Impact of Aging and Pathologies on Human Oral Mucosa: Preliminary Investigation of Biophysical Markers from Thermal and Vibrational Analyses
Abstract
1. Introduction
2. Materials and Methods
2.1. Biopsies Collection
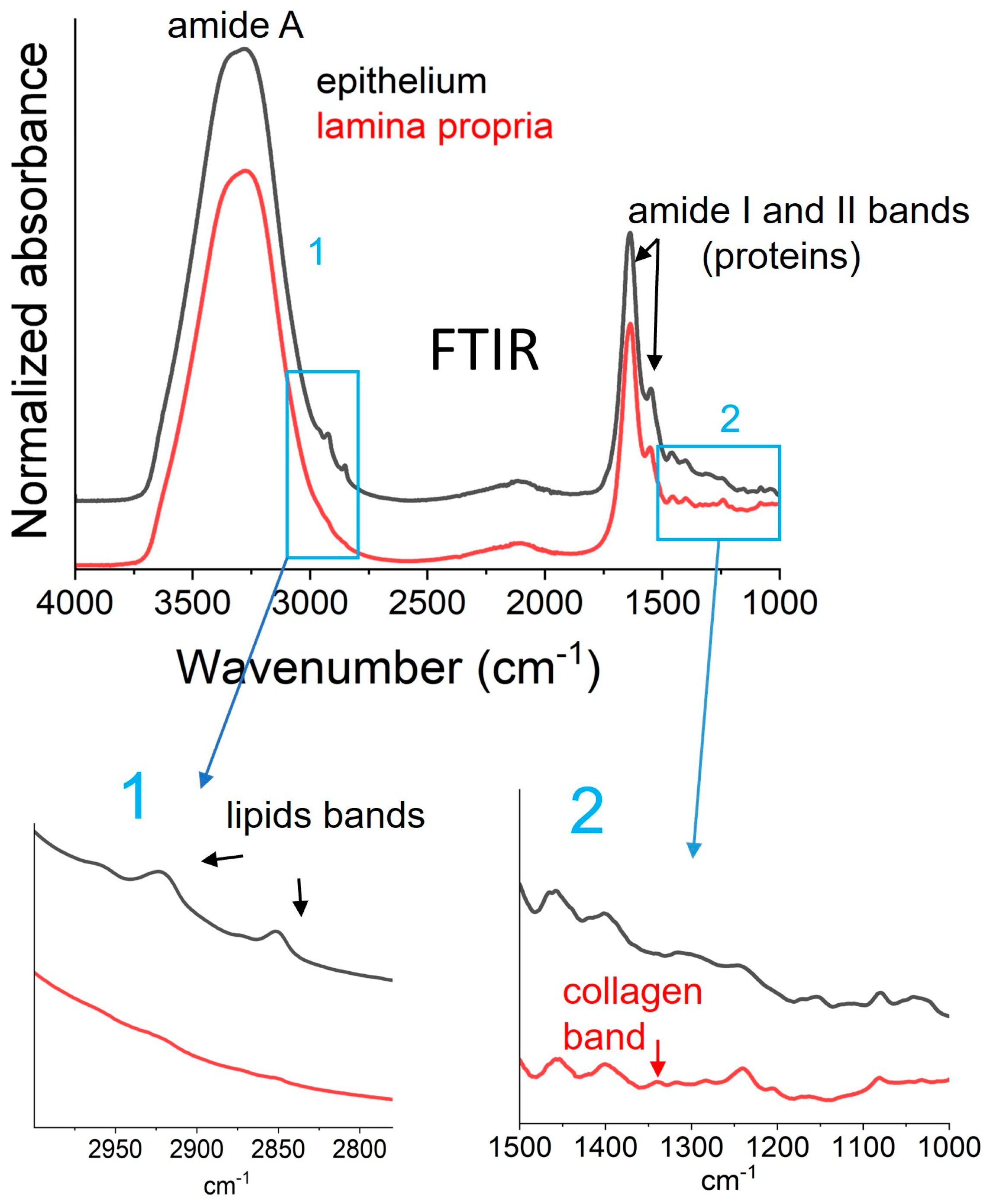
2.2. Vibrational Analysis of Both Lamina Propria and Epithelium from Oral Biopsies
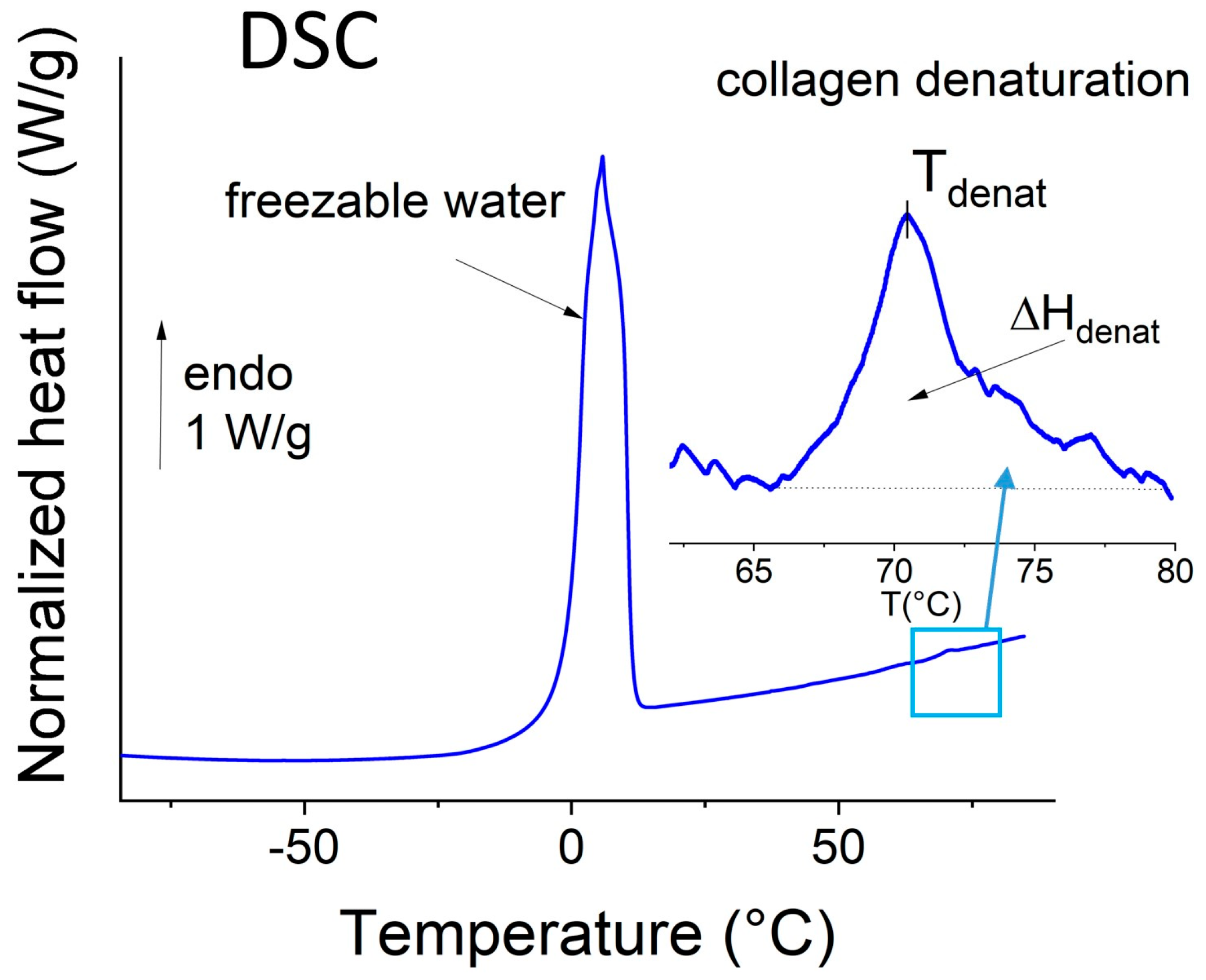
2.3. Thermal Analysis of the Whole Biopsies
2.4. Statistical Analysis
3. Results and Discussion
3.1. Description of the Population
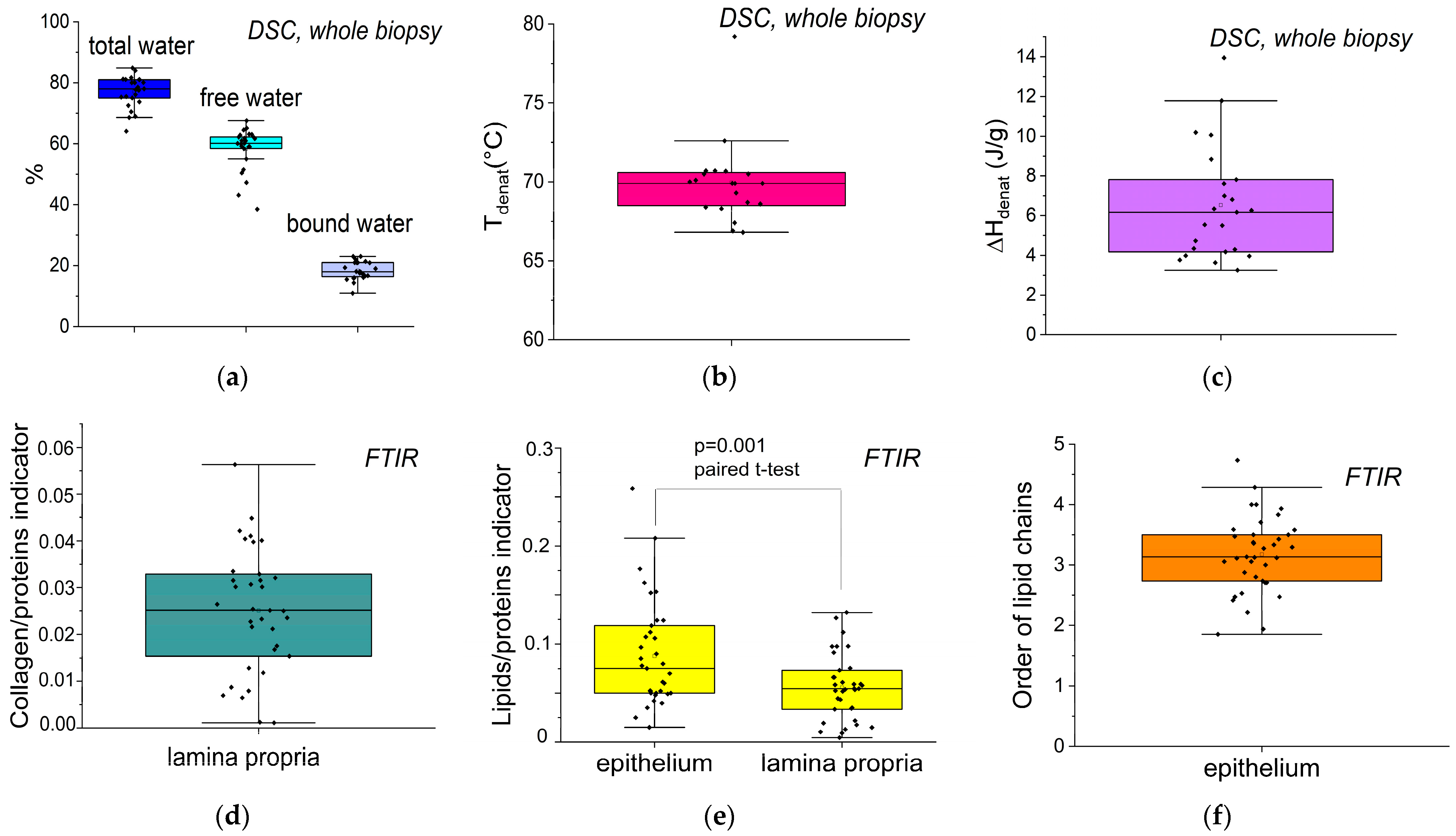
3.2. Extraction of Biophysical Markers from FTIR Spectra and DSC Thermograms
3.3. Links Between Biophysical Markers and Physio-Pathological Factors
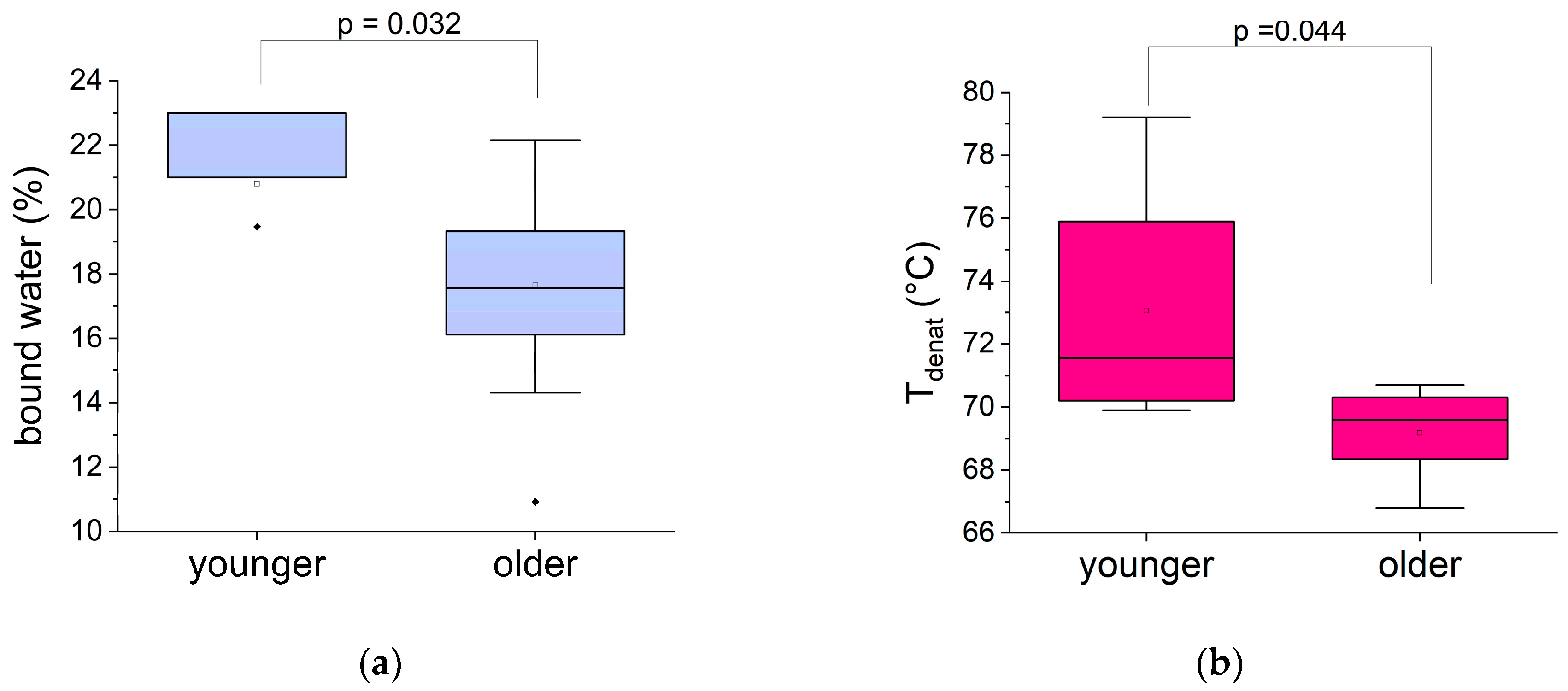
3.3.1. Impact of Age
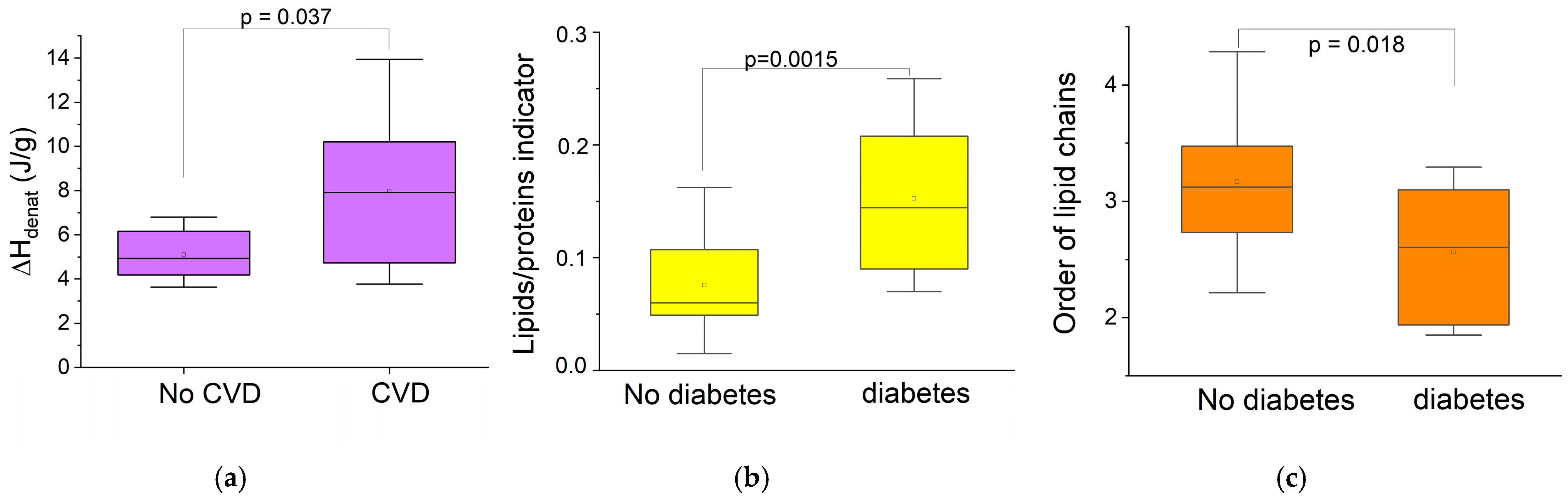
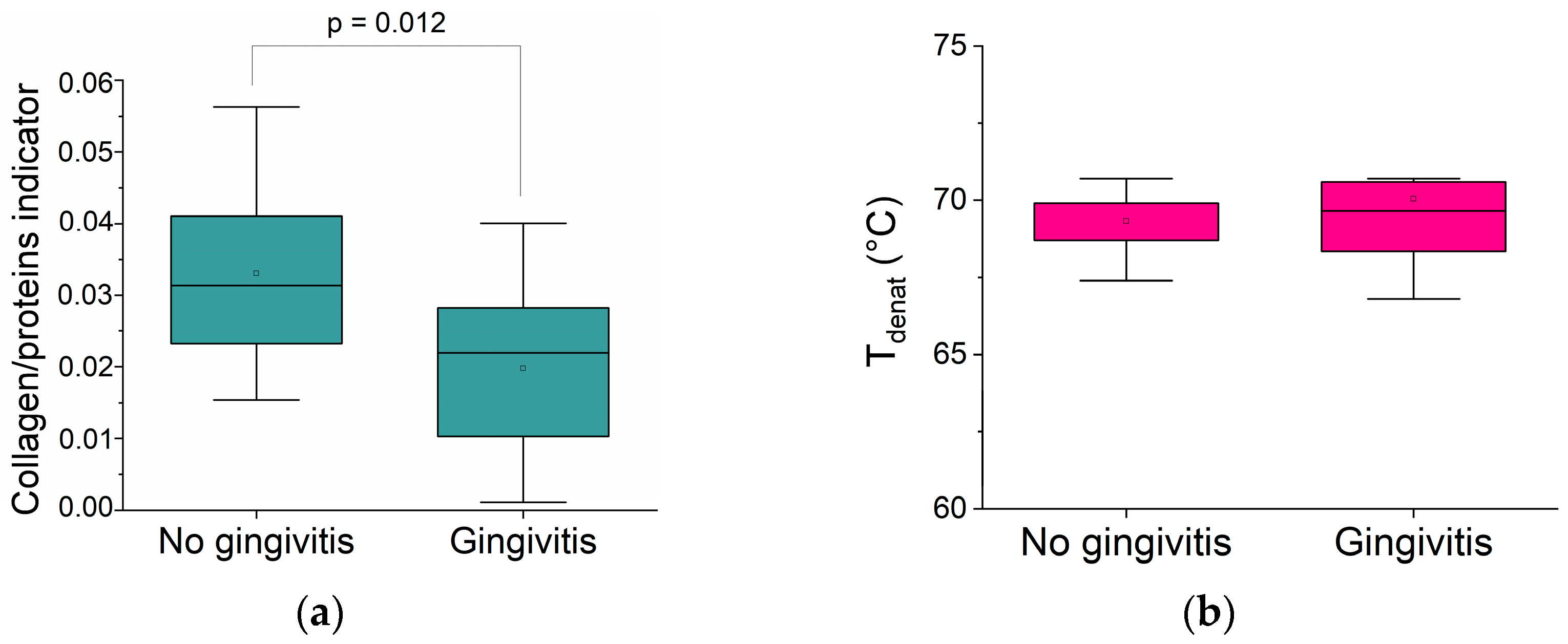
3.3.2. Links Between Biophysical Markers and Pathological Factors in the Older Group
- •
- Chronic pathologies
- •
- Local inflammation (gingivitis)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crimmins, E.; Vasunilashorn, S.; Kim, J.K.; Alley, D. Biomarkers Related to Ageing in Human Populations. Adv. Clin. Chem. 2008, 46, 161–216. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ahmad, R.; Li, W.; Swain, M.; Li, Q. Biomechanics of oral mucosa. J. R. Soc. Interface 2015, 12, 20150325. [Google Scholar] [CrossRef]
- Waasdorp, M.; Krom, B.P.; Bikker, F.J.; van Zuijlen, P.P.M.; Niessen, F.B.; Gibbs, S. The Bigger Picture: Why Oral Mucosa Heals Better Than Skin. Biomolecules 2021, 11, 1165. [Google Scholar] [CrossRef]
- Cruchley, A.T.; Bergmeier, L.A. Structure and Functions of the Oral Mucosa. In Oral Mucosa in Health and Disease; Bergmeier, L.A., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–18. [Google Scholar]
- Hamzah, Z.; Indartin, D.; Meilawaty, Z. The Progressive Low Chronic Inflammation on Oral Tissue in Elderly. In Proceedings of the 1st International Conference on Medicine and Health Sciences (ICMHS), East Java, Indonesia, 31 August–1 September 2016; pp. 177–181. [Google Scholar]
- Akimoto, K. Observations on the Structural Changes According to Aging of Oral Mucous Membrane in the Elderly-Structure of Buccal Mucous Membrane in the Vicinity of Angulus Oris-. J. Stomatol. Soc. 2004, 71, 80–94. [Google Scholar] [CrossRef] [PubMed]
- Quan, T.; Fisher, G.J. Role of Age-Associated Alterations of the Dermal Extracellular Matrix Microenvironment in Human Skin Aging: A Mini-Review. Gerontology 2015, 61, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Rajalalitha, P.; Vali, S. Molecular Pathogenesis of Oral Submucous Fibrosis—A Collagen Metabolic Disorder. J. Oral Pathol. Med. 2005, 34, 321–328. [Google Scholar] [CrossRef]
- Poser, M.; Sing, K.E.A.; Ebert, T.; Ziebolz, D.; Schmalz, G. The Rosetta Stone of Successful Ageing: Does Oral Health Have a Role? Biogerontology 2023, 24, 867–888. [Google Scholar] [CrossRef]
- Teker, H.T.; Ceylani, T.; Keskin, S.; Samgane, G.; Mansuroglu, S.; Baba, B.; Allahverdi, H.; Acıkgoz, E.; Gurbanov, R. Age-Related Differences in Response to Plasma Exchange in Male Rat Liver Tissues: Insights from Histopathological and Machine-Learning Assisted Spectrochemical Analyses. Biogerontology 2023, 24, 563–580. [Google Scholar] [CrossRef]
- Rehman, I.; Movasaghi, Z.; Rehman, S. Vibrational Spectroscopy for Tissue Analysis; CRC Press: Boca Raton, FL, USA, 2012; ISBN 9781439836095. [Google Scholar]
- Melin, A.M.; Perromat, A.; Deleris, G. Fourier-Transform Infrared Spectroscopy: A Pharmacotoxicologic Tool for in Vivo Monitoring Radical Aggression. Can. J. Physiol. Pharmacol. 2001, 79, 158–165. [Google Scholar] [CrossRef]
- Staniszewska, E.; Malek, K.; Baranska, M. Rapid Approach to Analyze Biochemical Variation in Rat Organs by ATR FTIR Spectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 118, 981–986. [Google Scholar] [CrossRef]
- Hynes, A.; Scott, D.A.; Man, A.; Singer, D.L.; Sowa, M.G.; Liu, K.Z. Molecular Mapping of Periodontal Tissues Using Infrared Microspectroscopy. BMC Med. Imaging 2005, 5, 2. [Google Scholar] [CrossRef][Green Version]
- Ober, C.; Samouillan, V.; Lacoste-Ferré, M.H.; Dandurand, J.; Lacabanne, C. Thermal and Vibrational Biomarkers of Porcine Oral Mucosa: Influence of Localization on Hydric Organization and Physical Structure. J. Therm. Anal. Calorim. 2020, 144, 1229–1238. [Google Scholar] [CrossRef]
- Lauritsen, A.K.; Pereira, J.E.M.; Juranyi, F.; Bordallo, H.N.; Larsen, L.; Benetti, A.R. Probing Water Mobility in Human Dentine with Neutron Spectroscopy. J. Dent. Res. 2018, 97, 1017–1022. [Google Scholar] [CrossRef]
- Greene, J.C.; Vermillion, J.R. The Simplified Oral Hygiene Index. J. Am. Dent. Assoc. 1964, 68, 7–13. [Google Scholar] [CrossRef]
- Newbrun, E. Indices to Measure Gingival Bleeding. J. Periodontol. 1996, 67, 555–561. [Google Scholar] [CrossRef]
- Chalmers, J.M.; King, P.L.; Spencer, A.J.; Wright, F.A.C.; Carter, K.D. The Oral Health Assessment Tool—Validity and Reliability. Aust. Dent. J. 2005, 50, 191–199. [Google Scholar] [CrossRef]
- Simsek Ozek, N.; Zeller, I.; Renaud, D.E.; Gümüs, P.; Nizam, N.; Severcan, F.; Buduneli, N.; Scott, D.A. Differentiation of Chronic and Aggressive Periodontitis by FTIR Spectroscopy. J. Dent. Res. 2016, 95, 1472–1478. [Google Scholar] [CrossRef]
- Samouillan, V.; Delaunay, F.; Dandurand, J.; Merbahi, N.; Gardou, J.-P.; Yousfi, M.; Gandaglia, A.; Spina, M.; Lacabanne, C. The Use of Thermal Techniques for the Characterization and Selection of Natural Biomaterials. J. Funct. Biomater. 2011, 2, 230–248. [Google Scholar] [CrossRef]
- Olsztyńska-Janus, S.; Pietruszka, A.; Kiełbowicz, Z.; Czarnecki, M.A. ATR-IR Study of Skin Components: Lipids, Proteins and Water. Part I: Temperature Effect. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 188, 37–49. [Google Scholar] [CrossRef]
- Tang, R.; Samouillan, V.; Dandurand, J.; Lacabanne, C.; Lacoste-Ferre, M.-H.; Bogdanowicz, P.; Bianchi, P.; Villaret, A.; Nadal-Wollbold, F. Identification of Ageing Biomarkers in Human Dermis Biopsies by Thermal Analysis (DSC) Combined with Fourier Transform Infrared Spectroscopy (FTIR/ATR). Ski. Res. Technol. 2017, 23, 573–580. [Google Scholar] [CrossRef]
- Miles, C.A.; Avery, N.C. Thermal Stabilization of Collagen in Skin and Decalcified Bone. Phys. Biol. 2011, 8, 026002. [Google Scholar] [CrossRef]
- Wiegand, N.; Naumov, I.; Nőt, L.G.; Vámhidy, L.; Lőrinczy, D. Differential Scanning Calorimetric Examination of Pathologic Scar Tissues of Human Skin. J. Therm. Anal. Calorim. 2012, 111, 1897–1902. [Google Scholar] [CrossRef]
- Samouillan, V.; Dandurand, J.; Lacabanne, C.; Thoma, R.J.; Adams, A.; Moore, M. Comparison of Chemical Treatments on the Chain Dynamics and Thermal Stability of Bovine Pericardium Collagen. J. Biomed. Mater. Res. A 2003, 64, 330–338. [Google Scholar] [CrossRef]
- Trębacz, H.; Szczęsna, A.; Arczewska, M. Thermal Stability of Collagen in Naturally Ageing and in Vitro Glycated Rabbit Tissues. J. Therm. Anal. Calorim. 2018, 134, 1903–1911. [Google Scholar] [CrossRef]
- Giannetti, G.; Matsumura, F.; Caporaletti, F.; Micha, D.; Koenderink, G.H.; Ilie, I.M.; Bonn, M.; Woutersen, S.; Giubertoni, G. Water and Collagen: A Mystery Yet to Unfold. Biomacromolecules 2025, 26, 2784–2799. [Google Scholar] [CrossRef]
- Kerch, G.; Zicans, J.; Merijs Meri, R.; Stunda-Ramava, A.; Jakobsons, E. The Use of Thermal Analysis in Assessing the Effect of Bound Water Content and Substrate Rigidity on Prevention of Platelet Adhesion. J. Therm. Anal. Calorim. 2015, 120, 533–539. [Google Scholar] [CrossRef]
- Shin, J.W.; Kwon, S.H.; Choi, J.Y.; Na, J.I.; Huh, C.H.; Choi, H.R.; Park, K.C. Molecular Mechanisms of Dermal Aging and Antiaging Approaches. Int. J. Mol. Sci. 2019, 20, 2126. [Google Scholar] [CrossRef]
- Wiegand, N.; Vámhidy, L.; Lőrinczy, D. Differential Scanning Calorimetric Examination of Ruptured Lower Limb Tendons in Human. J. Therm. Anal. Calorim. 2010, 101, 487–492. [Google Scholar] [CrossRef]
- Radzki, D.; Negri, A.; Kusiak, A.; Obuchowski, M. Matrix Metalloproteinases in the Periodontium-Vital in Tissue Turnover and Unfortunate in Periodontitis. Int. J. Mol. Sci. 2024, 25, 2763. [Google Scholar] [CrossRef]
- Ruiz Martínez, M.A.; Peralta Galisteo, S.; Castán, H.; Morales Hernández, M.E. Role of proteoglycans on skin ageing: A review. Int. J. Cosmet. Sci. 2020, 42, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Reigle, K.L.; Di Lullo, G.; Turner, K.R.; Last, J.A.; Chervoneva, I.; Birk, D.E.; Funderburgh, J.L.; Elrod, E.; Germann, M.W.; Surber, C.; et al. Non-Enzymatic Glycation of Type I Collagen Diminishes Collagen-Proteoglycan Binding and Weakens Cell Adhesion. J. Cell. Biochem. 2008, 104, 1684–1698. [Google Scholar] [CrossRef] [PubMed]
- Calleja-Agius, J.; Muscat-Baron, Y.; Brincat, M.P. Skin Ageing. Menopause Int. 2007, 13, 60–64. [Google Scholar] [CrossRef]
- Calleja-Agius, J.; Brincat, M.; Borg, M. Skin Connective Tissue and Ageing. Best Pract. Res. Clin. Obstet. Gynaecol. 2013, 27, 727–740. [Google Scholar] [CrossRef]
- Camilion, J.V.; Khanna, S.; Anasseri, S.; Laney, C.; Mayrovitz, H.N. Physiological, Pathological, and Circadian Factors Impacting Skin Hydration. Cureus 2022, 14, e27666. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Gallagher, J. Healthy Ageing and Oral Health: Priority, Policy and Public Health. BDJ Open 2024, 10, 79. [Google Scholar] [CrossRef]
- Travers, J.G.; Kamal, F.A.; Robbins, J.; Yutzey, K.E.; Blaxall, B.C. Cardiac Fibrosis: The Fibroblast Awakens. Circ. Res. 2016, 118, 1021–1040. [Google Scholar] [CrossRef]
- Mialet-Perez, J.; Douin-Echinard, V.; Cussac, D.; Bril, A.; Parini, A. Vieillissement—Une Question de Cœur? Med. Sci. 2015, 31, 1006–1013. [Google Scholar] [CrossRef]
- Chandrasekaran, P.; Weiskirchen, R. The Role of Obesity in Type 2 Diabetes Mellitus—An Overview. Int. J. Mol. Sci. 2024, 25, 1882. [Google Scholar] [CrossRef]
- Klaiss-Luna, M.C.; Manrique-Moreno, M. Infrared Spectroscopic Study of Multi-Component Lipid Systems: A Closer Approximation to Biological Membrane Fluidity. Membranes 2022, 12, 534. [Google Scholar] [CrossRef]
- Suda, M.; Paul, K.H.; Tripathi, U.; Minamino, T.; Tchkonia, T.; Kirkland, J.L. Targeting Cell Senescence and Senolytics: Novel Interventions for Age-Related Endocrine Dysfunction. Endocr. Rev. 2024, 12, 655–675. [Google Scholar] [CrossRef] [PubMed]
- Eid, S.; Sas, K.M.; Abcouwer, S.F.; Feldman, E.L.; Gardner, T.W.; Pennathur, S.; Fort, P.E. New Insights into the Mechanisms of Diabetic Complications: Role of Lipids and Lipid Metabolism. Diabetologia 2019, 62, 1539–1549. [Google Scholar] [CrossRef] [PubMed]
- Melling, M.; Pfeiler, W.; Karimian-Teherani, D.; Schnallinger, M.; Sobal, G.; Zangerle, C.; Menzel, E.J. Differential Scanning Calorimetry, Biochemical, and Biomechanical Analysis of Human Skin from Individuals with Diabetes Mellitus. Anat. Rec. 2000, 259, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Sakai, S.; Kikuchi, K.; Satoh, J.; Tagami, H.; Inoue, S. Functional Properties of the Stratum Corneum in Patients with Diabetes Mellitus: Similarities to Senile Xerosis. Br. J. Dermatol. 2005, 153, 319–323. [Google Scholar] [CrossRef]
- Lauritano, D.; Moreo, G.; Vella, F.D.; Stasio, D.D.; Carinci, F.; Lucchese, A.; Petruzzi, M. Oral Health Status and Need for Oral Care in an Aging Population: A Systematic Review. Int. J. Environ. Res. Public Health 2019, 16, 4558. [Google Scholar] [CrossRef]
- Luchian, I.; Goriuc, A.; Sandu, D.; Covasa, M. The Role of Matrix Metalloproteinases (MMP-8, MMP-9, MMP-13) in Periodontal and Peri-Implant Pathological Processes. Int. J. Mol. Sci. 2022, 23, 1806. [Google Scholar] [CrossRef]
- Lacoste-Ferré, M.H.; Ober, C.; Samouillan, V. Viscoelastic Behavior of Oral Mucosa. A Rheological Study Using Small-Amplitude Oscillatory Shear Tests. J. Mech. Behav. Biomed. Mater. 2023, 143, 105898. [Google Scholar] [CrossRef]
| Younger [20–40 Years Old] n = 5 | Older [70–90 Years Old] n = 33 | |
|---|---|---|
| Sociodemographic characteristics | ||
| Age (mean ± SD) | 27 ± 5 | 82 ± 5 |
| Gender M/F (%) | 4/1 | 19/14 |
| Oral Evaluation | ||
| Teeth number (mean ± SD) | 30 ± 2 | 19 ± 8 |
| OHAT (healthy/degraded) (%) | 4/1 | 10/23 |
| Gingivitis (OHI + SBI > 1) Yes/No (%) | 1/4 | 18/15 |
| Chronic diseases | ||
| Cardiovascular pathologies Yes/No (%) | 0/5 | 21/12 |
| Type II diabetes yes/no (%) | 0/5 | 6 1/27 |
| Biophysical Marker | Technique | Determination | |
|---|---|---|---|
| Hydration | Free water (%) | DSC Whole biopsy | ΔHice melting/334 |
| Total water (%) | Weighing Whole biopsy | (weight hydrated − weight dry)/weight hydrated | |
| Bound water (%) | DSC/weighing Whole biopsy | total water(%) − free water(%) | |
| Collagen | Thermal stability (°C) | DSC Whole biopsy | Tdenat (from denaturation peak) |
| Hydrogen bonds (J/g) | DSC Whole biopsy | ΔHdenat (from denaturation peak) | |
| Collagen/proteins | FTIR Lamina propria only | area (1338 cm−1)/area(1540 cm−1) | |
| Lipids | Lipids/proteins | FTIR Lamina propria Epithelium | area (2927 + 2850 cm−1)/area(1540 cm−1) |
| Order of lipid chains | FTIR Lamina propria only | intensity (2927 cm−1)/intensity (2850 cm−1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samouillan, V.; Ober, C.; Lacoste-Ferré, M.-H. Impact of Aging and Pathologies on Human Oral Mucosa: Preliminary Investigation of Biophysical Markers from Thermal and Vibrational Analyses. Biomolecules 2025, 15, 978. https://doi.org/10.3390/biom15070978
Samouillan V, Ober C, Lacoste-Ferré M-H. Impact of Aging and Pathologies on Human Oral Mucosa: Preliminary Investigation of Biophysical Markers from Thermal and Vibrational Analyses. Biomolecules. 2025; 15(7):978. https://doi.org/10.3390/biom15070978
Chicago/Turabian StyleSamouillan, Valérie, Camille Ober, and Marie-Hélène Lacoste-Ferré. 2025. "Impact of Aging and Pathologies on Human Oral Mucosa: Preliminary Investigation of Biophysical Markers from Thermal and Vibrational Analyses" Biomolecules 15, no. 7: 978. https://doi.org/10.3390/biom15070978
APA StyleSamouillan, V., Ober, C., & Lacoste-Ferré, M.-H. (2025). Impact of Aging and Pathologies on Human Oral Mucosa: Preliminary Investigation of Biophysical Markers from Thermal and Vibrational Analyses. Biomolecules, 15(7), 978. https://doi.org/10.3390/biom15070978







