Identification of a Novel Antibacterial Function of Mammalian Calreticulin
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains, Antibodies, and Reagents
2.2. Animals
2.3. Preparation of Recombinant Calreticulin
2.4. Antibacterial Activity Assay of Calreticulin
2.5. Determination of Bacterial Growth Curves
2.6. Calreticulin–Bacteria-Binding Assays
2.7. Western Blot Analysis
2.8. Bacterial Agglutination Assay
2.9. Histopathological Detection
2.10. Immunohistochemical Staining
2.11. In Vivo Evaluation of Calreticulin’s Protective Effect Against Pasteurella multocida
2.12. RT-qPCR
2.13. Statistical Analysis
3. Results
3.1. Evolutionary Conservation and Sequence Homology Analysis of Calreticulin Across Different Species
3.2. Expression Analysis of Calreticulin in Goat Tissues
3.3. Expression and Purification of Goat Calreticulin
3.4. Verification of Antibacterial Activity of Calreticulin
3.5. Calreticulin Mediates Bacterial Binding and Aggregation
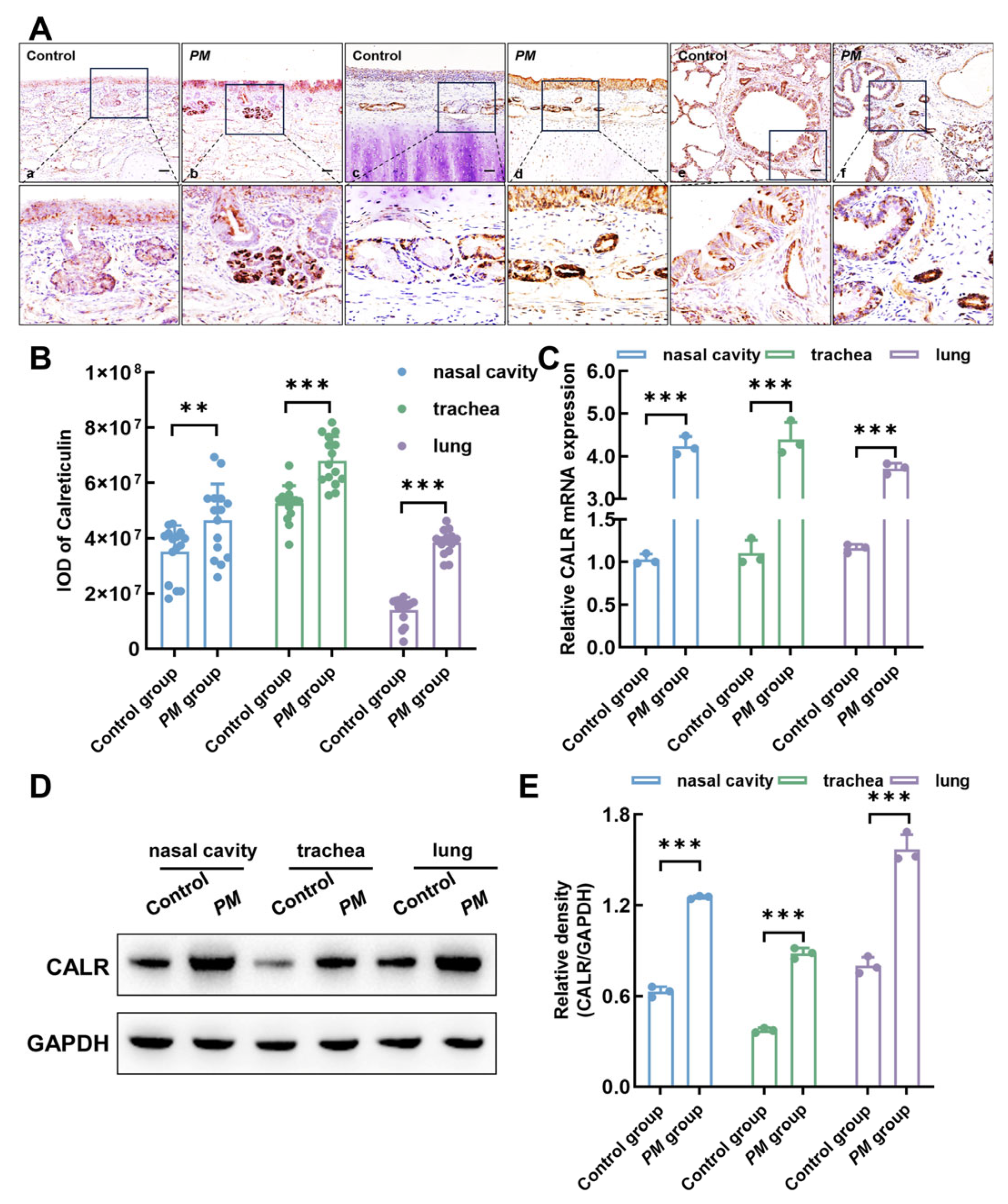
3.6. Upregulation of Calreticulin Expression in Respiratory Mucosa Following Pasteurella multocida Infection in Lambs
3.7. Intranasal Administration of Calreticulin Alleviates Pathological Damage and Promotes Pasteurella multocida Clearance in Lambs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Michalak, M.; Groenendyk, J.; Szabo, E.; Gold, L.I.; Opas, M. Calreticulin, a multi-process calcium-buffering chaperone of the endoplasmic reticulum. Biochem. J. 2009, 417, 651–666. [Google Scholar] [CrossRef]
- Okura, G.C.; Bharadwaj, A.G.; Waisman, D.M. Calreticulin-from the endoplasmic reticulum to the plasma membrane-adventures of a wandering protein. Cancers 2025, 17, 288. [Google Scholar] [CrossRef]
- Krause, K.H.; Michalak, M. Calreticulin. Cell 1997, 88, 439–443. [Google Scholar] [CrossRef]
- Michalak, M. Calreticulin: Endoplasmic reticulum Ca2+ gatekeeper. J. Cell. Mol. Med. 2024, 28, e17839. [Google Scholar] [CrossRef]
- Kwon, M.S.; Park, C.S.; Choi, K.; Ahnn, J.; Kim, J.I.; Eom, S.H.; Kaufman, S.J.; Song, W.K. Calreticulin couples calcium release and calcium influx in integrin-mediated calcium signaling. Mol. Biol. Cell 2000, 11, 1433–1443. [Google Scholar] [CrossRef]
- Schürch, P.M.; Malinovska, L.; Hleihil, M.; Losa, M.; Hofstetter, M.C.; Wildschut, M.H.E.; Lysenko, V.; Lakkaraju, A.K.K.; Maat, C.A.; Benke, D.; et al. Calreticulin mutations affect its chaperone function and perturb the glycoproteome. Cell Rep. 2022, 41, 111689. [Google Scholar] [CrossRef]
- Lum, R.; Ahmad, S.; Hong, S.J.; Chapman, D.C.; Kozlov, G.; Williams, D.B. Contributions of the lectin and polypeptide binding sites of calreticulin to its chaperone functions in vitro and in Cells. J. Biol. Chem. 2016, 291, 19631–19641. [Google Scholar] [CrossRef]
- Wang, X.; Liu, X.; Chen, Y.; Wang, H.; Zhang, R.; Zhang, Q.; Wei, Y.; Shi, S.; Li, X. Calreticulin regulated intrinsic apoptosis through mitochondria-dependent and independent pathways mediated by ER stress in arsenite exposed HT-22 cells. Chemosphere 2020, 251, 126466. [Google Scholar] [CrossRef]
- Ohkuro, M.; Kim, J.D.; Kuboi, Y.; Hayashi, Y.; Mizukami, H.; Kobayashi-Kuramochi, H.; Muramoto, K.; Shirato, M.; Michikawa-Tanaka, F.; Moriya, J.; et al. Calreticulin and integrin alpha dissociation induces anti-inflammatory programming in animal models of inflammatory bowel disease. Nat. Commun. 2018, 9, 1982. [Google Scholar] [CrossRef]
- Domnick, A.; Winter, C.; Sušac, L.; Hennecke, L.; Hensen, M.; Zitzmann, N.; Trowitzsch, S.; Thomas, C.; Tampé, R. Molecular basis of MHC I quality control in the peptide loading complex. Nat. Commun. 2022, 13, 4701. [Google Scholar] [CrossRef]
- Fucikova, J.; Spisek, R.; Kroemer, G.; Galluzzi, L. Calreticulin and cancer. Cell Res. 2021, 31, 5–16. [Google Scholar] [CrossRef]
- Huang, Y.; Li, Y.F.; Wang, R.X.; Xie, M.F.; Shi, Y.; Zhao, Z. Calreticulin functions in antimicrobial immunity of obscure puffer Takifugu obscurus. Mol. Immunol. 2021, 140, 77–86. [Google Scholar] [CrossRef]
- Sun, J.Q.; Zhao, K.Y.; Zhang, Z.X.; Li, X.P. Two novel teleost calreticulins PoCrt-1/2, with bacterial binding and agglutination activity, are involved in antibacterial immunity. Fish. Shellfish. Immunol. 2023, 143, 109203. [Google Scholar] [CrossRef]
- Liu, X.; Xu, N.; Zhang, S. Calreticulin is a microbial-binding molecule with phagocytosis-enhancing capacity. Fish. Shellfish. Immunol. 2013, 35, 776–784. [Google Scholar] [CrossRef]
- Wang, G.; Jiang, Z.; Zhang, M.; Yang, N.; Zhu, D. Identification of a new calreticulin homolog from Yesso scallop (Patinopecten yessoensis) and its role in innate immunity. Fish. Shellfish. Immunol. 2016, 58, 108–115. [Google Scholar] [CrossRef]
- Wang, G.; Jiang, Z.; Yang, N.; Zhu, D.; Zhang, M. Identification and characterization of a novel calreticulin involved in the immune response of the Zhikong scallop, Chlamys farreri. Fish. Shellfish. Immunol. 2017, 64, 251–259. [Google Scholar] [CrossRef]
- Huang, Y.; Hui, K.; Jin, M.; Yin, S.; Wang, W.; Ren, Q. Two endoplasmic reticulum proteins (calnexin and calreticulin) are involved in innate immunity in Chinese mitten crab (Eriocheir sinensis). Sci. Rep. 2016, 6, 27578. [Google Scholar] [CrossRef]
- Oliveira, M.; Antunes, W.; Mota, S.; Madureira-Carvalho, Á.; Dinis-Oliveira, R.J.; Dias da Silva, D. An overview of the recent advances in antimicrobial resistance. Microorganisms 2024, 12, 1920. [Google Scholar] [CrossRef]
- Elshobary, M.E.; Badawy, N.K.; Ashraf, Y.; Zatioun, A.A.; Masriya, H.H.; Ammar, M.M.; Mohamed, N.A.; Mourad, S.; Assy, A.M. Combating antibiotic resistance: Mechanisms, multidrug-resistant pathogens, and novel therapeutic approaches: An updated review. Pharmaceuticals 2025, 18, 402. [Google Scholar] [CrossRef]
- Haney, E.F.; Straus, S.K.; Hancock, R.E.W. Reassessing the host defense peptide landscape. Front. Chem. 2019, 7, 43. [Google Scholar] [CrossRef]
- Shibata, R.; Raita, Y.; Zhu, Z. Salivary polyreactive antibodies, airway bacteria, and recurrent respiratory infection severity. Eur. Respir. J. 2024, 64, 2401526. [Google Scholar] [CrossRef]
- Claassen-Weitz, S.; Lim, K.Y.L.; Mullally, C.; Zar, H.J.; Nicol, M.P. The association between bacteria colonizing the upper respiratory tract and lower respiratory tract infection in young children: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2021, 27, 1262–1270. [Google Scholar] [CrossRef]
- Mac Aogáin, M.; Dicker, A.J.; Mertsch, P.; Chotirmall, S.H. Infection and the microbiome in bronchiectasis. Eur. Respir. Rev. 2024, 33, 240038. [Google Scholar] [CrossRef]
- Fröhlich, E. Animals in respiratory research. Int. J. Mol. Sci. 2024, 25, 2903. [Google Scholar] [CrossRef]
- Alvites, R.D.; Branquinho, M.V.; Sousa, A.C.; Lopes, B.; Sousa, P.; Mendonça, C.; Atayde, L.M.; Maurício, A.C. Small ruminants and its use in regenerative medicine: Recent works and future perspectives. Biology 2021, 10, 249. [Google Scholar] [CrossRef]
- Gonzalez-Juarrero, M.; Bosco-Lauth, A.; Podell, B.; Soffler, C.; Brooks, E.; Izzo, A.; Sanchez-Campillo, J.; Bowen, R. Experimental aerosol Mycobacterium bovis model of infection in goats. Tuberculosis 2013, 93, 558–564. [Google Scholar] [CrossRef]
- Guillamón, M.D.L.; Clau, L.B. The sheep as a large animal experimental model in respiratory diseases research. Arch. Bronconeumol. 2010, 46, 499–501. [Google Scholar] [CrossRef]
- Wilson, B.A.; Ho, M. Pasteurella multocida: From zoonosis to cellular microbiology. Clin. Microbiol. Rev. 2013, 26, 631–655. [Google Scholar] [CrossRef]
- Peng, Z.; Lin, L.; Wang, X.; Chen, H.; Wu, B. The public health concern of Pasteurella multocida should not be ignored. Lancet Microbe. 2022, 3, e560. [Google Scholar] [CrossRef]
- Huang, Y.; Pan, J.; Li, X.; Ren, Q.; Zhao, Z. Molecular cloning and functional characterization of a short peptidoglycan recognition protein from triangle-shell pearl mussel (Hyriopsis cumingii). Fish. Shellfish. Immunol. 2019, 86, 571–580. [Google Scholar] [CrossRef]
- Mu, L.; Yin, X.; Wu, H.; Lei, Y.; Han, K.; Mo, J.; Guo, Z.; Li, J.; Ye, J. Mannose-binding lectin possesses agglutination activity and promotes opsonophagocytosis of macrophages with calreticulin interaction in an early vertebrate. J. Immunol. 2020, 205, 3443–3455. [Google Scholar] [CrossRef]
- Wu, M.; Jia, B.B.; Li, M.F. Complement C3 and activated fragment C3a are involved in complement activation and anti-bacterial immunity. Front. Immunol. 2022, 13, 813173. [Google Scholar] [CrossRef]
- Ebrahimi, S.B.; Samanta, D. Engineering protein-based therapeutics through structural and chemical design. Nat. Commun. 2023, 14, 2411. [Google Scholar] [CrossRef]
- Parayath, N.N.; Amiji, M.M. Therapeutic targeting strategies using endogenous cells and proteins. J. Control. Release 2017, 258, 81–94. [Google Scholar] [CrossRef]
- Escobar-Salom, M.; Torrens, G.; Jordana-Lluch, E.; Oliver, A.; Juan, C. Mammals’ humoral immune proteins and peptides targeting the bacterial envelope: From natural protection to therapeutic applications against multidrug-resistant Gram-negatives. Biol. Rev. Camb. Philos. Soc. 2022, 97, 1005–1037. [Google Scholar] [CrossRef]
- Mendlovic, F.; Conconi, M. Calreticulin: A multifaceted protein. Nat. Educ. 2010, 4, 1. [Google Scholar]
- Okura, G.C.; Bharadwaj, A.G.; Waisman, D.M. Calreticulin-Enigmatic discovery. Biomolecules 2024, 14, 866. [Google Scholar] [CrossRef]
- Opas, M.; Dziak, E.; Fliegel, L.; Michalak, M. Regulation of expression and intracellular distribution of calreticulin, a major calcium binding protein of nonmuscle cells. J. Cell. Physiol. 1991, 149, 160–171. [Google Scholar] [CrossRef]
- Johnson, S.; Michalak, M.; Opas, M.; Eggleton, P. The ins and outs of calreticulin: From the ER lumen to the extracellular space. Trends. Cell Biol. 2001, 11, 122–129. [Google Scholar] [CrossRef]
- Wang, G.H.; Li, Z.X.; Guo, E.M.; Wang, J.J.; Zhang, M.; Hu, Y.H. A novel calreticulin-related molecule that interacts with bacteria and enhances host resistance against bacterial infection in black rockfish, Sebastes schlegeli. Fish. Shellfish. Immunol. 2019, 93, 823–831. [Google Scholar] [CrossRef]
- Silerová, M.; Kauschke, E.; Procházková, P.; Josková, R.; Tucková, L.; Bilej, M. Characterization, molecular cloning and localization of calreticulin in Eisenia fetida earthworms. Gene 2007, 397, 169–177. [Google Scholar] [CrossRef]
- Karbalaei, M.; Rezaee, S.A.; Farsiani, H. Pichia pastoris: A highly successful expression system for optimal synthesis of heterologous proteins. J. Cell. Physiol. 2020, 235, 5867–5881. [Google Scholar] [CrossRef]
- Vijayakumar, V.E.; Venkataraman, K. A systematic review of the potential of Pichia pastoris (Komagataella phaffii) as an alternative host for biologics production. Mol. Biotechnol. 2024, 66, 1621–1639. [Google Scholar] [CrossRef]
- Varricchio, L.; Falchi, M.; Dall’Ora, M.; De Benedittis, C.; Ruggeri, A.; Uversky, V.N.; Migliaccio, A.R. Calreticulin: Challenges posed by the intrinsically disordered nature of calreticulin to the study of its function. Front. Cell Dev. Biol. 2017, 5, 96. [Google Scholar] [CrossRef]
- Bondos, S.E.; Dunker, A.K.; Uversky, V.N. Intrinsically disordered proteins play diverse roles in cell signaling. Cell Commun. Signal. 2022, 20, 20. [Google Scholar] [CrossRef]
- Kishore, U.; Eggleton, P.; Reid, K.B. Modular organization of carbohydrate recognition domains in animal lectins. Matrix. Biol. 1997, 15, 583–592. [Google Scholar] [CrossRef]
- Day, C.J.; Tran, E.N.; Semchenko, E.A.; Tram, G.; Hartley-Tassell, L.E.; Ng, P.S.; King, R.M.; Ulanovsky, R.; McAtamney, S.; Apicella, M.A.; et al. Glycan: Glycan interactions: High affinity biomolecular interactions that can mediate binding of pathogenic bacteria to host cells. Proc. Natl. Acad. Sci. USA 2015, 112, E7266–E7275. [Google Scholar] [CrossRef]
- Di Lorenzo, F.; Duda, K.A.; Lanzetta, R.; Silipo, A.; De Castro, C.; Molinaro, A. A Journey from structure to function of bacterial lipopolysaccharides. Chem. Rev. 2022, 122, 15767–15821. [Google Scholar] [CrossRef]
- Yin, X.; Shan, J.; Dou, L.; Cheng, Y.; Liu, S.; Hassan, R.; Wang, Y.; Wang, J.; Zhang, D. Multiple bacteria recognition mechanisms and their applications. Coord. Chem. Rev. 2024, 517, 216025. [Google Scholar] [CrossRef]
- Jannesari, M.; Ejehi, F.; English, N.J.; Mohammadpour, R.; Akhavan, O.; Shams, S. Triggering triboelectric nanogenerator antibacterial Activities: Effect of charge polarity and host material correlation. Chem. Eng. J. 2024, 486, 150036. [Google Scholar] [CrossRef]
- Gong, X.; Han, Y.; Wang, T.; Song, G.; Chen, H.; Tang, H.; Huang, X.; Deng, K.; Wang, S.; Wang, Y. Cell-Penetrating peptide induced superstructures triggering highly efficient antibacterial activity. Adv. Mater. 2025, 37, e2414357. [Google Scholar] [CrossRef]
- Jørgensen, C.S.; Ryder, L.R.; Steinø, A.; Højrup, P.; Hansen, J.; Beyer, N.H.; Heegaard, N.H.; Houen, G. Dimerization and oligomerization of the chaperone calreticulin. Eur. J. Biochem. 2003, 270, 4140–4148. [Google Scholar] [CrossRef]
- Huang, S.H.; Zhao, L.X.; Hong, C.; Duo, C.C.; Guo, B.N.; Zhang, L.J.; Gong, Z.; Xiong, S.D.; Gong, F.Y.; Gao, X.M. Self-oligomerization is essential for enhanced immunological activities of soluble recombinantf calreticulin. PLoS ONE 2013, 8, e64951. [Google Scholar]
- Liang, W.; Hu, L.; Dai, F.; Shi, Y.; Yang, L.; Li, C. Calreticulin from Apostichopus japonicus relieves endoplasmic reticulum stress induced by Vibrio splendidus through autophagy. Fish. Shellfish. Immunol. 2024, 153, 109798. [Google Scholar] [CrossRef]
- Li, Z.; Gu, J.; Huang, X.; Lu, Z.; Feng, Y.; Xu, X.; Yang, J. Transcriptome-based network analysis reveals hub immune genes and pathways of hepatopancreas against LPS in Amphioctopus fangsiao. Fish. Shellfish. Immunol. 2024, 151, 109696. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Liu, J.; Qin, X.; Cui, X.; Yang, Q. Identification of a Novel Antibacterial Function of Mammalian Calreticulin. Biomolecules 2025, 15, 966. https://doi.org/10.3390/biom15070966
Ma Y, Liu J, Qin X, Cui X, Yang Q. Identification of a Novel Antibacterial Function of Mammalian Calreticulin. Biomolecules. 2025; 15(7):966. https://doi.org/10.3390/biom15070966
Chicago/Turabian StyleMa, Yichao, Jiachen Liu, Xinming Qin, Xiaojing Cui, and Qian Yang. 2025. "Identification of a Novel Antibacterial Function of Mammalian Calreticulin" Biomolecules 15, no. 7: 966. https://doi.org/10.3390/biom15070966
APA StyleMa, Y., Liu, J., Qin, X., Cui, X., & Yang, Q. (2025). Identification of a Novel Antibacterial Function of Mammalian Calreticulin. Biomolecules, 15(7), 966. https://doi.org/10.3390/biom15070966





