1. Introduction
Idiopathic pulmonary fibrosis (IPF) is a chronic fibrotic interstitial pneumonia of unknown etiology, characterized by the radiological and histological features of usual interstitial pneumonia (UIP). IPF typically occurs in older individuals and is marked by progressive dyspnea and declining lung function, leading to a poor prognosis [
1]. The median survival time for patients with IPF is approximately 1 to 2 years [
2,
3,
4]. The global incidence of IPF is estimated to be between one to thirteen cases per 100,000 people, with a prevalence of three to forty-five cases per 100,000 [
5]. As the global population continues to age, increased awareness of IPF’s clinical presentation, along with the emergence of various risk factors, is expected to contribute to a rising global burden of the disease. The pathogenesis of IPF is thought to result from a complex interplay of genetic and environmental factors, as well as comorbid conditions [
6]. Viral infections (such as hepatitis C virus, adenovirus, SARS-CoV-2, and herpesvirus) are also significant risk factors that may lead to lung fibrosis, acute exacerbation, and disease progression. Furthermore, comorbidities like gastroesophageal reflux disease, diabetes, and obstructive sleep apnea can also increase the risk of developing IPF [
6,
7]. The disease course and progression of IPF are heterogeneous and unpredictable. Currently, two medications, pirfenidone and nintedanib, can slow the decline in lung function in IPF patients, but they do not halt disease progression or extend survival.
The pathogenesis of IPF is complex and involves interactions among various cells and signaling pathways. The initial stage of IPF usually involves abnormal repair of alveolar epithelial cells, which promotes inflammation and fibrosis. Overactivation of fibroblasts is also a key factor, as these cells play an important role in normal repair of lung tissue, while in IPF, they lead to abnormal deposition of collagen, which promotes the development of fibrosis. Functional abnormalities in fibroblasts are also closely linked to their resistance to apoptosis and reduced autophagy. Under normal circumstances, apoptosis is a key mechanism for maintaining tissue homeostasis; however, in IPF, fibroblasts exhibit resistance to apoptotic signals. This apoptotic resistance allows fibroblasts to persist and remain active in pathological conditions, further exacerbating the fibrotic process. Additionally, autophagy is an essential mechanism for the clearance of damaged components and maintenance of the energy balance. Its reduction in fibroblasts leads to the accumulation of harmful substances within the cells, impairing normal cellular functions and accelerating the onset of fibrosis.
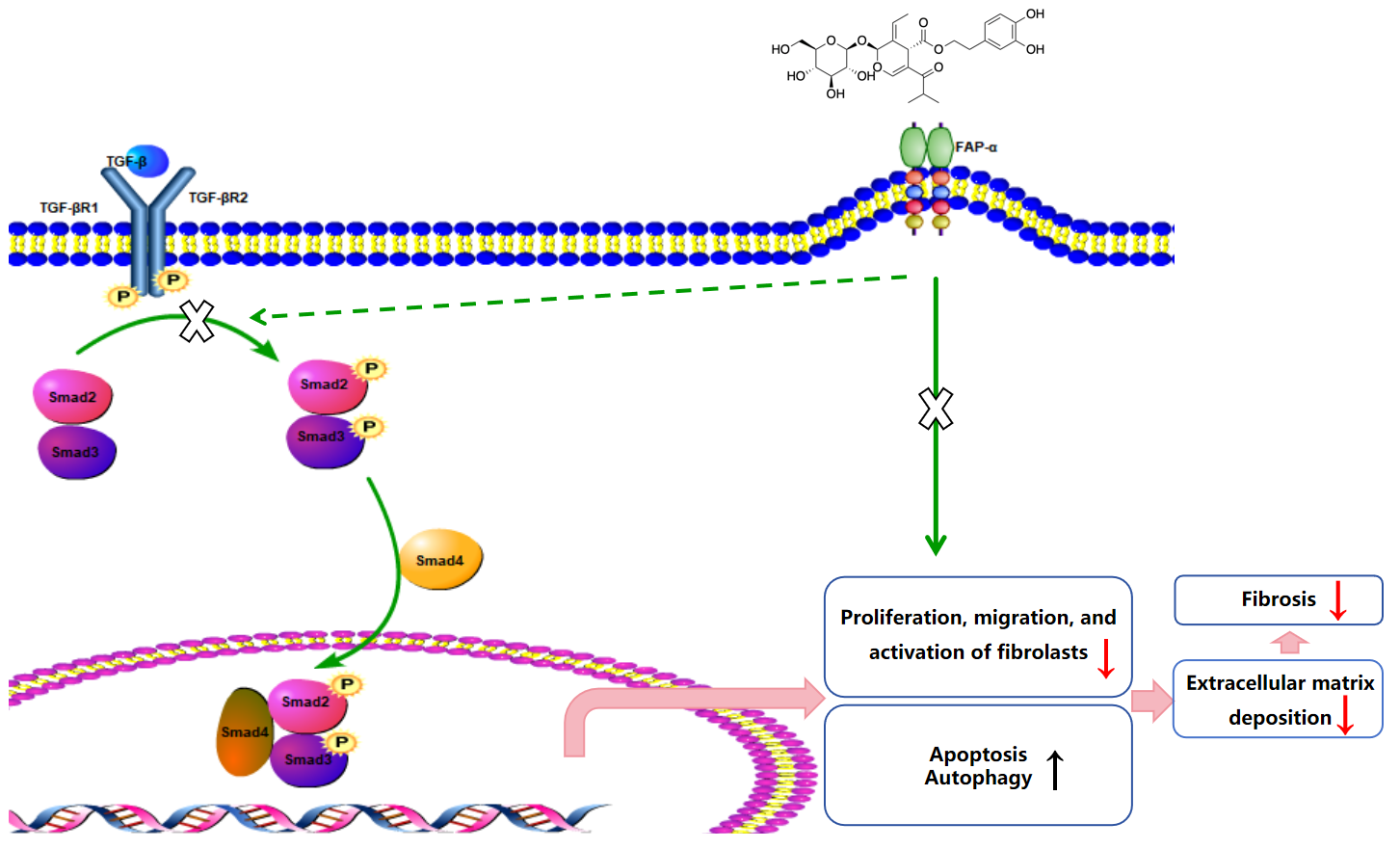
On the other hand, the aberrant activation of profibrotic factors, such as the TGF-β pathway, also plays a significant role in IPF. This pathway activates downstream Smad and non-Smad signaling pathways, including the MAPK and mTOR pathways. These pathways not only promote the proliferation and migration of fibroblasts but also further inhibit autophagy and enhance cellular resistance to apoptosis. Therefore, a deeper understanding of the functional changes in fibroblasts and the dysregulation of their associated signaling pathways will provide important insights into the pathological mechanisms of IPF and potential therapeutic targets [
8].
Oleuropein is a secoiridoid glycoside compound found in plants such as olive leaves, with the potential to prevent various age-related diseases, including those associated with steatohepatitis and fibrosis. Studies have shown that oleuropein can treat non-alcoholic steatohepatitis (NASH) and its progression to fibrosis, significantly reducing liver damage, pathological obesity, and lipid accumulation, while also alleviating hepatitis and fibrosis [
9]. In diabetic models, oleuropein has demonstrated the ability to improve cardiac fibrosis and hypertrophy, suppressing the expression of fibrosis-related genes [
10]. Additionally, research has indicated that oleuropein has protective effects on chronic obstructive pulmonary disease (COPD) and acute lung injury, primarily exerting its effects through anti-inflammatory and antioxidant mechanisms [
11,
12]. Industrial extracts of olive leaves have been shown to ameliorate bleomycin-induced pulmonary fibrosis in rats [
13]. Therefore, oleuropein plays a significant pharmacological role in the prevention and treatment of fibrosis-related diseases.
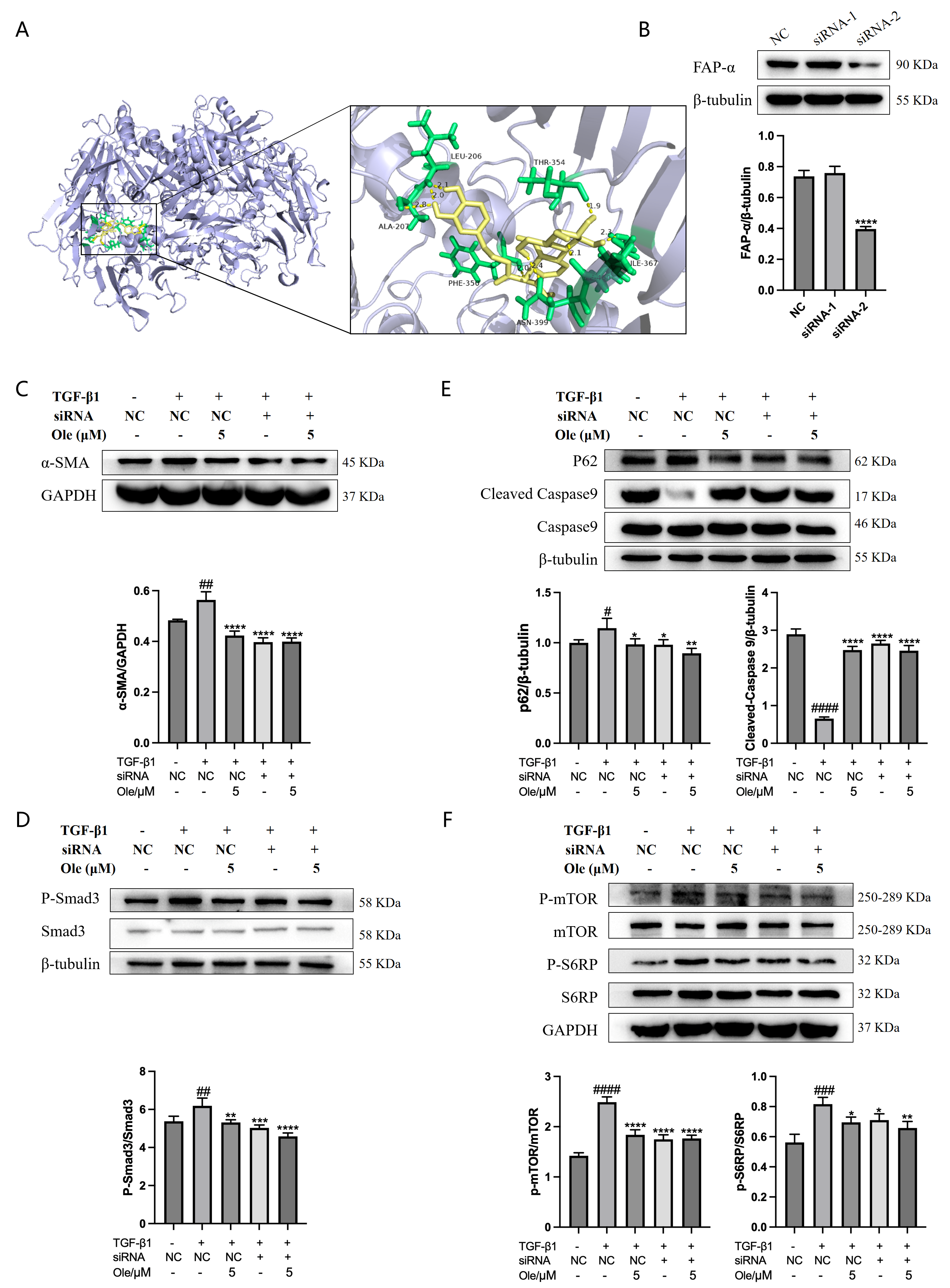
Given the central role of fibroblast overactivation in the pathogenesis of pulmonary fibrosis, the activation marker α-SMA has been utilized for the early screening of drugs with antifibrotic effects. Fibroblast activation was induced using TGF-β1, and the impact of compounds from a library of traditional Chinese medicine monomers on α-SMA expression was assessed. The screening results indicated that oleuropein dose-dependently inhibited TGF-β1-induced α-SMA expression. Furthermore, oleuropein demonstrated promising antifibrotic effects on animal models of pulmonary fibrosis. Therefore, this study aims to investigate the therapeutic effects and mechanisms of oleuropein in pulmonary fibrosis, providing new insights for the treatment of this condition.
2. Materials and Methods
2.1. Mice and Bleomycin Induction of Pulmonary Fibrosis
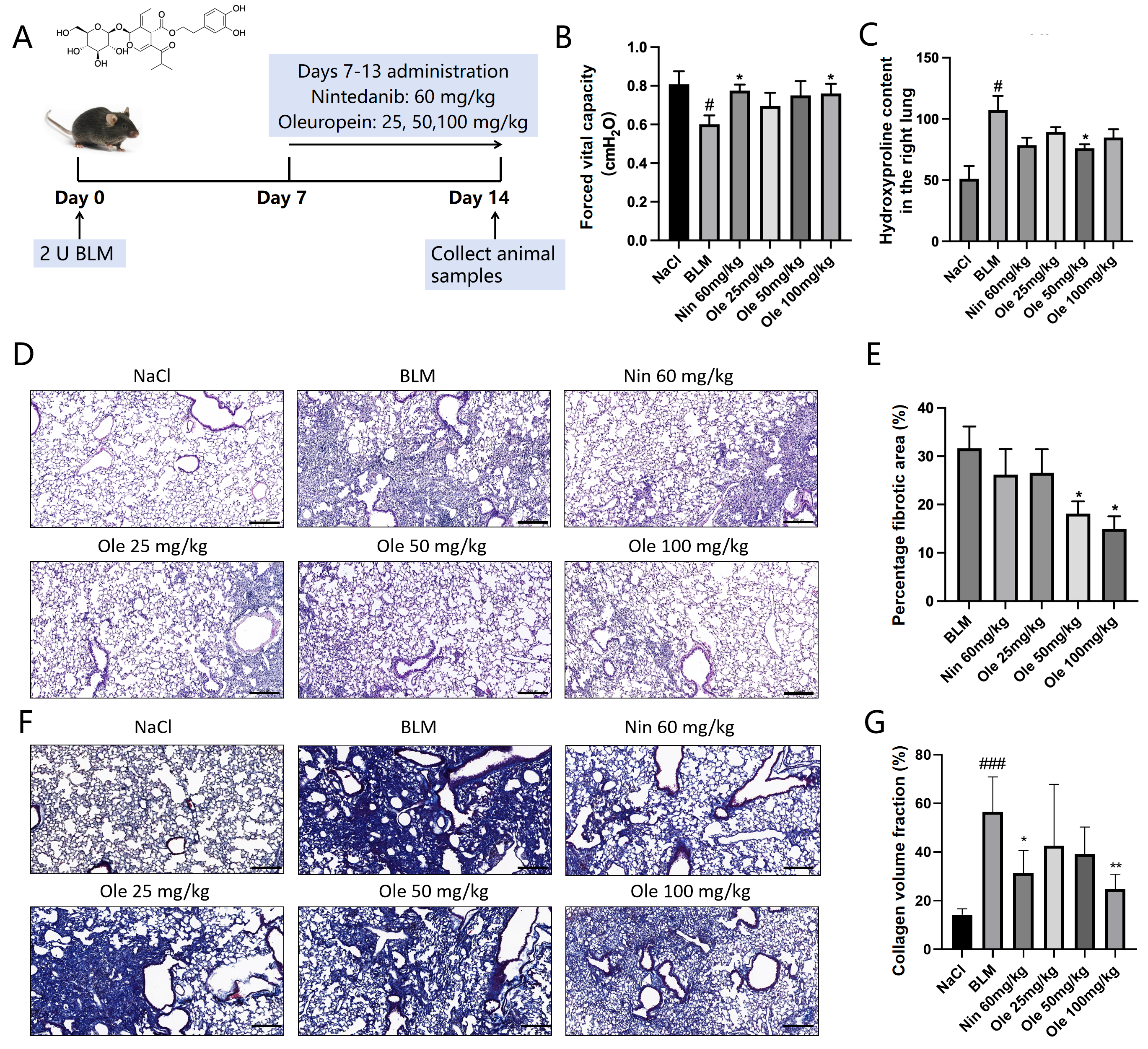
C57BL/6J male mice, aged 6 to 8 weeks, were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Shenzhen, China). (Production License Number: SCXK (Jing) 2021-0006). These mice were allowed free access to water and food. The mice were randomly divided into the following groups: NaCl group, bleomycin (BLM) group, nintedanib 60 mg/kg group, Ole 25 mg/kg group, Ole 50 mg/kg group, and Ole 100 mg/kg group, with 5 mice in each group. After acclimatization to the breeding environment, modeling was conducted. Following anesthesia, the mice were fixed and the area disinfected with alcohol, after which an incision was made in the neck using a surgical scalpel. The muscle tissue was separated with forceps to expose the trachea, and a syringe was inserted into the trachea toward the ribs to administer bleomycin (970592, Nippon Kayaku Co., Ltd., Tokyo, Japan) at a dosage of 2 U/kg. After the injection, the mice were held upright and gently tapped on the back to facilitate the distribution of bleomycin in the lungs. The sham surgery group received an injection of saline into the trachea.
Oleuropein is a monoterpenoid with the molecular formula C25H32O13, and was purchased from Chengdu Push Bio-technology Co., Ltd., Chengdu, China, with a purity of >98.0%.
2.2. Experimental Design
Thirty mice were included in the experiment. Using a random number table, the mice were divided into the control group, the model group, and the drug group based on their body weight. The entire grouping process was completely random. On the seventh day, we measured the degree of lesions in the mice after they received BLM stimulation using microCT and administered the drug. We chose the marketed drug nintedanib for idiopathic pulmonary fibrosis as the positive drug to measure the stability of the entire system.
2.3. Pulmonary Function Testing
After anesthetizing and securing the mice, the area was disinfected with alcohol, and an incision was made in the neck using a surgical scalpel. Muscle tissue was carefully dissected with forceps to expose the trachea. A surgical suture was placed beneath the trachea, and a long needle with a tracheal cannula was inserted. The needle tip carefully punctured the trachea, and the cannula was slowly twisted into place. A surgical suture was used to secure the cannula to the trachea. The tracheal cannula was then connected to a plethysmograph (AniRes2005, Beijing BoiLab Co., Ltd., Beijing, China) to measure pulmonary function data while the mice were in a stable breathing state. The relevant operations were carried out according to the steps provided in the manual of the lung function instrument.
2.4. Histological and Immunohistochemical Analyses
During lung tissue collection, the right lung was ligated with surgical sutures, and 4% paraformaldehyde was infused through the trachea to inflate the left lung. The left lung, along with the trachea, were then excised and immersed in 4% paraformaldehyde for 48 h for fixation. Subsequent procedures included dehydration and paraffin embedding. Paraffin sections with a thickness of 4 μm were prepared and placed in an oven at 60 °C for 2 h to ensure it can be used to start dyeing.
The HE staining procedure was conducted according to the manufacturer’s instructions (G1120, Beijing Solarbio Science & Technology Co., Ltd., Beijing, China). The Masson staining procedure was conducted according to the manufacturer’s instructions (G1340, Beijing Solarbio Science & Technology Co., Ltd.). Lung tissue sections were sequentially immersed in xylene, absolute ethanol, 95% ethanol, and distilled water for dewaxing. The staining process followed the protocol of hematoxylin for 5 min, distilled water for 5 min, 75% ethanol for 2 s, 95% ethanol for 2 min, and eosin for 3 min. The sections were then sequentially immersed in 95% ethanol, absolute ethanol, and xylene for transparency, and finally, mounted in neutral resin for preservation.
During the immunohistochemistry experiment, lung tissue sections were sequentially immersed in xylene, absolute ethanol, 95% ethanol, and distilled water for dewaxing. An antigen retrieval solution was prepared, and the sections were subjected to boiling for 2 min. After rinsing with PBS and removing the excess liquid, the sections were incubated with an endogenous peroxidase blocking agent at room temperature for 10 min. Following another PBS rinse and the removal of excess liquid, the sections were incubated with a non-specific blocking agent for 10 min. After removing the liquid from the sections, they were incubated with the primary antibody overnight at 4 °C. The primary antibody was then collected, and the sections were washed with PBST, with the excess liquid removed, before being incubated with a biotinylated secondary antibody at room temperature for 10 min. After another PBST rinse and the removal of excess liquid, the sections were incubated with streptavidin-horseradish peroxidase at room temperature for 10 min. Following a PBST rinse, freshly prepared DAB was used to incubate the sections for 3–5 min before discarding it. The sections were then rinsed with distilled water and counterstained with hematoxylin for approximately 10 s. Finally, the sections were sequentially immersed in 95% ethanol, absolute ethanol, and xylene for transparency, and mounted in neutral resin for preservation.
2.5. Hydroxyproline Assay
The right lung of the mouse was isolated and placed at the bottom of a 5 mL glass vial, which was then dried in a 120 °C oven for 18 h. Subsequently, 2 mL of 6 M hydrochloric acid was added, and the vial was tightly sealed to prevent the evaporation of the hydrochloric acid, followed by heating in a 120 °C oven for another 18 h. After that, 2 mL of 6 M sodium hydroxide solution was added, the mixture was filtered through a 0.45 µm filter, and the pH was adjusted to between 6.8 and 7.4 before diluting to a final volume of 10 mL.
A standard curve was established using hydroxyproline standard solutions ranging from 0.625 µg/mL to 10 µg/mL. For the prepared standard solutions, 400 µL was transferred to an Eppendorf tube, while 50 µL of the sample to be measured was added to another Eppendorf tube, followed by the addition of 350 µL of ultrapure water. Subsequently, 200 µL of chloramine T was added to each tube, and the mixture was vortexed and allowed to sit at room temperature for 20 min. After that, 200 µL of HClO4 was added to each tube, which was vortexed and left at room temperature for an additional 5 min. Next, 200 µL of P-DMAB was added, followed by vortexing and an incubation in a 50 °C water bath for 20 min to produce a red compound. After cooling, 200 µL was taken from each Eppendorf tube and transferred to a 96-well plate to measure the absorbance at 577 nm.
2.6. Cell Culture, Transfection, and Treatment
NIH-3T3 cells and MLg cells were purchased from the ATCC cell bank. Both cell types were cultured in complete RPMI 1640 medium supplemented with 10% FBS (10099-141, Gibco, GrandIsland, NY, USA).
Cells in the culture plates were transfected when they reached 80–90% confluence. Ten microliters of siRNA (10 µM) was added to 250 µL of Opti-MEM (1897175, Gibco) and gently mixed; then, 5 µL of transfection reagent (40802ES, Yeasen Biotechnology (Shanghai) Co., Ltd., Shanghai, China) was added to another 250 µL of Opti-MEM and incubated at room temperature for 5 min. The two solutions were then combined and gently mixed, followed by a 20 min incubation at room temperature to allow the formation of RNA–lipid complexes. The prepared complexes were uniformly added to different regions of each well, and the culture plates were gently agitated. After 6 h, the medium was replaced with fresh culture medium or treatment with drugs was initiated. Two pairs of siRNAs targeting the FAP-α mRNA were selected for RNA interference in NIH-3T3 cells. The expression levels of FAP-α were detected using Western blot analysis, and the pair of siRNAs with the most effective interference was chosen for subsequent experiments, with the sequences being the sense strand 5′-GAACCAGGAGCAUGUAGAA-3′ and the antisense strand 5′-UUCUACAUGCUCCUGGUUC-3′.
2.7. Rapid and Sensitive Detection of Cell Proliferation and Cytotoxicity
A suspension of the passaged cells was obtained, counted, and diluted to a concentration of 7.5 × 104 cells/mL. The suspension was mixed well and 100 µL was added to each well of a 96-well plate. After drug treatment of the cells for the expected duration, 10 µL of Cell Counting Kit-8 (CCK-8) reagent (C0005, Topscience Co., Ltd., Rizhao, China) was added to each well of the 96-well plate, and the mixture was thoroughly mixed to avoid bubble formation. The 96-well plate was then incubated in a cell culture incubator for 1 to 4 h, followed by shaking for 1 min. The absorbance at 450 nm was measured using a microplate reader.
2.8. Wound Healing Assay
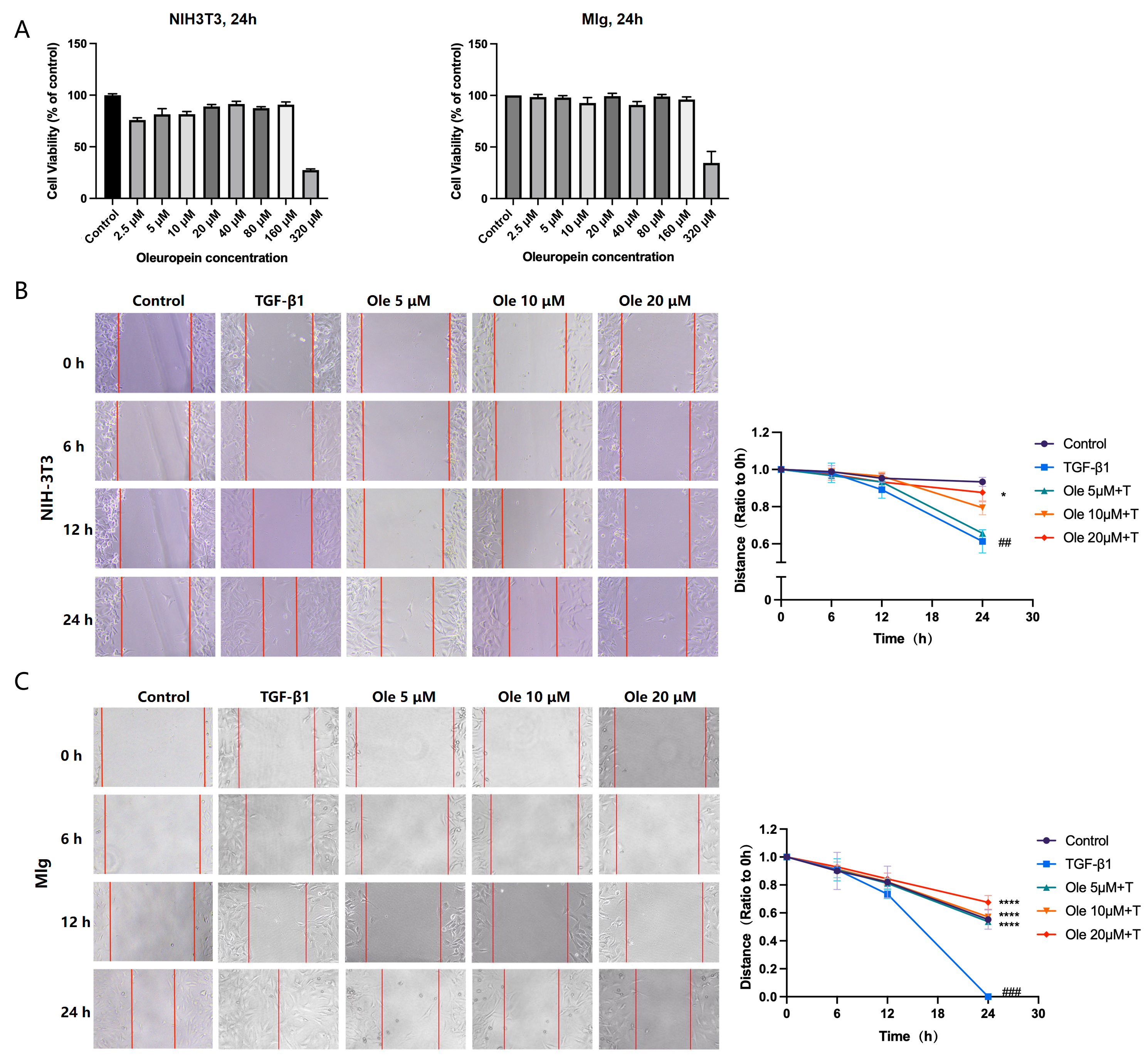
Primary lung fibroblasts were seeded in plates. Before inflicting the wound, the cells were fully confluent. A scratch was made in the center of the culture well using a sterile 200 μL micropipette tip. The wounds were observed using an inverted optical microscope, and multiple images were obtained at areas flanking the intersections of the wound and the marker lines after the scratch at 0, 12, and 24 h. Images were obtained for analysis using Image-Pro Plus software 6.0.
2.9. Quantitative Real-Time PCR
For cell samples, after drug treatment, the original medium in the cell culture plate was discarded, and the cells were washed with PBS and aspirated. Then, 1 mL of Trizol (10606ES60, Yeasen Biotechnology (Shanghai) Co., Ltd.) was added to each well, and the cells were transferred to Eppendorf tubes by pipetting. For tissue samples, lung tissue was minced in Eppendorf tubes, and 0.4 mL of Trizol was added, followed by multiple rounds of homogenization and ultrasonic disruption on ice, with Trizol brought up to a final volume of 1 mL. Chloroform was added to the Trizol in the sample tubes, mixed by vortexing for 30 s, allowed to sit at room temperature for 3 min, and then centrifuged at 12,000 rpm for 15 min at 4 °C. At this stage, the solution separated into three layers. The upper aqueous phase, approximately 390 µL, was carefully transferred to a new Eppendorf tube, and an equal volume of isopropanol was added, mixed gently, and allowed to sit at room temperature for 10 min. The mixture was then centrifuged at 12,000 rpm for 10 min at 4 °C, and the supernatant was discarded. To the white pellet, 1 mL of 75% ethanol was added, followed by centrifugation at 7000 rpm for 5 min at 4 °C. This step was repeated once more, with the supernatant being carefully aspirated using a white pipette tip during the second round. The pellet was dried for 20 min to allow for complete evaporation of the ethanol, and then 10–20 µL of RNase-free H2O was added to dissolve the RNA. After sitting at room temperature for 10 min to ensure complete dissolution, the RNA concentration was measured.
Reverse transcription was performed according to the instructions of the FastKing gDNA Dispelling RT SuperMix (KR118, TIANGEN, Beijing, China) kit, setting up a 20 µL reaction system. After establishing the system, the reaction was carried out at 42 °C for 15 min followed by 95 °C for 3 min.
A 20 µL reaction system was set up according to the instructions of the Hieff
® qPCR SYBR Green Master Mix (No Rox) (11201ES03, Yeasen Biotechnology (Shanghai) Co., Ltd.). The reaction program was as follows: 5 min of pre-denaturation at 95 °C, followed by 10 s of denaturation at 95 °C, 20 s of annealing at 60 °C, and 20 s of extension at 72 °C, for a total of 45 cycles. The primer sequences used in the experiment are listed in
Table 1.
2.10. Western Blotting
First, prepare an ice box and perform the subsequent operations on ice. For the cell culture plates after drug treatment, discard the original culture medium and rinse with PBS, then aspirate. To each well of a six-well plate, add 70 µL of RIPA lysis buffer (1% NaF, 1% PMSF), scrape the cells repeatedly with a pre-chilled cell scraper, and transfer the lysate to an Eppendorf tube. The cells are then subjected to multiple rounds of ultrasonic disruption. The samples are centrifuged at 12,000 rpm for 10 min at 4 °C. After centrifugation, the supernatant is carefully collected into a new Eppendorf tube, and the protein concentration is determined using the BCA assay. Based on the volume of the collected supernatant, add 5× loading buffer and mix thoroughly by pipetting. Heat the protein samples at 100 °C for 7 min.
Mouse lung tissues were dissected and separated, then snap-frozen in liquid nitrogen, and subsequently stored at −80 °C. A portion of the lung tissue was minced and then mixed with RIPA lysis buffer (1% NaF, 1% PMSF), followed by multiple rounds of tissue homogenization and ultrasonic disruption. The samples were centrifuged at 12,000 rpm for 10 min at 4 °C, with subsequent operations performed in accordance with the protocol for total protein extraction from cells.
In a 96-well plate, standard curve wells and sample wells were designed. The procedure involved sequentially adding PBS diluent, standard protein, or sample protein, and BCA working solution. The plate was incubated at 37 °C for 30 min, and the absorbance was measured at 562 nm.
Based on the protein concentration, the loading amount was calculated, with 30 µg of sample added to each well, flanked by pre-stained proteins on both sides. Appropriate-sized PVDF membranes were cut and activated in methanol. On the transfer membrane apparatus, layers of sponge, filter paper, gel, PVDF membrane, filter paper, and sponge were placed in order. After clamping, the assembly was inserted into the transfer tank. Transfer was conducted at 100 V for 150 min, ensuring that the transfer tank was placed in an ice-water bath. A TBST solution containing 5% non-fat milk was prepared and used to cover the PVDF membrane for a 1 h incubation.
Primary antibodies against collagen I (AF7001, Affinity), α-SMA (AF1032, Affinity), Fibronectin (AF0738, Affinity), P-Smad2 (Ser467) (AF3449, Affinity), Smad2 (AF6449, Affinity), P-Smad3 (Ser423 + Ser425) (AF8315, Affinity), Smad3 (AF6362, Affinity), SQSTM1/p62 (AF5384, Affinity), Beclin 1 (AF5128, Affinity), cleaved Caspase 3 (Asp175) (AF7022, Affinity), LC3A/B (D3U4C) (12741T, Cell Signaling Technology), cleaved Caspase 3 (Asp175) (AF7022, Affinity), Caspase 3/p17/p19 (19677-1-AP, Proteintech), Caspase 9 (AF6348, Affinity), cleaved Caspase 9 (Asp353) (AF5240, Affinity), P-mTOR (Ser2448) (5536S, Cell Signaling Technology), mTOR (2983S, Cell Signaling Technology), P-S6 Ribosomal Protein (Ser235/236) (4858S, Cell Signaling Technology), S6 Ribosomal Protein (AF6354, Affinity), P-ULK1 (Ser757) (14202T, Cell Signaling Technology), ULK1 (8054T, Cell Signaling Technology), P-p70 S6 Kinase (Thr389) (9234T, Cell Signaling Technology), p70 S6 Kinase (AF6226, Affinity), and FAP (66562, Cell Signaling Technology) were prepared according to the instruction manual (typically at a dilution of 1:1000) and dissolved in 5% non-fat milk. The PVDF membrane was incubated with the primary antibody overnight at 4 °C. After removal from 4 °C, the primary antibody was allowed to warm to room temperature before being collected, and the membrane was washed with TBST. Secondary antibodies were selected based on the origin of the primary antibody and incubated with the membrane at room temperature for 2 h. Following the collection of the secondary antibody, the membrane was washed with TBST, and detection reagent was added. Imaging was performed using a chemiluminescence apparatus (Beijing Sage Creation Science Co., Ltd., Beijing, China), and gray scale analysis was conducted using Image J 1.8.0 software.
2.11. Flow Cytometry
After drug exposure, the culture plates were collected, and the procedure was carried out according to the instructions of the apoptosis detection kit (CA1020, Beijing Solarbio Science & Technology Co., Ltd.). For adherent cells, the cell culture medium was collected into a centrifuge tube, and trypsin (without EDTA) was added to digest the cells. The previously collected culture medium was injected into the corresponding wells, and the cells were blown and collected into the centrifuge tube. The samples were centrifuged at 1000 rpm for 5 min, and the supernatant was carefully aspirated to avoid disturbing the cells. The cells were resuspended in 1 mL of pre-cooled PBS at 4 °C and centrifuged again to remove the supernatant. The cells were then resuspended in binding buffer, adjusting the concentration to 1–5 × 106 cells/mL. A volume of 100 µL of the cell suspension was transferred to a 5 mL flow cytometry tube, and 5 µL of Annexin V/FITC was added and mixed well, followed by a 5 min incubation at room temperature, avoiding light exposure. Then, 5 µL of propidium iodide (PI) was added, followed by the addition of 400 µL of PBS. The wavelengths for FITC and PI were selected, and the flow cytometry analysis was conducted immediately.
2.12. Molecular Docking
The crystal structure of the target protein FAP-α (ID: 1Z68, Organism: Homo sapiens) was downloaded from the Protein Data Bank website, and the molecular structure of oleuropein (Compound CID: 5281544) was downloaded from the PubChem website. Molecular docking was performed using AutoDock Tools 4.2.6 software, following the sequence of ligand preparation, receptor preparation, grid file setup, and docking file configuration (using the Genetic Algorithm). The docking results were obtained and analyzed accordingly. The specific interactions between oleuropein and the target were examined using PyMOL 3.1.0 software.
2.13. Statistical Analysis
The research data are presented as means ± SDs and analyzed using GraphPad Prism 9 software. Differences between the control group and the model group, as well as between the drug treatment group and the model group, were evaluated using one-way ANOVA, with p < 0.05 considered statistically significant. Cell experiments were repeated three times, and the sample size for animal experiments was no less than five subjects.
4. Discussion
The lung interstitium is the supportive connective tissue and spaces surrounding the bronchi or blood vessels, alveolar septa, and under the visceral pleura, comprising the alveolar septa, lobular septa, and the peripheral tissues of bronchi or vessels. Idiopathic pulmonary fibrosis (IPF) is a chronic, irreversible, and deadly lung disease characterized by scarring and thickening of pulmonary interstitial tissue, leading to respiratory difficulty and ultimately respiratory failure. The histological features of IPF are defined by a usual interstitial pneumonia (UIP) pattern, which includes honeycombing in the lower lobes and subpleural regions, as well as foci of myofibroblasts located within more densely fibrotic areas, with an increase in proliferative type II alveolar epithelial cells and a decrease in type I alveolar epithelial cells [
14,
15]. Currently, only pirfenidone and nintedanib have been approved for the treatment of IPF, and while they demonstrate some efficacy in slowing the decline in lung function over a year, their limited effectiveness and serious side effects indicate a continued urgent need for new drugs in the IPF field.
Oleuropein is widely distributed in the stems, leaves, and fruits of Oleaceae plants, such as
Olea europaea L. var, and exhibits various pharmacological activities, including anti-cancer, antioxidant, anti-inflammatory, cardiovascular-protective, neuroprotective, antiviral, skin-protective, and anti-aging effects [
16,
17]. Studies have shown that oleuropein exhibits anti-fibrotic effects on animal models of liver fibrosis arising from non-alcoholic steatohepatitis and cardiac fibrosis due to diabetes, although its impact on pulmonary fibrosis has been less frequently reported [
9,
10,
11,
12,
13]. In respiratory diseases, oleuropein has been shown to suppress lung inflammation in animal models of asthma and chronic obstructive pulmonary disease (COPD) and provides protective effects on acute lung injury through its anti-inflammatory and antioxidant properties [
11,
12,
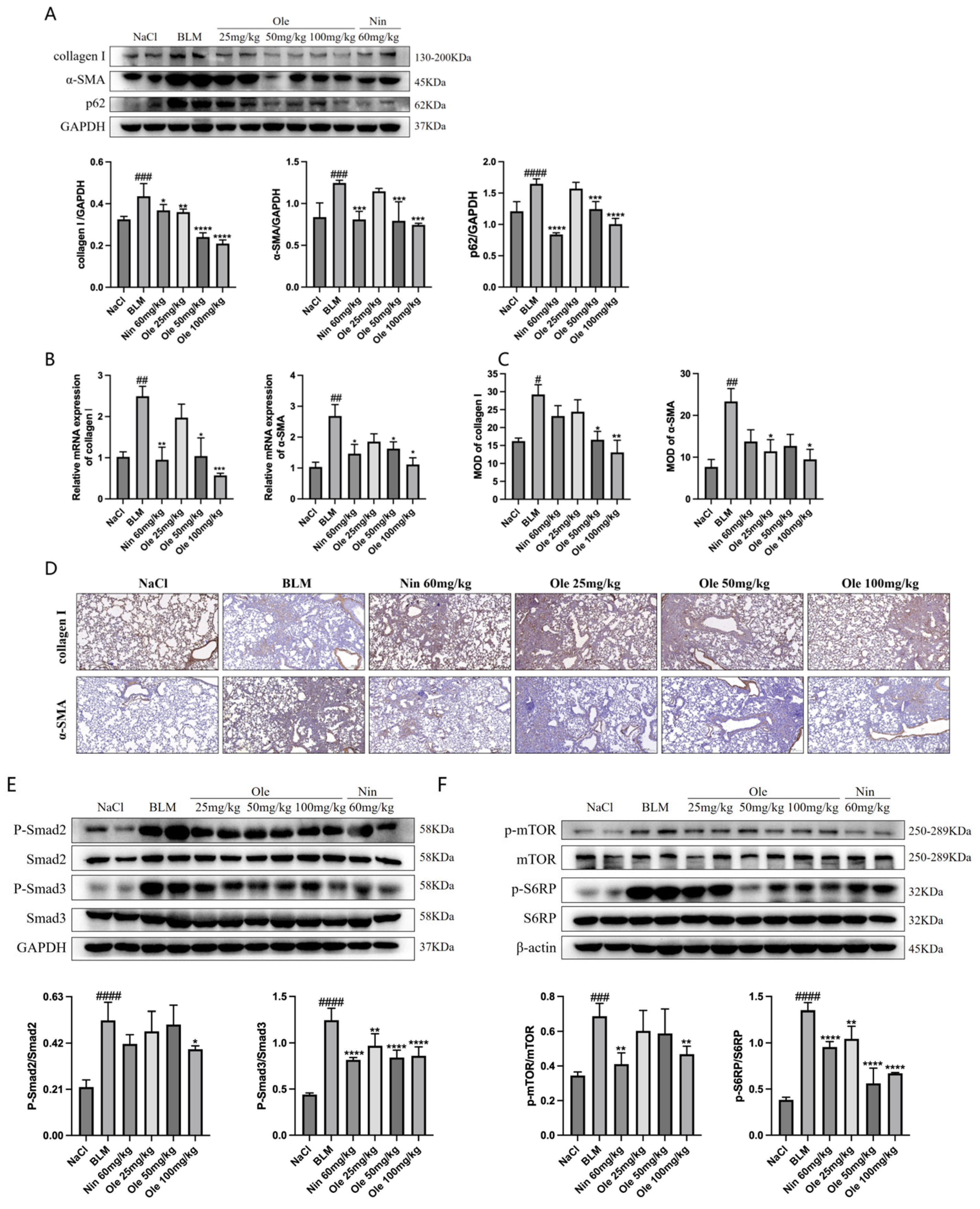
13]. Early screenings in our study demonstrated that oleuropein significantly inhibited the expression of α-SMA in fibroblasts induced by TGF-β1. In a bleomycin-induced mouse model of pulmonary fibrosis, oleuropein treatment dose-dependently reduced hydroxyproline levels in lung tissue and the proportion of lung fibrotic area, improved lung tissue pathological features, and significantly alleviated weight loss and pulmonary function impairment in mice.
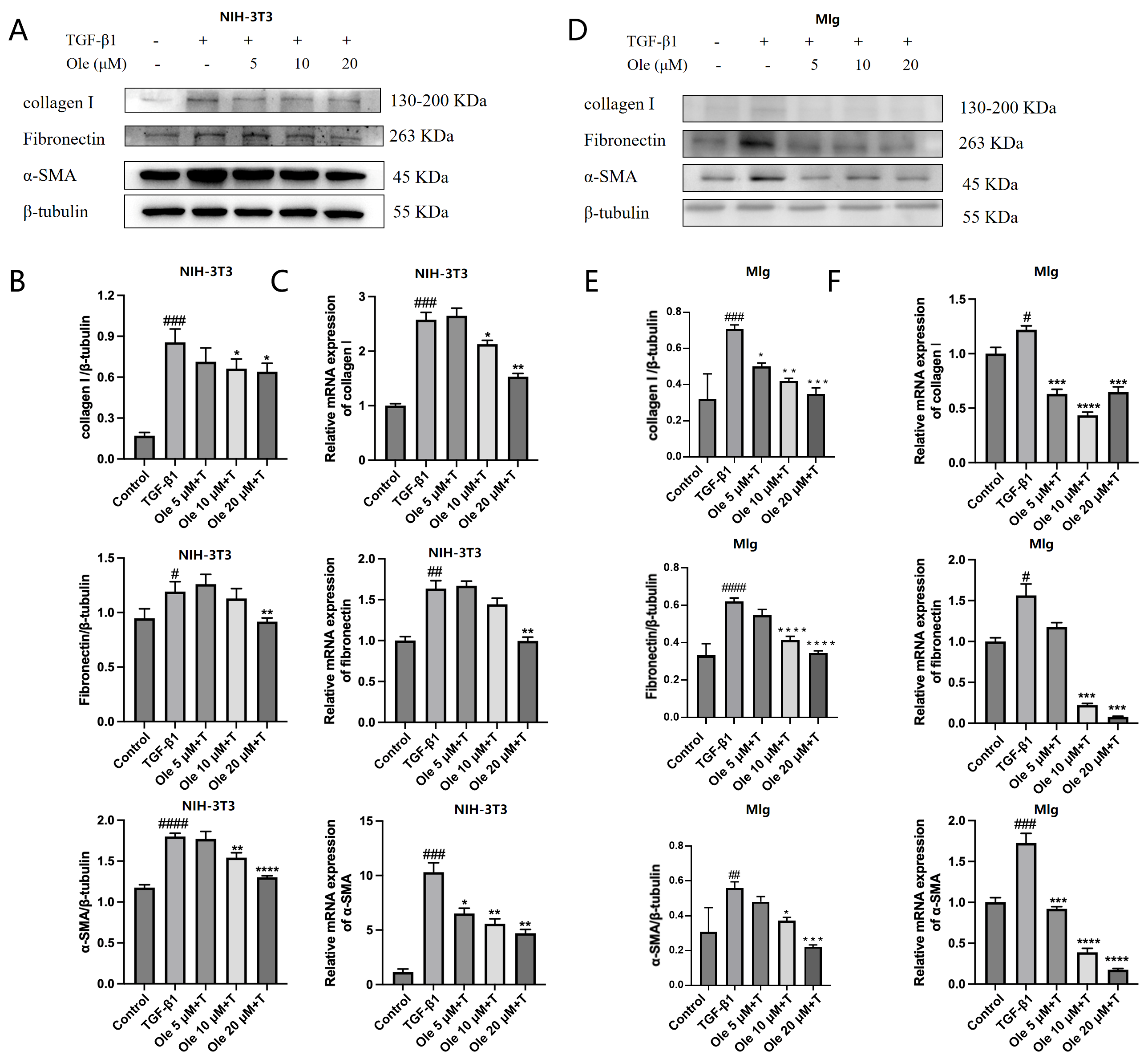
This study assessed the activation of fibroblasts to myofibroblasts using three markers: α-SMA, collagen I, and fibronectin. Various types of collagen and fibronectin are important extracellular matrix (ECM) proteins expressed by myofibroblasts. A prominent feature of myofibroblasts is the new expression of α-SMA in stress fibers, which serves as the molecular basis for their high contractile activity. During normal wound healing, myofibroblasts arise through various pathways, primarily through the differentiation of fibroblasts, and are regulated by factors such as TGF-β, WNT, fibronectin, and tissue stiffness. TGF-β is the principal growth factor guiding the formation of myofibroblasts, as it directly induces the production of extracellular matrix (ECM) and the expression of α-SMA. During the fibrotic process, TGF-β regulates multiple downstream signaling pathways, including the Smad3, PI3K/AKT, and p38 MAPK pathways, which promote the transdifferentiation of fibroblasts into myofibroblasts [
18,
19]. Notably, the overexpression of Smad3 enhances the production of α-SMA and ECM proteins by fibroblasts.
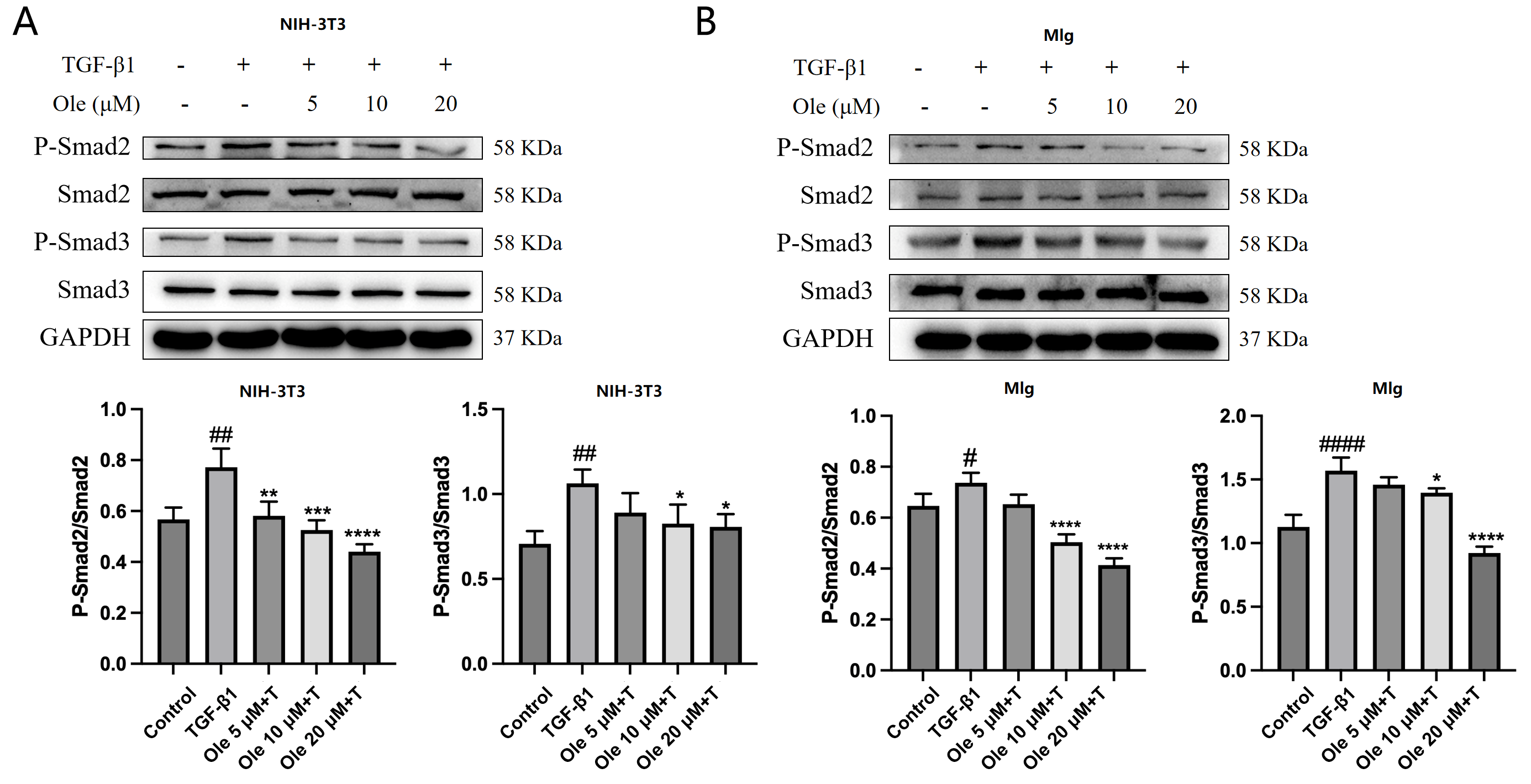
In vitro studies in this research demonstrated that oleuropein dose-dependently inhibited the TGF-β1-induced migration of fibroblasts and significantly suppressed the upregulation of the activation markers α-SMA, collagen I, and fibronectin at both the transcript and protein expression levels. Additionally, oleuropein dose-dependently inhibited the phosphorylation of Smad2 and Smad3 in TGF-β1-induced fibroblasts, indicating its inhibitory effect on the TGF-β1/Smad signaling pathway. Thus, oleuropein inhibits fibroblast migration, activation, and ECM deposition by suppressing the TGF-β1/Smad pathway.
Research also indicates that TGF-β can reduce autophagy by inhibiting the expression of several autophagy-related proteins (ATGs), including ATG 5 and ATG 7, as well as p62, in normal pulmonary fibroblasts [
20]. The inhibition of autophagy through the knockout of LC3B and ATG 5 was found to increase the expression of myofibroblast markers in fibroblasts from patients with idiopathic pulmonary fibrosis (IPF) [
21]. The fibroblasts from IPF patients naturally exhibit a sustained reduction in autophagy, which is associated with a propensity for fibrosis [
22]. Fibronectin, a core component of the extracellular matrix (ECM), primarily regulates cellular activities through interactions with integrin receptors on the cell surface. In fibroblasts, the binding of fibronectin to integrins can mediate fibroblast survival, whereas the absence of this binding can lead to autophagy. Studies have shown that fibronectin can inhibit autophagy by activating the AKT/mTOR signaling pathway [
23].
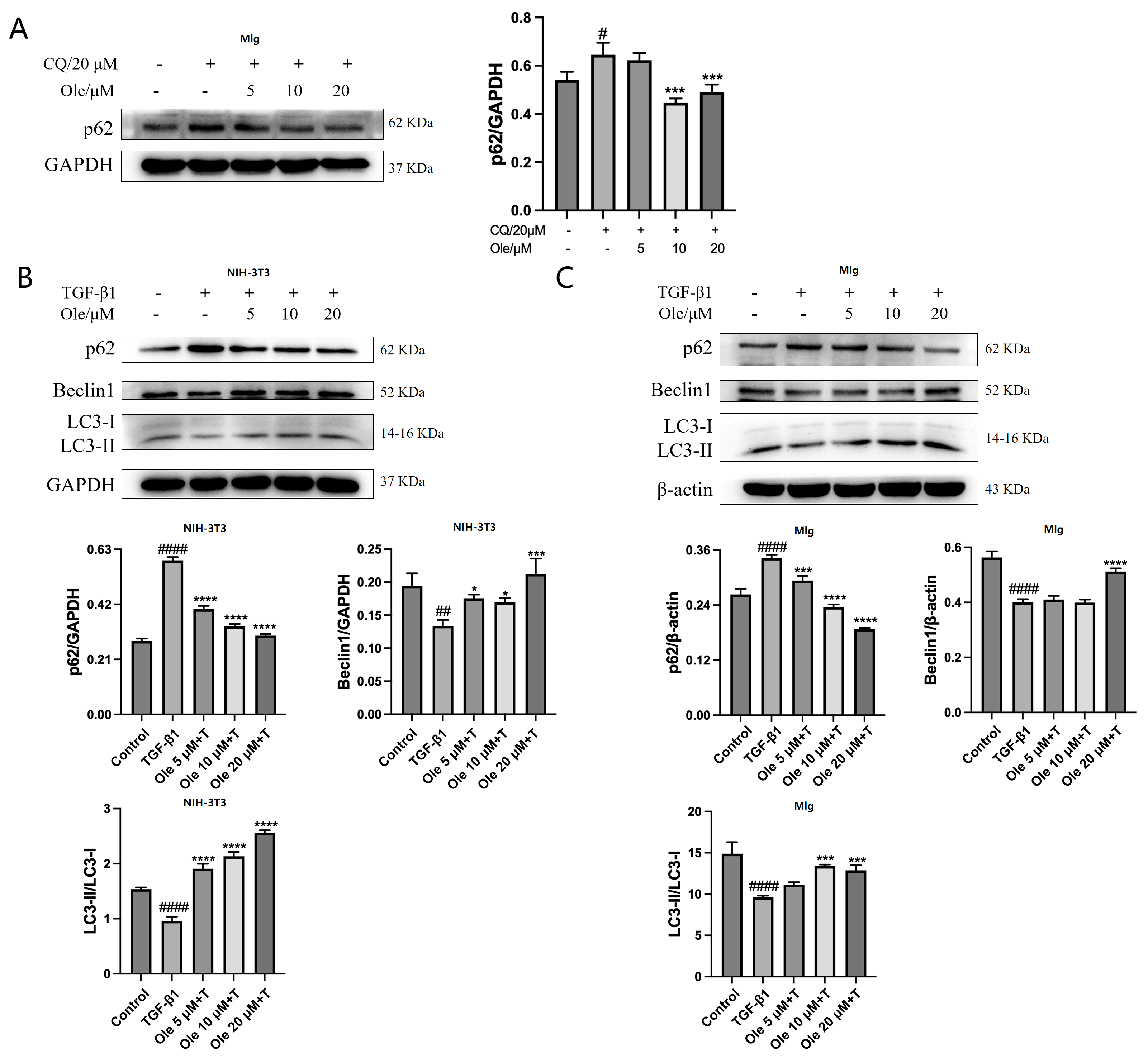
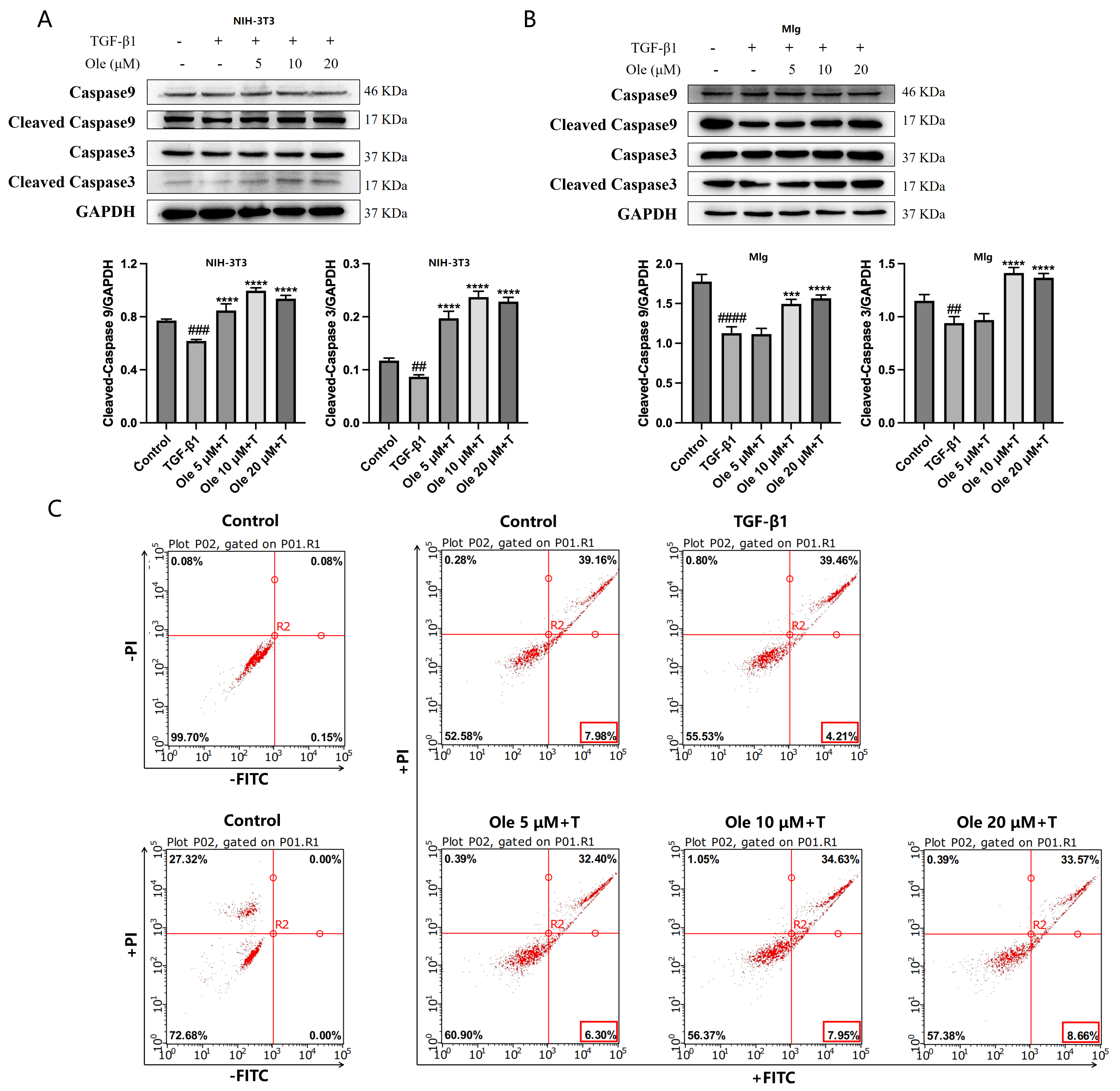
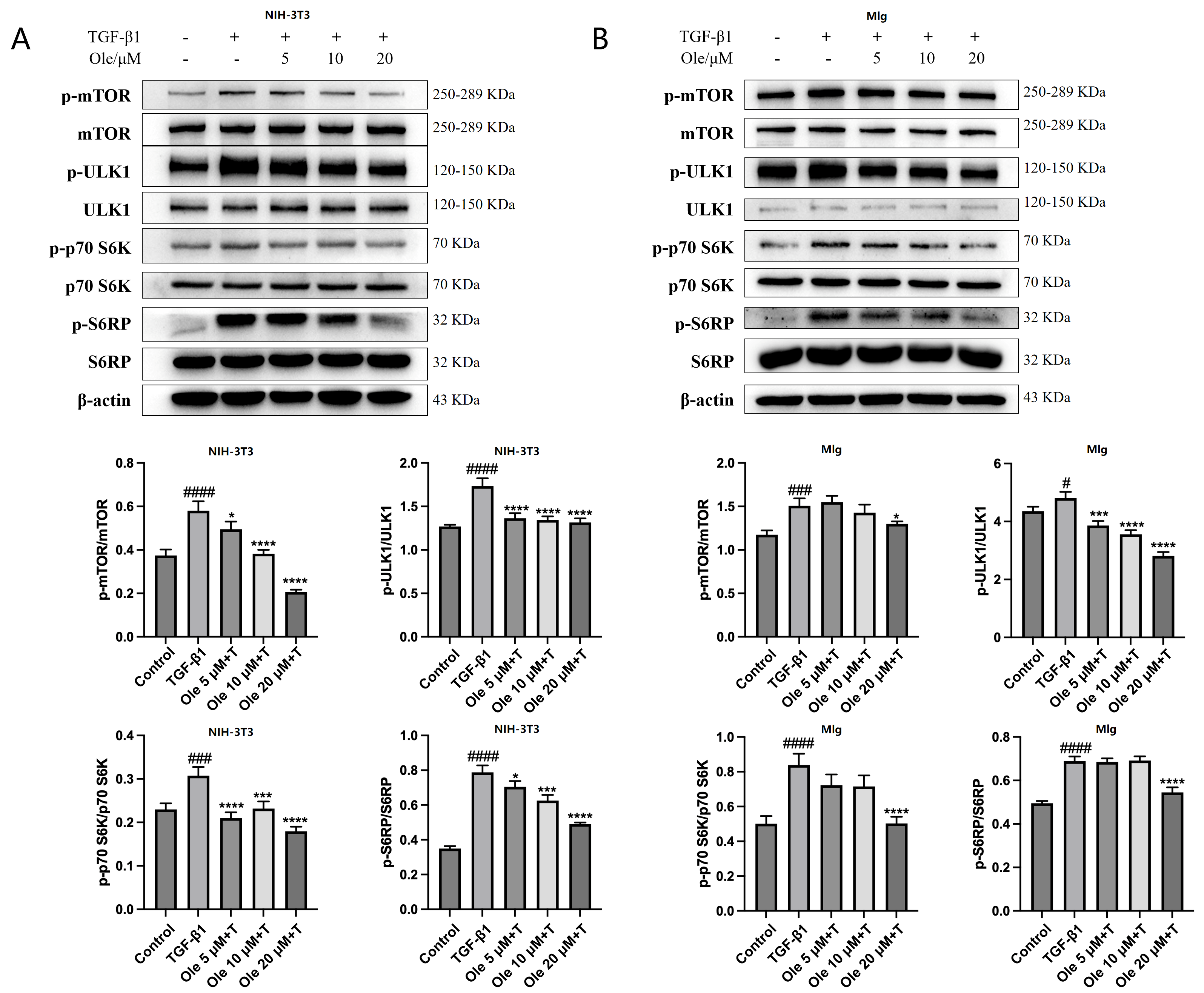
The in vitro studies conducted in this project demonstrated that oleuropein dose-dependently reduced p62 levels and increased the Beclin1 level and LC3-II/LC3-I ratio, indicating that oleuropein effectively improved TGF-β1-induced autophagy dysfunction in fibroblasts. Furthermore, oleuropein also dose-dependently elevated the protein levels of cleaved Caspase 9 and cleaved Caspase 3, as well as increased the proportion of early apoptotic cells in the Annexin V-FITC/PI apoptosis assay, suggesting that oleuropein alleviated TGF-β1-induced apoptotic resistance in fibroblasts. Additionally, oleuropein dose-dependently decreased the phosphorylation levels of mTOR, ULK1, p70S6K, and S6RP in fibroblasts, thereby inhibiting the TGF-β1/mTOR pathway. Consequently, oleuropein improves autophagic suppression and apoptotic resistance in fibroblasts during pulmonary fibrosis through the modulation of the TGF-β1/mTOR pathway.
FAP-α is a protein characterized by an α/β hydrolase domain and a β-spiral structure, playing a role in the fibrotic process. The levels of FAP-α regulate collagen integrity and ECM processing [
24,
25]. Typically absent in normal tissues, FAP-α is selectively expressed during tissue remodeling and repair, with its inhibition linked to cellular reprogramming and functional restoration [
26]. The expression of FAP-α is associated with TGF-β signaling and inflammatory pathways, although the mechanisms of its inhibition remain unclear and may involve estrogen signaling and specific microRNAs [
27,
28,
29]. Studies also indicate that the transcription of the FAP-α gene is regulated by various transcription factors, and its interaction with the chaperone protein BAG6/BAT3 may influence its degradation [
30]. In pathological tissues, elevated expression of FAP-α is typically detrimental; thus, inhibiting FAP-α is considered beneficial for the treatment of inflammatory diseases such as metabolic syndrome, cancer, and fibrosis [
24,
25].
Molecular docking studies have demonstrated that FAP-α exhibits a strong binding affinity for oleuropein, with a binding energy of −7.19. In vitro knockout of FAP-α using siRNAs indicates that oleuropein inhibits the activation of fibroblasts by targeting the TGF-β1/Smad pathway through the suppression of FAP-α. Additionally, oleuropein enhances fibroblast autophagy and apoptosis via the TGF-β1/mTOR pathway, which is also mediated by FAP-α. FAP-α activity exhibits pro-fibrogenic functions and acts as a regulator of cell apoptosis, adhesion, and migration, independent of its enzymatic activity; however, there are currently no reports detailing how FAP-α influences the regulation of the TGF-β1/Smad and TGF-β1/mTOR pathways. It remains unclear whether FAP-α directly regulates the proteins within these pathways or exerts an indirect effect, and the specific regulatory mechanisms warrant further investigation [
31,
32,
33].
In this study, we explored the therapeutic effects of oleuropein on a bleomycin-induced mouse model of pulmonary fibrosis. The results showed that oleuropein effectively alleviated weight loss and lung pathological changes caused by fibrosis, significantly reducing the fibrotic area and the tissue hydroxyproline content, while also improving lung function. These findings provide important experimental evidence for the potential of oleuropein as a therapeutic agent for pulmonary fibrosis. Further pharmacological studies, both in vitro and in vivo, indicated that oleuropein inhibits fibroblast migration, activation, and extracellular matrix deposition by suppressing the TGF-β1/Smad pathway. Additionally, oleuropein improves the suppression of autophagy and apoptotic resistance in fibroblasts by inhibiting the TGF-β1/mTOR pathway. This mechanism of action was further validated through molecular docking and in vitro studies, demonstrating a high affinity between oleuropein and FAP-α, with a binding energy of −7.19. The research suggests that oleuropein, by inhibiting FAP-α, not only acts on the TGF-β1/Smad pathway to suppress fibroblast activation but also enhances autophagy and apoptosis in fibroblasts through FAP-α.
A notable constraint of this investigation lies in its dependence on the bleomycin-induced pulmonary fibrosis model. Although this approach provides a robust platform for assessing therapeutic potential, it may not entirely recapitulate the intricate pathophysiology and phenotypic diversity observed in IPF or other clinical variants of the disease. Moreover, the relatively short treatment and observation windows could impede a comprehensive evaluation of the long-term therapeutic outcomes and potential adverse effects. This study’s focus on predefined endpoints may also have overlooked certain mechanistic or secondary biological responses associated with the interventions. Finally, while the sample size was adequate for initial hypothesis testing, it may limit the broader applicability and statistical power of the findings.