Role of Tpm Isoforms Produced by the TPM4 Gene in the Regulation of Actin Filament Dynamics by Cofilin
Abstract
1. Introduction
2. Materials and Methods
2.1. Protein Expression and Purification
2.2. Protein Labeling and Preparation for Fluorescence Applications
2.3. Viscosity Measurements
2.4. Actin Polymerization Assay
2.5. Binding of Cofilin to F-Actin and Displacement of Tropomyosin from Actin Surface
2.6. Rhodamin–Phalloidin Fluorescence Assay
2.7. Determination of F-Actin Lengths by Electron Microscopy
3. Results
3.1. Cofilin Severing and Depolymerizing Activity Determined Through Viscosity Measurements
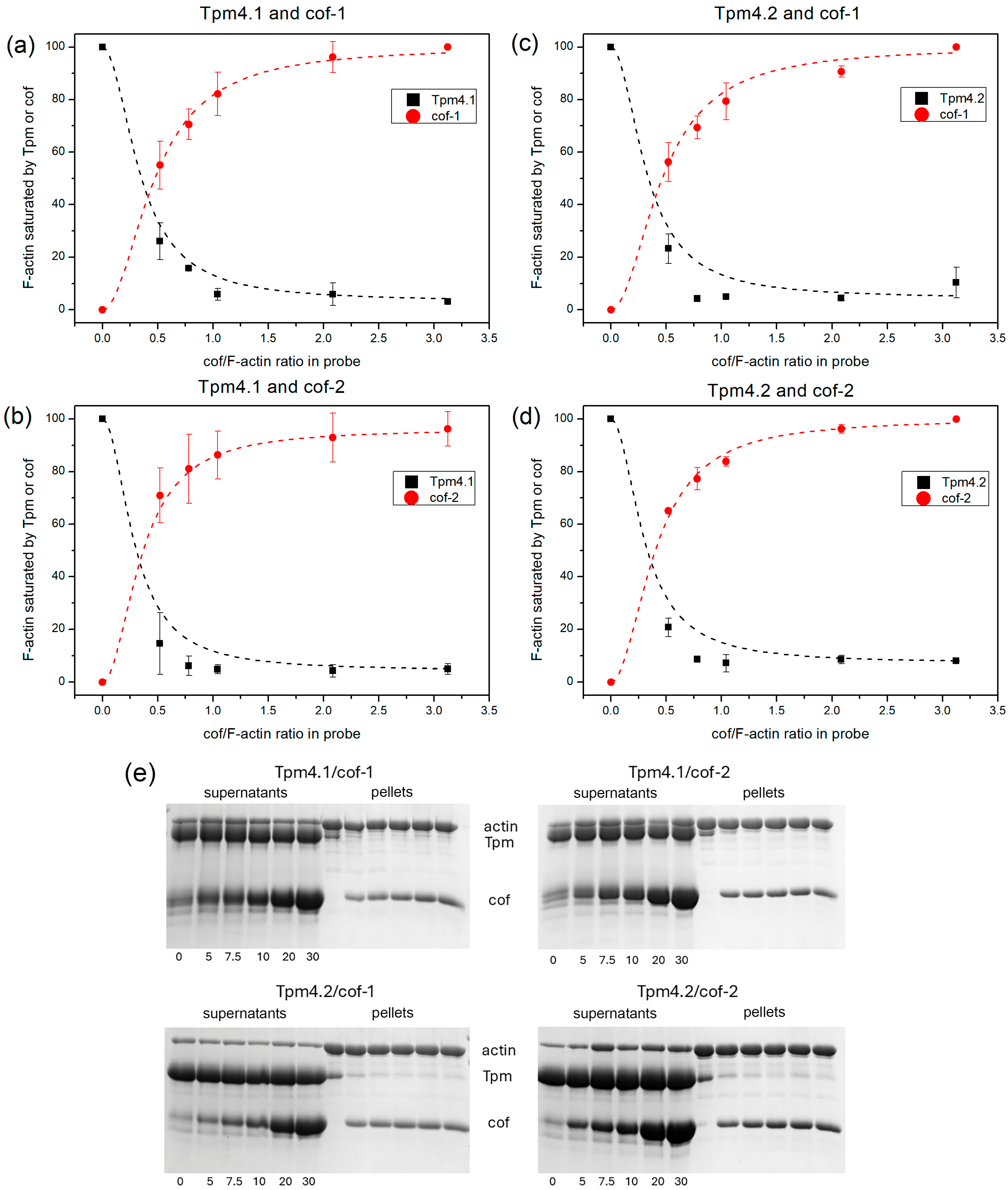
3.2. Binding of Cofilin to F-Actin and Displacement of Tpm from Actin Surface
3.3. Rhodamine–Phalloidin Decay Induced by Cofilins
3.4. Determination of Actin Filaments’ Lengths Through Electron Microscopy Experiments
3.5. Effect of Tropomyosin and Cofilin on Actin Polymerization Rate
4. Discussion
4.1. Effects of Different Cofilin Isoforms on F-Actin Covered by Tpm4.1 and Tpm4.2
4.2. Determination of Cofilin’s Function by Tpm4.1 and Tpm4.2 in Comparison with Other Tpm Isoforms
4.3. Potential Role of Tpm4.1 and Tpm4.2’s Interaction with Cofilin Isoforms
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HMW | High molecular weight |
| LMW | Low molecular weight |
| cof-1 | Cofilin-1 |
| cof-2 | Cofilin-2 |
| Tpm | Tropomyosin |
References
- Koestler, S.A.; Rottner, K.; Lai, F.; Block, J.; Vinzenz, M.; Small, J.V. F- and G-Actin Concentrations in Lamellipodia of Moving Cells. PLoS ONE 2009, 4, e4810. [Google Scholar] [CrossRef]
- Blanchoin, L.; Boujemaa-Paterski, R.; Sykes, C.; Plastino, J. Actin Dynamics, Architecture, and Mechanics in Cell Motility. Physiol. Rev. 2014, 94, 235–263. [Google Scholar] [CrossRef] [PubMed]
- Pollard, T.D.; Cooper, J.A. Actin, a Central Player in Cell Shape and Movement. Science 2009, 326, 1208–1212. [Google Scholar] [CrossRef] [PubMed]
- Pelham, R.J.; Chang, F. Actin Dynamics in the Contractile Ring during Cytokinesis in Fission Yeast. Nature 2002, 419, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Katsuta, H.; Sokabe, M.; Hirata, H. From Stress Fiber to Focal Adhesion: A Role of Actin Crosslinkers in Force Transmission. Front. Cell Dev. Biol. 2024, 12, 1444827. [Google Scholar] [CrossRef]
- Gefen, A.; Weihs, D. Mechanical Cytoprotection: A Review of Cytoskeleton-Protection Approaches for Cells. J. Biomech. 2016, 49, 1321–1329. [Google Scholar] [CrossRef]
- Dong, Y.; Quan, C. NPFs-Mediated Actin Cytoskeleton: A New Viewpoint on Autophagy Regulation. Cell Commun. Signal. 2024, 22, 111. [Google Scholar] [CrossRef]
- Ivanov, A.I.; Lechuga, S.; Marino-Melendez, A.; Naydenov, N.G. Unique and Redundant Functions of Cytoplasmic Actins and Nonmuscle Myosin II Isoforms at Epithelial Junctions. Ann. N. Y. Acad. Sci. 2022, 1515, 61–74. [Google Scholar] [CrossRef]
- Schevzov, G.; Curthoys, N.M.; Gunning, P.W.; Fath, T. Functional Diversity of Actin Cytoskeleton in Neurons and Its Regulation by Tropomyosin. In International Review of Cell and Molecular Biology; Elsevier: Amsterdam, The Netherlands, 2012; Volume 298, pp. 33–94. ISBN 978-0-12-394309-5. [Google Scholar]
- Gao, J.; Nakamura, F. Actin-Associated Proteins and Small Molecules Targeting the Actin Cytoskeleton. Int. J. Mol. Sci. 2022, 23, 2118. [Google Scholar] [CrossRef]
- Carlier, M.-F.; Shekhar, S. Global Treadmilling Coordinates Actin Turnover and Controls the Size of Actin Networks. Nat. Rev. Mol. Cell Biol. 2017, 18, 389–401. [Google Scholar] [CrossRef]
- Toshima, J.Y.; Horikomi, C.; Okada, A.; Hatori, M.N.; Nagano, M.; Masuda, A.; Yamamoto, W.; Siekhaus, D.E.; Toshima, J. Srv2/CAP Is Required for Polarized Actin Cable Assembly and Patch Internalization during Clathrin-Mediated Endocytosis. J. Cell Sci. 2015, 129, 367–379. [Google Scholar] [CrossRef]
- Rzadzinska, A.K.; Schneider, M.E.; Davies, C.; Riordan, G.P.; Kachar, B. An Actin Molecular Treadmill and Myosins Maintain Stereocilia Functional Architecture and Self-Renewal. J. Cell Biol. 2004, 164, 887–897. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, P.; Chatterton, P.; Ikeda, A.; Ikeda, S.; Corey, D.P.; Ervasti, J.M.; Perrin, B.J. Length Regulation of Mechanosensitive Stereocilia Depends on Very Slow Actin Dynamics and Filament-Severing Proteins. Nat. Commun. 2015, 6, 6855. [Google Scholar] [CrossRef]
- Lees, J.G.; Bach, C.T.T.; O’Neill, G.M. Interior Decoration: Tropomyosin in Actin Dynamics and Cell Migration. Cell Adhes. Migr. 2011, 5, 181–186. [Google Scholar] [CrossRef]
- Tokuraku, K.; Kuragano, M.; Uyeda, T.Q.P. Long-Range and Directional Allostery of Actin Filaments Plays Important Roles in Various Cellular Activities. Int. J. Mol. Sci. 2020, 21, 3209. [Google Scholar] [CrossRef]
- Bugyi, B.; Carlier, M.-F. Control of Actin Filament Treadmilling in Cell Motility. Annu. Rev. Biophys. 2010, 39, 449–470. [Google Scholar] [CrossRef]
- Bamburg, J.R. Proteins of the ADF/Cofilin Family: Essential Regulators of Actin Dynamics. Annu. Rev. Cell Dev. Biol. 1999, 15, 185–230. [Google Scholar] [CrossRef]
- De La Cruz, E.M. How Cofilin Severs an Actin Filament. Biophys. Rev. 2009, 1, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Suarez, C.; Roland, J.; Boujemaa-Paterski, R.; Kang, H.; McCullough, B.R.; Reymann, A.-C.; Guérin, C.; Martiel, J.-L.; De La Cruz, E.M.; Blanchoin, L. Cofilin Tunes the Nucleotide State of Actin Filaments and Severs at Bare and Decorated Segment Boundaries. Curr. Biol. 2011, 21, 862–868. [Google Scholar] [CrossRef]
- Ostrowska-Podhorodecka, Z.; Śliwinska, M.; Reisler, E.; Moraczewska, J. Tropomyosin Isoforms Regulate Cofilin 1 Activity by Modulating Actin Filament Conformation. Arch. Biochem. Biophys. 2020, 682, 108280. [Google Scholar] [CrossRef] [PubMed]
- McGough, A.; Pope, B.; Chiu, W.; Weeds, A. Cofilin Changes the Twist of F-Actin: Implications for Actin Filament Dynamics and Cellular Function. J. Cell Biol. 1997, 138, 771–781. [Google Scholar] [CrossRef]
- McCullough, B.R.; Grintsevich, E.E.; Chen, C.K.; Kang, H.; Hutchison, A.L.; Henn, A.; Cao, W.; Suarez, C.; Martiel, J.-L.; Blanchoin, L.; et al. Cofilin-Linked Changes in Actin Filament Flexibility Promote Severing. Biophys. J. 2011, 101, 151–159. [Google Scholar] [CrossRef]
- Prochniewicz, E.; Janson, N.; Thomas, D.D.; De La Cruz, E.M. Cofilin Increases the Torsional Flexibility and Dynamics of Actin Filaments. J. Mol. Biol. 2005, 353, 990–1000. [Google Scholar] [CrossRef]
- McCullough, B.R.; Blanchoin, L.; Martiel, J.-L.; De La Cruz, E.M. Cofilin Increases the Bending Flexibility of Actin Filaments: Implications for Severing and Cell Mechanics. J. Mol. Biol. 2008, 381, 550–558. [Google Scholar] [CrossRef]
- Galkin, V.E.; Orlova, A.; Kudryashov, D.S.; Solodukhin, A.; Reisler, E.; Schröder, G.F.; Egelman, E.H. Remodeling of Actin Filaments by ADF/Cofilin Proteins. Proc. Natl. Acad. Sci. USA 2011, 108, 20568–20572. [Google Scholar] [CrossRef] [PubMed]
- Carlier, M.-F.; Laurent, V.; Santolini, J.; Melki, R.; Didry, D.; Xia, G.-X.; Hong, Y.; Chua, N.-H.; Pantaloni, D. Actin Depolymerizing Factor (ADF/Cofilin) Enhances the Rate of Filament Turnover: Implication in Actin-Based Motility. J. Cell Biol. 1997, 136, 1307–1322. [Google Scholar] [CrossRef]
- Blanchoin, L.; Pollard, T.D. Interaction of Actin Monomers with AcanthamoebaActophorin (ADF/Cofilin) and Profilin. J. Biol. Chem. 1998, 273, 25106–25111. [Google Scholar] [CrossRef]
- Dedova, I.V.; Nikolaeva, O.P.; Mikhailova, V.V.; Dos Remedios, C.G.; Levitsky, D.I. Two Opposite Effects of Cofilin on the Thermal Unfolding of F-Actin: A Differential Scanning Calorimetric Study. Biophys. Chem. 2004, 110, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Pavlov, D.; Muhlrad, A.; Cooper, J.; Wear, M.; Reisler, E. Actin Filament Severing by Cofilin. J. Mol. Biol. 2007, 365, 1350–1358. [Google Scholar] [CrossRef]
- Kanellos, G.; Frame, M.C. Cellular Functions of the ADF/Cofilin Family at a Glance. J. Cell Sci. 2016, 129, 3211–3218. [Google Scholar] [CrossRef] [PubMed]
- Bamburg, J.R.; Bernstein, B.W. Actin Dynamics and Cofilin-actin Rods in Alzheimer Disease. Cytoskeleton 2016, 73, 477–497. [Google Scholar] [CrossRef]
- Vartiainen, M.K.; Mustonen, T.; Mattila, P.K.; Ojala, P.J.; Thesleff, I.; Partanen, J.; Lappalainen, P. The Three Mouse Actin-Depolymerizing Factor/Cofilins Evolved to Fulfill Cell-Type–Specific Requirements for Actin Dynamics. Mol. Biol. Cell 2002, 13, 183–194. [Google Scholar] [CrossRef]
- Thirion, C.; Stucka, R.; Mendel, B.; Gruhler, A.; Jaksch, M.; Nowak, K.J.; Binz, N.; Laing, N.G.; Lochmüller, H. Characterization of Human Muscle Type Cofilin (CFL2) in Normal and Regenerating Muscle. Eur. J. Biochem. 2001, 268, 3473–3482. [Google Scholar] [CrossRef] [PubMed]
- Kremneva, E.; Makkonen, M.H.; Skwarek-Maruszewska, A.; Gateva, G.; Michelot, A.; Dominguez, R.; Lappalainen, P. Cofilin-2 Controls Actin Filament Length in Muscle Sarcomeres. Dev. Cell 2014, 31, 215–226. [Google Scholar] [CrossRef]
- Ono, S.; Minami, N.; Abe, H.; Obinata, T. Characterization of a Novel Cofilin Isoform That Is Predominantly Expressed in Mammalian Skeletal Muscle. J. Biol. Chem. 1994, 269, 15280–15286. [Google Scholar] [CrossRef]
- Ohashi, K. Roles of Cofilin in Development and Its Mechanisms of Regulation. Dev. Growth Differ. 2015, 57, 275–290. [Google Scholar] [CrossRef]
- Bekker-Jensen, D.B.; Kelstrup, C.D.; Batth, T.S.; Larsen, S.C.; Haldrup, C.; Bramsen, J.B.; Sørensen, K.D.; Høyer, S.; Ørntoft, T.F.; Andersen, C.L.; et al. An Optimized Shotgun Strategy for the Rapid Generation of Comprehensive Human Proteomes. Cell Syst. 2017, 4, 587–599.e4. [Google Scholar] [CrossRef]
- Nakashima, K.; Sato, N.; Nakagaki, T.; Abe, H.; Ono, S.; Obinata, T. Two Mouse Cofilin Isoforms, Muscle-Type (MCF) and Non–Muscle Type (NMCF), Interact with F-Actin with Different Efficiencies. J. Biochem. 2005, 138, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Gunning, P.; O’Neill, G.; Hardeman, E. Tropomyosin-Based Regulation of the Actin Cytoskeleton in Time and Space. Physiol. Rev. 2008, 88, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Gunning, P.W.; Hardeman, E.C.; Lappalainen, P.; Mulvihill, D.P. Tropomyosin–Master Regulator of Actin Filament Function in the Cytoskeleton. J. Cell Sci. 2015, 128, 2965–2974. [Google Scholar] [CrossRef]
- Geeves, M.A.; Hitchcock-DeGregori, S.E.; Gunning, P.W. A Systematic Nomenclature for Mammalian Tropomyosin Isoforms. J. Muscle Res. Cell Motil. 2015, 36, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Bryce, N.S.; Schevzov, G.; Ferguson, V.; Percival, J.M.; Lin, J.J.-C.; Matsumura, F.; Bamburg, J.R.; Jeffrey, P.L.; Hardeman, E.C.; Gunning, P.; et al. Specification of Actin Filament Function and Molecular Composition by Tropomyosin Isoforms. Mol. Biol. Cell 2003, 14, 1002–1016. [Google Scholar] [CrossRef] [PubMed]
- Gallant, C.; Appel, S.; Graceffa, P.; Leavis, P.; Lin, J.J.-C.; Gunning, P.W.; Schevzov, G.; Chaponnier, C.; DeGnore, J.; Lehman, W.; et al. Tropomyosin Variants Describe Distinct Functional Subcellular Domains in Differentiated Vascular Smooth Muscle Cells. Am. J. Physiol.-Cell Physiol. 2011, 300, C1356–C1365. [Google Scholar] [CrossRef]
- Gunning, P.W.; Ghoshdastider, U.; Whitaker, S.; Popp, D.; Robinson, R.C. The Evolution of Compositionally and Functionally Distinct Actin Filaments. J. Cell Sci. 2015, 128, 2009–2019. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.J.-C.; Eppinga, R.D.; Warren, K.S.; McCrae, K.R. Human Tropomyosin Isoforms in the Regulation of Cytoskeleton Functions. Adv. Exp. Med. Biol. 2008, 644, 201–222. [Google Scholar] [CrossRef]
- Cagigas, M.L.; Ariotti, N.; Hook, J.; Rae, J.; Parton, R.G.; Bryce, N.S.; Gunning, P.W.; Hardeman, E.C. Single Molecule Visualization of Tropomyosin Isoform Organization in the Mammalian Actin Cytoskeleton. Cytoskeleton 2025, 82, 45–54. [Google Scholar] [CrossRef]
- Meiring, J.C.M.; Bryce, N.S.; Wang, Y.; Taft, M.H.; Manstein, D.J.; Liu Lau, S.; Stear, J.; Hardeman, E.C.; Gunning, P.W. Co-Polymers of Actin and Tropomyosin Account for a Major Fraction of the Human Actin Cytoskeleton. Curr. Biol. 2018, 28, 2331–2337.e5. [Google Scholar] [CrossRef]
- Ono, S.; Ono, K. Tropomyosin Inhibits ADF/Cofilin-Dependent Actin Filament Dynamics. J. Cell Biol. 2002, 156, 1065–1076. [Google Scholar] [CrossRef]
- Khaitlina, S.Y. Tropomyosin as a Regulator of Actin Dynamics. In International Review of Cell and Molecular Biology; Elsevier: Amsterdam, The Netherlands, 2015; Volume 318, pp. 255–291. ISBN 978-0-12-802279-5. [Google Scholar]
- Kuhn, T.B.; Bamburg, J.R. Tropomyosin and ADF/Cofilin as Collaborators and Competitors. Adv. Exp. Med. Biol. 2008, 644, 232–249. [Google Scholar] [CrossRef]
- Bernstein, B.W.; Bamburg, J.R. Tropomyosin Binding to F-actin Protects the F-actin from Disassembly by Brain Actin-depolymerizing Factor (ADF). Cell Motil. 1982, 2, 1–8. [Google Scholar] [CrossRef]
- Creed, S.J.; Desouza, M.; Bamburg, J.R.; Gunning, P.; Stehn, J. Tropomyosin Isoform 3 Promotes the Formation of Filopodia by Regulating the Recruitment of Actin-Binding Proteins to Actin Filaments. Exp. Cell Res. 2011, 317, 249–261. [Google Scholar] [CrossRef]
- Robaszkiewicz, K.; Ostrowska, Z.; Marchlewicz, K.; Moraczewska, J. Tropomyosin Isoforms Differentially Modulate the Regulation of Actin Filament Polymerization and Depolymerization by Cofilins. FEBS J. 2016, 283, 723–737. [Google Scholar] [CrossRef] [PubMed]
- Gateva, G.; Kremneva, E.; Reindl, T.; Kotila, T.; Kogan, K.; Gressin, L.; Gunning, P.W.; Manstein, D.J.; Michelot, A.; Lappalainen, P. Tropomyosin Isoforms Specify Functionally Distinct Actin Filament Populations In Vitro. Curr. Biol. 2017, 27, 705–713. [Google Scholar] [CrossRef]
- Ostrowska, Z.; Robaszkiewicz, K.; Moraczewska, J. Regulation of Actin Filament Turnover by Cofilin-1 and Cytoplasmic Tropomyosin Isoforms. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2017, 1865, 88–98. [Google Scholar] [CrossRef]
- Selvaraj, M.; Kokate, S.B.; Reggiano, G.; Kogan, K.; Kotila, T.; Kremneva, E.; DiMaio, F.; Lappalainen, P.; Huiskonen, J.T. Structural Basis Underlying Specific Biochemical Activities of Non-Muscle Tropomyosin Isoforms. Cell Rep. 2023, 42, 111900. [Google Scholar] [CrossRef]
- Robaszkiewicz, K.; Śliwinska, M.; Moraczewska, J. Regulation of Actin Filament Length by Muscle Isoforms of Tropomyosin and Cofilin. Int. J. Mol. Sci. 2020, 21, 4285. [Google Scholar] [CrossRef]
- Robaszkiewicz, K.; Wróbel, J.; Moraczewska, J. Troponin and a Myopathy-Linked Mutation in TPM3 Inhibit Cofilin-2-Induced Thin Filament Depolymerization. Int. J. Mol. Sci. 2023, 24, 16457. [Google Scholar] [CrossRef]
- Dube, D.K.; Dube, S.; Abbott, L.; Wang, J.; Fan, Y.; Alshiekh-Nasany, R.; Shah, K.K.; Rudloff, A.P.; Poiesz, B.J.; Sanger, J.M.; et al. Identification, Characterization, and Expression of Sarcomeric Tropomyosin Isoforms in Zebrafish. Cytoskeleton 2017, 74, 125–142. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Lim, S.; Schevzov, G.; Gunning, P.W.; Helfman, D.M. Loss of Tpm4.1 Leads to Disruption of Cell-Cell Adhesions and Invasive Behavior in Breast Epithelial Cells via Increased Rac1 Signaling. Oncotarget 2017, 8, 33544–33559. [Google Scholar] [CrossRef]
- Macwan, R.S.; Ferrero, G.; Pardini, B.; Naccarati, A.; Kozlowski, P.B.; Papetti, M.J. TPM4 Overexpression Drives Colon Epithelial Cell Tumorigenesis by Suppressing Differentiation and Promoting Proliferation. Neoplasia 2025, 59, 101093. [Google Scholar] [CrossRef] [PubMed]
- Suchowerska, A.K.; Fok, S.; Stefen, H.; Gunning, P.W.; Hardeman, E.C.; Power, J.; Fath, T. Developmental Profiling of Tropomyosin Expression in Mouse Brain Reveals Tpm4.2 as the Major Post-Synaptic Tropomyosin in the Mature Brain. Front. Cell. Neurosci. 2017, 11, 421. [Google Scholar] [CrossRef]
- Matyushenko, A.M.; Artemova, N.V.; Shchepkin, D.V.; Kopylova, G.V.; Bershitsky, S.Y.; Tsaturyan, A.K.; Sluchanko, N.N.; Levitsky, D.I. Structural and Functional Effects of Two Stabilizing Substitutions, D137L and G126R, in the Middle Part of A-tropomyosin Molecule. FEBS J. 2014, 281, 2004–2016. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, P.B.; Lataro, R.C.; Ferro, J.A.; Reinach, F.d.C. Functional Alpha-Tropomyosin Produced in Escherichia Coli. A Dipeptide Extension Can Substitute the Amino-Terminal Acetyl Group. J. Biol. Chem. 1994, 269, 10461–10466. [Google Scholar] [CrossRef]
- Pardee, J.D.; Aspudich, J. [18] Purification of Muscle Actin. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1982; Volume 85, pp. 164–181. ISBN 978-0-12-181985-9. [Google Scholar]
- Chou, S.Z.; Pollard, T.D. Cryo-Electron Microscopy Structures of Pyrene-Labeled ADP-Pi- and ADP-Actin Filaments. Nat. Commun. 2020, 11, 5897. [Google Scholar] [CrossRef] [PubMed]
- Marchenko, M.A.; Nefedova, V.V.; Yampolskaya, D.S.; Borzova, V.A.; Kleymenov, S.Y.; Nabiev, S.R.; Nikitina, L.V.; Matyushenko, A.M.; Levitsky, D.I. Comparative Structural and Functional Studies of Low Molecular Weight Tropomyosin Isoforms, the TPM3 Gene Products. Arch. Biochem. Biophys. 2021, 710, 108999. [Google Scholar] [CrossRef] [PubMed]
- Bobkov, A.A.; Muhlrad, A.; Shvetsov, A.; Benchaar, S.; Scoville, D.; Almo, S.C.; Reisler, E. Cofilin (ADF) Affects Lateral Contacts in F-Actin. J. Mol. Biol. 2004, 337, 93–104. [Google Scholar] [CrossRef]
- Marchenko, M.; Nefedova, V.; Artemova, N.; Kleymenov, S.; Levitsky, D.; Matyushenko, A. Structural and Functional Peculiarities of Cytoplasmic Tropomyosin Isoforms, the Products of TPM1 and TPM4 Genes. Int. J. Mol. Sci. 2021, 22, 5141. [Google Scholar] [CrossRef]
- Logvinov, A.S.; Nefedova, V.V.; Yampolskaya, D.S.; Kleymenov, S.Y.; Levitsky, D.I.; Matyushenko, A.M. Structural and Functional Properties of Tropomyosin Isoforms Tpm4.1 and Tpm2.1. Biochemistry 2023, 88, 801–809. [Google Scholar] [CrossRef]
- Muhlrad, A.; Ringel, I.; Pavlov, D.; Peyser, Y.M.; Reisler, E. Antagonistic Effects of Cofilin, Beryllium Fluoride Complex, and Phalloidin on Subdomain 2 and Nucleotide-Binding Cleft in F-Actin. Biophys. J. 2006, 91, 4490–4499. [Google Scholar] [CrossRef]
- Hitchcock-DeGregori, S.E.; Sampath, P.; Pollard, T.D. Tropomyosin Inhibits the Rate of Actin Polymerization by Stabilizing Actin Filaments. Biochemistry 1988, 27, 9182–9185. [Google Scholar] [CrossRef]
- Nishida, E.; Maekawa, S.; Sakai, H. Cofilin, a Protein in Porcine Brain That Binds to Actin Filaments and Inhibits Their Interactions with Myosin and Tropomyosin. Biochemistry 1984, 23, 5307–5313. [Google Scholar] [CrossRef] [PubMed]
- Umeki, N.; Hirose, K.; Uyeda, T.Q.P. Cofilin-Induced Cooperative Conformational Changes of Actin Subunits Revealed Using Cofilin-Actin Fusion Protein. Sci. Rep. 2016, 6, 20406. [Google Scholar] [CrossRef]
- Dedova, I.V.; Dedov, V.N.; Nosworthy, N.J.; Hambly, B.D.; Dos Remedios, C.G. Cofilin and DNase I Affect the Conformation of the Small Domain of Actin. Biophys. J. 2002, 82, 3134–3143. [Google Scholar] [CrossRef] [PubMed]
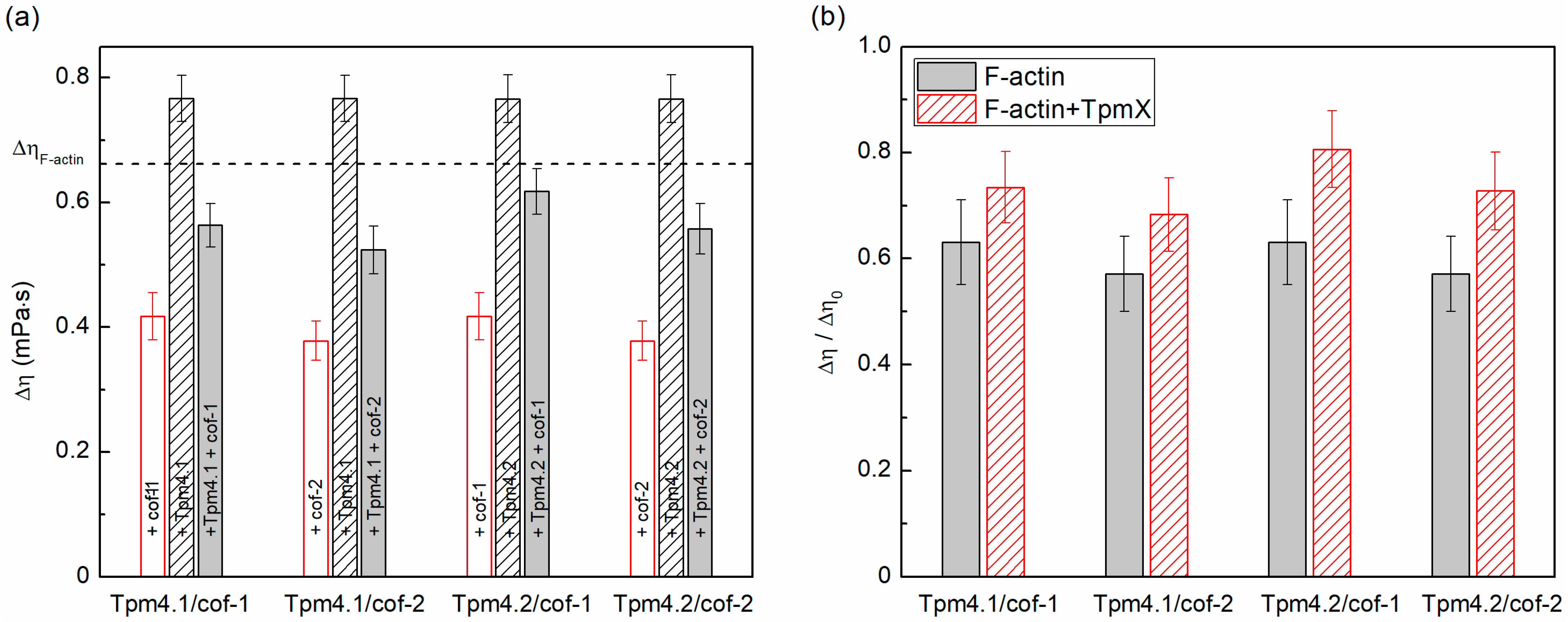



| W/o Cofilins | With cof-1 | With cof-2 | |||
|---|---|---|---|---|---|
| Δη01, mPa·s | Δη2, mPa·s | Δη/Δη0, % | Δη2, mPa·s | Δη/Δη0, % | |
| F-actin | 0.66 ± 0.04 | 0.42 ± 0.04 | 64 ± 8 | 0.38 ± 0.03 | 58 ± 7 |
| +Tpm4.1 | 0.77 ± 0.04 | 0.57 ± 0.03 | 74 ± 7 | 0.52 ± 0.04 | 68 ± 7 |
| +Tpm4.2 | 0.77 ± 0.04 | 0.62 ± 0.04 | 81 ± 7 | 0.56 ± 0.04 | 73 ± 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roman, S.G.; Nefedova, V.V.; Matyushenko, A.M. Role of Tpm Isoforms Produced by the TPM4 Gene in the Regulation of Actin Filament Dynamics by Cofilin. Biomolecules 2025, 15, 1206. https://doi.org/10.3390/biom15081206
Roman SG, Nefedova VV, Matyushenko AM. Role of Tpm Isoforms Produced by the TPM4 Gene in the Regulation of Actin Filament Dynamics by Cofilin. Biomolecules. 2025; 15(8):1206. https://doi.org/10.3390/biom15081206
Chicago/Turabian StyleRoman, Svetlana G., Victoria V. Nefedova, and Alexander M. Matyushenko. 2025. "Role of Tpm Isoforms Produced by the TPM4 Gene in the Regulation of Actin Filament Dynamics by Cofilin" Biomolecules 15, no. 8: 1206. https://doi.org/10.3390/biom15081206
APA StyleRoman, S. G., Nefedova, V. V., & Matyushenko, A. M. (2025). Role of Tpm Isoforms Produced by the TPM4 Gene in the Regulation of Actin Filament Dynamics by Cofilin. Biomolecules, 15(8), 1206. https://doi.org/10.3390/biom15081206






