Involvement of Pathogenesis-Related Proteins and Their Roles in Abiotic Stress Responses in Plants
Abstract
1. Introduction
2. Overview of PR Proteins
2.1. PR-1 Proteins
2.2. PR-2 Proteins
2.3. PR-3, PR-4, PR-8, and PR-11 Proteins
2.4. PR-5 Proteins
2.5. PR-6 Proteins
2.6. PR-7 Proteins
2.7. PR-9 Proteins
2.8. PR-10 Proteins
2.9. PR-12 Proteins
2.10. PR-13 Proteins
2.11. PR-14 Proteins
2.12. PR-15 and PR-16 Proteins
2.13. PR-17 Proteins
3. Response of Plant PR Proteins to Abiotic Stress
3.1. Drought Stress or Osmotic Stress
3.2. High Salinity Stress
3.3. Low Temperature Stress
3.4. High-Temperature Stress
3.5. Other Abiotic Stresses
3.5.1. Heavy Metal Stress
3.5.2. UV Radiation Stress
3.5.3. Waterlogging Stress
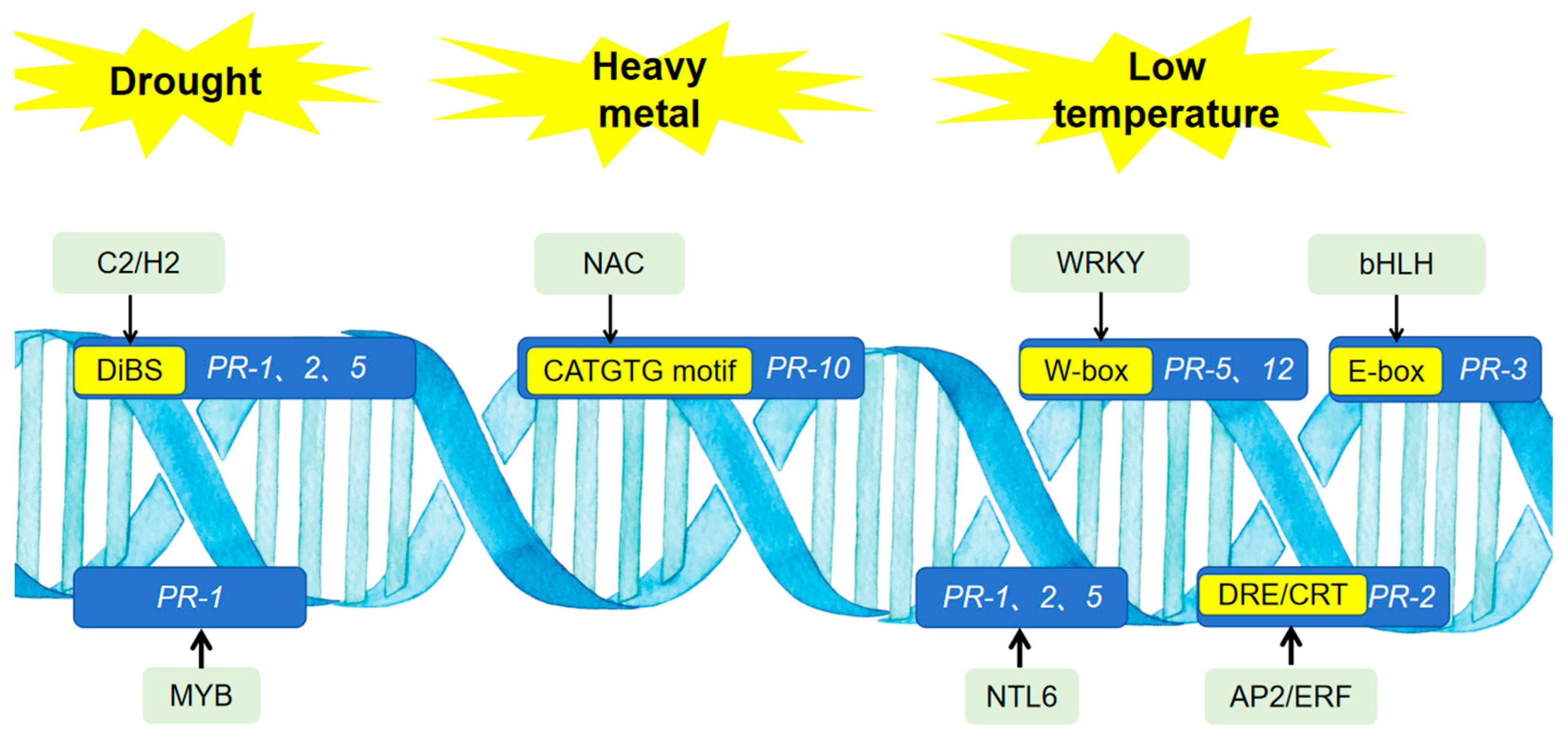
4. Regulation of PR Proteins Involved in Abiotic Stress Responses
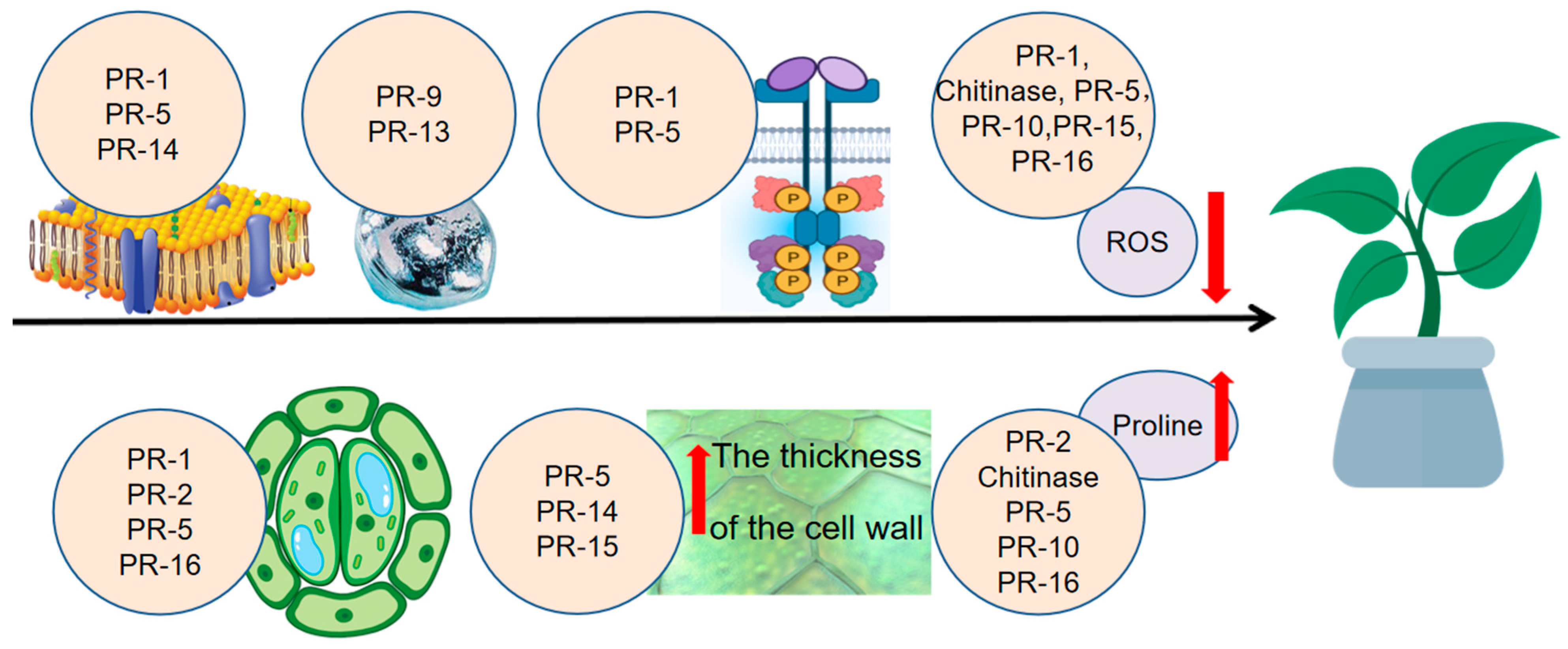
5. Biological Functions of PR Proteins in Abiotic Stress Responses
6. Conclusions
6.1. Critical Knowledge Gaps
6.2. Prospects for Study on PR Proteins
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sels, J.; Mathys, J.; De Coninck, B.M.A.; Cammue, B.P.A.; De Bolle, M.F.C. Plant pathogenesis-related (PR) proteins: A focus on PR peptides. Plant Physiol. Biochem. 2008, 46, 941–950. [Google Scholar] [CrossRef]
- Van Loon, L.C.; Van Kammen, A. Polyacrylamide disc electrophoresis of the soluble leaf proteins from Nicotiana tabacum var. “Samsun” and “Samsun NN”. II. Changes in protein constitution after infection with tobacco mosaic virus. Virology 1970, 40, 190–211. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-Y.; Gao, L.; Zhang, W.-H.; Liu, J.-K.; Zhang, Y.-J.; Wang, H.-Y.; Liu, D.-Q. Characteristic expression of wheat PR5 gene in response to infection by the leaf rust pathogen, Puccinia triticina. J. Plant Interact. 2015, 10, 132–141. [Google Scholar] [CrossRef]
- Van Loon, L.C.; Pierpoint, W.S.; Boller, T.; Conejero, V. Recommendations for naming plant pathogenesis-related proteins. Plant Mol. Biol. Rep. 1994, 12, 245–264. [Google Scholar] [CrossRef]
- Gordon-Weeks, R.; Sugars, J.; Antoniw, J.; White, R. Accumulation of a novel PR1 protein in Nicotiana langsdorfii leaves in response to virus infection or treatment with salicylic acid. Physiol. Mol. Plant Pathol. 1997, 50, 263–273. [Google Scholar] [CrossRef]
- Niki, T.; Mitsuhara, I.; Seo, S.; Ohtsubo, N.; Ohashi, Y. Antagonistic Effect of Salicylic Acid and Jasmonic Acid on the Expression of Pathogenesis-Related (PR) Protein Genes in Wounded Mature Tobacco Leaves. Plant Cell Physiol. 1998, 39, 500–507. [Google Scholar] [CrossRef]
- Fernández, C.; Szyperski, T.; Bruyère, T.; Ramage, P.; Mösinger, E.; Wüthrich, K. NMR solution structure of the pathogenesis-related protein P14a. J. Mol. Biol. 1997, 266, 576–593. [Google Scholar] [CrossRef]
- Sun, T.; Yan, N.; Liu, Q.; Bai, T.; Gao, H.; Chen, J. Re-Examination Characterization and Screening of Stripe Rust Resistance Gene of Wheat TaPR1 Gene Family Based on the Transcriptome in Xinchun 32. Int. J. Mol. Sci. 2025, 26, 640. [Google Scholar] [CrossRef]
- Kothari, K.S.; Dansana, P.K.; Giri, J.; Tyagi, A.K. Rice stress associated protein 1 (OsSAP1) interacts with aminotransferase (OsAMTR1) and pathogenesis-related 1a protein (OsSCP) and regulates abiotic stress responses. Front. Plant Sci. 2016, 7, 1057. [Google Scholar] [CrossRef]
- Liu, W.-X.; Zhang, F.-C.; Zhang, W.-Z.; Song, L.-F.; Wu, W.-H.; Chen, Y.-F. Arabidopsis Di19 functions as a transcription factor and modulates PR1, PR2, and PR5 expression in response to drought stress. Mol. Plant 2013, 6, 1487–1502. [Google Scholar] [CrossRef]
- Seo, P.J.; Kim, M.J.; Park, J.Y.; Kim, S.Y.; Jeon, J.; Lee, Y.H.; Kim, J.; Park, C.M. Cold activation of a plasma membrane-tethered NAC transcription factor induces a pathogen resistance response in Arabidopsis. Plant J. 2010, 61, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Akbudak, M.A.; Yildiz, S.; Filiz, E. Pathogenesis related protein-1 (PR-1) genes in tomato (Solanum lycopersicum L.): Bioinformatics analyses and expression profiles in response to drought stress. Genomics 2020, 112, 4089–4099. [Google Scholar] [CrossRef]
- Roy Choudhury, S.; Roy, S.; Singh, S.K.; Sengupta, D.N. Molecular characterization and differential expression of β-1, 3-glucanase during ripening in banana fruit in response to ethylene, auxin, ABA, wounding, cold and light–dark cycles. Plant Cell Rep. 2010, 29, 813–828. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, L.; Yang, S.; Liu, G.; Zeng, Q.; Liu, Y. Molecular characterization and functional analysis of a pathogenesis-related β-1, 3-glucanase gene in spruce (Picea asperata). Eur. J. Plant Pathol. 2022, 164, 177–192. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Keen, N.T.; Wang, M.-C. A Receptor on Soybean Membranes for a Fungal Elicitor of Phytoalexin Accumulation 1. Plant Physiol. 1983, 73, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Levy, A.; Erlanger, M.; Rosenthal, M.; Epel, B.L. A plasmodesmata-associated β-1,3-glucanase in Arabidopsis. Plant J. 2007, 49, 669–682. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-W.; Kumar, R.; Iswanto, A.B.B.; Kim, J.Y. Callose balancing at plasmodesmata. J. Exp. Bot. 2018, 69, 5325–5339. [Google Scholar] [CrossRef] [PubMed]
- Parveen, S.; Mazumder, M.; Bhattacharya, A.; Mukhopadhyay, S.; Saha, U.; Mukherjee, A.; Mondal, B.; Debnath, A.J.; Das, S.; Sikdar, S. Identification of anther-specific genes from sesame and functional assessment of the upstream region of a tapetum-specific β-1, 3-glucanase gene. Plant Mol. Biol. Rep. 2018, 36, 149–161. [Google Scholar] [CrossRef]
- Yaish, M.W.; Doxey, A.C.; McConkey, B.J.; Moffatt, B.A.; Griffith, M. Cold-active winter rye glucanases with ice-binding capacity. Plant Physiol. 2006, 141, 1459–1472. [Google Scholar] [CrossRef] [PubMed]
- Hincha, D.K.; Meins Jr, F.; Schmitt, J.M. β-1, 3-glucanase is cryoprotective in vitro and is accumulated in leaves during cold acclimation. Plant Physiol. 1997, 114, 1077–1083. [Google Scholar] [CrossRef][Green Version]
- Su, Y.-C.; Wang, Z.-Q.; Liu, F.; Li, Z.; Peng, Q.; Guo, J.-L.; Xu, L.-P.; Que, Y.-X. Isolation and Characterization of ScGluD 2, a New Sugarcane β-1, 3-Glucanase D Family Gene Induced by Sporisorium scitamineum, ABA, H2O2, NaCl, and CdCl2 Stresses. Front. Plant Sci. 2016, 7, 1348. [Google Scholar] [CrossRef]
- Kumar, M.; Brar, A.; Yadav, M.; Chawade, A.; Vivekanand, V.; Pareek, N. Chitinases—Potential candidates for enhanced plant resistance towards fungal pathogens. Agriculture 2018, 8, 88. [Google Scholar] [CrossRef]
- Hong, J.K.; Hwang, B.K. Induction by pathogen, salt and drought of a basic class II chitinase mRNA and its in situ localization in pepper (Capsicum annuum). Physiol. Plant. 2002, 114, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Bravo, J.M.; Campo, S.; Murillo, I.; Coca, M.; San Segundo, B. Fungus-and wound-induced accumulation of mRNA containing a class II chitinase of the pathogenesis-related protein 4 (PR-4) family of maize. Plant Mol. Biol. 2003, 52, 745–759. [Google Scholar] [CrossRef] [PubMed]
- Beintema, J.J. Structural features of plant chitinases and chitin-binding proteins. FEBS Lett. 1994, 350, 159–163. [Google Scholar] [CrossRef]
- Ohnuma, T.; Numata, T.; Osawa, T.; Mizuhara, M.; Lampela, O.; Juffer, A.H.; Skriver, K.; Fukamizo, T. A class V chitinase from Arabidopsis thaliana: Gene responses, enzymatic properties, and crystallographic analysis. Planta 2011, 234, 123–137. [Google Scholar] [CrossRef]
- Gomez, L.; Allona, I.; Casado, R.; Aragoncillo, C. Seed chitinases. Seed Sci. Res. 2002, 12, 217–230. [Google Scholar] [CrossRef]
- Maia, L.B.L.; Pereira, H.D.M.; Garratt, R.C.; Brandão-Neto, J.; Henrique-Silva, F.; Toyama, D.; Dias, R.O.; Bachega, J.F.R.; Peixoto, J.V.; Silva-Filho, M.C. Structural and Evolutionary Analyses of PR-4 SUGARWINs Points to a Different Pattern of Protein Function. Front. Plant Sci. 2021, 12, 734248. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, C.; Yokoyama, A.; Itoh, Y.; Hashimoto, M.; Watanabe, T.; Fukamizo, T. Comparative study of the reaction mechanism of eamily 18 chitinases from plants and microbes. J. Biochem. 2002, 131, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Nagpure, A.; Choudhary, B.; Gupta, R.K. Chitinases: In agriculture and human healthcare. Crit. Rev. Biotechnol. 2014, 34, 215–232. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.; Dutta, A.K.; Chandrashekara, K.N.; Acharya, K. In silico characterization, homology modeling of Camellia sinensis chitinase and its evolutionary analyses with other plant chitinases. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2017, 87, 685–695. [Google Scholar] [CrossRef]
- Šindelářová, M.; Šindelář, L. Isolation of pathogenesis-related proteins from TMV-infected tobacco and their influence on infectivity of TMV. Plant Prot. Sci. 2005, 41, 52–57. [Google Scholar] [CrossRef]
- Tapia, G.; Morales-Quintana, L.; Inostroza, L.; Acuna, H. Molecular characterisation of Ltchi7, a gene encoding a Class III endochitinase induced by drought stress in Lotus spp. Plant Biol. 2011, 13, 69–77. [Google Scholar] [CrossRef]
- Janská, A.; Maršík, P.; Zelenková, S.; Ovesná, J. Cold stress and acclimation–what is important for metabolic adjustment? Plant Biol. 2010, 12, 395–405. [Google Scholar] [CrossRef]
- Mészáros, P.; Rybanský, Ľ.; Spieß, N.; Socha, P.; Kuna, R.; Libantová, J.; Moravčíková, J.; Piršelová, B.; Hauptvogel, P.; Matušíková, I. Plant chitinase responses to different metal-type stresses reveal specificity. Plant Cell Rep. 2014, 33, 1789–1799. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.-C.; Lin, J.-H.; Chua, A.C.N.; Chung, T.-Y.; Tsai, I.C.; Tzen, J.T.C.; Chou, W.-M. Cloning and expression of pathogenesis-related protein 4 from jelly fig (Ficus awkeotsang Makino) achenes associated with ribonuclease, chitinase and anti-fungal activities. Plant Physiol. Biochem. 2012, 56, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Edens, L.; Heslinga, L.; Klok, R.; Ledeboer, A.M.; Maat, J.; Toonen, M.Y.; Visser, C.; Verrips, C.T. Cloning of cDNA encoding the sweet-tasting plant protein thaumatin and its expression in Escherichia coli. Gene 1982, 18, 1–12. [Google Scholar] [CrossRef]
- Kauffmann, S.; Legrand, M.; Fritig, B. Isolation and characterization of six pathogenesis-related (PR) proteins of Samsun NN tobacco. Plant Mol. Biol. 1990, 14, 381–390. [Google Scholar] [CrossRef]
- Brandazza, A.; Angeli, S.; Tegoni, M.; Cambillau, C.; Pelosi, P. Plant stress proteins of the thaumatin-like family discovered in animals. FEBS Lett. 2004, 572, 3–7. [Google Scholar] [CrossRef]
- Piggott, N.; Ekramoddoullah, A.K.; Liu, J.-J.; Yu, X. Gene cloning of a thaumatin-like (PR-5) protein of western white pine (Pinus monticola D. Don) and expression studies of members of the PR-5 group. Physiol. Mol. Plant Pathol. 2004, 64, 1–8. [Google Scholar] [CrossRef]
- Koiwa, H.; Kato, H.; Nakatsu, T.; Oda, J.i.; Yamada, Y.; Sato, F. Crystal structure of tobacco PR-5d protein at 1.8 Å resolution reveals a conserved acidic cleft structure in antifungal thaumatin-like proteins11Edited by R. Huber. J. Mol. Biol. 1999, 286, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, Y.; Xin, X.; Ding, C.; Lv, F.; Mo, W.; Xia, Y.; Wang, S.; Cai, J.; Sun, L.; et al. The Osmotin-Like Protein Gene PdOLP1 Is Involved in Secondary Cell Wall Biosynthesis during Wood Formation in Poplar. Int. J. Mol. Sci. 2020, 21, 3993. [Google Scholar] [CrossRef]
- Singh, S.; Tripathi, R.K.; Lemaux, P.G.; Buchanan, B.B.; Singh, J. Redox-dependent interaction between thaumatin-like protein and β-glucan influences malting quality of barley. Proc. Natl. Acad. Sci. USA 2017, 114, 7725–7730. [Google Scholar] [CrossRef]
- Kobayashi, K.; Fukuda, M.; Igarashi, D.; Sunaoshi, M. Cytokinin-binding proteins from tobacco callus share homology with osmotin-like protein and an endochitinase. Plant Cell Physiol. 2000, 41, 148–157. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stintzi, A.; Heitz, T.; Kauffmann, S.; Legrand, M.; Fritig, B. Identification of a basic pathogenesis-related, thaumatin-like protein of virus-infected tobacco as osmotin. Physiol. Mol. Plant Pathol. 1991, 38, 137–146. [Google Scholar] [CrossRef]
- Singh, N.K.; Bracker, C.A.; Hasegawa, P.M.; Handa, A.K.; Buckel, S.; Hermodson, M.A.; Pfankoch, E.; Regnier, F.E.; Bressan, R.A. Characterization of osmotin: A thaumatin-like protein associated with osmotic adaptation in plant cells. Plant Physiol. 1987, 85, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Breiteneder, H.; Radauer, C. A classification of plant food allergens. J. Allergy Clin. Immunol. 2004, 113, 821–831. [Google Scholar] [CrossRef]
- Myagmarjav, D.; Sukweenadhi, J.; Kim, Y.-J.; Jang, M.-G.; Rahimi, S.; Silva, J.; Choi, J.-Y.; Mohanan, P.; Kwon, W.-S.; Kim, C. Molecular characterization and expression analysis of pathogenesis related protein 6 from Panax ginseng. Russ. J. Genet. 2017, 53, 1211–1220. [Google Scholar] [CrossRef]
- Doares, S.H.; Syrovets, T.; Weiler, E.W.; Ryan, C.A. Oligogalacturonides and chitosan activate plant defensive genes through the octadecanoid pathway. P. Natl. A. Sci. India. B 1995, 92, 4095–4098. [Google Scholar] [CrossRef] [PubMed]
- Terras, F.R.; Schoofs, H.M.; Thevissen, K.; Osborn, R.W.; Vanderleyden, J.; Cammue, B.P.; Broekaert, W.F. Synergistic enhancement of the antifungal activity of wheat and barley thionins by radish and oilseed rape 2S albumins and by barley trypsin inhibitors. Plant Physiol. 1993, 103, 1311–1319. [Google Scholar] [CrossRef]
- Koiwa, H.; Bressan, R.A.; Hasegawa, P.M. Regulation of protease inhibitors and plant defense. Trends Plant Sci. 1997, 2, 379–384. [Google Scholar] [CrossRef]
- Umemoto, N.; Kakitani, M.; Iwamatsu, A.; Yoshikawa, M.; Yamaoka, N.; Ishida, I. The structure and function of a soybean β-glucan-elicitor-binding protein. PNAS 1997, 94, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Lavrova, V.; Matveeva, E.; Zinovieva, S. Expression of genes, encoded defense proteins, in potato plants infected with the cyst-forming nematode Globodera rostochiensis (Wollenweber 1923) Behrens, 1975 and modulation of their activity during short-term exposure to low temperatures. Biol. Bull. 2017, 44, 128–136. [Google Scholar] [CrossRef]
- Giacometti, R.; Barneto, J.; Barriga, L.G.; Sardoy, P.M.; Balestrasse, K.; Andrade, A.M.; Pagano, E.A.; Alemano, S.G.; Zavala, J.A. Early perception of stink bug damage in developing seeds of field-grown soybean induces chemical defences and reduces bug attack. Pest Manag. Sci. 2016, 72, 1585–1594. [Google Scholar] [CrossRef]
- Vera, P.; Conejero, V. The induction and accumulation of the pathogenesis-related P69 proteinase in tomato during citrus exocortis viroid infection and in response to chemical treatments. Physiol. Mol. Plant Pathol. 1989, 34, 323–334. [Google Scholar] [CrossRef]
- Tornero, P.; Conejero, V.; Vera, P. Primary structure and expression of a pathogen-induced protease (PR-P69) in tomato plants: Similarity of functional domains to subtilisin-like endoproteases. PNAS 1996, 93, 6332–6337. [Google Scholar] [CrossRef] [PubMed]
- Siezen, R.J.; de Vos, W.M.; Leunissen, J.A.; Dijkstra, B.W. Homology modelling and protein engineering strategy of subtilases, the family of subtilisin-like serine proteinases. Protein Eng. Des. Sel. 1991, 4, 719–737. [Google Scholar] [CrossRef]
- Ahmad, A.; Shafique, S.; Shafique, S. Molecular basis of antifungal resistance in tomato varieties. Pak. J. Agr. Sci. 2014, 51, 683–687. [Google Scholar]
- Campos, M.A.; Rosa, D.D.; Teixeira, J.É.C.; Targon, M.L.P.; Souza, A.A.; Paiva, L.V.; Stach-Machado, D.R.; Machado, M.A. PR gene families of citrus: Their organ specific-biotic and abiotic inducible expression profiles based on ESTs approach. Genet. Mol. Biol. 2007, 30, 917–930. [Google Scholar] [CrossRef]
- Baboulène, L.; Silvestre, J.; Pinelli, E.; Morard, P. Effect of Ca Deficiency on Growth and Leaf Acid Soluble Proteins of Tomato. J. Plant Nutr. 2007, 30, 497–515. [Google Scholar] [CrossRef][Green Version]
- Irigoyen, M.L.; Garceau, D.C.; Bohorquez-Chaux, A. Genome-wide analyses of cassava Pathogenesis-related (PR) gene families reveal core. BMC Genom. 2020, 21, 1. [Google Scholar] [CrossRef]
- Vera, P.; Conejero, V. Effect of Ethephon on Protein Degradation and the Accumulation of Pathogenesis-Related’(PR) Proteins in Tomato Leaf Discs. Plant Physiol. 1990, 92, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Barceló, A.R.; Ros, L.V.G.; Carrasco, A.E.J. Looking for syringyl peroxidases. Trends Plant Sci. 2007, 12, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Nawrot, R.; Barylski, J.; Nowicki, G.; Broniarczyk, J.; Buchwald, W.; Goździcka-Józefiak, A. Plant antimicrobial peptides. Folia Microbiol. 2014, 59, 181–196. [Google Scholar] [CrossRef] [PubMed]
- Lavid, N.; Schwartz, A.; Yarden, O.; Tel-Or, E. The involvement of polyphenols and peroxidase activities in heavy-metal accumulation by epidermal glands of the waterlily (Nymphaeaceae). Planta 2001, 212, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Ryu, S.H.; Kwon, S.Y.; Lee, H.S.; Kim, J.; Kwak, S.S. Differential expression of six novel peroxidase cDNAs from cell cultures of sweetpotato in response to stress. Mol. Gen. Genom. 2003, 269, 542–552. [Google Scholar] [CrossRef]
- Liu, J.-J.; Ekramoddoullah, A.K. The family 10 of plant pathogenesis-related proteins: Their structure, regulation, and function in response to biotic and abiotic stresses. Physiol. Mol. Plant Pathol. 2006, 68, 3–13. [Google Scholar] [CrossRef]
- Biesiadka, J.; Bujacz, G.; Sikorski, M.M.; Jaskolski, M. Crystal structures of two homologous pathogenesis-related proteins from yellow lupine. J. Mol. Biol. 2002, 319, 1223–1234. [Google Scholar] [CrossRef] [PubMed]
- Park, C.J.; Kim, K.J.; Shin, R.; Park, J.M.; Shin, Y.C.; Paek, K.H. Pathogenesis-related protein 10 isolated from hot pepper functions as a ribonuclease in an antiviral pathway. Plant J. 2004, 37, 186–198. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.-R.; Chen, Z.-Y.; Brown, R.L.; Bhatnagar, D. Expression and functional characterization of two pathogenesis-related protein 10 genes from Zea mays. J. Plant Physiol. 2010, 167, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Pühringer, H.; Moll, D.; Hoffmann-Sommergruber, K.; Watillon, B.; Katinger, H.; da Câmara Machado, M.L. The promoter of an apple Ypr10 gene, encoding the major allergen Mal d 1, is stress-and pathogen-inducible. Plant Sci. 2000, 152, 35–50. [Google Scholar] [CrossRef]
- Gonneau, M.; Pagant, S.; Brun, F.; Laloue, M. Photoaffinity labelling with the cytokinin agonist azido-CPPU of a 34 kDa peptide of the intracellular pathogenesis-related protein family in the moss Physcomitrella patens. Plant Mol. Biol. 2001, 46, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Odintsova, T.I.; Slezina, M.P.; Istomina, E.A. Defensins of Grasses: A Systematic Review. Biomolecules 2020, 10, 1029. [Google Scholar] [CrossRef] [PubMed]
- Jha, S.; Chattoo, B.B. Expression of a plant defensin in rice confers resistance to fungal phytopathogens. Transgenic Res. 2010, 19, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Yount, N.Y.; Yeaman, M.R. Peptide antimicrobials: Cell wall as a bacterial target. Ann. N. Y. Acad. Sci. 2013, 1277, 127–138. [Google Scholar] [CrossRef]
- Koike, M.; Okamoto, T.; Tsuda, S.; Imai, R. A novel plant defensin-like gene of winter wheat is specifically induced during cold acclimation. Biochem. Biophys. Res. Commun. 2002, 298, 46–53. [Google Scholar] [CrossRef]
- Do, H.M.; Lee, S.C.; Jung, H.W.; Sohn, K.H.; Hwang, B.K. Differential expression and in situ localization of a pepper defensin (CADEF1) gene in response to pathogen infection, abiotic elicitors and environmental stresses in Capsicum annuum. Plant Sci. 2004, 166, 1297–1305. [Google Scholar] [CrossRef]
- Mirouze, M.; Sels, J.; Richard, O.; Czernic, P.; Loubet, S.; Jacquier, A.; François, I.E.; Cammue, B.P.; Lebrun, M.; Berthomieu, P. A putative novel role for plant defensins: A defensin from the zinc hyper-accumulating plant, Arabidopsis halleri, confers zinc tolerance. Plant J. 2006, 47, 329–342. [Google Scholar] [CrossRef]
- Bohlmann, H.; Broekaert, W. The role of thionins in plant protection. Crit. Rev. Plant Sci. 1994, 13, 1–16. [Google Scholar] [CrossRef]
- Chan, Y.-L.; Prasad, V.; Sanjaya; Chen, K.H.; Liu, P.C.; Chan, M.-T.; Cheng, C.-P. Transgenic tomato plants expressing an Arabidopsis thionin (Thi2. 1) driven by fruit-inactive promoter battle against phytopathogenic attack. Planta 2005, 221, 386–393. [Google Scholar] [CrossRef]
- Leybourne, D.J.; Valentine, T.A.; Binnie, K.; Taylor, A.; Karley, A.J.; Bos, J.I.B. Drought stress increases the expression of barley defence genes with negative consequences for infesting cereal aphids. J. Exp. Bot. 2022, 73, 2238–2250. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Gong, X.; Zhou, D.; Jiang, Q.; Liang, Y.; Ye, R.; Zhang, S.; Wang, Y.; Tang, X.; Li, F.; et al. Plant Defensin-Dissimilar Thionin OsThi9 Alleviates Cadmium Toxicity in Rice Plants and Reduces Cadmium Accumulation in Rice Grains. J. Agric. Food Chem. 2023, 71, 8367–8380. [Google Scholar] [CrossRef] [PubMed]
- García-Olmedo, F.; Molina, A.; Alamillo, J.M.; Rodríguez-Palenzuéla, P. Plant defense peptides. Pept. Sci. 1998, 47, 479–491. [Google Scholar] [CrossRef]
- Wang, S.-Y.; Wu, J.-H.; Ng, T.; Ye, X.-Y.; Rao, P.-F. A non-specific lipid transfer protein with antifungal and antibacterial activities from the mung bean. Peptides 2004, 25, 1235–1242. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Zhang, C.; Shan, R.; Li, X.; Tseke Inkabanga, A.; Li, L.; Jiang, H.; Chai, Y. Genome-wide identification and expression analysis of nsLTP gene family in rapeseed (Brassica napus) reveals their critical roles in biotic and abiotic stress responses. Int. J. Mol. Sci. 2022, 23, 8372. [Google Scholar] [CrossRef] [PubMed]
- Govindan, G.; Sandhiya, K.R.; Alphonse, V.; Somasundram, S. Role of Germin-Like Proteins (GLPs) in Biotic and Abiotic Stress Responses in Major Crops: A Review on Plant Defense Mechanisms and Stress Tolerance. Plant Mol. Biol. Rep. 2024, 42, 450–468. [Google Scholar] [CrossRef]
- Islam, M.M.; El-Sappah, A.H.; Ali, H.M.; Zandi, P.; Huang, Q.; Soaud, S.A.; Alazizi, E.M.Y.; Wafa, H.A.; Hossain, M.A.; Liang, Y. Pathogenesis-related proteins (PRs) countering environmental stress in plants: A review. S. Afr. J. Bot. 2023, 160, 414–427. [Google Scholar] [CrossRef]
- Folkerts, O. Overexpression of a gene encoding H2O2-generating oxalate oxidase evoke defense responses in sunflower. Plant Physiol. 2003, 133, 170181. [Google Scholar]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Krishna, G.; Singh, B.K.; Kim, E.K.; Morya, V.K.; Ramteke, P.W. Progress in genetic engineering of peanut (Arachis hypogaea L.)—A review. Plant Biotechnol. J. 2015, 13, 147–162. [Google Scholar] [CrossRef]
- Druka, A.; Kudrna, D.; Kannangara, C.G.; von Wettstein, D.; Kleinhofs, A. Physical and genetic mapping of barley (Hordeum vulgare) germin-like cDNAs. Proc. Natl. Acad. Sci. USA 2002, 99, 850–855. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, D.; Li, W.; Mallano, A.I.; Zhang, Y.; Wang, T.; Lu, M.; Qin, Z.; Li, W. Expression study of soybean germin-like gene family reveals a role of GLP7 gene in various abiotic stress tolerances. Can. J. Plant Sci. 2016, 96, 296–304. [Google Scholar] [CrossRef][Green Version]
- Christensen, A.B.; Cho, B.H.; Naesby, M.; Gregersen, P.L.; Brandt, J.; Madriz-Ordenana, K.; Collinge, D.B.; Thordal-Christensen, H. The molecular characterization of two barley proteins establishes the novel PR-17 family of pathogenesis-related proteins. Mol. Plant Pathol. 2002, 3, 135–144. [Google Scholar] [CrossRef]
- Schweizer, P.; Pokorny, J.; Abderhalden, O.; Dudler, R. A transient assay system for the functional assessment of defense-related genes in wheat. Mol. Plant-Microbe Interact. 1999, 12, 647–654. [Google Scholar] [CrossRef]
- Benhamou, N.; Bélanger, R.R. Benzothiadiazole-Mediated Induced Resistance to Fusarium oxysporum f. sp.radicis-lycopersici in Tomato. Plant Physiol. 1998, 118, 1203–1212. [Google Scholar] [CrossRef]
- Luo, X.; Tian, T.; Feng, L.; Yang, X.; Li, L.; Tan, X.; Wu, W.; Li, Z.; Treves, H.; Serneels, F. Pathogenesis-related protein 1 suppresses oomycete pathogen by targeting against AMPK kinase complex. J. Adv. Res. 2023, 43, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Xiong, D.; Schneiter, R.; Tian, C. The function of plant PR1 and other members of the CAP protein superfamily in plant-pathogen interactions. Mol. Plant Pathol. 2023, 24, 651–668. [Google Scholar] [CrossRef] [PubMed]
- Breen, S.; Williams, S.J.; Outram, M.; Kobe, B.; Solomon, P.S. Emerging Insights into the Functions of Pathogenesis-Related Protein 1. Trends Plant Sci. 2017, 22, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Bai, Y.; Wei, Y.; Dong, Y.; Zeng, H.; Reiter, R.J.; Shi, H. Fine-tuning of pathogenesis-related protein 1 (PR1) activity by the melatonin biosynthetic enzyme ASMT2 in defense response to cassava bacterial blight. J. Pineal Res. 2022, 72, e12784. [Google Scholar] [CrossRef]
- Niderman, T.; Genetet, I.; Bruyère, T.; Gees, R.; Stintzi, A.; Legrand, M.; Fritig, B.; Mösinger, E. Pathogenesis-related PR-1 proteins are antifungal. Isolation and characterization of three 14-kilodalton proteins of tomato and of a basic PR-1 of tobacco with inhibitory activity against Phytophthora infestans. Plant Physiol. 1995, 108, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Almeida-Silva, F.; Venancio, T.M. Pathogenesis-related protein 1 (PR-1) genes in soybean: Genome-wide identification, structural analysis and expression profiling under multiple biotic and abiotic stresses. Gene 2022, 809, 146013. [Google Scholar] [CrossRef] [PubMed]
- Goyal, R.K.; Fatima, T.; Topuz, M.; Bernadec, A.; Sicher, R.; Handa, A.K.; Mattoo, A.K. Pathogenesis-Related Protein 1b1 (PR1b1) Is a Major Tomato Fruit Protein Responsive to Chilling Temperature and Upregulated in High Polyamine Transgenic Genotypes. Front. Plant Sci. 2016, 7, 901. [Google Scholar] [CrossRef] [PubMed]
- Zribi, I.; Ghorbel, M.; Haddaji, N.; Besbes, M.; Brini, F. Genome-wide identification and expression profiling of pathogenesis-related protein 1 (PR-1) genes in durum wheat (Triticum durum Desf.). Plants 2023, 12, 1998. [Google Scholar] [CrossRef] [PubMed]
- Sarowar, S.; Kim, Y.J.; Kim, E.N.; Kim, K.D.; Hwang, B.K.; Islam, R.; Shin, J.S. Overexpression of a pepper basic pathogenesis-related protein 1 gene in tobacco plants enhances resistance to heavy metal and pathogen stresses. Plant Cell Rep. 2005, 24, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Green, R.; Fluhr, R. UV-B-induced PR-1 accumulation is mediated by active oxygen species. Plant Cell 1995, 7, 203–212. [Google Scholar] [CrossRef]
- Leah, R.; Tommerup, H.; Svendsen, I.; Mundy, J. Biochemical and molecular characterization of three barley seed proteins with antifungal properties. J. Biol. Chem. 1991, 266, 1564–1573. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Xue, Q.; Zhang, Z.; Shu, P.; Deng, H.; Bouzayen, M.; Hong, Y.; Liu, M. β-1, 3-GLUCANASE10 regulates tomato development and disease resistance by modulating callose deposition. Plant Physiol. 2023, 192, 2785–2802. [Google Scholar] [CrossRef]
- Nawrath, C.; Métraux, J.-P. Salicylic acid induction–deficient mutants of Arabidopsis express PR-2 and PR-5 and accumulate high levels of camalexin after pathogen inoculation. Plant Cell 1999, 11, 1393–1404. [Google Scholar] [CrossRef]
- Sunpapao, A.; Pornsuriya, C. Overexpression of β-1, 3-glucanase gene in response to Phytophthora palmivora infection in leaves of Hevea brasiliensis clones. Wal. J. Sci. Technol. 2016, 13, 35–43. [Google Scholar]
- Li, Y.; Jiao, M.; Li, Y.; Zhong, Y.; Li, X.; Chen, Z.; Chen, S.; Wang, J. Penicillium chrysogenum polypeptide extract protects tobacco plants from tobacco mosaic virus infection through modulation of ABA biosynthesis and callose priming. J. Exp. Bot. 2021, 72, 3526–3539. [Google Scholar] [CrossRef]
- Rabie, M.; Aseel, D.G.; Younes, H.A.; Behiry, S.I.; Abdelkhalek, A. Transcriptional responses and secondary metabolites variation of tomato plant in response to tobacco mosaic virus infestation. Sci. Rep. 2024, 14, 19565. [Google Scholar] [CrossRef]
- Bozbuga, R. Commonalities of molecular response in tomato plants against parasitic nematodes. Biol. Bull. 2021, 48, S12–S21. [Google Scholar] [CrossRef]
- Wang, L.; Li, R.; Li, K.; Qu, Z.; Zhou, R.; Lu, G.; Li, P.; Li, G. Genome-wide identification of the grapevine β-1, 3-glucanase gene (VviBG) family and expression analysis under different stresses. BMC Plant Biol. 2024, 24, 911. [Google Scholar] [CrossRef] [PubMed]
- Sock, J.; Rohringer, R.; Kang, Z. Extracellular beta-1,3-Glucanases in Stem Rust-Affected and Abiotically Stressed Wheat Leaves: Immunocytochemical Localization of the Enzyme and Detection of Multiple Forms in Gels by Activity Staining with Dye-Labeled Laminarin. Plant Physiol. 1990, 94, 1376–1389. [Google Scholar] [CrossRef] [PubMed]
- Surplus, S.; Jordan, B.; Murphy, A.; Carr, J.; Thomas, B.; Mackerness, S.H. Ultraviolet-B-induced responses in Arabidopsis thaliana: Role of salicylic acid and reactive oxygen species in the regulation of transcripts encoding photosynthetic and acidic pathogenesis-related proteins. Plant Cell Environ. 1998, 21, 685–694. [Google Scholar] [CrossRef]
- Barre, A.; Damme, E.; Simplicien, M.; Benoist, H.; Rouge, P. Are Dietary Lectins Relevant Allergens in Plant Food Allergy? Foods 2020, 9, 1724. [Google Scholar] [CrossRef]
- Schlumbaum, A.; Mauch, F.; Vögeli, U.; Boller, T. Plant chitinases are potent inhibitors of fungal growth. Nature 1986, 324, 365–367. [Google Scholar] [CrossRef]
- Chandrashekar, N.; Ali, S.; Grover, A. Exploring expression patterns of PR-1, PR-2, PR-3, and PR-12 like genes in Arabidopsis thaliana upon Alternaria brassicae inoculation. 3 Biotech 2018, 8, 230. [Google Scholar] [CrossRef]
- Omar, A.Z.; Hamdy, E.; Hamed, E.A.; Hafez, E.; Abdelkhalek, A. The curative activity of some arylidene dihydropyrimidine hydrazone against Tobacco mosaic virus infestation. J. Saudi Chem. Soc. 2022, 26, 101504. [Google Scholar] [CrossRef]
- Kumar, S.A.; Kumari, P.H.; Jawahar, G.; Prashanth, S.; Suravajhala, P.; Katam, R.; Sivan, P.; Rao, K.S.; Kirti, P.B.; Kishor, P.B.K. Beyond just being foot soldiers—osmotin like protein (OLP) and chitinase (Chi11) genes act as sentinels to confront salt, drought, and fungal stress tolerance in tomato. Environ. Exp. Bot. 2016, 132, 53–65. [Google Scholar] [CrossRef]
- Yun, H.-K.; Yi, S.-Y.; Yu, S.-H.; Choi, D. Cloning of a Pathogenesis-Related Protein-1 Gene from Nicotians glutinosa L. and Its Salicylic Acid-Independent Induction by Copper and β-Aminobutyric Acid. J. Plant Physiol. 1999, 154, 327–333. [Google Scholar] [CrossRef]
- Guevara-Morato, M.A.; García de Lacoba, M.; García-Luque, I.; Serra, M.T. Characterization of a pathogenesis-related protein 4 (PR-4) induced in Capsicum chinense L3 plants with dual RNase and DNase activities. J. Exp. Bot. 2010, 61, 3259–3271. [Google Scholar] [CrossRef]
- Chouhan, R.; Ahmed, S.; Gandhi, S.G. Over-expression of PR proteins with chitinase activity in transgenic plants for alleviation of fungal pathogenesis. J. Plant Pathol. 2023, 105, 69–81. [Google Scholar] [CrossRef]
- Wang, N.; Xiao, B.; Xiong, L. Identification of a cluster of PR4-like genes involved in stress responses in rice. J. Plant Physiol. 2011, 168, 2212–2224. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, H.; Jang, M.; Kwon, W.; Kim, S.; Yang, D. Cloning and characterization of pathogenesis-related protein 4 gene from Panax ginseng. Russ. J. Plant Physl. 2014, 61, 664–671. [Google Scholar] [CrossRef]
- Bashir, M.A.; Silvestri, C.; Ahmad, T.; Hafiz, I.A.; Abbasi, N.A.; Manzoor, A.; Cristofori, V.; Rugini, E. Osmotin: A Cationic Protein Leads to Improve Biotic and Abiotic Stress Tolerance in Plants. Plants 2020, 9, 992. [Google Scholar] [CrossRef]
- de Jesus-Pires, C.; Ferreira-Neto, J.R.C.; Pacifico Bezerra-Neto, J.; Kido, E.A.; de Oliveira Silva, R.L.; Pandolfi, V.; Wanderley-Nogueira, A.C.; Binneck, E.; da Costa, A.F.; Pio-Ribeiro, G.; et al. Plant Thaumatin-like Proteins: Function, Evolution and Biotechnological Applications. Curr. Protein Pept. Sci. 2020, 21, 36–51. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Hussain, A.; Shaban, M.; Khan, A.H.; Alariqi, M.; Gul, S.; Jun, Z.; Lin, S.; Li, J.; Jin, S. Osmotin: A plant defense tool against biotic and abiotic stresses. Plant Physiol. Biochem. 2018, 123, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Medrano, R.; Jimenez-Moraila, B.; Herrera-Estrella, L.; Rivera-Bustamante, R.F. Nucleotide sequence of an osmotin-like cDNA induced in tomato during viroid infection. Plant Mol. Biol. 1992, 20, 1199–1202. [Google Scholar] [CrossRef] [PubMed]
- Aseel, D.G.; Abdelkhalek, A.; Alotibi, F.O.; Samy, M.A.; Al-Askar, A.A.; Arishi, A.A.; Hafez, E.E. Foliar application of nanoclay promotes potato (Solanum tuberosum L.) growth and induces systemic resistance against potato virus Y. Viruses 2022, 14, 2151. [Google Scholar] [CrossRef]
- Viktorova, J.; Krasny, L.; Kamlar, M.; Novakova, M.; Mackova, M.; Macek, T. Osmotin, a pathogenesis-related protein. Curr. Protein Pept. Sci. 2012, 13, 672–681. [Google Scholar] [CrossRef]
- Rajam, M.; Chandola, N.; Saiprasad Goud, P.; Singh, D.; Kashyap, V.; Choudhary, M.; Sihachakr, D. Thaumatin gene confers resistance to fungal pathogens as well as tolerance to abiotic stresses in transgenic tobacco plants. Biol. Plant. 2007, 51, 135–141. [Google Scholar] [CrossRef]
- Singh, V.; Hallan, V.; Pati, P.K. Withania somnifera osmotin (WsOsm) confers stress tolerance in tobacco and establishes novel interactions with the defensin protein (WsDF). Physiol. Plant. 2024, 176, e14513. [Google Scholar] [CrossRef] [PubMed]
- Patade, V.Y.; Khatri, D.; Kumari, M.; Grover, A.; Mohan Gupta, S.; Ahmed, Z. Cold tolerance in Osmotin transgenic tomato (Solanum lycopersicum L.) is associated with modulation in transcript abundance of stress responsive genes. SpringerPlus 2013, 2, 117. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhou, Q.; Wang, X.; Cai, J.; Dai, T.; Cao, W.; Jiang, D. Physiological and transcriptional analyses of induced post-anthesis thermo-tolerance by heat-shock pretreatment on germinating seeds of winter wheat. Environ. Exp. Bot. 2016, 131, 181–189. [Google Scholar] [CrossRef]
- Djemal, R.; Bahloul, O.; Khoudi, H. A novel thaumatin-like protein from durum wheat, TdPR-5, is homologous to known plant allergens, responsive to stress exposure, and confers multiple-abiotic stress tolerances to transgenic yeast. Plant Gene 2022, 31, 100360. [Google Scholar] [CrossRef]
- Yarullina, L.; Cherepanova, E.A.; Burkhanova, G.F.; Sorokan, A.V.; Zaikina, E.A.; Tsvetkov, V.O.; Mardanshin, I.S.; Fatkullin, I.Y.; Kalatskaja, J.N.; Yalouskaya, N.A.; et al. Stimulation of the Defense Mechanisms of Potatoes to a Late Blight Causative Agent When Treated with Bacillus subtilis Bacteria and Chitosan Composites with Hydroxycinnamic Acids. Microorganisms 2023, 11, 1993. [Google Scholar] [CrossRef]
- Sabnam, N.; Hussain, A.; Saha, P. The secret password: Cell death-inducing proteins in filamentous phytopathogens—As versatile tools to develop disease-resistant crops. Microb. Pathog. 2023, 183, 106276. [Google Scholar] [CrossRef] [PubMed]
- Abdelkhalek, A.; Al-Askar, A.A.; Alsubaie, M.M.; Behiry, S.I. First report of protective activity of Paronychia argentea extract against tobacco mosaic virus infection. Plants 2021, 10, 2435. [Google Scholar] [CrossRef] [PubMed]
- Bozbuga, R. Molecular analysis of nematode-responsive defence genes CRF1, WRKY45, and PR7 in Solanum lycopersicum tissues during the infection of plant-parasitic nematode species of the genus Meloidogyne. Genome 2022, 65, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Derikvand, F.; Bazgir, E.; Darvishnia, M.; Mirzaei Najafgholi, H. Evaluation the activity of peroxidase and catalase enzymes and the expression level of PR1 and PR8 genes in apple fruit following brown rot (Monilinia laxa) disease. Plant Genet. Res. 2023, 10, 29–42. [Google Scholar]
- Fagerstedt, K.V.; Kukkola, E.M.; Koistinen, V.V.; Takahashi, J.; Marjamaa, K. Cell wall lignin is polymerised by class III secretable plant peroxidases in Norway spruce. J. Integr. Plant Biol. 2010, 52, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; El Hadrami, A.; Adam, L.R.; Daayf, F. Genes encoding pathogenesis-related proteins PR-2, PR-3 and PR-9, are differentially regulated in potato leaves inoculated with isolates from US-1 and US-8 genotypes of Phytophthora infestans (Mont.) de Bary. Physiol. Mol. Plant Pathol. 2005, 67, 49–56. [Google Scholar] [CrossRef]
- Lambais, M.R. In silico differential display of defense-related expressed sequence tags from sugarcane tissues infected with diazotrophic endophytes. Genet. Mol. Biol. 2001, 24, 103–111. [Google Scholar] [CrossRef]
- Carrillo, M.G.C.; Martin, F.; Variar, M.; Bhatt, J.C.; Perez-Quintero, A.L.; Leung, H.; Leach, J.E.; Vera Cruz, C.M. Accumulating candidate genes for broad-spectrum resistance to rice blast in a drought-tolerant rice cultivar. Sci. Rep. 2021, 11, 21502. [Google Scholar] [CrossRef]
- Morris, J.S.; Caldo, K.M.P.; Liang, S.; Facchini, P.J. PR10/Bet v1-like Proteins as Novel Contributors to Plant Biochemical Diversity. ChemBioChem 2021, 22, 264–287. [Google Scholar] [CrossRef]
- Lopes, N.D.S.; Santos, A.S.; de Novais, D.P.S.; Pirovani, C.P.; Micheli, F. Pathogenesis-related protein 10 in resistance to biotic stress: Progress in elucidating functions, regulation and modes of action. Front. Plant Sci. 2023, 14, 1193873. [Google Scholar] [CrossRef] [PubMed]
- Barreto, L.R.; Barreto, T.; Melo, S.; Pungartnik, C.; Brendel, M. Sensitivity of Yeast Mutants Deficient in Mitochondrial or Vacuolar ABC Transporters to Pathogenesis-Related Protein TcPR-10 of Theobroma cacao. Biology 2018, 7, 35. [Google Scholar] [CrossRef] [PubMed]
- Regner, A.; Szepannek, N.; Wiederstein, M.; Fakhimahmadi, A.; Paciosis, L.F.; Blokhuis, B.R.; Redegeld, F.A.; Hofstetter, G.; Dvorak, Z.; Jensen-Jarolim, E.; et al. Binding to Iron Quercetin Complexes Increases the Antioxidant Capacity of the Major Birch Pollen Allergen Bet v 1 and Reduces Its Allergenicity. Antioxidants 2022, 12, 42. [Google Scholar] [CrossRef]
- Besbes, F.; Franz-Oberdorf, K.; Schwab, W. Phosphorylation-dependent ribonuclease activity of Fra a 1 proteins. J. Plant Physiol. 2019, 233, 1–11. [Google Scholar] [CrossRef]
- Wang, T.; Xie, M.; Hou, S.; Ma, J.; Lin, Y.; Chen, S.; Li, D.; Yang, G. Walnut PR10/Bet v1-like proteins interact with chitinase in response to anthracnose stress. J. Evol. Biol. 2025, 38, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Maitirouzi, A.; Feng, Y.; Zhang, H.; Heng, Y.; Zhang, J.; Wang, Y. Heterologous expression of Halostachys caspica pathogenesis-related protein 10 increases salt and drought resistance in transgenic Arabidopsis thaliana. Plant Mol. Biol. 2024, 115, 5. [Google Scholar] [CrossRef]
- Jomová, K.; Feszterová, M.; Morovič, M. Expression of pathogenesis-related protein genes and changes of superoxide dismutase activity induced by toxic elements in Lupinus luteus L. J. Microb. Biotec. Food 2011, 1, 437–445. [Google Scholar]
- Pinto, M.P.; Ricardo, C.P. Lupinus albus L. pathogenesis-related proteins that show similarity to PR-10 proteins. Plant Physiol. 1995, 109, 1345–1351. [Google Scholar] [CrossRef]
- Han, Z.; Schneiter, R. Dual functionality of pathogenesis-related proteins: Defensive role in plants versus immunosuppressive role in pathogens. Front. Plant Sci. 2024, 15, 1368467. [Google Scholar] [CrossRef]
- Heitz, T.; Segond, S.; Kauffmann, S.; Geoffroy, P.; Prasad, V.; Brunner, F.; Fritig, B.; Legrand, M. Molecular characterization of a novel tobacco pathogenesis-related (PR) protein: A new plant chitinase/lysozyme. Mol. Gen. Genet. 1994, 245, 246–254. [Google Scholar] [CrossRef]
- Zribi, I.; Ghorbel, M.; Brini, F. Pathogenesis Related Proteins (PRs): From Cellular Mechanisms to Plant Defense. Curr. Protein Pept. Sci. 2021, 22, 396–412. [Google Scholar] [CrossRef] [PubMed]
- Scala, E.; Villalta, D.; Meneguzzi, G.; Giani, M.; Asero, R. Storage molecules from tree nuts, seeds and legumes: Relationships and amino acid identity among homologue molecules. Eur. Ann. Allergy Clin. Immunol. 2018, 50, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Pandey, S.; Pati, P.K. Interaction between pathogenesis-related (PR) proteins and phytohormone signaling pathways in conferring disease tolerance in plants. Physiol. Plant 2025, 177, e70174. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Ganai, B.A.; Kamili, A.N.; Bhat, A.A.; Mir, Z.A.; Bhat, J.A.; Tyagi, A.; Islam, S.T.; Mushtaq, M.; Yadav, P. Pathogenesis-related proteins and peptides as promising tools for engineering plants with multiple stress tolerance. Microbiol. Res. 2018, 212, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Hawamda, A.I.; Reichert, S.; Ali, M.A.; Nawaz, M.A.; Austerlitz, T.; Schekahn, P.; Abbas, A.; Tenhaken, R.; Bohlmann, H. Characterization of an Arabidopsis defensin-like gene conferring resistance against nematodes. Plants 2022, 11, 280. [Google Scholar] [CrossRef] [PubMed]
- Singiri, J.R.; Swetha, B.; Sikron-Persi, N.; Grafi, G. Differential response to single and combined salt and heat stresses: Impact on accumulation of proteins and metabolites in dead pericarps of Brassica juncea. Int. J. Mol. Sci. 2021, 22, 7076. [Google Scholar] [CrossRef]
- Srivastava, S.; Dashora, K.; Ameta, K.L.; Singh, N.P.; El-Enshasy, H.A.; Pagano, M.C.; Hesham, A.E.; Sharma, G.D.; Sharma, M.; Bhargava, A. Cysteine-rich antimicrobial peptides from plants: The future of antimicrobial therapy. Phytother. Res. 2021, 35, 256–277. [Google Scholar] [CrossRef]
- Rayapuram, C.; Wu, J.; Haas, C.; Baldwin, I.T. PR-13/Thionin but not PR-1 mediates bacterial resistance in Nicotiana attenuata in nature, and neither influences herbivore resistance. Mol. Plant-Microbe Interact. 2008, 21, 988–1000. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gaudet, D.A.; Laroche, A.; Frick, M.; Huel, R.; Puchalski, B. Cold induced expression of plant defensin and lipid transfer protein transcripts in winter wheat. Physiol. Plant. 2003, 117, 195–205. [Google Scholar] [CrossRef]
- Finkina, E.I.; Melnikova, D.N.; Bogdanov, I.V.; Ovchinnikova, T.V. Plant Pathogenesis-Related Proteins PR-10 and PR-14 as Components of Innate Immunity System and Ubiquitous Allergens. Curr. Med. Chem. 2017, 24, 1772–1787. [Google Scholar] [CrossRef]
- Bao, Y.; Pu, Y.; Yu, X.; Gregory, B.D.; Srivastava, R.; Howell, S.H.; Bassham, D.C. IRE1B degrades RNAs encoding proteins that interfere with the induction of autophagy by ER stress in Arabidopsis thaliana. Autophagy 2018, 14, 1562–1573. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Jiang, J.; Wang, N. Control of Citrus Huanglongbing via Trunk Injection of Plant Defense Activators and Antibiotics. Phytopathology 2018, 108, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Liu, Y.; Xu, X.; Liu, D.; Zhu, G.; Yan, X.; Wang, Z.; Yan, Y. Comparative Proteome Analysis of Wheat Flag Leaves and Developing Grains Under Water Deficit. Front. Plant Sci. 2018, 9, 425. [Google Scholar] [CrossRef] [PubMed]
- Woo, E.J.; Dunwell, J.M.; Goodenough, P.W.; Marvier, A.C.; Pickersgill, R.W. Germin is a manganese containing homohexamer with oxalate oxidase and superoxide dismutase activities. Nat. Struct. Mol. Biol. 2000, 7, 1036–1040. [Google Scholar] [CrossRef]
- Park, C.J.; An, J.M.; Shin, Y.C.; Kim, K.J.; Lee, B.J.; Paek, K.H. Molecular characterization of pepper germin-like protein as the novel PR-16 family of pathogenesis-related proteins isolated during the resistance response to viral and bacterial infection. Planta 2004, 219, 797–806. [Google Scholar] [CrossRef]
- Edreva, A. Pathogenesis-related Proteins: Research Progress in the last 15 Years. Gen. Appl. Plant Physiol. 2005, 35, 105–124. [Google Scholar]
- Breen, J.; Bellgard, M. Germin-like proteins (GLPs) in cereal genomes: Gene clustering and dynamic roles in plant defence. Funct. Integr. Genom. 2010, 10, 463–476. [Google Scholar] [CrossRef]
- Zaynab, M.; Peng, J.; Sharif, Y.; Fatima, M.; Albaqami, M.; Al-Yahyai, R.; Khan, K.A.; Alotaibi, S.S.; Alaraidh, I.A.; Shaikhaldein, H.O. Genome-wide identification and expression profiling of germin-like proteins reveal their role in regulating abiotic stress response in potato. Front. Plant Sci. 2022, 12, 831140. [Google Scholar]
- Okushima, Y.; Koizumi, N.; Kusano, T.; Sano, H. Secreted proteins of tobacco cultured BY2 cells: Identification of a new member of pathogenesis-related proteins. Plant Mol. Biol. 2000, 42, 479–488. [Google Scholar] [CrossRef]
- Zhang, H.-M.; Zhu, J.-H.; Gong, Z.-Z.; Zhu, J.-K. Abiotic stress responses in plants. Nat. Rev. Genet. 2022, 23, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Mao, X.; Wang, R.; Li, A.; Zhao, G.; Zhao, J.; Jing, R. Identification of wheat stress-responding genes and TaPR-1-1 function by screening a cDNA yeast library prepared following abiotic stress. Sci. Rep. 2019, 9, 141. [Google Scholar] [CrossRef]
- Kosova, K.; Vitamvas, P.; Urban, M.O.; Klima, M.; Roy, A.; Prasil, I.T. Biological Networks Underlying Abiotic Stress Tolerance in Temperate Crops--A Proteomic Perspective. Int. J. Mol. Sci. 2015, 16, 20913–20942. [Google Scholar] [CrossRef]
- Wu, J.; Kim, S.G.; Kang, K.Y.; Kim, J.G.; Park, S.R.; Gupta, R.; Kim, Y.H.; Wang, Y.; Kim, S.T. Overexpression of a Pathogenesis-Related Protein 10 Enhances Biotic and Abiotic Stress Tolerance in Rice. Plant Pathol. J. 2016, 32, 552–562. [Google Scholar] [CrossRef]
- Wu, X.; Gong, F.; Cao, D.; Hu, X.; Wang, W. Advances in crop proteomics: PTMs of proteins under abiotic stress. Proteomics 2016, 16, 847–865. [Google Scholar] [CrossRef] [PubMed]
- Misra, R.C.; Sandeep; Kamthan, M.; Kumar, S.; Ghosh, S. A thaumatin-like protein of Ocimum basilicum confers tolerance to fungal pathogen and abiotic stress in transgenic Arabidopsis. Sci. Rep. 2016, 6, 25340. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Liu, Q.; Wu, R.; Songbuerbatu; Zhu, M.; Dorjee, T.; Zhou, Y.; Gao, F. The alteration of proteins and metabolites in leaf apoplast and the related gene expression associated with the adaptation of Ammopiptanthus mongolicus to winter freezing stress. Int. J. Biol. Macromol. 2023, 240, 124479. [Google Scholar] [CrossRef] [PubMed]
- Archambault, A.; Strömvik, M.V. PR-10, defensin and cold dehydrin genes are among those over expressed in Oxytropis (Fabaceae) species adapted to the arctic. Funct. Integr. Genom. 2011, 11, 497–505. [Google Scholar] [CrossRef]
- Alhaithloul, H.A.S. Impact of combined heat and drought stress on the potential growth responses of the desert grass Artemisia sieberi alba: Relation to biochemical and molecular adaptation. Plants 2019, 8, 416. [Google Scholar] [CrossRef] [PubMed]
- Carbonell-Bejerano, P.; Santa María, E.; Torres-Pérez, R.; Royo, C.; Lijavetzky, D.; Bravo, G.; Aguirreolea, J.; Sánchez-Díaz, M.; Antolín, M.C.; Martínez-Zapater, J.M. Thermotolerance Responses in Ripening Berries of Vitis vinifera L. cv Muscat Hamburg. Plant Cell Physiol. 2013, 54, 1200–1216. [Google Scholar] [CrossRef]
- Domingo, G.; Locato, V.; Cimini, S.; Ciceri, L.; Marsoni, M.; De Gara, L.; Bracale, M.; Vannini, C. A comprehensive characterization and expression profiling of defensin family peptides in Arabidopsis thaliana with a focus on their abiotic stress-specific transcriptional modulation. Curr. Plant Biol. 2024, 39, 100376. [Google Scholar] [CrossRef]
- Pós, V.; Hunyadi-Gulyás, É.; Caiazzo, R.; Jócsák, I.; Medzihradszky, K.; Lukács, N. Induction of pathogenesis-related proteins in intercellular fluid by cadmium stress in barley (Hordeum vulgare L.)—A proteomic analysis. Acta Aliment. 2011, 40, 164–175. [Google Scholar] [CrossRef]
- Dana, M.d.l.M.; Pintor-Toro, J.A.; Cubero, B. Transgenic tobacco plants overexpressing chitinases of fungal origin show enhanced resistance to biotic and abiotic stress agents. Plant Physiol. 2006, 142, 722–730. [Google Scholar] [CrossRef]
- Cho, U.H.; Park, J.O. Mercury-induced oxidative stress in tomato seedlings. Plant Sci. 2000, 156, 1–9. [Google Scholar] [CrossRef]
- Utriainen, M.; Kokko, H.; Auriola, S.; Sarrazin, O.; Kärenlampi, S. PR-10 protein is induced by copper stress in roots and leaves of a Cu/Zn tolerant clone of birch, Betula pendula. Plant Cell Environ. 1998, 21, 821–828. [Google Scholar] [CrossRef]
- Berna, A.; Bernier, F. Regulation by biotic and abiotic stress of a wheat germin gene encoding oxalate oxidase, a H2O2-producing enzyme. Plant Mol. Biol. 1999, 39, 539–549. [Google Scholar] [CrossRef]
- Ota, E.; Nishimura, F.; Mori, M.; Tanaka, M.; Kanto, T.; Hosokawa, M.; Osakabe, M.; Satou, M.; Takeshita, M. Up-regulation of pathogenesis-related genes in strawberry leaves treated with powdery mildew-suppressing ultraviolet irradiation. Plant Pathol. 2021, 70, 1378–1387. [Google Scholar] [CrossRef]
- Chen, H.; Wu, Q.; Ni, M.; Chen, C.; Han, C.; Yu, F. Transcriptome Analysis of Endogenous Hormone Response Mechanism in Roots of Styrax tonkinensis Under Waterlogging. Front. Plant Sci. 2022, 13, 896850. [Google Scholar] [CrossRef]
- Cid, G.A.; Francioli, D.; Kolb, S.; Moya, Y.A.T.; Wirén, N.V.; Hajirezaei, M.R. Transcriptomic and metabolomic approaches elucidate the systemic response of wheat plants under waterlogging. J. Exp. Bot. 2022, 75, 1510–1529. [Google Scholar] [CrossRef] [PubMed]
- Gedam, P.A.; Khandagale, K.; Shirsat, D.; Thangasamy, A.; Kulkarni, O.; Kulkarni, A.; Patil, S.S.; Barvkar, V.T.; Mahajan, V.; Gupta, A.J.; et al. Elucidating the molecular responses to waterlogging stress in onion (Allium cepa L.) leaf by comparative transcriptome profiling. Front. Plant Sci. 2023, 14, 1150909. [Google Scholar] [CrossRef]
- Kesarwani, M.; Yoo, J.; Dong, X. Genetic interactions of TGA transcription factors in the regulation of pathogenesis-related genes and disease resistance in Arabidopsis. Plant Physiol. 2007, 144, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Delessert, C.; Kazan, K.; Wilson, I.W.; Van Der Straeten, D.; Manners, J.; Dennis, E.S.; Dolferus, R. The transcription factor ATAF2 represses the expression of pathogenesis-related genes in Arabidopsis. Plant J. 2005, 43, 745–757. [Google Scholar] [CrossRef] [PubMed]
- Cheong, Y.H.; Moon, B.C.; Kim, J.K.; Kim, C.Y.; Kim, M.C.; Kim, I.H.; Park, C.Y.; Kim, J.C.; Park, B.O.; Koo, S.C.; et al. BWMK1, a rice mitogen-activated protein kinase, locates in the nucleus and mediates pathogenesis-related gene expression by activation of a transcription factor. Plant Physiol. 2003, 132, 1961–1972. [Google Scholar] [CrossRef] [PubMed]
- Kumari, D.; Prasad, B.D.; Dwivedi, P.; Sahni, S.; Kumar, M.; Alamri, S.; Adil, M.F.; Alakeel, K.A. Comprehensive analysis of transcription factor binding sites and expression profiling of rice pathogenesis related genes (OsPR1). Front. Plant Sci. 2024, 15, 1463147. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Chen, T.; Kan, J.; Yao, Y.; Guo, D.; Yang, Y.; Ling, X.; Wang, J.; Zhang, B. The GhMYB36 transcription factor confers resistance to biotic and abiotic stress by enhancing PR1 gene expression in plants. Plant Biotechnol. J. 2022, 20, 722–735. [Google Scholar] [CrossRef]
- Ito, Y.; Katsura, K.; Maruyama, K.; Taji, T.; Kobayashi, M.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Functional analysis of rice DREB1/CBF-type transcription factors involved in cold-responsive gene expression in transgenic rice. Plant Cell Physiol. 2006, 47, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yan, H.; Zhang, Z.; Zheng, L.; Zhou, Y.; Gao, F. AmDEF2. 7, a tandem duplicated defensin gene from Ammopiptanthus mongolicus, activated by AmWRKY14, enhances the tolerance of Arabidopsis to low temperature and osmotic stress. Environ. Exp. Bot. 2024, 227, 105956. [Google Scholar] [CrossRef]
- Zheng, L.; Li, B.; Zhou, Y.; Gao, F. A WRKY-regulated TLP gene mediates the response to cold, drought, and wound stress in jojoba. Ind. Crop Prod. 2024, 220, 119224. [Google Scholar] [CrossRef]
- Zheng, L.-M.; Li, B.-J.; Liu, Q.; Richardson, J.E.; Zhou, Y.-J.; Gao, F. Proteomic analysis of leaf apoplast reveals that a jasmonate-regulated CHIA participates in the response to cold and drought stress in jojoba. Hortic. Plant J. 2025, early view, online version. [Google Scholar] [CrossRef]
- Hu, S.; Shinwari, K.I.; Song, Y.; Xia, J.; Xu, H.; Du, B.; Luo, L.; Zheng, L. OsNAC300 positively regulates cadmium stress responses and tolerance in rice roots. Agronomy 2021, 11, 95. [Google Scholar] [CrossRef]
- Zhao, Y.; Gong, J.; Shi, R.; Wu, Z.; Liu, S.; Chen, S.; Tao, Y.; Li, S.; Tian, J. Application of proteomics in investigating the responses of plant to abiotic stresses. Planta 2025, 261, 128. [Google Scholar] [CrossRef]
- Lu, S.; Faris, J.D.; Edwards, M.C. Molecular cloning and characterization of two novel genes from hexaploid wheat that encode double PR-1 domains coupled with a receptor-like protein kinase. Mol. Genet. Genom. 2017, 292, 435–452. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Shi, W.L.; You, J.F.; Bian, M.D.; Qin, X.M.; Yu, H.; Liu, Q.; Ryan, P.R.; Yang, Z.M. Transgenic Arabidopsis thaliana plants expressing a β-1,3-glucanase from sweet sorghum (Sorghum bicolor L.) show reduced callose deposition and increased tolerance to aluminium toxicity. Plant Cell Environ. 2015, 38, 1178–1188. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Ballesta, M.T.; Gosalbes, M.J.; Rodrigo, M.J.; Granell, A.; Zacarias, L.; Lafuente, M.T. Characterization of a β-1,3-glucanase from citrus fruit as related to chilling-induced injury and ethylene production. Postharvest. Biol. Technol. 2006, 40, 133–140. [Google Scholar] [CrossRef]
- Wan, K.; Buitrago, S.; Cheng, B.; Zhang, W.; Pan, R. Analysis of chitinase gene family in barley and function study of HvChi22 involved in drought tolerance. Mol. Biol. Rep. 2024, 51, 731. [Google Scholar] [CrossRef]
- Mandal, A.B.; Biswas, A.; Mukherjee, P.; Mondal, R.; Mandal, C. Osmotin: A PR gene imparts tolerance to excess salt in indica rice. Adv. Life Sci. 2018, 8, 39–50. [Google Scholar]
- Husaini, A.M.; Abdin, M.Z. Overexpression of tobacco osmotin gene leads to salt stress tolerance in strawberry (Fragaria x ananassa Duch.) plants. Indian J. Biotechnol. 2008, 7, 465–471. [Google Scholar]
- Takemoto, D.; Furuse, K.; Doke, N.; Kawakita, K. Identification of chitinase and osmotin-like protein as actin-binding proteins in suspension-cultured potato cells. Plant Cell Physiol. 1997, 38, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Abad, L.R.; D’Urzo, M.P.; Liu, D.; Narasimhan, M.L.; Reuveni, M.; Zhu, J.K.; Niu, X.; Singh, N.K.; Hasegawa, P.M.; Bressan, R.A. Antifungal activity of tobacco osmotin has specificity and involves plasma membrane permeabilization. Plant Sci. 1996, 118, 11–23. [Google Scholar] [CrossRef]
- Priya, M.; Dhanker, O.P.; Siddique, K.H.; HanumanthaRao, B.; Nair, R.M.; Pandey, S.; Singh, S.; Varshney, R.K.; Prasad, P.V.; Nayyar, H. Drought and heat stress-related proteins: An update about their functional relevance in imparting stress tolerance in agricultural crops. Theor. Appl. Genet. 2019, 132, 1607–1638. [Google Scholar] [CrossRef]
- Wang, X.; Zafian, P.; Choudhary, M.; Lawton, M. The PR5K receptor protein kinase from Arabidopsis thaliana is structurally related to a family of plant defense proteins. Proc. Natl. Acad. Sci. USA 1996, 93, 2598–2602. [Google Scholar] [CrossRef]
- Baek, D.; Kim, M.C.; Kumar, D.; Park, B.; Cheong, M.S.; Choi, W.; Park, H.C.; Chun, H.J.; Park, H.J.; Lee, S.Y.; et al. AtPR5K2, a PR5-Like Receptor Kinase, Modulates Plant Responses to Drought Stress by Phosphorylating Protein Phosphatase 2Cs. Front. Plant Sci. 2019, 10, 1146. [Google Scholar] [CrossRef] [PubMed]
- Barthakur, S.; Babu, V.; Bansa, K. Over-expression of osmotin induces proline accumulation and confers tolerance to osmotic stress in transgenic tobacco. J. Plant Biochem. Biotechnol. 2001, 10, 31–37. [Google Scholar] [CrossRef]
- Hmida-Sayari, A.; Gargouri-Bouzid, R.; Bidani, A.; Jaoua, L.; Savouré, A.; Jaoua, S. Overexpression of Δ1-pyrroline-5-carboxylate synthetase increases proline production and confers salt tolerance in transgenic potato plants. Plant Sci. 2005, 169, 746–752. [Google Scholar] [CrossRef]
- Jung, H.W.; Kim, K.D.; Hwang, B.K. Identification of pathogen-responsive regions in the promoter of a pepper lipid transfer protein gene (CALTPI) and the enhanced resistance of the CALTPI transgenic Arabidopsis against pathogen and environmental stresses. Planta 2005, 221, 361–373. [Google Scholar] [CrossRef]
- Cui, S.; Takeda, K.Y.; Wakatake, T.; Luo, J.; Tobimatsu, Y.; Yoshida, S. Striga hermonthica induces lignin deposition at the root tip to facilitate prehaustorium formation and obligate parasitism. Plant Commun. 2025, 6, 101294. [Google Scholar] [CrossRef] [PubMed]
- Yamahara, T.; Shiono, T.; Suzuki, T.; Tanaka, K.; Takio, S.; Sato, K.; Yamazaki, S.; Satoh, T. Isolation of a germin-like protein with manganese superoxide dismutase activity from cells of a moss, Barbula unguiculata. J. Biol. Chem. 1999, 274, 33274–33278. [Google Scholar] [CrossRef] [PubMed]

| Property | Molecular Function | Biotic Stress | Abiotic Stress | |
|---|---|---|---|---|
| PR-1 | Antifungal | Antimicrobial activity [96] Pathogen toxin degradation Viral coat protein-receptor binding inhibition [8] Recruitment of PR proteins (PR-5/PR-14) [97] Sterol binding [98] | Bacteria [99] Fungi [100] Viruses [8] Oomycetes Insects Nematodes [101] | Drought [12] High salinity [9] Low temperature [102] High temperature [103] Heavy metals [104] UV radiation [105] |
| PR-2 | β-1,3-glucanase | Microbial growth suppression [106] Fungal cell wall degradation [14] Callose deposition modulation [107] | Bacteria [108] Fungi [14] Oomycetes [109] Viruses [110,111] Nematodes [112] | Drought [18] High salinity [21] Low temperature [113] Heavy metals [114] UV radiation [115] |
| PR-3 | Endochitinase (Classes I, II, IV, V, VI, VII) | Chitinase activity [116] Fungal cell wall decomposition [117] | Fungi [118] Viruses [119] Nematodes [112] | Drought [59] High salinity [120] High temperature [36] Heavy metals [121] |
| PR-4 | Endochitinase (Classes I, II) | Spore germination inhibition Hyphal growth suppression Cell wall degradation assistance Ribonuclease/DNase activity [28,122] | Fungi [123] Insect and pests [28] | Drought [124] High salinity [23] Low temperature [125] Heavy metals [24] |
| PR-5 | Thaumatin-like protein | Microbial growth inhibition [106] Fungal membrane permeabilization Membrane potential dissipation [126] Fruit ripening promotion [127] | Fungi [128] Viruses [129,130] Bacteria [131] Nematodes [112] | Drought [132] High salinity [133] Osmotic stress [46] Low temperature [134] High temperature [135] Heavy metals [136] UV radiation [115] |
| PR-6 | Protease inhibitor | Pathogen protease inhibition Host cell degradation prevention [137] | Bacteria, Fungi [137] | Low temperature [53] |
| PR-7 | Alkaline endoprotease | Fungal structural proteins degradation Cell wall integrity impairment [138] | Fungi [58] Bacteria [59] Viruses [139] Nematodes [140] | - |
| PR-8 | Class III chitinase | Bacterial cell wall hydrolysis [123] | Fungi [141] | Drought [33] |
| PR-9 | Peroxidase | ROS concentration modulation Lignin-mediated cell wall reinforcement Cytotoxic radical production [142] | Fungi [143] Bacteria [144] Viruses [64] | Drought [145] High salinity [66] Heavy metals [65] |
| PR-10 | Ribonuclease-like proteins | Ribonuclease activity [70] Small hydrophobic ligand binding [146] Fungal/bacterial growth inhibition [147] Secondary metabolite biosynthesis regulation [148] Stress response participation Iron chelation [149] Growth/development promotion [150] | Viruses Bacteria [151] Fungi Nematodes Insects [147] | High salinity Drought [152] Heavy metals [153] Low temperature [70] UV radiation [154] |
| PR-11 | Class I chitinase | β-1,4-chitin glycosidic bond hydrolysis Hyphal structure disruption Spore germination inhibition [155] Pathogen defense enhancement [123] | Bacteria [59] Viruses [156] | Drought [59] |
| PR-12 | Plant defensin | Antimicrobial activity Systemic defense potentiation [157] Human IgE-mediated allergenicity [158] Membrane pore formation Pathogen protease inhibition [159] | Fungi [160] Viruses [64] Nematodes [161] | Drought [77] High-salt/high-temperature stress [162] Low temperature [76] Heavy metals [78] |
| PR-13 | Thionin | Phospholipid binding Transmembrane pore formation Ion leakage induction [163] Damage-associated molecular pattern function [1] | Bacteria [164] Fungi [80] Viruses [64] | Drought [81] High-salt/high-temperature stress [162] Low temperature [165] Heavy metals [82] |
| PR-14 | Lipid transfer proteins | Lipid binding/transport Membrane biosynthesis participation Pathogen membrane disruption [166] Lipid signaling modulation [166,167] SAR network synergy [81] | Bacteria Fungi [84] Viruses [64] | Drought High salinity Low temperature [166] |
| PR-15 | Oxalate oxidase | ROS-controlled antimicrobial activity [168] | Fungi [88] Bacteria [59] Viruses Insect and pests [90] | Drought [169] Low temperature [91] Heavy metals [86] High temperature High salinity Waterlogging [170] |
| PR-16 | Oxalate oxidase-like | Fungal toxin inhibition [171] SOD-mediated superoxide scavenging Defense gene activation [92,172] H2O2-dependent cell wall fortification [173] ABA sensitivity enhancement [92] Pathogen wall hydrolysis [86] | Fungi [88] Bacteria Viruses [59] | Drought [59] High salinity [174] |
| PR-17 | Antifungal and antiviral | Extracellular protease activity [93] | Viruses Fungi [94] Bacteria [59] | Drought [175] |
| PR Family | Enzymatic Activity Type | Biological Functions |
|---|---|---|
| PR-2 | β-1,3-Glucanase | Hydrolysis of β-1,3-glucans in fungal cell walls Release of elicitors Carbohydrate metabolism Osmotic adjustment |
| PR-3, 4, 8, 11 | Chitinase | Degradation of chitin (fungal cell walls/insect exoskeletons) Partial lysozyme activity Antioxidant response COS generation |
| PR-9 | Peroxidase | H2O2-dependent oxidative burst catalysis Lignification promotion ROS scavenging Phenolic compound metabolism |
| PR-10 | Ribonuclease | RNA degradation Signal transduction Phytohormone binding Modulation of antioxidant enzyme activities |
| PR-15 | Oxalate oxidase | Oxalate degradation with H2O2 generation Cell wall lignification ROS signaling |
| PR-16 | Oxalate oxidase-like protein | SOD activity ROS scavenging Ion homeostasis maintenance |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Gao, F. Involvement of Pathogenesis-Related Proteins and Their Roles in Abiotic Stress Responses in Plants. Biomolecules 2025, 15, 1103. https://doi.org/10.3390/biom15081103
Zhu Y, Gao F. Involvement of Pathogenesis-Related Proteins and Their Roles in Abiotic Stress Responses in Plants. Biomolecules. 2025; 15(8):1103. https://doi.org/10.3390/biom15081103
Chicago/Turabian StyleZhu, Yilin, and Fei Gao. 2025. "Involvement of Pathogenesis-Related Proteins and Their Roles in Abiotic Stress Responses in Plants" Biomolecules 15, no. 8: 1103. https://doi.org/10.3390/biom15081103
APA StyleZhu, Y., & Gao, F. (2025). Involvement of Pathogenesis-Related Proteins and Their Roles in Abiotic Stress Responses in Plants. Biomolecules, 15(8), 1103. https://doi.org/10.3390/biom15081103






