Promotion of Bone Defect Repair Using Decellularized Antler Cancellous Bone Loaded with Deer Osteoglycin
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Purification of dOGN
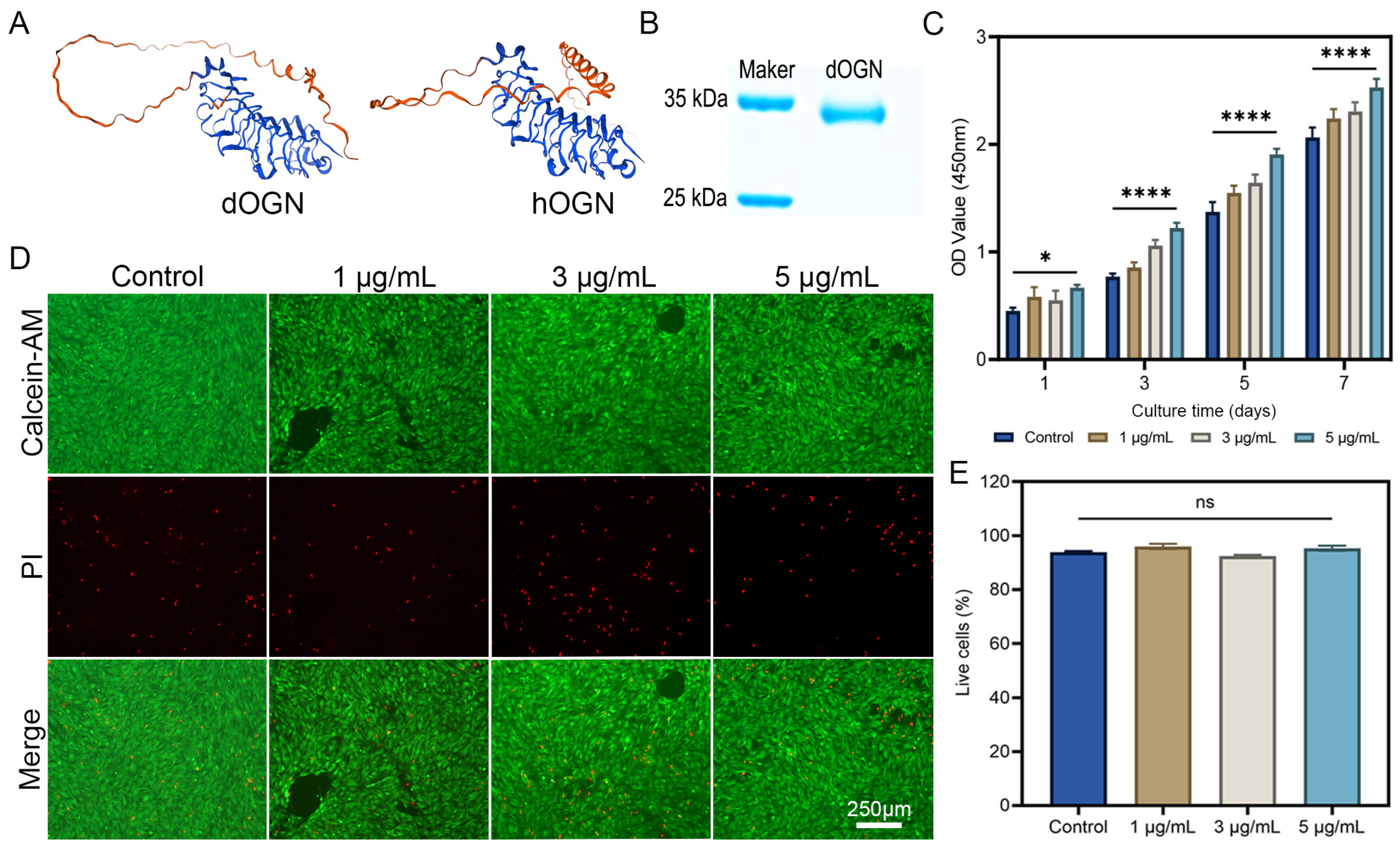
2.3. Bioactivity Assay of dOGN In Vitro
2.3.1. Cell Proliferation and Toxicity Assay
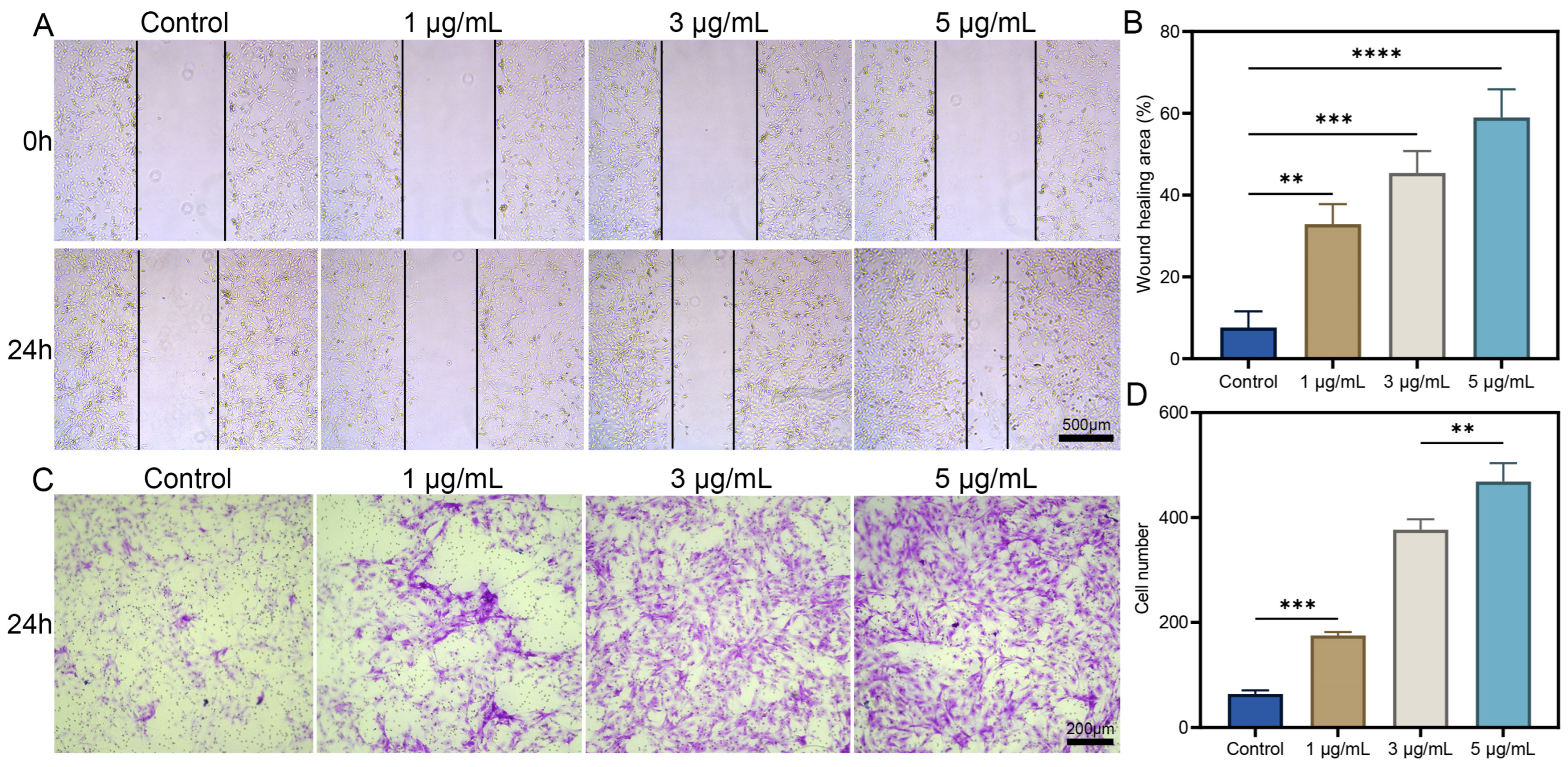
2.3.2. Cell Migration
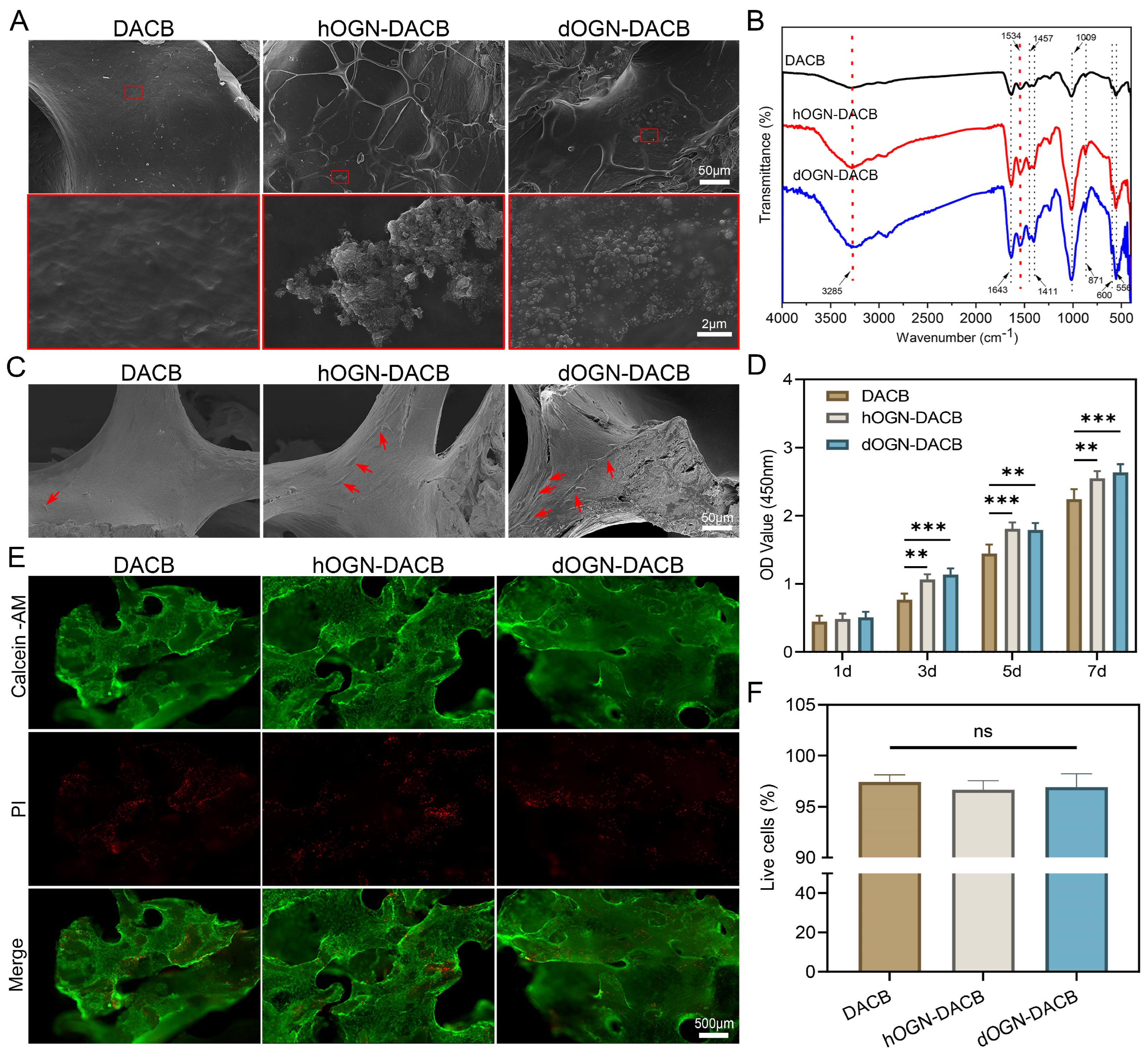
2.4. OGN Loading and Characterization on DACB
2.5. In Vitro Release Assay
2.6. Attachment and Proliferation of Cells
2.7. Cytotoxicity Assessment
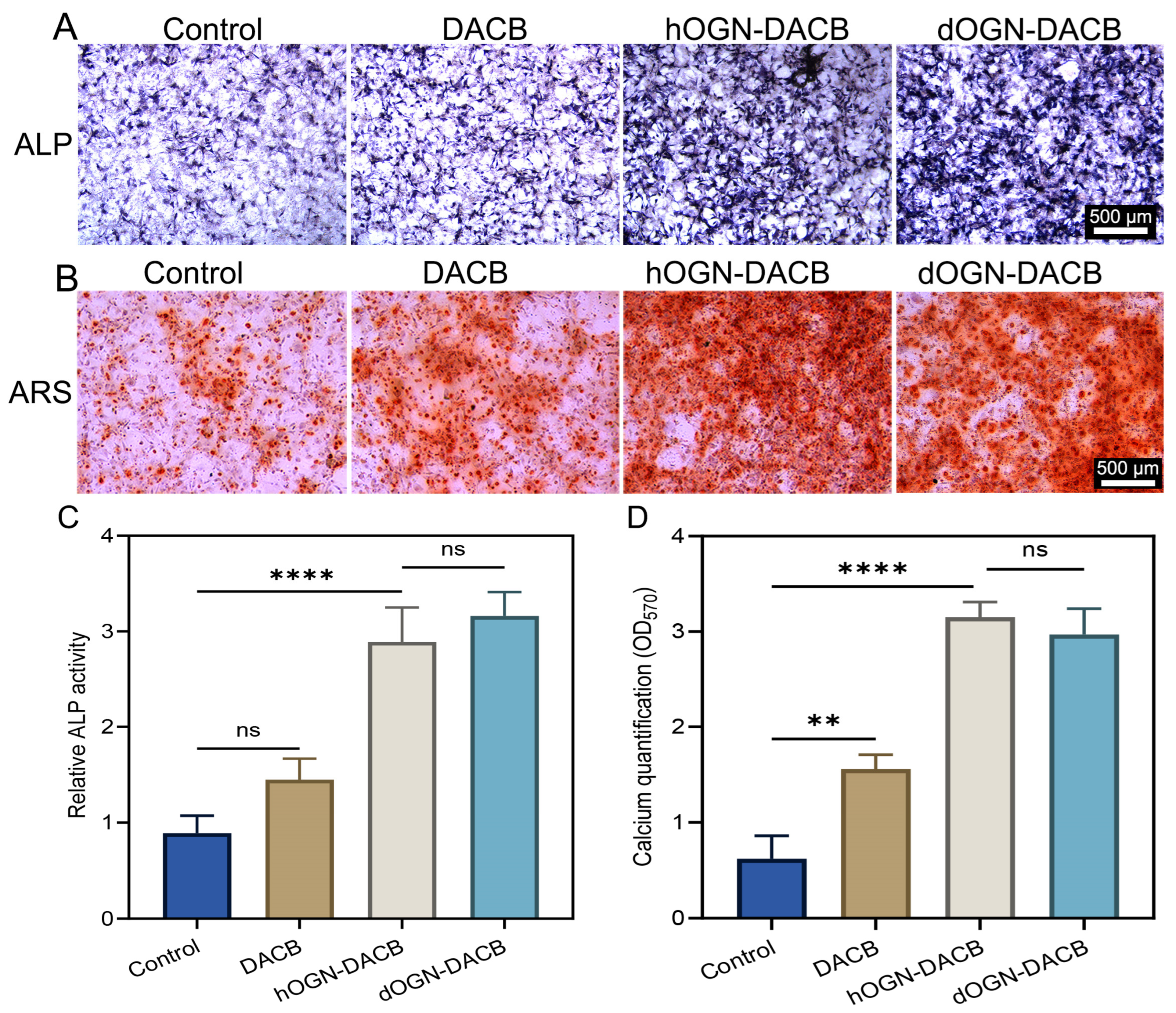
2.8. Osteogenic Differentiation In Vitro
2.9. In Vivo Osteogenic Assessment
2.10. Statistical Analysis
3. Results and Discussion
3.1. Preparation and Bioactivity Assays of dOGN In Vitro
3.2. Loading Characteristics of Bioactive Factors onto DACB
3.3. Cytocompatibility Test
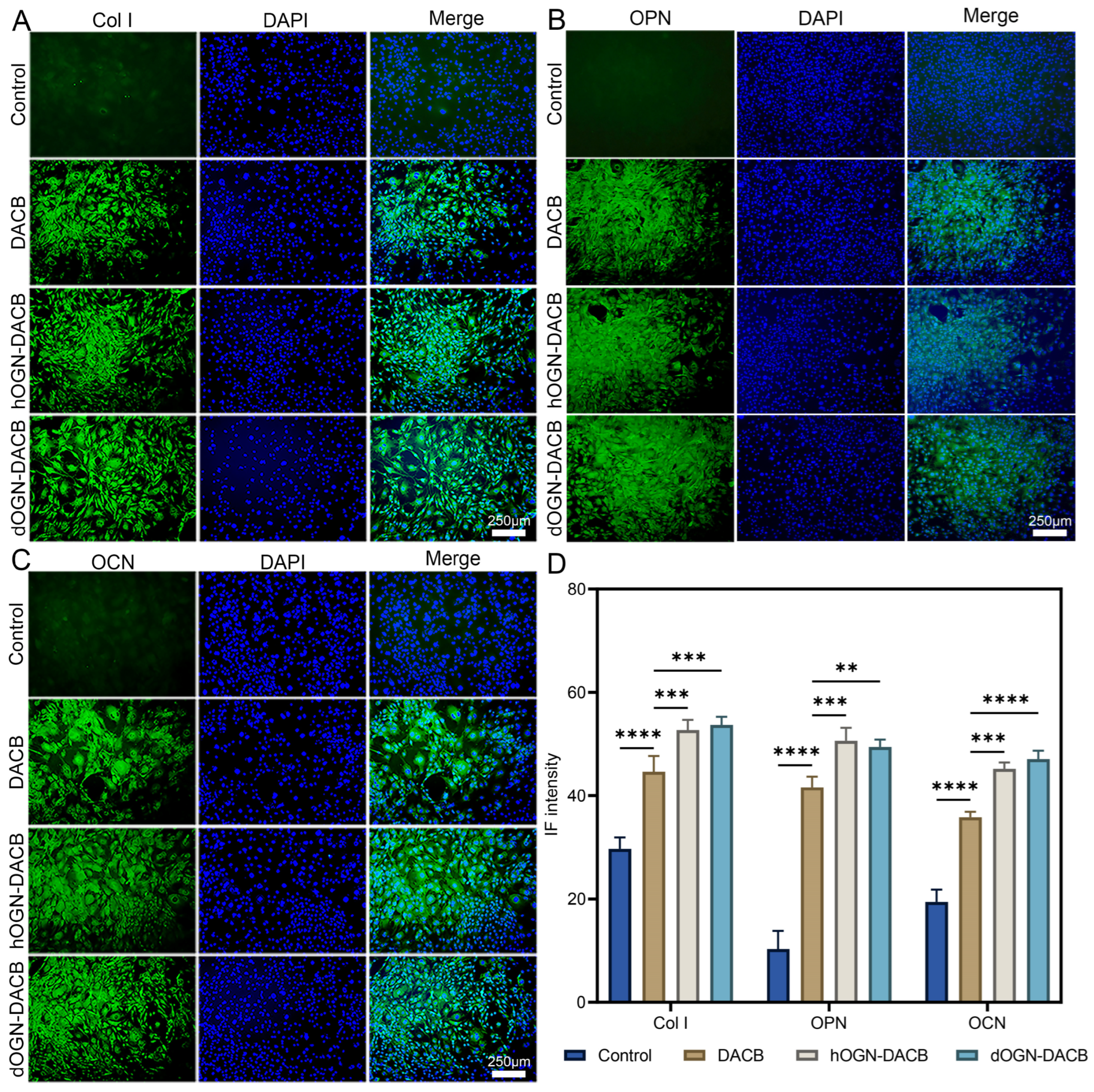
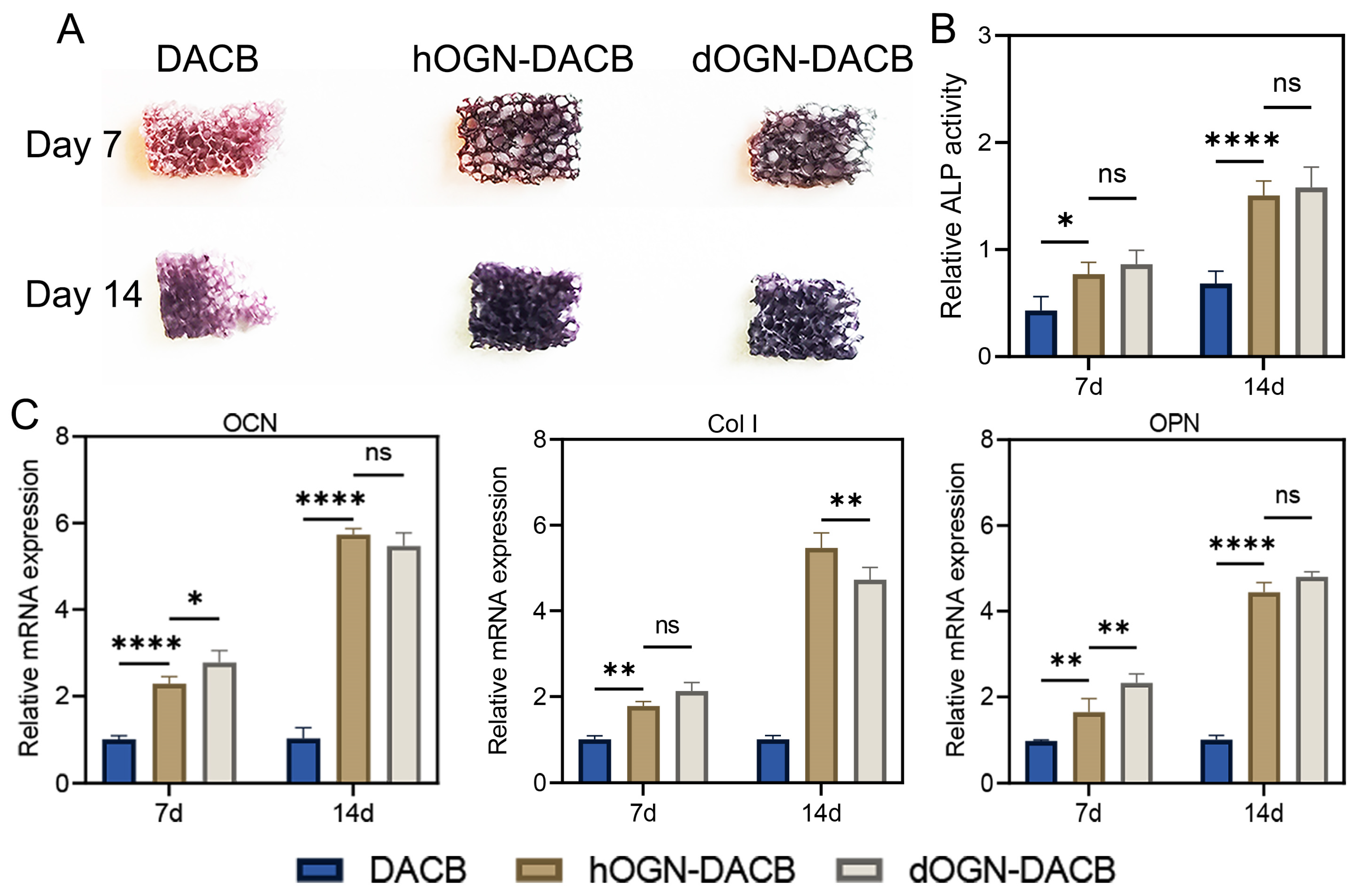
3.4. Osteogenic Differentiation Effect In Vitro
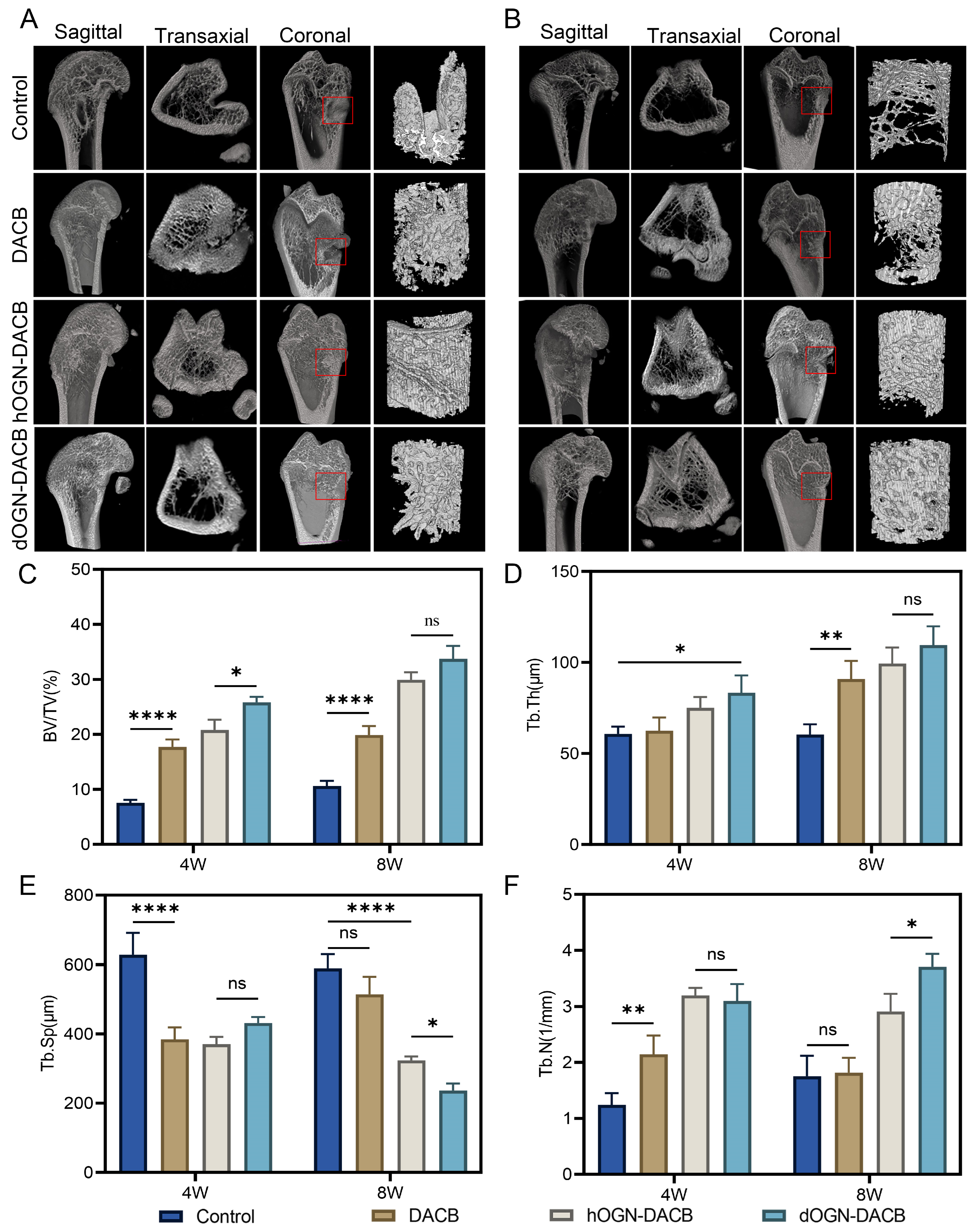
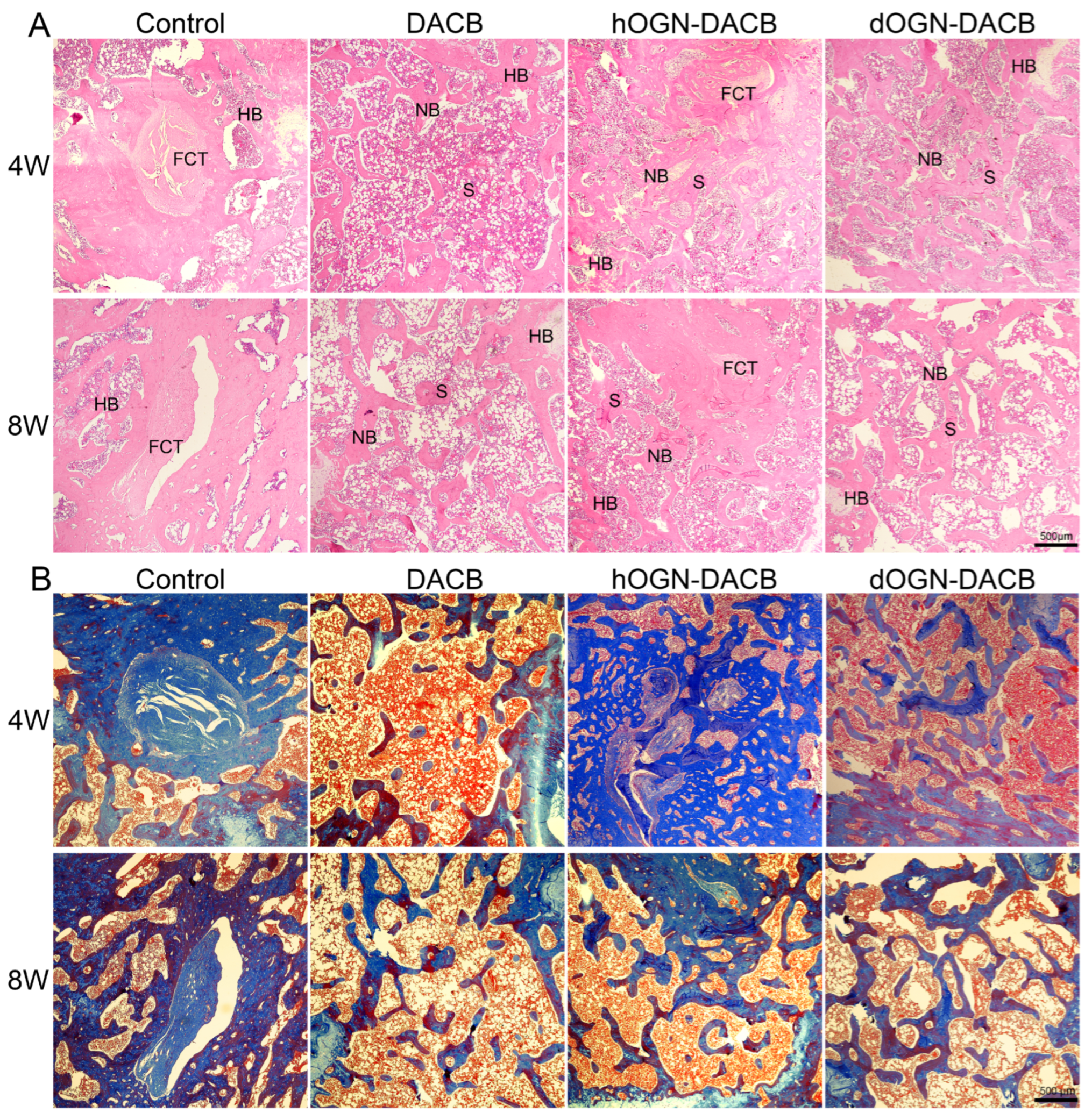
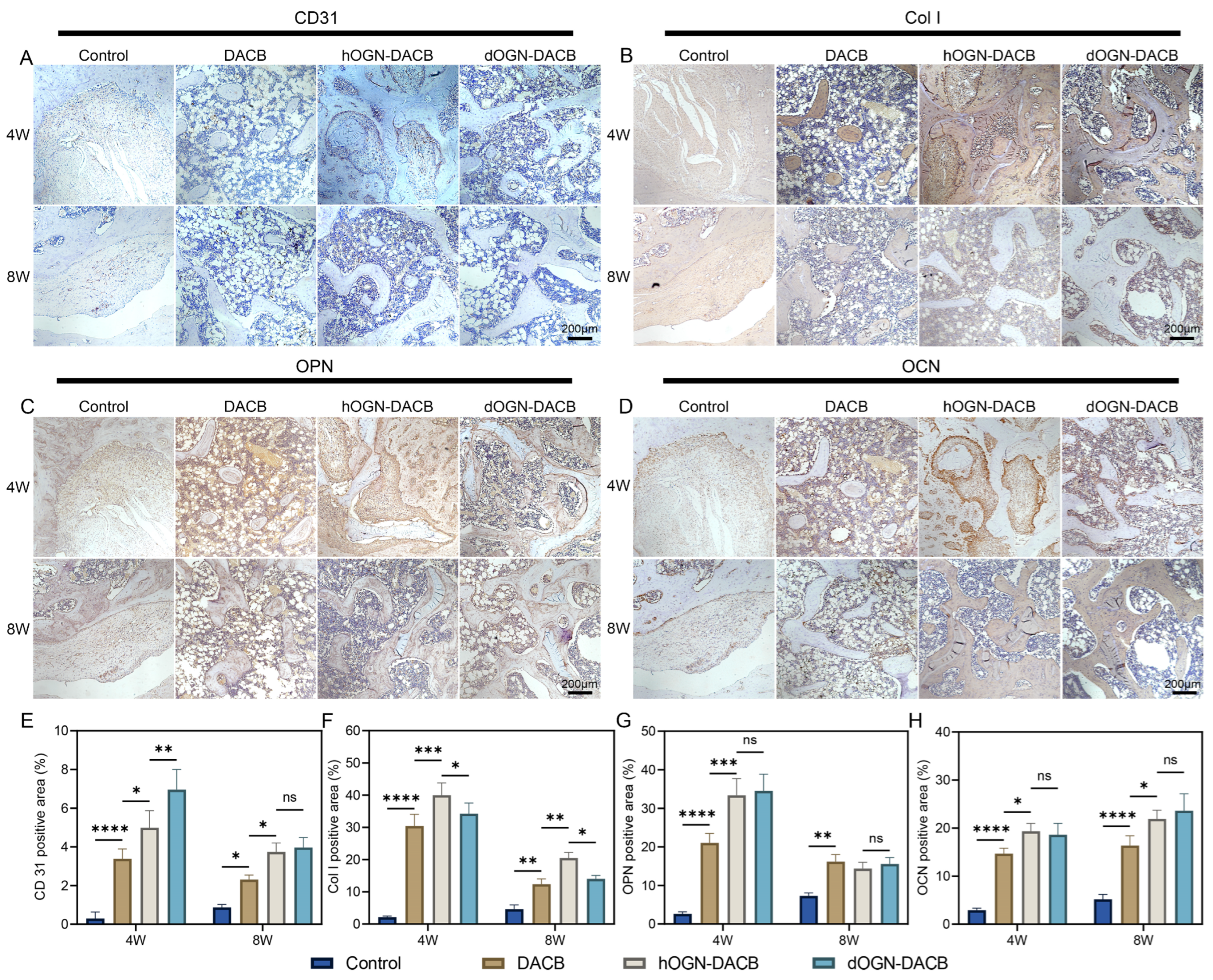
3.5. Bone Healing Effect In Vivo
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, J.J.; Ebied, M.; Xu, J.; Zreiqat, H. Current approaches to bone tissue engineering: The interface between biology and engineering. Adv. Healthc. Mater. 2018, 7, 1701061. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, J.; Mouser, V.H.M.; Roumans, N.; Moroni, L.; Habibovic, P. Biomimetic mechanically strong one-dimensional hydroxyapatite/poly (D, L-lactide) composite inducing formation of anisotropic collagen matrix. Polymers 2021, 15, 17480–17498. [Google Scholar] [CrossRef]
- Schmidt, A.H. Autologous bone graft: Is it still the gold standard? Injury 2021, 52, S18–S22. [Google Scholar] [CrossRef] [PubMed]
- Aslankoohi, N.; Mondal, D.; Rizkalla, A.S.; Mequanint, K. Bone repair and regenerative biomaterials: Towards recapitulating the microenvironment. Polymers 2019, 11, 1437. [Google Scholar] [CrossRef]
- Bauer, T.W.; Muschler, G.F. Bone graft materials: An overview of the basic science. Clin. Orthop. Relat. Res. 2000, 371, 10–27. [Google Scholar] [CrossRef]
- Wang, W.; Yeung, K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef]
- Feroz, S.; Cathro, P.; Ivanovski, S.; Muhammad, N. Biomimetic bone grafts and substitutes: A review of recent advancements and applications. Biomed. Eng. Adv. 2023, 6, 100107. [Google Scholar] [CrossRef]
- Carmagnola, D.; Adriaens, P.; Berglundh, T. Healing of human extraction sockets filled with Bio-Oss®. Clin. Orthop. Relat. Res. 2003, 14, 137–143. [Google Scholar] [CrossRef]
- Bracey, D.N.; Seyler, T.M.; Jinnah, A.H.; Smith, T.L.; Ornelles, D.A.; Deora, R.; Parks, G.D.; Van Dyke, M.E.; Whitlock, P.W. A porcine xenograft-derived bone scaffold is a biocompatible bone graft substitute: An assessment of cytocompatibility and the alpha-Gal epitope. Xenotransplantation 2019, 26, e12534. [Google Scholar] [CrossRef]
- Malagón-Escandón, A.; Hautefeuille, M.; Jimenez-Díaz, E.; Arenas-Alatorre, J.; Saniger, J.M.; Badillo-Ramírez, I.; Vazquez, N.; Piñón-Zarate, G.; Castell-Rodríguez, A. Three-Dimensional porous scaffolds derived from bovine cancellous bone matrix promote osteoinduction, osteoconduction, and osteogenesis. Polymers 2021, 13, 4390. [Google Scholar] [CrossRef]
- Zhao, J.; Chao, T.; Zhou, M.; Yue, K.; Xu, F.; Wang, H.; Guo, J.; Gao, Z. Decellularization techniques pave the way for tissue engineering and regenerative medicine: A narrative review. Regen. Med. Rep. 2024, 1, 117–130. [Google Scholar] [CrossRef]
- Wang, Y.; Zong, Y.; Chen, W.; Diao, N.; Zhao, Q.; Li, C.; Jia, B.; Zhang, M.; Li, J.; Zhao, Y.; et al. Decellularized antler cancellous bone matrix material can serve as potential bone tissue scaffold. Biomolecules 2024, 14, 907. [Google Scholar] [CrossRef] [PubMed]
- Ba, H.; Wang, D.; Yau, T.O.; Shang, Y.; Li, C. Transcriptomic analysis of different tissue layers in antler growth Center in Sika Deer (Cervus nippon). BMC Genom. 2019, 20, 173. [Google Scholar] [CrossRef]
- Zhang, W.; Chu, W.; Liu, Q.; Coates, D.; Shang, Y.; Li, C. Deer thymosin beta 10 functions as a novel factor for angiogenesis and chondrogenesis during antler growth and regeneration. Stem Cell Res. Ther. 2018, 9, 166. [Google Scholar] [CrossRef]
- Kierdorf, U.; Stock, S.R.; Gomez, S.; Antipova, O.; Kierdorf, H. Distribution, structure, and mineralization of calcified cartilage remnants in hard antlers. Bone Rep. 2022, 16, 101571. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yang, Y.; Yu, B.; Gao, X.; Gao, X.; Nie, S.; Qin, T.; Hao, Y.; Guo, L.; Wu, H.; et al. A novel deer antler-Inspired bone graft triggers rapid bone regeneration. Adv. Mater. 2025, 37, 2411571. [Google Scholar] [CrossRef]
- Stegen, S.; Carmeliet, G. The skeletal vascular system—Breathing life into bone tissue. Bone 2018, 115, 50–58. [Google Scholar] [CrossRef]
- Gao, J.; Ren, J.; Ye, H.; Chu, W.; Ding, X.; Ding, L.; Fu, Y. Thymosin beta 10 loaded ZIF-8/sericin hydrogel promoting angiogenesis and osteogenesis for bone regeneration. Int. J. Biol. Macromol. 2024, 267, 131562. [Google Scholar] [CrossRef]
- Plowman, G.; Rosen, D.; Segarini, P.; Dasch, J.; Thompson, A.; Ziman, J.; Bentz, H.; Purchio, A. Molecular cloning of a novel bone-forming compound: Osteoinductive factor. DNA Cell Biol. 1990, 9, 303–309. [Google Scholar]
- Tanaka, K.-I.; Matsumoto, E.; Higashimaki, Y.; Katagiri, T.; Sugimoto, T.; Seino, S.; Kaji, H. Role of osteoglycin in the linkage between muscle and bone. Cell Mol. Life Sci. 2012, 287, 11616–11628. [Google Scholar] [CrossRef]
- Chen, S.-H.; Wang, X.-L.; Xie, X.-H.; Zheng, L.-Z.; Yao, D.; Wang, D.-P.; Leng, Y.; Zhang, G.; Qin, L. Comparative study of osteogenic potential of a composite scaffold incorporating either endogenous bone morphogenetic protein-2 or exogenous phytomolecule icaritin: An in vitro efficacy study. Acta Biomater. 2012, 8, 3128–3137. [Google Scholar] [CrossRef] [PubMed]
- Rienks, M.; Papageorgiou, A.; Wouters, K.; Verhesen, W.; van Leeuwen, R.; Carai, P.; Summer, G.; Westermann, D.; Heymans, S. A novel 72-kDa leukocyte-derived osteoglycin enhances the activation of toll-like receptor 4 and exacerbates cardiac inflammation during viral myocarditis. Cell Mol. Life Sci. 2017, 74, 1511–1525. [Google Scholar] [CrossRef]
- Dimitriou, R.; Jones, E.; McGonagle, D.; Giannoudis, P.V. Bone regeneration: Current concepts and future directions. BMC Med. 2011, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.J.; Ali, N.; Zhang, L.; Qi, Y.; Clarke, I.; Enriquez, R.F.; Brzozowska, M.; Lee, I.C.; Rogers, M.J.; Laybutt, D.R.; et al. Osteoglycin, a novel coordinator of bone and glucose homeostasis. Bioact. Mater. 2018, 13, 30–44. [Google Scholar]
- Jóźwiak, T.; Filipkowska, U.; Bakuła, T. The use of exoskeletons and molts of farmed mealworm (Tenebrio molitor) for the removal of reactive dyes from aqueous solutions. Appl. Sci. 2023, 13, 7379. [Google Scholar] [CrossRef]
- Boutinguiza, M.; Pou, J.; Comesaña, R.; Lusquiños, F.; de Carlos, A.; León, B. Biological hydroxyapatite obtained from fish bones. Mater. Sci. Eng. C 2012, 32, 478–486. [Google Scholar] [CrossRef]
- Dehghani, N.; Haghiralsadat, F.; Yazdian, F.; Sadeghian-Nodoushan, F.; Ghasemi, N.; Mazaheri, F.; Pourmadadi, M.; Naghib, S.M. Chitosan/silk fibroin/nitrogen-doped carbon quantum dot/α-tricalcium phosphate nanocomposite electrospinned as a scaffold for wound healing application: In vitro and in vivo studies. Int. J. Biol. Macromol. 2023, 238, 124078. [Google Scholar] [CrossRef]
- Lee, Y.-H.; Wu, H.-C.; Yeh, C.-W.; Kuan, C.-H.; Liao, H.-T.; Hsu, H.-C.; Tsai, J.-C.; Sun, J.-S.; Wang, T.-W. Enzyme-crosslinked gene-activated matrix for the induction of mesenchymal stem cells in osteochondral tissue regeneration. Acta Biomater. 2017, 63, 210–226. [Google Scholar] [CrossRef]
- Wang, R.; Hua, Y.; Wu, H.; Wang, J.; Xiao, Y.-C.; Chen, X.; Ao, Q.; Zeng, Q.; Zhu, X.; Zhang, X. Hydroxyapatite nanoparticles promote TLR4 agonist-mediated anti-tumor immunity through synergically enhanced macrophage polarization. Acta Biomater. 2023, 164, 626–640. [Google Scholar] [CrossRef]
- Luginbuehl, V.; Meinel, L.; Merkle, H.P.; Gander, B. Localized delivery of growth factors for bone repair. Eur. J. Pharm. Biopharm. 2004, 58, 197–208. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, M.; Song, L.; Wei, Y.; Lin, Y.; Liu, W.; Heng, B.C.; Peng, H.; Wang, Y.; Deng, X. Effects of compatibility of deproteinized antler cancellous bone with various bioactive factors on their osteogenic potential. Biomaterials 2013, 34, 9103–9114. [Google Scholar] [CrossRef]
- Li, C.; Lv, H.; Du, Y.; Zhu, W.; Yang, W.; Wang, X.; Wang, J.; Chen, W. Biologically modified implantation as therapeutic bioabsorbable materials for bone defect repair. Regen. Ther. 2022, 19, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Granéli, C.; Thorfve, A.; Ruetschi, U.; Brisby, H.; Thomsen, P. Novel markers of osteogenic and adipogenic differentiation of human bone marrow stromal cells identified using a quantitative proteomics approach. Stem Cell Res. 2014, 12, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Chen, F.; Liu, H.; Wu, P.; Yang, Z.; Zhang, Z.; Su, J.; Cai, L.; Zhang, Y. Bioinspired sandwich-like hybrid surface functionalized scaffold capable of regulating osteogenesis, angiogenesis, and osteoclastogenesis for robust bone regeneration. Mater. Today Bio 2022, 17, 100458. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Li, H. Overexpressed microRNA-140 inhibits pulmonary fibrosis in interstitial lung disease via the Wnt signaling pathway by downregulating osteoglycin. Am. J. Physiol. Cell 2020, 319, C895–C905. [Google Scholar] [CrossRef]
- Diomede, F.; Marconi, G.D.; Fonticoli, L.; Pizzicanella, J.; Merciaro, I.; Bramanti, P.; Mazzon, E.; Trubiani, O. Functional relationship between osteogenesis and angiogenesis in tissue regeneration. Int. J. Mol. Sci. 2020, 21, 3242. [Google Scholar] [CrossRef]
- Saran, U.; Piperni, S.G.; Chatterjee, S. Role of angiogenesis in bone repair. Arch. Biochem. Biophys. 2014, 561, 109–117. [Google Scholar] [CrossRef]
- Chavez, T.; Gerecht, S. Engineering of the microenvironment to accelerate vascular regeneration. Trends Mol. Med. 2023, 29, 35–47. [Google Scholar] [CrossRef]
- Filipowska, J.; Tomaszewski, K.A.; Niedźwiedzki, Ł.; Walocha, J.A.; Niedźwiedzki, T. The role of vasculature in bone development, regeneration and proper systemic functioning. Angiogenesis 2017, 20, 291–302. [Google Scholar] [CrossRef]
- Ibne Mahbub, M.S.; Park, M.; Park, S.; Won, M.; Lee, B.; Kim, H.; Lee, B. dECM and β-TCP incorporation effect on the highly porous injectable bio-glass bead for enhanced bone regeneration: In-vitro, in-vivo insights. Int. J. Biol. Macromol. 2025, 305, 141040. [Google Scholar] [CrossRef]
- Boskey, A.; Coleman, L.R. Aging and bone. J. Dent. Res. 2010, 89, 1333–1348. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Gill, G.; Kaur, H.; Amhmed, M.; Jakhu, H. Role of osteopontin in bone remodeling and orthodontic tooth movement: A review. Prog. Orthod. 2018, 19, 18. [Google Scholar] [CrossRef] [PubMed]
- Depalle, B.; McGilvery, C.M.; Nobakhti, S.; Aldegaither, N.; Shefelbine, S.J.; Porter, A.E. Osteopontin regulates type I collagen fibril formation in bone tissue. Acta Biomater. 2021, 120, 194–202. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zong, Y.; Chen, W.; Diao, N.; Zhao, Q.; Jia, B.; Zhang, M.; Li, J.; Zhao, Y.; He, Z.; et al. Promotion of Bone Defect Repair Using Decellularized Antler Cancellous Bone Loaded with Deer Osteoglycin. Biomolecules 2025, 15, 1124. https://doi.org/10.3390/biom15081124
Wang Y, Zong Y, Chen W, Diao N, Zhao Q, Jia B, Zhang M, Li J, Zhao Y, He Z, et al. Promotion of Bone Defect Repair Using Decellularized Antler Cancellous Bone Loaded with Deer Osteoglycin. Biomolecules. 2025; 15(8):1124. https://doi.org/10.3390/biom15081124
Chicago/Turabian StyleWang, Yusu, Ying Zong, Weijia Chen, Naichao Diao, Quanmin Zhao, Boyin Jia, Miao Zhang, Jianming Li, Yan Zhao, Zhongmei He, and et al. 2025. "Promotion of Bone Defect Repair Using Decellularized Antler Cancellous Bone Loaded with Deer Osteoglycin" Biomolecules 15, no. 8: 1124. https://doi.org/10.3390/biom15081124
APA StyleWang, Y., Zong, Y., Chen, W., Diao, N., Zhao, Q., Jia, B., Zhang, M., Li, J., Zhao, Y., He, Z., & Du, R. (2025). Promotion of Bone Defect Repair Using Decellularized Antler Cancellous Bone Loaded with Deer Osteoglycin. Biomolecules, 15(8), 1124. https://doi.org/10.3390/biom15081124




