Abstract
Diabetes is a risk factor for periodontitis. Increasing evidence suggests that a central player in this link is the vacuolar H+-ATPase (V-ATPase), which provides a physical and functional core for regulation by the catabolic lysosomal AMP-activated protein kinase complex (L-AMPK) and the anabolic mammalian target of rapamycin complex 1 (mTORC1). These complexes detect levels of various cellular nutrients, including glucose at the lysosome, and promote cellular responses to restore homeostasis. The high-glucose conditions of diabetes foster anabolic mTORC1 signaling that increases inflammation and inflammatory bone resorption in response to periodontal infections. Here, we review the structure and composition of V-ATPase, L-AMPK, mTORC1, and other elements of the energy-sensing platform. Mechanisms by which V-ATPase passes signals to the complexes are examined and recent data are reviewed. Current anti-bone resorptive therapeutics, bisphosphonates and denosumab, enhance the risk of medicine-related osteonecrosis of the jaw (MRONJ) and are not used to treat periodontal bone loss. Accumulating data suggest that it may be possible to target inflammatory bone resorption through agents that stimulate L-AMPK, including metformin and glucagon-like peptide-1 agonists. This approach may reduce inflammatory bone resorption without major effects on overall bone remodeling or increased risk of MRONJ.
1. Introduction
Irving Glickman, who is known as the “father of modern periodontology”, observed a heightened incidence of periodontitis in people suffering from diabetes [1,2,3]. We increasingly understand the underlying mechanisms for this connection. One component is cellular energy sensing at the lysosome [4]. During the past thirty-five years, a series of advances have led to the view that in addition to being an essential proton pump, vacuolar H+-ATPase (V-ATPase) is also vital for lysosomal energy sensing [4]. As a proton pump, V-ATPase is required for duties including acidifying lysosomes, late endosomes, phagosomes, autophagosomes, and compartments of the Golgi in eukaryotic cells [5]. In addition, V-ATPase pumps protons out of specialized cells in the kidney to help maintain systemic acid–base balance [6]. In osteoclasts, V-ATPase pumps protons into an extracellular resorption compartment that allows the removal of bone, the first step in bone remodeling [7]. In neurons, V-ATPase is crucial for generating an energy gradient that allows the loading of neurotransmitters into synaptic vesicles [8].
V-ATPases also play a central and ancient role in energy sensing [6,7,9]. Table 1 shows the percentage identity of selected components of the lysosomal energy-sensing apparatus in different eukaryotic organisms. Current understanding of these components and their interactions in mammals will be described in the article. V-ATPase interacts with two protein complexes, the catabolic lysosomal AMPK complex (L-AMPK) [10] and the anabolic mammalian target of rapamycin complex 1 (mTORC1) [11]. These interactions are in part through the Ragulator/Ras-related GTPase (RAG) complex, which is itself an amino acid sensor [12,13], and the scaffolding protein, axis inhibition protein (AXIN) [14]. The history of the discovery of mTORC1 and lysosomal AMPK and their characterization has recently been described authoritatively, and we will direct readers to these excellent review articles for detailed background [4,15,16]. In addition, V-ATPase and the Ragulator/RAG complex have also been recently excellently reviewed [5,6,17,18]. In this article, we will provide brief introductions to the central players, V-ATPase, mTORC1, L-AMPK, the Ragulator/RAG complex, and associated proteins. We will discuss evidence that lysosomal energy sensing is crucial in periodontal disease. We will focus on the interactions between the lysosomal energy-sensing platform components in the setting of periodontal disease and examine crucial open questions regarding the complexes and their functions.

Table 1.
Conservation of elements of lysosomal energy-sensing apparatus in eukaryotes.
2. Lysosomal Energy Sensing and Periodontitis
Sensing nutrients in cells occurs at multiple levels. Is it reasonable to hypothesize that energy sensing at the lysosome has an important or crucial role in periodontitis? The best evidence to date is that inhibitors that specifically or selectively affect lysosomal energy sensing have effects on the pathology of periodontal disease.
2.1. Rapamycin/Sirolimus
Rapamycin (also known as sirolimus) is a specific inhibitor of mTORC1, part of the lysosomal energy-sensing complex [19]. Various studies in animal models show that rapamycin ameliorates some of the pathological effects of periodontal disease, including bone loss [20,21,22]. This is likely due to reduced inflammatory signaling, decreased oxidative stress, and direct inhibition of the activity of osteoclasts [22]. A recent examination of the effects of off-label rapamycin use provides some evidence that it reduces periodontitis-linked bone loss in humans [23]. However, rapamycin has been reported to increase the risk of medicine-related osteonecrosis of the jaw (MRONJ), so caution is indicated in the use of this drug [24].
2.2. Metformin
Metformin less explicitly targets lysosomal energy sensing compared with rapamycin [25,26,27]. It affects the overall glucose supply and has direct effects on V-ATPases involved in energy sensing, as will be discussed in greater detail later in the article [28]. Various studies in animal models and a few human studies report that metformin is efficacious for the treatment of periodontitis [29,30,31,32,33,34,35,36,37,38]. Unlike rapamycin, there is no evidence that metformin triggers MRONJ, and indeed, some studies suggest that it may reduce the pathology of MRONJ [39,40,41]. The differences between metformin and rapamycin, which both result in a reduction in mTORC1 activity, may be due to metformin functioning to simultaneously increase AMPK activity while inhibiting mTORC1, serving to dial the natural rheostat toward catabolism, whereas rapamycin blocks one pathway without directly affecting other branches of the nutrient signaling network.
A challenge for the use of metformin or other L-AMPK modulators for periodontitis is that they affect all parts of the body. An exciting approach is to deliver metformin locally to the periodontium. Various approaches have been reported recently, including the use of synthetic scaffolds or the inclusion of metformin in organic matrices [31,42,43,44,45,46,47,48,49,50]. Another potential approach is based on the finding that metformin stimulates certain cell types to produce extracellular vesicles or cytokines that reduce the pathology of periodontitis [33,36]. These extracellular vesicles or cytokines could be used to treat periodontitis. Recent studies have examined the mechanisms by which metformin ameliorates periodontitis [33,42,50,51,52,53,54,55,56,57,58].
2.3. Glucagon-like Peptide-1 (GLP-1) Agonists
GLP-1 agonists, including Ozempic, Wegovy, Rybelsus, Saxenda, Victoza, Trulicity, Byetta, and Bydureon, have stormed the market [59,60]. Since the introduction of the first of this class, Byetta [61,62], in 2005, GLP-1 agonists have become mega drugs. Although these therapeutics are approved for treating diabetes, they have become popular for off-label use to reduce obesity. The world market in 2024 was USD 53.5 billion and is projected to rise to USD 156 billion by 2030 [63]. These drugs work by increasing insulin secretion and reducing glucagon [59]. This reduces blood glucose. Consequently, they stimulate L-AMPK and inhibit mTORC1 [64]. They are not thought of as agents to treat periodontal disease or MRONJ but would be expected to do so if lysosomal energy sensing played an important role. Several studies now show that they do reduce periodontal infections and associated pathology [65,66,67]. There is also some evidence that periodontal infection alters regulation through the GLP-1 receptor [68,69,70,71,72]. There is no evidence to date regarding the potential effects of GLP-1 agonists on MRONJ.
2.4. Salicylate
Salicylate is the active compound in aspirin. In addition to its well-known role as a cyclooxygenase inhibitor, salicylate is a direct stimulator of AMPK [73]. Evidence suggests it may be beneficial for the treatment of periodontal disease [74,75]. Efforts are underway to develop methods to apply salicylate directly to the periodontium as an approach to treating periodontal disease [74,76]. To date, salicylate has not been implicated in the development of MRONJ.
2.5. Resveratrol
This small molecule, which is famously present in red wine, stimulates Sirtuin-1, a deacetylase that promotes anti-inflammatory pathways [77,78,79,80,81]. Two mechanisms that have been described are likely to impact periodontal bone loss. Resveratrol stimulates Sirtuin-1 to deacetylate Forkhead Box Os (FOXOs), transcription factors involved in processes including energy metabolism [82]. Deacetylation of FOXOs by this route has been described to inhibit osteoclastic bone resorption [82]. As will be described in detail below, Sirtuins were recently shown to inactivate V-ATPase activity by deacetylating several residues on the E-subunit of V-ATPase. This promotes L-AMPK activity [83]. This would also be expected to directly inhibit V-ATPase activity in osteoclasts, which is required for bone resorption [84]. Resveratrol has been described to have therapeutic effects on periodontal disease [81,85,86,87,88]. Of particular importance, it has recently been shown that genetic variants of the Sirtuin-1 gene increase the risk of the development of MRONJ [89,90]. Menaquinone prevented MRONJ in a mouse model by a Sirtuin-dependent mechanism [91].
2.6. 2-Deoxy-D-Glucose
Data were presented supporting the glycolysis inhibitor 2-Deoxy-d-glucose (2-DG) as a therapeutic agent to reduce the pathological effects of periodontal disease [92,93]. 2-DG enters cells through the same transporters as glucose but does not enter glycolysis and accumulates in cells, reducing glycolytic production of ATP and stimulating AMPK [94]. While this requires being used carefully, as it can also trigger apoptosis and is not cell-type-specific, it provides additional evidence linking glycolytic metabolism and therapeutic effects that are useful for treating periodontitis.
2.7. Dorsomorphin (Compound C)
There has been less interest in developing inhibitors of AMPK or stimulators of mTOR. The data that is available is consistent with the overall premise that, in periodontal disease, stimulating catabolic pathways is the best target for reducing the pathology associated with periodontal infections. Perhaps the most obvious of these results is Dr. Glickman’s observation that diabetics have an increased risk of periodontal disease [1]. Dorsomorphin is an inhibitor of AMPK. It has been shown to reduce osteoclast differentiation and activity in vitro [95]. It has not been tested on periodontal disease, but would be expected to exacerbate the pathology.
3. Key Players in Lysosomal Energy Sensing
Energy sensing in general and lysosomal energy sensing in particular is extraordinarily complex. The primary components are described below. These include V-ATPase, which serves as the central element of the lysosomal energy-sensing machinery, mTORC1, which interacts with V-ATPase when energy is abundant, and L-AMPK, which interacts with a different activation state of V-ATPase when energy is depleted. We will also discuss the Ragulator/RAG complex, which regulates the activation state of mTORC1, and what is known about how mTORC1 and L-AMPK interact with V-ATPase. We will discuss how the glycolytic enzyme aldolase binds V-ATPase. Finally, we will address what is known about the reversible assembly of V-ATPase and how this may provide crucial signals for determining the balance between mTORC1 and L-AMPK activity.
3.1. V-ATPase
To understand lysosomal energy sensing, it is crucial to be familiar with V-ATPase. It interacts with both mTORC1 and L-AMPK, and its activation state and (probably) its assembly state are linked to mTORC1 and L-AMPK activity. We will review the overall structure of V-ATPase in this section.
Mammalian V-ATPases are composed of 15 subunits divided into a peripheral domain called V1 and an integral domain called V0 (Figure 1) [5,6]. As shown in Table 2, many of the subunits are present as isoforms that include one that is ubiquitous and one or more isoforms that are found in specialized V-ATPases in specific cell types. V1 contains eight proteins with the following stoichiometry: three A-subunits, three B-subunits, one C-subunit, one D-subunit, three E-subunits, one F-subunit, three G-subunits, and one H-subunit.
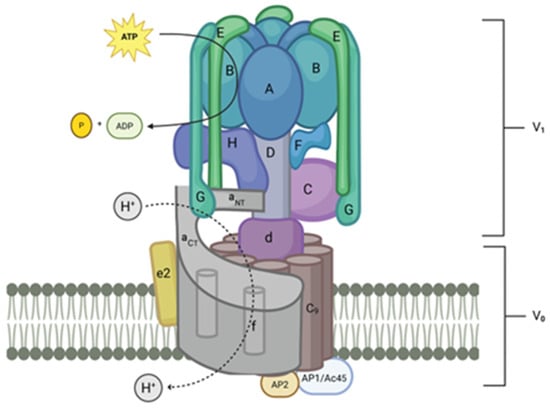
Figure 1.
Model of mammalian V-ATPase. The V1 component is in the cytosol of the cell. V0 is composed of membrane-spanning elements. Rotary movement of the D-subunit and d-subunit powered by ATP hydrolysis in the A-subunit causes rotation of the c-subunit, c″-subunit ring. This rotating ring forms with the a-subunit, a rotation-dependent channel for protons to move through the membrane. Protons are pumped into lumen of vesicles or organelles. In the case of specialized cells, including osteoclasts and renal intercalated cells, protons are pumped out of the cell through the plasma membrane (created with BioRender.com).
The structure of V-ATPases from budding yeast and mammalian brains was recently determined by cryo-electron microscopy and X-ray crystallography, providing excellent models and new insight into these enzymes [8,96,97]. The A-subunits, the site of ATP hydrolysis, are organized into a hetero-hexagon with three B-subunits. Three extended dimers of the E-subunit and G-subunit attach to the top of each B-subunit designated EG1, EG2, and EG3 based on their differential association with the membrane proximal collar domain, which is composed of an a-subunit, C-subunit and H-subunit. EG1 binds the H-subunit and a-subunit. EG2 binds the C-subunit foot domain and a-subunit and EG3 binds the C-subunit head domain and a-subunit [5]. The C-subunit and the H-subunit form a platform at the membrane side of the A/B hetero-hexagon and interact with both V1 and V0. The D-subunit forms a rotor that transduces conformational changes in the hetero-hexagon to spin; it is linked to the V0 through the d-subunit. The V0 domain includes the largest subunit, the a-subunit, which has a membrane-spanning region that contains nine transmembrane domains and a cytosolic region that forms a base interacting directly with various subunits, including the E-subunit/G-subunit stators. It functions in conjunction with a ring of nine c-subunits plus one c″-subunit (the gen is called ATP6V0b) in mammals, which is spun by the D-subunit/d-subunit rotor.

Table 2.
V-ATPase subunits, their isoforms, isoform expression, and diseases caused by mutations in the genes.
Table 2.
V-ATPase subunits, their isoforms, isoform expression, and diseases caused by mutations in the genes.
| Gene | Protein | Location | Disease |
|---|---|---|---|
| ATP6V1A | A | ubiquitous | Developmental and epileptic encephalopathies, cutis laxa, cardiac abnormalities, dysmorphic facial features, and severe hypotonia lysosome dysfunction [98,99] |
| ATP6V1B1 | B1 | kidney, epididymis | Renal tubular acidosis with deafness [100] |
| ATP6V1B2 | B2 | ubiquitous | Dominant Deafness–Onychodystrophy Syndrome (DDOD), Zimmermann–Laband Syndrome 2 (ZLS2), DOORS (Deafness, Onychodystrophy, Osteodystrophy, Intellectual Disability, and Seizures [101,102,103] |
| ATP6V1C1 | C1 | ubiquitous | DOORS syndrome (Deafness, Onychodystrophy, Osteodystrophy, Impaired Intellectual Development, And Seizures Syndrome), autosomal recessive osteopetrosis, and other lysosomal storage disorders [102] |
| ATP6V1C2 | C2 | lung, kidney | Renal tubular acidosis with deafness [104] |
| ATP6V1D | D | ubiquitous | |
| ATP6V1E1 | E1 | testis | Autosomal recessive cutis laxa type Iic [103] |
| ATP6V1E2 | E2 | ubiquitous | |
| ATP6V1F | F | ubiquitous | |
| ATP6V1G1 | G1 | ubiquitous | |
| ATP6V1G2 | G2 | neural | |
| ATP6V1G3 | G3 | kidney, epididymis | |
| ATP6V!H | H | ubiquitous | lysosome dysfunction [98,99] |
| ATP6V0a1 | a1 | ubiquitous | Developmental and epileptic encephalopathy: early-onset seizures, developmental delays, intellectual disabilities, neurodegenerative disorders bone, kidney disorders. Neurodegenerative disorders: Parkinson’s disease, Alzheimer’s disease, and other conditions affecting the brain [105,106] |
| ATP6V0a2 | a2 | ubiquitous | Autosomal recessive cutis laxa type IIA (ARCL2A) and, in some cases, wrinkly skin syndrome [107] |
| ATP6V0a3 | a3 | osteoclast (ubiquitous?) | Autosomal recessive osteopetrosis [108] |
| ATP6V0a4 | a4 | kidney, epididymis | Autosomal recessive distal renal tubular acidosis, renal tubular acidosis with deafness [109] |
| ATP6V0b | b, c″ | ubiquitous | |
| ATP6V0c | c | ubiquitous | |
| ATP6V0d1 | d1 | ubiquitous | |
| ATP6V0d2 | d2 | kidney, epididymis | |
| ATP6V0e | e | ubiquitous | |
| ATP6AP1 | AP1, AC45 | ubiquitous | Congenital Disorder of Glycosylation type, Follicular lymphoma, immunodeficiency with hepatopathy, cognitive impairment [110,111,112] |
| ATP6AP2 | AP2, Prorenin Receptor | ubiquitous | X-linked syndromic intellectual disability (Hedera type), X-linked Parkinsonism–spasticity syndrome, and congenital disorder of glycosylation type 2R autophagic liver disease [113,114] |
With the a-subunit’s transmembrane domain, this spinning creates a route for protons through the membrane [115]. V0 also contains an e-subunit that is located in association with the a-subunit. In addition, two “accessory” proteins, AC45 (ATP6AP1) and the pro-renin receptor (ATP6AP2), were recently identified as having transmembrane domains that are within the c-subunit ring in intact bovine brain V-ATPases and seem to be true mammalian V-ATPase subunits [8,97]. As we will see, ATP6AP1 plays a crucial role in energy sensing in the activation of mTORC1 [116,117].
Because V-ATPases have multiple isoforms of numerous subunits, and many cell types express multiple isoforms, the question is whether those V-ATPases that interact with energy-sensing complexes contain specific isoforms that are involved in their interaction with the energy-sensing machinery. This is not yet clear. One candidate for a lysosomal sensing subunit is the a3-isoform of the a-subunit [118,119]. The a3-subunit is overexpressed in osteoclasts, where it is part of the subset of V-ATPases that are targeted to the ruffled border subdomain of the plasma membrane, and it is required for bone resorption [120,121]. The a3-subunit is often described as the lysosomal isoform of the a-subunit [118]. Null mutations in the gene lead to a very severe form of osteopetrosis, but children with the mutation often survive several years and die from complications of the bone defects that result from inactive osteoclasts [120,121]. Bone marrow transplants can extend life for extended periods [122,123]. Five-year survival with a 100% compatible donor is reported to be 73% [122,123,124]. No metabolic syndrome pathology has been reported.
The hypothesis that interactions between mTORC1 and L-AMPK might be mediated by specific isoforms of V-ATPase subunits is supported by a recent study that shows that TBC/LysM-Associated Domain Containing 2 (TDLc2) binds selectively to the B1-isoform of the B-subunit, which is expressed in the kidney. TDLc2 binds B1 and inactivates the V-ATPase through the interaction [125]. Knockout of TDLc2 in mouse kidneys impairs urine alkalinization [126].
Mammalian Enhancer-of-Akt-1–7 (mEAK7) binds the A-, B-, E- and D-subunits [127]. It may serve as a V-ATPase activator and as a non-canonical means of linking mTOR signaling to V-ATPase activity [125]. In Section 3.7, we will discuss reversible assembly of V-ATPase as a regulatory mechanism and how it may be linked to the formation of the mTORC1/V-ATPase and L-AMPK/V-ATPase complexes.
3.2. mTORC1
The mTORC1 complex stimulates anabolic signaling when nutrients are plentiful. The central component is the enzyme mTOR. Active mTORC1 complexes form and are maintained in association with active V-ATPase in the lysosome. In this section, we will discuss how mTORC1 fits into mTOR signaling in general and describe the composition of the mTORC1 complex. We will use the second mTOR complex, mTORC2, for comparison.
mTOR was discovered through studies of the activity of rapamycin, a bacterial toxin discovered on Easter Island (Rapa Nui is its native name), which was first described as having strong anti-fungal properties [128]. It was later shown to also have anti-cancer and anti-inflammatory activities [129,130]. This led to the concept and demonstration that rapamycin had the same target in yeast and mammals and the discovery of the conserved kinase, mTOR [129,131,132,133]. Subsequent studies have shown that rapamycin targets only one of two mTOR-containing complexes in mammals, mTORC1, which is composed of the catalytic mTOR subunit, regulatory-associated protein of mTOR (RAPTOR), mammalian lethal with sec-13 protein 8 (MLST8), proline-rich Akt substrate of 40 kDa (PRAS40), Tel2 interacting protein 1 (TTI1)/ Telomere maintenance 2 interacting protein 2 (TEL2) and DEP domain containing mTOR-interacting protein (DEPTOR) [134]. mTORC1 binds to the cytosolic region of V-ATPase through the Ragulator/RAG complex and AXIN in an amino-acid-dependent manner [11].
Active mTORC1 stimulates downstream signaling pathways that favor cell growth and proliferation. For example, many cancer cells display inappropriate mTORC1 activation under metabolic conditions where the catabolic L-AMPK signaling pathway should be favored [135,136,137]. mTORC1 is primarily involved in regulating protein synthesis, cell growth, and metabolism, and is activated by nutrients and growth factors [11].
mTORC2 is a second mTOR-containing complex that is composed of seven protein subunits [138,139]. These include the catalytic mTOR subunit, DEPTOR, mLST8, and TTI1/TEL2 complex, which are shared by both mTORC2 and mTORC1. Rapamycin-insensitive companion of mTOR (RICTOR), mammalian stress-activated protein kinase interacting protein 1 (mSIN1), and protein observed with Rictor 1 and 2 (Protor1/2) can only be found in mTORC2. As indicated by its name, RICTOR serves to protect mTOR in mTORC2 from rapamycin [140]. mTORC2 plays a more prominent role in cell survival and proliferation and is activated by stress and growth factors [139].
3.3. L-AMPK
The L-AMPK complex has at its core the enzyme AMPK. In this section, we will discuss the composition of L-AMPK and discuss how it can be activated, comparing it with the canonical AMPK stimulation pathway [4,141].
AMPK is a heterotrimeric protein complex formed by the catalytic α-subunit and regulatory β- and γ-subunits [10]. AMPK stimulates several signaling pathways that lead to catabolic responses. Canonical AMPK activation is triggered by an increase in the AMP/ATP and ADP/ATP ratios, which cause the binding of AMP to the AMPK γ-subunit. This activates the kinase through phosphorylation by liver kinase B1 (LKB1). It is now known that there are several non-canonical routes for AMPK activation that are linked to a V-ATPase-associated L-AMPK complex [142].
L-AMPK activation pathways were only recently elucidated [28,142]. When energy levels are low, myristoylated AMPK associated with lysosomes binds V-ATPase in a complex that includes aldolase, the Ragulator, AXIN, and the kinase, LKB1 [4,9,10,142,143]. Upon glucose starvation, AMPK is activated by phosphorylation on threonine 172, and its kinase activity triggers signaling pathways that promote catabolism and inhibit anabolic processes. Low-dose metformin primarily stimulates the L-AMPK pathway [28]. This occurs through the interaction between metformin and a protein called presenilin enhancer 2 (PEN2), which in turn interacts with AC45 to inhibit V-ATPase activity [28], activating L-AMPK. Important consequences of low-dose metformin include reducing glucose production in the liver, increasing insulin sensitivity in various body tissues, and reducing Growth Differentiation Factor 15 (GDF15) secretion, which reduces appetite [28].
Fructose-bisphosphate aldolase (Aldolase) was recently identified as an important component of L-AMPK signaling. The binding between Aldolase and the V-ATPase was first identified in the early 2000s [144]. The interaction was initially detected in yeast two-hybrid assays, then confirmed by various methods, including immunoprecipitations from osteoclasts. It was suggested that coupling glycolysis directly to the V-ATPases might be a means for preventing alkalinization of the cytosol [144]. Because glycolysis produces protons as a byproduct of ATP synthesis, having both ATP and protons produced in the microenvironment of V-ATPase might be very useful (Figure 2A) [144]. It is important to consider the steps immediately after step 5 of glycolysis, which is catalyzed by aldolase. Step 6 is catalyzed by glyceraldehyde 3-phosphate dehydrogenase (GAPDH), and phosphoglycerate kinase catalyzes step 7. Steps 6 and 7 are coupled reactions and produce a free proton, derived from inorganic phosphate. The phosphate is attached to glyceraldehyde 3-phosphate to give 1,3-bisphosphoglycerate. In step 7, an ATP produced as a phosphate from 1,3-bisphosphoglycerate is added to ADP (Figure 2B). Step 6 has a free energy of +6.3 KJ/mol, while step 7 has a favorable free energy of −18.8 KJ/mol. Coupling the two reactions allows them to proceed and links proton and ATP production. Finally, it was reported that GAPDH is bound to V-ATPase in addition to aldolase, further supporting the coupling of glycolysis to V-ATPase [145].
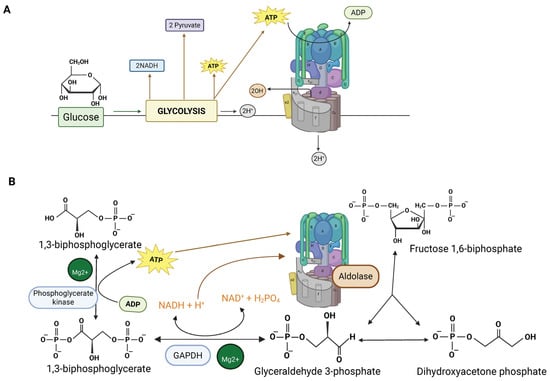
Figure 2.
(A) Glycolysis produces both ATP to power the proton pump and protons. Local production of both protons and ATP by glycolytic enzymes linked to V-ATPase may reduce cytosolic alkalinization. This figure was adapted from reference [114]. (B) Focus on steps 5–7 of glycolysis which are proposed to be crucial for coupling glycolysis to V-ATPase. Aldolase, which is bound to V-ATPase, produces glyceraldehyde 3-phosphate and dihydroxyacetone phosphate (which interconvert). Glyceraldehyde 3-phosphate is converted to 1,3-biphosphate in step 6 by GAPDH, producing a free proton from phosphate. Step 7, which is coupled to step 6, produces 3-phosphoglycerate and an ATP. The step-6 and -7 reactions occur twice, giving a total of two ATPs and two free protons (created with BioRender.com).
Carbonic anhydrase plays a role in maintaining cytosolic pH by converting CO2 produced by mitochondrial metabolism, plus water, to bicarbonate and protons. Mutations in carbonic anhydrase II result in distal renal tubular acidosis and mild osteopetrosis [112,146]. However, osteoclasts still function, although less efficiently, without carbonic anhydrase II, suggesting that other proton-generating strategies are available. Consistent with a role for localized glycolysis, Aldolase B was localized to the ruffled border of osteoclasts, the region rich in V-ATPases where protons are pumped into an extracellular resorption compartment during bone resorption [144].
Subsequent studies showed that aldolase is required in yeast for V-ATPase assembly, suggesting that the link between aldolase and V-ATPase, and indeed the link between V-ATPase and glycolysis, has ancient evolutionary origins [145,147]. Coupling V-ATPase to glycolysis may be a crucial means to prevent alkalinization of the cytosol whenever V-ATPase is active. In Figure 3, we provide a plausible binding interaction between V-ATPase and aldolase. This model is based on previous findings showing aldolase binding interactions with three subunits, the B-subunit and E-subunits of the V1 a-subunit [145].
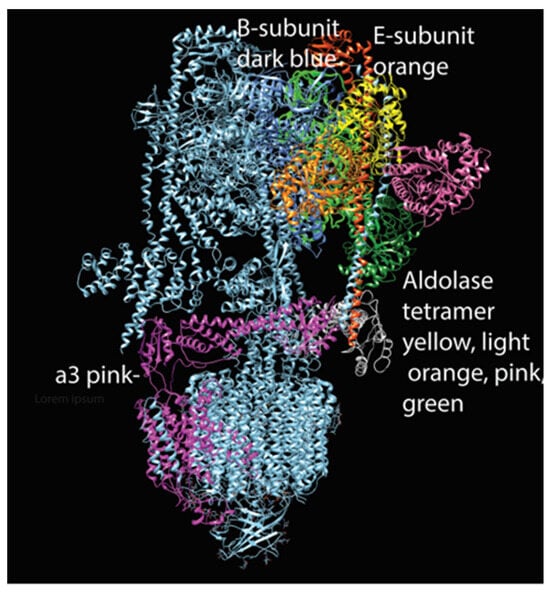
Figure 3.
Model of aldolase tetramer attached to V-ATPase (created with Chimera). This hypothetical model suggests that an aldolase tetramer may be able to interact simultaneously with the three subunits shown to bind in in vitro studies [114].
Activated AMPK inhibits mTORC1 primarily by phosphorylating and activating the mTORC1-negative regulator TSC2 and by phosphorylating and inhibiting the mTORC1 subunit RAPTOR [148]. Activated L-AMPK promotes catabolic pathways, including pathways that break down molecules to produce ATP, such as fatty acid oxidation and glycolysis. L-AMPK stimulates autophagy and mitophagy to cannibalize cell components to provide basic nutrients. AMPK turns off pathways that require energy to build products, including gluconeogenesis (glucose production), lipogenesis (fat synthesis), and protein synthesis [4,142,149,150].
3.4. Ragulator/RAG Complex
The Ragulator/RAG complex serves to activate mTORC1 and to link mTORC1 to V-ATPase both physically and functionally. The Ragulator complex recruits the RAGs, small GTPases that in turn interact with another small GTPase, Ras homolog enriched in brain (Rheb). These receive signals from an amino acid transporter and the V-ATPase.
The Ragulator is composed of five Late endosomal/lysosomal adaptor and MAPK and mTOR activator (LAMTOR) subunits [12,151]. A crystal structure shows that LAMTOR 2 and LAMTOR 3 form an obligate heterodimer and LAMTOR 4 and LAMTOR 5 form a second obligate heterodimer [152]. These two heterodimers interact. LAMTOR1 also forms a dimer with a different structure, which wraps the two heterodimers, stabilizing the structure. The Ragulator then recruits Ras-related-GTP binding (RAG) GTPases (Figure 4) [153].
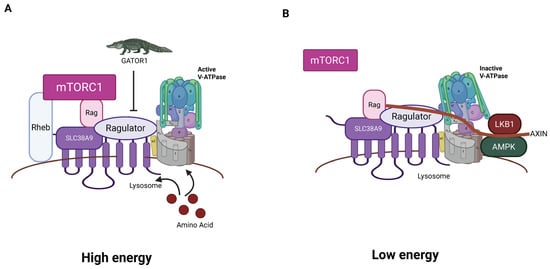
Figure 4.
Model of mTORC1/Ragulator/RAG/L-AMPK complex. (A) In the high-energy condition in the presence of high sugars and amino acids, RAGS and Rheb are active and facilitate recruitment and activation of mTOR, driving anabolic pathways. The GAP GATOR1 acts as an inhibitor. (B) In the low-energy condition, the Ragulator is inactive, mTor is inactive, and the L-AMPK is recruited to V-ATPase through AXIN and activated by LKB1. The active AMPK stimulates catabolic pathways (created with BioRender.com).
The RAG GTPases represent a particularly complicated variation in the theme of regulation by the Ras family of small GTPases. Like other members of the Ras family, RAG GTPases have two states, GTP-bound or GDP-bound [154]. Transitioning occurs through guanine exchange factors (GEFs) that promote the exchange of the nucleotide. Because most free guanine nucleotides in the cytosol are GTP, GEFs promote activation, but as we will see below, there is a crucial variation on this theme. RAG GTPases, like other members of the family, require interaction with GTPase-activating proteins (GAPs) to convert GTP to GDP to change the activation state. RAG GTPases add additional levels of complexity to this basic scheme [153].
There are four RAG GTPases: the small RAGs, RAGA and RAGB, and the large RAGS, RAGC and RAGD [151]. Small RAGs form heterodimers with large RAGs. Small RAGs are activated by the Ragulator, which serves as a GEF for RAGA/B but only triggers release of GTP from RAGC/D. Heterodimers in which the small RAG is GTP-bound and the large RAG is GDP-bound are active. When the large RAG is in its GTP-bound conformation, that heterodimer is locked into that state. Therefore, interaction with the Ragulator activates RAG GTPases by promoting GDP to GTP transition in the small RAGs by the typical strategy but triggers loss of GTP, not GDP, in the large RAGs [153] (Figure 5). Different RAG heterodimers have different activities; this regulation was the focus of a recent excellent review [17].
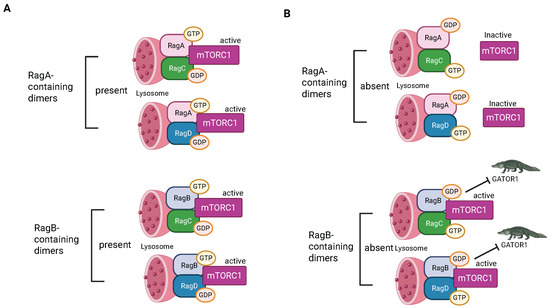
Figure 5.
Regulation by RAG GTPases. (A) Instances where small RAGs are GTP-bound. (B) Instances when small RAGs are GDP-bound (created with BioRender.com).
RAG heterodimers can also be activated by Solute carrier family 38 member 9 (SLC38A9) [153] (Figure 4). It binds and acts as a GEF for the small RAGs in the presence of the amino acid arginine. When the small RAG binds GTP, it breaks its interaction with SLC38A9, allowing SLC38A9 to activate more RAGs, acting like an enzyme. In the absence of amino acids arginine and leucine, GTPase-activating protein toward Rags 2 (GATOR2) promotes GTPase activity in the small RAGS, leading to inactivation [151]. This provides a pathway to inhibit Ragulator/RAG signaling and consequent activation of mTORC1 (as well as other anabolic signaling) when amino acids are limited.
The Ragulator with activated RAG heterodimer recruits mTORC1 to the lysosome [151]. It also recruits Rheb, another small GTPase, which allosterically activates the mTORC1 complex [155]. Activated mTOR in mTORC1 then activates pathways that result in anabolic processes [11]. These include protein synthesis by activating downstream effectors like S6K and 4E-BP, which promote translation and protein production [156]. Lipid synthesis is stimulated through SREBP transcription factors [134]. mTORC1 also stimulates nucleotide synthesis and mitochondrial biogenesis through transcriptional and translational regulation [157].
Like other members of the Ras family, a GEF is required to activate Rheb [100]. Recent data, surprisingly, identified the (or a) GEF as AC45, the newly identified subunit of the V-ATPase [116]. This may be related to the finding that concurrent mutations in RAGC and ATP6AP1 occur in follicular lymphoma [158]. These make mTORC1 resistant to amino acid deprivation. The finding that AC45 is a GEF for Rheb will be discussed in more detail in the next section. The GAP for Rheb is the Tuberous Sclerosis Complex (TSC) 2-subunit of the TSC [159]. The TSC’s GAP activity is negatively regulated by insulin and other growth factors through PI 3-kinase/AKT signaling, keeping mTORC1 signaling on [160]. The GAP activity is positively regulated by LKB1/AMPK signaling, turning off mTORC1 signaling [148].
3.5. Associations Between V-ATPase, Ragulator, L-AMPK, and mTORC1
There remains considerable uncertainty regarding the binding interactions between components of the lysosomal energy-sensing apparatus. In this section, we will describe what is known and indicate areas that are unclear. The known structure of the V-ATPase serves as a guide to where interactions may occur.
There is an intrinsic asymmetry in the structure of V-ATPases [8,97]. The V-ATPase is mobile when V-ATPase is active, swaying gently as the stator arms resist the force generated by conformational changes associated with ATP hydrolysis and turning of the internal rotor [96,161]. This means that in an active pump, proteins that will be subjected to binding sites that may change rapidly in the active pump.
The three stator arms, composed of E- and G-subunits, connect the catalytic V1 domain to the membrane-embedded V0 domain [8,97]. As described in a previous section, the three stators have a unique membrane proximal attachment: EG1 binds the H-subunit and a-subunit, EG2 binds the C-subunit foot and a-subunit, and EG3 binds the C-subunit head and the N-terminus of the a-subunit [8,97]. The three attachment sites have the potential to be differentially regulated.
The V0 is dramatically asymmetrical. The most prominent element is the largest subunit, the a-subunit, which has an extensive membrane-embedded region which functions with the rotating ring of nine c-subunits with a single c″ in mammals to form the rotating membrane-embedded ring [8,97].
Current data suggest that mTORC1 interacts indirectly with V-ATPase through Ragulator/RAG, and that Ragulator interacts with AXIN, a scaffolding protein that also binds the B-subunit and C-subunit of V-ATPase and microfilaments [162], through binding between LAMTOR1 and AXIN [162]. As described above, AC45 was recently described as serving as a GEF for Rheb [117]. Rheb is recruited to the lysosome by active RAG heterodimers, which in turn are activated by interaction with the Ragulator complex and SLC38A9 [153].
The version of AC45 found in brain V-ATPases lacked its extracellular/luminal domain, which had been cleaved, leaving its C-terminal tail associated with V-ATPase. This included a transmembrane domain, with a short cytosolic element embedded in the c-ring of the V0 [8,97]. The pro-renin receptor also lacked its extracellular/luminal domain. The cleavage of the pro-renin receptor is through the action of furin and/or other proteases early in the secretory pathway, and this cleavage is crucial for V-ATPase function [163]. The short cytosolic domain of AC45 stimulates Rheb. The atomic level structure of the V0 component of brain V-ATPase shows AC45 off-center in the ring opposite the a-subunit. It is homologous with that of the yeast V-ATPase subunit Voa1 [164]. A challenge with the hypothesis that AC45 serves as a GEF for Rheb is that this region seems blocked by its location in the V-ATPase. The experiment in which AC45 was identified as a GEF for Rheb used overexpressed proteins [116]; it is possible that the interaction detected is not physiologically relevant. Alternatively, it may be exposed by a disassembled state of the V-ATPase. In any event, the activated Rheb then binds mTORC1 to activate the mTOR activity.
There is evidence that regulatory interactions between V-ATPase and mTORC1 may involve both activation of mTORC1 through V-ATPase activity and assembly of V-ATPase triggered by mTORC1 and associated proteins [9,165]. This will be examined in greater detail in the next section.
Recently it was reported that metformin acts directly on V-ATPase through an interaction with PEN2, a subunit of the γ-secretase [28]. This directly inhibits V-ATPase by binding AC45 and leads to activation of AMPK. This also might block the ability of AC45 to act as a GEF for Rheb, resulting in inhibition of mTORC1. Given the location of AC45 in V-ATPase, it is also possible that the association of PEN2 with AC45 triggered by metformin could disrupt the V0 domain of V-ATPase, inactivating the V-ATPase.
The γ-secretase is a multi-subunit transmembrane protease that cleaves the transmembrane domain of proteins [166]. Metformin had been shown to slightly increase the activity of γ-secretase, which is consistent with an interaction between the two [167]. Because γ-secretase activity contributes to the deposition of amyloid-β protein, this raised concerns regarding long-term use of metformin [167].
L-AMPK phosphorylates the A-subunit of V-ATPase [168]. This implies proximity to V-ATPase. It has been proposed that L-AMPK binds indirectly to V-ATPase through AXIN, which binds directly to V-ATPase and interacts with both L-AMPK and the Ragulator. AXIN binds the AMPK and LKB1 elements of the L-AMPK and serves to recruit them to V-ATPase in the lysosome in low-glucose conditions and to facilitate phosphorylation of AMPK by LKB1 in conjunction with inactivation of V-ATPase [14,146]. It must be noted that knockout of one of the two AXIN proteins, AXIN1, surprisingly, did not affect AMPK/mTORC1 signaling [169]. Separately, AXIN also has a role as an inhibitor of Wnt/β-catenin signaling, which is crucial for regulating osteoblast activity [14].
Both the C-subunit and the B-subunit of V-ATPase have been shown to bind microfilaments [170,171,172,173,174,175]. The binding site in the B-subunit is blocked in active V-ATPases by the stator arms containing an E-subunit and G-subunit, which contact the same region [8,96,97]. The C-subunit has been described as binding and crosslinking microfilaments in its disassembled state. It is possible that disassembly of V-ATPase leads to binding of microfilaments to B- and C-subunits and binding of AXIN to the partially disassembled V-ATPase, and then recruitment of AMPK and LKB1 to activate AMPK activity. The models that have been proposed rely on AXIN to serve as a scaffold for both the connection of L-AMPK and mTORC1, through the Ragulator [162]. AXIN was not detected associated with brain V-ATPases [8,97]. AXIN may only bind lysosomal V-ATPases, perhaps through a lysosome-specific isoform of V-ATPase.
Aldolase, as described above, is a key element of L-AMPK and was shown to bind three V-ATPase subunits: the a-subunit, the amino terminus of the B-subunit and the E-subunit [145]. For aldolase to utilize these three binding sites would suggest that binding is in the expected tetrameric form of active aldolase and probably only one tetramer is associated. The a-subunit would serve as an asymmetrical component to limit binding. Such interaction could account for its link to V-ATPase assembly, with aldolase triggering or stabilizing association between the a-subunit and elements of V0 and the E-subunit and B-subunit, a stator component with the membrane-distal attachment of the stator in V0.
3.6. Sugar Sensing by Aldolase
As described above, Aldolase binds V-ATPase and may serve to couple glycolysis to V-ATPase activity. In this section, we will describe the proposed mechanism by which aldolase is linked to energy sensing.
Aldolase acts as a sensor for glucose availability by binding to fructose 1,6-bisphosphate (FBP), an intermediate of glucose metabolism [143,176]. Under high-glucose conditions, FBP is available for Aldolase, and this maintains the activity of the V-ATPase. While V-ATPase is active, mTORC1 is indirectly kept active as described above. When FBP is low, the Aldolase changes its conformation, and V-ATPase is no longer active [143]. V-ATPase may undergo some version of reversible assembly, as will be described below. Recently, data have emerged linking this process to calcium flux through Transient Receptor Potential V channels (TRPVs). V-ATPase-associated aldolase, when inactive due to low FBP, binds and inhibits TRPV channels in the endoplasmic reticulum [176,177] (Figure 6). This reduces local calcium and allows inactive TRPV to bind V-ATPase, which relays the FBP-free status of aldolase to the V-ATPase. In support of this mechanism, genetic depletion of TRPVs blocked glucose starvation-induced AMPK activation in cells and the liver of mice and in nematodes [177]. The pharmacological inhibitor of TRPV1–4 (AMG-9810) activated AMPK in the mice without altering the adenylate ratios and elevated NAD+ levels in aged muscles, rejuvenating the animals’ running capacity [177].
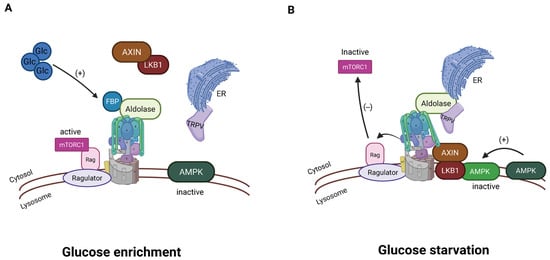
Figure 6.
Aldolase associated with V-ATPase is involved in energy sensing. (A) In a high-glucose environment, V-ATPase is active, and aldolase is associated with the active V-ATPase. (B) When glucose is not abundant, V-ATPase is inactive; aldolase interacts with TRPV channels in the endoplasmic reticulum, inhibiting calcium release. The reduced calcium is involved in passing the signal of low glucose to the sensing system (created with BioRender.com).
3.7. Role of V-ATPase Assembly and Disassembly
At the heart of current thinking regarding the role of V-ATPase in energy sensing is the hypothesis that V-ATPase activity is regulated by reversible assembly of the V-ATPase. The intact V-ATPase and disassembled subcomplexes are thought to display different binding specificities; the intact active V-ATPase stimulates mTORC1 while disassembled subcomplexes promote L-AMPK. Currently, a model system capable of studying the molecular details of this system has not yet been identified.
In the yeast Saccharomyces cerevisiae, V-ATPase undergoes reversible assembly that has been characterized in detail [178,179,180]. Reversible assembly of V-ATPase in yeast is linked to lysosome function [181]. The simplest model suggests that disassembly of V-ATPase involves release of the V1 subdomain into the cytosol with V0 left in the membrane. Both subdomains are locked into inactive forms; V1’s enzymatic activity is autoinhibited due to the action of the H-subunit, which prevents ATP hydrolysis [96,182]. V0 is likewise locked to prevent protons from crossing the membrane [183]. Many of the intermediate steps and the final structures have been characterized enzymatically and structurally [180,184,185,186]. In mammalian cells, characterization has been less straightforward, and though reversible assembly is proposed to regulate mammalian V-ATPases, the data are less clear [5] (Figure 7).
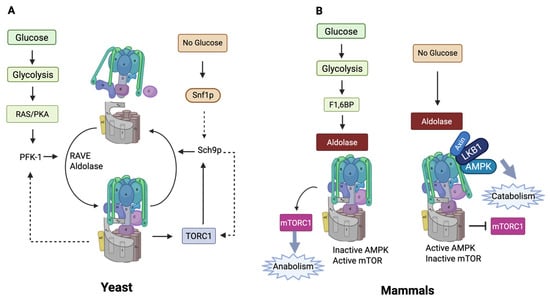
Figure 7.
(A) Reversible assembly in yeast and its links to energy sensing. (B) Energy sensing in mammals. In mammals, V-ATPase inactivation seems to involve some form of disassembly. This is represented in the figure by a tilted V1, but the exact nature of the disassembly is not known (created with BioRender.com).
Consider the volume of Saccharomyces cerevisiae, 40–90 µM3 [187], versus a typical mammalian cell (4000 µM3) [188], which is 50–100 times larger. This makes clear the increased challenge in mammalian cells of reuniting a truly free V1 in the cytosol with a membrane-incorporated V0. It seems likely that scaffolding, perhaps involving microfilaments and AXIN, keeps the V-ATPase subcomplexes tethered when they are disassembled. This could conveniently provide scaffolding for the L-AMPK and LKB1. This also suggests that energy sensing may depend on the regulation of cytoskeletal dynamics. Polymerization–depolymerization and myosin-powered contraction of the cytoskeleton could have a vital role in reversible assembly. Supporting this idea, V-ATPase from osteoclasts, immunoisolated with an anti-E-subunit antibody, had both actin and myosin II bound to it [171]. Myosin II is the conventional myosin that causes contraction of the microfilament cytoskeleton.
Understanding how the activation and inactivation of V-ATPases occur in mammals is a vital challenge. Such understanding may well provide new targets for pharmacological agents to treat the myriads of pathologies associated with V-ATPase activity. Such understanding may require the identification of a model where large amounts of the energy-sensing complexes are present, or the generation of such a model to allow its isolation and structural analysis.
4. Energy Sensing and Periodontal Disease: How Does Inflammation Result from Energy Sensing?
4.1. RAGE Activation and AMPK
It is well established that increased inflammation is associated with diabetes [189]. The underlying mechanism is not clear. Several hypotheses have been presented. Hyperglycemia can stimulate inflammation [190]. High levels can cause increased levels of advanced glycation end (AGE) products on surface proteins on cells in the bloodstream [191]. The AGEs stimulate the receptor of advanced glycation end products (RAGE), which triggers oxidative stress, inflammation, and damage to organs [191]. There is a substantial body of literature that demonstrates regulatory relationships between AGE/RAGE signaling and energy sensing.
RAGE activation triggered by AGEs has been reported to downregulate AMPK activity [192]. In diabetic mice, increased AGEs and RAGE activation reduce phosphorylated AMPK. RAGE deficiency has been linked to autophagy induction through the AMPK signaling pathway [192]. AICAR, an AMPK activator, promotes the activity of the protease, ADAM10 [193]. This can lead to RAGE ectodomain shedding, inactivating the RAGE as a receptor. Likewise, metformin activates AMPK and inhibits AGE-induced RAGE activation of NF-κB [194,195].
4.2. Pattern Recognition Receptors (PRRs)
Innate pattern recognition receptor systems are involved in the response to periodontal infections. These include toll-like receptors (TLRs), which have been the subject of much research [196]. More recently, nucleotide-binding oligomerization domain-containing protein 1 (NOD1) was implicated in periodontal bone loss and evidence surprisingly suggested that MYD88, a molecule downstream of TLRs, was not involved [197]. Considerable evidence indicates that signaling from PRRs is entangled with energy sensing in general and with lysosomal energy sensing in particular [93,198].
There is evidence that responses triggered by TLRs are modulated by components of the lysosomal energy-sensing apparatus. For example, in response to viral double-stranded RNAs, TLR3 triggers cleavage of a regulatory portion of cellular SQSTM1/p62 [199]. SQSTM1/p62 is a ubiquitin ligase that when mutated can lead to Paget’s Disease of the bone. This cleavage is through TLR3 activation of RIPK1 and CASP8 (caspase 8). The cleaved molecule called SQSTM1/p62TRM (amino acids 1–329) is an mTORC1 regulator whose production correlates with the sustained activation of mTORC1 and RPS6KB1/p70S6K1 phosphorylation. This pathway may stimulate mTORC1 activity in excess of what is required based on nutrient status, which may lead to increased inflammatory signaling [199]. In cases where nutrient levels are already high, this enhances the anabolic signaling that leads to inflammation. However, therapeutic activation of AMPK would be expected to reduce this mTORC1 signaling by phosphorylating and activating TCS2 and phosphorylating and inhibiting RAPTOR.
NOD1 recognizes peptidoglycan fragments from bacteria in the cytosol and triggers innate immune responses [200]. The notion that a cytosolic bacterial component sensor would play a crucial role in periodontal bone loss is not surprising. One of the archetypical periodontal pathogens is Porphyromonas gingivalis (P. gingivalis), which thrives in the cytosol of periodontal cells [201].
Recently, a new mechanism has emerged that may be regulated through lysosomal energy sensing. Over time, mitochondrial damage occurs, eventually leading to leakage of double-stranded DNA from the mitochondria into the cellular cytosol. This leads to the recruitment and activation of cyclic GMP-AMP-synthase (cGAS). cGAS produces 2′3′-cGAMP (cGMP-AMP), which functions as a second messenger to activate stimulator of interferon genes (STING) [199,202]. This triggers signaling pathways resulting in the production of interferons and activation of nuclear factor kappa B (NFKB), which stimulates the production of inflammatory molecules [203] (Figure 7).
5. Energy Sensing and MRONJ
MRONJ is a severe pathology of the jaw that is linked primarily, but not exclusively, to agents that alter bone remodeling. The condition was first identified as bisphosphonate-related osteonecrosis of the jaw (BRONJ) that appeared in the early 2000s, caused by nitrogen-containing bisphosphonates that had recently been introduced as anti-resorptives to treat osteoporosis and bone cancer [204]. Various other agents have now been linked to the condition, including the antiresorptive, denosumab, a humanized monoclonal antibody that binds receptor activator of nuclear factor κ B (RANK) ligand and competitively inhibits its binding to its receptor RANK [205]. A second biologic, Romosozumab, which inhibits sclerostin, thereby triggering bone formation and inhibiting bone resorption, also triggers MRONJ, as does rapamycin/sirolimus [24].
For osteoporosis and bone cancer, first-line therapeutics are nitrogen-containing bisphosphonates, including zoledronate and ibandronate or denosumab [206]. Bisphosphonates function by being targeted to sites of active bone resorption, where they are incorporated into the bone [207]. When osteoclasts begin to resorb the bone, the bisphosphonate is mobilized and blocks a step in the mevalonate pathway, which prevents prenylation of Rho small GTPases and triggers apoptosis by osteoclasts [208]. Denosumab prevents osteoclast formation. The therapeutic impact of the two agents, despite their very different mechanisms of action, is similar [98,99,100]. Both also increase the risk of MRONJ about the same [24]. MRONJ occurs rarely even when these antiresorptives are given at high doses. It is hypothesized that specific genetic variations may make people susceptible, and that bisphosphonates and denosumab may have different underlying genetic susceptibilities [101]. To test this idea, studies have looked for susceptibility markers. For bisphosphonate-induced MRONJ, two genes have been identified to date. One encodes Farnesyl-Diphosphate Farnesyltransferase 1, which is an enzyme in the mevalonate pathway [102]. Altered activity of the enzyme would change the pool of substrates for the enzyme inhibited by nitrogen-containing bisphosphonates and thus alter their impact on cells. The second is Sirtuin 1 [89]. As discussed previously, this deacetylase affects lysosomal energy sensing, at least in part through inhibiting V-ATPase activity by deacetylating residues on the E-subunit [83]. The latter suggests that MRONJ susceptibility may be entangled with lysosomal energy sensing, and that agents that stimulate L-AMPK may prevent or treat MRONJ.
Bisphosphonates and denosumab inhibit bone resorption by targeting osteoclasts, while metformin and GLP-1 agonists affect not just osteoclasts but also osteoblasts, osteocytes, and all other cells in the body (Figure 8). Additive or synergistic effects could result when these different drug classes are given together. There have been several studies examining the consequences of antiresorptives in combination with metformin. Metformin and alendronate were tested in an animal model for effects on osteoporosis in a diabetic mouse model. Although both agents reduced signs of osteoporosis, there was no obvious synergy or additive effects [104]. The same combination was useful in the treatment of glucocorticoid-induced osteoporosis [103]. Promising results show that metformin given in combination with alendronate reduces cartilage damage in a mouse model of osteoarthritis [105]. Combinations of bisphosphonates with metformin have been suggested as a treatment for osteoarthritis [106]. Metformin and bisphosphonates have been tested for effects on cancer [107,108]. A practical effect of metformin is that it reduces gastric damage associated with oral bisphosphonates in rat models [109]. Surprisingly, there is very limited data regarding the use of the combination of bisphosphonates with denosumab/GLP-1 agonists with antiresorptives [209]. Interestingly, denosumab alone increases intrinsic GLP-1 levels and improves glucose control in postmenopausal women with type 2 diabetes [210].
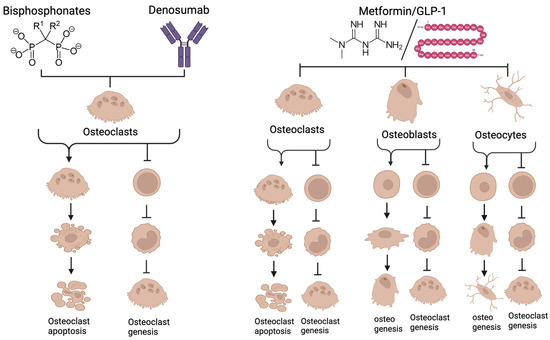
Figure 8.
Bisphosphonates and Denosumab act directly on osteoclasts, the bone-resorbing cells. Bisphosphonates are incorporated into bone then released as osteoclasts begin resorbing. The bisphosphonate perturbs the mevalonate pathway in osteoclasts, triggering apoptosis of the osteoclast. Denosumab both blocks osteoclastogenesis and inhibits mature osteoclasts. Metformin and GLP-1 agonists have effects on all types of bone cells. They reduce differentiation and at high concentrations trigger apoptosis of osteoclasts. Both stimulate osteogenesis toward osteoblasts and osteocytes and inhibit pro-osteoclastic signals from these cells. They also affect other cells involved in periodontitis including immune cells and fibroblasts. Because of the different mechanisms of action between conventional antiresorptives and metformin/GLP-1 agonists, there may be additive or synergistic therapeutic effects. See text for more discussion (created with BioRender.com).
There is some evidence supporting the use of metformin for treating MRONJ [40,41]. There is also ample evidence that diabetes is a risk factor for MRONJ [110,142,207]. Based on this evidence, further study of agents or practices that encourage L-AMPK activation for preventing and treating MRONJ is warranted.
It must be noted, however, that rapamycin has been implicated in the development of MRONJ, although with low risk [24]. More detailed understanding of lysosomal energy sensing and how it interacts with periodontitis and other diseases is urgently needed. Lysosomal energy sensing is of great importance to the cell and to the mammalian organism. The machinery is complex and in many areas is not understood [4]. The complexity of the signaling may provide targets for future therapeutic agents or therapeutic approaches.
6. Conclusions
Remarkable progress in understanding how cells respond to nutrients and how this impacts the health of the body in general and periodontal health has been accomplished. The nutrient sensing machinery is very ancient. In budding yeast and plants, it is significantly different from that in mammalian cells, but V-ATPase has served as the core of the machinery since the rise of eukaryotic cells. Shifting cellular responses to varying nutrient availability allows cells to adapt to the environment. In humans, an overabundance of nutrients can lead to increased inflammation. Activation of cGAS/STING downstream of mitochondrial damage and insufficient mitophagy were recently described and add to oxidative stress and AGE/RAGE signaling associated with high levels of carbohydrates.
Diabetes and obesity were identified as risk factors for periodontal disease before the dawn of molecular cell biology. Lysosomal energy sensing provides a mechanistic basis to at least partially explain this link.
Much is unknown regarding lysosomal energy sensing, including fundamentally important elements. Perhaps most important is the fact that reversible assembly, or whatever changes in V-ATPase that occur in mammals to regulate its activity, is not well understood. It is also not known whether and how V-ATPase isoforms influence association with nutrient-sensing machinery. The interactions between V-ATPase and the energy-sensing complexes that signal high and low energy states are not yet fully understood. Indeed, a central focus appears to be ATP6AP1. This subunit was recently considered to be an accessory protein to V-ATPase but now appears to be a core subunit.
Therapeutics, including metformin and GLP-1 agonists, show promise for the prevention and treatment of periodontal disease and MRONJ, particularly in diabetic patients. However, these medications have been associated with off-target effects. For example, GLP-1 agonists have been linked to gastrointestinal issues, pancreatitis, increased risk of depression and suicide, nephrolithiasis or kidney disease, and, in a few cases, acute kidney disease downstream of dehydration from gastrointestinal issues [113,114]. The effectiveness of metformin and GLP-1 agonists serves as a mechanistic basis for clinicians to encourage patients to engage in activities that promote a healthy blend of L-AMPK and mTORC1 activity. In many, this can be obtained and maintained by modest increases in exercise and improvements in diet [211].
While we typically think of advancing medicine as developing new therapeutic agents, lysosomal energy-sensing pathways and their role in health offer an example where behavioral changes obviously offer opportunities to prevent or treat periodontal disease and possibly MRONJ. For example, the underlying mechanistic basis for increased health and longevity observed on a calorically restricted diet was recently uncovered, and it involved a pathway to inhibit V-ATPase and thereby activate L-AMPK [83]. While it is possible that lithocholic acid, the agonist for sirtuins that was induced by caloric restriction, might be developed into a therapeutic agent, caloric restriction might be a simpler and safer solution. Treatment of periodontitis and MRONJ might be easily and naturally accomplished by eating healthier, staying away from sweets, reducing caloric intake, getting plenty of exercise, and perhaps practicing intermittent fasting [212]. Because of the complexity of the lysosomal energy-sensing machinery, the safest and most efficient approach may be finding ways to adjust the natural energy rheostat through changes in behavior.
Author Contributions
Conceptualization, X.Y. and L.S.H.; methodology, X.Y. and L.S.H.; software, X.Y.; validation, X.Y., and L.S.H.; formal analysis, X.Y. and L.S.H.; investigation, X.Y. and L.S.H.; writing—original draft preparation, L.S.H.; writing—review and editing, X.Y.; visualization, X.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the American Association of Orthodontists Foundation.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| V-ATPase | vacuolar H+-ATPase |
| L-AMPK | lysosomal AMP-activated protein kinase complex |
| mTORC1 | mammalian target of rapamycin complex 1 |
| MRONJ | medicine-related osteonecrosis of the jaw |
| RAG | Ras-related GTPase |
| AXIN | axis inhibition protein |
| GLP-1 | Glucagon-like Peptide-1 |
| FOXO | Forkhead Box O |
| 2-DG | 2-Deoxy-d-glucose |
| TDLc2 | TBC/LysM-Associated Domain Containing 2 |
| mEAK7 | Mammalian Enhancer-of-Akt-1–7 |
| RAPTOR | regulatory-associated protein of mTOR |
| MLST8 | mammalian lethal with sec-13 protein 8 |
| PRAS40 | proline-rich Akt substrate of 40 kDa |
| TTI1 | Tel2 interacting protein 1 |
| TEL2 | Telomere maintenance 2 interacting protein 2 |
| DEPTOR DEP | domain containing mTOR-interacting protein |
| RICTOR | Rapamycin-insensitive companion of mTOR |
| PROCTOR | protein observed with RICTOR |
| LKB-1 | Liver Kinase B1 |
| PEN2 | presenilin enhancer 2 |
| TSC2 | Tuberous sclerosis complex 2 |
| GEF | Guanine exchange factor |
| GAP | GTPase-activating protein |
| SLC38A9 | Solute carrier family 38 member 9 |
| GATOR | GTPase-activating protein toward Rags |
| Rheb | Ras homolog enriched in brain |
| PI-3 kinase | Phosphatidylinositol 3-kinase |
| AKT | Protein kinase B |
| LAMTOR | Late endosomal/lysosomal adaptor and MAPK and mTOR activator |
| TRPV | Transient Receptor Potential V |
| AGE | Advanced glycation end product |
| PRR | Pattern Recognition Receptors |
| TLR | Toll-like receptor |
| NOD1 | nucleotide-binding oligomerization domain-containing protein 1 |
| cGAS | cyclic GMP-AMP-synthase |
| STING | Stimulator of Interferon Genes |
| BRONJ | bisphosphonate-related osteonecrosis of the jaw |
| RANK | receptor activator of nuclear factor κ B |
References
- Glickman, I. The Relation of Experimental Diabetes to Periodontal Disease. Am. J. Orthod. 1947, 33, 703–722. [Google Scholar] [CrossRef]
- Glickman, I. The Periodontium, Pancreas and Blood Sugar Levels in Experimental Diabetes. J. Dent. Res. 1946, 25, 169. [Google Scholar] [PubMed]
- Glickman, I. The Periodontal Structures in Experimental Diabetes. N. Y. J. Dent. 1946, 16, 226–251. [Google Scholar]
- Gonzalez, A.; Hall, M.N.; Lin, S.C.; Hardie, D.G. Ampk and Tor: The Yin and Yang of Cellular Nutrient Sensing and Growth Control. Cell Metab. 2020, 31, 472–492. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.P.; Forgac, M. Regulation and Function of V-Atpases in Physiology and Disease. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183341. [Google Scholar] [CrossRef]
- Eaton, A.F.; Merkulova, M.; Brown, D. The H(+)-Atpase (V-Atpase): From Proton Pump to Signaling Complex in Health and Disease. Am. J. Physiol.-Cell Physiol. 2021, 320, C392–C414. [Google Scholar] [CrossRef]
- Holliday, L.S. Vacuolar H(+)-Atpases (V-Atpases) as Therapeutic Targets: A Brief Review and Recent Developments. Biotarget 2017, 1, 675430. [Google Scholar] [CrossRef]
- Abbas, Y.M.; Wu, D.; Bueler, S.A.; Robinson, C.V.; Rubinstein, J.L. Structure of V-Atpase from the Mammalian Brain. Science 2020, 367, 1240–1246. [Google Scholar] [CrossRef]
- Collins, M.P.; Forgac, M. Regulation of V-Atpase Assembly in Nutrient Sensing and Function of V-Atpases in Breast Cancer Metastasis. Front. Physiol. 2018, 9, 902. [Google Scholar] [CrossRef]
- Hardie, D.G.; Lin, S.C. Amp-Activated Protein Kinase-Not Just an Energy Sensor. F1000Research 2017, 6, 1724. [Google Scholar] [CrossRef]
- Szwed, A.; Kim, E.; Jacinto, E. Regulation and Metabolic Functions of Mtorc1 and Mtorc2. Physiol. Rev. 2021, 101, 1371–1426. [Google Scholar] [CrossRef] [PubMed]
- Bar-Peled, L.; Sabatini, D.M. Regulation of Mtorc1 by Amino Acids. Trends Cell Biol. 2014, 24, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Tsujimoto, K.; Takamatsu, H.; Kumanogoh, A. The Ragulator Complex: Delving Its Multifunctional Impact on Metabolism and Beyond. Inflamm. Regen. 2023, 43, 28. [Google Scholar] [CrossRef]
- Qiu, L.; Sun, Y.; Ning, H.; Chen, G.; Zhao, W.; Gao, Y. The Scaffold Protein Axin1: Gene Ontology, Signal Network, and Physiological Function. Cell Commun. Signal. 2024, 22, 77. [Google Scholar] [CrossRef]
- Condon, K.J.; Sabatini, D.M. Nutrient Regulation of Mtorc1 at a Glance. J. Cell Sci. 2019, 132, jcs222570. [Google Scholar] [CrossRef] [PubMed]
- Saxton, R.A.; Sabatini, D.M. Mtor Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef]
- Lama-Sherpa, T.D.; Jeong, M.H.; Jewell, J.L. Regulation of Mtorc1 by the Rag Gtpases. Biochem. Soc. Trans. 2023, 51, 655–664. [Google Scholar] [CrossRef]
- Jewell, J.L. Rag-Ulating Mtorc1 with Amino Acids. Nat. Rev. Mol. Cell Biol. 2021, 22, 587. [Google Scholar] [CrossRef]
- Lamming, D.W. Inhibition of the Mechanistic Target of Rapamycin (Mtor)-Rapamycin and Beyond. Cold Spring Harb. Perspect. Med. 2016, 6, a25924. [Google Scholar] [CrossRef]
- An, J.Y.; Quarles, E.K.; Mekvanich, S.; Kang, A.; Liu, A.; Santos, D.; Miller, R.A.; Rabinovitch, P.S.; Cox, T.C.; Kaeberlein, M. Rapamycin Treatment Attenuates Age-Associated Periodontitis in Mice. Geroscience 2017, 39, 457–463. [Google Scholar] [CrossRef]
- Li, X.; Chang, B.; Wang, B.; Bu, W.; Zhao, L.; Liu, J.; Meng, L.; Wang, L.; Xin, Y.; Wang, D.; et al. Rapamycin Promotes Osteogenesis under Inflammatory Conditions. Mol. Med. Rep. 2017, 16, 8923–8929. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Liu, Y.; Zhang, B.Y.; Zhang, H.; Shan, F.Y.; Li, T.Q.; Zhao, Z.N.; Wang, X.X.; Zhang, X.Y. Rapamycin Inhibits Osteoclastogenesis and Prevents Lps-Induced Alveolar Bone Loss by Oxidative Stress Suppression. ACS Omega 2023, 8, 20739–20754. [Google Scholar] [CrossRef] [PubMed]
- Hudson, J.; Kaeberlein, T.; Mahal, A.; Wong, N.; Ghorbanifarajzadeh, M.; Radella, F.; Isman, A.; Nyquist, A.; Zalzala, S.; Haddad, G.; et al. Evaluation of Off-Label Rapamycin Use on Oral Health. Geroscience 2024, 46, 4135–4146. [Google Scholar] [CrossRef]
- King, R.; Tanna, N.; Patel, V. Medication-Related Osteonecrosis of the Jaw Unrelated to Bisphosphonates and Denosumab—A Review. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2019, 127, 289–299. [Google Scholar] [CrossRef]
- Kim, J.; You, Y.J. Regulation of Organelle Function by Metformin. IUBMB Life 2017, 69, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Foretz, M.; Guigas, B.; Viollet, B. Metformin: Update on Mechanisms of Action and Repurposing Potential. Nat. Rev. Endocrinol. 2023, 19, 460–476. [Google Scholar] [CrossRef]
- Isop, L.M.; Neculau, A.E.; Necula, R.D.; Kakucs, C.; Moga, M.A.; Dima, L. Metformin: The Winding Path from Understanding Its Molecular Mechanisms to Proving Therapeutic Benefits in Neurodegenerative Disorders. Pharmaceuticals 2023, 16, 1714. [Google Scholar] [CrossRef]
- Ma, T.; Tian, X.; Zhang, B.; Li, M.; Wang, Y.; Yang, C.; Wu, J.; Wei, X.; Qu, Q.; Yu, Y.; et al. Low-Dose Metformin Targets the Lysosomal Ampk Pathway through Pen2. Nature 2022, 603, 159–165. [Google Scholar] [CrossRef]
- Neves, V.C.M.; Okajima, L.S.; Elbahtety, E.; Joseph, S.; Daly, J.; Menon, A.; Fan, D.; Volkyte, A.; Mainas, G.; Fung, K.; et al. Repurposing Metformin for Periodontal Disease Management as a Form of Oral-Systemic Preventive Medicine. J. Transl. Med. 2023, 21, 655. [Google Scholar] [CrossRef]
- Silva, F.F.V.E.; Chauca-Bajana, L.; Caponio, V.C.A.; Cueva, K.A.S.; Velasquez-Ron, B.; Padin-Iruegas, M.E.; Almeida, L.L.; Lorenzo-Pouso, A.I.; Suarez-Penaranda, J.M.; Perez-Sayans, M. Regeneration of Periodontal Intrabony Defects Using Platelet-Rich Fibrin (Prf): A Systematic Review and Network Meta-Analysis. Odontology 2024, 112, 1047–1068. [Google Scholar] [CrossRef]
- Miron, R.J.; Moraschini, V.; Estrin, N.E.; Shibli, J.A.; Cosgarea, R.; Jepsen, K.; Jervoe-Storm, P.M.; Sculean, A.; Jepsen, S. Periodontal Regeneration Using Platelet-Rich Fibrin. Furcation Defects: A Systematic Review with Meta-Analysis. Periodontology 2000, 2024; Online ahead of print. [Google Scholar]
- Neves, V.C.M.; Savchenko, V.; Daly, J.; Sharpe, P. Periodontal Ageing and Its Management Via Pharmacological Glucose Modulation. Front. Dent. Med. 2024, 5, 1415960. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Song, J.H.; Kim, J.W.; Kwon, S.H.; Piao, X.; Oh, S.H.; Park, S.G.; Kim, S.H.; Ryu, J.H.; Kim, O.S.; et al. Metformin Reverses Periodontal Destruction Caused by Experimental Periodontitis by Inhibiting Interleukin-1β Activity. J. Periodontol. 2025; Online ahead of print. [Google Scholar]
- Liu, F.; Han, R.; Nie, S.; Cao, Y.; Zhang, X.; Gao, F.; Wang, Z.; Xing, L.; Ouyang, Z.; Sui, L.; et al. Metformin Rejuvenates Nap1l2-Impaired Immunomodulation of Bone Marrow Mesenchymal Stem Cells Via Metabolic Reprogramming. Cell Prolif. 2024, 57, e13612. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Xiong, Y.; Zhang, W.; Ma, X.; Xu, X. Metformin Promotes Osteogenic Differentiation and Protects against Oxidative Stress-Induced Damage in Periodontal Ligament Stem Cells Via Activation of the Akt/Nrf2 Signaling Pathway. Exp. Cell Res. 2020, 386, 111717. [Google Scholar] [CrossRef]
- Xiang, M.; Liu, Y.; Guo, Q.; Liao, C.; Xiao, L.; Xiang, M.; Guan, X.; Liu, J. Metformin Enhances the Therapeutic Effects of Extracellular Vesicles Derived from Human Periodontal Ligament Stem Cells on Periodontitis. Sci. Rep. 2024, 14, 19940. [Google Scholar] [CrossRef]
- Ren, C.; Hao, X.; Wang, L.; Hu, Y.; Meng, L.; Zheng, S.; Ren, F.; Bu, W.; Wang, H.; Li, D.; et al. Metformin Carbon Dots for Promoting Periodontal Bone Regeneration Via Activation of Erk/Ampk Pathway. Adv. Health Mater. 2021, 10, e2100196. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.H. Metformin and Risk of Gingival/Periodontal Diseases in Diabetes Patients: A Retrospective Cohort Study. Front. Endocrinol. 2022, 13, 1036885. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Kang, M.A.; Moon, Y.J.; Jang, K.Y.; Kim, J.R. Metformin Coordinates Osteoblast/Osteoclast Differentiation Associated with Ischemic Osteonecrosis. Aging 2020, 12, 4727–4741. [Google Scholar] [CrossRef]
- Nakagawa, T.; Tsuka, S.; Aonuma, F.; Nodai, T.; Munemasa, T.; Tamura, A.; Mukaibo, T.; Kondo, Y.; Masaki, C.; Hosokawa, R. Effects of Metformin on the Prevention of Bisphosphonate-Related Osteonecrosis of the Jaw-Like Lesions in Rats. J. Prosthodont. Res. 2021, 65, 219–224. [Google Scholar] [CrossRef]
- Zhuang, J.; Zu, J.; Zhou, C.; Sun, Y.; Kong, P.; Jing, Y. Bioinformatic Data Mining for Candidate Drugs Affecting Risk of Bisphosphonate-Related Osteonecrosis of the Jaw (Bronj) in Cancer Patients. Dis. Markers 2022, 2022, 3348480. [Google Scholar] [CrossRef]
- Drucker, D.J. Mechanisms of Action and Therapeutic Application of Glucagon-Like Peptide-1. Cell Metab. 2018, 27, 740–756. [Google Scholar] [CrossRef]
- Muller, T.D.; Finan, B.; Bloom, S.R.; D’Alessio, D.; Drucker, D.J.; Flatt, P.R.; Fritsche, A.; Gribble, F.; Grill, H.J.; Habener, J.F.; et al. Glucagon-Like Peptide 1 (Glp-1). Mol. Metab. 2019, 30, 72–130. [Google Scholar] [CrossRef] [PubMed]
- Joy, S.V.; Rodgers, P.T.; Scates, A.C. Incretin Mimetics as Emerging Treatments for Type 2 Diabetes. Ann. Pharmacother. 2005, 39, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A.; Meier, J.J. Glucagon-Like Peptide 1 and Its Derivatives in the Treatment of Diabetes. Regul. Pept. 2005, 128, 135–148. [Google Scholar] [CrossRef]
- Lu, W.; Zhou, Z.; Jiang, N.; Han, J. An Updated Patent Review of Glp-1 Receptor Agonists (2020-Present). Expert. Opin. Ther. Pat. 2023, 33, 597–612. [Google Scholar] [CrossRef]
- Birajdar, S.V.; Mazahir, F.; Alam, M.I.; Kumar, A.; Yadav, A.K. Repurposing and Clinical Attributes of Antidiabetic Drugs for the Treatment of Neurodegenerative Disorders. Eur. J. Pharmacol. 2023, 961, 176117. [Google Scholar] [CrossRef]
- Sawada, N.; Adachi, K.; Nakamura, N.; Miyabe, M.; Ito, M.; Kobayashi, S.; Miyajima, S.I.; Suzuki, Y.; Kikuchi, T.; Mizutani, M.; et al. Glucagon-Like Peptide-1 Receptor Agonist Liraglutide Ameliorates the Development of Periodontitis. J. Diabetes Res. 2020, 2020, 8843310. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, X.; Zhang, L.; Wang, B.; Xu, B.; Zhang, J. Glp-1 Inhibits Pkcbeta2 Phosphorylation to Improve the Osteogenic Differentiation Potential of Hpdlscs in the Age Microenvironment. J. Diabetes Complicat. 2020, 34, 107495. [Google Scholar] [CrossRef]
- Gheonea, T.C.; Surlin, P.; Nicolae, F.M.; Gheorghe, D.N.; Popescu, D.M.; Rogoveanu, I. Dipeptidyl-Peptidase-4 and Glucagon-Like-Peptide-1, a Link in the Connection between Periodontitis and Diabetes Mellitus-What Do We Know So Far?—A Scoping Review. J. Clin. Med. 2024, 13, 903. [Google Scholar] [CrossRef] [PubMed]
- Solini, A.; Suvan, J.; Santini, E.; Gennai, S.; Seghieri, M.; Masi, S.; Petrini, M.; D’Aiuto, F.; Graziani, F. Periodontitis Affects Glucoregulatory Hormones in Severely Obese Individuals. Int. J. Obes. 2019, 43, 1125–1129. [Google Scholar] [CrossRef]
- Mohamed, H.G.; Idris, S.B.; Mustafa, M.; Ahmed, M.F.; Astrom, A.N.; Mustafa, K.; Ibrahim, S.O. Impact of Chronic Periodontitis on Levels of Glucoregulatory Biomarkers in Gingival Crevicular Fluid of Adults with and without Type 2 Diabetes. PLoS ONE 2015, 10, e0127660. [Google Scholar] [CrossRef]
- Suvan, J.; Masi, S.; Harrington, Z.; Santini, E.; Raggi, F.; D’Aiuto, F.; Solini, A. Effect of Treatment of Periodontitis on Incretin Axis in Obese and Nonobese Individuals: A Cohort Study. J. Clin. Endocrinol. Metab. 2021, 106, e74–e82. [Google Scholar] [CrossRef] [PubMed]
- Ohara-Nemoto, Y.; Nakasato, M.; Shimoyama, Y.; Baba, T.T.; Kobayakawa, T.; Ono, T.; Yaegashi, T.; Kimura, S.; Nemoto, T.K. Degradation of Incretins and Modulation of Blood Glucose Levels by Periodontopathic Bacterial Dipeptidyl Peptidase 4. Infect. Immun. 2017, 85, e00277-17. [Google Scholar] [CrossRef]
- Ahmad, P.; Estrin, N.; Farshidfar, N.; Zhang, Y.; Miron, R.J. Glucagon-Like Peptide 1 Receptor Agonists (Glp-1ras) Improve Periodontal and Peri-Implant Health in Type 2 Diabetes Mellitus. J. Periodontal. Res. 2025; Online ahead of print. [Google Scholar]
- Hawley, S.A.; Fullerton, M.D.; Ross, F.A.; Schertzer, J.D.; Chevtzoff, C.; Walker, K.J.; Peggie, M.W.; Zibrova, D.; Green, K.A.; Mustard, K.J.; et al. The Ancient Drug Salicylate Directly Activates Amp-Activated Protein Kinase. Science 2012, 336, 918–922. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, R.; Da, N.; Wang, Y.; Yang, J.; Li, B.; He, X. Aspirin Loaded Extracellular Vesicles Inhibit Inflammation of Macrophages Via Switching Metabolic Phenotype in Periodontitis. Biochem. Biophys. Res. Commun. 2023, 667, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Luo, Z.; Liu, Y.; Jia, L.; Jiang, Y.; Du, J.; Guo, L.; Bai, Y.; Liu, Y. Aspirin Inhibits Rankl-Induced Osteoclast Differentiation in Dendritic Cells by Suppressing Nf-Kappab and Nfatc1 Activation. Stem Cell Res. Ther. 2019, 10, 375. [Google Scholar] [CrossRef]
- Hasan, F.; Ikram, R.; Simjee, S.U.; Iftakhar, K.; Asadullah, K.; Usman, M. The Effects of Aspirin Gel and Mouthwash on Levels of Salivary Biomarkers Pge2, Tnf-Alpha and Nitric Oxide in Patients with Periodontal Diseases. Pak. J. Pharm. Sci. 2019, 32, 2019–2023. [Google Scholar] [PubMed]
- Rogina, B.; Tissenbaum, H.A. Sirt1, Resveratrol and Aging. Front. Genet. 2024, 15, 1393181. [Google Scholar] [CrossRef]
- Zhou, D.D.; Cheng, J.; Li, J.; Wu, S.X.; Xiong, R.G.; Huang, S.Y.; Cheung, P.C.; Li, H.B. Resveratrol and Its Analogues: Anti-Ageing Effects and Underlying Mechanisms. Subcell Biochem. 2024, 107, 183–203. [Google Scholar]
- Zhu, L.; Yang, M.; Fan, L.; Yan, Q.; Zhang, L.; Mu, P.; Lu, F. Interaction between Resveratrol and Sirt1: Role in Neurodegenerative Diseases. Naunyn Schmiedebergs Arch. Pharmacol. 2025, 398, 89–101. [Google Scholar] [CrossRef]
- AlHayani, D.A.; Kubaev, A.; Uthirapathy, S.; Mandaliya, V.; Ballal, S.; Kalia, R.; Arya, R.; Gabble, B.C.; Alasheqi, M.Q.; Kadhim, A.J. Insights into the Therapeutic Potential of Sirt1-Modifying Compounds for Alzheimer’s Disease: A Focus on Molecular Mechanisms. J. Mol. Neurosci. 2025, 75, 29. [Google Scholar] [CrossRef]
- Nikniaz, S.; Vaziri, F.; Mansouri, R. Impact of Resveratrol Supplementation on Clinical Parameters and Inflammatory Markers in Patients with Chronic Periodontitis: A Randomized Clinical Trail. BMC Oral. Health 2023, 23, 177. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.N.; Han, L.; Iyer, S.; de Cabo, R.; Zhao, H.; O’Brien, C.A.; Manolagas, S.C.; Almeida, M. Sirtuin1 Suppresses Osteoclastogenesis by Deacetylating Foxos. Mol. Endocrinol. 2015, 29, 1498–1509. [Google Scholar] [CrossRef]
- Qu, Q.; Chen, Y.; Wang, Y.; Wang, W.; Long, S.; Yang, H.Y.; Wu, J.; Li, M.; Tian, X.; Wei, X.; et al. Lithocholic Acid Binds Tulp3 to Activate Sirtuins and Ampk to Slow down Ageing. Nature, 2024; Online ahead of print. [Google Scholar]
- Blair, H.C.; Teitelbaum, S.L.; Ghiselli, R.; Gluck, S. Osteoclastic Bone Resorption by a Polarized Vacuolar Proton Pump. Science 1989, 245, 855–857. [Google Scholar] [CrossRef]
- Huang, C.Y.; Chen, S.H.; Lin, T.; Liao, Y.W.; Chang, Y.C.; Chen, C.C.; Yu, C.C.; Chen, C.J. Resveratrol Attenuates Advanced Glycation End Product-Induced Senescence and Inflammation in Human Gingival Fibroblasts. J. Dent. Sci. 2024, 19, 580–586. [Google Scholar] [CrossRef]
- Shi, J.; Zhang, Y.; Zhang, X.; Chen, R.; Wei, J.; Hou, J.; Wang, B.; Lai, H.; Huang, Y. Remodeling Immune Microenvironment in Periodontitis Using Resveratrol Liposomes as an Antibiotic-Free Therapeutic Strategy. J. Nanobiotechnol. 2021, 19, 429. [Google Scholar] [CrossRef]
- Jiang, K.; Li, J.; Jiang, L.; Li, H.; Lei, L. Pink1-Mediated Mitophagy Reduced Inflammatory Responses to Porphyromonas Gingivalis in Macrophages. Oral Dis. 2023, 29, 3665–3676. [Google Scholar] [CrossRef] [PubMed]
- Hashim, N.T.; Babiker, R.; Chaitanya, N.; Mohammed, R.; Priya, S.P.; Padmanabhan, V.; Ahmed, A.; Dasnadi, S.P.; Islam, M.S.; Gismalla, B.G.; et al. New Insights in Natural Bioactive Compounds for Periodontal Disease: Advanced Molecular Mechanisms and Therapeutic Potential. Molecules 2025, 30, 807. [Google Scholar] [CrossRef]
- Yang, G.; Collins, J.M.; Rafiee, R.; Singh, S.; Langaee, T.; McDonough, C.W.; Holliday, L.S.; Wang, D.; Lamba, J.K.; Kim, Y.S.; et al. Sirt1 Gene Snp Rs932658 Is Associated with Medication-Related Osteonecrosis of the Jaw. J. Bone Miner. Res. 2021, 36, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Bojtor, B.; Vaszilko, M.; Armos, R.; Tobias, B.; Podani, J.; Szentpeteri, S.; Balla, B.; Lengyel, B.; Piko, H.; Illes, A.; et al. Analysis of Sirt1 Gene Snps and Clinical Characteristics in Medication-Related Osteonecrosis of the Jaw. Int. J. Mol. Sci. 2024, 25, 3646. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, W.; Yang, P.; Zhu, S.; Luo, S.; Li, M. Menaquinone-4 Prevents Medication-Related Osteonecrosis of the Jaw through the Sirt1 Signaling-Mediated Inhibition of Cellular Metabolic Stresses-Induced Osteoblast Apoptosis. Free Radic. Biol. Med. 2023, 206, 33–49. [Google Scholar] [CrossRef]
- Su, W.; Li, J.; Jiang, L.; Lei, L.; Li, H. Hexokinase 2-Mediated Glycolysis Supports Inflammatory Responses to Porphyromonas Gingivalis in Gingival Fibroblasts. BMC Oral. Health 2023, 23, 103. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, Y.; Jia, X.; Li, Y.; Yang, Y.; Pan, L.; Zhao, R.; Han, Y.; Wang, F.; Guan, X.; et al. Glycolytic Reprogramming Controls Periodontitis-Associated Macrophage Pyroptosis Via Ampk/Sirt1/Nf-Kappab Signaling Pathway. Int. Immunopharmacol. 2023, 119, 110192. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, F.; Zhu, C.; Zhang, C.; Le, Y.; Li, Z.; Wan, Q. The Glycolytic Enzyme Pkm2 Regulates Inflammatory Osteoclastogenesis by Modulating Stat3 Phosphorylation. J. Biol. Chem. 2025, 301, 108389. [Google Scholar] [CrossRef]
- Lee, Y.S.; Kim, Y.S.; Lee, S.Y.; Kim, G.H.; Kim, B.J.; Lee, S.H.; Lee, K.U.; Kim, G.S.; Kim, S.W.; Koh, J.M. Amp Kinase Acts as a Negative Regulator of Rankl in the Differentiation of Osteoclasts. Bone 2010, 47, 926–937. [Google Scholar] [CrossRef]
- Wang, H.; Rubinstein, J.L. Cryoem of V-Atpases: Assembly, Disassembly, and Inhibition. Curr. Opin. Struct. Biol. 2023, 80, 102592. [Google Scholar] [CrossRef]
- Wang, R.; Long, T.; Hassan, A.; Wang, J.; Sun, Y.; Xie, X.S.; Li, X. Cryo-Em Structures of Intact V-Atpase from Bovine Brain. Nat. Commun. 2020, 11, 3921. [Google Scholar] [CrossRef]
- Cotter, K.; Stransky, L.; McGuire, C.; Forgac, M. Recent Insights into the Structure, Regulation, and Function of the V-Atpases. Trends Biochem. Sci. 2015, 40, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Liu, F.; Li, R.; Zhou, Z.; Lin, Z.; Lin, S.; Deng, S.; Li, Y.; Nong, B.; Xia, Y.; et al. The Rapid Proximity Labeling System Phastid Identifies Atp6ap1 as an Unconventional Gef for Rheb. Cell Res. 2024, 34, 355–369. [Google Scholar] [CrossRef]
- Li, S.; Ouyang, X.; Su, B. Atp6ap1 Was Phast-Id’ed as a Long-Sought Gef for Rheb. Cell Res. 2024, 34, 397–398. [Google Scholar] [CrossRef]
- Chu, A.; Zirngibl, R.A.; Manolson, M.F. The V-Atpase A3 Subunit: Structure, Function and Therapeutic Potential of an Essential Biomolecule in Osteoclastic Bone Resorption. Int. J. Mol. Sci. 2021, 22, 6934. [Google Scholar] [CrossRef]
- Nakanishi-Matsui, M.; Matsumoto, N. V-Atpase A3 Subunit in Secretory Lysosome Trafficking in Osteoclasts. Biol. Pharm. Bull. 2022, 45, 1426–1431. [Google Scholar] [CrossRef] [PubMed]
- Susani, L.; Pangrazio, A.; Sobacchi, C.; Taranta, A.; Mortier, G.; Savarirayan, R.; Villa, A.; Orchard, P.; Vezzoni, P.; Albertini, A.; et al. Tcirg1-Dependent Recessive Osteopetrosis: Mutation Analysis, Functional Identification of the Splicing Defects, and in Vitro Rescue by U1 Snrna. Hum. Mutat. 2004, 24, 225–235. [Google Scholar] [CrossRef]
- Frattini, A.; Orchard, P.J.; Sobacchi, C.; Giliani, S.; Abinun, M.; Mattsson, J.P.; Keeling, D.J.; Andersson, A.K.; Wallbrandt, P.; Zecca, L.; et al. Defects in Tcirg1 Subunit of the Vacuolar Proton Pump Are Responsible for a Subset of Human Autosomal Recessive Osteopetrosis. Nat. Genet. 2000, 25, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Shadur, B.; Zaidman, I.; NaserEddin, A.; Lokshin, E.; Hussein, F.; Oron, H.C.; Avni, B.; Grisariu, S.; Stepensky, P. Successful Hematopoietic Stem Cell Transplantation for Osteopetrosis Using Reduced Intensity Conditioning. Pediatr. Blood Cancer 2018, 65, e27010. [Google Scholar] [CrossRef]
- Kantaputra, P.N.; Thawanaphong, S.; Issarangporn, W.; Klangsinsirikul, P.; Ohazama, A.; Sharpe, P.; Supanchart, C. Long-Term Survival in Infantile Malignant Autosomal Recessive Osteopetrosis Secondary to Homozygous P.Arg526gln Mutation in Clcn7. Am. J. Med. Genet. A 2012, 158A, 909–916. [Google Scholar] [CrossRef]
- Even-Or, E.; Stepensky, P. How We Approach Malignant Infantile Osteopetrosis. Pediatr. Blood Cancer 2021, 68, e28841. [Google Scholar] [CrossRef]
- Oot, R.A.; Wilkens, S. Human V-Atpase Function Is Positively and Negatively Regulated by Tldc Proteins. Structure 2024, 32, 989–1000.e6. [Google Scholar] [CrossRef] [PubMed]
- Eaton, A.F.; Danielson, E.C.; Tu, L.J.; Brown, D.; Merkulova, M. Knockout of the V-Atpase Interacting Protein Tldc2 in B-Type Kidney Intercalated Cells Impairs Urine Alkalinization. Am. J. Physiol. Ren. Physiol. 2025, 328, F890–F906. [Google Scholar] [CrossRef]
- Tan, Y.Z.; Abbas, Y.M.; Wu, J.Z.; Wu, D.; Keon, K.A.; Hesketh, G.G.; Bueler, S.A.; Gingras, A.C.; Robinson, C.V.; Grinstein, S.; et al. Cryoem of Endogenous Mammalian V-Atpase Interacting with the Tldc Protein Meak-7. Life Sci. Alliance 2022, 5, e202201527. [Google Scholar] [CrossRef]
- Heitman, J.; Movva, N.R.; Hall, M.N. Targets for Cell Cycle Arrest by the Immunosuppressant Rapamycin in Yeast. Science 1991, 253, 905–909. [Google Scholar] [CrossRef]
- Heitman, J.; Koller, A.; Kunz, J.; Henriquez, R.; Schmidt, A.; Movva, N.R.; Hall, M.N. The Immunosuppressant Fk506 Inhibits Amino Acid Import in Saccharomyces Cerevisiae. Mol. Cell Biol. 1993, 13, 5010–5019. [Google Scholar] [PubMed]
- Arriola Apelo, S.I.; Lamming, D.W. Rapamycin: An Inhibitor of Aging Emerges from the Soil of Easter Island. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Sabatini, D.M.; Erdjument-Bromage, H.; Lui, M.; Tempst, P.; Snyder, S.H. Raft1: A Mammalian Protein That Binds to Fkbp12 in a Rapamycin-Dependent Fashion and Is Homologous to Yeast Tors. Cell 1994, 78, 35–43. [Google Scholar] [CrossRef]
- Sirchia, F.; Taietti, I.; Donesana, M.; Bassanese, F.; Clemente, A.M.; Barbato, E.; Orsini, A.; Ferretti, A.; Marseglia, G.L.; Savasta, S.; et al. Expanding the Spectrum of Autosomal Dominant ATP6V1A-Related Disease: Case Report and Literature Review. Genes 2024, 15, 1219. [Google Scholar] [CrossRef]
- Li, B.; Song, L.; Liu, X.; Ji, J.-J.; He, Y.-Y.; Zhang, D.-M.; Xu, J.; Sun, H.; Shi, Z.; Wang, J.; et al. ATP6V1A variants are associated with childhood epilepsy with favorable outcome. Seizure Eur. J. Epilepsy 2024, 116, 81–86. [Google Scholar] [CrossRef]
- Stover, E.H.; Borthwick, K.J.; Bavalia, C.; Eady, N.; Fritz, D.M.; Rungroj, N.; Giersch, A.B.; Morton, C.C.; Axon, P.R.; Akil, I.; et al. Novel Atp6v1b1 and Atp6v0a4 Mutations in Autosomal Recessive Distal Renal Tubular Acidosis with New Evidence for Hearing Loss. J. Med. Genet. 2002, 39, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yang, Y.; Klionsky, D.J.; Malek, S.N. Mutations in V-Atpase in Follicular Lymphoma Activate Autophagic Flux Creating a Targetable Dependency. Autophagy 2023, 19, 716–719. [Google Scholar] [CrossRef]
- Carpentieri, G.; Cecchetti, S.; Bocchinfuso, G.; Radio, F.C.; Leoni, C.; Onesimo, R.; Calligari, P.; Pietrantoni, A.; Ciolfi, A.; Ferilli, M.; et al. Dominantly Acting Variants In atp6v1c1 and atp6v1b2 Cause a Multisystem Phenotypic Spectrum by Altering Lysosomal and/or Autophagosome Function. Hum. Genet. Genom. Adv. 2024, 5, 100349. [Google Scholar] [CrossRef]
- Van Damme, T.; Gardeitchik, T.; Mohamed, M.; Guerrero-Castillo, S.; Freisinger, P.; Guillemyn, B.; Kariminejad, A.; Dalloyaux, D.; van Kraaij, S.; Lefeber, D.J.; et al. Mutations in Atp6v1e1 or Atp6v1a Cause Autosomal-Recessive Cutis Laxa. Am. J. Hum. Genet. 2017, 100, 216–227. [Google Scholar] [CrossRef]
- Corniere, N.; Eladari, D. Identification of Atp6v1c2 as a Novel Candidate Gene for Distal Tubular Acidosis. Kidney Int. 2020, 97, 452–455. [Google Scholar] [CrossRef]
- Aoto, K.; Kato, M.; Akita, T.; Nakashima, M.; Mutoh, H.; Akasaka, N.; Tohyama, J.; Nomura, Y.; Hoshino, K.; Ago, Y.; et al. Atp6v0a1 Encoding the A1-Subunit of the V0 Domain of Vacuolar H(+)-Atpases Is Essential for Brain Development in Humans and Mice. Nat. Commun. 2021, 12, 2107. [Google Scholar] [CrossRef]
- Bott, L.C.; Forouhan, M.; Lieto, M.; Sala, A.J.; Ellerington, R.; Johnson, J.O.; Speciale, A.A.; Criscuolo, C.; Filla, A.; Chitayat, D.; et al. Variants in Atp6v0a1 Cause Progressive Myoclonus Epilepsy and Developmental and Epileptic Encephalopathy. Brain Commun. 2021, 3, fcab245. [Google Scholar] [CrossRef] [PubMed]
- Hucthagowder, V.; Morava, E.; Kornak, U.; Lefeber, D.J.; Fischer, B.; Dimopoulou, A.; Aldinger, A.; Choi, J.; Davis, E.C.; Abuelo, D.N.; et al. Loss-of-Function Mutations in Atp6v0a2 Impair Vesicular Trafficking, Tropoelastin Secretion and Cell Survival. Hum. Mol. Genet. 2009, 18, 2149–2165. [Google Scholar] [CrossRef] [PubMed]
- Scimeca, J.C.; Quincey, D.; Parrinello, H.; Romatet, D.; Grosgeorge, J.; Gaudray, P.; Philip, N.; Fischer, A.; Carle, G.F. Novel Mutations in the Tcirg1 Gene Encoding the A3 Subunit of the Vacuolar Proton Pump in Patients Affected by Infantile Malignant Osteopetrosis. Hum. Mutat. 2003, 21, 151–157. [Google Scholar] [CrossRef]
- Smith, A.N.; Skaug, J.; Choate, K.A.; Nayir, A.; Bakkaloglu, A.; Ozen, S.; Hulton, S.A.; Sanjad, S.A.; Al-Sabban, E.A.; Lifton, R.P.; et al. Mutations in Atp6n1b, Encoding a New Kidney Vacuolar Proton Pump 116-Kd Subunit, Cause Recessive Distal Renal Tubular Acidosis with Preserved Hearing. Nat. Genet. 2000, 26, 71–75. [Google Scholar] [CrossRef]
- Morales-Romero, B.; Munoz-Pujol, G.; Artuch, R.; Garcia-Cazorla, A.; O’Callaghan, M.; Sykut-Cegielska, J.; Campistol, J.; Moreno-Lozano, P.J.; Oud, M.M.; Wevers, R.A.; et al. Genome and Rna Sequencing Were Essential to Reveal Cryptic Intronic Variants Associated to Defective Atp6ap1 Mrna Processing. Mol. Genet. Metab. 2024, 142, 108511. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, B.; Himmelreich, N.; Ederveen, A.L.H.; Luchtenborg, C.; Okun, J.G.; Breuer, M.; Hutter, A.M.; Carl, M.; Guglielmi, L.; Hellwig, A.; et al. Cutis Laxa, Exocrine Pancreatic Insufficiency and Altered Cellular Metabolomics as Additional Symptoms in a New Patient with Atp6ap1-Cdg. Mol. Genet. Metab. 2018, 123, 364–374. [Google Scholar] [CrossRef]
- Jansen, E.J.; Timal, S.; Ryan, M.; Ashikov, A.; van Scherpenzeel, M.; Graham, L.A.; Mandel, H.; Hoischen, A.; Iancu, T.C.; Raymond, K.; et al. Atp6ap1 Deficiency Causes an Immunodeficiency with Hepatopathy, Cognitive Impairment and Abnormal Protein Glycosylation. Nat. Commun. 2016, 7, 11600. [Google Scholar] [CrossRef]
- Hirose, T.; Cabrera-Socorro, A.; Chitayat, D.; Lemonnier, T.; Feraud, O.; Cifuentes-Diaz, C.; Gervasi, N.; Mombereau, C.; Ghosh, T.; Stoica, L.; et al. Atp6ap2 Variant Impairs Cns Development and Neuronal Survival to Cause Fulminant Neurodegeneration. J. Clin. Investig. 2019, 129, 2145–2162. [Google Scholar] [CrossRef]
- Cannata Serio, M.; Rujano, M.A.; Simons, M. Mutations in Atp6ap2 Cause Autophagic Liver Disease in Humans. Autophagy 2018, 14, 1088–1089. [Google Scholar] [CrossRef]
- Lorenz, M.C.; Heitman, J. Tor Mutations Confer Rapamycin Resistance by Preventing Interaction with Fkbp12-Rapamycin. J. Biol. Chem. 1995, 270, 27531–27537. [Google Scholar] [CrossRef]
- Alarcon, C.M.; Cardenas, M.E.; Heitman, J. Mammalian Raft1 Kinase Domain Provides Rapamycin-Sensitive Tor Function in Yeast. Genes. Dev. 1996, 10, 279–288. [Google Scholar] [CrossRef]
- Laplante, M.; Sabatini, D.M. Regulation of Mtorc1 and Its Impact on Gene Expression at a Glance. J. Cell Sci. 2013, 126, 1713–1719. [Google Scholar] [CrossRef] [PubMed]
- Sabatini, D.M. Mtor and Cancer: Insights into a Complex Relationship. Nat. Rev. Cancer 2006, 6, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Almeida, L.O.; Silva, L.C.; Emerick, C.; Santos, J.A.D.; Castilho, R.M.; Squarize, C.H. Head and Neck Cancer Stem Cell Maintenance Relies on Mtor Signaling, Specifically Involving the Mechanistic Target of Rapamycin Complexes 1 and 2 (Mtorc1 and Mtorc2). Arch. Oral. Biol. 2024, 157, 105840. [Google Scholar] [CrossRef]
- Efeyan, A.; Zoncu, R.; Sabatini, D.M. Amino Acids and Mtorc1: From Lysosomes to Disease. Trends Mol. Med. 2012, 18, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Chen, H.; Xiao, H. Mtorc2: A Neglected Player in Aging Regulation. J. Cell Physiol. 2024, 239, e31363. [Google Scholar] [CrossRef]
- Ragupathi, A.; Kim, C.; Jacinto, E. The Mtorc2 Signaling Network: Targets and Cross-Talks. Biochem. J. 2024, 481, 45–91. [Google Scholar] [CrossRef]
- Sarbassov, D.D.; Ali, S.M.; Kim, D.H.; Guertin, D.A.; Latek, R.R.; Erdjument-Bromage, H.; Tempst, P.; Sabatini, D.M. Rictor, a Novel Binding Partner of Mtor, Defines a Rapamycin-Insensitive and Raptor-Independent Pathway That Regulates the Cytoskeleton. Curr. Biol. 2004, 14, 1296–1302. [Google Scholar] [CrossRef]
- Zong, Y.; Zhang, C.S.; Li, M.; Wang, W.; Wang, Z.; Hawley, S.A.; Ma, T.; Feng, J.W.; Tian, X.; Qi, Q.; et al. Hierarchical Activation of Compartmentalized Pools of Ampk Depends on Severity of Nutrient or Energy Stress. Cell Res. 2019, 29, 460–473. [Google Scholar] [CrossRef]
- Lin, S.C.; Hardie, D.G. Ampk: Sensing Glucose as Well as Cellular Energy Status. Cell Metab. 2018, 27, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.S.; Hawley, S.A.; Zong, Y.; Li, M.; Wang, Z.; Gray, A.; Ma, T.; Cui, J.; Feng, J.W.; Zhu, M.; et al. Fructose-1,6-Bisphosphate and Aldolase Mediate Glucose Sensing by Ampk. Nature 2017, 548, 112–116. [Google Scholar] [CrossRef]
- Lu, M.; Holliday, L.S.; Zhang, L.; Dunn, W.A., Jr.; Gluck, S.L. Interaction between Aldolase and Vacuolar H+-Atpase: Evidence for Direct Coupling of Glycolysis to the Atp-Hydrolyzing Proton Pump. J. Biol. Chem. 2001, 276, 30407–30413. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.M.; O’Hara, P.V.; Kelleher, E.M.; Casserly, L.F. Osteopetrosis with Renal Tubular Acidosis and Cerebral Calcification. Kidney Int. 2018, 93, 1020. [Google Scholar] [CrossRef] [PubMed]
- Whyte, M.P. Carbonic Anhydrase Ii Deficiency. Bone 2023, 169, 116684. [Google Scholar] [CrossRef]
- Lu, M.; Ammar, D.; Ives, H.; Albrecht, F.; Gluck, S.L. Physical Interaction between Aldolase and Vacuolar H+-Atpase Is Essential for the Assembly and Activity of the Proton Pump. J. Biol. Chem. 2007, 282, 24495–24503. [Google Scholar] [CrossRef]
- Lu, M.; Sautin, Y.Y.; Holliday, L.S.; Gluck, S.L. The Glycolytic Enzyme Aldolase Mediates Assembly, Expression, and Activity of Vacuolar H+-Atpase. J. Biol. Chem. 2004, 279, 8732–8739. [Google Scholar] [CrossRef]
- Van Nostrand, J.L.; Hellberg, K.; Luo, E.C.; Van Nostrand, E.L.; Dayn, A.; Yu, J.; Shokhirev, M.N.; Dayn, Y.; Yeo, G.W.; Shaw, R.J. Ampk Regulation of Raptor and Tsc2 Mediate Metformin Effects on Transcriptional Control of Anabolism and Inflammation. Genes. Dev. 2020, 34, 1330–1344. [Google Scholar] [CrossRef]
- Wang, S.; Li, H.; Yuan, M.; Fan, H.; Cai, Z. Role of Ampk in Autophagy. Front. Physiol. 2022, 13, 1015500. [Google Scholar] [CrossRef]
- Herzig, S.; Shaw, R.J. Ampk: Guardian of Metabolism and Mitochondrial Homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef]
- Valenstein, M.L.; Lalgudi, P.V.; Gu, X.; Kedir, J.F.; Taylor, M.S.; Chivukula, R.R.; Sabatini, D.M. Rag-Ragulator Is the Central Organizer of the Physical Architecture of the Mtorc1 Nutrient-Sensing Pathway. Proc. Natl. Acad. Sci. USA 2024, 121, e2322755121. [Google Scholar] [CrossRef] [PubMed]
- Mu, Z.; Wang, L.; Deng, W.; Wang, J.; Wu, G. Structural Insight into the Ragulator Complex Which Anchors Mtorc1 to the Lysosomal Membrane. Cell Discov. 2017, 3, 17049. [Google Scholar] [CrossRef]
- Shen, K.; Sabatini, D.M. Ragulator and Slc38a9 Activate the Rag Gtpases through Noncanonical Gef Mechanisms. Proc. Natl. Acad. Sci. USA 2018, 115, 9545–9550. [Google Scholar] [CrossRef] [PubMed]
- Rogala, K.B.; Gu, X.; Kedir, J.F.; Abu-Remaileh, M.; Bianchi, L.F.; Bottino, A.M.S.; Dueholm, R.; Niehaus, A.; Overwijn, D.; Fils, A.P.; et al. Structural Basis for the Docking of Mtorc1 on the Lysosomal Surface. Science 2019, 366, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Napolitano, G.; de Araujo, M.E.G.; Esposito, A.; Monfregola, J.; Huber, L.A.; Ballabio, A.; Hurley, J.H. Structure of the Lysosomal Mtorc1-Tfeb-Rag-Ragulator Megacomplex. Nature 2023, 614, 572–579. [Google Scholar] [CrossRef]
- Zoncu, R.; Efeyan, A.; Sabatini, D.M. Mtor: From Growth Signal Integration to Cancer, Diabetes and Ageing. Nat. Rev. Mol. Cell Biol. 2011, 12, 21–35. [Google Scholar] [CrossRef]
- Hees, J.T.; Harbauer, A.B. Metabolic Regulation of Mitochondrial Protein Biogenesis from a Neuronal Perspective. Biomolecules 2022, 12, 1595. [Google Scholar] [CrossRef]
- Yang, H.; Yu, Z.; Chen, X.; Li, J.; Li, N.; Cheng, J.; Gao, N.; Yuan, H.X.; Ye, D.; Guan, K.L.; et al. Structural Insights into Tsc Complex Assembly and Gap Activity on Rheb. Nat. Commun. 2021, 12, 339. [Google Scholar] [CrossRef]
- Sutera, P.; Kim, J.; Kumar, R.; Deek, R.A.; Stephenson, R.; Mayer, T.; Saraiya, B.; Ghodoussipour, S.; Jang, T.; Golombos, D.; et al. Pik3/Akt/Mtor Pathway Alterations in Metastatic Castration-Sensitive Prostate Cancer. Prostate 2024, 84, 1301–1308. [Google Scholar] [CrossRef]
- Coupland, C.E.; Karimi, R.; Bueler, S.A.; Liang, Y.; Courbon, G.M.; Di Trani, J.M.; Wong, C.J.; Saghian, R.; Youn, J.Y.; Wang, L.Y.; et al. High-Resolution Electron Cryomicroscopy of V-Atpase in Native Synaptic Vesicles. Science 2024, 385, 168–174. [Google Scholar] [CrossRef]
- Zhang, C.S.; Jiang, B.; Li, M.; Zhu, M.; Peng, Y.; Zhang, Y.L.; Wu, Y.Q.; Li, T.Y.; Liang, Y.; Lu, Z.; et al. The Lysosomal V-Atpase-Ragulator Complex Is a Common Activator for Ampk and Mtorc1, Acting as a Switch between Catabolism and Anabolism. Cell Metab. 2014, 20, 526–540. [Google Scholar] [CrossRef] [PubMed]
- Jansen, E.J.; van Bakel, N.H.; Loohuis, N.F.O.; Hafmans, T.G.; Arentsen, T.; Coenen, A.J.; Scheenen, W.J.; Martens, G.J. Identification of Domains within the V-Atpase Accessory Subunit Ac45 Involved in V-Atpase Transport and Ca2+-Dependent Exocytosis. J. Biol. Chem. 2012, 287, 27537–27546. [Google Scholar] [CrossRef] [PubMed]
- Ratto, E.; Chowdhury, S.R.; Siefert, N.S.; Schneider, M.; Wittmann, M.; Helm, D.; Palm, W. Direct Control of Lysosomal Catabolic Activity by Mtorc1 through Regulation of V-Atpase Assembly. Nat. Commun. 2022, 13, 4848. [Google Scholar] [CrossRef]
- Wolfe, M.S. Presenilin, Gamma-Secretase, and the Search for Pathogenic Triggers of Alzheimer’s Disease. Biochemistry 2025, 64, 1662–1672. [Google Scholar] [CrossRef] [PubMed]
- Son, S.M.; Shin, H.J.; Byun, J.; Kook, S.Y.; Moon, M.; Chang, Y.J.; Mook-Jung, I. Metformin Facilitates Amyloid-β Generation by β- and Gamma-Secretases via Autophagy Activation. J. Alzheimer’s Dis. 2016, 51, 1197–1208. [Google Scholar] [CrossRef]
- Alzamora, R.; Al-Bataineh, M.M.; Liu, W.; Gong, F.; Li, H.; Thali, R.F.; Joho-Auchli, Y.; Brunisholz, R.A.; Satlin, L.M.; Neumann, D.; et al. Amp-Activated Protein Kinase Regulates the Vacuolar H+-Atpase Via Direct Phosphorylation of the a Subunit (Atp6v1a) in the Kidney. Am. J. Physiol. Ren. Physiol. 2013, 305, F943–F956. [Google Scholar] [CrossRef]
- Li, J.; Knudsen, J.R.; Henriquez-Olguin, C.; Li, Z.; Birk, J.B.; Persson, K.W.; Hellsten, Y.; Offergeld, A.; Jarassier, W.; Le Grand, F.; et al. Axin1 Knockout Does Not Alter Ampk/Mtorc1 Regulation and Glucose Metabolism in Mouse Skeletal Muscle. J. Physiol. 2021, 599, 3081–3100. [Google Scholar] [CrossRef]
- Chen, S.H.; Bubb, M.R.; Yarmola, E.G.; Zuo, J.; Jiang, J.; Lee, B.S.; Lu, M.; Gluck, S.L.; Hurst, I.R.; Holliday, L.S. Vacuolar H+-Atpase Binding to Microfilaments: Regulation in Response to Phosphatidylinositol 3-Kinase Activity and Detailed Characterization of the Actin-Binding Site in Subunit B. J. Biol. Chem. 2004, 279, 7988–7998. [Google Scholar] [CrossRef]
- Lee, B.S.; Gluck, S.L.; Holliday, L.S. Interaction between Vacuolar H(+)-Atpase and Microfilaments during Osteoclast Activation. J. Biol. Chem. 1999, 274, 29164–29171. [Google Scholar] [CrossRef]
- Holliday, L.S.; Lu, M.; Lee, B.S.; Nelson, R.D.; Solivan, S.; Zhang, L.; Gluck, S.L. The Amino-Terminal Domain of the B Subunit of Vacuolar H+-Atpase Contains a Filamentous Actin Binding Site. J. Biol. Chem. 2000, 275, 32331–32337. [Google Scholar] [CrossRef]
- Zuo, J.; Jiang, J.; Chen, S.H.; Vergara, S.; Gong, Y.; Xue, J.; Huang, H.; Kaku, M.; Holliday, L.S. Actin Binding Activity of Subunit B of Vacuolar H+-Atpase Is Involved in Its Targeting to Ruffled Membranes of Osteoclasts. J. Bone Min. Res. 2006, 21, 714–721. [Google Scholar] [CrossRef] [PubMed]
- Vitavska, O.; Merzendorfer, H.; Wieczorek, H. The V-Atpase Subunit C Binds to Polymeric F-Actin as Well as to Monomeric G-Actin and Induces Cross-Linking of Actin Filaments. J. Biol. Chem. 2005, 280, 1070–1076. [Google Scholar] [CrossRef]
- Vitavska, O.; Wieczorek, H.; Merzendorfer, H. A Novel Role for Subunit C in Mediating Binding of the H+-V-Atpase to the Actin Cytoskeleton. J. Biol. Chem. 2003, 278, 18499–18505. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, C.S.; Feng, J.W.; Wei, X.; Zhang, C.; Xie, C.; Wu, Y.; Hawley, S.A.; Atrih, A.; Lamont, D.J.; et al. Aldolase Is a Sensor for Both Low and High Glucose, Linking to Ampk and Mtorc1. Cell Res. 2021, 31, 478–481. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, C.S.; Zong, Y.; Feng, J.W.; Ma, T.; Hu, M.; Lin, Z.; Li, X.; Xie, C.; Wu, Y.; et al. Transient Receptor Potential V Channels Are Essential for Glucose Sensing by Aldolase and Ampk. Cell Metab. 2019, 30, 508–524.e12. [Google Scholar] [CrossRef]
- Kane, P.M. Targeting Reversible Disassembly as a Mechanism of Controlling V-Atpase Activity. Curr. Protein Pept. Sci. 2012, 13, 117–123. [Google Scholar] [CrossRef]
- Parra, K.J.; Kane, P.M. Reversible Association between the V1 and V0 Domains of Yeast Vacuolar H+-Atpase Is an Unconventional Glucose-Induced Effect. Mol. Cell Biol. 1998, 18, 7064–7074. [Google Scholar] [CrossRef]
- Kane, P.M.; Smardon, A.M. Assembly and Regulation of the Yeast Vacuolar H+-Atpase. J. Bioenerg. Biomembr. 2003, 35, 313–321. [Google Scholar] [CrossRef]
- Jaskolka, M.C.; Kane, P.M. Interaction between the Yeast Rave Complex and Vph1-Containing V(O) Sectors Is a Central Glucose-Sensitive Interaction Required for V-Atpase Reassembly. J. Biol. Chem. 2020, 295, 2259–2269. [Google Scholar] [CrossRef]
- Oot, R.A.; Kane, P.M.; Berry, E.A.; Wilkens, S. Crystal Structure of Yeast V1-Atpase in the Autoinhibited State. EMBO J. 2016, 35, 1694–1706. [Google Scholar] [CrossRef]
- Kishikawa, J.I.; Nakanishi, A.; Furuta, A.; Kato, T.; Namba, K.; Tamakoshi, M.; Mitsuoka, K.; Yokoyama, K. Mechanical Inhibition of Isolated V(O) from V/a-Atpase for Proton Conductance. Elife 2020, 9, e56862. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Tarsio, M.; Kane, P.M.; Rubinstein, J.L. Structure of Yeast Rave Bound to a Partial V(1) Complex. Proc. Natl. Acad. Sci. USA 2024, 121, e2414511121. [Google Scholar] [CrossRef]
- Jaskolka, M.C.; Winkley, S.R.; Kane, P.M. Rave and Rabconnectin-3 Complexes as Signal Dependent Regulators of Organelle Acidification. Front. Cell Dev. Biol. 2021, 9, 698190. [Google Scholar] [CrossRef] [PubMed]
- Kane, P.M.; Tarsio, M.; Liu, J. Early Steps in Assembly of the Yeast Vacuolar H+-Atpase. J. Biol. Chem. 1999, 274, 17275–17283. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, P.; Nishikawa, J.L.; Breitkreutz, B.J.; Tyers, M. Systematic Identification of Pathways That Couple Cell Growth and Division in Yeast. Science 2002, 297, 395–400. [Google Scholar] [CrossRef]
- Luby-Phelps, K. Cytoarchitecture and Physical Properties of Cytoplasm: Volume, Viscosity, Diffusion, Intracellular Surface Area. Int. Rev. Cytol. 2000, 192, 189–221. [Google Scholar]
- Esser, N.; Legrand-Poels, S.; Piette, J.; Scheen, A.J.; Paquot, N. Inflammation as a Link between Obesity, Metabolic Syndrome and Type 2 Diabetes. Diabetes Res. Clin. Pract. 2014, 105, 141–150. [Google Scholar] [CrossRef]
- Deng, T.; Lyon, C.J.; Bergin, S.; Caligiuri, M.A.; Hsueh, W.A. Obesity, Inflammation, and Cancer. Annu. Rev. Pathol. 2016, 11, 421–449. [Google Scholar] [CrossRef]
- Khalid, M.; Petroianu, G.; Adem, A. Advanced Glycation End Products and Diabetes Mellitus: Mechanisms and Perspectives. Biomolecules 2022, 12, 542. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Han, Y.; Chen, Y.; Liu, L.; Lang, J.; Yang, C.; Luo, H.; Ning, J. Advanced Glycation End-Products Suppress Autophagy by Ampk/Mtor Signaling Pathway to Promote Vascular Calcification. Mol. Cell Biochem. 2020, 471, 91–100. [Google Scholar] [CrossRef]
- Baek, C.H.; Kim, H.; Moon, S.Y.; Yang, W.S. Ampk Boosts Adam10 Shedding Activity in Human Aortic Endothelial Cells by Promoting Rab14-Dependent Adam10 Cell Surface Translocation. Biochem. Biophys. Res. Commun. 2023, 675, 54–60. [Google Scholar] [CrossRef]
- Beisswenger, P.J.; Howell, S.K.; Touchette, A.D.; Lal, S.; Szwergold, B.S. Metformin Reduces Systemic Methylglyoxal Levels in Type 2 Diabetes. Diabetes 1999, 48, 198–202. [Google Scholar] [CrossRef]
- Araujo, A.A.; Pereira, A.; Medeiros, C.; Brito, G.A.C.; Leitao, R.F.C.; Araujo, L.S.; Guedes, P.M.M.; Hiyari, S.; Pirih, F.Q.; Junior, R.F.A. Effects of Metformin on Inflammation, Oxidative Stress, and Bone Loss in a Rat Model of Periodontitis. PLoS ONE 2017, 12, e0183506. [Google Scholar] [CrossRef]
- Song, B.; Zhang, Y.L.; Chen, L.J.; Zhou, T.; Huang, W.K.; Zhou, X.; Shao, L.Q. The Role of Toll-Like Receptors in Periodontitis. Oral. Dis. 2017, 23, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Kittaka, M.; Yoshimoto, T.; Levitan, M.E.; Urata, R.; Choi, R.B.; Teno, Y.; Xie, Y.; Kitase, Y.; Prideaux, M.; Dallas, S.L.; et al. Osteocyte Rankl Drives Bone Resorption in Mouse Ligature-Induced Periodontitis. J. Bone Min. Res. 2023, 38, 1521–1540. [Google Scholar] [CrossRef] [PubMed]
- Portes, J.; Bullon, B.; Quiles, J.L.; Battino, M.; Bullon, P. Diabetes Mellitus and Periodontitis Share Intracellular Disorders as the Main Meeting Point. Cells 2021, 10, 2411. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Garrido, J.; Shenoy, A.R. Regulation and Repurposing of Nutrient Sensing and Autophagy in Innate Immunity. Autophagy 2021, 17, 1571–1591. [Google Scholar] [CrossRef]
- Trindade, B.C.; Chen, G.Y. Nod1 and Nod2 in Inflammatory and Infectious Diseases. Immunol. Rev. 2020, 297, 139–161. [Google Scholar] [CrossRef]
- Singhrao, S.K.; Harding, A.; Poole, S.; Kesavalu, L.; Crean, S. Porphyromonas Gingivalis Periodontal Infection and Its Putative Links with Alzheimer’s Disease. Mediators Inflamm 2015, 2015, 137357. [Google Scholar] [CrossRef]
- Kim, Y.; Li, C.; Gu, C.; Fang, Y.; Tycksen, E.; Puri, A.; Pietka, T.A.; Sivapackiam, J.; Kidd, K.; Park, S.J.; et al. Manf Stimulates Autophagy and Restores Mitochondrial Homeostasis to Treat Autosomal Dominant Tubulointerstitial Kidney Disease in Mice. Nat. Commun. 2023, 14, 6493. [Google Scholar] [CrossRef]
- Bi, R.; Yang, Y.; Liao, H.; Ji, G.; Ma, Y.; Cai, L.; Li, J.; Yang, J.; Sun, M.; Liang, J.; et al. Porphyromonas Gingivalis Induces an Inflammatory Response Via the Cgas-Sting Signaling Pathway in a Periodontitis Mouse Model. Front. Microbiol. 2023, 14, 1183415. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, S.L.; Mehrotra, B.; Rosenberg, T.J.; Engroff, S.L. Osteonecrosis of the Jaws Associated with the Use of Bisphosphonates: A Review of 63 Cases. J. Oral Maxillofac. Surg. 2004, 62, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Nagy, V.; Penninger, J.M. The Rankl-Rank Story. Gerontology 2015, 61, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Gogakos, A.I.; Anastasilakis, A.D. Current and Emerging Bone Resorption Inhibitors for the Treatment of Osteoporosis. Expert. Opin. Pharmacother. 2025, 26, 265–278. [Google Scholar] [CrossRef]
- Drake, M.T.; Clarke, B.L.; Khosla, S. Bisphosphonates: Mechanism of Action and Role in Clinical Practice. Mayo Clin. Proc. 2008, 83, 1032–1045. [Google Scholar] [CrossRef]
- Coxon, F.P.; Thompson, K.; Roelofs, A.J.; Ebetino, F.H.; Rogers, M.J. Visualizing Mineral Binding and Uptake of Bisphosphonate by Osteoclasts and Non-Resorbing Cells. Bone 2008, 42, 848–860. [Google Scholar] [CrossRef]
- Dong, L.; Jiang, L.; Xu, Z.; Zhang, X. Denosumab, Teriparatide and Bisphosphonates for Glucocorticoid-Induced Osteoporosis: A Bayesian Network Meta-Analysis. Front. Pharmacol. 2024, 15, 1336075. [Google Scholar] [CrossRef]
- Son, S.; Oh, M.Y.; Yoo, B.R.; Park, H.B. Comparison of the Efficacy of Zoledronate and Denosumab in Patients with Acute Osteoporotic Vertebral Compression Fractures: A Randomized Controlled Trial. J. Clin. Med. 2024, 13, 2040. [Google Scholar] [CrossRef]
- Tang, Y.; Jin, Z.; Lu, Y.; Chen, L.; Lv, S.; Xu, T.; Tong, P.; Chen, G. Comparing the Efficacy of Antiosteoporotic Drugs in Preventing Periprosthetic Bone Loss Following Total Hip Arthroplasty: A Systematic Review and Bayesian Network Meta-Analysis. Orthop. Surg. 2024, 16, 2344–2354. [Google Scholar] [CrossRef]
- Yang, G.; Williams, R.; Wang, L.; Farhadfar, N.; Chen, Y.; Loiacono, A.T.; Bian, J.; Holliday, L.S.; Katz, J.; Gong, Y. Medication-Related Osteonecrosis of the Jaw in Cancer Patients: Result from the Oneflorida Clinical Research Consortium. J. Bone Min. Res. 2022, 37, 2466–2471. [Google Scholar] [CrossRef]
- Yang, G.; Singh, S.; McDonough, C.W.; Lamba, J.K.; Hamadeh, I.; Holliday, L.S.; Wang, D.; Katz, J.; Lakatos, P.A.; Balla, B.; et al. Genome-Wide Association Study Identified Chromosome 8 Locus Associated with Medication-Related Osteonecrosis of the Jaw. Clin. Pharmacol. Ther. 2021, 110, 1558–1569. [Google Scholar] [CrossRef] [PubMed]
- Otsuru, M.; Fujiki, Y.; Soutome, S.; Nakamura, N.; Miyoshi, T.; Naruse, T.; Ohnuma, M.; Hotokezaka, Y.; Rokutanda, S.; Umeda, M. Risk Factors for Dental Findings of the Development of Medication-Related Osteonecrosis of the Jaw: Investigation of 3734 Teeth in Cancer Patients Receiving High Dose Antiresorptive Agents. J. Dent. Sci. 2024, 19, 203–210. [Google Scholar] [CrossRef]
- Castillo, E.J.; Messer, J.G.; Abraham, A.M.; Jiron, J.M.; Alekseyenko, A.V.; Israel, R.; Thomas, S.; Gonzalez-Perez, G.M.; Croft, S.; Gohel, A.; et al. Preventing or Controlling Periodontitis Reduces the Occurrence of Osteonecrosis of the Jaw (Onj) in Rice Rats (Oryzomys Palustris). Bone 2021, 145, 115866. [Google Scholar] [CrossRef] [PubMed]
- Bone, H.G.; Wagman, R.B.; Brandi, M.L.; Brown, J.P.; Chapurlat, R.; Cummings, S.R.; Czerwinski, E.; Fahrleitner-Pammer, A.; Kendler, D.L.; Lippuner, K.; et al. 10 Years of Denosumab Treatment in Postmenopausal Women with Osteoporosis: Results from the Phase 3 Randomised Freedom Trial and Open-Label Extension. Lancet Diabetes Endocrinol. 2017, 5, 513–523. [Google Scholar] [CrossRef]
- Narayan, N.; Vadde, T.; Sandesara, M.; Divity, S.; Mamytova, A.; Tagaev, T. Efficacy and Safety of Efpeglenatide in Patients with Type 2 Diabetes and Obesity: A Systematic Review. Cureus 2025, 17, e77089. [Google Scholar] [CrossRef] [PubMed]
- Bushi, G.; Khatib, M.N.; Rohilla, S.; Singh, M.P.; Uniyal, N.; Ballal, S.; Bansal, P.; Bhopte, K.; Gupta, M.; Gaidhane, A.M.; et al. Association of Glp-1 Receptor Agonists with Risk of Suicidal Ideation and Behaviour: A Systematic Review and Meta-Analysis. Diabetes Metab. Res. Rev. 2025, 41, e70037. [Google Scholar] [CrossRef]
- Bednarczyk, M.; Dabrowska-Szeja, N.; Letowski, D.; Dziegielewska-Gesiak, S.; Waniczek, D.; Muc-Wierzgon, M. Relationship between Dietary Nutrient Intake and Autophagy-Related Genes in Obese Humans: A Narrative Review. Nutrients 2024, 16, 4003. [Google Scholar] [CrossRef]
- Wan, K.; Jin, Y.; Fan, R.; Xu, Q.; Li, X.; Yan, H.; Wang, R. Exploring Molecular Mechanisms of Exercise on Metabolic Syndrome: A Bibliometric and Visualization Study Using Citespace. Front. Endocrinol. 2024, 15, 1408466. [Google Scholar] [CrossRef]
- Lin, J.; Zhang, X.; Sun, Y.; Xu, H.; Li, N.; Wang, Y.; Tian, X.; Zhao, C.; Wang, B.; Zhu, B.; et al. Exercise Ameliorates Muscular Excessive Mitochondrial Fission, Insulin Resistance and Inflammation in Diabetic Rats Via Irisin/Ampk Activation. Sci. Rep. 2024, 14, 10658. [Google Scholar] [CrossRef]
- Kolnes, K.J.; Nilsen, E.T.F.; Brufladt, S.; Meadows, A.M.; Jeppesen, P.B.; Skattebo, O.; Johansen, E.I.; Birk, J.B.; Hojlund, K.; Hingst, J.; et al. Effects of Seven Days’ Fasting on Physical Performance and Metabolic Adaptation during Exercise in Humans. Nat. Commun. 2025, 16, 122. [Google Scholar] [CrossRef]
- Qu, Q.; Chen, Y.; Wang, Y.; Long, S.; Wang, W.; Yang, H.Y.; Li, M.; Tian, X.; Wei, X.; Liu, Y.H.; et al. Lithocholic Acid Phenocopies Anti-Ageing Effects of Calorie Restriction. Nature, 2024; Online ahead of print. [Google Scholar]
- Yuliyanasari, N.; Rejeki, P.S.; Hidayati, H.B.; Subsomwong, P.; Miftahussurur, M. The Effect of Intermittent Fasting on Preventing Obesity-Related Early Aging from a Molecular and Cellular Perspective. J. Med. Life 2024, 17, 261–272. [Google Scholar] [PubMed]
- Paoli, A.; Tinsley, G.M.; Mattson, M.P.; De Vivo, I.; Dhawan, R.; Moro, T. Common and Divergent Molecular Mechanisms of Fasting and Ketogenic Diets. Trends Endocrinol. Metab. 2024, 35, 125–141. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zhang, H.; Lv, Y.; Gao, M.; Gai, Z.; Liu, Y. Clinical and Genetic Characteristics of Two Cases with Developmental and Epileptic Encephalopathy 93 Caused by Novel ATP6V1A Mutations and Literature Review. Hum. Mutat. 2024, 2024, 4678670. [Google Scholar] [CrossRef]
- Mucha, B.E.; Banka, S.; Ajeawung, N.F.; Molidperee, S.; Chen, G.G.; Koenig, M.K.; Adejumo, R.B.; Till, M.; Harbord, M.; Perrier, R.; et al. A New Microdeletion Syndrome Involving Tbc1d24, Atp6v0c, and Pdpk1 Causes Epilepsy, Microcephaly, and Developmental Delay. Genet. Med. 2019, 21, 1058–1064. [Google Scholar] [CrossRef] [PubMed]
- Mattison, K.A.; Tossing, G.; Mulroe, F.; Simmons, C.; Butler, K.M.; Schreiber, A.; Alsadah, A.; Neilson, D.E.; Naess, K.; Wedell, A.; et al. Atp6v0c Variants Impair V-Atpase Function Causing a Neurodevelopmental Disorder Often Associated with Epilepsy. Brain 2023, 146, 1357–1372. [Google Scholar] [CrossRef]
- Rujano, M.A.; Serio, M.C.; Panasyuk, G.; Peanne, R.; Reunert, J.; Rymen, D.; Hauser, V.; Park, J.H.; Freisinger, P.; Souche, E.; et al. Mutations in the X-Linked Atp6ap2 Cause a Glycosylation Disorder with Autophagic Defects. J. Exp. Med. 2017, 214, 3707–3729. [Google Scholar] [CrossRef]
- Knüppel, H. Dental Power: How We Can Live Longer and Better and What Role Teeth Play as a Reflection of Health; BoD–Books on Demand: Hamburg, Germany, 2024. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).