Recent Advances in the Role of Fibroblast Growth Factors in Hair Follicle Growth
Abstract
1. Introduction
2. Hair Follicle
2.1. Hair Follicle Formation
2.2. Structure and Morphology of Hair Follicles
2.3. Classification of Hair Follicles
2.4. Periodic Changes in Hair Follicles
2.5. Cell Toxicity-Induced Hair Loss Mechanisms
3. Expression and Distribution of FGFs and Their Receptors in Hair Follicles
3.1. Expression and Distribution of FGFs in Hair Follicles
3.2. Expression and Distribution of FGF Receptors in Hair Follicles
4. The Regulatory Role of FGFs and FGFRs in Hair Follicle Cycles
5. Recent Research on FGFs Detected During Growth
5.1. FGFs That Are Found During Growth and Promote Hair Growth
5.2. FGFs That Are Found During Growth and Inhibit Hair Growth
6. Recent Research on FGFs Detected During Telogen
6.1. FGFs That Are Found During Telogen and Promote Hair Growth
6.2. FGFs That Are Found During Telogen and Inhibit Hair Growth
7. Recent Research on FGFs Detected in All Periods
8. Regulatory Roles of Other FGFs in the Hair Follicle Cycle
9. Regulation of FGF Signaling and Androgenic Alopecia
10. Conclusions and Perspectives
10.1. Conclusions
10.2. Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rabata, A.; Fedr, R.; Soucek, K.; Hampl, A.; Koledova, Z. 3D Cell Culture Models Demonstrate a Role for FGF and WNT Signaling in Regulation of Lung Epithelial Cell Fate and Morphogenesis. Front. Cell. Dev. Biol. 2020, 8, 574. [Google Scholar] [CrossRef]
- Weber, E.L.; Lai, Y.C.; Lei, M.; Jiang, T.X.; Chuong, C.M. Human Fetal Scalp Dermal Papilla Enriched Genes and the Role of R-Spondin-1 in the Restoration of Hair Neogenesis in Adult Mouse Cells. Front. Cell. Dev. Biol. 2020, 8, 583434. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Xu, Q.; Meng, X.; Liu, X.; Liu, J. Status of research on the development and regeneration of hair follicles. Int. J. Med. Sci. 2024, 21, 80–94. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.; Suh, W.; Sung, J.H. Hair Growth Regulation by Fibroblast Growth Factor 12 (FGF12). Int. J. Mol. Sci. 2022, 23, 9467. [Google Scholar] [CrossRef]
- Zhang, B.; He, X.; Guo, Y.; Zhao, S.; Lan, J.; Chen, F.; Wan, L.; Tian, H.; Xu, X. Human Fibroblast Growth Factor 9 Induces Hair Follicle Cycle Transition via TGF-beta/BMP/Smad Pathway. Clin. Cosmet. Investig. Dermatol. 2025, 18, 845–857. [Google Scholar] [CrossRef]
- Kim, J.; Song, S.Y.; Sung, J.H. Recent Advances in Drug Development for Hair Loss. Int. J. Mol. Sci. 2025, 26, 3461. [Google Scholar] [CrossRef]
- Zhou, S.Y.; Giang, N.N.; Kim, H.; Chien, P.N.; Le, L.T.T.; Trinh, T.T.; Nga, P.T.; Kwon, H.J.; Ham, J.R.; Lee, W.K.; et al. Assessing the efficacy of mesotherapy products: Ultra Exo Booster, and Ultra S Line Plus in hair growth: An ex vivo study. Ski. Res. Technol. 2024, 30, e13780. [Google Scholar] [CrossRef]
- Garcia, A.; Navarro, M.R.; Ramirez, A.; Pino, A.; Navarro, A.; Moles, I.; Gallego, E.; Anitua, E. Plasma Rich in Growth Factors as an Adjuvant Treatment for the Management of Frontal Fibrosing Alopecia: A Retrospective Observational Clinical Study. J. Cutan. Med. Surg. 2023, 27, 340–349. [Google Scholar] [CrossRef]
- Garg, S.; Thirumalaiswamy, A. Clinical and Trichoscopic Analysis of PRP Versus GFC Used for Male and Female Pattern Hair Loss. Dermatol. Surg. 2025, 10, 1097. [Google Scholar] [CrossRef]
- Castro, A.R.; Portinha, C.; Logarinho, E. The booming business of hair loss. Trends Biotechnol. 2023, 41, 731–735. [Google Scholar] [CrossRef]
- Tian, J.; Yao, G.; Tian, T.; Li, X.; Li, S.; Wu, C.; Zhang, S. Comparison of the efficacy and safety of different growth factors in the treatment of diabetic foot ulcers: An updated network meta-analysis. Front. Endocrinol. 2025, 16, 1614597. [Google Scholar] [CrossRef]
- Li, X.; Guo, F.; Deng, J.; Li, J.; Zhang, J.; Fu, M.; Fan, H. Leukocyte Platelet-Rich Plasma-Derived Exosomes Restrained Macrophages Viability and Induced Apoptosis, NO Generation, and M1 Polarization. Immun. Inflamm. Dis. 2024, 12, e70064. [Google Scholar] [CrossRef] [PubMed]
- Bakadia, B.M.; Qaed Ahmed, A.A.; Lamboni, L.; Shi, Z.; Mutu Mukole, B.; Zheng, R.; Pierre Mbang, M.; Zhang, B.; Gauthier, M.; Yang, G. Engineering homologous platelet-rich plasma, platelet-rich plasma-derived exosomes, and mesenchymal stem cell-derived exosomes-based dual-crosslinked hydrogels as bioactive diabetic wound dressings. Bioact. Mater. 2023, 28, 74–94. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, S.; Tehrani, L.; Koechle, B.; Chandramohan, P.; Hilburn, B.; Aoki, K.C.; Jacobs, R.J. A Scoping Review of Exosome Delivery Applications in Hair Loss. Cureus 2025, 17, e81152. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Seo, J.; Tu, S.; Nanmo, A.; Kageyama, T.; Fukuda, J. Exosomes for hair growth and regeneration. J. Biosci. Bioeng. 2024, 137, 1–8. [Google Scholar] [CrossRef]
- Lu, C.; Ding, Y.; Zhang, R.; Du, Y.; Bi, L.; Zhao, M.; Wang, C.; Wu, Q.; Jing, H.; Fan, W. Platelet-rich plasma-derived exosomes stimulate hair follicle growth through activation of the Wnt/beta-Catenin signaling pathway. Regen. Ther. 2025, 29, 435–446. [Google Scholar] [CrossRef]
- Rahman, E.; Sayed, K.; Rao, P.; Abu-Farsakh, H.; Sadeghi-Esfahlani, S.; Garcia, P.E.; Ioannidis, S.; Nassif, A.D.; Goodman, G.; Webb, W.R. Exosome Revolution or Marketing Mirage? AI-Based Multi-domain Evaluation of Claims, Scientific Evidence, Transparency, Public Sentiment, and Media Narratives. Aesthetic Plast. Surg. 2025, 49, 3454–3479. [Google Scholar] [CrossRef]
- Huang, X.; Zhao, P.; Zhang, G.; Su, X.; Li, H.; Gong, H.; Ma, X.; Liu, F. Application of Non-Pharmacologic Therapy in Hair Loss Treatment and Hair Regrowth. Clin. Cosmet. Investig. Dermatol. 2024, 17, 1701–1710. [Google Scholar] [CrossRef]
- Welle, M.M. Basic principles of hair follicle structure, morphogenesis, and regeneration. Vet. Pathol. 2023, 60, 732–747. [Google Scholar] [CrossRef]
- Zhao, J.; Lin, H.; Wang, L.; Guo, K.; Jing, R.; Li, X.; Chen, Y.; Hu, Z.; Gao, S.; Xu, N. Suppression of FGF5 and FGF18 Expression by Cholesterol-Modified siRNAs Promotes Hair Growth in Mice. Front. Pharmacol. 2021, 12, 666860. [Google Scholar] [CrossRef]
- Colin-Pierre, C.; El Baraka, O.; Danoux, L.; Bardey, V.; André, V.; Ramont, L.; Brézillon, S. Regulation of stem cell fate by HSPGs: Implication in hair follicle cycling. NPJ Regen. Med. 2022, 7, 77. [Google Scholar] [CrossRef]
- Tao, Y.; Yang, Q.; Wang, L.; Zhang, J.; Zhu, X.; Sun, Q.; Han, Y.; Luo, Q.; Wang, Y.; Guo, X.; et al. β-catenin activation in hair follicle dermal stem cells induces ectopic hair outgrowth and skin fibrosis. J. Mol. Cell Biol. 2019, 11, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Platt, S.; Lim, C.H.; Ito, M.; Myung, P. The development of hair follicles and nail. Dev. Biol. 2024, 513, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Klufa, J.; Bauer, T.; Hanson, B.; Herbold, C.; Starkl, P.; Lichtenberger, B.; Srutkova, D.; Schulz, D.; Vujic, I.; Mohr, T.; et al. Hair eruption initiates and commensal skin microbiota aggravate adverse events of anti-EGFR therapy. Sci. Transl. Med. 2019, 11, eaax2693. [Google Scholar] [CrossRef]
- Chen, J.K.; Wiedemann, J.; Nguyen, L.; Lin, Z.; Tahir, M.; Hui, C.C.; Plikus, M.V.; Andersen, B. IRX5 promotes DNA damage repair and activation of hair follicle stem cells. Stem Cell Rep. 2023, 18, 1227–1243. [Google Scholar] [CrossRef]
- Ge, W.; Zhang, W.; Zhang, Y.; Zheng, Y.; Li, F.; Wang, S.; Liu, J.; Tan, S.; Yan, Z.; Wang, L.; et al. A Single-cell Transcriptome Atlas of Cashmere Goat Hair Follicle Morphogenesis. Genom. Proteom. Bioinform. 2021, 19, 437–451. [Google Scholar] [CrossRef]
- Jiao, Q.; Wang, Y.R.; Zhao, J.Y.; Wang, Z.Y.; Guo, D.; Bai, W.L. Identification and molecular analysis of cashmere goat lncRNAs reveal their integrated regulatory network and potential roles in secondary hair follicle. Anim. Biotechnol. 2021, 32, 719–732. [Google Scholar] [CrossRef]
- Lv, X.; Chen, L.; He, S.; Liu, C.; Han, B.; Liu, Z.; Yusupu, M.; Blair, H.; Kenyon, P.; Morris, S.; et al. Effect of Nutritional Restriction on the Hair Follicles Development and Skin Transcriptome of Chinese Merino Sheep. Animals 2020, 10, 1058. [Google Scholar] [CrossRef]
- Yang, C.H.; Xu, J.H.; Ren, Q.C.; Duan, T.; Mo, F.; Zhang, W. Melatonin promotes secondary hair follicle development of early postnatal cashmere goat and improves cashmere quantity and quality by enhancing antioxidant capacity and suppressing apoptosis. J. Pineal Res. 2019, 67, e12569. [Google Scholar] [CrossRef]
- Li, K.N.; Tumbar, T. Hair follicle stem cells as a skin-organizing signaling center during adult homeostasis. Embo J. 2021, 40, e107135. [Google Scholar] [CrossRef]
- Meng, X.; Zheng, L.; Xiao, Y.; Ding, X.; Wang, K.; Kang, Y.J. A novel method for histological examination of hair follicles. Histochem. Cell Biol. 2022, 158, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Harshuk-Shabso, S.; Dressler, H.; Niehrs, C.; Aamar, E.; Enshell-Seijffers, D. Fgf and Wnt signaling interaction in the mesenchymal niche regulates the murine hair cycle clock. Nat. Commun. 2020, 11, 5114. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Xiang, F.; Guo, H.; Gong, H.; Li, Y. Reversibly immortalization establishes a hair follicle stem cell line with hair follicle reconstruction ability. Exp. Dermatol. 2024, 33, e14999. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Zhu, L.; He, J. Morphogenesis, Growth Cycle and Molecular Regulation of Hair Follicles. Front. Cell Dev. Biol. 2022, 10, 899095. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Y.X.; Xiong, Y.Y.; Li, Y.M. Pathogenesis and regenerative therapy in vitiligo and alopecia areata: Focus on hair follicle. Front. Med. 2024, 11, 1510363. [Google Scholar] [CrossRef]
- Ma, S.; Ji, D.; Wang, X.; Yang, Y.; Shi, Y.; Chen, Y. Transcriptomic Analysis Reveals Candidate Ligand-Receptor Pairs and Signaling Networks Mediating Intercellular Communication between Hair Matrix Cells and Dermal Papilla Cells from Cashmere Goats. Cells 2023, 12, 1645. [Google Scholar] [CrossRef]
- Hamida, O.B.; Kim, M.K.; Sung, Y.K.; Kim, M.K.; Kwack, M.H. Hair Regeneration Methods Using Cells Derived from Human Hair Follicles and Challenges to Overcome. Cells 2024, 14, 7. [Google Scholar] [CrossRef]
- Diao, X.; Yao, L.; Duan, T.; Qin, J.; He, L.; Zhang, W. Melatonin promotes the development of the secondary hair follicles by regulating circMPP5. J. Anim. Sci. Biotechnol. 2023, 14, 51. [Google Scholar] [CrossRef] [PubMed]
- Naeem, Z.; Zukunft, S.; Günther, S.; Liebner, S.; Weigert, A.; Hammock, B.D.; Frömel, T.; Fleming, I. Role of the soluble epoxide hydrolase in the hair follicle stem cell homeostasis and hair growth. Pflug. Arch. 2022, 474, 1021–1035. [Google Scholar] [CrossRef]
- Hinnant, T.; Lechler, T. Hair follicle stem cells feel the pressure. Cell Stem Cell 2022, 29, 1–2. [Google Scholar] [CrossRef]
- Adav, S.S.; Ng, K.W. Recent omics advances in hair aging biology and hair biomarkers analysis. Ageing Res. Rev. 2023, 91, 102041. [Google Scholar] [CrossRef]
- Lee, D.S.; Schrader, A.; Zou, J.; Ang, W.H.; Warchol, M.E.; Sheets, L. Direct targeting of mitochondria by cisplatin leads to cytotoxicity in zebrafish lateral-line hair cells. iScience 2024, 27, 110975. [Google Scholar] [CrossRef]
- Kearney, C.A.; Maguire, C.A.; Oza, V.S.; Oh, C.S.; Occidental, M.A.; Shapiro, J.; Orlow, S.J.; Glasser, C.L.; Lacouture, M.E.; Lakdawala, N.R.; et al. Alopecia in Children with Cancer: A Review from Pathophysiology to Management. Am. J. Clin. Dermatol. 2025. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.B.; Wang, W.H.; Hsu, Y.W.; Tee, S.Y.; Wu, Y.F.; Huang, W.Y.; Lai, S.F.; Lin, S.J. Hair Follicle Transit-Amplifying Cells Phagocytose Dead Cells after Radiotherapeutic and Chemotherapeutic Injuries for Timely Regeneration. J. Invest. Dermatol. 2024, 144, 243–251.e242. [Google Scholar] [CrossRef]
- Gezer, A.; Üstündağ, H.; Kılıç Baygutalp, N.; Erbaş, E.; Özkaraca, M. The Protective Effect of Gallic Acid Against Bisphenol A-Induced Ovarian Toxicity and Endocrine Disruption in Female Rats. J. Med. Food 2024, 27, 651–660. [Google Scholar] [CrossRef]
- Du, F.; Li, J.; Zhang, S.; Zeng, X.; Nie, J.; Li, Z. Oxidative stress in hair follicle development and hair growth: Signalling pathways, intervening mechanisms and potential of natural antioxidants. J. Cell Mol. Med. 2024, 28, e18486. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Liu, X.; Wang, Y.; Sui, Y.; Liu, F.; Liu, Z.; Zou, F.; Zuo, K.; Wang, Z.; Sun, W.; et al. Intrinsic ROS Drive Hair Follicle Cycle Progression by Modulating DNA Damage and Repair and Subsequently Hair Follicle Apoptosis and Macrophage Polarization. Oxid. Med. Cell Longev. 2022, 2022, 8279269. [Google Scholar] [CrossRef]
- Dong, T.R.; Li, Y.J.; Jin, S.Y.; Yang, F.L.; Xiong, R.X.; Dai, Y.Q.; Song, X.Z.; Guan, C.P. Progress on mitochondria and hair follicle development in androgenetic alopecia: Relationships and therapeutic perspectives. Stem Cell Res. Ther. 2025, 16, 44. [Google Scholar] [CrossRef]
- Lou, J.; Wu, F.; He, W.; Hu, R.; Cai, Z.; Chen, G.; Zhao, W.; Zhang, Z.; Si, Y. Hesperidin activates Nrf2 to protect cochlear hair cells from cisplatin-induced damage. Redox Rep. 2024, 29, 2341470. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Chen, X.; Ni, N.; Zhuang, C.; Yu, Z.; Xu, Z.; Li, Y.; Lin, C.; Huang, K. Corticotropin-releasing hormone inhibits autophagy by suppressing PTEN to promote apoptosis in dermal papilla cells. Ann. Med. 2025, 57, 2490823. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Su, N.; Yang, J.; Tan, Q.; Huang, S.; Jin, M.; Ni, Z.; Zhang, B.; Zhang, D.; Luo, F.; et al. FGF/FGFR signaling in health and disease. Signal Transduct. Target. Ther. 2020, 5, 181. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Bertolini, M.; Gherardini, J.; Keren, A.; Ponce, L.; Chéret, J.; Alenfall, J.; Dunér, P.; Nilsson, A.H.; Gilhar, A.; et al. An osteopontin-derived peptide inhibits human hair growth at least in part by decreasing fibroblast growth factor-7 production in outer root sheath keratinocytes. Br. J. Dermatol. 2020, 182, 1404–1414. [Google Scholar] [CrossRef] [PubMed]
- Kawano, M.; Komi-Kuramochi, A.; Asada, M.; Suzuki, M.; Oki, J.; Jiang, J.; Imamura, T. Comprehensive analysis of FGF and FGFR expression in skin: FGF18 is highly expressed in hair follicles and capable of inducing anagen from telogen stage hair follicles. J. Invest. Dermatol. 2005, 124, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Takabayashi, Y.; Nambu, M.; Ishihara, M.; Kuwabara, M.; Fukuda, K.; Nakamura, S.; Hattori, H.; Kiyosawa, T. Enhanced effect of fibroblast growth factor-2-containing dalteparin/protamine nanoparticles on hair growth. Clin. Cosmet. Investig. Dermatol. 2016, 9, 127–134. [Google Scholar] [CrossRef]
- Hébert, J.M.; Rosenquist, T.; Götz, J.; Martin, G.R. FGF5 as a regulator of the hair growth cycle: Evidence from targeted and spontaneous mutations. Cell 1994, 78, 1017–1025. [Google Scholar] [CrossRef]
- Suzuki, S.; Kato, T.; Takimoto, H.; Masui, S.; Oshima, H.; Ozawa, K.; Suzuki, S.; Imamura, T. Localization of rat FGF-5 protein in skin macrophage-like cells and FGF-5S protein in hair follicle: Possible involvement of two Fgf-5 gene products in hair growth cycle regulation. J. Invest. Dermatol. 1998, 111, 963–972. [Google Scholar] [CrossRef]
- Choi, N.; Shin, S.; Song, S.U.; Sung, J.H. Minoxidil Promotes Hair Growth through Stimulation of Growth Factor Release from Adipose-Derived Stem Cells. Int. J. Mol. Sci. 2018, 19, 691. [Google Scholar] [CrossRef]
- Hamada, K.; Ozawa, K.; Itami, S.; Yoshikawa, K. Human fibroblast growth factor 10 expression in dermal papilla cells, outer root sheath cells and keratinocytes. Exp. Dermatol. 1999, 8, 347–349. [Google Scholar]
- Lin, W.H.; Xiang, L.J.; Shi, H.X.; Zhang, J.; Jiang, L.P.; Cai, P.T.; Lin, Z.L.; Lin, B.B.; Huang, Y.; Zhang, H.L.; et al. Fibroblast growth factors stimulate hair growth through β-catenin and Shh expression in C57BL/6 mice. Biomed. Res. Int. 2015, 2015, 730139. [Google Scholar] [CrossRef]
- Gay, D.; Kwon, O.; Zhang, Z.; Spata, M.; Plikus, M.V.; Holler, P.D.; Ito, M.; Yang, Z.; Treffeisen, E.; Kim, C.D.; et al. Fgf9 from dermal γδ T cells induces hair follicle neogenesis after wounding. Nat. Med. 2013, 19, 916–923. [Google Scholar] [CrossRef]
- Imamura, T. Physiological functions and underlying mechanisms of fibroblast growth factor (FGF) family members: Recent findings and implications for their pharmacological application. Biol. Pharm. Bull. 2014, 37, 1081–1089. [Google Scholar] [CrossRef]
- Kimura-Ueki, M.; Oda, Y.; Oki, J.; Komi-Kuramochi, A.; Honda, E.; Asada, M.; Suzuki, M.; Imamura, T. Hair cycle resting phase is regulated by cyclic epithelial FGF18 signaling. J. Invest. Dermatol. 2012, 132, 1338–1345. [Google Scholar] [CrossRef]
- Nakatake, Y.; Hoshikawa, M.; Asaki, T.; Kassai, Y.; Itoh, N. Identification of a novel fibroblast growth factor, FGF-22, preferentially expressed in the inner root sheath of the hair follicle. Biochim. Biophys. Acta 2001, 1517, 460–463. [Google Scholar] [CrossRef]
- Jarosz, M.; Robbez-Masson, L.; Chioni, A.M.; Cross, B.; Rosewell, I.; Grose, R. Fibroblast growth factor 22 is not essential for skin development and repair but plays a role in tumorigenesis. PLoS ONE 2012, 7, e39436. [Google Scholar] [CrossRef]
- Bertin, A.C.J.; Vilarinho, A.; Junqueira, A.L.A. Fractional non-ablative laser-assisted drug delivery leads to improvement in male and female pattern hair loss. J. Cosmet. Laser Ther. 2018, 20, 391–394. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, R.; Shome, D. Intradermal injections of a hair growth factor formulation for enhancement of human hair regrowth—safety and efficacy evaluation in a first-in-man pilot clinical study. J. Cosmet. Laser Ther. 2018, 20, 369–379. [Google Scholar] [CrossRef]
- Ornitz, D.M.; Itoh, N. New developments in the biology of fibroblast growth factors. WIREs Mech. Dis. 2022, 14, e1549. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, S.; Du, C.; Wang, R.; Han, C.; Che, Y.; Feng, W.; Wang, C.; Gao, S.; Zhao, W. Novel PLCL nanofibrous/keratin hydrogel bilayer wound dressing for skin wound repair. Colloids Surf. B Biointerfaces 2023, 222, 113119. [Google Scholar] [CrossRef]
- Takahashi, R.; Takahashi, G.; Kameyama, Y.; Sato, M.; Ohtsuka, M.; Wada, K. Gender-Difference in Hair Length as Revealed by Crispr-Based Production of Long-Haired Mice with Dysfunctional FGF5 Mutations. Int. J. Mol. Sci. 2022, 23, 11855. [Google Scholar] [CrossRef]
- Jain, R.; De-Eknamkul, W. Potential targets in the discovery of new hair growth promoters for androgenic alopecia. Expert. Opin. Ther. Targets 2014, 18, 787–806. [Google Scholar] [CrossRef]
- Rosenquist, T.A.; Martin, G.R. Fibroblast growth factor signalling in the hair growth cycle: Expression of the fibroblast growth factor receptor and ligand genes in the murine hair follicle. Dev. Dyn. 1996, 205, 379–386. [Google Scholar] [CrossRef]
- Geyfman, M.; Plikus, M.V.; Treffeisen, E.; Andersen, B.; Paus, R. Resting no more: Re-defining telogen, the maintenance stage of the hair growth cycle. Biol. Rev. Camb. Philos. Soc. 2015, 90, 1179–1196. [Google Scholar] [CrossRef] [PubMed]
- Kawano, M.; Suzuki, S.; Suzuki, M.; Oki, J.; Imamura, T. Bulge- and basal layer-specific expression of fibroblast growth factor-13 (FHF-2) in mouse skin. J. Invest. Dermatol. 2004, 122, 1084–1090. [Google Scholar] [CrossRef] [PubMed]
- Mok, K.W.; Saxena, N.; Heitman, N.; Grisanti, L.; Srivastava, D.; Muraro, M.J.; Jacob, T.; Sennett, R.; Wang, Z.; Su, Y.; et al. Dermal Condensate Niche Fate Specification Occurs Prior to Formation and Is Placode Progenitor Dependent. Dev. Cell 2019, 48, 32–48.e35. [Google Scholar] [CrossRef] [PubMed]
- Diao, X.; Yao, L.; Wang, X.; Li, S.; Qin, J.; Yang, L.; He, L.; Zhang, W. Hair Follicle Development and Cashmere Traits in Albas Goat Kids. Animals 2023, 13, 617. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, W.D.; Zhong, Z.Y.; Zhou, X.B.; Shi, X.R.; Wang, X. FGF7 secreted from dermal papillae cell regulates the proliferation and differentiation of hair follicle stem cell1. J. Integr. Agric. 2023, 22, 1–28. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, L.; Zhang, Y.; Cao, M.; Wang, C.; Hu, A.; Cao, L.; Luo, Q.; You, Z.; Ma, X.; et al. FGF7 and FGF10 Promote Fate Transition of Human Epidermal Cell-derived Organoids to an Eccrine Gland Phenotype. Int. J. Biol. Sci. 2024, 20, 4162–4177. [Google Scholar] [CrossRef]
- Lee, C.Y.; Yang, C.Y.; Lin, C.C.; Yu, M.C.; Sheu, S.J.; Kuan, Y.H. Hair growth is promoted by BeauTop via expression of EGF and FGF-7. Mol. Med. Rep. 2018, 17, 8047–8052. [Google Scholar] [CrossRef]
- Hu, M.S.; Borrelli, M.R.; Hong, W.X.; Malhotra, S.; Cheung, A.T.M.; Ransom, R.C.; Rennert, R.C.; Morrison, S.D.; Lorenz, H.P.; Longaker, M.T. Embryonic skin development and repair. Organogenesis 2018, 14, 46–63. [Google Scholar] [CrossRef]
- Madaan, A.; Verma, R.; Singh, A.T.; Jaggi, M. Review of Hair Follicle Dermal Papilla cells as in vitro screening model for hair growth. Int. J. Cosmet. Sci. 2018, 40, 429–450. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, Y.; Fan, C.; Liu, R.; Huang, J.; Zhang, Y.; Tang, C.; Zhou, B.; Chen, X.; Ju, W.; et al. Cell-subpopulation alteration and FGF7 activation regulate the function of tendon stem/progenitor cells in 3D microenvironment revealed by single-cell analysis. Biomaterials 2022, 280, 121238. [Google Scholar] [CrossRef] [PubMed]
- Abreu, C.M.; Marques, A.P. Recreation of a hair follicle regenerative microenvironment: Successes and pitfalls. Bioeng. Transl. Med. 2022, 7, e10235. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Song, W.; Hao, F.; Duo, L.; Zhe, X.; Gao, C.; Guo, X.; Liu, D. Effect of Fibroblast Growth Factor 10 and an Interacting Non-Coding RNA on Secondary Hair Follicle Dermal Papilla Cells in Cashmere Goats’ Follicle Development Assessed by Whole-Transcriptome Sequencing Technology. Animals 2023, 13, 2234. [Google Scholar] [CrossRef]
- Calvo-Sánchez, M.I.; Fernández-Martos, S.; Carrasco, E.; Moreno-Bueno, G.; Bernabéu, C.; Quintanilla, M.; Espada, J. A role for the Tgf-β/Bmp co-receptor Endoglin in the molecular oscillator that regulates the hair follicle cycle. J. Mol. Cell Biol. 2019, 11, 39–52. [Google Scholar] [CrossRef]
- Kong, J.; Qiang, W.; Jiang, J.; Hu, X.; Chen, Y.; Guo, Y.; Liu, H.; Sun, S.; Gao, H.; Zhang, Y.; et al. Safflower oil body nanoparticles deliver hFGF10 to hair follicles and reduce microinflammation to accelerate hair regeneration in androgenetic alopecia. Int. J. Pharm. 2022, 616, 121537. [Google Scholar] [CrossRef]
- Tan, X.; Zhu, H.; Tao, Q.; Guo, L.; Jiang, T.; Xu, L.; Yang, R.; Wei, X.; Wu, J.; Li, X.; et al. FGF10 Protects Against Renal Ischemia/Reperfusion Injury by Regulating Autophagy and Inflammatory Signaling. Front. Genet. 2018, 9, 556. [Google Scholar] [CrossRef]
- Beach, R.A.; McDonald, K.A.; Barrett, B.M.; Abdel-Qadir, H. Side effects of low-dose oral minoxidil for treating alopecia. J. Am. Acad. Dermatol. 2021, 84, e239–e240. [Google Scholar] [CrossRef]
- Sanabria, B.; Vanzela, T.N.; Miot, H.A.; Müller Ramos, P. Adverse effects of low-dose oral minoxidil for androgenetic alopecia in 435 patients. J. Am. Acad. Dermatol. 2021, 84, 1175–1178. [Google Scholar] [CrossRef]
- Lv, X.; Chen, W.; Sun, W.; Hussain, Z.; Wang, S.; Wang, J. Analysis of lncRNAs Expression Profiles in Hair Follicle of Hu Sheep Lambskin. Animals 2020, 10, 1035. [Google Scholar] [CrossRef]
- Lv, X.; Chen, W.; Wang, S.; Cao, X.; Yuan, Z.; Getachew, T.; Mwacharo, J.M.; Haile, A.; Sun, W. Integrated Hair Follicle Profiles of microRNAs and mRNAs to Reveal the Pattern Formation of Hu Sheep Lambskin. Genes 2022, 13, 324. [Google Scholar] [CrossRef]
- Anaya, G.; Laseca, N.; Granero, A.; Ziadi, C.; Arrebola, F.; Domingo, A.; Molina, A. Genomic Characterization of Quality Wool Traits in Spanish Merino Sheep. Genes 2024, 15, 795. [Google Scholar] [CrossRef]
- Shin, D.W. The Molecular Mechanism of Natural Products Activating Wnt/beta-Catenin Signaling Pathway for Improving Hair Loss. Life 2022, 12, 1856. [Google Scholar] [CrossRef]
- Zou, Y.; Tang, F.; Li, P.; Qiu, W.; Lei, M. Wnt10b Regulation of Hair Follicle Development, Regeneration, and Skin Diseases. Stem Cell Rev. Rep. 2025. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.; Lee, K.W.; Chung, O.; Yim, H.S.; Cha, S.S.; Lee, S.W.; Jun, J.; Cho, Y.S.; Bhak, J.; Magalhães, J.P.; et al. Analysis of the FGF gene family provides insights into aquatic adaptation in cetaceans. Sci. Rep. 2017, 7, 40233. [Google Scholar] [CrossRef]
- Wambier, C.G.; Vaño-Galván, S.; McCoy, J.; Pai, S.; Dhurat, R.; Goren, A. Androgenetic alopecia in COVID-19: Compared to age-matched epidemiologic studies and hospital outcomes with or without the Gabrin sign. J. Am. Acad. Dermatol. 2020, 83, e453–e454. [Google Scholar] [CrossRef] [PubMed]
- Furuta, R.; Miyake, A. Fibroblast growth factor 22. Differentiation 2025, 143, 100860. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, N.; Jung, A.R.; Jang, J.H.; Lee, J.S.; Bae, J.T. Effect of Plantago asiatica L. extract on the anagen phase in human hair follicle dermal papilla cells. J. Cosmet. Dermatol. 2023, 22, 2324–2332. [Google Scholar] [CrossRef]
- Zhu, M.; Fang, Y.; Huang, Y.; Qiu, W.; Ning, L.; Li, Y.; Zhu, C.; Song, X.; Wu, Y.; Zou, W.; et al. Transcriptomics sequencing reveals Qu-shi-yu-fa Decoction promotes hair cycle and keratinization by upregulating FOXN1 and TGM3 to treat androgenetic alopecia. Phytomedicine 2025, 143, 156837. [Google Scholar] [CrossRef]
- Nasseri, S.; Parsa, S.; Vahabzadeh, Z.; Baban, B.; Khademerfan, M.B.; Nikkhoo, B.; Rastegar Khosravi, M.; Bahrami, S.; Fathi, F. CRISPR/Cas9-Induced Fam83h Knock-out Leads to Impaired Wnt/beta-Catenin Pathway and Altered Expression of Tooth Mineralization Genes in Mice. Iran. J. Biotechnol. 2023, 21, e3673. [Google Scholar] [CrossRef]
- Horibe, I.; Izumi, S.; Ke, Y.; Tanahashi, N.; Takagi, Y.; Ishihara, R.; Nakano, T.; Sumiyoshi, T.; Nagaoka, Y. Acquired curved hair is caused by fusion of multiple hair matrix cells. J. Dermatol. Sci. 2024, 113, 130–137. [Google Scholar] [CrossRef]
- Duran, C.; Barcenas, M.; Wang, Q. Modeling of ionizing radiation induced hair follicle regenerative dynamics. J. Theor. Biol. 2022, 555, 111283. [Google Scholar] [CrossRef]
- Carrion, E.A.; Moses, M.M.; Behringer, R.R. FGF5. Differentiation 2024, 139, 100736. [Google Scholar] [CrossRef]
- Avigad Laron, E.; Aamar, E.; Enshell-Seijffers, D. The Mesenchymal Niche of the Hair Follicle Induces Regeneration by Releasing Primed Progenitors from Inhibitory Effects of Quiescent Stem Cells. Cell Rep. 2018, 24, 909–921.e903. [Google Scholar] [CrossRef]
- Premanand, A.; Reena Rajkumari, B. Androgen modulation of Wnt/β-catenin signaling in androgenetic alopecia. Arch. Dermatol. Res. 2018, 310, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wu, H.; Lian, Z. Bioinformatics analysis of evolutionary characteristics and biochemical structure of FGF5 Gene in sheep. Gene 2019, 702, 123–132. [Google Scholar] [CrossRef]
- Zhang, R.; Li, Y.; Jia, K.; Xu, X.; Li, Y.; Zhao, Y.; Zhang, X.; Zhang, J.; Liu, G.; Deng, S.; et al. Crosstalk between androgen and Wnt/β-catenin leads to changes of wool density in FGF5-knockout sheep. Cell Death Dis. 2020, 11, 407. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.H.; Zhang, L.; Gong, G.; Yan, X.C.; Zhang, L.T.; Zhang, F.T.; Liu, H.F.; Lv, Q.; Wang, Z.Y.; Wang, R.J.; et al. Genome-wide association study of fleece traits in Inner Mongolia Cashmere goats. Anim. Genet. 2021, 52, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Yao, F.; Zhao, B.; Hu, S.; Bai, S.; Jin, R.; Zhang, C.; Chen, Y.; Wu, X. miR-129-5p Participates in Hair Follicle Growth by Targeting HOXC13 in Rabbit. Genes. 2022, 13, 679. [Google Scholar] [CrossRef]
- Kazi, T.; Niibe, I.; Nishikawa, A.; Matsuzaki, T. Optimal stimulation toward the dermal papilla lineage can be promoted by combined use of osteogenic and adipogenic inducers. FEBS Open Bio 2020, 10, 197–210. [Google Scholar] [CrossRef]
- Rivas, L.J.; Uribe, R.A. Fibroblast Growth Factor (FGF) 13. Differentiation 2024, 140, 100814. [Google Scholar] [CrossRef] [PubMed]
- Shwartz, Y.; Gonzalez-Celeiro, M.; Chen, C.-L.; Pasolli, H.A.; Sheu, S.H.; Fan, S.M.Y.; Shamsi, F.; Assaad, S.; Lin, E.T.Y.; Zhang, B.; et al. Cell Types Promoting Goosebumps Form a Niche to Regulate Hair Follicle Stem Cells. Cell 2020, 182, 578–593.e19. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhang, J.; Bao, Q.; Zhong, J.; Wang, X.; Tao, Y.; Xu, X.; Lv, K.; Wang, Y.; Li, B.; et al. Foxp1 and Foxp4 Deletion Causes the Loss of Follicle Stem Cell Niche and Cyclic Hair Shedding by Inducing Inner Bulge Cell Apoptosis. Stem Cells 2022, 40, 843–856. [Google Scholar] [CrossRef]
- Heilmann-Heimbach, S.; Herold, C.; Hochfeld, L.M.; Hillmer, A.M.; Nyholt, D.R.; Hecker, J.; Javed, A.; Chew, E.G.; Pechlivanis, S.; Drichel, D.; et al. Meta-analysis identifies novel risk loci and yields systematic insights into the biology of male-pattern baldness. Nat. Commun. 2017, 8, 14694. [Google Scholar] [CrossRef]
- Choi, K.; Park, S.H.; Park, S.Y.; Yoon, S.K. The stem cell quiescence and niche signaling is disturbed in the hair follicle of the hairpoor mouse, an MUHH model mouse. Stem Cell Res. Ther. 2022, 13, 211. [Google Scholar] [CrossRef]
- Cai, J.; Wen, R.; Li, W.; Wang, X.; Tian, H.; Yi, S.; Zhang, L.; Li, X.; Jiang, C.; Li, H. Oil body bound oleosin-rhFGF9 fusion protein expressed in safflower (Carthamus tinctorius L.) stimulates hair growth and wound healing in mice. BMC Biotechnol. 2018, 18, 51. [Google Scholar] [CrossRef]
- Jia, Q.; Zhang, S.; Wang, D.; Liu, J.; Luo, X.; Liu, Y.; Li, X.; Sun, F.; Xia, G.; Zhang, L. Regulatory Effects of FGF9 on Dermal Papilla Cell Proliferation in Small-Tailed Han Sheep. Genes 2023, 14, 1106. [Google Scholar] [CrossRef]
- Zhang, Y.; Sheng, Y.; Yang, Q.; Zeng, Y. Homocysteine in androgenetic alopecia: A case control study and observational experiments on mice. J. Cosmet. Dermatol. 2024, 23, 3608–3615. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, Y.; Chen, M.; Zhang, Y. Overexpression of Fgf8 in the epidermis inhibits hair follicle development. Exp. Dermatol. 2021, 30, 494–502. [Google Scholar] [CrossRef]
- Telerman, S.B.; Rognoni, E.; Sequeira, I.; Pisco, A.O.; Lichtenberger, B.M.; Culley, O.J.; Viswanathan, P.; Driskell, R.R.; Watt, F.M. Dermal Blimp1 Acts Downstream of Epidermal TGFβ and Wnt/β-Catenin to Regulate Hair Follicle Formation and Growth. J. Investig. Dermatol. 2017, 137, 2270–2281. [Google Scholar] [CrossRef] [PubMed]
- Ratzan, E.M.; Moon, A.M.; Deans, M.R. Fgf8 genetic labeling reveals the early specification of vestibular hair cell type in mouse utricle. Development 2020, 147, dev192849. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.G.; Gong, L.; Jiang, T.L.; Li, Y.H.; Gao, X.H.; Tian, H.; Chen, H.D. Stimulation of mouse vibrissal follicle growth by recombinant human fibroblast growth factor 20. Biotechnol. Lett. 2018, 40, 1009–1014. [Google Scholar] [CrossRef] [PubMed]
- Biggs, L.C.; Mäkelä, O.J.; Myllymäki, S.M.; Das Roy, R.; Närhi, K.; Pispa, J.; Mustonen, T.; Mikkola, M.L. Hair follicle dermal condensation forms via Fgf20 primed cell cycle exit, cell motility, and aggregation. Elife 2018, 7, e36468. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, P.; Zhang, X.; Li, X.; Bai, Y.; Ao, Y.; Hexig, B.; Guo, X.; Liu, D. Fgf21 knockout mice generated using CRISPR/Cas9 reveal genetic alterations that may affect hair growth. Gene 2020, 733, 144242. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, P.; Zhang, X.F.; Li, X.; Bai, Y.; Jia, K.R.; Guo, X.D.; Zhang, H.; Ma, X.Y.; Cang, M.; et al. Construction of FGF21 knockout mouse models by the CRISPR/Cas9 system. Yi Chuan 2018, 40, 66–74. [Google Scholar] [CrossRef]
- Li, Y.; Ao, Y.; Xie, X.; Ulan, T.; Liu, D.; Guo, X. Fgf21 Deficiency Delays Hair Follicle Cycling and Modulates miRNA-Target Gene Interactions in Mice. Biology 2025, 14, 526. [Google Scholar] [CrossRef]
- Wambier, C.G.; Vaño-Galván, S.; McCoy, J.; Gomez-Zubiaur, A.; Herrera, S.; Hermosa-Gelbard, Á.; Moreno-Arrones, O.M.; Jiménez-Gómez, N.; González-Cantero, A.; Fonda-Pascual, P.; et al. Androgenetic alopecia present in the majority of patients hospitalized with COVID-19: The "Gabrin sign". J. Am. Acad. Dermatol. 2020, 83, 680–682. [Google Scholar] [CrossRef]
- Wambier, C.G.; McCoy, J.; Goren, A. Male balding as a major risk factor for severe COVID-19: A possible role for targeting androgens and transmembrane protease serine 2 to protect vulnerable individuals. J. Am. Acad. Dermatol. 2020, 83, e401–e402. [Google Scholar] [CrossRef]
- Moravvej, H.; Pourani, M.R.; Baghani, M.; Abdollahimajd, F. Androgenetic alopecia and COVID-19: A review of the hypothetical role of androgens. Dermatol. Ther. 2021, 34, e15004. [Google Scholar] [CrossRef] [PubMed]
- Desai, D.D.; Nohria, A.; Sikora, M.; Anyanwu, N.; Shapiro, J.; Lo Sicco, K.I. Assessing low-dose oral minoxidil efficacy in androgenetic alopecia: A comparative study of AGA and AGA unmasked by telogen effluvium. Arch. Dermatol. Res. 2024, 316, 514. [Google Scholar] [CrossRef] [PubMed]
- Devjani, S.; Ezemma, O.; Kelley, K.J.; Stratton, E.; Senna, M. Androgenetic Alopecia: Therapy Update. Drugs 2023, 83, 701–715. [Google Scholar] [CrossRef] [PubMed]
- Egger, A.; Resnik, S.R.; Aickara, D.; Maranda, E.; Kaiser, M.; Wikramanayake, T.C.; Jimenez, J.J. Examining the Safety and Efficacy of Low-Level Laser Therapy for Male and Female Pattern Hair Loss: A Review of the Literature. Ski. Appendage Disord. 2020, 6, 259–267. [Google Scholar] [CrossRef]
- Herz-Ruelas, M.E.; Álvarez-Villalobos, N.A.; Millán-Alanís, J.M.; de León-Gutiérrez, H.; Ocampo-Garza, S.S.; Gómez-Flores, M.; Grimalt, R. Efficacy of Intralesional and Oral Dutasteride in the Treatment of Androgenetic Alopecia: A Systematic Review. Ski. Appendage Disord. 2020, 6, 338–345. [Google Scholar] [CrossRef]
- Panchaprateep, R.; Lueangarun, S. Efficacy and Safety of Oral Minoxidil 5 mg Once Daily in the Treatment of Male Patients with Androgenetic Alopecia: An Open-Label and Global Photographic Assessment. Dermatol. Ther. 2020, 10, 1345–1357. [Google Scholar] [CrossRef]
- Pereira, A.; Coelho, T.O.A. Post-finasteride syndrome. Bras. Dermatol. 2020, 95, 271–277. [Google Scholar] [CrossRef]
- Gentile, P.; Garcovich, S. Advances in Regenerative Stem Cell Therapy in Androgenic Alopecia and Hair Loss: Wnt pathway, Growth-Factor, and Mesenchymal Stem Cell Signaling Impact Analysis on Cell Growth and Hair Follicle Development. Cells 2019, 8, 466. [Google Scholar] [CrossRef]
- Quan, R.; Du, W.; Zheng, X.; Xu, S.; Li, Q.; Ji, X.; Wu, X.; Shao, R.; Yang, D. VEGF165 induces differentiation of hair follicle stem cells into endothelial cells and plays a role in in vivo angiogenesis. J. Cell Mol. Med. 2017, 21, 1593–1604. [Google Scholar] [CrossRef]
- Dai, R.; Xu, Q.; Shao, Z.; Wu, X. The co-expression pattern of VEGFR-2 with indicators related to proliferation, apoptosis, and differentiation of anagen hair follicles. Open Life Sci. 2023, 18, 20220723. [Google Scholar] [CrossRef] [PubMed]
- Cooper, F.; Souilhol, C.; Haston, S.; Gray, S.; Boswell, K.; Gogolou, A.; Frith, T.J.R.; Stavish, D.; James, B.M.; Bose, D.; et al. Notch signalling influences cell fate decisions and HOX gene induction in axial progenitors. Development 2024, 151, dev202098. [Google Scholar] [CrossRef]
- Niehrs, C.; Zapparoli, E.; Lee, H. ‘Three Signals—Three Body Axes’ as Patterning Principle in Bilaterians. Cells Dev. 2024, 203944. [Google Scholar] [CrossRef]
- Cooper, E.J.; Scholpp, S. Transport and gradient formation of Wnt and Fgf in the early zebrafish gastrula. Curr. Top. Dev. Biol. 2024, 157, 125–153. [Google Scholar] [CrossRef]
- Ryu, Y.C.; Park, J.; Kim, Y.R.; Choi, S.; Kim, G.U.; Kim, E.; Hwang, Y.; Kim, H.; Han, G.; Lee, S.H.; et al. CXXC5 Mediates DHT-Induced Androgenetic Alopecia via PGD(2). Cells 2023, 12, 555. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, S.; Tan, L.; Li, X.; Long, D.; Lu, J.; Wang, D. Necrosulfonamide promotes hair growth and ameliorates DHT-induced hair growth inhibition. J. Dermatol. Sci. 2024, 115, 64–74. [Google Scholar] [CrossRef]
- Horton, C.; Liu, Y.; Wang, J.; Green, J.; Tsyporin, J.; Chen, B.; Wang, Z.A. Modulation of the canonical Wnt activity by androgen signaling in prostate epithelial basal stem cells. Stem Cell Rep. 2023, 18, 1355–1370. [Google Scholar] [CrossRef]
- Lim, H.W.; Kim, H.J.; Jeon, C.Y.; Lee, Y.; Kim, M.; Kim, J.; Kim, S.R.; Lee, S.; Lim, D.C.; Park, H.D.; et al. Hair Growth Promoting Effects of 15-Hydroxyprostaglandin Dehydrogenase Inhibitor in Human Follicle Dermal Papilla Cells. Int. J. Mol. Sci. 2024, 25, 7485. [Google Scholar] [CrossRef]
- Fu, H.; Li, W.; Liu, J.; Tang, Q.; Weng, Z.; Zhu, L.; Ding, B. Ellagic acid inhibits dihydrotestosterone-induced ferroptosis and promotes hair regeneration by activating the wnt/beta-catenin signaling pathway. J. Ethnopharmacol. 2024, 330, 118227. [Google Scholar] [CrossRef] [PubMed]
- Pei, F.; Guo, T.; Zhang, M.; Ma, L.; Jing, J.; Feng, J.; Ho, T.V.; Wen, Q.; Chai, Y. FGF signaling modulates mechanotransduction/WNT signaling in progenitors during tooth root development. Bone Res. 2024, 12, 37. [Google Scholar] [CrossRef] [PubMed]
- Nakazato, H.; Onodera, S.; Aida, N.; Furusawa, M.; Azuma, T. Comprehensive analysis of transcription factors involved in odontoblast differentiation mechanism. Med. Mol. Morphol. 2024, 57, 253–267. [Google Scholar] [CrossRef]
- Mehta, A.; Motavaf, M.; Raza, D.; McLure, A.J.; Osei-Opare, K.D.; Bordone, L.A.; Gru, A.A. Revolutionary Approaches to Hair Regrowth: Follicle Neogenesis, Wnt/ss-Catenin Signaling, and Emerging Therapies. Cells 2025, 14, 779. [Google Scholar] [CrossRef]
- Im, S.T.; Mun, H.; Kang, N.; Heo, S.J.; Lee, S.H. Anti-androgenetic effect of diphlorethohydroxycarmalol on testosterone-induced hair loss by inhibiting 5alpha-reductase and promoting Wnt/beta-catenin signaling pathway in human dermal papilla cells. Toxicol. Vitr. 2025, 104, 106017. [Google Scholar] [CrossRef]
- Han, S.H.; Jo, K.W.; Kim, Y.; Kim, K.T. Piperonylic Acid Promotes Hair Growth by Activation of EGFR and Wnt/beta-Catenin Pathway. Int. J. Mol. Sci. 2024, 25, 10774. [Google Scholar] [CrossRef]
- Strobl, K.; Klufa, J.; Jin, R.; Artner-Gent, L.; Krauss, D.; Novoszel, P.; Strobl, J.; Stary, G.; Vujic, I.; Griss, J.; et al. JAK-STAT1 as therapeutic target for EGFR deficiency-associated inflammation and scarring alopecia. EMBO Mol. Med. 2024, 16, 3142–3168. [Google Scholar] [CrossRef]
- Kageyama, T.; Seo, J.; Yan, L.; Hamano, S.; Fukuda, J. Serotonin activates dermal papilla cells and promotes hair growth. Sci. Rep. 2025, 15, 24525. [Google Scholar] [CrossRef]
- Olczak, A.; Pieczonka, T.D.; Lawicki, S.; Lukaszyk, K.; Pulawska-Czub, A.; Cambier, L.; Kobielak, K. The overexpression of R-spondin 3 affects hair morphogenesis and hair development along with the formation and maturation of the hair follicle stem cells. Front. Physiol. 2024, 15, 1424077. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, Z.; Feng, J.; Chen, Z.; Liu, Z.; Wang, X.; Yan, H.; Gao, C. Novel recombinant R-spondin1 promotes hair regeneration by targeting the Wnt/beta-catenin signaling pathway. Acta Biochim. Biophys. Sin. 2023, 55, 1213–1221. [Google Scholar] [CrossRef]
- Zhao, Y.; Liang, W.; Liu, Z.; Chen, X.; Lin, C. Impact of SDF-1 and AMD3100 on Hair Follicle Dynamics in a Chronic Stress Model. Biomolecules 2024, 14, 1206. [Google Scholar] [CrossRef]
- Muangsanguan, A.; Ruksiriwanich, W.; Arjin, C.; Jamjod, S.; Prom, U.T.C.; Jantrawut, P.; Rachtanapun, P.; Hnorkaew, P.; Satsook, A.; Sainakham, M.; et al. Comparison of In Vitro Hair Growth Promotion and Anti-Hair Loss Potential of Thai Rice By-Product from Oryza sativa L. cv. Buebang 3 CMU and Sanpatong. Plants 2024, 13, 3079. [Google Scholar] [CrossRef] [PubMed]
- Muangsanguan, A.; Ruksiriwanich, W.; Linsaenkart, P.; Jantrawut, P.; Rachtanapun, P.; Jantanasakulwong, K.; Sommano, S.R.; Sringarm, K.; Arjin, C.; Sainakham, M.; et al. Synergistic Phytochemical and Pharmacological Actions of Hair Rise(TM) Microemulsion: A Novel Herbal Formulation for Androgenetic Alopecia and Hair Growth Stimulation. Plants 2024, 13, 2802. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.Y.; Kim, B.H.; Kim, D.W.; Lee, W.Y.; Kim, C.E.; Kim, H.Y.; Pyo, J.; Park, E.S.; Kang, K.S. Hair Growth Effect of DN106212 in C57BL/6 Mouse and Its Network Pharmacological Mechanism of Action. Curr. Issues Mol. Biol. 2023, 45, 5071–5083. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, T.; Anakama, R.; Togashi, H.; Fukuda, J. Impacts of manipulating cell sorting on in vitro hair follicle regeneration. J. Biosci. Bioeng. 2022, 134, 534–540. [Google Scholar] [CrossRef]
- Fu, J.; Zhang, X.; Wang, D.; Liu, W.; Zhang, C.; Wang, W.; Fan, W.; Zhang, L.; Sun, F. Analysis of the Long Non-Coding and Messenger RNA Expression Profiles in the Skin Tissue of Super Merino and Small-Tailed Han Sheep. Curr. Issues Mol. Biol. 2024, 46, 9588–9606. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.; Chang, J.; Zhang, R.; Liu, Z.; Liang, J.; Wang, D.; Feng, J.; Zhao, W.; Xiao, H. Shh Gene Regulates the Proliferation and Apoptosis of Dermal Papilla Cells to Affect Its Differential Expression in Secondary Hair Follicle Growth Cycle of Cashmere Goats. Animals 2024, 14, 2049. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Y.; Ding, P.; Lin, Z.; Sun, Z.; Jin, M.; Li, C.; Zhao, Z.; Bi, H. The SHH-GLI1 pathway is required in skin expansion and angiogenesis. Exp. Dermatol. 2023, 32, 1085–1095. [Google Scholar] [CrossRef]
- Jaiswal, A.; Singh, R. Homeostases of epidermis and hair follicle, and development of basal cell carcinoma. Biochim. Biophys. Acta Rev. Cancer 2022, 1877, 188795. [Google Scholar] [CrossRef]
- Sun, S.; Zhao, B.; Li, J.; Zhang, X.; Yao, S.; Bao, Z.; Cai, J.; Yang, J.; Chen, Y.; Wu, X. Regulation of Hair Follicle Growth and Development by Different Alternative Spliceosomes of FGF5 in Rabbits. Genes 2024, 15, 409. [Google Scholar] [CrossRef]
- Song, Y.; Li, Y.; Lu, Z.; Yue, L.; Xiao, T.; Yang, B.; Liu, J.; Yuan, C.; Guo, T. FGF20 Secreted From Dermal Papilla Cells Regulate the Proliferation and Differentiation of Hair Follicle Stem Cells in Fine-Wool Sheep. J. Anim. Physiol. Anim. Nutr. 2025, 109, 655–666. [Google Scholar] [CrossRef]
- Qian, H.; Ye, Z.; Hu, Y.; Chen, L.; Li, L.; Qin, K.; Ye, Q.; Zuo, X. Dahuang-Gancao decoction ameliorates testosterone-induced androgenetic alopecia in mice. J. Ethnopharmacol. 2025, 341, 119347. [Google Scholar] [CrossRef] [PubMed]
- Thanasarnaksorn, W.; Limsuchaiwat, N.; Sirithanabadeekul, P.; Charoensuksira, S.; Suwanchinda, A.; Meephansan, J. Polynucleotides as a novel therapeutic approach in androgenetic alopecia: An analysis of effectiveness and safety. Arch. Dermatol. Res. 2025, 317, 399. [Google Scholar] [CrossRef]
- He, G.; Liu, M.; Wang, F.; Sun, S.; Cao, Y.; Sun, Y.; Ma, S.; Wang, Y. Non-invasive assessment of hair regeneration in androgenetic alopecia mice in vivo using two-photon and second harmonic generation imaging. Biomed. Opt. Express 2023, 14, 5870–5885. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.X.; Yu, Q.; Zhou, H.Q.; Fan, W.H.; Zheng, J.J.; Yang, Y.L.; Zhang, W.Z.; Cao, X.; Yang, H. TMT-based quantitative proteomics reveals the genetic mechanisms of secondary hair follicle development in fine-wool sheep. PLoS ONE 2025, 20, e0315637. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Zhang, X.; Bao, P.; Zhou, X.; Chu, M.; Guo, X.; Liang, C.; Pan, H.; Yan, P. Understanding Mammalian Hair Follicle Ecosystems by Single-Cell RNA Sequencing. Animals 2022, 12, 2409. [Google Scholar] [CrossRef] [PubMed]
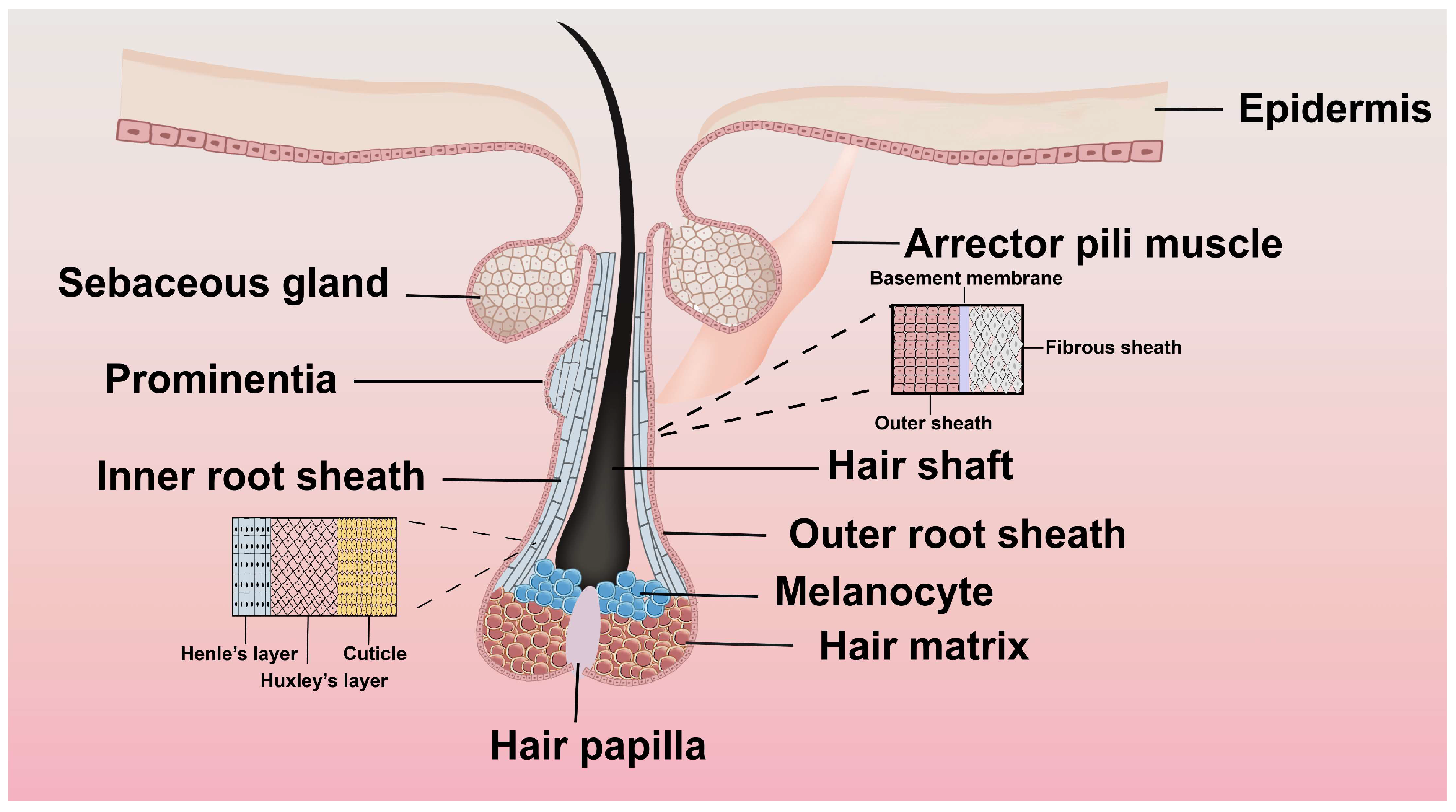
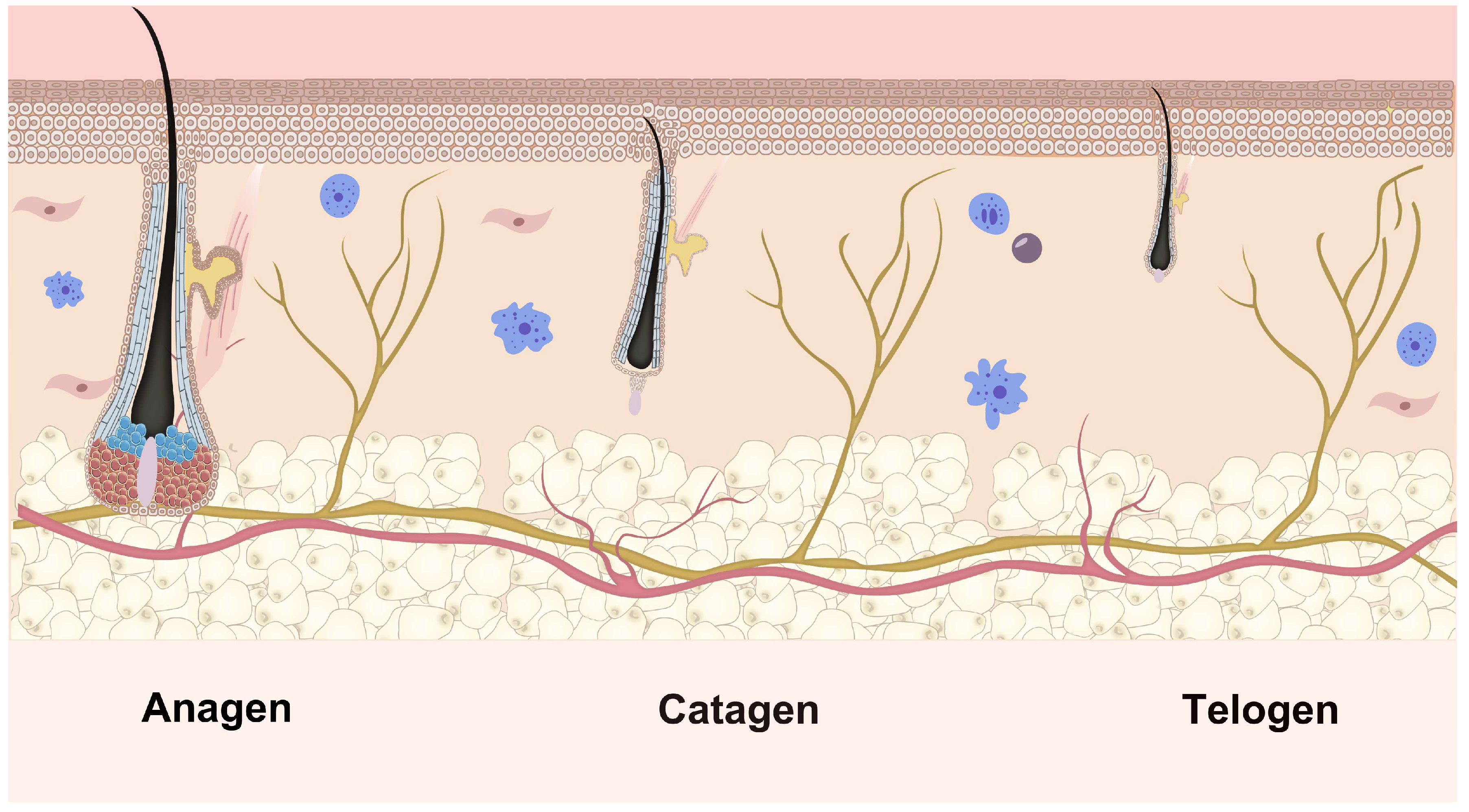
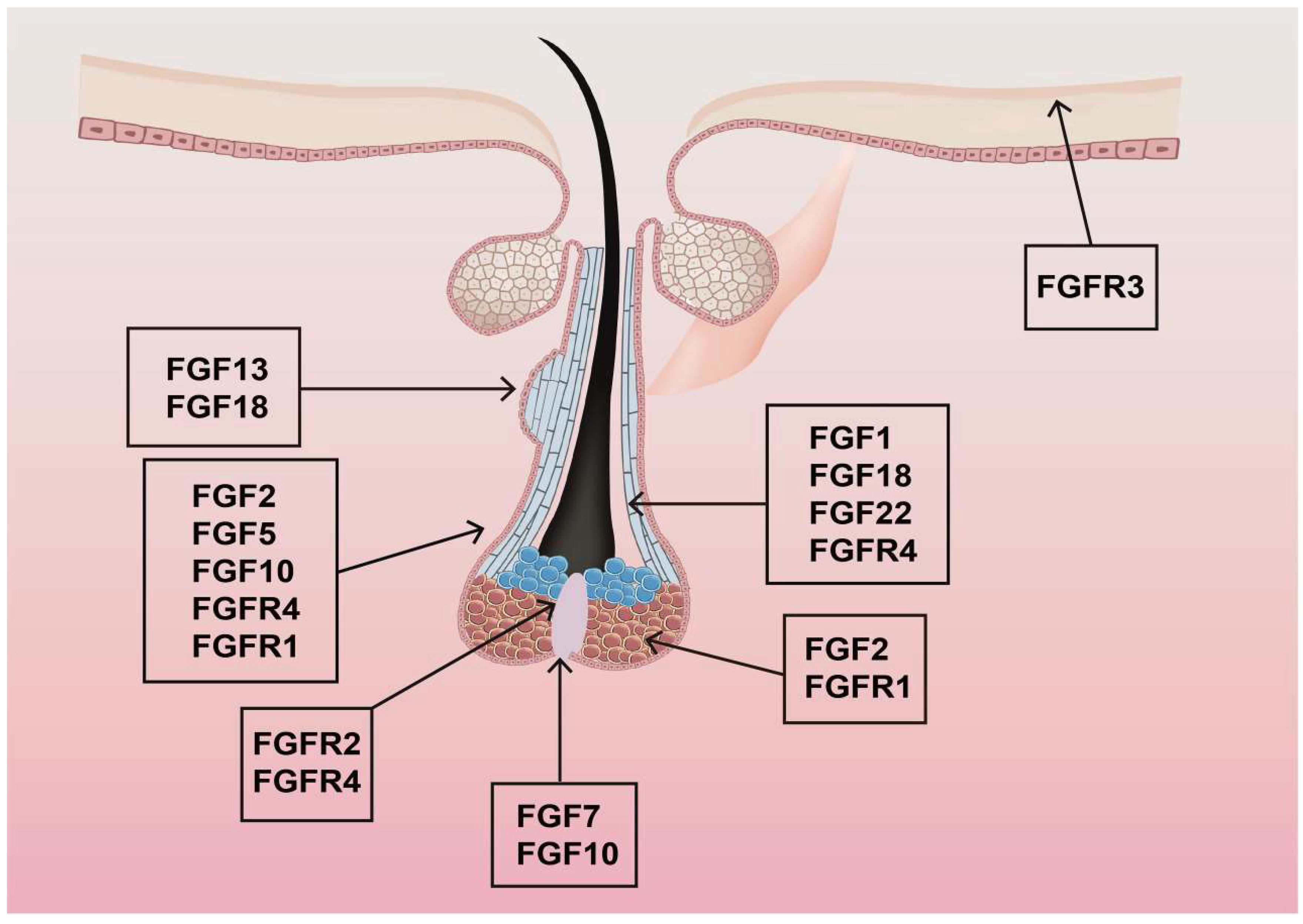
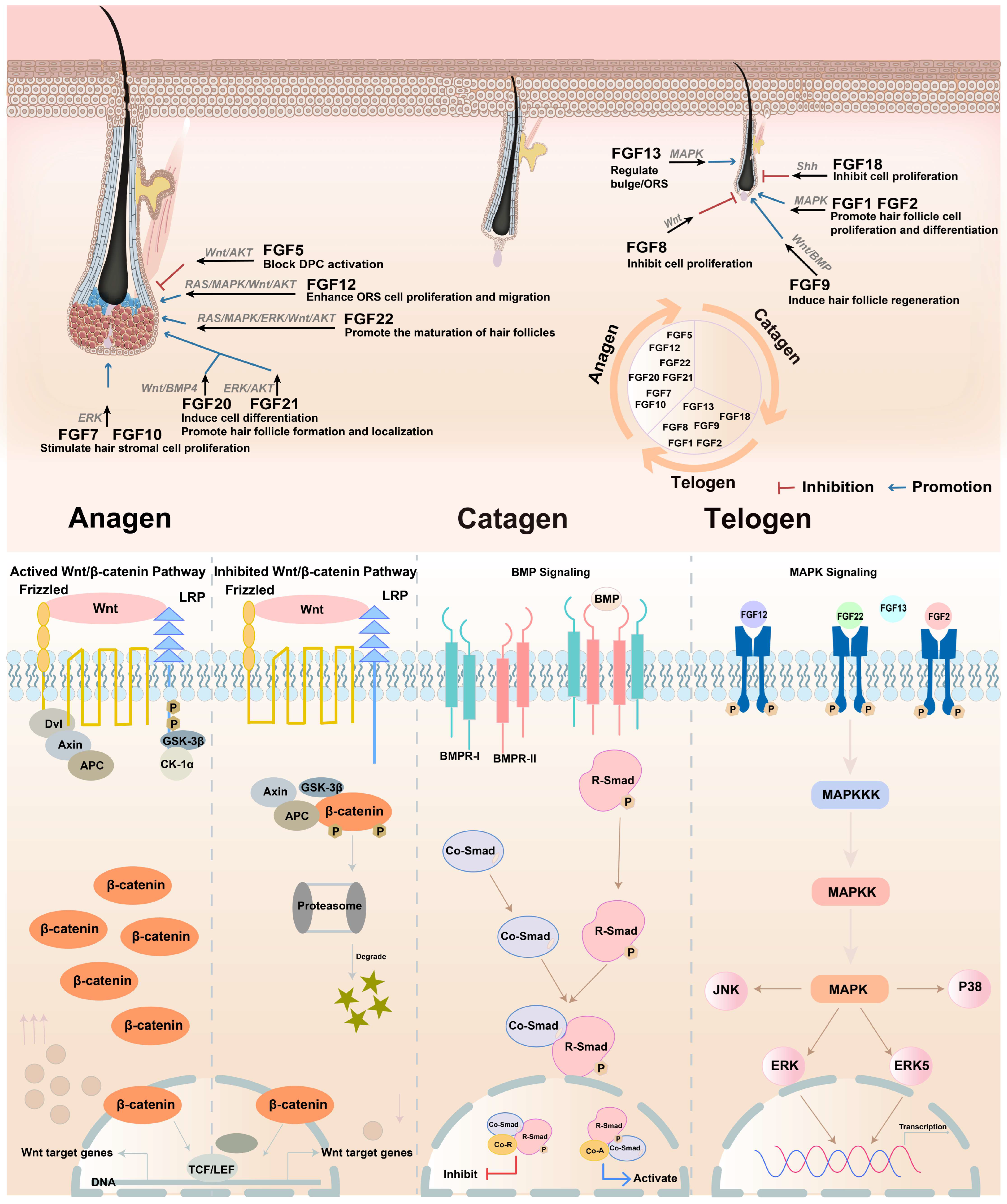

| FGF | Sites of mRNA Distribution | Peak Expression |
|---|---|---|
| FGF1 | Internal root sheath of the hair follicle [52] | Quiescent phase [53] |
| FGF2 | Outer root sheath of the hair follicle, hair parent material [54] | Quiescent phase [54] |
| FGF5 | Outer root sheath of the hair follicle [55] | Growth stage VI [56] |
| FGF7 | Hairy papillae [52] | Growth stage V [57] |
| FGF10 | Hairy papillae, outer root sheath of hair follicles [58] | Growth stage V [59] |
| FGF13 | Hair follicle bulge area [53] | Quiescent phase [60,61] |
| FGF18 | Internal root sheath of the hair follicle, hair follicle bulge area [53] | Quiescent phase [62] |
| FGF22 | Internal root sheath of the hair follicle [63] | Growth stage VI [64] |
| Function | ||
|---|---|---|
| FGFs | Action | |
| FGF1 | + | Hair follicle differentiation and prevention of radiation-induced apoptosis in hair follicles [4] |
| FGF2 | + | Proliferation of hair follicle cells and prolonging of the growth period [4,68] |
| FGF5 | – | Blocking of DPC activation during the growth phase [69] |
| FGF7 | + | Prolonging of the growth period, proliferation of hair embryo, activation of stem cells [70] |
| FGF8 | – | Inhibition of epidermal cell proliferation [71] |
| FGF9 | + | Induction of hair follicle regeneration after trauma [72] |
| FGF10 | + | Hair follicle formation and morphogenesis [58] |
| FGF13 | + | Regulation of bulge/ORS cells with reduced expression in hyperplasia [73] |
| FGF18 | – | Induction of the rest period and maintenance of stem cells at rest [53] |
| FGF20 | + | Formation of dermal condensate and development of substrate [74] |
| FGF21 | + | Development of secondary hair follicles [75] |
| Year | FGF Target | Key Finding | Model System |
|---|---|---|---|
| 2023 | FGF7 | Enhances hair shaft growth and elongation in xenograft-cultured hair follicles and improves the survival of hair follicles following chemotherapy and other cytotoxic injuries [76] | Organ-cultured human HFs and scalp skin |
| 2023 | FGF10 | MiR-184 within DPCs (SHF-DPCs) influences hair growth by modulating FGF10 levels [83] | Cashmere goat embryos |
| 2022 | FGF12 | Predominantly expressed in ORS cells, FGF12 shows high expression levels during the growth phase of hair follicles [4] | Mice |
| 2023 | FGF22 | Dual role: Drives hair shaft keratinization (late anagen) and induces catagen via FGFR2b/MAPK/TGF-β2 synergy [99] | Mice |
| 2024 | FGF5 | FGF5 alternative spliceosomes inhibit DPC proliferation [164] | Rabbit |
| 2021 | FGF1 | FGF1 links to yield, fineness, and length of Inner Mongolia cashmere goats [107] | Mongolia cashmere goats |
| 2022 | FGF2 | Intradermal injections increase terminal hairs (80% patients) and hair shaft diameter (+3.03 μm) in the Phase I trial [108] | AGA patients |
| 2024 | FGF13 | Facilitate the transition of hair follicles from the quiescent phase to the regenerative phase [110] | Mice |
| 2022 | FGF18 | A subcutaneous injection of FGF18 at the end of the hair follicle growth stage immediately stops progenitor cell proliferation and significantly suppresses growth [112] | Mice |
| 2023 | FGF9 | Fgf9 improves DPC proliferation and cell cycle and regulates hair follicle growth and development via the Wnt/β-catenin signaling pathway [116] | Small-Tailed Han Sheep |
| 2021 | FGF8 | Excessive FGF8 not only inhibits epidermal cell proliferation but also promotes apoptosis, thereby obstructing hair follicle development [118] | Mice |
| 2024 | FGF20 | FGF20 secreted from dermal papilla Cells regulates the proliferation and differentiation of hair follicle stem cells [165] | Fine-Wool Sheep |
| 2025 | FGF21 | Fgf21 induces the expression and phosphorylation of ERK and Akt proteins, thus influencing a complex signaling network that regulates hair growth [125] | Mice |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Wang, L.; Gao, S.; Li, X. Recent Advances in the Role of Fibroblast Growth Factors in Hair Follicle Growth. Biomolecules 2025, 15, 1198. https://doi.org/10.3390/biom15081198
Wang J, Wang L, Gao S, Li X. Recent Advances in the Role of Fibroblast Growth Factors in Hair Follicle Growth. Biomolecules. 2025; 15(8):1198. https://doi.org/10.3390/biom15081198
Chicago/Turabian StyleWang, Junchao, Lusheng Wang, Shuang Gao, and Xiaokun Li. 2025. "Recent Advances in the Role of Fibroblast Growth Factors in Hair Follicle Growth" Biomolecules 15, no. 8: 1198. https://doi.org/10.3390/biom15081198
APA StyleWang, J., Wang, L., Gao, S., & Li, X. (2025). Recent Advances in the Role of Fibroblast Growth Factors in Hair Follicle Growth. Biomolecules, 15(8), 1198. https://doi.org/10.3390/biom15081198






