PRDM2—The Key Research Targets for the Development of Diseases in Various Systems
Abstract
1. Introduction
2. Structure and Biological Functions of PRDM2
2.1. Structure of PRDM2
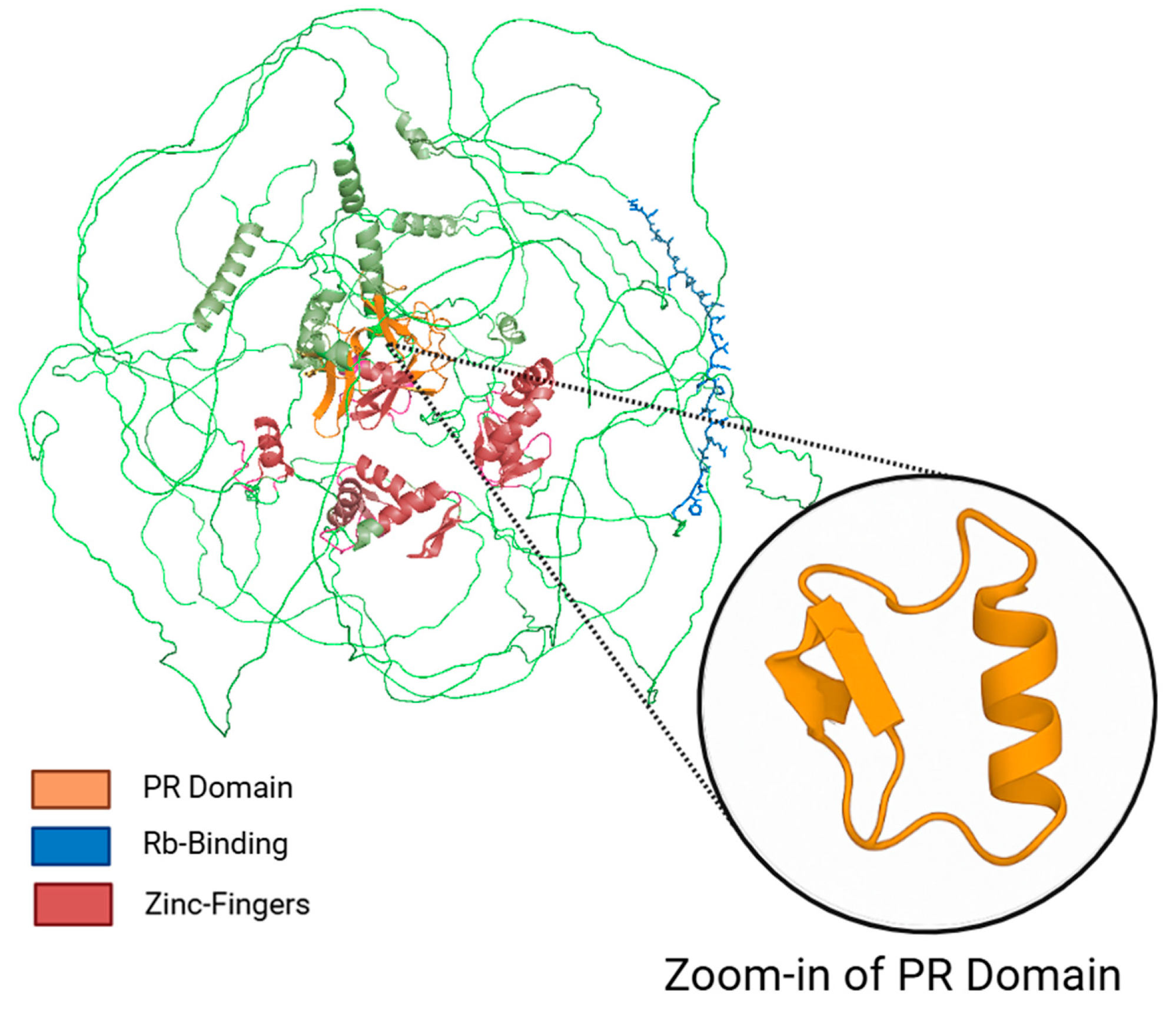
| Isoform Name | UniProt ID | Structural Domains | Key Features | Notes |
|---|---|---|---|---|
| RIZ1 (PRDM2 Isoform 1) | Q13029-1 | PR domain (N-terminal), Rb-binding motif, 8 C2H2 zinc fingers | Full-length isoform; exhibits H3K9 methyltransferase activity; functions as tumor suppressor | Nuclear localization; involved in chromatin remodeling and cell cycle regulation |
| RIZ2 (PRDM2 Isoform 3) | Q13029-3 | Rb-binding motif, 8 C2H2 zinc fingers (lacks PR domain) | Shorter isoform via alternative promoter; lacks methyltransferase activity | May antagonize RIZ1; often upregulated in cancers |
| Transcript 3/RIZ2 isoform c | Not assigned | Similar to RIZ2 (lacks PR domain) | Variant transcript regulated by estrogen receptor α in monocytic leukemia cells | Functional role remains unclear; may overlap with RIZ2 but differs in hormonal responsiveness |
| MTB-Zf (PRDM2 Isoform 2) | Q13029-2 | 8 C2H2 zinc fingers (lacks PR domain) | Variant identified from monocytic leukemia cells; MTE-responsive | Limited functional data; classified as PRDM2 isoform 2 in UniProt |
2.2. Biological Function of PRDM2
2.2.1. The Role of PRDM2 in Cellular Metabolic Processes
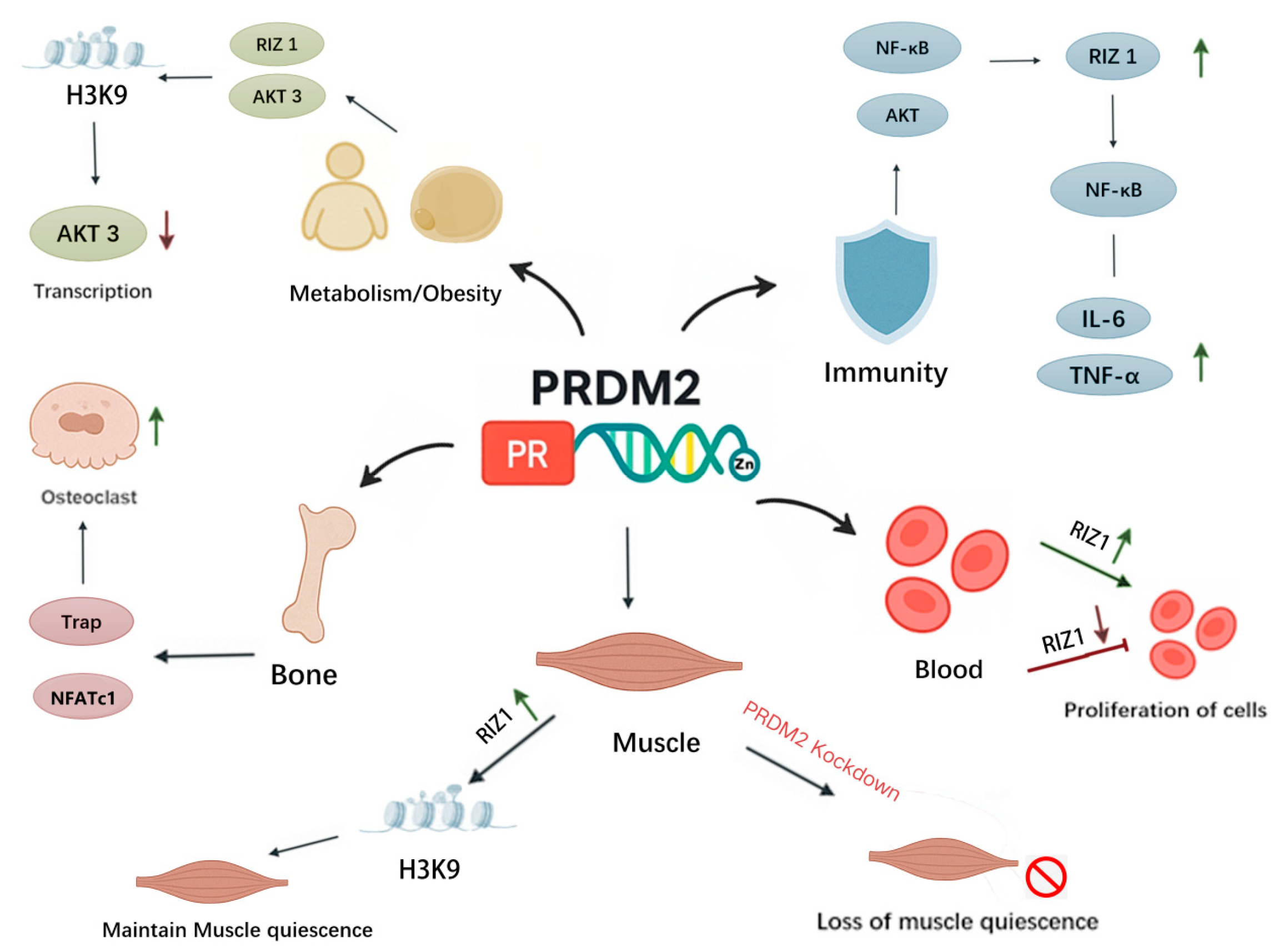
2.2.2. PRDM2 and Estrogen Receptor-Specific Binding
3. PRDM2 Is the Key Research Target for Disease Development in Various Systems
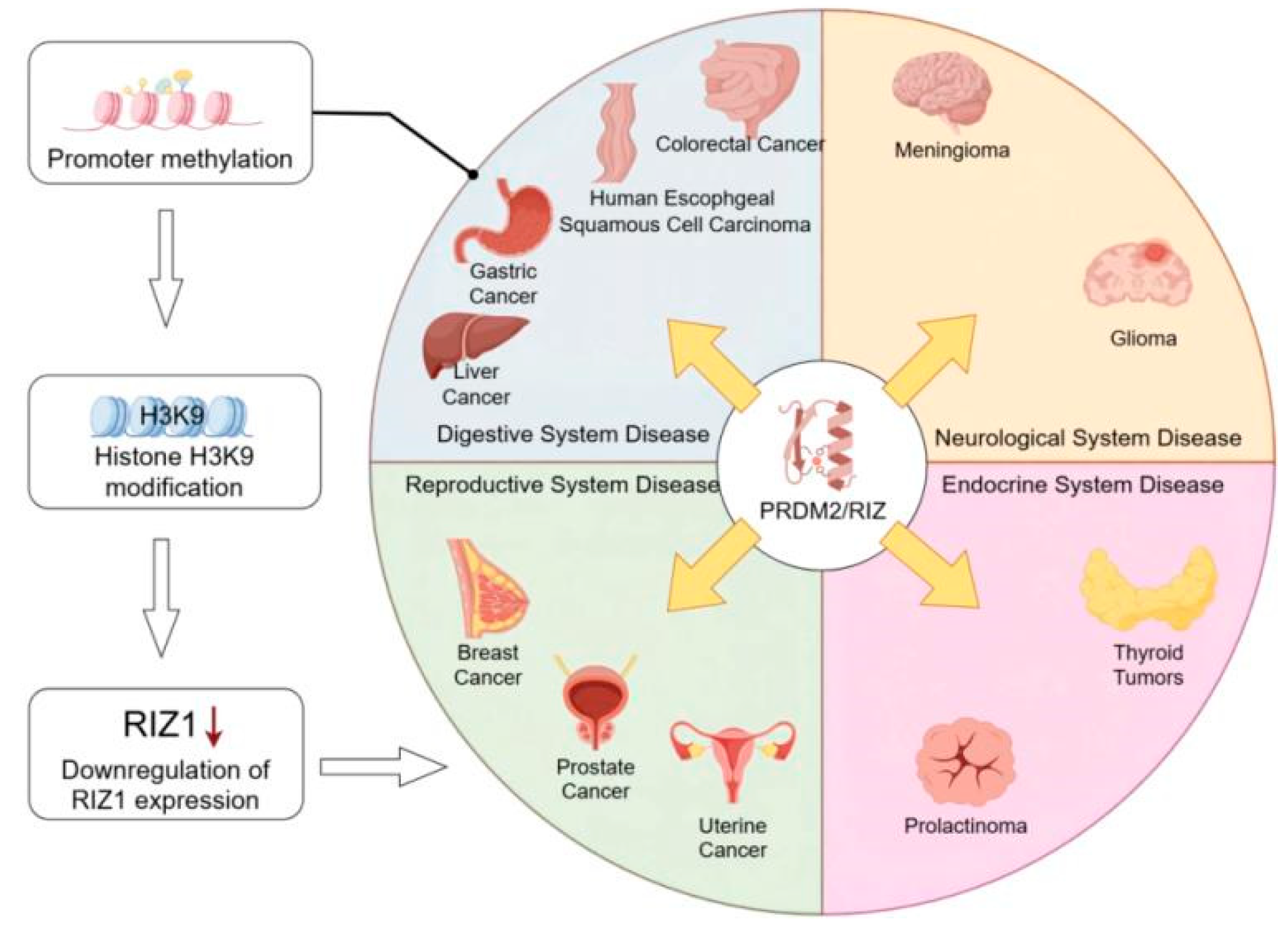
3.1. Role of PRDM2 in Digestive Diseases
3.1.1. PRDM2 and Liver Cancer
3.1.2. PRDM2 and Esophageal Squamous Carcinoma
3.1.3. PRDM2 and Gastric Cancer
3.2. Role of PRDM2 in Neurological Diseases
3.3. Role of PRDM2 in Reproductive Disorders
3.4. Role of PRDM2 in Endocrine System Diseases
4. Perspectives
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Vervoort, M.; Meulemeester, D.; Béhague, J.; Kerner, P. Evolution of Prdm Genes in Animals: Insights from Comparative Genomics. Mol. Biol. Evol. 2015, 33, 679–696. [Google Scholar] [CrossRef]
- Casamassimi, A.; Rienzo, M.; Di Zazzo, E.; Sorrentino, A.; Fiore, D.; Proto, M.C.; Moncharmont, B.; Gazzerro, P.; Bifulco, M.; Abbondanza, C. Multifaceted Role of PRDM Proteins in Human Cancer. Int. J. Mol. Sci. 2020, 21, 2648. [Google Scholar] [CrossRef]
- Sorrentino, A.; Rienzo, M.; Ciccodicola, A.; Casamassimi, A.; Abbondanza, C. Human PRDM2: Structure, function and pathophysiology. Biochim. Biophys. Acta BBA-Gene Regul. Mech. 2018, 1861, 657–671. [Google Scholar] [CrossRef] [PubMed]
- Buyse, I.M.; Shao, G.; Huang, S. The retinoblastoma protein binds to RIZ, a zinc-finger protein that shares an epitope with the adenovirus E1A protein. Proc. Natl. Acad. Sci. USA 1995, 92, 4467–4471. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, V.S.; Lee, P.; Winoto, A. Identification and cloning of the G3B cDNA encoding a 3′ segment of a protein binding to GATA-3. Gene 1995, 163, 329–330. [Google Scholar] [CrossRef]
- Muraosa, Y.; Takahashi, K.; Yoshizawa, M.; Shibahara, S. cDNA cloning of a novel protein containing two zinc-finger domains that may function as a transcription factor for the human heme-oxygenase-1 gene. Eur. J. Biochem. 1996, 235, 471–479. [Google Scholar] [CrossRef]
- Derunes, C.; Briknarová, K.; Geng, L.; Li, S.; Gessner, C.R.; Hewitt, K.; Wu, S.; Huang, S.; Woods, V.I., Jr.; Ely, K.R. Characterization of the PR domain of RIZ1 histone methyltransferase. Biochem. Biophys. Res. Commun. 2005, 333, 925–934. [Google Scholar] [CrossRef]
- Huang, S.; Shao, G.; Liu, L. The PR domain of the Rb-binding zinc finger protein RIZ1 is a protein binding interface and is related to the SET domain functioning in chromatin-mediated gene expression. J. Biol. Chem. 1998, 273, 15933–15939. [Google Scholar] [CrossRef] [PubMed]
- Abbondanza, C.; De Rosa, C.; D’Arcangelo, A.; Pacifico, M.; Spizuoco, C.; Piluso, G.; Di Zazzo, E.; Gazzerro, P.; Medici, N.; Moncharmont, B.; et al. Identification of a functional estrogen-responsive enhancer element in the promoter 2 of PRDM2 gene in breast cancer cell lines. J. Cell. Physiol. 2012, 227, 964–975. [Google Scholar] [CrossRef]
- Liu, L.; Shao, G.; Steele-Perkins, G.; Huang, S. The retinoblastoma interacting zinc finger gene RIZ produces a PR domain-lacking product through an internal promoter. J. Biol. Chem. 1997, 272, 2984–2991. [Google Scholar] [CrossRef]
- Xie, M.; Shao, G.; Buyse, I.M.; Huang, S. Transcriptional repression mediated by the PR domain zinc finger gene RIZ. J. Biol. Chem. 1997, 272, 26360–26366. [Google Scholar] [CrossRef] [PubMed]
- Huang, S. The retinoblastoma protein-interacting zinc finger gene RIZ in 1p36-linked cancers. Front. Biosci. 1999, 4, D528–D532. [Google Scholar] [CrossRef] [PubMed]
- Piao, Z.; Fang, W.; Malkhosyan, S.; Kim, H.; Horii, A.; Perucho, M.; Huang, S. Frequent frameshift mutations of RIZ in sporadic gastrointestinal and endometrial carcinomas with microsatellite instability. Cancer Res. 2000, 60, 4701–4704. [Google Scholar]
- Steele-Perkins, G.; Fang, W.; Yang, X.-H.; Van Gele, M.; Carling, T.; Gu, J.; Buyse, I.M.; Fletcher, J.A.; Liu, J.; Bronson, R.; et al. Tumor formation and inactivation of RIZ1, an Rb-binding member of a nuclear protein–methyltransferase superfamily. Genes Dev. 2001, 15, 2250–2262. [Google Scholar] [CrossRef] [PubMed]
- Congdon, L.M.; Sims, J.K.; Tuzon, C.T.; Rice, J.C. The PR-Set7 binding domain of Riz1 is required for the H4K20me1-H3K9me1 trans-tail ‘histone code’and Riz1 tumor suppressor function. Nucleic Acids Res. 2014, 42, 3580–3589. [Google Scholar] [CrossRef]
- Du, Y.; Carling, T.; Fang, W.; Piao, Z.; Sheu, J.-C.; Huang, S. Hypermethylation in human cancers of the RIZ1 tumor suppressor gene, a member of a histone/protein methyltransferase superfamily. Cancer Res. 2001, 61, 8094–8099. [Google Scholar]
- Gazzerro, P.; Bontempo, P.; Schiavone, E.M.; Abbondanza, C.; Moncharmont, B.; Armetta, I.; Medici, N.; Simone, M.D.; Nola, E.; Puca, G.A.; et al. Differentiation of myeloid cell lines correlates with a selective expression of RIZ protein. Mol. Med. 2001, 7, 552–560. [Google Scholar] [CrossRef]
- Xie, W.; Li, X.; Chen, X.; Huang, S.; Huang, S. Decreased expression of PRDM2 (RIZ1) and its correlation with risk stratification in patients with myelodysplastic syndrome. Br. J. Haematol. 2010, 150, 242–244. [Google Scholar] [CrossRef]
- Zhao, F.; Chen, Y.; Zeng, L.-l.; Li, R.; Zeng, R.; Wen, L.; Liu, Y.; Zhang, C. Effects of triptolide on RIZ1 expression, proliferation, and apoptosis in multiple myeloma U266 cells. Acta Pharmacol. Sin. 2010, 31, 733–740. [Google Scholar] [CrossRef]
- Pastural, E.; Takahashi, N.; Dong, W.; Bainbridge, M.; Hull, A.; Pearson, D.; Huang, S.; Lowsky, R.; DeCoteau, J.; Geyer, C. RIZ1 repression is associated with insulin-like growth factor-1 signaling activation in chronic myeloid leukemia cell lines. Oncogene 2007, 26, 1586–1594. [Google Scholar] [CrossRef]
- Lakshmikuttyamma, A.; Takahashi, N.; Pastural, E.; Torlakovic, E.; Amin, H.M.; Garcia-Manero, G.; Voralia, M.; Czader, M.; DeCoteau, J.F.; Geyer, C.R. RIZ1 is potential CML tumor suppressor that is down-regulated during disease progression. J. Hematol. Oncol. 2009, 2, 28. [Google Scholar] [CrossRef]
- Cheedipudi, S.; Puri, D.; Saleh, A.; Gala, H.P.; Rumman, M.; Pillai, M.S.; Sreenivas, P.; Arora, R.; Sellathurai, J.; Schrøder, H.D.; et al. A fine balance: Epigenetic control of cellular quiescence by the tumor suppressor PRDM2/RIZ at a bivalent domain in the cyclin a gene. Nucleic Acids Res. 2015, 43, 6236–6256. [Google Scholar] [CrossRef]
- Noman, A.S.M.; Koide, N.; Iftakhar-E-Khuda, I.; Dagvadorj, J.; Tumurkhuu, G.; Naiki, Y.; Komatsu, T.; Yoshida, T.; Yokochi, T. Retinoblastoma protein-interacting zinc finger 1, a tumor suppressor, augments lipopolysaccharide-induced proinflammatory cytokine production via enhancing nuclear factor-κB activation. Cell. Immunol. 2010, 264, 114–118. [Google Scholar] [CrossRef]
- Noman, A.S.; Koide, N.; Iftakhar, E.K.I.; Dagvadorj, J.; Tumurkhuu, G.; Naiki, Y.; Komatsu, T.; Yoshida, T.; Yokochi, T. Retinoblastoma protein-interacting zinc finger 1 (RIZ1) participates in RANKL-induced osteoclast formation via regulation of NFATc1 expression. Immunol. Lett. 2010, 131, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Man, X.; Zhu, Z.; Yuan, D.; Huang, S. Tumor suppressor RIZ1 in obesity and the PI3K/AKT/mTOR pathway. Obesity 2016, 24, 389–397. [Google Scholar] [CrossRef]
- Liu, Q.; Qu, X.; Xie, X.; He, P.; Huang, S. Repression of Akt3 gene transcription by the tumor suppressor RIZ1. Sci. Rep. 2018, 8, 1528. [Google Scholar] [CrossRef]
- Carling, T.; Kim, K.C.; Yang, X.H.; Gu, J.; Zhang, X.K.; Huang, S. A histone methyltransferase is required for maximal response to female sex hormones. Mol. Cell. Biol. 2004, 24, 7032–7042. [Google Scholar] [CrossRef]
- Gazzerro, P.; Abbondanza, C.; D’Arcangelo, A.; Rossi, M.; Medici, N.; Moncharmont, B.; Puca, G.A. Modulation of RIZ gene expression is associated to estradiol control of MCF-7 breast cancer cell proliferation. Exp. Cell Res. 2006, 312, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Abbondanza, C.; D’Arcangelo, A.; Gazzerro, P.; Medici, N.; Moncharmont, B.; Puca, G.A. The Zn-finger domain of RIZ protein promotes MCF-7 cell proliferation. Cancer Lett. 2004, 215, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Rossi, V.; Staibano, S.; Abbondanza, C.; Pasquali, D.; De Rosa, C.; Mascolo, M.; Bellastella, G.; Visconti, D.; De Bellis, A.; Moncharmont, B.; et al. Expression of RIZ1 protein (Retinoblastoma-interacting zinc-finger protein 1) in prostate cancer epithelial cells changes with cancer grade progression and is modulated in vitro by DHT and E2. J. Cell. Physiol. 2009, 221, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Rienzo, M.; Sorrentino, A.; Di Zazzo, E.; Di Donato, M.; Carafa, V.; Marino, M.M.; De Rosa, C.; Gazzerro, P.; Castoria, G.; Altucci, L.; et al. Searching for a putative mechanism of RIZ2 tumor-promoting function in cancer models. Front. Oncol. 2021, 10, 583533. [Google Scholar] [CrossRef]
- Grundberg, E.; Carling, T.; Brandstrom, H.; Huang, S.; Ribom, E.; Ljunggren, O.; Mallmin, H.; Kindmark, A. A deletion polymorphism in the RIZ gene, a female sex steroid hormone receptor coactivator, exhibits decreased response to estrogen in vitro and associates with low bone mineral density in young Swedish women. J. Clin. Endocrinol. Metab. 2004, 89, 6173–6178. [Google Scholar] [CrossRef]
- Grundberg, E.; Åkesson, K.; Kindmark, A.; Gerdhem, P.; Holmberg, A.; Mellström, D.; Ljunggren, Ö.; Orwoll, E.; Mallmin, H.; Ohlsson, C.; et al. The impact of estradiol on bone mineral density is modulated by the specific estrogen receptor-α cofactor retinoblastoma-interacting zinc finger protein-1 insertion/deletion polymorphism. J. Clin. Endocrinol. Metab. 2007, 92, 2300–2306. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chung, S.J.; Armasu, S.M.; Biernacka, J.M.; Lesnick, T.G.; Rider, D.N.; Cunningham, J.M.; Maraganore, D.M. Variants in estrogen-related genes and risk of Parkinson’s disease. Mov. Disord. 2011, 26, 1234–1242. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.l.; Liu, L.; Buyse, I.M.; Simon, D.; Huang, S. Decreased RIZ1 expression but not RIZ2 in hepatoma and suppression of hepatoma tumorigenicity by RIZ1. Int. J. Cancer 1999, 83, 541–546. [Google Scholar] [CrossRef]
- Chen, L.-B.; Xu, J.-Y.; Yang, Z.; Wang, G.-B. Silencing SMYD3 in hepatoma demethylates RIZI promoter induces apoptosis and inhibits cell proliferation and migration. World J. Gastroenterol. WJG 2007, 13, 5718. [Google Scholar] [CrossRef]
- Zhang, C.; Li, H.; Wang, Y.; Liu, W.; Zhang, Q.; Zhang, T.; Zhang, X.; Han, B.; Zhou, G. Epigenetic inactivation of the tumor suppressor gene RIZ1 in hepatocellular carcinoma involves both DNA methylation and histone modifications. J. Hepatol. 2010, 53, 889–895. [Google Scholar] [CrossRef]
- Nishida, N.; Kudo, M.; Nagasaka, T.; Ikai, I.; Goel, A. Characteristic patterns of altered DNA methylation predict emergence of human hepatocellular carcinoma. Hepatology 2012, 56, 994–1003. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-Q.; Sun, N.; Zhang, C.-S.; Li, N.; Wu, B.; Zhang, J.-L. Inactivation of lncRNA HOTAIRM1 caused by histone methyltransferase RIZ1 accelerated the proliferation and invasion of liver cancer. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 8767–8777. [Google Scholar]
- Zhao, Z.; Hu, Y.; Shen, X.; Lao, Y.; Zhang, L.; Qiu, X.; Hu, J.; Gong, P.; Cui, H.; Lu, S. HBx represses RIZ1 expression by DNA methyltransferase 1 involvement in decreased miR-152 in hepatocellular carcinoma. Oncol. Rep. 2017, 37, 2811–2818. [Google Scholar] [CrossRef]
- Abnet, C.C.; Arnold, M.; Wei, W.Q. Epidemiology of Esophageal Squamous Cell Carcinoma. Gastroenterology 2018, 154, 360–373. [Google Scholar] [CrossRef]
- Dong, S.-W.; Zhang, P.; Liu, Y.-M.; Cui, Y.-T.; Wang, S.; Liang, S.-J.; He, Z.; Sun, P.; Wang, Y.-G. Study on RIZ1 gene promoter methylation status in human esophageal squamous cell carcinoma. World J. Gastroenterol. WJG 2012, 18, 576. [Google Scholar] [CrossRef]
- Dong, S.-W.; Li, D.; Xu, C.; Sun, P.; Wang, Y.-G.; Zhang, P. Alteration in gene expression profile and oncogenicity of esophageal squamous cell carcinoma by RIZ1 upregulation. World J. Gastroenterol. WJG 2013, 19, 6170. [Google Scholar] [CrossRef]
- Dong, S.-W.; Zhang, H.; Wang, B.-L.; Sun, P.; Wang, Y.-G.; Zhang, P. Effect of the downregulation of SMYD3 expression by RNAi on RIZ1 expression and proliferation of esophageal squamous cell carcinoma. Oncol. Rep. 2014, 32, 1064–1070. [Google Scholar] [CrossRef]
- Crew, K.D.; Neugut, A.I. Epidemiology of gastric cancer. World J. Gastroenterol. 2006, 12, 354–362. [Google Scholar] [CrossRef]
- Tokumaru, Y.; Nomoto, S.; Jeronimo, C.; Henrique, R.; Harden, S.; Trink, B.; Sidransky, D. Biallelic inactivation of the RIZ1 gene in human gastric cancer. Oncogene 2003, 22, 6954–6958. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Oshimo, Y.; Oue, N.; Mitani, Y.; Nakayama, H.; Kitadai, Y.; Yoshida, K.; Chayama, K.; Yasui, W. Frequent epigenetic inactivation of RIZ1 by promoter hypermethylation in human gastric carcinoma. Int. J. Cancer 2004, 110, 212–218. [Google Scholar] [CrossRef]
- Jiang, G.-L.; Huang, S. Adenovirus expressing RIZ1 in tumor suppressor gene therapy of microsatellite-unstable colorectal cancers. Cancer Res. 2001, 61, 1796–1798. [Google Scholar] [PubMed]
- Chadwick, R.B.; Jiang, G.-L.; Bennington, G.A.; Yuan, B.; Johnson, C.K.; Stevens, M.W.; Niemann, T.H.; Peltomaki, P.; Huang, S.; De La Chapelle, A. Candidate tumor suppressor RIZ is frequently involved in colorectal carcinogenesis. Proc. Natl. Acad. Sci. USA 2000, 97, 2662–2667. [Google Scholar] [CrossRef]
- van Toledo, D.E.; Bleijenberg, A.G.; Venema, A.; de Wit, M.J.; van Eeden, S.; Meijer, G.A.; Carvalho, B.; Dekker, E.; Henneman, P.; IJspeert, J.E.; et al. Aberrant PRDM2 methylation as an early event in serrated lesions destined to evolve into microsatellite-instable colorectal cancers. J. Pathol. Clin. Res. 2024, 10, e348. [Google Scholar] [CrossRef] [PubMed]
- Di Donato, M.; Di Zazzo, E.; Salvati, A.; Sorrentino, C.; Giurato, G.; Fiore, D.; Proto, M.C.; Rienzo, M.; Casamassimi, A.; Gazzerro, P.; et al. RIZ2 at the crossroad of the EGF/EGFR signaling in colorectal cancer. J. Transl. Med. 2023, 21, 736. [Google Scholar] [CrossRef]
- Geli, J.; Nord, B.; Frisk, T.; Edström Elder, E.; Ekström, T.J.; Carling, T.; Bäckdahl, M.; Larsson, C. Deletions and altered expression of the RIZ1 tumour suppressor gene in 1p36 in pheochromocytomas and abdominal paragangliomas. Int. J. Oncol. 2005, 26, 1385–1391. [Google Scholar] [CrossRef] [PubMed]
- Geli, J.; Kiss, N.; Kogner, P.; Larsson, C. Suppression of RIZ in biologically unfavourable neuroblastomas. Int. J. Oncol. 2010, 37, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, J.; Liu, H.; Ma, X.; Wang, C.; Zhang, X.; Tao, Y.; Lu, Y.; Liao, J.; Hu, G. Retinoblastoma protein-interacting zinc-finger gene 1 (RIZ1) dysregulation in human malignant meningiomas. Oncogene 2013, 32, 1216–1222. [Google Scholar] [CrossRef]
- Zhang, C.; Zhu, Q.; He, H.; Jiang, L.; Qiang, Q.; Hu, L.; Hu, G.; Jiang, Y.; Ding, X.; Lu, Y. RIZ1: A potential tumor suppressor in glioma. BMC Cancer 2015, 15, 990. [Google Scholar] [CrossRef]
- Ding, M.-H.; Wang, Z.; Jiang, L.; Fu, H.-L.; Gao, J.; Lin, X.-B.; Zhang, C.-L.; Liu, Z.-Y.; Shi, Y.-F.; Qiu, G.-Z.; et al. The transducible TAT-RIZ1-PR protein exerts histone methyltransferase activity and tumor-suppressive functions in human malignant meningiomas. Biomaterials 2015, 56, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Meng, W.; Wang, J.; Lu, Y.; Hu, G.; Hu, L.; Ma, J. Methylation status of the RIZ1 gene promoter in human glioma tissues and cell lines. Cell. Mol. Neurobiol. 2017, 37, 1021–1027. [Google Scholar] [CrossRef]
- He, L.; Yu, J.X.; Liu, L.; Buyse, I.M.; Wang, M.-s.; Yang, Q.-c.; Nakagawara, A.; Brodeur, G.M.; Shi, Y.E.; Huang, S. RIZ1, but not the alternative RIZ2 product of the same gene, is underexpressed in breast cancer, and forced RIZ1 expression causes G2-M cell cycle arrest and/or apoptosis. Cancer Res. 1998, 58, 4238–4244. [Google Scholar]
- Hasegawa, Y.; Matsubara, A.; Teishima, J.; Seki, M.; Mita, K.; Usui, T.; Oue, N.; Yasui, W. DNA methylation of the RIZ1 gene is associated with nuclear accumulation of p53 in prostate cancer. Cancer Sci. 2007, 98, 32–36. [Google Scholar] [CrossRef]
- Di Zazzo, E.; Porcile, C.; Bartollino, S.; Moncharmont, B. Critical function of PRDM2 in the neoplastic growth of testicular germ cell tumors. Biology 2016, 5, 54. [Google Scholar] [CrossRef]
- Cheng, H.; Zhang, T.; Qu, Y.; Shi, W.; Lou, G.; Liu, Y.; Zhang, Y.; Cheng, L. Synergism between RIZ1 gene therapy and paclitaxel in SiHa cervical cancer cells. Cancer Gene Ther. 2016, 23, 392–395. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Q.; Sun, Q.; Tang, J.; Chen, J.; Ji, N.; Zheng, Y.; Fang, F.; Lei, W.; Li, P.; et al. Genome evolution analysis of recurrent testicular malignant mesothelioma by whole-genome sequencing. Cell. Physiol. Biochem. 2018, 45, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Carling, T.; Du, Y.; Fang, W.; Correa, P.; Huang, S. Intragenic allelic loss and promoter hypermethylation of the RIZ1 tumor suppressor gene in parathyroid tumors and pheochromocytomas. Surgery 2003, 134, 932–939. [Google Scholar] [CrossRef] [PubMed]
- Lal, G.; Padmanabha, L.; Smith, B.J.; Nicholson, R.M.; Howe, J.R.; O’Dorisio, M.S.; Domann, F.E., Jr. RIZ1 is epigenetically inactivated by promoter hypermethylation in thyroid carcinoma. Cancer 2006, 107, 2752–2759. [Google Scholar] [CrossRef]
- Gao, H.; Wang, F.; Lan, X.; Li, C.; Feng, J.; Bai, J.; Cao, L.; Gui, S.; Hong, L.; Zhang, Y. Lower PRDM2 expression is associated with dopamine-agonist resistance and tumor recurrence in prolactinomas. BMC Cancer 2015, 15, 272. [Google Scholar] [CrossRef]
- Chang, H.W.; Chan, A.; Kwong, D.L.W.; Wei, W.I.; Sham, J.S.T.; Yuen, A.P.W. Detection of hypermethylated RIZ1 gene in primary tumor, mouth, and throat rinsing fluid, nasopharyngeal swab, and peripheral blood of nasopharyngeal carcinoma patient. Clin. Cancer Res. 2003, 9, 1033–1038. [Google Scholar]
- Yoon, K.-A.; Park, S.; Hwangbo, B.; Shin, H.D.; Cheong, H.S.; Shin, H.-R.; Lee, J.S. Genetic polymorphisms in the Rb-binding zinc finger gene RIZ and the risk of lung cancer. Carcinogenesis 2007, 28, 1971–1977. [Google Scholar] [CrossRef]
- Abbondanza, C.; de Nigris, F.; De Rosa, C.; Rossiello, R.; Puca, G.A.; Napoli, C. Silencing of YY1 downregulates RIZ1 promoter in human osteosarcoma. Oncol. Res. 2008, 17, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Johansson, P.; Klein-Hitpass, L.; Choidas, A.; Habenberger, P.; Mahboubi, B.; Kim, B.; Bergmann, A.; Scholtysik, R.; Brauser, M.; Lollies, A.; et al. SAMHD1 is recurrently mutated in T-cell prolymphocytic leukemia. Blood Cancer J. 2018, 8, 11. [Google Scholar] [CrossRef]
- Poetsch, M.; Dittberner, T.; Woenckhaus, C. Frameshift mutations of RIZ, but no point mutations in RIZ1 exons in malignant melanomas with deletions in 1p36. Oncogene 2002, 21, 3038–3042. [Google Scholar] [CrossRef]
- Di Zazzo, E.; Rienzo, M.; Casamassimi, A.; De Rosa, C.; Medici, N.; Gazzerro, P.; Bifulco, M.; Abbondanza, C. Exploring the putative role of PRDM1 and PRDM2 transcripts as mediators of T lymphocyte activation. J. Transl. Med. 2023, 21, 217. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, S.; Li, H.; Zhu, C.; Zhang, L.; Zou, J. PRDM2—The Key Research Targets for the Development of Diseases in Various Systems. Biomolecules 2025, 15, 1170. https://doi.org/10.3390/biom15081170
Deng S, Li H, Zhu C, Zhang L, Zou J. PRDM2—The Key Research Targets for the Development of Diseases in Various Systems. Biomolecules. 2025; 15(8):1170. https://doi.org/10.3390/biom15081170
Chicago/Turabian StyleDeng, Shiqi, Hui Li, Chenyu Zhu, Lingli Zhang, and Jun Zou. 2025. "PRDM2—The Key Research Targets for the Development of Diseases in Various Systems" Biomolecules 15, no. 8: 1170. https://doi.org/10.3390/biom15081170
APA StyleDeng, S., Li, H., Zhu, C., Zhang, L., & Zou, J. (2025). PRDM2—The Key Research Targets for the Development of Diseases in Various Systems. Biomolecules, 15(8), 1170. https://doi.org/10.3390/biom15081170





