Valproic Acid Promotes the Differentiation of Satellite Glial Cells into Neurons via the pH-Dependent Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. DRG (Dorsal Root Ganglia)-SGCs Culture
2.2. SVGp12, U87, and U251 Cell Culture
2.3. Neuronal Induction
2.4. Cytosolic pH Measurement
2.5. Immunofluorescence
2.6. Western Blot
2.7. Statistical Analysis
3. Results
3.1. The Process of VPA-Induced Differentiation of SGCs into Neurons Is Accompanied by a Sustained Increase in the Intracellular PH
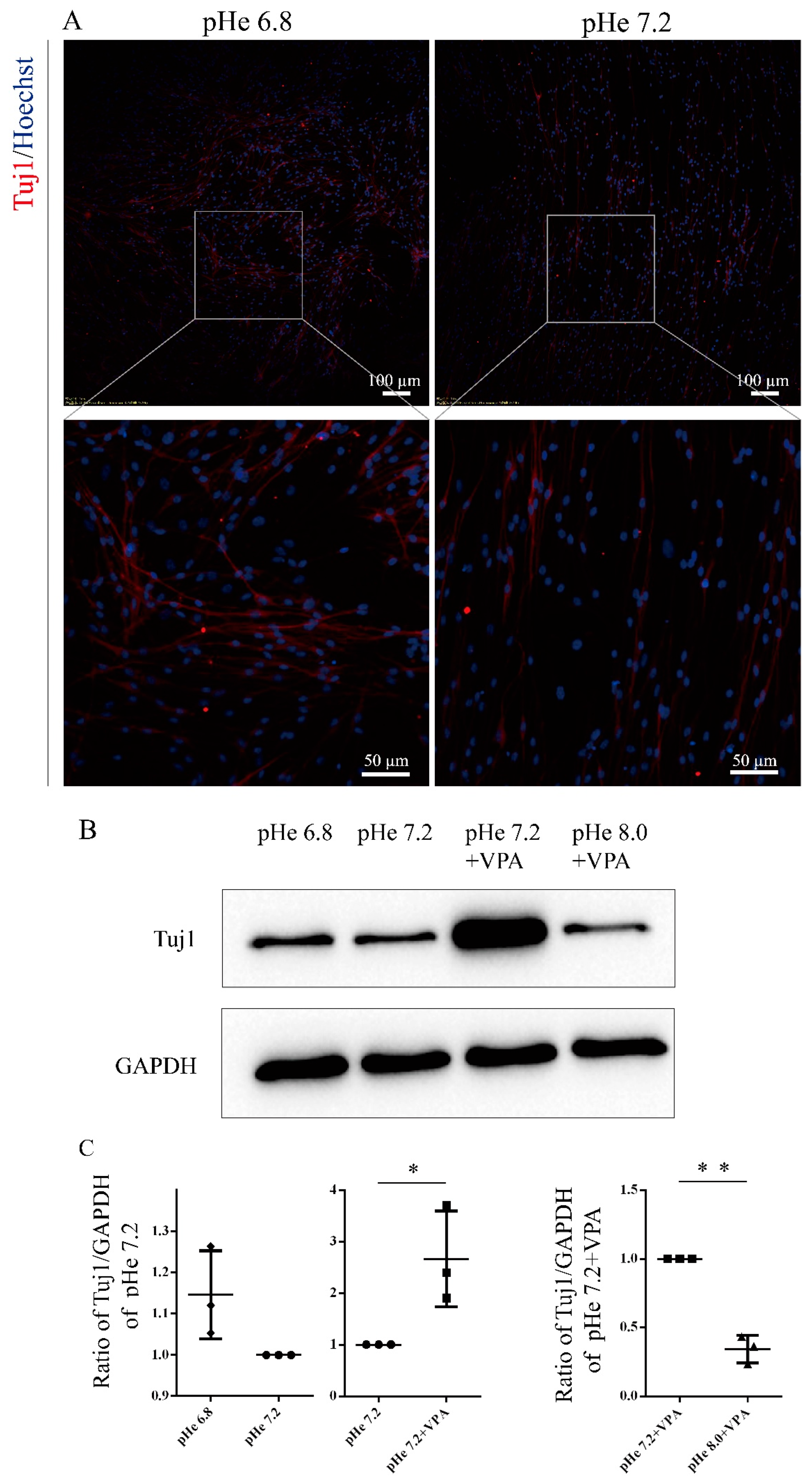
3.2. Intracellular pH Elevation Mediates VPA-Induced Neuronal Differentiation
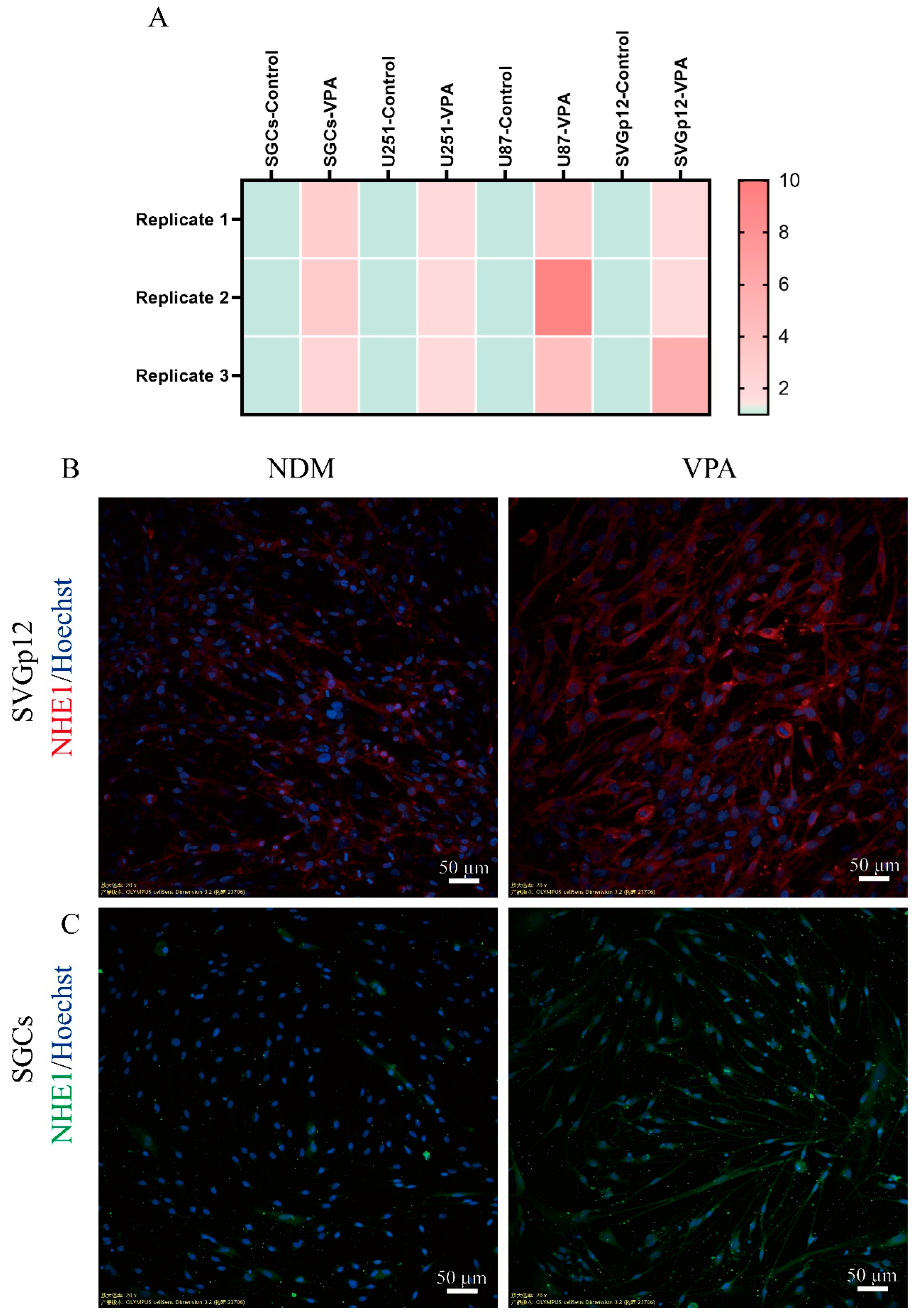
3.3. VPA Increases Intracellular PH by Enhancing the Expression of NHE1
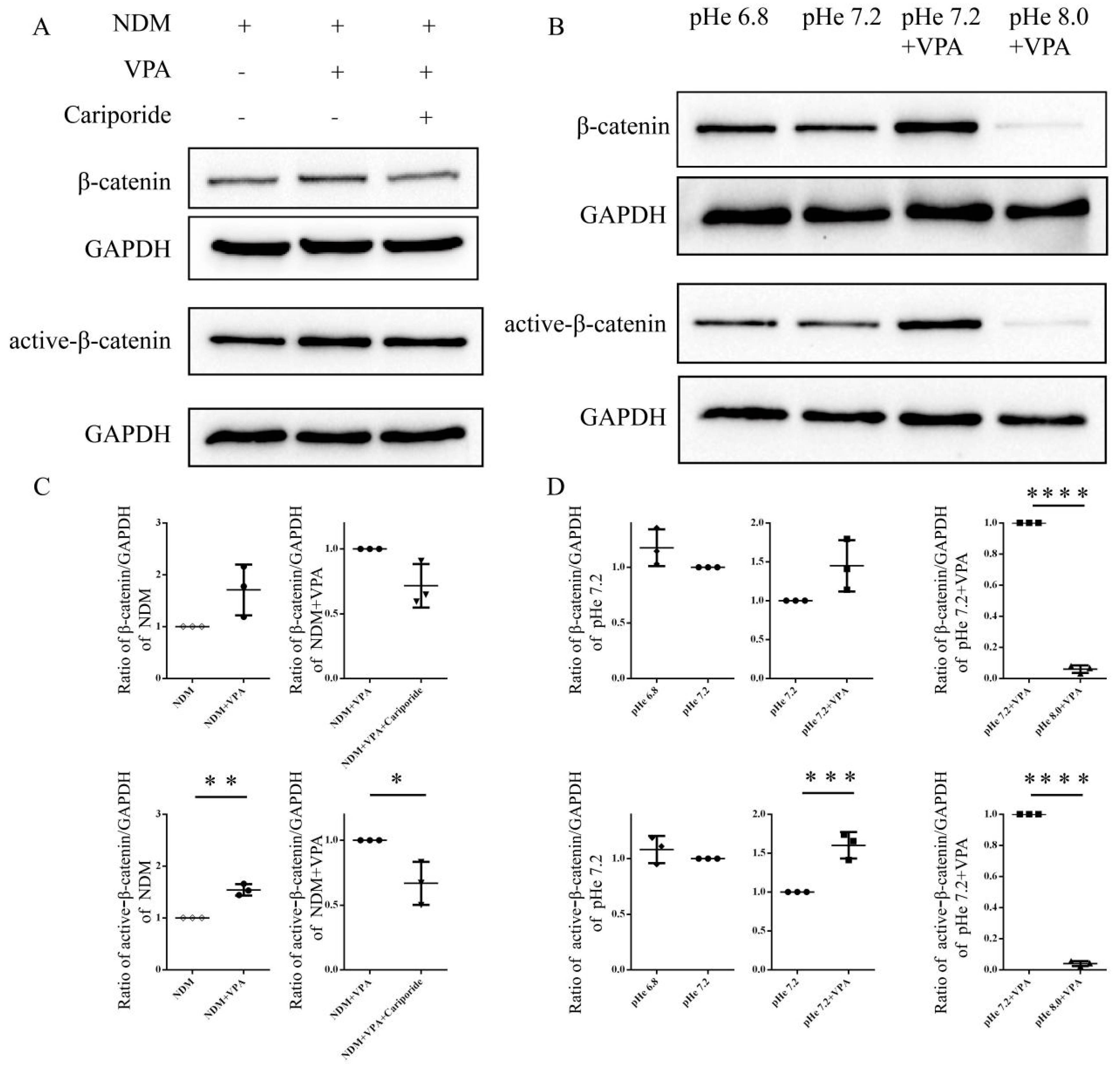
3.4. VPA Activates β-Catenin Signaling by Increasing the Intracellular PH
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Go, H.S.; Seo, J.E.; Kim, K.C.; Han, S.M.; Kim, P.; Kang, Y.S.; Han, S.H.; Shin, C.Y.; Ko, K.H. Valproic acid inhibits neural progenitor cell death by activation of NF-kappaB signaling pathway and up-regulation of Bcl-XL. J. Biomed. Sci. 2011, 18, 48. [Google Scholar]
- He, X.; Yuan, R.; Chen, Y.; Huang, W.; Xu, Z.; Wang, B.; Liu, C.; Xiong, T. Mechanism of valproic acid-induced hepatic steatosis via enhancing NRF2-FATP2-mediated fatty acid uptake. Theranostics 2025, 15, 5258–5276. [Google Scholar]
- Yu, I.T.; Park, J.Y.; Kim, S.H.; Lee, J.S.; Kim, Y.S.; Son, H. Valproic acid promotes neuronal differentiation by induction of proneural factors in association with H4 acetylation. Neuropharmacology 2009, 56, 473–480. [Google Scholar] [PubMed]
- Zhang, J.; Zhang, J.X.; Zhang, Q.L. PI3K/AKT/mTOR-mediated autophagy in the development of autism spectrum disorder. Brain Res. Bull. 2016, 125, 152–158. [Google Scholar]
- Cheng, L.; Gao, L.; Guan, W.; Mao, J.; Hu, W.; Qiu, B.; Zhao, J.; Yu, Y.; Pei, G. Direct conversion of astrocytes into neuronal cells by drug cocktail. Cell Res. 2015, 25, 1269–1272. [Google Scholar] [PubMed]
- Hu, W.; Qiu, B.; Guan, W.; Wang, Q.; Wang, M.; Li, W.; Gao, L.; Shen, L.; Huang, Y.; Xie, G.; et al. Direct Conversion of Normal and Alzheimer’s Disease Human Fibroblasts into Neuronal Cells by Small Molecules. Cell Stem Cell 2015, 17, 204–212. [Google Scholar]
- Wang, D.; Lu, J.; Xu, X.; Yuan, Y.; Zhang, Y.; Xu, J.; Chen, H.; Liu, J.; Shen, Y.; Zhang, H. Satellite Glial Cells Give Rise to Nociceptive Sensory Neurons. Stem Cell Rev. Rep. 2021, 17, 999–1013. [Google Scholar]
- Tatapudy, S.; Aloisio, F.; Barber, D.; Nystul, T. Cell fate decisions: Emerging roles for metabolic signals and cell morphology. EMBO Rep. 2017, 18, 2105–2118. [Google Scholar] [PubMed]
- Liu, Y.; White, K.A.; Barber, D.L. Intracellular pH Regulates Cancer and Stem Cell Behaviors: A Protein Dynamics Perspective. Front. Oncol. 2020, 10, 1401. [Google Scholar]
- Ulmschneider, B.; Grillo-Hill, B.K.; Benitez, M.; Azimova, D.R.; Barber, D.L.; Nystul, T.G. Increased intracellular pH is necessary for adult epithelial and embryonic stem cell differentiation. J. Cell Biol. 2016, 215, 345–355. [Google Scholar]
- McBrian, M.A.; Behbahan, I.S.; Ferrari, R.; Su, T.; Huang, T.W.; Li, K.; Hong, C.S.; Christofk, H.R.; Vogelauer, M.; Seligson, D.B.; et al. Histone acetylation regulates intracellular pH. Mol. Cell 2013, 49, 310–321. [Google Scholar]
- Lu, J.; Zhang, Y.; Wang, D.; Xu, X.; Xu, J.; Yang, X.; Qian, H.; Zhang, H. Basic Fibroblast Growth Factor Promotes Mesenchymal Stem Cell Migration by Regulating Glycolysis-Dependent beta-Catenin Signaling. Stem Cells 2023, 41, 628–642. [Google Scholar]
- Wang, N.; Zhou, L.; Shao, C.Y.; Wang, X.T.; Zhang, N.; Ma, J.; Hu, H.L.; Wang, Y.; Qiu, M.; Shen, Y. Potassium channel K(ir) 4.1 regulates oligodendrocyte differentiation via intracellular pH regulation. Glia 2022, 70, 2093–2107. [Google Scholar]
- Oginuma, M.; Harima, Y.; Tarazona, O.A.; Diaz-Cuadros, M.; Michaut, A.; Ishitani, T.; Xiong, F.; Pourquié, O. Intracellular pH controls WNT downstream of glycolysis in amniote embryos. Nature 2020, 584, 98–101. [Google Scholar] [PubMed]
- Hendus-Altenburger, R.; Kragelund, B.B.; Pedersen, S.F. Structural dynamics and regulation of the mammalian SLC9A family of Na(+)/H(+) exchangers. Curr. Top. Membr. 2014, 73, 69–148. [Google Scholar] [PubMed]
- Wang, L.; Liu, Y.; Li, S.; Long, Z.Y.; Wu, Y.M. Wnt signaling pathway participates in valproic acid-induced neuronal differentiation of neural stem cells. Int. J. Clin. Exp. Pathol. 2015, 8, 578–585. [Google Scholar]
- Kelkawi, A.H.A.; Hashemzadeh, H.; Pashandi, Z.; Tiraihi, T.; Naderi-Manesh, H. Differentiation of PC12 cell line into neuron by Valproic acid encapsulated in the stabilized core-shell liposome-chitosan Nano carriers. Int. J. Biol. Macromol. 2022, 210, 252–260. [Google Scholar]
- Hsieh, J.; Nakashima, K.; Kuwabara, T.; Mejia, E.; Gage, F.H. Histone deacetylase inhibition-mediated neuronal differentiation of multipotent adult neural progenitor cells. Proc. Natl. Acad. Sci. USA 2004, 101, 16659–16664. [Google Scholar] [PubMed]
- Souza, V.d.O.e.; Taboada, T.B.; Ramalho, B.D.S.; Pires, G.N.; Da Costa, T.P.; El-Cheikh, M.C.; Carneiro, K.; Martinez, A.M.B. Valproic acid ameliorates morpho-dysfunctional effects triggered by Ischiatic nerve crush injury-induced by compression model in mice: Nerve regeneration and immune-modulatory pathway. Brain Res. Bull. 2025, 220, 111140. [Google Scholar]
- Kowalski, T.W.; Lord, V.O.; Sgarioni, E.; Gomes, J.D.A.; Mariath, L.M.; Recamonde-Mendoza, M.; Vianna, F.S.L. Transcriptome meta-analysis of valproic acid exposure in human embryonic stem cells. Eur. Neuropsychopharmacol. 2022, 60, 76–88. [Google Scholar]
- Sandvik, D.; Vianca, E.; Anderson, A.; Javaid, M.S.; O’Brien, T.J.; Antonic-Baker, A. In vitro models of valproic acid to assess neurodevelopmental toxicity: A scoping review. Epilepsia 2025. Epub ahead of print. [Google Scholar] [CrossRef]
- Zhang, X.; He, X.; Li, Q.; Kong, X.; Ou, Z.; Zhang, L.; Gong, Z.; Long, D.; Li, J.; Zhang, M.; et al. PI3K/AKT/mTOR Signaling Mediates Valproic Acid-Induced Neuronal Differentiation of Neural Stem Cells through Epigenetic Modifications. Stem Cell Rep. 2017, 8, 1256–1269. [Google Scholar]
- Chen, A.; Wang, M.; Xu, C.; Zhao, Y.; Xian, P.; Li, Y.; Zheng, W.; Yi, X.; Wu, S.; Wang, Y. Glycolysis mediates neuron specific histone acetylation in valproic acid-induced human excitatory neuron differentiation. Front. Mol. Neurosci. 2023, 16, 1151162. [Google Scholar]
- Amith, S.R.; Fliegel, L. Regulation of the Na+/H+ Exchanger (NHE1) in Breast Cancer Metastasis. Cancer Res. 2013, 73, 1259–1264. [Google Scholar] [PubMed]
- Chen, A.; Zhang, J.; Yan, Z.; Lu, Y.; Chen, W.; Sun, Y.; Gu, Q.; Li, F.; Yang, Y.; Qiu, S.; et al. Acidic preconditioning induced intracellular acid adaptation to protect renal injury via dynamic phosphorylation of focal adhesion kinase-dependent activation of sodium hydrogen exchanger 1. Cell Commun. Signal 2024, 22, 393. [Google Scholar]
- Liao, S.; Wu, G.; Xie, Z.; Lei, X.; Yang, X.; Huang, S.; Deng, X.; Wang, Z.; Tang, G. pH regulators and their inhibitors in tumor microenvironment. Eur. J. Med. Chem. 2024, 267, 116170. [Google Scholar] [PubMed]
- Wang, H.; Singh, D.; Fliegel, L. The Na+/H+ antiporter potentiates growth and retinoic acid-induced differentiation of P19 embryonal carcinoma cells. J. Biol. Chem. 1997, 272, 26545–26549. [Google Scholar]
- Walker, N.M.; Liu, J.; Stein, S.R.; Stefanski, C.D.; Strubberg, A.M.; Clarke, L.L. Cellular chloride and bicarbonate retention alters intracellular pH regulation in Cftr KO crypt epithelium. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 310, G70–G80. [Google Scholar]
- Strubberg, A.M.; Liu, J.; Walker, N.M.; Stefanski, C.D.; MacLeod, R.J.; Magness, S.T.; Clarke, L.L. Cftr Modulates Wnt/beta-Catenin Signaling and Stem Cell Proliferation in Murine Intestine. Cell Mol. Gastroenterol. Hepatol. 2018, 5, 253–271. [Google Scholar]
- Amiri, M.; Seidler, U.E.; Nikolovska, K. The Role of pH(i) in Intestinal Epithelial Proliferation-Transport Mechanisms, Regulatory Pathways, and Consequences. Front. Cell Dev. Biol. 2021, 9, 618135. [Google Scholar]
- Simons, M.; Gault, W.J.; Gotthardt, D.; Rohatgi, R.; Klein, T.J.; Shao, Y.; Lee, H.-J.; Wu, A.-L.; Fang, Y.; Satlin, L.M.; et al. Electrochemical cues regulate assembly of the Frizzled/Dishevelled complex at the plasma membrane during planar epithelial polarization. Nat. Cell Biol. 2009, 11, 286–294. [Google Scholar] [PubMed]
- Hari, L.; Brault, V.; Kléber, M.; Lee, H.-Y.; Ille, F.; Leimeroth, R.; Paratore, C.; Suter, U.; Kemler, R.; Sommer, L. Lineage-specific requirements of beta-catenin in neural crest development. J. Cell Biol. 2002, 159, 867–880. [Google Scholar]
- Lee, H.Y.; Kleber, M.; Hari, L.; Brault, V.; Suter, U.; Taketo, M.M.; Kemler, R.; Sommer, L. Instructive role of Wnt/beta-catenin in sensory fate specification in neural crest stem cells. Science 2004, 303, 1020–1023. [Google Scholar]
- Doyen, D.; Poët, M.; Jarretou, G.; Pisani, D.F.; Tauc, M.; Cougnon, M.; Argentina, M.; Bouret, Y.; Counillon, L. Intracellular pH Control by Membrane Transport in Mammalian Cells. Insights Into the Selective Advantages of Functional Redundancy. Front. Mol. Biosci. 2022, 9, 825028. [Google Scholar]
- Ritter, J.M.; Doktor, H.S.; Benjamin, N. Paradoxical effect of bicarbonate on cytoplasmic pH. Lancet 1990, 335, 1243–1246. [Google Scholar]
- Goldsmith, D.J.; Forni, L.G.; Hilton, P.J. Bicarbonate therapy and intracellular acidosis. Clin. Sci. 1997, 93, 593–598. [Google Scholar]
- Persi, E.; Duran-Frigola, M.; Damaghi, M.; Roush, W.R.; Aloy, P.; Cleveland, J.L.; Gillies, R.J.; Ruppin, E. Systems analysis of intracellular pH vulnerabilities for cancer therapy. Nat. Commun. 2018, 9, 2997. [Google Scholar] [PubMed]
- Harguindey, S.; Arranz, J.L.; Polo Orozco, J.D.; Rauch, C.; Fais, S.; Cardone, R.A.; Reshkin, S.J. Cariporide and other new and powerful NHE1 inhibitors as potentially selective anticancer drugs—An integral molecular/biochemical/metabolic/clinical approach after one hundred years of cancer research. J. Transl. Med. 2013, 11, 282. [Google Scholar]
- Bogdanov, A.; Bogdanov, A.; Chubenko, V.; Volkov, N.; Moiseenko, F.; Moiseyenko, V. Tumor acidity: From hallmark of cancer to target of treatment. Front. Oncol. 2022, 12, 979154. [Google Scholar]
- Helmy, M.M.; Ruusuvuori, E.; Watkins, P.V.; Voipio, J.; Kanold, P.O.; Kaila, K. Acid extrusion via blood-brain barrier causes brain alkalosis and seizures after neonatal asphyxia. Brain 2012, 135 Pt 11, 3311–3319. [Google Scholar]
- Bhuiyan, S.A.; Xu, M.; Yang, L.; Semizoglou, E.; Bhatia, P.; Pantaleo, K.I.; Tochitsky, I.; Jain, A.; Erdogan, B.; Blair, S.; et al. Harmonized cross-species cell atlases of trigeminal and dorsal root ganglia. Sci. Adv. 2024, 10, eadj9173. [Google Scholar] [PubMed]
- Avraham, O.; Chamessian, A.; Feng, R.; Yang, L.; Halevi, A.E.; Moore, A.M.; Gereau, R.W.I.; Cavalli, V. Profiling the molecular signature of satellite glial cells at the single cell level reveals high similarities between rodents and humans. Pain 2022, 163, 2348–2364. [Google Scholar] [PubMed]
- Song, D.; Li, B.; Yan, E.; Man, Y.; Wolfson, M.; Chen, Y.; Peng, L. Chronic treatment with anti-bipolar drugs causes intracellular alkalinization in astrocytes, altering their functions. Neurochem. Res. 2012, 37, 2524–2540. [Google Scholar] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Kang, W.; Zhang, J.; Xu, J.; Wang, R.; Xiao, X.; Wei, C.; Yu, W.; Lu, J. Valproic Acid Promotes the Differentiation of Satellite Glial Cells into Neurons via the pH-Dependent Pathway. Biomolecules 2025, 15, 986. https://doi.org/10.3390/biom15070986
Wang D, Kang W, Zhang J, Xu J, Wang R, Xiao X, Wei C, Yu W, Lu J. Valproic Acid Promotes the Differentiation of Satellite Glial Cells into Neurons via the pH-Dependent Pathway. Biomolecules. 2025; 15(7):986. https://doi.org/10.3390/biom15070986
Chicago/Turabian StyleWang, Dongyan, Wenrun Kang, Jinhui Zhang, Jianwei Xu, Ruyi Wang, Xiangdan Xiao, Chao Wei, Wenfeng Yu, and Junhou Lu. 2025. "Valproic Acid Promotes the Differentiation of Satellite Glial Cells into Neurons via the pH-Dependent Pathway" Biomolecules 15, no. 7: 986. https://doi.org/10.3390/biom15070986
APA StyleWang, D., Kang, W., Zhang, J., Xu, J., Wang, R., Xiao, X., Wei, C., Yu, W., & Lu, J. (2025). Valproic Acid Promotes the Differentiation of Satellite Glial Cells into Neurons via the pH-Dependent Pathway. Biomolecules, 15(7), 986. https://doi.org/10.3390/biom15070986






