, K. A Multi-Enzyme Cascade Reaction for the Production of 2′3′-cGAMP. Biomolecules 2021, 11, 590. [Google Scholar] [CrossRef]
- Alcántara, A.R.; Domínguez de María, P.; Littlechild, J.A.; Schürmann, M.; Sheldon, R.A.; Wohlgemuth, R. Biocatalysis as Key to Sustainable Industrial Chemistry. ChemSusChem 2022, 15, e202102709. [Google Scholar] [CrossRef]
- Amatto, I.V.D.S.; Rosa-Garzon, N.G.D.; Simões, F.A.D.O.; Santiago, F.; Leite, N.P.D.S.; Martins, J.R.; Cabral, H. Enzyme engineering and its industrial applications. Biotechnol. Appl. Biochem. 2022, 69, 389–409. [Google Scholar] [CrossRef]
- Wu, S.; Snajdrova, R.; Moore, J.C.; Baldenius, K.; Bornscheuer, U.T. Biocatalysis: Enzymatic Synthesis for Industrial Applications. Angew. Chem. Int. Ed. Engl. 2021, 60, 88–119. [Google Scholar] [CrossRef]
- Maina, S.; Prabhu, A.A.; Vivek, N.; Vlysidis, A.; Koutinas, A.; Kumar, V. Prospects on bio-based 2,3-butanediol and acetoin production: Recent progress and advances. Biotechnol. Adv. 2022, 54, 107783. [Google Scholar] [CrossRef]
- Xiao, Z.; Lu, J.R. Generation of acetoin and its derivatives in foods. J. Agric. Food. Chem. 2014, 62, 6487–6497. [Google Scholar] [CrossRef]
- Zhou, C.; Shen, H.; Yan, S.; Ma, C.; Leng, J.; Song, Y.; Gao, N. Acetoin Promotes Plant Growth and Alleviates Saline Stress by Activating Metabolic Pathways in Lettuce Seedlings. Plants 2024, 13, 3312. [Google Scholar] [CrossRef]
- Qin, Y.; Han, Y.; Shang, Q.; Li, P. Complete genome sequence of Bacillus amyloliquefaciens L-H15, a plant growth promoting rhizobacteria isolated from cucumber seedling substrate. J. Biotechnol. 2015, 200, 59–60. [Google Scholar] [CrossRef]
- Li, T.; Liu, P.; Guo, G.; Liu, Z.; Zhong, L.; Guo, L.; Chen, C.; Hao, N.; Ouyang, P. Production of acetoin and its derivative tetramethylpyrazine from okara hydrolysate with Bacillus subtilis. AMB Express 2023, 13, 25. [Google Scholar] [CrossRef]
- Cui, Z.; Wang, Z.; Zheng, M.; Chen, T. Advances in biological production of acetoin: A comprehensive overview. Crit. Rev. Biotechnol. 2022, 42, 1135–1156. [Google Scholar] [CrossRef]
- Zhu, P.; Zhang, C.; Chen, J.; Zeng, X. Multilevel systemic engineering of Bacillus licheniformis for efficient production of acetoin from lignocellulosic hydrolysates. Int. J. Biol. Macromol. 2024, 279, 135142. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Lu, J.R. Strategies for enhancing fermentative production of acetoin: A review. Biotechnol. Adv. 2014, 32, 492–503. [Google Scholar] [CrossRef]
- Cui, Z.; Zheng, M.; Ding, M.; Dai, W.; Wang, Z.; Chen, T. Efficient production of acetoin from lactate by engineered Escherichia coli whole-cell biocatalyst. Appl. Microbiol. Biotechnol. 2023, 107, 3911–3924. [Google Scholar] [CrossRef]
- Bae, S.J.; Kim, S.; Park, H.J.; Kim, J.; Jin, H.; Kim, B.G.; Hahn, J.S. High-yield production of (R)-acetoin in Saccharomyces cerevisiae by deleting genes for NAD(P)H-dependent ketone reductases producing meso-2,3-butanediol and 2,3-dimethylglycerate. Metab. Eng. 2021, 66, 68–78. [Google Scholar] [CrossRef]
- Li, S.; Liu, L.; Chen, J. Compartmentalizing metabolic pathway in Candida glabrata for acetoin production. Metab. Eng. 2015, 28, 1–7. [Google Scholar] [CrossRef]
- Moxley, W.C.; Brown, R.E.; Eiteman, M.A. Escherichia coli aceE variants coding pyruvate dehydrogenase improve the generation of pyruvate-derived acetoin. Eng. Life. Sci. 2023, 23, e2200054. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, Q.; Zhan, S.; Li, Y.; Lin, H.; Sun, S.; Sha, L.; Hu, K.; Guan, X.; Shen, Y. A new NAD(H)-dependent meso-2,3-butanediol dehydrogenase from an industrially potential strain Serratia marcescens H30. Appl. Microbiol. Biotechnol. 2014, 98, 1175–1184. [Google Scholar] [CrossRef]
- Rehman, S.; Islam, M.K.; Khanzada, N.K.; Kyoungjin An, A.; Chaiprapat, S.; Leu, S.Y. Whole sugar 2,3-butanediol fermentation for oil palm empty fruit bunches biorefinery by a newly isolated Klebsiella pneumoniae PM2. Bioresour. Technol. 2021, 333, 125206. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Mao, Y.; Kou, M.; Cui, Z.; Jin, B.; Chang, Z.; Wang, Z.; Ma, H.; Chen, T. Engineering central pathways for industrial-level (3R)-acetoin biosynthesis in Corynebacterium glutamicum. Microb. Cell. Fact. 2020, 19, 102. [Google Scholar] [CrossRef]
- Maina, S.; Schneider, R.; Alexandri, M.; Papapostolou, H.; Nychas, G.J.; Koutinas, A.; Venus, J. Volumetric oxygen transfer coefficient as fermentation control parameter to manipulate the production of either acetoin or D-2,3-butanediol using bakery waste. Bioresour. Technol. 2021, 335, 125155. [Google Scholar] [CrossRef]
- Tsigoriyna, L.; Petrova, P.; Petrov, K. High production of acetoin from glycerol by Bacillus subtilis 35. Appl. Microbiol. Biotechnol. 2023, 107, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, J.; Fu, Q.; Liu, H.; Li, M.; Wang, Z.; Gu, W.; Zhu, X.; Lin, R.; Dai, L.; et al. Metabolic engineering of Paenibacillus polymyxa for effective production of 2,3-butanediol from poplar hydrolysate. Bioresour. Technol. 2024, 392, 130002. [Google Scholar] [CrossRef]
- Meng, D.; Wei, X.; Bai, X.; Zhou, W.; You, C. Artificial in Vitro Synthetic Enzymatic Biosystem for the One-Pot Sustainable Biomanufacturing of Glucosamine from Starch and Inorganic Ammonia. ACS Catal. 2020, 10, 13809–13819. [Google Scholar] [CrossRef]
- Zhao, Q.; Ansorge-Schumacher, M.B.; Haag, R.; Wu, C. Living whole-cell catalysis in compartmentalized emulsion. Bioresour. Technol. 2020, 295, 122221. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Sánchez, D.; Carceller, A.; Álvaro, G.; Romero, O.; Guillén, M. Artificial cell-free system for the sustainable production of acetoin from bioethanol. Bioresour. Technol. 2025, 419, 132059. [Google Scholar] [CrossRef]
- Jia, X.; Kelly, R.M.; Han, Y. Simultaneous biosynthesis of (R)-acetoin and ethylene glycol from D-xylose through in vitro metabolic engineering. Metab. Eng. Commun. 2018, 7, e00074. [Google Scholar] [CrossRef]
- Jia, X.; Liu, Y.; Han, Y. A thermophilic cell-free cascade enzymatic reaction for acetoin synthesis from pyruvate. Sci. Rep. 2017, 7, 4333. [Google Scholar] [CrossRef]
- Siegel, J.B.; Smith, A.L.; Poust, S.; Wargacki, A.J.; Bar-Even, A.; Louw, C.; Shen, B.W.; Eiben, C.B.; Tran, H.M.; Noor, E.; et al. Computational protein design enables a novel one-carbon assimilation pathway. Proc. Natl. Acad. Sci. USA 2015, 112, 3704–3709. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.; Guo, D.; Lou, Q.; Lu, X.; Cheng, J.; Qiao, J.; Lu, L.; Cai, T.; Liu, Y.; Jiang, H. Synthesis of Ligustrazine from Acetaldehyde by a Combined Biological-Chemical Approach. ACS. Synth. Biol. 2020, 9, 2902–2908. [Google Scholar] [CrossRef]
- Zhang, L.; Singh, R.; Sivakumar, D.; Zewang, G.; Li, J.; Chen, F.; He, Y.; Guan, X.; Kang, Y.; Lee, J.-K. An artificial synthetic pathway for acetoin, 2,3-butanediol, and 2-butanol production from ethanol using cell free multi-enzyme catalysis. Green Chem. 2017, 20, 230–242. [Google Scholar] [CrossRef]
- Zhang, J.; Lin, H.; Zheng, C.; Yang, B.; Liang, M.; Lin, Y.; Zhang, L. Efficient 2,3-Butanediol Production from Ethanol by a Modified Four-Enzyme Synthetic Biosystem. Molecules 2024, 29, 3934. [Google Scholar] [CrossRef]
- Tian, X.; Feng, M.; Wei, X.; Cheng, C.; He, K.; Jiang, T.; He, B.; Gu, Z. In situ formed depot of elastin-like polypeptide-hirudin fusion protein for long-acting antithrombotic therapy. Proc. Natl. Acad. Sci. USA 2024, 121, e2314349121. [Google Scholar] [CrossRef] [PubMed]
- Yeboah, A.; Cohen, R.I.; Rabolli, C.; Yarmush, M.L.; Berthiaume, F. Elastin-like polypeptides: A strategic fusion partner for biologics. Biotechnol. Bioeng. 2016, 113, 1617–1627. [Google Scholar] [CrossRef] [PubMed]
- Mills, C.E.; Michaud, Z.; Olsen, B.D. Elastin-like Polypeptide (ELP) Charge Influences Self-Assembly of ELP-mCherry Fusion Proteins. Biomacromolecules 2018, 19, 2517–2525. [Google Scholar] [CrossRef]
- Du, K.; Sun, J.; Song, X.; Song, C.; Feng, W. Enhancement of the solubility and stability of D-amino acid oxidase by fusion to an elastin like polypeptide. J. Biotechnol. 2015, 212, 50–55. [Google Scholar] [CrossRef]
- Han, J.; Fang, S.; He, X.; Wang, L.; Li, C.; Wu, J.; Cai, Y.; Wang, Y. Combination of aqueous two-phase flotation and inverse transition cycling: Strategies for separation and purification of recombinant β-glucosidase from cell lysis solution. Food. Chem. 2022, 373, 131543. [Google Scholar] [CrossRef]
- Lin, Y.; Li, C.; Wei, C.; Lin, H.; Zhang, L. Mining, Identification, and Characterization of Three Xylanases from the Microbiota of T. fuciformis with Its Companion Strains. Catalysts 2024, 14, 15. [Google Scholar] [CrossRef]
- Trabbic-Carlson, K.; Liu, L.; Kim, B.; Chilkoti, A. Expression and purification of recombinant proteins from Escherichia coli: Comparison of an elastin-like polypeptide fusion with an oligohistidine fusion. Protein Sci. 2004, 13, 3274–3284. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, X.; Yan, D.; Addai Peprah, F.; Ji, X.; Fletcher, E.E.; Wang, Y.; Wang, Y.; Gu, J.; Lin, F.; et al. Multifunctional elastin-like polypeptide renders β-glucosidase enzyme phase transition and high stability. Biotechnol. Biofuels 2019, 12, 157. [Google Scholar] [CrossRef]
- Dall, N.R.; Mendonça, C.; Torres Vera, H.L.; Marqusee, S. The importance of the location of the N-terminus in successful protein folding in vivo and in vitro. Proc. Natl. Acad. Sci. USA 2024, 121, e2321999121. [Google Scholar] [CrossRef]
- Tang, N.C.; Su, J.C.; Shmidov, Y.; Kelly, G.; Deshpande, S.; Sirohi, P.; Peterson, N.; Chilkoti, A. Synthetic intrinsically disordered protein fusion tags that enhance protein solubility. Nat. Commun. 2024, 15, 3727. [Google Scholar] [CrossRef]
- Zou, Z.; Cao, L.; Zhou, P.; Su, Y.; Sun, Y.; Li, W. Hyper-acidic protein fusion partners improve solubility and assist correct folding of recombinant proteins expressed in Escherichia coli. J. Biotechnol. 2008, 135, 333–339. [Google Scholar] [CrossRef]
- Peprah Addai, F.; Wang, T.; Kosiba, A.A.; Lin, F.; Zhen, R.; Chen, D.; Gu, J.; Shi, H.; Zhou, Y. Integration of elastin-like polypeptide fusion system into the expression and purification of Lactobacillus sp. B164 β-galactosidase for lactose hydrolysis. Bioresour. Technol. 2020, 311, 123513. [Google Scholar] [CrossRef]
- Liu, D.; Du, K.; Feng, W. Immobilization of enzymes using a multifunctional fusion polypeptide. Biotechnol. Lett. 2018, 40, 181–187. [Google Scholar] [CrossRef]
- Qin, G.; Perez, P.M.; Mills, C.E.; Olsen, B.D. Effect of ELP Sequence and Fusion Protein Design on Concentrated Solution Self-Assembly. Biomacromolecules 2016, 17, 928–934. [Google Scholar] [CrossRef]
- Paraskevopoulou, V.; Falcone, F.H. Polyionic Tags as Enhancers of Protein Solubility in Recombinant Protein Expression. Microorganisms 2018, 6, 47. [Google Scholar] [CrossRef]
- Fu, Y.; Mao, S.; Liao, T.; Feng, W. Simultaneous production of linear α-olefins and 2,5-furandicarboxylic acid by combining two recombinant enzymes OleT-ELP and HMFO-ELP. Enzyme Microb. Technol. 2025, 188, 110637. [Google Scholar] [CrossRef]
- Chen, N.; Chang, B.; Shi, N.; Yan, W.; Lu, F.; Liu, F. Cross-linked enzyme aggregates immobilization: Preparation, characterization, and applications. Crit. Rev. Biotechnol. 2023, 43, 369–383. [Google Scholar] [CrossRef]


| Enzyme | Vmax (U/mg) | Km (mM) a | kcat (s−1) a | kcat/Km (s−1·M−1) |
|---|---|---|---|---|
| His-ALS: I28V/L482E | 8.21 ± 1.72 | 343.84 ± 6.84 | 8.45 ± 0.26 | 24.63 ± 4.28 |
| ELP-ALS: I28V/L482E | 15.25 ± 3.10 | 210.39 ± 14.13 | 15.37 ± 3.12 | 73.05 ± 6.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, H.; Zhang, J.; Hu, J.; Ma, L.; Lai, K.; Zheng, C.; Yang, Q.; Zhang, L. Improvement of Soluble Expression, Stability, and Activity of Acetaldehyde Lyase by Elastin-like Polypeptides Fusion for Acetoin Production from Acetaldehyde. Biomolecules 2025, 15, 1216. https://doi.org/10.3390/biom15091216
Lin H, Zhang J, Hu J, Ma L, Lai K, Zheng C, Yang Q, Zhang L. Improvement of Soluble Expression, Stability, and Activity of Acetaldehyde Lyase by Elastin-like Polypeptides Fusion for Acetoin Production from Acetaldehyde. Biomolecules. 2025; 15(9):1216. https://doi.org/10.3390/biom15091216
Chicago/Turabian StyleLin, Hui, Jiming Zhang, Jie Hu, Lu Ma, Kaili Lai, Chaosong Zheng, Qiuhua Yang, and Liaoyuan Zhang. 2025. "Improvement of Soluble Expression, Stability, and Activity of Acetaldehyde Lyase by Elastin-like Polypeptides Fusion for Acetoin Production from Acetaldehyde" Biomolecules 15, no. 9: 1216. https://doi.org/10.3390/biom15091216
APA StyleLin, H., Zhang, J., Hu, J., Ma, L., Lai, K., Zheng, C., Yang, Q., & Zhang, L. (2025). Improvement of Soluble Expression, Stability, and Activity of Acetaldehyde Lyase by Elastin-like Polypeptides Fusion for Acetoin Production from Acetaldehyde. Biomolecules, 15(9), 1216. https://doi.org/10.3390/biom15091216







