1. Introduction
The Bactrian camel (
Camelus bactrianus), a critically important livestock species in arid and semi-arid ecosystems, is facing a global decline in reproductive efficiency. Although recent investigations employing Bactrian camels have identified several biomarkers associated with reproductive and metabolic processes [
1,
2], the comprehensive physiological and molecular mechanisms regulating camel reproduction remain incompletely characterized. The camel’s reproductive physiology represents a complex adaptive system shaped by desert selection pressures [
3], integrating multiple specialized features: seasonal breeding cyclicity, induced ovulation mechanisms, one of the longest gestation periods among terrestrial mammals, and evolutionarily constrained reproductive rates [
3,
4,
5]. These specialized adaptations, while optimizing individual fitness under xeric conditions with limited resources, create fundamental constraints on reproductive output that increasingly jeopardize population sustainability amid growing climatic challenges and human-induced pressures [
6,
7]. Male camels have a characteristic poll gland (occipital gland or neck occipital gland), a V-shaped structure located bilaterally at the C1 vertebra within the dermis [
8]. Composed of pyramidal lobules with almond-like pigmentation, this gland undergoes seasonal enlargement during rutting seasons, secreting a viscous, amber-colored fluid with a potent odor [
8,
9]. The poll gland’s secretions, rich in bioactive compounds, play pivotal roles in estrus induction and mating facilitation by stimulating female camels and enhancing male sexual activity [
10].
During rutting, males exhibit distinct behavioral displays, including tail-urine flapping and defecation, vocalizations (“beeping”), mucus secretion, and territorial aggression [
6,
11]. These energetically demanding activities correlate with increased gland development and pheromone output, suggesting a direct link between poll gland function and reproductive success [
10,
12]. Additionally, behaviors such as foaming at the mouth, teeth grinding, and reduced appetite during rutting may reflect metabolic trade-offs between reproductive effort and energy conservation. Although the poll gland’s functional significance is well-established, the underlying molecular mechanisms coordinating its activity with pheromone production, metabolic processes, and mating behaviors are yet to be fully elucidated. Addressing these knowledge gaps may contribute to the development of strategies aimed at improving camel fertility and supporting the species’ ecological and economic roles. Fatty acids provide a more sustained energy source than carbohydrates and proteins, making fatty acid metabolism especially important during energy-demanding physiological states such as the rutting season in male Bactrian camels, when prolonged physical activity and poll gland secretory function are pronounced [
13]. This study focuses on fatty acid catabolism, particularly the mitochondrial β-oxidation pathway, to investigate how metabolic energy supports reproductive behaviors.
Fatty acid catabolism, a central energy-producing pathway, involves sequential enzymatic steps: lipolysis, mitochondrial transport via carnitine palmitoyltransferase 1A (CPT1A), β-oxidatiolhyufon, and acetyl-CoA generation for ATP synthesis [
14,
15]. CPT1A, a rate-limiting enzyme, facilitates fatty acid entry into mitochondria, while acyl-CoA synthetases (ACSLs) activate long-chain fatty acids [
16]. Hydroxyacyl-CoA dehydrogenase (HADH) and other β-oxidation enzymes contribute to acetyl-CoA production, linking lipid catabolism to the tricarboxylic acid (TCA) cycle [
17,
18]. CPT1A serves as a master regulator of this pathway, ensuring metabolic flexibility and energy homeostasis, particularly during fasting or high energy demand [
19]. CPT1A activity is tightly controlled by nutritional status and hormonal cues such as glucagon insulin and malonyl-CoA, allowing cells to adapt fuel usage to metabolic needs [
20]. In brown adipose tissue, CPT1A-mediated fatty acid oxidation contributes to thermogenesis through uncoupling protein 1 (UCP1), while in the liver, it supports ketogenesis and helps maintain systemic glucose balance during energy deprivation [
2,
3]. In brown adipose tissue, CPT1A-mediated fatty acid oxidation contributes to thermogenesis through uncoupling protein 1 (UCP1), while in the liver, it supports ketogenesis and helps maintain systemic glucose balance during energy deprivation [
2,
3]. These functions highlight the cross-tissue relevance of CPT1A in maintaining energy homeostasis. Moreover, dysregulation of CPT1A has been implicated in various metabolic disorders, including hepatic steatosis and insulin resistance, underscoring its clinical significance. Pharmacological activation of CPT1A, for example via AMPK/mTORC1 pathway modulators, has shown therapeutic promise in improving lipid metabolism and alleviating metabolic syndromes [
4,
21,
22].
This study aims to investigate the molecular mechanisms of fatty acid metabolism, particularly the role of CPT1A, in the poll gland of male Bactrian camels during the rutting season. Specifically, proteomic and metabolomic analyses were employed to identify differentially expressed proteins (DEPs) and metabolites associated with reproductive behaviors. The expression and localization changes of CPT1A in the glandular tissues were evaluated to understand its role in metabolic fueling and reproductive activities. Our findings provide new insights into the patterns of fatty acid metabolism in the poll gland and reproduction of male Bactrian camels.
2. Materials and Methods
2.1. Animals and Samples
For this study, healthy male Bactrian camels (Camelus bactrianus) of similar age (8 years old) and body weight (480 ± 5 kg) were selected from a farm in Zhangye (Gansu, China). During the non-breeding season (April to November), the camels were allowed to roam and feed freely, while they were kept in captivity during the breeding season (December to March of the following year). To minimize animal suffering, candidate camels were deeply sedated by intravenous injection of Xylazine (0.3 mg/kg, Lanzhou, China) and then induced to painless death via intravenous injection of pentobarbital sodium (140 mg/kg, Dechra Veterinary Products, Shrewsbury, UK). Samples of neck mane and urine were collected in June (non-breeding season, n = 3) and January (breeding season, n = 3), serving as the non-breeding season group (NBS group) and breeding season group (BS group), respectively. Due to significant atrophy of the poll gland during the non-breeding season, tissue collection was only feasible during the breeding season. Poll gland tissues with high and low secretion levels were collected accordingly. All samples were processed as follows: flash-frozen in liquid nitrogen for proteomic analysis and fixed in 4% paraformaldehyde for histochemical examination. Then, neck mane, urine, and poll gland samples from different reproductive stages were used for a non-targeted metabolomic analysis. All procedures strictly adhered to the ethical guidelines approved by the Animal Protection Committee of Gansu Agricultural University (Approval No. GSU-LC-2020-39).
2.2. Metabolite Extraction and Liquid Chromatography–Mass Spectrometry (LC-MS) Analysis
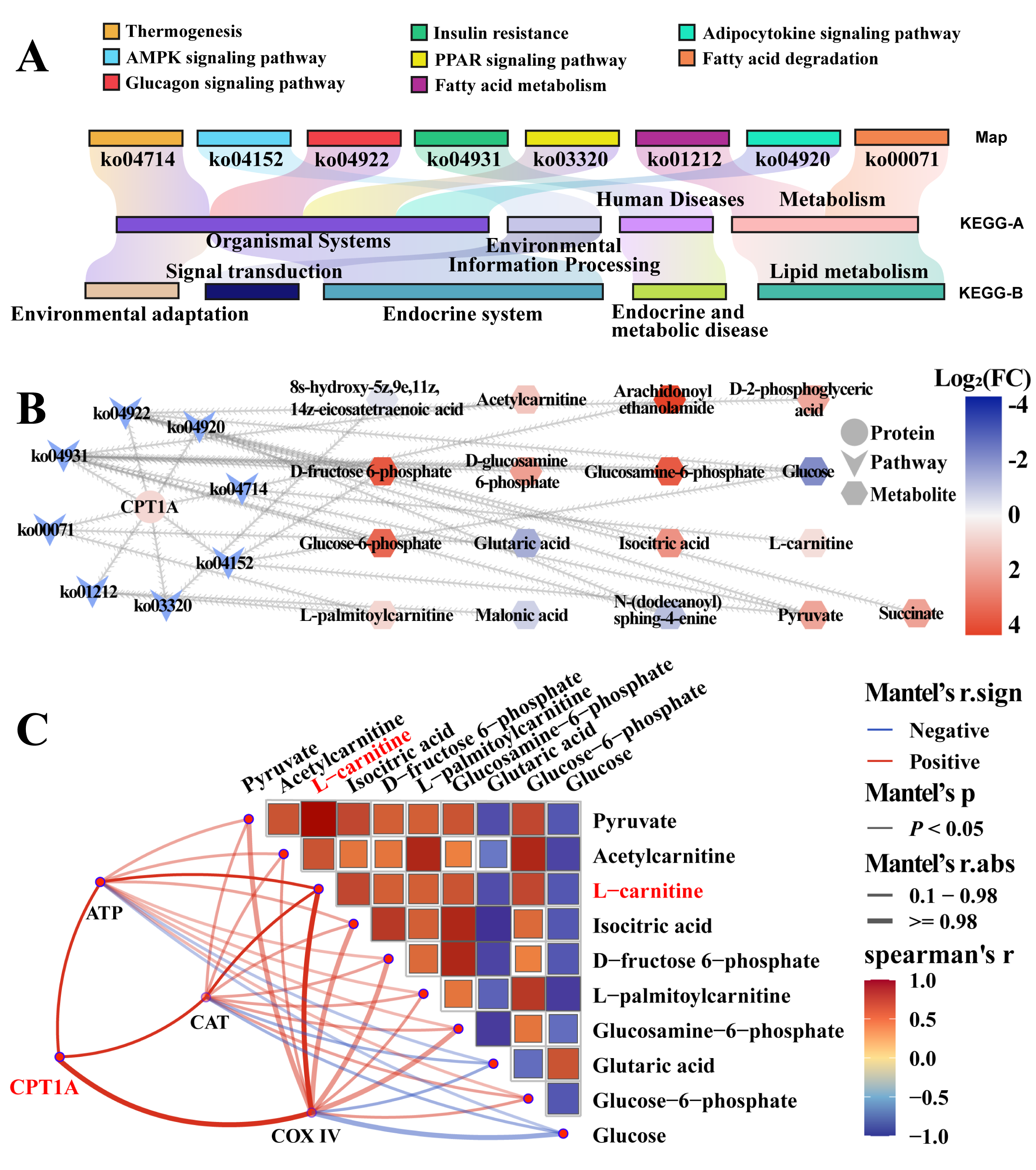
Poll gland tissue or neck mane samples (80 mg) were homogenized in 200 μL of ultrapure water (H2O) using a homogenizer (Wonbio, Shanghai, China) equipped with five ceramic beads. For metabolite extraction, 800 μL of a methanol–acetonitrile (1:1, v/v) solution was added to the homogenate or urine samples. The mixture was vortexed thoroughly and then centrifuged at 12,000× g for 15 min. The resulting supernatant was collected and dried under vacuum using a centrifuge (Bioridge, Shanghai, China). The dried residue was reconstituted in 100 μL of acetonitrile–water (1:1, v/v) for subsequent LC-MS analysis. Metabolite profiling was performed using ultra-high-performance liquid chromatography (UHPLC; 1290 Infinity LC, Agilent Technologies, Santa Clara, CA, USA) coupled with a high-resolution mass spectrometer (AB Sciex TripleTOF 6600, Waters, Redwood City, CA, USA). Chromatographic separation was achieved on an ACQUITY UPLC HSS T3 column (100 mm × 2.1 mm, 1.8 μm; Waters, Redwood City, CA, USA). To ensure analytical reproducibility, quality control (QC) samples were prepared by pooling equal volumes of all extracted samples and analyzed intermittently throughout the run. Raw LC-MS data were processed using Progenesis QI software v.4.2 (Nonlinear Dynamics, Newcastle, Newcastle upon Tyne, UK) for baseline correction, peak alignment, deconvolution, and peak area integration. Metabolite identification was performed by matching mass spectra against the Human Metabolome Database (HMDB). Differentially expressed metabolites (DEMs) were screened based on a false discovery rate (FDR) < 0.05 and a variable importance in projection (VIP) score > 1. Identified metabolites were functionally annotated and subjected to pathway enrichment analysis using the Kyoto Encyclopedia of Genes and Genomes (KEGG) database.
2.3. Proteomic Sequencing and Bioinformatics Analysis
Tissue samples were processed according to the manufacturer’s protocol for the iST Sample Preparation Kit (PreOmics, Tübingen, Germany). The workflow included protein denaturation, reduction with dithiothreitol (DTT), alkylation with iodoacetamide (IAA), tryptic digestion, and peptide cleanup using C18 spin columns. For spectral library generation, digested peptides were reconstituted in buffer (20 mM ammonium formate in water, pH 10.0) and fractionated by high-pH reversed-phase chromatography on an Ultimate 3000 system (ThermoFisher, Waltham, MA, USA) equipped with an XBridge C18 column (3.5 μm, 4.6 × 150 mm; Waters, Waltham, MA, USA). Peptides were eluted using a 5–45% gradient of buffer (20 mM ammonium formate in 80% acetonitrile, pH 10.0) over 60 min, with collection of six fractions. Fractionated peptides were analyzed by LC-MS using an Orbitrap Lumos mass spectrometer (ThermoFisher, Waltham, MA, USA) in data-independent acquisition (DIA) mode. Raw DIA files were processed using Spectronaut X (Biognosys AG, Schlieren, Switzerland) with default settings. DEPs were identified using thresholds of a false discovery rate (FDR) < 0.05 and a log2 fold change > 0.58. The complete DIA proteomic dataset was deposited into the ProteomeXchange Consortium via the PRIDE repository with the dataset identifier PXD047457.
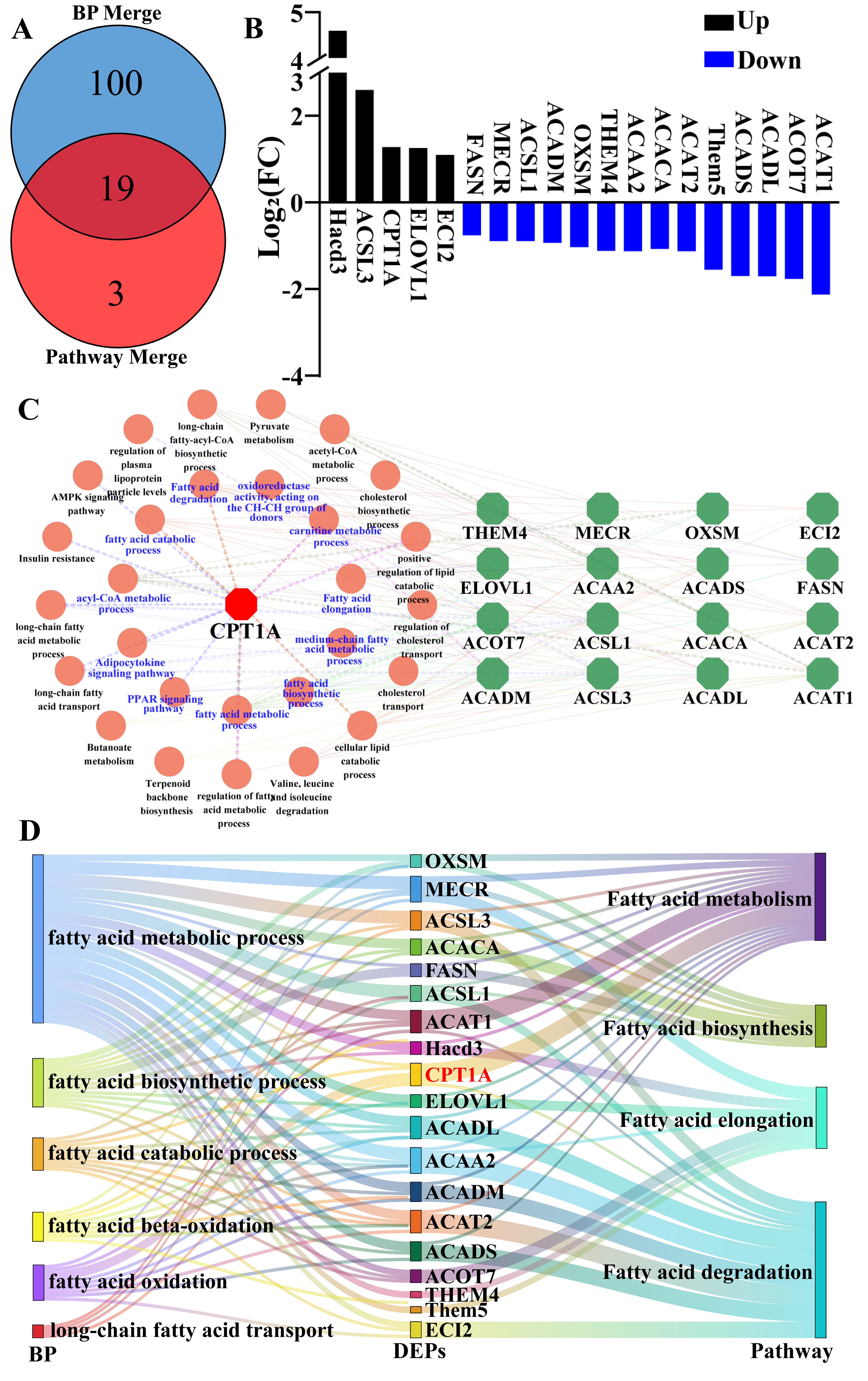
To characterize the biological functions of DEPs, Gene Ontology (GO) annotation and KEGG enrichment analyses were performed with particular focus on proteins associated with fatty acid catabolism. For data visualization, hierarchical clustering heatmaps and Venn diagrams were generated using the OmicShare network (
https://www.omicshare.com/tools/). Protein–protein interaction networks were constructed by integrating data from the STRING database (version 11.5,
https://cn.string-db.org/) and visualized using Cytoscape software (version 3.9.1).
2.4. RNA Isolation and Quantitative Gene Expression Analysis
Total RNA was isolated from tissue samples using the TRIzol reagent (Invitrogen) according to the manufacturer’s protocol. RNA concentration and purity were determined spectrophotometrically (NanoDrop 2000, ThermoFisher, Waltham, MA, USA). First-strand cDNA was synthesized from 1 μg total RNA using a PrimeScript RT reagent kit (Takara, Dalian, China) following the manufacturer’s instructions. Quantitative real-time PCR (qPCR) was performed using SYBR Premix Ex Taq (Takara) on a QuantStudio 6 Flex Real-Time PCR System (Applied Biosystems). The thermal cycling conditions consisted of 95 °C for 30 s followed by 40 cycles of 95 °C for 5 s and 60 °C for 30 s. A melt curve analysis was performed to verify amplification specificity. The qPCR primer sequences used in this study were as follows: CPT1A F, 5′-ATTTCCTCCCGGTCCAGTTT-3′; CPT1A R, 5′-GGACAGCAAGCACATAGTCG-3′; β-actin F, 5′-CCAAGGCCAACCGTGAGAA-3′; β-actin R, 5′-CCAGAGGCATACAGGGACAG-3′. All primers were custom-synthesized by Tsingke Biotechnology (Yangling, China). β-actin served as the endogenous control for normalization. Relative gene expression levels were calculated using the 2−ΔΔCT method. All experiments were performed in triplicate with three biological replicates.
2.5. Histochemical Analysis
Paraffin-embedded fixed tissues were sectioned at 5 µm thickness using a rotary microtome (Leica RM2235, Wetzlar, Germany). Tissue sections were subjected to standard hematoxylin and eosin (H&E) staining and immunohistochemical (IHC) analysis following established protocols [
2,
12]. For IHC staining, sections were incubated with the following primary antibodies: rabbit anti-COX IV (1:500 dilution; Proteintech, Wuhan, China) and rabbit anti-CPT1A (1:400 dilution; Proteintech). Stained sections were digitally imaged using a high-resolution whole-slide scanner (Pannoramic, 3D HISTECH, Budapest, Hungary). All experiments were performed in triplicate to ensure reproducibility.
2.6. Immunofluorescence (IF) Analysis
IF staining was performed following established protocols [
12]. Tissue sections were incubated with the following primary antibodies: rabbit anti-COX IV (1:300 dilution; Proteintech), rabbit anti-CPT1A (1:300 dilution; Proteintech), and mouse monoclonal anti-CK7 (1:500; Bioss, Beijing, China). After secondary antibody incubation, cell nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI; 1 μg/mL, Solarbio, Beijing, China) for 10 min at room temperature. Fluorescence images were acquired using a high-resolution fluorescence microscope (Echo Revolve R4, Echo Laboratories, San Diego, CA, USA) equipped with appropriate filter sets. All experiments were repeated independently at least three times.
2.7. Western Blot Analysis
Protein expression levels were analyzed by Western blotting following established protocols. In brief, tissue samples were homogenized in RIPA lysis buffer containing protease inhibitors (Beyotime, Beijing, China), and protein concentrations were determined using the BCA assay (Beyotime). Equal amounts of protein (30 μg per lane) were separated by 10% SDS-PAGE and transferred to PVDF membranes (Millipore, Billerica, MA, USA). Membranes were blocked with 5% skimmed milk in TBST for 1 h at room temperature then incubated overnight at 4 °C with the following primary antibodies: rabbit polyclonal anti-CPT1A (1:5000; Proteintech) and mouse monoclonal anti-β-actin (1:8000; Bioss). After incubation with HRP-conjugated secondary antibodies (1:3000; Proteintech) for 1 h at room temperature, protein bands were visualized using an enhanced chemiluminescence (ECL) substrate (Beyotime). Band intensities were quantified using ImageJ software v1.44p (NIH; Bethesda, MD, USA). β-actin served as the loading control for normalization. All experiments were repeated three times with independent biological replicates.
2.8. Quantitative Biochemical Analyses
ATP concentration in tissues was quantified using an ATP content detection kit (chemiluminescence method, G4309, Servicebio, Wuhan, China) following the manufacturer’s instructions. Catalase (CAT) activity in tissues was assessed with a CAT detection kit (G4307, Servicebio) in accordance with the manufacture’s protocols.
2.9. Statistical Analysis
All statistical analyses were conducted using SPSS statistical software (version 26.0, IBM Corporation, Chicago, IL, USA). Continuous variables were expressed as mean ± standard deviation (SD) and analyzed using two-tailed Student’s t-tests for comparisons between groups. For multiple comparisons, one-way ANOVA followed by Tukey’s post hoc test was applied. Data visualization was performed using OriginPro software (version 9.1, OriginLab Corporation, Northampton, MA, USA). A probability value (p) of less than 0.05 was considered statistically significant for all analyses.
4. Discussion
As seasonal breeders, Bactrian camels exhibit distinct seasonal breeding patterns, with reproductive activity primarily occurring during the winter months. This reproductive seasonality is particularly evident in male camels, where the development and secretory activity of poll glands show marked cyclical changes synchronized with sexual activity [
4,
10,
24]. The poll gland, a specialized sebaceous structure located in the cranial region, produces pheromone-rich secretions that serve as crucial chemical signals in camel reproduction [
8,
9]. These secretions contain bioactive compounds that not only enhance male libido but also play a key role in stimulating estrus behavior and mating receptivity in female camels [
25]. However, the precise biochemical composition and mechanistic functions of poll gland secretions remain poorly understood. The specific pheromonal compounds responsible for mediating reproductive behaviors and their mode of action at the molecular level have yet to be fully characterized.
Seasonal breeding in male animals is typically associated with increased energy demands due to courtship displays, mate competition, and reproductive behaviors [
26]. This general trend is also evident in male Bactrian camels, whose poll gland undergoes marked seasonal activation. Our group is committed to exploring the metabolites of the camel poll gland and their roles in regulating the breeding season via identifying DEMs and DEPS. Our previous study identified some differential metabolites and clarified the metabolic mechanism in the poll gland, such as hydrogen sulfide (H
2S) and creatine [
1,
10,
12]. In this study, we found that fatty acid is another differential metabolite in the poll gland during the breeding season based on non-targeted metabolomics. Our proteomic analysis revealed that the key protein CPT1A, a master regulator of fatty acid metabolism, was significantly activated, and signaling pathways related to fatty acid metabolism, including fatty acid biosynthesis, elongation, metabolism, and degradation pathways, were stimulated in the poll gland during the breeding season. Consistently, the expression pattern revealed that the central regulator CPT1A involved in fatty acid metabolism was widely distributed in the acinar cells of the poll gland, and its expression increased significantly during the breeding season, together with the results that the expression of COX IV, a well-established marker for cellular energy metabolism levels [
23], ATP content, and CAT activity increased significantly during the breeding season, which may lead to vigorous fatty acid metabolism and energy generation.
Fatty acid metabolism is a central energy-producing pathway and is closely related to various physiological processes in mammals, including estrus and breeding [
27,
28]. Similar metabolic shifts, such as weight loss, hormone elevation, and enhanced glandular activity, have been observed in other seasonal breeders like male yaks and sheep during the breeding season [
29,
30]. Unlike Bactrian camels, which rely on social interactions and territorial defense, yaks and sheep engage in more direct competition, with male yaks showing significant fat and muscle breakdown due to high energy expenditure [
31]. Male sheep also exhibit increased fat oxidation and elevated testosterone, fueling their aggressive behavior [
32]. These shifts in fatty acid metabolism and energy supply support their reproductive efforts, while Bactrian camels show more moderate changes, reflecting different reproductive strategies. CPT1A, a mitochondrial enzyme, is a master regulator of fatty acid β-oxidation by catalyzing the transport of long-chain fatty acids into mitochondria for energy production [
19]. These metabolic adjustments are key to supporting reproductive efforts across species, including courtship and mating. In Bactrian camels, exploration of the role of CPT1A-mediated fatty acid metabolism in the camel poll gland would help further understand the function of the poll gland and its important role in breeding, while also highlighting unique camel-specific features such as the importance of the poll gland in pheromone secretion [
10,
12]. By comparing these metabolic processes in different seasonal breeders, we can better understand which features are specific to camels and which reflect broader reproductive strategies among seasonal breeders. The elevated expression of CPT1A and COX IV and increased ATP production in the poll gland during the breeding season support the hypothesis that male Bactrian camels undergo significant metabolic reprogramming to meet the energy demands of reproduction.
Some limitations of this study should be acknowledged. While we focused primarily on fatty acid metabolism in the poll gland, exploring lipid metabolism and mitochondrial activity in other tissues, such as liver and muscle, could provide additional evidence of systemic energy mobilization during the rutting season. Investigating the expression of CPT1A and other lipid metabolism markers in these tissues would offer a broader view of metabolic shifts and their role in supporting the energy demands of reproduction.
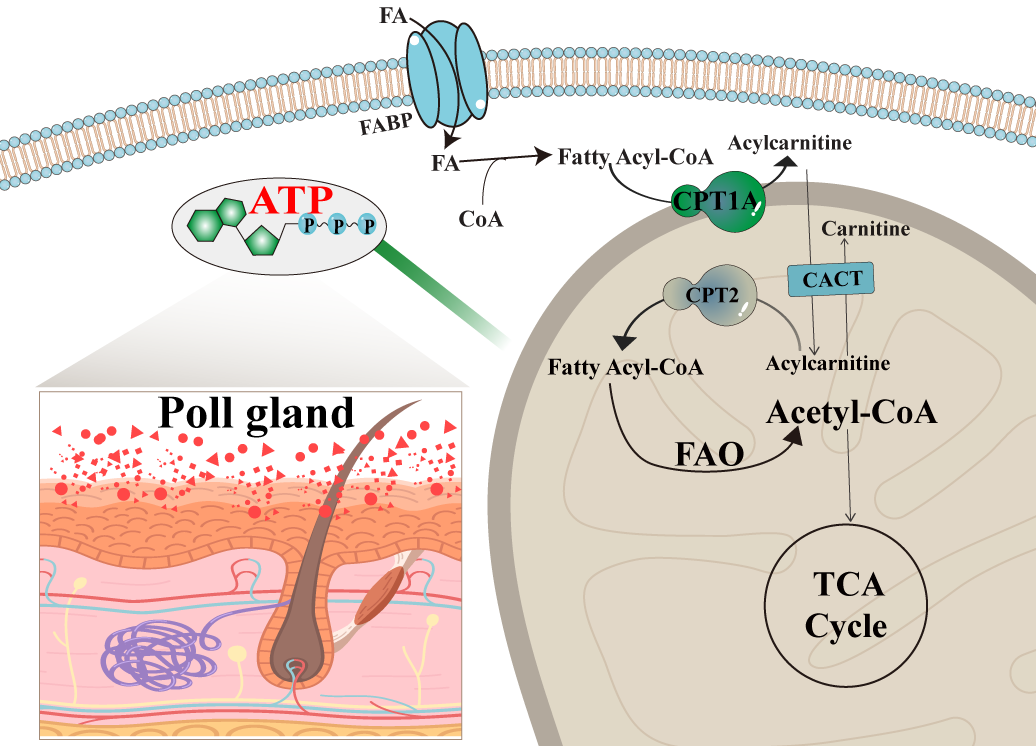
In summary, as shown in
Figure 7, in the rutting season, male camels undergo significant metabolic changes to support reproductive physiology. Fatty acids from the circulatory system are taken up by poll gland acinar cells and activated into fatty acyl-CoA. This activated form is then converted to acylcarnitine via CPT1A in the presence of carnitine at the mitochondrial membrane. The resulting acylcarnitine is transported into the mitochondrial matrix by carnitine-acylcarnitine translocase (CACT). Inside the mitochondria, acylcarnitine is reconverted to fatty acyl-CoA, which undergoes β-oxidation to generate ATP. This energy production sustains the high metabolic demands of the poll gland during periods of heightened secretory and reproductive activity. Such findings provide a theoretical basis for understanding the reproductive patterns of camels, improving their reproductive functions, and increasing fertility rates.