Abstract
Plant parasitic nematodes cause huge economic losses to agriculture and forestry every year, and chemical insecticides destroy the ecological environment. Researching the mechanism by which small-molecule signaling substances regulate nematode behavior and development is important for developing environmentally friendly biological control agents. Nematode pheromones are essential chemicals signaling intraspecies and interspecies communication, regulating development, reproduction, and social behavior. Their structural diversity enables ecological adaptation and cross-kingdom interactions, influencing fungal predation and plant immunity. This review focuses on the classification, function, and regulatory mechanisms of nematode pheromones, interspecific signal transmission, and biosynthesis pathways. We pay special attention to their potential as environmentally friendly biological control agents as well as the challenges currently encountered in their application.
1. Introduction
Chemical communication, a ubiquitous information transfer mechanism, is particularly critical for Nematoda [1]. Plant-parasitic nematodes impose substantial ecological and economic burdens, contributing to over USD 175.0 billion in annual crop losses worldwide through direct parasitism and soil ecosystem degradation [2]. As chemical signaling mediates diverse ecological interactions (e.g., pheromones [3,4,5], plant volatiles [6,7,8,9], microbial cues [10,11,12,13]), nematode-derived signaling molecules, especially ascarosides, have emerged as pivotal targets for sustainable pest control due to their functional versatility. These evolutionarily conserved molecules precisely modulate nematode behavior (e.g., aggregation, dispersal) and development (e.g., Dauer formation), as well as cross-species interactions (e.g., plant immune activation) [3,4,5,14], as shown in Figure 1. Deciphering the molecular mechanisms underlying these chemical signals will provide a crucial theoretical foundation for developing novel strategies in agricultural pest control, environmental restoration, and biomedical applications.
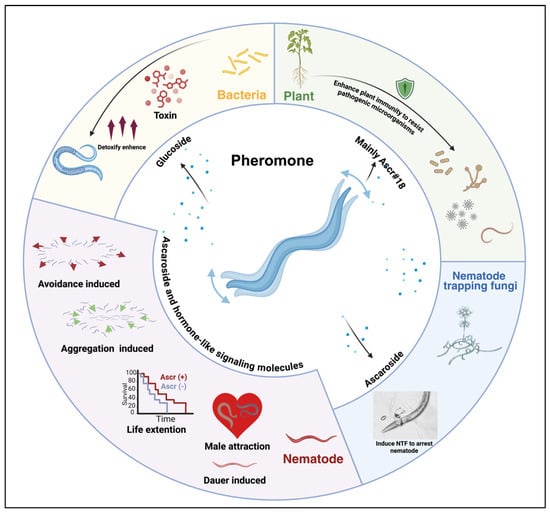
Figure 1.
Nematode pheromones as key mediators of behavior, development, and ecological interactions.
Nematodes orchestrate intra- and interspecies interactions through ascarosides, structurally diverse glycoside–lipid hybrids that function as multimodal signaling molecules. These compounds mediate critical ecological processes, population dynamics (e.g., ascr#3/5-driven aggregation vs. ascr#2-induced dispersal), reproductive strategies (male chemotaxis by ascr#3), and developmental plasticity (Dauer formation via DAF-7/TGF-β) [15,16]. Notably, plants and microbes exploit ascarosides for defense modulation (e.g., ascr#18-triggered jasmonate immunity), suggesting coevolutionary arms races [17,18,19]. The conservation of DAF/TGF-β and insulin pathways across species positions ascarosides as prime targets for sustainable nematode control and aging research.
In this review, we comprehensively examine nematode pheromones. We focus on their classification, functional roles, autoregulatory mechanisms, interspecies signaling, and biosynthesis pathways. Special attention is given to their potential as eco-friendly biocontrol agents, alongside the current challenges in their application. A key future research direction involves the identification of novel nematode signaling molecules and the investigation of their functions and regulatory mechanisms. Such efforts will expand the repertoire of known nematode pheromones and their regulatory networks. This will establish a discipline studying nematode signaling molecule-based biocontrol ecology and lay the foundation for developing environmentally friendly biocontrol formulations.
2. Nematode Pheromone-Mediated Communication in Ecosystems
Chemical communication releases signaling molecules (e.g., pheromones, secondary metabolites) that diffuse into the air, water, or soil, which are detected by other organisms and processed by them to elicit behavioral, physiological, or ecological responses [20]. This form of communication includes interactions between individuals and groups. Individual-level regulation involves mate selection, territorial marking, aggregation, and danger warnings, which involve signals such as sex pheromones, aggregation pheromones, and alarm pheromones [21]. Plant–insect or microbe–host interactions are common instances of interspecies communication, which plays an important role in the regulation of ecosystem functions [22,,23]. Nematodes release glycoside pheromones to coordinate individual behavior and group interaction [24]. Chemical communication regulates predator–prey relationships, interspecies competition, and symbiotic associations. For instance, predatory fungi on nematodes intercept nematode-released pheromones to form infection structures that capture nematodes, thereby balancing fungal and nematode populations in soil habitats [25,26,27]. Similarly, when Meloidogyne incognita infect plants, the plants detect long-chain ascarosides released by the nematodes and metabolically edit them into clusters of short-chain ascarosides that inhibit nematode activity [19,28]. Chemical communication is of paramount importance for various organisms, from simple to complex.
3. Classification and Function of Nematode Pheromones
An important role of nematode pheromones is to regulate development, reproduction, social behavior, and population dynamics through small-molecule chemical signals produced and secreted by nematodes. They also serve as a language system for communication across species and between species [24,29,30,31], as shown in Table 1. Therefore, pheromone-mediated conserved chemical communication is critical for the survival and reproduction of nematodes [32].

Table 1.
Classification and function of nematode pheromones.
3.1. Nematode Ascaroside Pheromones
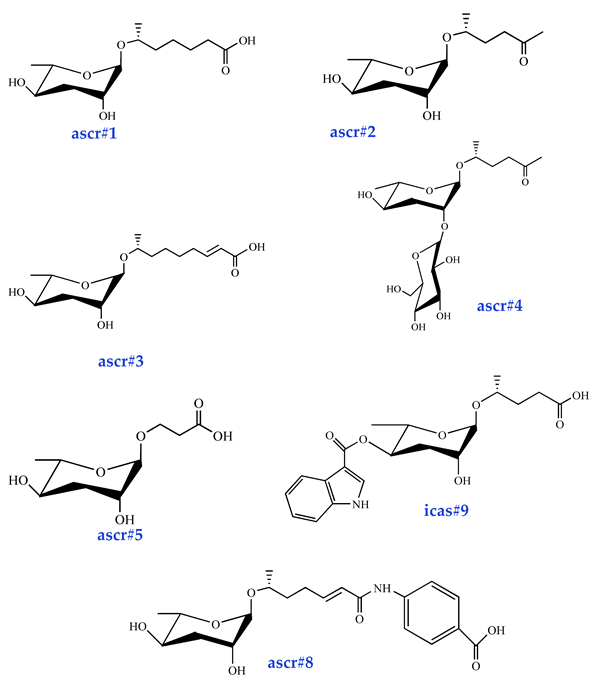
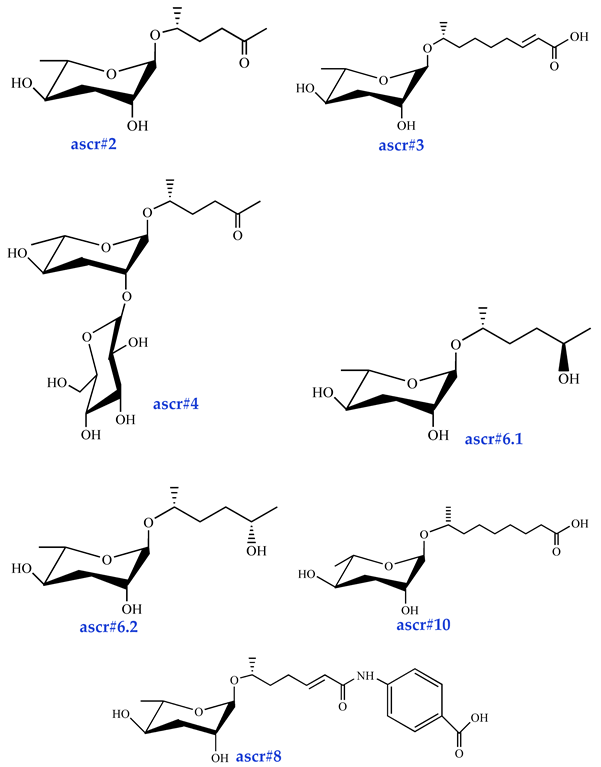
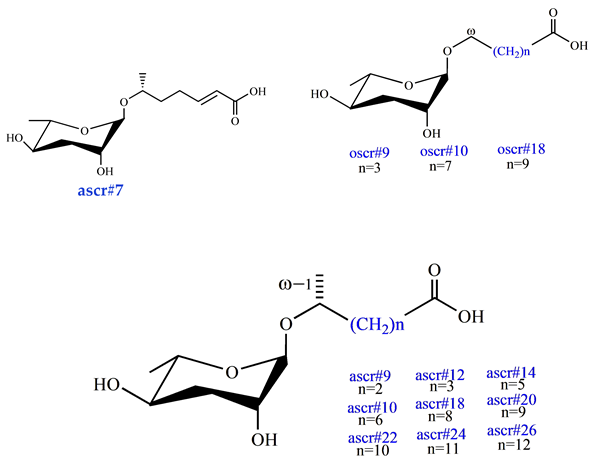
Ascarosides are the primary constituents of nematode pheromones, representing a class of highly conserved small-molecule signaling compounds. Their chemical structure consists of a glycuronic sugar (3,6-dideoxy-L-ascarylose) linked to fatty acid-derived side chains of varying lengths, which are further modified with additional moieties derived from amino acids, folate, and other primary metabolites, forming a diverse and extensive signaling system (Figure 2). To date, more than 200 distinct ascarosides have been identified across over 20 species of free-living and parasitic nematodes, with their biological functions spanning developmental regulation and social behaviors [17,47]. The chemical composition, structural diversity, and relative abundance of ascarosides vary significantly depending on developmental stage, lifestyle, and species [48,49,50]. The model organism Caenorhabditis elegans has been instrumental in elucidating the nematode pheromone network. Comparative genomics and metabolomics studies have revealed that the modular assembly of ascaroside derivatives endows them with functional diversity in biological activity [51]. In addition, mammals, plants, and microorganisms share a biosynthetic pathway that produces ascaroside-like signals. These organisms can also diversify the nematode ascaroside signaling system, generating structurally distinct variants [52].
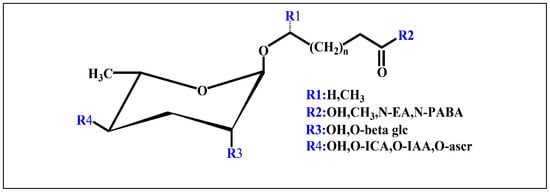
Figure 2.
Basic structure of ascarosides.
3.2. Nematode Glucoside Pheromones
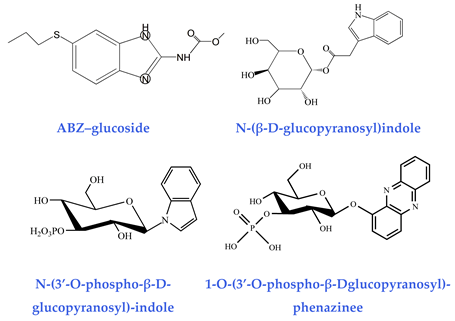
As well as ascaroside pheromones, nematodes can produce hundreds of glucosides by conjugating different sugar molecules (e.g., glucose, galactose) with certain fatty acids. Representative examples include ABZ–glucoside generated during xenobiotic detoxification [41], indole–glucoside produced from bacterial indole metabolism [42], and phenazine glucoside synthesized in response to the pathogenic toxin 1-hydroxyphenazine (1-HP) from Pseudomonas aeruginosa [43]. The glycosylation-mediated conjugation of xenotoxic small molecules into glycoside derivatives constitutes a crucial survival strategy for nematodes. Recent metabolic and biosynthesis studies demonstrate that C. elegans and other nematodes employ a streamlined assembly strategy involving ester/amide bond formation. This is performed to generate numerous modular glucoside metabolites, using glycosides as central scaffolds. Some of these compounds have been functionally validated as signaling molecules that modulate nematode behavior and development [53].
3.3. Other Nematode Pheromones
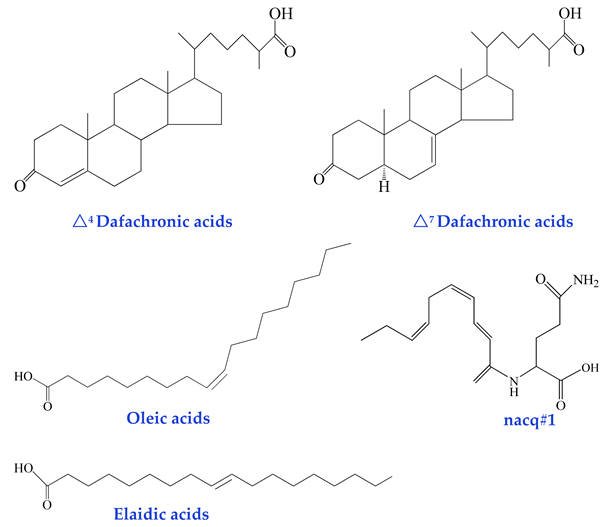
In recent years, several non-glycosidic compounds have been identified as pheromones, including dafachronic acids, unsaturated fatty acids, and other small-molecule metabolites [54,55]. Dafachronic acids are derived from 3-keto-cholenoic acid and feature a terminal carboxylic acid group on the sterol side chain, structurally resembling bile acids and steroid hormones. These compounds function as ligands for the DAF-12 nuclear receptor, playing a pivotal role in regulating nematode life cycles, particularly in Dauer larva formation and recovery. Thus, they represent a class of hormone-like signaling molecules critically involved in developmental and behavioral modulation [44].
Emerging evidence indicates that unsaturated fatty acids (UFAs), primarily derived from the lipid metabolism and peroxisomal β-oxidation pathways, actively participate in nematode lifespan modulation [45]. Nematodes also secrete amino acid-conjugated long-chain fatty acids as signaling molecules in response to dynamic social environments. Male nematodes (and to a lesser extent, hermaphrodites) secrete nacq#1—a small-molecule signaling substance belonging to the N-acylated glutamine class—which regulates a conserved gene regulatory network via chemosensory pathways and nuclear hormone receptors (NHRs). This signaling cascade counteracts diapause, accelerates developmental progress, and promotes sexual maturation, albeit at the cost of a reduced lifespan. At concentrations ranging from picomolar (pM) to low nanomolar (nM), nacq#1 significantly enhances reproductive development, particularly during the transition from the L4 larval stage to young adulthood (YA), resulting in a ~30% increase in egg-laying output on the first day of reproduction [46]. Notably, nacq#1 exhibits an antagonistic relationship with ascaroside pheromones (e.g., acr#2 and acr#3) in governing nematode reproductive strategies: while nacq#1 promotes accelerated reproductive, Ascarosides induce developmental arrest (Dauer formation). Together, these small-molecule systems fine-tune the Dauer development trade-off, ensuring adaptive responses to environmental cues. Intriguingly, nacq#1 is evolutionarily conserved and detected across diverse animal phyla, including human sweat. This suggests that amino acid-derived metabolites may serve as universal chemo-effectors to modulate physiological processes. This functional conservation implies that such signals likely operate through specialized neural circuits. This highlights potential parallels in the inter- and intra-tissue communication mechanisms between vertebrates and invertebrates. Furthermore, nematodes employ fatty acid metabolism and the mevalonate pathway to biosynthesize an array of non-glycosylated small-molecule signals, including fatty acids, terpenoids, and peptides. These compounds are typically labile and volatile, enabling rapid response coordination in dynamic environments [56].
Nematodes exhibit remarkable genome size variation (tens of Mb to >1 Gb), with plant-parasitic species typically exceeding 80 Mb while C. elegans maintains a compact ~100 Mb genome [57,58]. Hundreds of signaling molecules—particularly ascarosides—have been identified via MS/MS and GC-EI/MS. Their bioactivity is finely modulated by structural variations (e.g., side chain length, ascarylose linkage, and terminal modifications, as seen in ascr#18-ascr#25 and hbas#3/mbas#3) and they typically function as concentration-dependent mixtures [59,60,61]. Despite the genomic complexity observed in nematodes and other model organisms (Drosophila, mice), known signaling chemicals remain limited, underscoring the need for integrated comparative genomics, metabolomics, and trace compound isolation to systematically unravel nematode pheromone systems.
4. Regulatory Functions and Mechanisms of Nematode Pheromones
Nematode pheromones, characterized by highly conserved chemical structures, serve as essential signaling molecules for inter- and intraspecies communication [30,40]. These pheromones exert multifaceted regulatory effects throughout the nematode life cycle, modulating behaviors such as aggregation, avoidance, developmental progression, and reproduction [24,61].
4.1. Regulation of Nematode Behavior by Pheromones
The chemical modulation of nematode social behaviors—including chemotaxis, avoidance, aggregation, dispersal, and foraging—represents a critical research focus in neurobiology and chemical ecology, with significant implications for pest control strategies [62,63]. Chemotactic interactions, such as attraction and repulsion, are mediated by 12 pairs of specialized chemosensory neurons in C. elegans’ head, as shown in Table 2 [64,65]. Each pair expresses distinct receptors that recognize specific pheromone molecules, activating downstream signaling cascades to generate appropriate behavioral outputs, as shown in Figure 3 [65,66]. Among nematode pheromones, ascr#8 has been identified as the principal male-attracting chemosignal [37]. Notably, nematodes employ complex behavioral strategies involving synergistic effects among multiple pheromone components, demonstrating the functional versatility of these chemical mediators. The ascaroside pheromones (ascr#2, ascr#3, and ascr#4) exhibit potent male attractant activity at sub-Dauer-inducing concentrations, with this response mediated by the cephalic chemosensory neurons ASK and CEM [55]. Genetic evidence indicates that mutations in NPR-1, encoding a neuropeptide Y receptor homolog, enhance nematode responsiveness to specific Ascarosides while attenuating aversive behaviors [67]. The RMG interneuron functions as a central processing unit in NPR-1-mediated signaling by integrating multiple sensory inputs to differentiate between attractive and aggregative chemical cues, depending on molecular specificity and concentration profiles [68].

Table 2.
Sensory neurons, receptors, and signal transduction pathways in C. elegans.
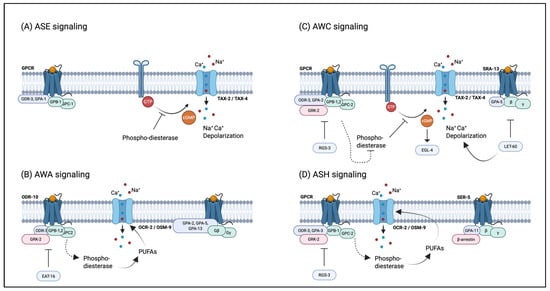
Figure 3.
Signal transduction pathways of major chemosensory neurons in C. elegans. (A) Signaling of the water-soluble attractant neuron ASE; (B) signaling of the volatile attractant neuron AWA; (C) signaling of the volatile attractant neuron AWC; (D) signaling of the volatile attractant neuron ASH.
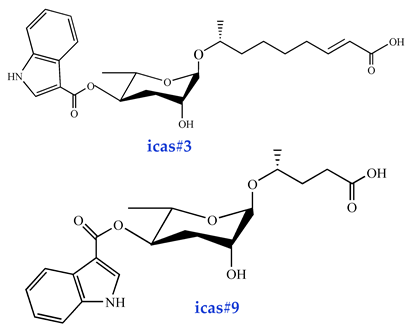
Moreover, both food availability and TGF-signaling perturbations can drive aggregation behavior in nematodes. The Indoleascarosides (icas#3, icas#9, icas#10, icas#1, icas#7) function as pheromonal regulators of this social behavior [35,37]. Notably, icas#3 exhibits strong attraction toward hermaphroditic nematodes at exceptionally low concentrations (1 pM). Structurally, icas#3 closely resembles ascr#3 but contains an additional indole moiety derived from tryptophan. Despite this similarity, their behavioral effects differ significantly: while ascr#3 attracts male nematodes, it repels hermaphrodites, highlighting distinct sex-specific regulatory roles [16]. Both indole glycosides and indole ascarosides modulate nematode behavioral responses through the NPR-1 receptor, further underscoring its pivotal role in pheromone-mediated neuroregulation [68].
Amphid single-ciliated chemosensory neuron type K (ASK) plays a critical role in mediating behavioral responses in C. elegans. Previous studies have demonstrated that ASK neurons are essential for behavioral responses to non-indole ascarosides in both males and hermaphrodites [68]. ASK sensory neurons form gap junction connections with the RMG interneuron. This interneuron functions as a central hub to integrate inputs from ASK and other sensory neurons to regulate aggregation and related social behaviors [69]. However, icas#3-mediated behavioral modulation operates independently of RMG but requires the AIA interneuron. Thus, the ASK-RMG circuit transduces ascr-type ascarosides, while the ASK-AIA pathway processes indole-modified ascarosides to govern attraction and aggregation behavior.
4.2. Dauer Larva Induction and Regulatory Mechanisms
Each nematode undergoes four distinct larval stages, with each transition marked by the development of a new cuticle and the molting of the previous cuticle [70,71]. Dauer larvae constitute an evolutionarily conserved developmental adaptation that has emerged as a specialized survival and dispersal strategy within the Rhabditida order and numerous parasitic nematode species [72,73]. This metabolically quiescent, development-arresting state is triggered by environmental stressors, including nutrient deprivation, thermal stress, or high population density [74]. The Dauer phase of nematodes is characterized by profound physiological changes, including changes in metabolism, cell stress tolerance, and lifespan extension. Collectively, these adaptations allow long-term persistence (spanning months or longer) under hostile conditions until favorable environmental cues reactivate development [75].
The regulation of Dauer entry and exit remains a central focus in developmental biology, and inhibiting these processes serves as a crucial target for nematode control [73]. Besides unfavorable environmental cues, ascarosides also play a vital role in Dauer formation. The concept of nematode Dauer pheromones was proposed by Golden and Riddle in 1982 [76], but its chemical identity was not determined until 2005 when the bioactivity-guided purification of ascr#1 revealed its identity. However, its active concentrations were toxic [33]. By 2008, ascr#2 and ascr#3 were demonstrated to exhibit potent Dauer-inducing activity at nM–µM levels, with a 10 nM equimolar mixture significantly increasing Dauer formation in populations [33,77]. Furthermore, ascr#5 synergizes with ascr#2 and ascr#3 to enhance Dauer induction [34,36,76]. Notably, while ascr#2 and ascr#3 have also been identified in other nematodes (e.g., Caenorhabditis briggsae), only ascr#2 is required to trigger Dauer formation in this species [78].
Nematodes’ entry into the Dauer stage involves complex interactions between multiple signaling pathways. These interactions are primarily triggered by insulin and TGF-signaling downregulation to initiate Dauer development [18]. Under environmental stressors such as crowding, food limitations, or elevated temperature, the chemosensory neurons of ASK detect changes in the pheromones ascr#2 and ascr#3 via a pair of pheromone receptors, SRBC-64 (sterpentine receptor, class BC-64) and SRBC-66, located at the ciliary tips. These receptors activate the inhibitory G protein GPA-3, which interacts with membrane-associated guanyl cyclase DAF-11 to convert GTP into cGMP. The TAX-2/TAX-4 cyclic nucleotide-gated (CNG) ion channels then transduce cGMP levels into an ionic influx, leading to membrane depolarization and subsequent Ca2+ and Na+ entry. This cascade activates downstream endocrine signaling pathways that modulate TGF-β and insulin-like signaling [79]. In C. elegans, mutations in srbC-64, srbC-66, or gpa-3 disrupt the nematode’s ability to respond to ascaroside pheromones (C6, C9, C7) and enter the Dauer stage. Since daf-7 (a TGF-β ortholog) negatively regulates Dauer formation, exposure to Dauer-inducing pheromones downregulates the expression of the GPCR str-3 in ASI neurons [80,81]. Thus, the G protein GPA-3 signaling pathway orchestrates the transcriptional regulation of downstream genes governing Dauer formation. Downstream from pheromone signaling, reduced cGMP levels suppress the production of insulin-like peptides (ILPs) and TGF-β, promoting the nuclear translocation of DAF-16/FOXO and the DAF-3/DAF-5/SMAD complex. This inhibits the biosynthesis of steroid ligands for DAF-12, a nuclear hormone receptor homologous to mammalian steroid receptors. Consequently, the unliganded DAF-12 binds to its corepressor DIN-1/SHARP, activating Dauer-specific gene expression while directly or indirectly suppressing hormone biosynthesis-related genes. This transcriptional reprogramming ultimately prevents development at the Dauer stage [18]. Furthermore, Dauer’s entry and exit are regulated by steroid hormone signaling pathways, which are crucial for fully exiting developmental arrest and actively promote this process. Notably, the spatiotemporal dynamics of steroid hormone regulation during exit from developmental arrest resemble those observed during entry into developmental arrest [82].
4.3. Pheromonal Regulation of Nematode Mating Behavior
Ascarosides serve as key chemosensory signals mediating intra- and interspecific communication in nematodes, which are primarily responsible for sexual recognition. An ascr#2, ascr#3, and ascr#4 mixture at ultra-low femtomolar (fM) to picomolar (pM) concentrations attracts males in the nematode population [16,83]. The relative abundance of these ascaroside molecules varies significantly, with individual compound assays revealing that ascr#3 exhibits stronger male-attracting activity than ascr#2. Interestingly, this contrasts sharply with their roles in Dauer formation. Ascr#2 demonstrates higher potency than ascr#3 in inducing Dauer developmental arrest. Conversely, male nematodes attract hermaphrodites through indole carboxyl (IC)-modified ascarosides (ICAS#). Structurally, these IC-ascarosides are characterized by an indole-3-carbonyl moiety conjugated to the C-4 position of the ascarylose sugar. Behavioral assays demonstrated that icas#1, icas#3, and icas#9 effectively elicit hermaphrodite attraction, with icas#3 and icas#9 exhibiting particularly pronounced effects at 100 fM concentrations [16]. Furthermore, IC-ascarosides (fM-pM) significantly enhance aggregation behaviors in both hermaphroditic and male nematodes.
The male-attracting effects of ascr#2, ascr#3, and ascr#4 are mediated by two specific sensory neurons: the cephalic companion neurons (CEM) and the amphid single-cilium sensory neuron K (ASK) in males. As previously described, ASK neurons regulate Dauer entry through G protein-coupled signaling, while indoleascarosides modulate nematode aggregation behaviors via responses in ASK and its downstream interneuron AIA. Thus, nematodes coordinate alternative behavioral outputs—reproduction vs. developmental arrestthrough shared chemosensory pathways that process overlapping ascaroside signals [16]. This neural mechanism enables the context-dependent modulation of population dynamics by the same family of signaling molecules.
In response to P. aeruginosa, the hermaphroditic C. elegans AWA sensory neurons are inhibited, leading to str-44 induction within these neurons. The AWA neurons are regulated by the transcription factor zip-5, which is upregulated by the pathogen. STR-44 is a pheromone receptor that inhibits the avoidance of pheromones ascr#2, ascr#3, and ascr#5. Its activity in AWA neurons reduces pheromone responses, increasing the likelihood of mating with males [84].
4.4. Pheromone-Mediated Modulation of Nematode Lifespan
C. elegans lifespan extension is regulated through the DAF-16/insulin signaling pathway and the DAF-12 pathway. However, the Schroeder research team introduced the concept of ascr-mediated increase in lifespan (AMILS), demonstrating that the ascaroside receptor DAF-37, upon activation by the Dauer phenomone ascr#2, extends nematode lifespan by nearly 20% through a Srtuin-dependent mechanism [85]. Unlike the insulin/IGF-1 pathway, which governs Dauer formation, AMILS promotes longevity without compromising reproductive fitness or feeding rates. This suggests a distinct regulatory mechanism. Additionally, the N-acyl glutamine-derived metabolite nacq#1, excreted by nematodes, accelerates reproductive development in C. elegans by hastening sexual maturation in hermaphrodites and shortening lifespan. Intriguingly, this nacq#1-induced developmental acceleration depends on chemosensory perception mediated by conserved nuclear hormone receptors (NHRs). Notably, structurally similar fatty-acylated amino acid derivatives have been identified in both vertebrates and invertebrates, implying that related signaling compounds may function as evolutionarily conserved endocrine regulators across metazoans [46].
5. Nematic Pheromones Mediate Interspecies Interactions
Beyond functioning as “self-beneficial compounds” to regulate nematode development and stress resistance, accumulating evidence reveals that nematodes employ highly conserved ascaroside pheromones for “cross-kingdom communication”, predominantly occurring in soil ecosystems where they interact with other organisms, such as nematode–fungi [86], nematode–bacteria [87,88], and nematode–plant systems [89,90,91]. Consequently, through long-term coevolutionary interactions, plants, animals, and microorganisms have developed conserved genetic mechanisms to perceive and respond to nematode pheromones, playing a pivotal role in maintaining soil communities’ ecological equilibrium [92].
5.1. Nematode Pheromones Modulate the Interaction Between Nematodes and Fungi
In ecosystems, predator recognition of specific pathogens or food resources plays a crucial role in predator–prey coevolution. Nematophagous fungi and nematodes represent a well-documented interaction system in soil environments. With over 380 reported species, nematophagous fungi are widely distributed and have evolved a conserved coexistence with ubiquitous nematodes [93]. These fungi employ diverse predatory structures—such as adhesive networks, constricting rings, and sticky knobs—to capture and digest nematodes for nutrients [94,95]. Nematode-derived ascarosides, a class of small-molecule pheromones, have been identified as key signaling molecules that induce fungal predatory structures. Through long-term coexistence with nematodes, fungi have evolved specialized molecular recognition mechanisms to detect nematode presence and initiate trap formation. Arthrobotrys oligospora, a model nematophagous fungus, responds to live nematodes and specific ascarosides (e.g., ascr#5, ascr#7, ascr#18) by producing adhesive networks for nematode capture [27]. Notably, both native and parasitic nematodes secrete ascarosides [40], suggesting that fungal–nematode interactions represent an evolutionarily conserved paradigm, offering foundational insights into nematode interactions with other organisms [96].
In two predacious fungi species (A. oligospora and Arthrobotrys flagrans), the mechanism by which G protein-coupled receptors (GPCRs) respond to nematode pheromones to trigger trap formation has been elucidated. In A. flagrans, the GPCR GprC exhibits dual localization on both the plasma membrane and the outer mitochondrial membrane. Mitochondrial GprC enhances respiratory metabolism. Upon nematode presence, GprC binds to the G protein α subunit GasA, initiating downstream signaling to activate fungal predatory behavior. Notably, spatial heterogeneity exists in GprC-GasA interactions: at the hyphal tip, their interaction primarily occurs on mitochondrial membranes, whereas at the hyphal base, it predominantly occurs on the plasma membrane. This spatial regulation may reflect functional specialization across the hyphal regions. Under nematode exposure, the GprC-GasA pathway suppresses the expression of the nuclear gene artA, inhibiting the production of the secondary metabolite 6-methylsalicylic acid (6-MSA). Concurrently, nematodes induce an increase in intracellular ROS levels and oxygen consumption, a process dependent on the GprC-GasA axis. Enhanced respiratory metabolism via this pathway likely provides energy for fungal predation. Unlike typical G protein signaling that relies on downstream MAPK or cAMP-PKA central pathways, the GprC-GasA pathway operates independently. Intriguingly, A. flagrans GprC shares conserved ascr# (ascarosides)-binding motifs with nematode GPCRs (SRBC64 and SRBC66). Functional complementation experiments demonstrated that replacing the nematode receptor’s last three transmembrane domains with those of GprC restored predation defects in GprC-deficient fungi, suggesting convergent evolution between fungal and nematode GPCRs [97,98].
The nematophagous fungus A. oligospora detects nematode-specific pheromones (ascarosides) through specialized G protein-coupled receptors (Gpr1-like GPCRs), triggering the formation of predatory structures. Concurrently, the additional 69 Pth11-like GPCRs in this fungus sense other small-molecule signals derived from nematodes. Upon exposure to C. elegans, several GPCRs are upregulated, particularly prior to trap development. Specifically, the glucose-sensing receptors Gpr2 and Gpr3 form a complex with the Gα subunit Gpa2, activating the cAMP-PKA signaling pathway to regulate trap morphogenesis. This intricate fungal–nematode interaction system reveals a sophisticated cross-kingdom communication mechanism, wherein fungal GPCRs serve as key mediators of predator–prey recognition. These findings highlight the potential for targeting nematophagous fungal GPCRs in developing novel biocontrol strategies against parasitic nematodes [25]. Moreover, A. oligospora produces various volatile compounds that attract nematodes, such as methyl 3-methyl-2-butenoate (MMB), which interferes with the mating of nematodes. These volatile compounds are primarily recognized by G protein-coupled receptors (GPCRs) expressed in nematode AWCs [99].
5.2. Regulation of Nematode–Pathogen Interactions by Nematode Pheromones
Nematode pheromones play a crucial regulatory role in mediating interactions between nematodes and pathogenic microbes. For instance, P. aeruginosa PA14, upon infecting nematodes, induces the expression of the chemoreceptor STR-44 in AWA sensory neurons, thereby suppressing the nematode’s avoidance response to ascarosides (e.g., ascr#2, ascr#3). STR-44 functions as a pheromone receptor, with its expression regulated by the transcription factor ZIP-5 and the TRPV channel OCR-2, ultimately modulating behavioral responses. Interestingly, exposure to PA14 shifts nematode behavior from pheromone avoidance to attraction, promoting mating behavior—an effect potentially linked to pathogen virulence, as attenuated strains (e.g., PA14-ΔgacA) exert weak modulation [84]. Beyond pheromone detection, nematodes also sense pathogen-derived volatile compounds (e.g., 2-heptanone) via AW-Con neurons, activating the receptor STR-2. This process relies on PLC-mediated signaling rather than the canonical TRPV or cGMP-dependent pathways, demonstrating an alternative mechanism for pathogen avoidance behavior [100].
Pathogen-derived signaling molecules mediate transgenerational immune priming in nematodes. Parental worms perceive volatile compounds such as cyanide released by pathogenic bacteria, which activates the enzyme CYSL-2 to convert cyanide into β-cyanoalanine. This metabolite functions as a transgenerational signal transmitted to offspring, where it activates the transcription factors MDT-15 and SKN-1, thereby enhancing the expression of stress-responsive genes (e.g., gst-4, cysl-2) and improving offspring’s resistance to pathogens [101].
5.3. Nematode Pheromones Modulate Nematode-Plant Interactions
In plant–parasitic nematode interactions, ascarosides, present in phytoparasitic nematodes, can trigger immune responses in crops such as maize, rice, wheat, and soybean. Plants employ pattern recognition receptors (PRRs) to detect nematode-associated molecular patterns (NAMPs) invading nematodes. When the plant roots or leaf tissues are exposed to ascr#18 during nematode infection, this molecule acts as a defense signal that primes plant resistance against pathogen threats. Notably, ascr#18, which is highly abundant in many phytoparasitic nematodes, is currently the only reported plant-associated molecular pattern (pAMP) in this context. The leucine-rich repeat receptor-like kinase NILR1 (NEMATODE-INDUCED LRR-RLK 1) serves as the immune receptor for ascr#18 [102], mediating defense responses in both monocots and dicots (e.g., Arabidopsis, tomato, potato, and barley). Ascr#18-induced signaling enhances resistance against pathogens and pests, including root–knot interactions and cyst nematodes, by activating hallmark defense mechanisms. Upon perception, ascr#18 elicits canonical plant immune pathways, such as MAPK cascades, SA/JA phytohormone signaling, and defense-related gene expression [19,103]. This response strengthens plant resistance against diverse pathogens, including viruses, fungi, bacteria, oomycetes, and nematodes [104].
Notably, both microbial communities and host plants possess evolutionary-conserved capacities to perceive and catabolize nematode pheromones. Genetic evidence demonstrates that Arabidopsis metabolizes ascr#18 through the peroxisomal β-oxidation pathway, converting it into structural derivatives (e.g., ascr#9) that exhibit potent nematode-repellent activity [19]. Intriguingly, while unidentified proteinaceous components in nematode culture filtrates elicit similar defense responses, their chemical identities remain uncharacterized. Functional studies confirm that these unknown elicitors predominantly activate immunity through the leucine-rich repeat receptor-like kinase (LRR-RLK) pathway, with NILR1 serving as the central receptor module [103]. These findings collectively establish a mechanistic framework for developing pheromone-based biocontrol strategies against phytoparasitic nematodes [19,52,103,105].
5.4. Other Aspects of Communication Through Nematode Pheromones
Most parasitic nematodes possess an infective juvenile stage (IJ or IL3), which is critical for survival outside of the host. In Bursaphelenchus xylophilus, the L3 larvae produce ascarosides (asc-C5 and asc-ΔC6) that promote beetle pupation upon reaching the adult stage; the beetles synthesize fatty acid ethyl esters, which facilitate the nematodes’ development into fourth-stage (LIV) larvae. Notably, adult beetles secrete specific ascarosides (particularly asc-C9, ascr#10) that attract LIV nematodes, potentially inducing their entry into the beetle tracheal systems for subsequent transmission to new pine hosts [106].
Originally recognized as pheromone-like signaling molecules in nematodes, ascarosides have now been identified in diverse higher organisms, including the Japanese pine sawyer (Monochamus alternatus) and mice, highlighting their broad functional significance. Studies on B. xylophilus control revealed that the vector beetle M. alternatus produces structurally distinct ascarosides (e.g., asc-C9), synthesized via β-oxidation, which synchronize the developmental cycles of the nematode and beetle, reinforcing their symbiotic relationship. This coordination contributes to the widespread dispersal and epidemic outbreaks of pine wilt disease in China []. Furthermore, ascarosides delay beetle development during winter, aiding overwintering survival. In addition, they regulate the life history alignment of M. alternatus and B. xylophilus, exacerbating disease transmission [107,108].
Although ascarosides are highly conserved as small-molecule signals in nematodes, recent studies have uncovered their biosynthesis in plants, animals, and microbes, expanding their ecological and biological relevance. In plant-parasitic nematode interactions, ascarosides trigger immune responses in crops such as maize, rice, wheat, and soybean. Exposure of root or leaf tissues to ascr#18, a molecule analogous to microbial PAMPs, rapidly activates pattern-triggered immunity (PTI) via membrane-bound receptors like NEMATODE-INDUCED LRR-RLK1 (NILR1), triggering MAPK cascades, SA/JA signaling, and defense-related genes, a defense strategy exploited in Arabidopsis and tomato [19,103]. Plants metabolically process long-chain ascr#18 via β-oxidation to generate short-chain ascaroside clusters (ascr#10, ascr#1, and ascr#9), which enhance systemic immunity against pathogens such as Turnip crinkle virus, Pseudomonas syringae and PV, as well as tomatoes, fungi (Blumeria graminis f. sp. hordei, Phytophthora infestans), and nematodes (Heterodera schachtii, Meloidogyne incognita) []. These findings offer novel strategies for sustainable agriculture by reducing reliance on harmful agrochemicals, with implications for global food security.
Ascarosides govern diverse nematode behaviors (e.g., aggregation, avoidance) and developmental processes. Recent research employing optogenetics, RNAi screening, and genetic validation demonstrates that nematode pheromones can remodel neurodevelopment. The two most abundant pheromones, ascr#3 and ascr#10, are detected by chemosensory neurons ASK and ASI via specific receptors, respectively. These neurons subsequently release neuropeptides and glutamate, integrating signals into the interneuron AIA. This cascade suppresses nuclear translocation of the DAF-16/FOXO transcription factor in AIA, which then secretes insulin-like peptide INS-1 to non-autonomously activate the insulin signaling pathway across neurons, inhibiting macroautophagy and accelerating neurodegeneration. Notably, brief exposure to pheromones during juvenility primes adult nematodes for insulin pathway activation, marked by diminished autolysosome formation and impaired macroautophagy [109]. These insights provide a framework for understanding human neurodegenerative disorders, such as Alzheimer’s and Parkinson’s diseases.
6. Nematode Pheromone Biosynthesis
The biosynthesis of the two major types of nematode pheromones—ascarosides and glucosides—follows a similar modular assembly pattern: ascarylose, paratose, and glucose serve as core scaffolds, to which small molecules derived from primary metabolism (e.g., lipids, amino acids, neurotransmitters, and nucleotides) are attached [53,108]. Diverse combinatorial arrangements of these modules generate hundreds of structurally distinct ascarosides and glucosides [51]. A key advantage of modular assembly lies in its efficiency: a limited number of transferase genes or conserved biosynthetic pathways can produce a vast library of small-molecule compounds, enabling these metabolites to serve as versatile signaling molecules [110]. Identifying novel variants of primary metabolic derivatives that function as critical signals in C. elegans and other nematodes will provide a powerful impetus for comprehensively analyzing metabolism in higher animals.
6.1. Biosynthesis of Nematode Ascaroside
Ascaroside pheromone biosynthesis demonstrates remarkable evolutionary conservation, relying on the peroxisomal β-oxidation (PβO) pathway involving four key enzymes—ACOX-1, MAOC-1, DHS-28 and DAF-22 [17,31,40,61]—as shown in Figure 4. Among the primary fatty acid degradation pathways in eukaryotes, PβO generates metabolic byproducts, including heat, NADH, and either acetyl-CoA or propionyl-CoA. In nematodes, the synthesis begins with long-chain fatty acid precursors (approximately C30) that are progressively shortened through iterative cycles of PβO to generate ascaroside derivatives with C3-C11 side chains [111]. The biochemical pathway initiates with ACOX-1 (acyl-CoA oxidase) catalyzing the formation of α, β-unsaturated bonds in long fatty acid chains. This is followed by MAOC-1 (2-enoyl-CoA hydratase) mediating the hydration of these unsaturated bonds, DHS-28 (β-hydroxyacyl-CoA dehydrogenase) producing β-hydroxyacyl-CoA intermediates, and finally DAF-22 (thiolase) completing each β-oxidation cycle [39,112,113,114]. Each full cycle results in the removal of two carbon units from the fatty acid side chain through coordinated enzymatic actions: ACOX enzymes (functioning as homo- or heterodimers) convert α-β single bonds into double bonds; MAOC-1 hydroxylates the resultant double bonds; DHS-28 oxidizes β-hydroxyacyl-CoA to β-ketoacyl-CoA; and DAF-22 cleaves the thioester bond to terminate the cycle. The C. elegans genome contains seven ACOX genes (cel-acox-1.1 to cel-acox1.6 and cel-acox-3) that display distinct substrate specificities. The ACOX-1.1 homodimer preferentially acts on ascarosides with side chains of C9 or longer, while the ACOX-1.1/ACOX-3 heterodimer processes shorter chain substrates (C7 or less). The ACOX-1.2 homodimer regulates ω-ascaroside production for side chains containing fewer than 5 carbons [114,]. An interesting comparative observation comes from the Japanese pine sawyer beetle (M. alternatus), where the multifunctional enzyme Mfe2 simultaneously performs the biochemical roles of both MAOC-1 and DHS-28 [108]. Despite significant progress in understanding ascaroside biosynthesis, critical questions remain unanswered regarding the biosynthetic origin of long-chain ascaroside precursors and the precise mechanisms governing functional group assembly on ascaroside derivatives.
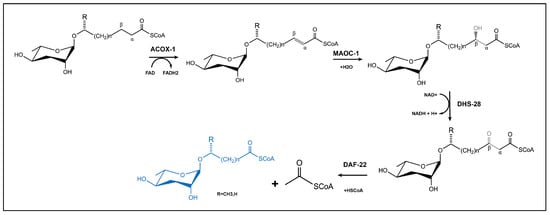
Figure 4.
β-oxidation pathway is involved in biosynthesis of ascarosides in nematodes.
In the β-oxidation pathway, functional specialization can be achieved between short-chain molecules (such as acr#3) and long-chain molecules (such as acr#18) [112]. Nematode behavior, such as aggregation, dispersal, Dauer formation, and sex ratio modulation, is controlled by this process. Importantly, β-oxidation integrates energy metabolism with signaling synthesis. For instance, food scarcity promotes the production of ascr#2/ascr#3, which activates the DAF-7/TGF-β pathway to induce Dauer larvae development. Moreover, this pathway mediates dynamic cross-species interactions: bacteria can degrade β-oxidation byproducts to disrupt nematode communication, while plants recognize specific short-chain ascarosides (e.g., ascr#18) to trigger immune responses. Notably, β-oxidation-derived end products (e.g., ascr#1) extend lifespan via the conserved DAF-16/FOXO pathway, highlighting their potential relevance in aging research. From a translational perspective, targeting β-oxidation enzymes (e.g., ACOX-1) to disrupt ascaroside biosynthesis offers novel strategies for nematode control, while the pathway’s metabolic reprogramming mechanisms provide a unique model for studying host–microbe interactions and longevity regulation. Ultimately, β-oxidation serves as a central metabolic hub that integrates chemical diversity, environmental adaptation, and interspecies communication, underpinning nematode ecological competitiveness and survival strategies.
6.2. Biosynthesis of Nematode Glucosides
Nematode glucosides are synthesized through the catalytic activity of UDP-glycosyltransferases (UGTs), which transfer a glucose moiety from uridine diphosphate glucose (UDP-glucose) to specific glycosyl acceptors, thereby forming the glucoside backbone structure. Identified acceptor molecules include indole, 3-aminobenzoic acid, phenylacetic acid, pyrrole-2-carboxylic acid, alkanes, isoprenoids, and others [53,108,115]. Although both ascarosides and glucosides are assembled modularly, the sugar donors for glucosides exhibit greater flexibility. This suggests that nematode glucosides may convey more complex and diverse biological signals [116]. UGTs are widely distributed across various nematode species, including parasitic nematodes such as Haemonchus contortus, B. xylophilus, and M. incognita [117]. The C. elegans genome encodes 67 UDP-glycosyltransferases, involved in the structural modification of endogenous and xenobiotic metabolites. These glucosylated products are further diversified by carboxylesterases, which regulate nematode behavior, development, and immune responses [118]. Although partial progress has been made in elucidating the biosynthesis of ascarosides and glucosides, their metabolic composition is dynamically influenced by habitat complexity, dietary sources, and nutritional status [119]. This highlights the remarkable adaptability of nematode glucoside production, serving as a critical strategy for nematodes to cope with nutrient fluctuations and environmental toxins.
Glucosylated glycosides are more commonly found in plants, representing a ubiquitous and important class of compound formed by the glycosidic linkage of glucose with other molecules (such as phenolics, steroids, terpenoids, etc.) [120]. These compounds play critical roles in plant growth, development, defense mechanisms, and ecological adaptation, with xenobiotic metabolism serving as a key contributor to the synthesis of glucosidic compounds in both plants and animals [121]. When C. elegans or parasitic nematodes such as Haemonchus contortus are exposed to a range of xenobiotic toxicants—including benzimidazole drugs (ABZ, mebendazole, fenbendazole, thiabendazole, and oxfendazole)—they rapidly upregulate Phase I and Phase II detoxification enzymes. Notably, a series of glycosyltransferase genes (ugt) are induced, leading to enhanced glucoside synthesis as a detoxification mechanism [122]. In nematodes, the partial inhibition of UGT activity can be achieved using the inhibitor chrysin, while the truncation of glucoside biosynthesis occurs upon the mutation of the transcription factor SKN-1 [123].
Genome-wide association studies (GWASs) have identified carboxylesterases as key enzymes facilitating the formation of ester and amide bonds, playing a pivotal role in mediating modular assembly [53]. Untargeted metabolomics enables the rapid discovery of glucoside derivatives, revealing their biosynthetic origin through the “hijacking” of conserved detoxification mechanisms. Modular metabolites represent an innovative biosynthetic strategy, repurposing cellular waste products to generate novel bioactive compounds that mediate critical biological functions. These metabolites contribute to the structural and functional diversification of pheromones and play essential roles in regulating behavior, development, and aging. Studies on C. elegans and other nematodes demonstrate their ability to repurpose biochemical degradation products in complex modular structures with diverse signaling functions. These structures incorporate neurotransmitters (e.g., tyramine, octopamine), indole derivatives, amino acids, nucleotides, and fatty acid metabolites linked to ascarosyl or glucosyl scaffolds. The attachment of diverse acyl groups at the C2 or C6 positions of glucose is mediated by enzymes such as Cel-cest-1.2, Cel-cest-4, and their homologs. The addition of a second acyl group to monoacylglucosides (MOGLs) to form diacylglucosides likely involves additional cest homologs, yielding hundreds of modular ascarosides and glucosides [53]. Wrobel et al. demonstrated that C. elegans carboxylesterase Cel-CEST-1.2 and C. briggsae carboxylesterase Cbr-CEST-2 collectively produce >150 modular glucosides, with 74 overlapping compounds [124]. This paradigm-shifting biochemical recycling mechanism fundamentally alters our understanding of animal and human metabolism, enabling the continuous generation of novel compounds under variable conditions. By accelerating the structural and functional annotation of unknown metabolites, this discovery opens new research avenues to explore over 100,000 uncharacterized chemical metabolites.
The insulin/IGF-1 and TGF-β signaling pathways promote the biosynthesis of cholesterol into dafachronic acids (DAs). Dafachronic acids—specifically Δ7-dafachronic acid (Δ7-DA) and Δ1,7-dafachronic acid (Δ1,7-DA)—serve as ligands for the nuclear hormone receptor DAF-12. Upon activation, DAF-12 governs the decision of whether C. elegans enters the Dauer diapause stage and regulates adult lifespan in response to gonadal signaling [125]. Among the dafachronic acids, the biosynthetic pathway of Δ7-DA is the most thoroughly characterized. It begins with the desaturation of cholesterol to 7-dehydrocholesterol, catalyzed by the Rieske-like oxygenase DAF-36. Subsequently, a Δ5-reductase (yet to be molecularly identified) is proposed in order to convert 7-dehydrocholesterol into lathosterol. This intermediate is then oxidized at the C-3 position by the short-chain dehydrogenase DHS-16 to yield lathosterone. Finally, DAF-9, a cytochrome P450 enzyme, oxidizes the side chain of lathosterone to a carboxyl group, completing Δ7-DA biosynthesis. In contrast, the synthesis of Δ4-dafachronic acid (Δ4-DA) remains poorly understood [126].
7. Application of Pheromones in Biological Control
Agricultural pheromones, characterized by their structural conservation, low toxicity, and high specificity, have emerged as a promising eco-friendly pest management approach [4,127]. Currently, insect pheromones are widely applied in agricultural and forestry pest control, while nematode pheromones (e.g., ascarosides) demonstrate considerable potential for developing novel biopesticides.
7.1. Pheromone-Based Pest Control
Currently, pheromone-based formulations consist of insect sexual pheromones or semiochemicals. These signals convey various signals relating to areas such as aggregation, foraging, mating, and alarm among insects, serving as their chemical and molecular language for communication. These compounds have been effectively employed as biocontrol agents for targeted pest management against agricultural pests, including the cotton bollworm (Helicoverpa armigera), tobacco cutworm (Spodoptera litura), and Japanese pine sawyer beetle (M. alternatus), with notable efficacy [128]. Furthermore, modern agriculture has integrated chemical ecology principles and CRISPR-based genetic engineering to introduce pheromone synthesis genes into plants, enabling the endogenous production of pest pheromones to recruit natural enemies. For instance, the ectopic synthesis of the aphid alarm pheromone (E)-β-farnesene in Arabidopsis enhances the foraging efficiency of aphid parasitoid wasps, while the introduction of (E)-β-caryophyllene synthase genes in corn attracts entomopathogenic nematodes to combat Western corn rootworm (Diabrotica virgifera) [129,130]. However, to date, the application of nematode pheromones to plant protection against parasitic nematodes has not yet been widely adopted or commercialized.
Insect sex pheromones have emerged as a cornerstone of modern integrated pest management (IPM) due to their exceptional species specificity, environmental safety, and operational efficiency. As a well-established international monitoring tool, they demonstrate particular efficacy against lepidopteran pests including Cydia pomonella, Grapholita molesta, and Spodoptera frugiperda, providing precise data for timely intervention strategies [131,132]. Their application extends to quarantine systems where tracking population dynamics of invasive species such as Hyphantria cunea and Lymantria dispar enables proactive risk mitigation. Beyond monitoring, pheromone-based control methods show remarkable field performance: mass trapping techniques effectively suppress populations of Helicoverpa armigera, Tuta absoluta, and C. pomonella through the direct removal of adult stages [133,134,135,136], while mating disruption strategies targeting G. molesta, Chilo suppressalis, and Cossus cossus achieve sustainable control by interfering with reproductive behavior [137,138,139,140]. This dual approach of population monitoring coupled with biologically targeted intervention establishes pheromone technology as both an environmentally benign and scientifically rigorous solution for contemporary agricultural pest challenges.
7.2. Pheromone-Based Nematode Control
Biological control ecology views nematode pheromones as an important tool for developing new nematode-based formulations for pest management. Studies have shown that ascaroside mixtures prepared from Caenorhabditis elegans can enhance the dispersal of Steinernema feliae infective juveniles (IJs) in Petri dish assays. Further greenhouse experiments revealed that these ascaroside mixtures not only promote nematode dispersal but also improve lethal efficiency against pecan weevils (Curculio caryae) and black soldier flies (Hermetia illucens) [141]. This suggests that nematode pheromones can enhance the biocontrol potential of entomopathogenic nematodes (EPNs), opening new avenues for their use in insect pest control. Research shows that EPN-infected host extracts, particularly ascaroside pheromones, improve dispersal, and efficacy of infective juveniles (IJs), which are critical factors for biocontrol success [49]. Recently, the U.S. agricultural biotech company Pheronym patented two nematode pheromone-based products—Nemastim™ and Pherocoat they plans to commercialize them for sustainable pest management by manipulating insect behavior [142,143]. Pheronym employs a natural communication platform by utilizing pheromones from commercially available beneficial nematodes to improve their efficacy. These pheromones optimize beneficial nematodes into more efficient predators while simultaneously deceiving parasitic nematodes into perceiving a lack of nearby food sources. This innovation facilitates the broader adoption of organic farming practices by providing an eco-friendly approach to agricultural pest control.
8. Challenges and Unresolved Issues in Nematode Pheromone Research
Establishing a comprehensive profile of nematode pheromones is a critical challenge in nematode pheromone research. These pheromones regulate diverse physiological responses, behavioral changes, or phenotypic adaptations at concentrations ranging from femtomolar to nanomolar. Even slight modifications in their chemical structure or functional groups can significantly alter their bioactivity. Traditional isolation methods are often inadequate for purifying individual pheromone compounds, as simple behavioral outputs in organisms are frequently governed by complex pathways or mixtures of signals. In fact, nematodes employ a sophisticated chemical communication strategy where multiple compounds act synergistically in a concentration-dependent manner, exhibiting diverse functional roles. This complexity renders conventional separation techniques unsuitable for pheromone identification. The first ascaroside pheromone, ascr#1, was isolated from 300 L of nematode culture through Dauer formation activity-guided fractionation, yielding only trace amounts [33]. Due to their extremely low secretion levels and functional reliance on synergistic interactions, isolating individual nematode pheromones remains highly challenging. Moreover, commercially available synthetic nematode pheromones—such as ascr#18 and ascr#9, despite their promising biocontrol potential—are prohibitively expensive (costing tens of thousands of RMB per gram), hindering their practical application in agricultural settings.”
Advances in comparative genomics, transcriptomics, metabolomics, and biosynthetic studies, combined with biochemical analyses and CRISPR-Cas9 genome editing, have enabled mechanistic validation in whole organisms [144]. Such integrative approaches facilitate both the discovery of novel metabolite families and the visualization of their biological context through synergistic interactions. By leveraging defective mutants in β-oxidation pathways and correlating their metabolomic profiles with nematode phenotypic variants, researchers have expanded the repertoire of known ascaroside pheromones. NMR-based methods offer notable advantages in linking naturally occurring small molecules to their biological functions, enabling the detection of cooperative signaling and the characterization of chemically labile metabolites. Currently, the dominant approach for pheromone identification is Differential Analysis of NMR Spectra (DANS) [145]. By applying DANS to wild-type (WT) and daf-22 mutant metabolomes, researchers identified ascarosides #6–8, with ascr#8 being the primary male-attracting component. Reverse-phase chromatography coupled with 2D NMR further isolated icas#3 from the daf-22 mutant metabolome, alongside predominant indole ascarosides (icas#3, icas#9, icas#10) and minor derivatives (icas#1, icas#7). Alternatively, capillary electrophoresis–mass spectrometry (CE-MS) has emerged as a complementary technique for profiling polar and charged metabolites in nematodes. For instance, CE-MS analysis of the long-lived daf-2 mutant revealed novel longevity-associated markers—such as nicotinic acid and NAD+—that were undetected by HILIC-MS or NMR-based methods [146].
Modular synthesis offers a distinctive strategy for expanding the nematode signaling molecule library, though key aspects of biosynthetic mechanisms and functional regulation remain unresolved. Comparative metabolomics studies on mutants that are defective in β-oxidation and carboxylase esterase pathways have successfully led to the discovery of new pheromone variants. Beyond the model organism C. elegans, exploring non-model nematodes has proven effective for identifying structurally novel metabolites. For instance, an integrated MS/MS and NMR approach enabled the identification of >30 previously unknown compounds in Pristionchus species [147]. Notably, nematodes dynamically remodel their metabolic networks in response to dietary shifts and environmental stressors. Untargeted metabolomics coupled with genomic analyses provides a powerful synergistic approach for annotating these ‘metabolic dark matter’.
In signal transduction research, C. elegans serves as a key model for studying pheromones, hormones, nutrient-sensing pathways, and their associated mechanisms. GPCRs (G protein-coupled receptors) mediate the detection of pheromonal cues, activating downstream signaling cascades that drive diverse physiological responses. However, while the nematode genome encodes over 1000 GPCRs, mostly expressed in chemosensory neurons, only a handful of receptor–ligand pairs have been functionally characterized. Similarly, among the 284 nuclear receptors identified in C. elegans, few have known activating ligands [148]. Notably, no structural data for any nematode GPCR has been resolved to date, highlighting that the regulatory networks governing pheromone signaling remain largely unexplored.
9. Bridging the Lab-to-Field Gap: Practical Challenges and Emerging Frontiers in Ascaroside-Based Nematode Management
While laboratory studies demonstrated the remarkable potential of ascarosides for nematode control [149], translating these findings into practical field applications presents significant challenges. First, the chemical stability of these signaling molecules under variable environmental conditions, such as UV exposure, soil pH fluctuations, and microbial degradation [150], must be optimized to ensure sustained efficacy in diverse agricultural settings. Second, scaling up production while maintaining cost-effectiveness remains a hurdle, particularly for smallholder farmers, as synthetic ascaroside production requires complex organic chemical pathways. Additionally, efficient delivery mechanisms [151], such as controlled-release formulations, soil-embedded microcapsules, or seed coatings [152], must be developed to ensure the precise spatiotemporal distribution of these compounds without the rapid dissipation or off-target effects.
Beyond these translational challenges, key unanswered questions in ascaroside research present exciting avenues for future investigation. For instance, how do soil microbiomes influence ascaroside persistence and function? Could engineered microbes be used as “live delivery systems” to enhance their bioavailability? Furthermore, while some ascarosides trigger plant immunity [19,103], others suppress it. Could antagonists of harmful nematode signals disrupt infestation? Finally, the potential for ascaroside resistance in nematode populations necessitates studies of long-term evolutionary dynamics and adaptive management strategies. Addressing these gaps will not only advance fundamental science but also accelerate the development of a scalable, next-generation nematode control solution.
Studies on nematode-derived ascarosides have unveiled three synergistic strategies for sustainable nematode management: (i) the plant defense activator ascr#18, which primes systemic resistance and metabolic reprogramming in crops, reducing root–knot nematode infections [103,153]; (ii) ascarosides like ascr#7 and ascr#5 that enhance fungal biocontrol by recruiting nematode-trapping fungi, increasing their predatory efficiency [27]; and (iii) seed pretreatment with entomopathogenic nematodes (EPNs) to establish early immune memory in developing plants [154]. Together, these approaches harness the natural signaling systems of nematodes to develop targeted, ecologically sound solutions that simultaneously exploit plant immunity, manipulate nematode behavior, and bolster beneficial soil microbiomes for integrated pest management.
Future research could exploit nematode ascarosides—key pheromone mediators—for targeted biocontrol by manipulating aggregation, dispersal, and mating behaviors. Aggregation signals (e.g., icas#3, icas#9) [38,155] could lure parasitic nematodes into fungal trap zones, while repulsive ascarosides (ascr#5, ascr#10) [40] might shield crops by dispersing pests toward fallow areas. Mating disruption using synthetic analogs (ascr#3, ascr#8) [34,] could impair reproduction by exaggerating mate-finding or prematurely inducing Dauer states. A coordinated “attract–kill–disrupt” strategy—combining fungal trap nodes, repellent root coatings, and pheromone confusion—could sustainably reduce infestations while minimizing non-target effects. Early field trials must optimize ascaroside stability and delivery (microcapsules, seed coatings) while assessing ecological risks. Addressing these challenges could unlock precision biocontrol tools aligning with integrated pest management, but scalability, cost, and resistance evolution remain critical hurdles. Theoretical frameworks now require empirical validation under real-world conditions to translate lab insights into scalable solutions.
10. Conclusions
Chemical communication is a ubiquitous information transfer mechanism in the biological world. It plays a crucial role in regulating the survival, reproductive, defense, and social behaviors of organisms. Ascarosides, a class of multifunctional signaling molecules in nematodes, mediate behaviors such as aggregation and avoidance, reproductive development, and interspecies interactions. They also serve as a key survival strategy for regulating population density and adapting to complex environments through chemosensory perception. Although significant progress has been made in the structural identification of ascarosides and glycosidic pheromones, major challenges remain in the discovery of novel signaling molecules. These challenges include the elucidation of functional mechanisms and the investigation of synergistic effects among mixed signals. Nematode behavior and development regulation often involves the coordinated actions of multiple pheromones. However, the underlying mechanisms of molecular pathways and regulatory networks remain incompletely understood. Future research should integrate multi-omics approaches, including comparative genomics, transcriptomics, and metabolomics, to identify novel signaling molecules in nematodes and systematically elucidate their biosynthetic pathways and regulatory mechanisms. Such efforts will not only deepen our understanding of nematode chemical communication networks but also lay a theoretical foundation for developing environmentally friendly, nematode-targeted biocontrol agents.
Author Contributions
Conceptualization, X.W.; writing—original draft preparation, X.Z.; writing—review and editing, X.W., X.Z. and J.L.; supervision, X.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Science and Technology Major Project from Yunnan Province (202201BC070004), the National Natural Science Foundation of China (32370138 and 32260029), the Applied Basic Research Foundation of Yunnan Province (202201AT070089), The National Key Research and Development Program (2023YFD1400013), Yunnan Provincial Higher Education Institutions Serving Key Industries Technology Project: Doctoral Student Serving Industry Scientific Research Innovation Training Program (FWCY-BSPY2024020).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ullah, A.; Bano, A.; Khan, N. Climate change and salinity effects on crops and chemical communication between plants and plant growth-promoting microorganisms under stress. Front. Sustain. Food Syst. 2021, 5, 618092. [Google Scholar] [CrossRef]
- Khan, M.R. Nematode pests of agricultural crops, a global overview. Nov. Biol. Biotechnol. Appl. Plant Nematode Manag. 2023. [Google Scholar] [CrossRef]
- Basu, S.; Clark, R.E.; Fu, Z.; Lee, B.W.; Crowder, D.W. Insect alarm pheromones in response to predators: Ecological trade-offs and molecular mechanisms. Insect Biochem. Mol. Biol. 2021, 128, 103514. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.A.H.; George, J.; Reddy, G.V.; Zeng, X.; Guerrero, A. Latest developments in insect sex pheromone research and its application in agricultural pest management. Insects 2021, 12, 484. [Google Scholar] [CrossRef]
- Kannan, K.; Galizia, C.G.; Nouvian, M. Olfactory strategies in the defensive behaviour of insects. Insects 2022, 13, 470. [Google Scholar] [CrossRef]
- Zhou, S.; Jander, G. Molecular ecology of plant volatiles in interactions with insect herbivores. J. Exp. Bot. 2022, 73, 449–462. [Google Scholar] [CrossRef]
- Dicke, M.; Lucas-Barbosa, D. Herbivore-induced plant volatiles as a source of information in plant–insect networks. In Biology of Plant Volatiles; CRC Press: Boca Raton, FL, USA, 2020; pp. 327–346. [Google Scholar]
- Kessler, A.; Chautá, A. The ecological consequences of herbivore-induced plant responses on plant–pollinator interactions. Emerg. Top. Life Sci. 2020, 4, 33–43. [Google Scholar] [PubMed]
- Moreira, X.; Abdala-Roberts, L.; Gols, R.; Lago-Núñez, B.; Rasmann, S.; Röder, G.; Soengas, P.; Vázquez-González, C.; Cartea, M.E. Insect herbivory but not plant pathogen infection drive floral volatile-mediated indirect effects on pollinators and plant fitness in Brassica rapa. J. Ecol. 2024, 112, 402–415. [Google Scholar] [CrossRef]
- Rattray, J.B.; Thomas, S.A.; Wang, Y.; Molotkova, E.; Gurney, J.; Varga, J.J.; Brown, S.P. Bacterial quorum sensing allows graded and bimodal cellular responses to variations in population density. MBio 2022, 13, e0074522. [Google Scholar] [CrossRef]
- Li, X.; Jin, J.; Zhang, X.; Xu, F.; Zhong, J.; Yin, Z.; Qi, H.; Wang, Z.; Shuai, J. Quantifying the optimal strategy of population control of quorum sensing network in Escherichia coli. Npj Syst. Biol. Appl. 2021, 7, 35. [Google Scholar] [CrossRef]
- Striednig, B.; Hilbi, H. Bacterial quorum sensing and phenotypic heterogeneity: How the collective shapes the individual. Trends Microbiol. 2022, 30, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Liu, J.; Liu, C.; Yang, A.; Qiao, J. Quorum sensing for population-level control of bacteria and potential therapeutic applications. Cell. Mol. Life Sci. 2020, 77, 1319–1343. [Google Scholar] [CrossRef] [PubMed]
- Buchinger, T.J.; Li, W. Chemical communication and its role in sexual selection across Animalia. Commun. Biol. 2023, 6, 1178. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, F.; Alborn, H.; von Reuss, S.; Ajredini, R.; Ali, J. Interspecific Nematode Signals Regulate Dispersal Behavior. PLoS ONE 2012, 7, e38735. [Google Scholar] [CrossRef]
- Srinivasan, J.; von Reuss, S.H.; Bose, N.; Zaslaver, A.; Mahanti, P.; Ho, M.C.; O’Doherty, O.G.; Edison, A.S.; Sternberg, P.W.; Schroeder, F.C. A modular library of small molecule signals regulates social behaviors in Caenorhabditis elegans. PLoS Biol. 2012, 10, e1001237. [Google Scholar] [CrossRef]
- Ludewig, A.H.; Schroeder, F.C. Ascaroside signaling in C. elegans. In WormBook: The Online Review of C. elegans Biology [Internet]; Cold Spring Harbor Laboratory: Pasadena, CA, USA, 2018. [Google Scholar] [CrossRef]
- Fielenbach, N.; Antebi, A. C. elegans dauer formation and the molecular basis of plasticity. Genes Dev. 2008, 22, 2149–2165. [Google Scholar] [CrossRef]
- Manohar, M.; Tenjo-Castano, F.; Chen, S.; Zhang, Y.K.; Kumari, A.; Williamson, V.M.; Wang, X.; Klessig, D.F.; Schroeder, F.C. Plant metabolism of nematode pheromones mediates plant-nematode interactions. Nat. Commun. 2020, 11, 208. [Google Scholar] [CrossRef]
- Michailidu, J.; Maťátková, O.; Čejková, A.; Masák, J. Chemical Conversations. Molecules 2025, 30, 431. [Google Scholar] [CrossRef]
- Law, J.H.; Regnier, F.E. Pheromones. Annu. Rev. Biochem. 1971, 40, 533–548. [Google Scholar] [CrossRef]
- Combarnous, Y.; Nguyen, T.M.D. Cell communications among microorganisms, plants, and animals: Origin, evolution, and interplays. Int. J. Mol. Sci. 2020, 21, 8052. [Google Scholar] [CrossRef]
- Abd El-Ghany, N.M. Pheromones and chemical communication in insects. Pests Weeds Dis. Agric. Crop Anim. Husb. Prod. 2020, 1–13. [Google Scholar]
- Yang, B.; Wang, J.; Zheng, X.; Wang, X. Nematode pheromones: Structures and functions. Molecules 2023, 28, 2409. [Google Scholar] [CrossRef]
- Kuo, C.-Y.; Tay, R.J.; Lin, H.-C.; Juan, S.-C.; Vidal-Diez de Ulzurrun, G.; Chang, Y.-C.; Hoki, J.; Schroeder, F.C.; Hsueh, Y.-P. The nematode-trapping fungus Arthrobotrys oligospora detects prey pheromones via G protein-coupled receptors. Nat. Microbiol. 2024, 9, 1738–1751. [Google Scholar] [CrossRef]
- Kuo, T.-H.; Yang, C.-T.; Chang, H.-Y.; Hsueh, Y.-P.; Hsu, C.-C. Nematode-trapping fungi produce diverse metabolites during predator–prey interaction. Metabolites 2020, 10, 117. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, Y.-P.; Mahanti, P.; Schroeder, F.C.; Sternberg, P.W. Nematode-trapping fungi eavesdrop on nematode pheromones. Curr. Biol. 2013, 23, 83–86. [Google Scholar] [CrossRef]
- Zou, J.; Kyndt, T.; Yu, J.; Zhou, J. Plant–nematode battle: Engagement of complex signaling network. Trends Parasitol. 2024, 40, 846–857. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, Y.-P.; Leighton, D.H.; Sternberg, P.W. Nematode Communication. In Biocommunication of Animals; Springer: Berlin/Heidelberg, Germany, 2013; pp. 383–407. [Google Scholar]
- Braendle, C. Pheromones: Evolving language of chemical communication in nematodes. Curr. Biol. 2012, 22, R294–R296. [Google Scholar] [CrossRef]
- Machado, R.A.; Von Reuss, S.H. Chemical ecology of nematodes. Chimia 2022, 76, 945–953. [Google Scholar]
- Srinivasan, J.; Durak, O.; Sternberg, P.W. Evolution of a polymodal sensory response network. BMC Biol. 2008, 6, 1–15. [Google Scholar] [CrossRef]
- Jeong, P.-Y.; Jung, M.; Yim, Y.-H.; Kim, H.; Park, M.; Hong, E.; Lee, W.; Kim, Y.H.; Kim, K.; Paik, Y.-K. Chemical structure and biological activity of the Caenorhabditis elegans dauer-inducing pheromone. Nature 2005, 433, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Butcher, R.A.; Fujita, M.; Schroeder, F.C.; Clardy, J. Small-molecule pheromones that control dauer development in Caenorhabditis elegans. Nat. Chem. Biol. 2007, 3, 420–422. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, J.; Kaplan, F.; Ajredini, R.; Zachariah, C.; Alborn, H.T.; Teal, P.E.; Malik, R.U.; Edison, A.S.; Sternberg, P.W.; Schroeder, F.C. A blend of small molecules regulates both mating and development in Caenorhabditis elegans. Nature 2008, 454, 1115–1118. [Google Scholar] [CrossRef]
- Butcher, R.A.; Ragains, J.R.; Kim, E.; Clardy, J. A potent dauer pheromone component in Caenorhabditis elegans that acts synergistically with other components. Proc. Natl. Acad. Sci. USA 2008, 105, 14288–14292. [Google Scholar] [CrossRef]
- Pungaliya, C.; Srinivasan, J.; Fox, B.W.; Malik, R.U.; Ludewig, A.H.; Sternberg, P.W.; Schroeder, F.C. A shortcut to identifying small molecule signals that regulate behavior and development in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2009, 106, 7708–7713. [Google Scholar] [CrossRef]
- Butcher, R.A.; Ragains, J.R.; Clardy, J. An indole-containing dauer pheromone component with unusual dauer inhibitory activity at higher concentrations. Org. Lett. 2009, 11, 3100–3103. [Google Scholar] [CrossRef]
- Izrayelit, Y.; Srinivasan, J.; Campbell, S.L.; Jo, Y.; von Reuss, S.H.; Genoff, M.C.; Sternberg, P.W.; Schroeder, F.C. Targeted metabolomics reveals a male pheromone and sex-specific ascaroside biosynthesis in Caenorhabditis elegans. ACS Chem. Biol. 2012, 7, 1321–1325. [Google Scholar] [CrossRef]
- Choe, A.; von Reuss, S.H.; Kogan, D.; Gasser, R.B.; Platzer, E.G.; Schroeder, F.C.; Sternberg, P.W. Ascaroside signaling is widely conserved among nematodes. Curr. Biol. 2012, 22, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Laing, S.T.; Ivens, A.; Laing, R.; Ravikumar, S.; Butler, V.; Woods, D.J.; Gilleard, J.S. Characterization of the xenobiotic response of Caenorhabditis elegans to the anthelmintic drug albendazole and the identification of novel drug glucoside metabolites. Biochem. J. 2010, 432, 505–516. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, Y.G.; Kim, M.; Kim, E.; Choi, H.; Kim, Y.; Lee, J. Indole-associated predator–prey interactions between the nematode Caenorhabditis elegans and bacteria. Environ. Microbiol. 2017, 19, 1776–1790. [Google Scholar] [CrossRef]
- Stupp, G.S.; von Reuss, S.H.; Izrayelit, Y.; Ajredini, R.; Schroeder, F.C.; Edison, A.S. Chemical detoxification of small molecules by Caenorhabditis elegans. ACS Chem. Biol. 2013, 8, 309–313. [Google Scholar] [CrossRef]
- Motola, D.L.; Cummins, C.L.; Rottiers, V.; Sharma, K.K.; Li, T.; Li, Y.; Suino-Powell, K.; Xu, H.E.; Auchus, R.J.; Antebi, A. Identification of ligands for DAF-12 that govern dauer formation and reproduction in C. elegans. Cell 2006, 124, 1209–1223. [Google Scholar] [CrossRef] [PubMed]
- Papsdorf, K.; Miklas, J.W.; Hosseini, A.; Cabruja, M.; Morrow, C.S.; Savini, M.; Yu, Y.; Silva-García, C.G.; Haseley, N.R.; Murphy, L.M. Lipid droplets and peroxisomes are co-regulated to drive lifespan extension in response to mono-unsaturated fatty acids. Nat. Cell Biol. 2023, 25, 672–684. [Google Scholar] [CrossRef]
- Ludewig, A.H.; Artyukhin, A.B.; Aprison, E.Z.; Rodrigues, P.R.; Pulido, D.C.; Burkhardt, R.N.; Panda, O.; Zhang, Y.K.; Gudibanda, P.; Ruvinsky, I. An excreted small molecule promotes C. elegans reproductive development and aging. Nat. Chem. Biol. 2019, 15, 838–845. [Google Scholar] [CrossRef] [PubMed]
- Chantab, K.; Rao, Z.; Zheng, X.; Han, R.; Cao, L. Ascarosides and Symbiotic Bacteria of Entomopathogenic Nematodes Regulate Host Immune Response in Galleria mellonella Larvae. Insects 2024, 15, 514. [Google Scholar] [CrossRef]
- Kaplan, F.; Srinivasan, J.; Mahanti, P.; Ajredini, R.; Durak, O.; Nimalendran, R.; Sternberg, P.W.; Teal, P.E.; Schroeder, F.C.; Edison, A.S. Ascaroside expression in Caenorhabditis elegans is strongly dependent on diet and developmental stage. PLoS ONE 2011, 6, e17804. [Google Scholar] [CrossRef] [PubMed]
- Hartley, C.J.; Lillis, P.E.; Owens, R.A.; Griffin, C.T. Infective juveniles of entomopathogenic nematodes (Steinernema and Heterorhabditis) secrete ascarosides and respond to interspecific dispersal signals. J. Invertebr. Pathol. 2019, 168, 107257. [Google Scholar] [CrossRef]
- Dong, C. Small Molecule Signaling in Nematodes; Friedrich-Schiller-Universität Jena: Jena, Germany, 2017. [Google Scholar]
- Von Reuss, S.H.; Schroeder, F.C. Combinatorial chemistry in nematodes: Modular assembly of primary metabolism-derived building blocks. Nat. Prod. Rep. 2015, 32, 994–1006. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, Y.K.; Manohar, M.; Artyukhin, A.B.; Kumari, A.; Tenjo-Castano, F.J.; Nguyen, H.; Routray, P.; Choe, A.; Klessig, D.F. Nematode signaling molecules are extensively metabolized by animals, plants, and microorganisms. ACS Chem. Biol. 2021, 16, 1050–1058. [Google Scholar] [CrossRef]
- Wrobel, C.J.; Schroeder, F.C. Repurposing degradation pathways for modular metabolite biosynthesis in nematodes. Nat. Chem. Biol. 2023, 19, 676–686. [Google Scholar] [CrossRef]
- Salzer, L.; Witting, M. Quo vadis Caenorhabditis elegans metabolomics—A review of current methods and applications to explore metabolism in the nematode. Metabolites 2021, 11, 284. [Google Scholar] [CrossRef] [PubMed]
- Bento, G.; Ogawa, A.; Sommer, R.J. Co-option of the hormone-signalling module dafachronic acid–DAF-12 in nematode evolution. Nature 2010, 466, 494–497. [Google Scholar] [CrossRef] [PubMed]
- Kotowska, A.M.; Hiramatsu, F.; Alexander, M.R.; Scurr, D.J.; Lightfoot, J.W.; Chauhan, V.M. Surface Lipids in Nematodes are Influenced by Development and Species-specific Adaptations. J. Am. Chem. Soc. 2025, 147, 6439–6449. [Google Scholar] [CrossRef]
- C. elegans Sequencing Consortium. Genome sequence of the nematode C. elegans: A platform for investigating biology. Science 1998, 282, 2012–2018. [Google Scholar] [CrossRef] [PubMed]
- Abad, P.; Gouzy, J.; Aury, J.-M.; Castagnone-Sereno, P.; Danchin, E.G.; Deleury, E.; Perfus-Barbeoch, L.; Anthouard, V.; Artiguenave, F.; Blok, V.C. Genome sequence of the metazoan plant-parasitic nematode Meloidogyne incognita. Nat. Biotechnol. 2008, 26, 909–915. [Google Scholar] [CrossRef]
- Dong, C.; Reilly, D.K.; Bergame, C.; Dolke, F.; Srinivasan, J.; von Reuss, S.H. Comparative ascaroside profiling of Caenorhabditis exometabolomes reveals species-specific (ω) and (ω–2)-hydroxylation downstream of peroxisomal β-oxidation. J. Org. Chem. 2018, 83, 7109–7120. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Perez, D.H.; Jones Lipinski, R.A.; Butcher, R.A.; Lipinski, R. Acyl-CoA Oxidases Fine-Tune the Production of Ascaroside Pheromones with Specific Side Chain Lengths. ACS Chem. Biol. 2018, 13, 1048–1056. [Google Scholar] [CrossRef]
- Park, J.Y.; Joo, H.-J.; Park, S.; Paik, Y.-K. Ascaroside pheromones: Chemical biology and pleiotropic neuronal functions. Int. J. Mol. Sci. 2019, 20, 3898. [Google Scholar] [CrossRef]
- Bono, M.d.; Villu Maricq, A. Neuronal substrates of complex behaviors in C. elegans. Annu. Rev. Neurosci. 2005, 28, 451–501. [Google Scholar] [CrossRef]
- Wang, J.; Cao, L.; Huang, Z.; Gu, X.; Cui, Y.; Li, J.; Li, Y.; Xu, C.; Han, R. Influence of the ascarosides on the recovery, yield and dispersal of entomopathogenic nematodes. J. Invertebr. Pathol. 2022, 188, 107717. [Google Scholar] [CrossRef]
- Bargmann, C. Chemosensation in C. elegans. In WormBook: The Online Review of C. elegans Biology; Cold Spring Harbor Laboratory: Pasadena, CA, USA, 2006. [Google Scholar] [CrossRef]
- Ferkey, D.M.; Sengupta, P.; Noelle, D.L. Chemosensory signal transduction in Caenorhabditis elegans. Genetics 2021, 217, iyab004. [Google Scholar] [CrossRef]
- Hart, A.C.; Chao, M.Y. From odors to behaviors in Caenorhabditis elegans. In The Neurobiology of Olfaction; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2010; p. 21882435. [Google Scholar]
- De Bono, M.; Bargmann, C.I. Natural variation in a neuropeptide Y receptor homolog modifies social behavior and food response in C. elegans. Cell 1998, 94, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Macosko, E.Z.; Pokala, N.; Feinberg, E.H.; Chalasani, S.H.; Butcher, R.A.; Clardy, J.; Bargmann, C.I. A hub-and-spoke circuit drives pheromone attraction and social behaviour in C. elegans. Nature 2009, 458, 1171–1175. [Google Scholar] [CrossRef]
- White, J.G.; Southgate, E.; Thomson, J.N.; Brenner, S. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos. Trans. R. Soc. B Biol. Sci. 1986, 314, 1–340. [Google Scholar] [PubMed]
- Poinar Jr, G.O. Nematoda and nematomorpha. In Ecology and Classification of North American Freshwater Invertebrates; Elsevier: Amsterdam, The Netherlands, 2010; pp. 237–276. [Google Scholar]
- Godoy, L.F.; Hochbaum, D. Transcriptional and spatiotemporal regulation of the dauer program. Transcription 2023, 14, 27–48. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.J. Dauer. In WormBook: The Online Review of C. elegans Biology [Internet]; Cold Spring Harbor Laboratory: Pasadena, CA, USA, 2018; doi: 10.1895/wormbook.1.144.1. [Google Scholar]
- Vlaar, L.E.; Bertran, A.; Rahimi, M.; Dong, L.; Kammenga, J.E.; Helder, J.; Goverse, A.; Bouwmeester, H.J. On the role of dauer in the adaptation of nematodes to a parasitic lifestyle. Parasites Vectors 2021, 14, 1–20. [Google Scholar] [CrossRef]
- Golden, J.W.; Riddle, D.L. The Caenorhabditis elegans dauer larva: Developmental effects of pheromone, food, and temperature. Dev. Biol. 1984, 102, 368–378. [Google Scholar] [CrossRef]
- Crook, M. The dauer hypothesis and the evolution of parasitism: 20 years on and still going strong. Int. J. Parasitol. 2014, 44, 1–8. [Google Scholar] [CrossRef]
- Golden, J.W.; Riddle, D.L. A pheromone influences larval development in the nematode Caenorhabditis elegans. Science 1982, 218, 578–580. [Google Scholar] [CrossRef]
- Diaz, S.A.; Brunet, V.; Lloyd-Jones, G.C.; Spinner, W.; Wharam, B.; Viney, M. Diverse and potentially manipulative signalling with ascarosides in the model nematode C. elegans. BMC Evol. Biol. 2014, 14, 1–8. [Google Scholar] [CrossRef]
- Cohen, S.M.; Wrobel, C.J.; Prakash, S.J.; Schroeder, F.C.; Sternberg, P.W. Formation and function of dauer ascarosides in the nematodes Caenorhabditis briggsae and Caenorhabditis elegans. G3 2022, 12, jkac014. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Sato, K.; Shibuya, M.; Zeiger, D.M.; Butcher, R.A.; Ragains, J.R.; Clardy, J.; Touhara, K.; Sengupta, P. Two chemoreceptors mediate developmental effects of dauer pheromone in C. elegans. Science 2009, 326, 994–998. [Google Scholar] [CrossRef] [PubMed]
- Ren, P.; Lim, C.-S.; Johnsen, R.; Albert, P.S.; Pilgrim, D.; Riddle, D.L. Control of C. elegans larval development by neuronal expression of a TGF-β homolog. Science 1996, 274, 1389–1391. [Google Scholar] [CrossRef]
- Schackwitz, W.S.; Inoue, T.; Thomas, J.H. Chemosensory neurons function in parallel to mediate a pheromone response in C. elegans. Neuron 1996, 17, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.G.; Sternberg, P.W. Entry to and exit from diapause arrest in Caenorhabditis elegans are both regulated by a steroid hormone pathway. BioRxiv 2021, 149, dev200173. [Google Scholar] [CrossRef]
- Simon, J.M.; Sternberg, P.W. Evidence of a mate-finding cue in the hermaphrodite nematode Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2002, 99, 1598–1603. [Google Scholar] [CrossRef]
- Wu, T.; Ge, M.; Wu, M.; Duan, F.; Liang, J.; Chen, M.; Gracida, X.; Liu, H.; Yang, W.; Dar, A.R. Pathogenic bacteria modulate pheromone response to promote mating. Nature 2023, 613, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Ludewig, A.H.; Izrayelit, Y.; Park, D.; Malik, R.U.; Zimmermann, A.; Mahanti, P.; Fox, B.W.; Bethke, A.; Doering, F.; Riddle, D.L. Pheromone sensing regulates Caenorhabditis elegans lifespan and stress resistance via the deacetylase SIR-2.1. Proc. Natl. Acad. Sci. USA 2013, 110, 5522–5527. [Google Scholar] [CrossRef]
- Vidal-Diez de Ulzurrun, G.; Hsueh, Y.-P. Predator-prey interactions of nematode-trapping fungi and nematodes: Both sides of the coin. Appl. Microbiol. Biotechnol. 2018, 102, 3939–3949. [Google Scholar] [CrossRef]
- Clarke, D.J.; Eberl, L. Interactions between bacteria and nematodes. Intest. Microorg. Termit. Other Invertebr. 2006, 55–64. [Google Scholar]
- Horiuchi, J.-i.; Prithiviraj, B.; Bais, H.P.; Kimball, B.A.; Vivanco, J.M. Soil nematodes mediate positive interactions between legume plants and rhizobium bacteria. Planta 2005, 222, 848–857. [Google Scholar] [CrossRef] [PubMed]
- Williamson, V.M.; Gleason, C.A. Plant–nematode interactions. Curr. On. Plant Biol. 2003, 6, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Eves-van den Akker, S. Plant–nematode interactions. Curr. Opin. Plant Biol. 2021, 62, 102035. [Google Scholar] [CrossRef]
- Abd-Elgawad, M.M. Understanding molecular plant–nematode interactions to develop alternative approaches for nematode control. Plants 2022, 11, 2141. [Google Scholar] [CrossRef]
- Akhtar, M.S. Opportunistic Fungi, Nematode and Plant Interactions: Interplay and Mechanisms; Springer Nature: Berlin/Heidelberg, Germany, 2024. [Google Scholar]
- Zhang, Y.; Li, G.; Zhang, K. A review on the research of nematophagous fungal species. Mycosystema 2011, 30, 836–845. [Google Scholar]
- Karakaş, M. Nematode-destroying fungi: Infection structures, interaction mechanisms and biocontrol. Commun. Fac. Sci. Univ. Ank. Ser. C Biol. 2020, 29, 176–201. [Google Scholar]
- Nordbring-Hertz, B.; Jansson, H.-B.; Tunlid, A. Nematophagous fungi. Encycl. Life Sci. 2006, 10. [Google Scholar]
- Yadav, B.; Singh, U.B.; Malviya, D.; Vishwakarma, S.K.; Ilyas, T.; Shafi, Z.; Shahid, M.; Singh, H.V. Nematophagous fungi: Biology, ecology and potential application. In Detection, Diagnosis and Management of Soil-Borne Phytopathogens; Springer: Berlin/Heidelberg, Germany, 2023; pp. 309–328. [Google Scholar]
- Hu, X.; Hoffmann, D.S.; Wang, M.; Schuhmacher, L.; Stroe, M.C.; Schreckenberger, B.; Elstner, M.; Fischer, R. GprC of the nematode-trapping fungus Arthrobotrys flagrans activates mitochondria and reprograms fungal cells for nematode hunting. Nat. Microbiol. 2024, 9, 1752–1763. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Shen, Q. Dual function of a fungal GPCR in activating mitochondrial respiration and initiating prey hunting in fungus-nematode interactions. Innov. Life 2024, 2, 100087. [Google Scholar] [CrossRef]
- Hsueh, Y.-P.; Gronquist, M.R.; Schwarz, E.M.; Nath, R.D.; Lee, C.-H.; Gharib, S.; Schroeder, F.C.; Sternberg, P.W. Nematophagous fungus Arthrobotrys oligospora mimics olfactory cues of sex and food to lure its nematode prey. Elife 2017, 6, e20023. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, N.; Chen, Y.; Zhang, D.; Yan, J.; Zou, W.; Zhang, K.; Huang, X. The signaling pathway of Caenorhabditis elegans mediates chemotaxis response to the attractant 2-heptanone in a trojan horse-like pathogenesis. J. Biol. Chem. 2016, 291, 23618–23627. [Google Scholar] [CrossRef] [PubMed]
- Pender, C.L.; Dishart, J.G.; Gildea, H.K.; Nauta, K.M.; Page, E.M.; Siddiqi, T.F.; Cheung, S.S.; Joe, L.; Burton, N.O.; Dillin, A. Perception of a pathogenic signature initiates intergenerational protection. Cell 2025, 188, 594–605.e510. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Yuan, Y.; Lewis, C.; Kud, J.; Kuhl, J.C.; Caplan, A.; Dandurand, L.-M.; Zasada, I.; Xiao, F. NILR1 perceives a nematode ascaroside triggering immune signaling and resistance. Curr. Biol. 2023, 33, 3992–3997.e3993. [Google Scholar] [CrossRef] [PubMed]
- Klessig, D.F.; Manohar, M.; Baby, S.; Koch, A.; Danquah, W.B.; Luna, E.; Park, H.J.; Kolkman, J.M.; Turgeon, B.G.; Nelson, R. Nematode ascaroside enhances resistance in a broad spectrum of plant–pathogen systems. J. Phytopathol. 2019, 167, 265–272. [Google Scholar] [CrossRef]
- Kamboj, A.; Thielmann, J.; Delfan, S.; Kloppe, T.; Schulz, P.; Manohar, M.; Schroeder, F.C.; Klessig, D.F.; Kogel, K.-H. The Nematode Signaling Molecule ascr# 18 Induces Prehaustorial Defenses in Wheat Against a Leaf Rust Fungus. J. Plant Dis. Prot. 2024, 131, 2053–2062. [Google Scholar]
- Mendy, B.; Wang’ombe, M.W.; Radakovic, Z.S.; Holbein, J.; Ilyas, M.; Chopra, D.; Holton, N.; Zipfel, C.; Grundler, F.M.; Siddique, S. Arabidopsis leucine-rich repeat receptor–like kinase NILR1 is required for induction of innate immunity to parasitic nematodes. PLoS Pathog. 2017, 13, e1006284. [Google Scholar] [CrossRef]
- Zhao, M.; Wickham, J.D.; Zhao, L.; Sun, J. Major ascaroside pheromone component asc-C5 influences reproductive plasticity among isolates of the invasive species pinewood nematode. Integr. Zool. 2021, 16, 893–907. [Google Scholar] [CrossRef]
- Zhao, L.; Ahmad, F.; Lu, M.; Zhang, W.; Wickham, J.D.; Sun, J. Ascarosides promote the prevalence of ophiostomatoid fungi and an invasive pathogenic nematode, Bursaphelenchus xylophilus. J. Chem. Ecol. 2018, 44, 701–710. [Google Scholar] [CrossRef]
- von Reuss, S.H. Exploring modular glycolipids involved in nematode chemical communication. Chimia 2018, 72, 297–303. [Google Scholar] [CrossRef]
- Peng, J.-Y.; Liu, X.; Zeng, X.-T.; Hao, Y.; Zhang, J.-H.; Li, Q.; Tong, X.-J. Early pheromone perception remodels neurodevelopment and accelerates neurodegeneration in adult C. elegans. Cell Rep. 2023, 42, 112598. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, F.C. Modular assembly of primary metabolic building blocks: A chemical language in C. elegans. Chem. Biol. 2015, 22, 7–16. [Google Scholar] [CrossRef]
- von Reuss, S.H.; Dolke, F.; Dong, C. Ascaroside profiling of Caenorhabditis elegans using gas chromatography–electron ionization mass spectrometry. Anal. Chem. 2017, 89, 10570–10577. [Google Scholar] [CrossRef] [PubMed]
- Von Reuss, S.H.; Bose, N.; Srinivasan, J.; Yim, J.J.; Judkins, J.C.; Sternberg, P.W.; Schroeder, F.C. Comparative metabolomics reveals biogenesis of ascarosides, a modular library of small-molecule signals in C. elegans. J. Am. Chem. Soc. 2012, 134, 1817–1824. [Google Scholar] [CrossRef]
- Zagoriy, V.; Matyash, V.; Kurzchalia, T. Long-Chain O-Ascarosyl-alkanediols Are Constitutive Components of Caenorhabditis elegans but Do Not Induce Dauer Larva Formation. Chem. Biodivers. 2010, 7, 2016–2022. [Google Scholar] [CrossRef]
- Zhang, X.; Feng, L.; Chinta, S.; Singh, P.; Wang, Y.; Nunnery, J.K.; Butcher, R.A. Acyl-CoA oxidase complexes control the chemical message produced by Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2015, 112, 3955–3960. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Vogt, M.C.; Fox, B.W.; Wrobel, C.J.; Fajardo Palomino, D.; Curtis, B.J.; Zhang, B.; Le, H.H.; Tauffenberger, A.; Hobert, O. Parallel pathways for serotonin biosynthesis and metabolism in C. elegans. Nat. Chem. Biol. 2023, 19, 141–150. [Google Scholar] [CrossRef]
- Le, H.H.; Wrobel, C.J.; Cohen, S.M.; Yu, J.; Park, H.; Helf, M.J.; Curtis, B.J.; Kruempel, J.C.; Rodrigues, P.R.; Hu, P.J. Modular metabolite assembly in Caenorhabditis elegans depends on carboxylesterases and formation of lysosome-related organelles. Elife 2020, 9, e61886. [Google Scholar] [CrossRef]
- Espada, M.; Silva, A.C.; Eves van den Akker, S.; Cock, P.J.; Mota, M.; Jones, J.T. Identification and characterization of parasitism genes from the pinewood nematode Bursaphelenchus xylophilus reveals a multilayered detoxification strategy. Mol. Plant Pathol. 2016, 17, 286–295. [Google Scholar] [CrossRef]
- Wrobel, C.J.J. Combinatorial Assembly of Modular Metabolites via Carboxylesterases in Nematodes; Cornell University: Ithaca, NY, USA, 2023. [Google Scholar]
- Szeto, S.S.; Reinke, S.N.; Lemire, B.D. 1 H NMR-based metabolic profiling reveals inherent biological variation in yeast and nematode model systems. J. Biomol. NMR 2011, 49, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Kowsalya, K.; Vidya, N.; Halka, J.; Preetha, J.S.Y.; Saradhadevi, M.; Sahayarayan, J.J.; Gurusaravanan, P.; Arun, M. Plant glycosides and glycosidases: Classification, sources, and therapeutic insights in current medicine. Glycoconj. J. 2025, 42, 107–124. [Google Scholar] [CrossRef] [PubMed]
- Stasiuk, S.; MacNevin, G.; Workentine, M.; Gray, D.; Redman, E.; Bartley, D.; Morrison, A.; Sharma, N.; Colwell, D.; Ro, D. Similarities and differences in the biotransformation and transcriptomic responses of Caenorhabditis elegans and Haemonchus contortus to five different benzimidazole drugs. Int. J. Parasitol. Drugs Drug Resist. 2019, 11, 13–29. [Google Scholar] [CrossRef] [PubMed]
- Kellerová, P.; Raisová Stuchlíková, L.; Matoušková, P.; Štěrbová, K.; Lamka, J.; Navrátilová, M.; Vokřál, I.; Szotáková, B.; Skálová, L. Sub-lethal doses of albendazole induce drug metabolizing enzymes and increase albendazole deactivation in Haemonchus contortus adults. Vet. Res. 2020, 51, 1–17. [Google Scholar] [CrossRef]
- Fontaine, P.; Choe, K. The transcription factor SKN-1 and detoxification gene ugt-22 alter albendazole efficacy in Caenorhabditis elegans. Int. J. Parasitol. Drugs Drug Resist. 2018, 8, 312–319. [Google Scholar] [CrossRef]
- Wrobel, C.J.; Yu, J.; Rodrigues, P.R.; Ludewig, A.H.; Curtis, B.J.; Cohen, S.M.; Fox, B.W.; O’Donnell, M.P.; Sternberg, P.W.; Schroeder, F.C. Combinatorial assembly of modular glucosides via carboxylesterases regulates C. elegans starvation survival. J. Am. Chem. Soc. 2021, 143, 14676–14683. [Google Scholar] [CrossRef]
- Mahanti, P.; Bose, N.; Bethke, A.; Judkins, J.C.; Wollam, J.; Dumas, K.J.; Zimmerman, A.M.; Campbell, S.L.; Hu, P.J.; Antebi, A. Comparative metabolomics reveals endogenous ligands of DAF-12, a nuclear hormone receptor, regulating C. elegans development and lifespan. Cell Metab. 2014, 19, 73–83. [Google Scholar] [CrossRef]
- Wollam, J.; Magner, D.B.; Magomedova, L.; Rass, E.; Shen, Y.; Rottiers, V.; Habermann, B.; Cummins, C.L.; Antebi, A. A novel 3-hydroxysteroid dehydrogenase that regulates reproductive development and longevity. PLoS Biol. 2012, 10, e1001305. [Google Scholar] [CrossRef]
- Cui, G.Z.; Zhu, J.J. Pheromone-based pest management in China: Past, present, and future prospects. J. Chem. Ecol. 2016, 42, 557–570. [Google Scholar] [CrossRef]
- Mota, M.M.; Vieira, P. Pine Wilt Disease: A Worldwide Threat to Forest Ecosystems; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Degenhardt, J.; Hiltpold, I.; Köllner, T.G.; Frey, M.; Gierl, A.; Gershenzon, J.; Hibbard, B.E.; Ellersieck, M.R.; Turlings, T.C. Restoring a maize root signal that attracts insect-killing nematodes to control a major pest. Proc. Natl. Acad. Sci. USA 2009, 106, 13213–13218. [Google Scholar] [CrossRef]
- Bruce, T.J.; Aradottir, G.I.; Smart, L.E.; Martin, J.L.; Caulfield, J.C.; Doherty, A.; Sparks, C.A.; Woodcock, C.M.; Birkett, M.A.; Napier, J.A. The first crop plant genetically engineered to release an insect pheromone for defence. Sci. Rep. 2015, 5, 11183. [Google Scholar] [CrossRef] [PubMed]
- Knight, A.; Barros-Parada, W.; Bosch, D.; Escudero-Colomar, L.A.; Fuentes-Contreras, E.; Hernández-Sánchez, J.; Yung, C.; Kim, Y.; Kovanci, O.; Levi, A. Similar worldwide patterns in the sex pheromone signal and response in the oriental fruit moth, Grapholita molesta (Lepidoptera: Tortricidae). Bull. Entomol. Res. 2015, 105, 23–31. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Wang, L.; Lv, C.; Ge, S.; Zhang, H.; Jiang, S.; Chu, B.; Yang, X.; Wyckhuys, K.A.; Wu, K. Use of food attractants to monitor and forecast Spodoptera frugiperda (JE Smith) seasonal abundance in southern China. J. Pest Sci. 2023, 96, 1509–1521. [Google Scholar] [CrossRef]
- Sullivan, N.; Butler, R.; Salehi, L.; Twidle, A.; Baker, G.; Suckling, D. Deployment of the sex pheromone of Pseudococcus calceolariae (Hemiptera: Pseudococcidae) as a potential new tool for mass trapping in citrus in South Australia. N. Zealand Entomol. 2019, 42, 1–12. [Google Scholar] [CrossRef]
- Cherif, A.; Harbaoui, K.; Zappala, L.; Grissa-Lebdi, K. Efficacy of mass trapping and insecticides to control Tuta absoluta in Tunisia. J. Plant Dis. Prot. 2018, 125, 51–61. [Google Scholar] [CrossRef]
- Madhu, T.; Shah, V.; Prabhulinga, T.; Chakravarthy, A.; Ashok Kumar, C. Optimization of pheromone trap densities and impact of insecticides on pheromone catches for mass trapping Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae) in chickpea. J. Entomol. Zool. Stud. 2019, 7, 78–84. [Google Scholar]
- Piñero, J.C.; Dudenhoeffer, A.P. Mass trapping designs for organic control of the Japanese beetle, Popillia japonica (Coleoptera: Scarabaeidae). Pest Manag. Sci. 2018, 74, 1687–1693. [Google Scholar] [CrossRef]
- Harari, A.R.; Zahavi, T.; Steinitz, H. Female detection of the synthetic sex pheromone contributes to the efficacy of mating disruption of the European grapevine moth, Lobesia botrana. Pest Manag. Sci. 2015, 71, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Walgenbach, J.F.; Schoof, S.C.; Bosch, D.; Escudero-Colomar, L.-A.; Lingren, B.; Krawczyk, G. Comparison of sex pheromone and kairomone-enhanced pheromone lures for monitoring oriental fruit moth (Lepidoptera: Tortricidae) in mating disruption and non-disruption tree fruit orchards. Environ. Entomol. 2021, 50, 1063–1074. [Google Scholar] [CrossRef]
- Liang, Y.-y.; Luo, M.; Fu, X.-g.; Zheng, L.-x.; Wei, H.-y. Mating disruption of Chilo suppressalis from sex pheromone of another pyralid rice pest Cnaphalocrocis medinalis (Lepidoptera: Pyralidae). J. Insect Sci. 2020, 20, 19. [Google Scholar] [CrossRef]
- Ricciardi, R.; Di Giovanni, F.; Cosci, F.; Ladurner, E.; Savino, F.; Iodice, A.; Benelli, G.; Lucchi, A. Mating Disruption for Managing the Honeydew Moth, Cryptoblabes gnidiella (Millière), in Mediterranean Vineyards. Insects 2021, 12, 390. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Hofman, C.; Kaplan, F.; Stevens, G.; Lewis, E.; Wu, S.; Alborn, H.T.; Perret-Gentil, A.; Shapiro-Ilan, D.I. Pheromone extracts act as boosters for entomopathogenic nematodes efficacy. J. Invertebr. Pathol. 2019, 164, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Villarreal, G.U.; Campos, E.V.R.; de Oliveira, J.L.; Fraceto, L.F. Development and commercialization of pheromone-based biopesticides: A global perspective. In Development and Commercialization of Biopesticides; Elsevier: Amsterdam, The Netherlands, 2023; pp. 37–56. [Google Scholar]
- Koul, O. Development and Commercialization of Biopesticides: Costs and Benefits; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar]
- Fox, B.W.; Ponomarova, O.; Lee, Y.-U.; Zhang, G.; Giese, G.E.; Walker, M.; Roberto, N.M.; Na, H.; Rodrigues, P.R.; Curtis, B.J. C. elegans as a model for inter-individual variation in metabolism. Nature 2022, 607, 571–577. [Google Scholar] [CrossRef]
- Robinette, S.L.; Brüschweiler, R.; Schroeder, F.C.; Edison, A.S. NMR in metabolomics and natural products research: Two sides of the same coin. Acc. Chem. Res. 2012, 45, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Salzer, L.; Schmitt-Kopplin, P.; Witting, M. Capillary electrophoresis-mass spectrometry as a tool for Caenorhabditis elegans metabolomics research. Metabolomics 2023, 19, 61. [Google Scholar] [CrossRef]
- Dong, C.; Weadick, C.J.; Truffault, V.; Sommer, R.J. Convergent evolution of small molecule pheromones in Pristionchus nematodes. Elife 2020, 9, e55687. [Google Scholar] [CrossRef]
- Butcher, R.A. Small-molecule pheromones and hormones controlling nematode development. Nat. Chem. Biol. 2017, 13, 577–586. [Google Scholar] [CrossRef]
- Zhang, Y.K.; Sanchez-Ayala, M.A.; Sternberg, P.W.; Srinivasan, J.; Schroeder, F.C. Improved synthesis for modular ascarosides uncovers biological activity. Org. Lett. 2017, 19, 2837–2840. [Google Scholar] [CrossRef]
- Pathak, V.M. Review on the current status of polymer degradation: A microbial approach. Bioresour. Bioprocess. 2017, 4, 1–31. [Google Scholar] [CrossRef]
- Vega-Vásquez, P.; Mosier, N.S.; Irudayaraj, J. Nanoscale drug delivery systems: From medicine to agriculture. Front. Bioeng. Biotechnol. 2020, 8, 79. [Google Scholar] [CrossRef]
- Paravar, A.; Piri, R.; Balouchi, H.; Ma, Y. Microbial seed coating: An attractive tool for sustainable agriculture. Biotechnol. Rep. 2023, 37, e00781. [Google Scholar] [CrossRef] [PubMed]
- Manosalva, P.; Manohar, M.; Von Reuss, S.H.; Chen, S.; Koch, A.; Kaplan, F.; Choe, A.; Micikas, R.J.; Wang, X.; Kogel, K.-H. Conserved nematode signalling molecules elicit plant defenses and pathogen resistance. Nat. Commun. 2015, 6, 7795. [Google Scholar] [CrossRef] [PubMed]
- González-Guzmán, A.; Sánchez-Rodríguez, A.R.; Quesada-Moraga, E.; del Campillo, M.C.; Yousef-Yousef, M. Optimizing wheat seed treatment with entomopathogenic fungi for improving plant growth at early development stages. Span. J. Agric. Res. 2021, 19, e1004. [Google Scholar] [CrossRef]
- Muirhead, C.S.; Srinivasan, J. Small molecule signals mediate social behaviors in C. elegans. J. Neurogenet. 2020, 34, 395–403. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).