The Role of Myeloid Differentiation Factor 2 in Stroke: Mechanisms and Therapeutic Potential
Abstract
1. Introduction
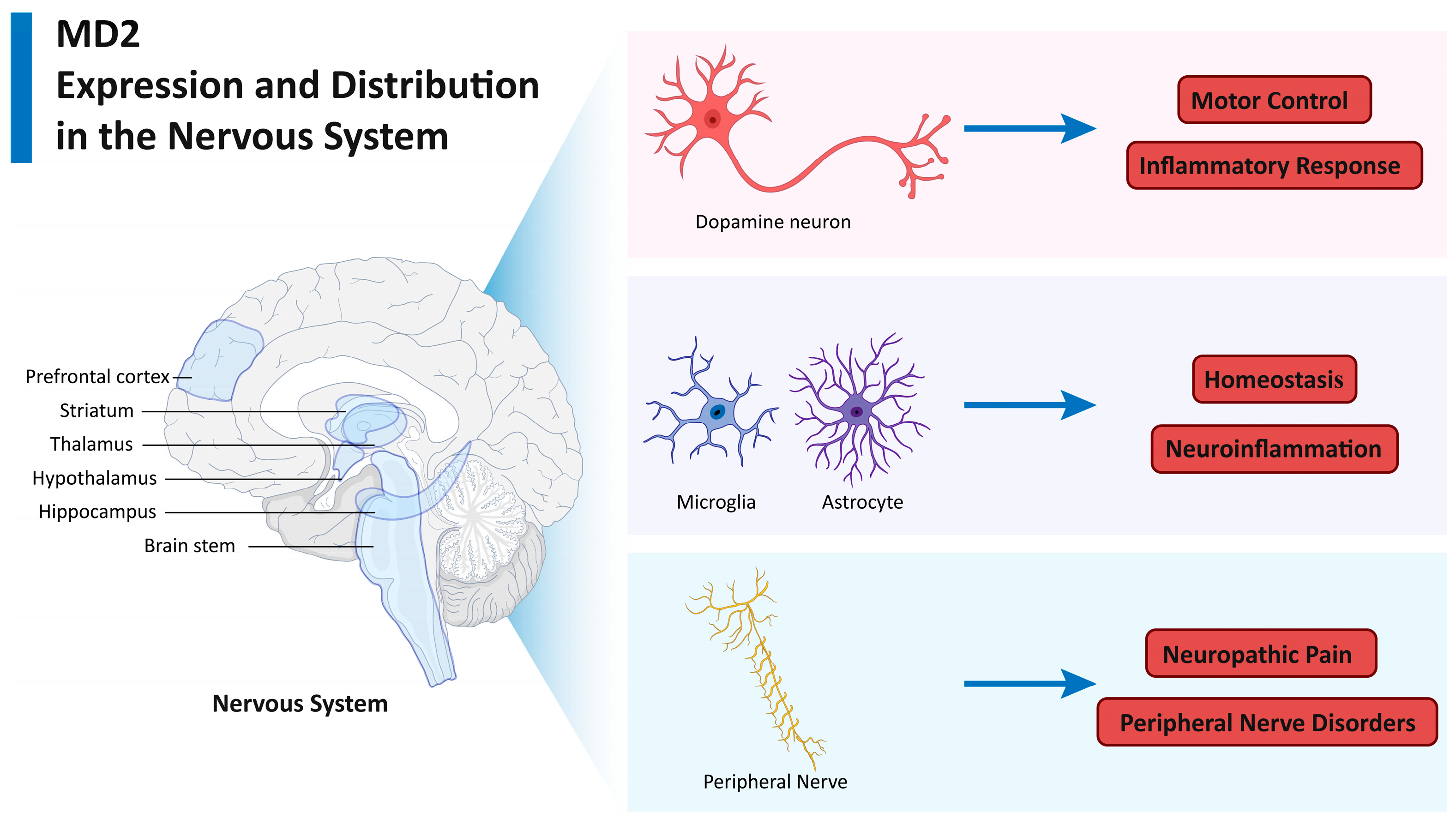
2. MD2 Expression and Distribution in the Nervous System
2.1. MD2 in Neurons
2.2. MD2 in Glial Cells
2.3. MD2 in Peripheral Nerve Cells
3. TLR4-MD2 Complex and Stroke
3.1. Molecular Mechanisms
3.2. Neuroinflammation
3.3. Neuronal Death
4. TLR4-Independent Mechanisms of MD2 in Stroke
4.1. MD2 and Sam68 Interaction
4.2. MD2 and Clec7a Interaction
4.3. MD2 and ROS
4.4. MD2 and Blood–Brain Barrier Disruption
5. Therapeutic Targeting of MD2 in Stroke
5.1. Small-Molecule Inhibitors
5.2. Peptide Inhibitor
5.3. Natural Compound
6. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MD2 | Myeloid Differentiation Factor 2 |
| TLR4 | Toll-like receptor 4 |
| DAMPs | Damage-associated molecular patterns |
| PAMPs | Pathogen-associated molecular patterns |
| NF-κB | Nuclear factor-kappa B |
| ROS | Reactive oxygen species |
| CNS | Central nervous system |
| BBB | Blood–brain barrier |
| TNF-α | Tumor necrosis factor-alpha |
| IL-1β | Interleukin-1 beta |
| IL-6 | Interleukin-6 |
| LPS | Lipopolysaccharide |
| TRIF | TIR-domain-containing adapter-inducing interferon-β |
| IRF | Interferon regulatory factor |
| MyD88 | Myeloid differentiation primary response 88 |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| RIPK | Receptor-interacting protein kinase |
| HMGB1 | High-mobility group box 1 |
| NLRP3 | NOD-like receptor family pyrin domain-containing 3 |
| RIPK1/RIPK3 | Receptor-interacting serine/threonine–protein kinase 1/3 |
| DRG | Dorsal root ganglion |
| NMDA | N-methyl-d-aspartate |
| FN-EDA | Extra domain A of fibronectin |
| Tat-CIRP | Tat–cold-inducible RNA binding protein |
| MCAO | Middle cerebral artery occlusion |
| CIRI | Cerebral ischemia–reperfusion injury |
References
- Khandelwal, P.; Yavagal, D.R.; Sacco, R.L. Acute Ischemic Stroke Intervention. J. Am. Coll. Cardiol. 2016, 67, 2631–2644. [Google Scholar] [CrossRef] [PubMed]
- Rotaru-Zavaleanu, A.D.; Dinescu, V.C.; Aldea, M.; Gresita, A. Hydrogel-Based Therapies for Ischemic and Hemorrhagic Stroke: A Comprehensive Review. Gels 2024, 10, 476. [Google Scholar] [CrossRef] [PubMed]
- Rocha, E.A.; Singhal, A.B. Recurrent Ischemic and Hemorrhagic Strokes in a Young Adult. JAMA Neurol. 2018, 75, 628–629. [Google Scholar] [CrossRef]
- Lambertsen, K.L.; Finsen, B.; Clausen, B.H. Post-stroke inflammation-target or tool for therapy? Acta Neuropathol. 2019, 137, 693–714. [Google Scholar] [CrossRef]
- Degterev, A.; Huang, Z.; Boyce, M.; Li, Y.; Jagtap, P.; Mizushima, N.; Cuny, G.D.; Mitchison, T.J.; Moskowitz, M.A.; Yuan, J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat. Chem. Biol. 2005, 1, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.; Dong, W.; Zhang, M.; Wang, Z.; Wang, Y.; Wang, T.; Gao, Y.; Meng, H.; Luo, B.; Luo, C.; et al. Anti-necroptosis chemical necrostatin-1 can also suppress apoptotic and autophagic pathway to exert neuroprotective effect in mice intracerebral hemorrhage model. J. Mol. Neurosci. 2014, 52, 242–249. [Google Scholar] [CrossRef]
- Yuan, Q.; Yuan, Y.; Zheng, Y.; Sheng, R.; Liu, L.; Xie, F.; Tan, J. Anti-cerebral ischemia reperfusion injury of polysaccharides: A review of the mechanisms. Biomed. Pharmacother. 2021, 137, 111303. [Google Scholar] [CrossRef]
- Xu, D.; Kong, T.; Shao, Z.; Liu, M.; Zhang, R.; Zhang, S.; Kong, Q.; Chen, J.; Cheng, B.; Wang, C. Orexin-A alleviates astrocytic apoptosis and inflammation via inhibiting OX1R-mediated NF-kappaB and MAPK signaling pathways in cerebral ischemia/reperfusion injury. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 166230. [Google Scholar] [CrossRef]
- Herz, J.; Bendix, I.; Felderhoff-Muser, U. Peripheral immune cells and perinatal brain injury: A double-edged sword? Pediatr. Res. 2022, 91, 392–403. [Google Scholar] [CrossRef]
- Chen, Q.; Xu, L.; Du, T.; Hou, Y.; Fan, W.; Wu, Q.; Yan, H. Enhanced Expression of PD-L1 on Microglia After Surgical Brain Injury Exerts Self-Protection from Inflammation and Promotes Neurological Repair. Neurochem. Res. 2019, 44, 2470–2481. [Google Scholar] [CrossRef]
- Bai, S.; Lu, X.; Pan, Q.; Wang, B.; Pong, U.K.; Yang, Y.; Wang, H.; Lin, S.; Feng, L.; Wang, Y.; et al. Cranial Bone Transport Promotes Angiogenesis, Neurogenesis, and Modulates Meningeal Lymphatic Function in Middle Cerebral Artery Occlusion Rats. Stroke, J. Cereb. Circ. 2022, 53, 1373–1385. [Google Scholar] [CrossRef]
- Thammisetty, S.S.; Pedragosa, J.; Weng, Y.C.; Calon, F.; Planas, A.; Kriz, J. Age-related deregulation of TDP-43 after stroke enhances NF-kappaB-mediated inflammation and neuronal damage. J. Neuroinflamm. 2018, 15, 312. [Google Scholar] [CrossRef] [PubMed]
- Nalamolu, K.R.; Smith, N.J.; Chelluboina, B.; Klopfenstein, J.D.; Pinson, D.M.; Wang, D.Z.; Vemuganti, R.; Veeravalli, K.K. Prevention of the Severity of Post-ischemic Inflammation and Brain Damage by Simultaneous Knockdown of Toll-like Receptors 2 and 4. Neuroscience 2018, 373, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Tse, K.H.; Chow, K.B.; Leung, W.K.; Wong, Y.H.; Wise, H. Primary sensory neurons regulate Toll-like receptor-4-dependent activity of glial cells in dorsal root ganglia. Neuroscience 2014, 279, 10–22. [Google Scholar] [CrossRef]
- Oladiran, O.; Shi, X.Q.; Yang, M.; Fournier, S.; Zhang, J. Inhibition of TLR4 signaling protects mice from sensory and motor dysfunction in an animal model of autoimmune peripheral neuropathy. J. Neuroinflamm. 2021, 18, 77. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, K.; Huang, Q.; Meng, F.; Deng, S. TLR4 signalling in ischemia/reperfusion injury: A promising target for linking inflammation, oxidative stress and programmed cell death to improve organ transplantation outcomes. Front. Immunol. 2024, 15, 1447060. [Google Scholar] [CrossRef] [PubMed]
- Nakatomi, K.; Ueno, H.; Ishikawa, Y.; Salim, R.C.; Mori, Y.; Kanemoto, I.; Tancharoen, S.; Kikuchi, K.; Miura, N.; Omori, T.; et al. TLR4/MD-2 is a receptor for extracellular nucleophosmin 1. Biomed. Rep. 2021, 14, 21. [Google Scholar] [CrossRef]
- Park, B.S.; Song, D.H.; Kim, H.M.; Choi, B.S.; Lee, H.; Lee, J.O. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature 2009, 458, 1191–1195. [Google Scholar] [CrossRef]
- Nagai, Y.; Akashi, S.; Nagafuku, M.; Ogata, M.; Iwakura, Y.; Akira, S.; Kitamura, T.; Kosugi, A.; Kimoto, M.; Miyake, K. Essential role of MD-2 in LPS responsiveness and TLR4 distribution. Nat. Immunol. 2002, 3, 667–672. [Google Scholar] [CrossRef]
- Chen, L.; Fu, W.; Zheng, L.; Wang, Y.; Liang, G. Recent progress in the discovery of myeloid differentiation 2 (MD2) modulators for inflammatory diseases. Drug Discov. Today 2018, 23, 1187–1202. [Google Scholar] [CrossRef]
- Kim, H.M.; Park, B.S.; Kim, J.I.; Kim, S.E.; Lee, J.; Oh, S.C.; Enkhbayar, P.; Matsushima, N.; Lee, H.; Yoo, O.J.; et al. Crystal structure of the TLR4-MD-2 complex with bound endotoxin antagonist Eritoran. Cell 2007, 130, 906–917. [Google Scholar] [CrossRef] [PubMed]
- Stover, A.G.; Da Silva Correia, J.; Evans, J.T.; Cluff, C.W.; Elliott, M.W.; Jeffery, E.W.; Johnson, D.A.; Lacy, M.J.; Baldridge, J.R.; Probst, P.; et al. Structure-activity relationship of synthetic toll-like receptor 4 agonists. J. Biol. Chem. 2004, 279, 4440–4449. [Google Scholar] [CrossRef]
- Beg, A.A. Endogenous ligands of Toll-like receptors: Implications for regulating inflammatory and immune responses. Trends Immunol. 2002, 23, 509–512. [Google Scholar] [CrossRef]
- Lucas, K.; Maes, M. Role of the Toll Like receptor (TLR) radical cycle in chronic inflammation: Possible treatments targeting the TLR4 pathway. Mol. Neurobiol. 2013, 48, 190–204. [Google Scholar] [CrossRef]
- Imai, Y.; Kuba, K.; Neely, G.G.; Yaghubian-Malhami, R.; Perkmann, T.; van Loo, G.; Ermolaeva, M.; Veldhuizen, R.; Leung, Y.H.; Wang, H.; et al. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell 2008, 133, 235–249. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, P.M.; Gottfried-Blackmore, A.; Anandasabapathy, N.; Bulloch, K. Brain dendritic cells: Biology and pathology. Acta Neuropathol. 2012, 124, 599–614. [Google Scholar] [CrossRef]
- De Laere, M.; Berneman, Z.N.; Cools, N. To the Brain and Back: Migratory Paths of Dendritic Cells in Multiple Sclerosis. J. Neuropathol. Exp. Neurol. 2018, 77, 178–192. [Google Scholar] [CrossRef]
- Bowman, C.C.; Rasley, A.; Tranguch, S.L.; Marriott, I. Cultured astrocytes express toll-like receptors for bacterial products. Glia 2003, 43, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Bsibsi, M.; Ravid, R.; Gveric, D.; van Noort, J.M. Broad expression of Toll-like receptors in the human central nervous system. J. Neuropathol. Exp. Neurol. 2002, 61, 1013–1021. [Google Scholar] [CrossRef]
- Olson, J.K.; Miller, S.D. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J. Immunol. 2004, 173, 3916–3924. [Google Scholar] [CrossRef]
- Wadachi, R.; Hargreaves, K.M. Trigeminal nociceptors express TLR-4 and CD14: A mechanism for pain due to infection. J. Dent. Res. 2006, 85, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Wu, D.; Deng, J.; Yang, Q.; Zhang, X.; Chen, J.; Wang, S.; Hu, S.; Hou, W.; Ning, S.; et al. An MD2-perturbing peptide has therapeutic effects in rodent and rhesus monkey models of stroke. Sci. Transl. Med. 2021, 13, eabb6716. [Google Scholar] [CrossRef]
- Li, Z.; Chen, A.; Wan, H.; Gao, X.; Li, C.; Xiong, L.; Liang, H. Immunohistochemical Localization of MD2, a Co-Receptor of TLR4, in the Adult Mouse Brain. ACS Chem. Neurosci. 2023, 14, 400–417. [Google Scholar] [CrossRef]
- Fakhoury, M. Microglia and Astrocytes in Alzheimer’s Disease: Implications for Therapy. Curr. Neuropharmacol. 2018, 16, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, S.; Agrawal-Rajput, R. Lipopolysaccharide from Rhodobacter sphaeroides Attenuates Microglia-Mediated Inflammation and Phagocytosis and Directs Regulatory T Cell Response. Int. J. Inflam. 2015, 2015, 361326. [Google Scholar] [PubMed]
- Lehnardt, S.; Massillon, L.; Follett, P.; Jensen, F.E.; Ratan, R.; Rosenberg, P.A.; Volpe, J.J.; Vartanian, T. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proc. Natl. Acad. Sci. USA 2003, 100, 8514–8519. [Google Scholar] [CrossRef]
- Tse, K.H.; Chow, K.B.; Leung, W.K.; Wong, Y.H.; Wise, H. Lipopolysaccharide differentially modulates expression of cytokines and cyclooxygenases in dorsal root ganglion cells via Toll-like receptor-4 dependent pathways. Neuroscience 2014, 267, 241–251. [Google Scholar] [CrossRef]
- Due, M.R.; Piekarz, A.D.; Wilson, N.; Feldman, P.; Ripsch, M.S.; Chavez, S.; Yin, H.; Khanna, R.; White, F.A. Neuroexcitatory effects of morphine-3-glucuronide are dependent on Toll-like receptor 4 signaling. J. Neuroinflamm. 2012, 9, 200. [Google Scholar] [CrossRef]
- Takeda, K.; Akira, S. TLR signaling pathways. Semin. Immunol. 2004, 16, 3–9. [Google Scholar] [CrossRef]
- Yamamoto, M.; Sato, S.; Hemmi, H.; Uematsu, S.; Hoshino, K.; Kaisho, T.; Takeuchi, O.; Takeda, K.; Akira, S. TRAM is specifically involved in the Toll-like receptor 4-mediated MyD88-independent signaling pathway. Nat. Immunol. 2003, 4, 1144–1150. [Google Scholar] [CrossRef]
- Lu, Y.C.; Yeh, W.C.; Ohashi, P.S. LPS/TLR4 signal transduction pathway. Cytokine 2008, 42, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Oganesyan, G.; Saha, S.K.; Guo, B.; He, J.Q.; Shahangian, A.; Zarnegar, B.; Perry, A.; Cheng, G. Critical role of TRAF3 in the Toll-like receptor-dependent and -independent antiviral response. Nature 2006, 439, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Ali, A.; Pandey, S.; Khan, I.A.; Prakash, P. Fibronectin containing alternatively spliced extra domain A interacts at the central and c-terminal domain of Toll-like receptor-4. Sci. Rep. 2022, 12, 9662. [Google Scholar] [CrossRef]
- Doddapattar, P.; Gandhi, C.; Prakash, P.; Dhanesha, N.; Grumbach, I.M.; Dailey, M.E.; Lentz, S.R.; Chauhan, A.K. Fibronectin Splicing Variants Containing Extra Domain A Promote Atherosclerosis in Mice Through Toll-Like Receptor 4. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 2391–2400. [Google Scholar] [CrossRef]
- Rajak, S.; Hussain, Y.; Singh, K.; Tiwari, S.; Ahmad, B.; Bharti, S.; Prakash, P. Cellular Fibronectin Containing Extra Domain A Causes Insulin Resistance via Toll-like Receptor 4. Sci. Rep. 2020, 10, 9102. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, E.; Sobrino, T.; Martins, V.C.; Lopez-Loureiro, I.; Campos, F.; Germano, J.; Rodriguez-Perez, M.; Cardoso, S.; Petrovykh, D.Y.; Castillo, J.; et al. Point-of-care quantification of serum cellular fibronectin levels for stratification of ischemic stroke patients. Nanomedicine 2020, 30, 102287. [Google Scholar] [CrossRef]
- Prakash, P.; Kulkarni, P.P.; Lentz, S.R.; Chauhan, A.K. Cellular fibronectin containing extra domain A promotes arterial thrombosis in mice through platelet Toll-like receptor 4. Blood 2015, 125, 3164–3172. [Google Scholar] [CrossRef]
- Khan, M.M.; Gandhi, C.; Chauhan, N.; Stevens, J.W.; Motto, D.G.; Lentz, S.R.; Chauhan, A.K. Alternatively-spliced extra domain A of fibronectin promotes acute inflammation and brain injury after cerebral ischemia in mice. Stroke J. Cereb. Circ. 2012, 43, 1376–1382. [Google Scholar] [CrossRef]
- Zhao, N.; Yi, M.; Zhang, L.J.; Zhang, Q.X.; Yang, L. 4-Octyl Itaconate Attenuates Neuroinflammation in Experimental Autoimmune Encephalomyelitis Via Regulating Microglia. Inflammation 2024, 48, 151–164. [Google Scholar] [CrossRef]
- Wang, H.; Liu, S.; Sun, Y.; Chen, C.; Hu, Z.; Li, Q.; Long, J.; Yan, Q.; Liang, J.; Lin, Y.; et al. Target modulation of glycolytic pathways as a new strategy for the treatment of neuroinflammatory diseases. Ageing Res. Rev. 2024, 101, 102472. [Google Scholar] [CrossRef]
- Buchanan, M.M.; Hutchinson, M.; Watkins, L.R.; Yin, H. Toll-like receptor 4 in CNS pathologies. J. Neurochem. 2010, 114, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Ashayeri Ahmadabad, R.; Khaleghi Ghadiri, M.; Gorji, A. The role of Toll-like receptor signaling pathways in cerebrovascular disorders: The impact of spreading depolarization. J. Neuroinflamm. 2020, 17, 108. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Zhang, X.; Zhang, T.; Grace, P.M.; Li, H.; Wang, Y.; Li, H.; Chen, H.; Watkins, L.R.; Hutchinson, M.R.; et al. Lovastatin inhibits Toll-like receptor 4 signaling in microglia by targeting its co-receptor myeloid differentiation protein 2 and attenuates neuropathic pain. Brain Behav. Immun. 2019, 82, 432–444. [Google Scholar] [CrossRef] [PubMed]
- MacDowell, K.S.; Caso, J.R.; Martin-Hernandez, D.; Madrigal, J.L.; Leza, J.C.; Garcia-Bueno, B. Paliperidone prevents brain toll-like receptor 4 pathway activation and neuroinflammation in rat models of acute and chronic restraint stress. Int. J. Neuropsychopharmacol. 2014, 18, pyu070. [Google Scholar] [CrossRef]
- Molagoda, I.M.N.; Athapaththu, A.; Choi, Y.H.; Park, C.; Jin, C.Y.; Kang, C.H.; Lee, M.H.; Kim, G.Y. Fisetin Inhibits NLRP3 Inflammasome by Suppressing TLR4/MD2-Mediated Mitochondrial ROS Production. Antioxidants 2021, 10, 1215. [Google Scholar] [CrossRef]
- Rehman, S.U.; Ali, T.; Alam, S.I.; Ullah, R.; Zeb, A.; Lee, K.W.; Rutten, B.P.F.; Kim, M.O. Ferulic Acid Rescues LPS-Induced Neurotoxicity via Modulation of the TLR4 Receptor in the Mouse Hippocampus. Mol. Neurobiol. 2019, 56, 2774–2790. [Google Scholar] [CrossRef]
- Shabab, T.; Khanabdali, R.; Moghadamtousi, S.Z.; Kadir, H.A.; Mohan, G. Neuroinflammation pathways: A general review. Int. J. Neurosci. 2017, 127, 624–633. [Google Scholar] [CrossRef] [PubMed]
- Trotta, T.; Porro, C.; Calvello, R.; Panaro, M.A. Biological role of Toll-like receptor-4 in the brain. J. Neuroimmunol. 2014, 268, 1–12. [Google Scholar] [CrossRef]
- Fan, Z.; Ma, H.; Li, Y.; Wu, Y.; Wang, J.; Xiong, L.; Fang, Z.; Zhang, X. Neuronal MD2 induces long-term mental impairments in septic mice by facilitating necroptosis and apoptosis. Front. Pharmacol. 2022, 13, 884821. [Google Scholar] [CrossRef]
- Sumneang, N.; Oo, T.T.; Singhanat, K.; Maneechote, C.; Arunsak, B.; Nawara, W.; Pratchayasakul, W.; Benjanuwattra, J.; Apaijai, N.; Liang, G.; et al. Inhibition of myeloid differentiation factor 2 attenuates cardiometabolic impairments via reducing cardiac mitochondrial dysfunction, inflammation, apoptosis and ferroptosis in prediabetic rats. Biochim. Biophys. Acta Mol. Basis Dis. 2022, 1868, 166301. [Google Scholar] [CrossRef]
- Kavinda, M.H.D.; Choi, Y.H.; Kang, C.H.; Lee, M.H.; Kim, G.Y. 2,4′-Dihydroxybenzophenone: A Promising Anti-Inflammatory Agent Targeting Toll-like Receptor 4/Myeloid Differentiation Factor 2-Mediated Mitochondrial Reactive Oxygen Species Production during Lipopolysaccharide-Induced Systemic Inflammation. ACS Pharmacol. Transl. Sci. 2024, 7, 1320–1334. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, Y.; Zhu, F. Myeloid differentiation protein 2 regulates the innate immunity and the disease resistant against Vibrio alginolyticus in Scylla paramamosain. Fish. Shellfish. Immunol. 2024, 154, 109896. [Google Scholar] [CrossRef]
- Zhan, H.; Pu, Q.; Long, X.; Lu, W.; Wang, G.; Meng, F.; Liao, Z.; Lan, X.; Chen, M. Oxybaphus himalaicus Mitigates Lipopolysaccharide-Induced Acute Kidney Injury by Inhibiting TLR4/MD2 Complex Formation. Antioxidants 2022, 11, 2307. [Google Scholar] [CrossRef] [PubMed]
- Parada, E.; Casas, A.I.; Palomino-Antolin, A.; Gomez-Rangel, V.; Rubio-Navarro, A.; Farre-Alins, V.; Narros-Fernandez, P.; Guerrero-Hue, M.; Moreno, J.A.; Rosa, J.M.; et al. Early toll-like receptor 4 blockade reduces ROS and inflammation triggered by microglial pro-inflammatory phenotype in rodent and human brain ischaemia models. Br. J. Pharmacol. 2019, 176, 2764–2779. [Google Scholar] [CrossRef]
- Wan, H.; He, M.; Cheng, C.; Yang, K.; Wu, H.; Cong, P.; Huang, X.; Zhang, Q.; Shi, Y.; Hu, J.; et al. Clec7a Worsens Long-Term Outcomes after Ischemic Stroke by Aggravating Microglia-Mediated Synapse Elimination. Adv. Sci. 2024, 11, e2403064. [Google Scholar] [CrossRef]
- Ramakrishnan, P.; Baltimore, D. Sam68 is required for both NF-kappaB activation and apoptosis signaling by the TNF receptor. Mol. Cell 2011, 43, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Fu, K.; Sun, X.; Zheng, W.; Wier, E.M.; Hodgson, A.; Tran, D.Q.; Richard, S.; Wan, F. Sam68 modulates the promoter specificity of NF-kappaB and mediates expression of CD25 in activated T cells. Nat. Commun. 2013, 4, 1909. [Google Scholar] [CrossRef]
- Matter, N.; Herrlich, P.; Konig, H. Signal-dependent regulation of splicing via phosphorylation of Sam68. Nature 2002, 420, 691–695. [Google Scholar] [CrossRef]
- Zheng, H.N.; Zhi, Y.R.; Su, Y.S.; Jiang, J.Y.; Zhang, H.Z.; Cao, F.; Wang, Y.; Chi, Y.; Zhang, Y. Dectin-1 induces TRPV1 sensitization and contributes to visceral hypersensitivity of irritable bowel syndrome in male mice. Eur. J. Pain. 2024, 28, 1811–1826. [Google Scholar] [CrossRef]
- Wang, S.; Sudan, R.; Peng, V.; Zhou, Y.; Du, S.; Yuede, C.M.; Lei, T.; Hou, J.; Cai, Z.; Cella, M.; et al. TREM2 drives microglia response to amyloid-beta via SYK-dependent and -independent pathways. Cell 2022, 185, 4153–4169.e19. [Google Scholar] [CrossRef]
- Lian, X.; Wang, X.; Xie, Y.; Sheng, H.; He, J.; Peng, T.; Xie, N.; Wang, C.; Lian, Y. ATF5-regulated Mitochondrial Unfolded Protein Response Attenuates Neuronal Damage in Epileptic Rat by Reducing Endoplasmic Reticulum Stress Through Mitochondrial ROS. Neurochem. Res. 2024, 49, 388–401. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Aran, K.R.; Paswan, R. A potential role of gut microbiota in stroke: Mechanisms, therapeutic strategies and future prospective. Psychopharmacology 2024, 241, 2409–2430. [Google Scholar] [CrossRef] [PubMed]
- Lyman, M.; Lloyd, D.G.; Ji, X.; Vizcaychipi, M.P.; Ma, D. Neuroinflammation: The role and consequences. Neurosci. Res. 2014, 79, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Carson, M.J.; Thrash, J.C.; Walter, B. The cellular response in neuroinflammation: The role of leukocytes, microglia and astrocytes in neuronal death and survival. Clin. Neurosci. Res. 2006, 6, 237–245. [Google Scholar] [CrossRef]
- Zusso, M.; Lunardi, V.; Franceschini, D.; Pagetta, A.; Lo, R.; Stifani, S.; Frigo, A.C.; Giusti, P.; Moro, S. Ciprofloxacin and levofloxacin attenuate microglia inflammatory response via TLR4/NF-kB pathway. J. Neuroinflamm. 2019, 16, 148. [Google Scholar] [CrossRef]
- Li, S.; Yang, P.; Wu, Z.; Huang, W.; Zhu, X.; Zhong, L. The effects and mechanisms of AM1241 in alleviating cerebral ischemia-reperfusion injury. Brain Res. Bull. 2024, 215, 111025. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; Wang, Y.; Li, H.; Wu, S.; Gao, J.; Zhang, T.; Xie, J.; Wang, X. Nalmefene non-enantioselectively targets myeloid differentiation protein 2 and inhibits toll-like receptor 4 signaling: Wet-lab techniques and in silico simulations. Phys. Chem. Chem. Phys. 2021, 23, 12260–12269. [Google Scholar] [CrossRef]
- Michalska, P.; Buendia, I.; Duarte, P.; FernandezMendivil, C.; Negredo, P.; Cuadrado, A.; Lopez, M.G.; Leon, R. Melatonin-sulforaphane hybrid ITH12674 attenuates glial response in vivo by blocking LPS binding to MD2 and receptor oligomerization. Pharmacol. Res. 2020, 152, 104597. [Google Scholar] [CrossRef]
- Zuo, W.; Zhao, J.; Zhang, J.; Fang, Z.; Deng, J.; Fan, Z.; Guo, Y.; Han, J.; Hou, W.; Dong, H.; et al. MD2 contributes to the pathogenesis of perioperative neurocognitive disorder via the regulation of alpha5GABA(A) receptors in aged mice. J. Neuroinflamm. 2021, 18, 204. [Google Scholar] [CrossRef]
- Hao, T.; Yang, Y.; Li, N.; Mi, Y.; Zhang, G.; Song, J.; Liang, Y.; Xiao, J.; Zhou, D.; He, D.; et al. Inflammatory mechanism of cerebral ischemia-reperfusion injury with treatment of stepharine in rats. Phytomedicine 2020, 79, 153353. [Google Scholar] [CrossRef]
- Parlar, A.; Arslan, S.O. CB2 Agonist (AM1241) Improving Effect on Ovalbumin-Induced Asthma in Rats. Iran. J. Pharm. Res. 2020, 19, 3–17. [Google Scholar] [PubMed]
- Zhang, X.; Zhang, Q.; Huang, L.; Liu, M.; Cheng, Z.; Zheng, Y.; Xu, W.; Lu, J.; Liu, J.; Huang, M. Pien-Tze-Huang attenuates neuroinflammation in cerebral ischaemia-reperfusion injury in rats through the TLR4/NF-kappaB/MAPK pathway. Pharm. Biol. 2021, 59, 828–839. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ma, W.; Zang, C.H.; Wang, G.D.; Zhang, S.J.; Wu, H.J.; Zhu, K.W.; Xiang, X.L.; Li, C.Y.; Liu, K.P.; et al. Salidroside inhibits NLRP3 inflammasome activation and apoptosis in microglia induced by cerebral ischemia/reperfusion injury by inhibiting the TLR4/NF-kappaB signaling pathway. Ann. Transl. Med. 2021, 9, 1694. [Google Scholar] [CrossRef]
- Villanueva, M.T. A shared target for stroke subtypes. Nat. Rev. Drug Discov. 2021, 20, 588. [Google Scholar] [CrossRef]
- Sepulveda, C.; Hernandez, B.; Burgos, C.F.; Fuentes, E.; Palomo, I.; Alarcon, M. The cAMP/PKA Pathway Inhibits Beta-amyloid Peptide Release from Human Platelets. Neuroscience 2019, 397, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Gorpenchenko, T.Y.; Grigorchuk, V.P.; Bulgakov, D.V.; Tchernoded, G.K.; Bulgakov, V.P. Tempo-Spatial Pattern of Stepharine Accumulation in Stephania Glabra Morphogenic Tissues. Int. J. Mol. Sci. 2019, 20, 808. [Google Scholar] [CrossRef]
- Michell-Robinson, M.A.; Touil, H.; Healy, L.M.; Owen, D.R.; Durafourt, B.A.; Bar-Or, A.; Antel, J.P.; Moore, C.S. Roles of microglia in brain development, tissue maintenance and repair. Brain J. Neurol. 2015, 138, 1138–1159. [Google Scholar] [CrossRef]

| Inhibitors | Type | Function | Effects | Models | Reference |
|---|---|---|---|---|---|
| Tat-CIRP | Peptide | Competitively binds to MD2 | Disrupts MD2-TLR4 binding, reduces neuroinflammation and neuronal death | MCAO mice, brain hemorrhage mice, I/R injury rhesus monkey | [32] |
| AM1241 | Small Molecule | Directly binds to MD2 | Inhibits the formation of the TLR4-MD2 complex, alleviates the inflammatory response and neuronal apoptosis | CIRI mice | [76] |
| Nalmefene | Small Molecule | Binds to MD2 | Prevents neuroinflammation and brain damage by blocking the interaction between TLR4 and MD2 | Microglia BV-2 cell line | [77] |
| Stepharine | Natural Compound | Binds to TLR4-MD2 complex | Improved outcomes in MCAO rats, reduces neuronal loss, suppresses microglial overactivation via the inhibition of the TLR4/NF-κB pathway | MCAO rats | [80] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, D.; Zhao, J.; Chen, Q.; Liu, Q.; Fang, Y. The Role of Myeloid Differentiation Factor 2 in Stroke: Mechanisms and Therapeutic Potential. Biomolecules 2025, 15, 961. https://doi.org/10.3390/biom15070961
Zhu D, Zhao J, Chen Q, Liu Q, Fang Y. The Role of Myeloid Differentiation Factor 2 in Stroke: Mechanisms and Therapeutic Potential. Biomolecules. 2025; 15(7):961. https://doi.org/10.3390/biom15070961
Chicago/Turabian StyleZhu, Deyuan, Jihu Zhao, Qian Chen, Qiong Liu, and Yibin Fang. 2025. "The Role of Myeloid Differentiation Factor 2 in Stroke: Mechanisms and Therapeutic Potential" Biomolecules 15, no. 7: 961. https://doi.org/10.3390/biom15070961
APA StyleZhu, D., Zhao, J., Chen, Q., Liu, Q., & Fang, Y. (2025). The Role of Myeloid Differentiation Factor 2 in Stroke: Mechanisms and Therapeutic Potential. Biomolecules, 15(7), 961. https://doi.org/10.3390/biom15070961






