Craniomaxillofacial-Derived MSCs in Congenital Defect Reconstruction
Abstract
1. Introduction
2. Safety and Efficacy of Mesenchymal Stem Cells
3. The Senescence of Mesenchymal Stem Cells
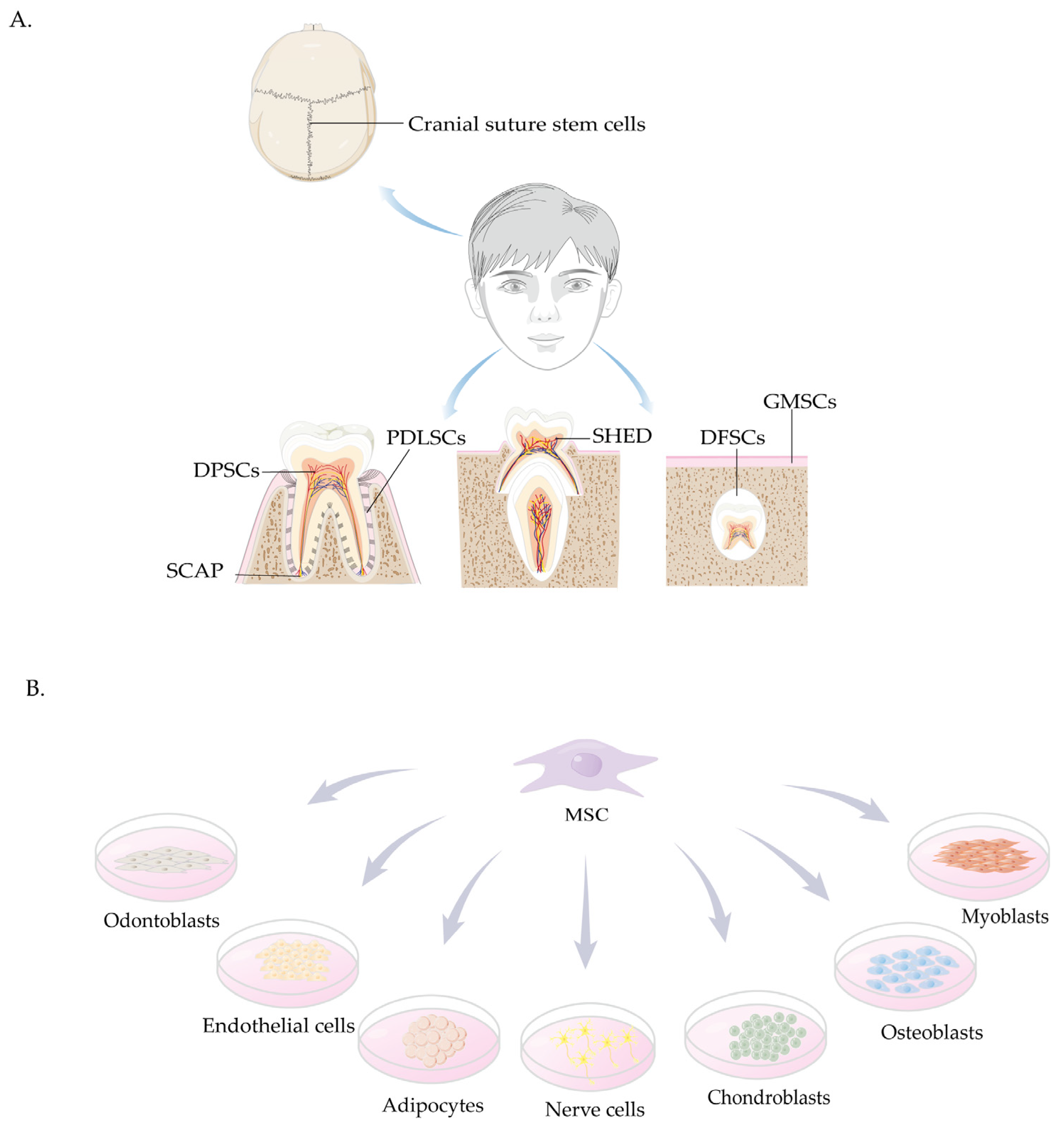
4. Sources and Characteristics of Craniomaxillofacial-Derived Mesenchymal Stem Cells
4.1. Cranial Suture Stem Cells
4.2. Stem Cells Derived from the Oral Cavity
4.2.1. DPSCs
4.2.2. SHED
4.2.3. SCAP
4.2.4. PDLSCs
4.2.5. GMSCs
4.2.6. DFSCs
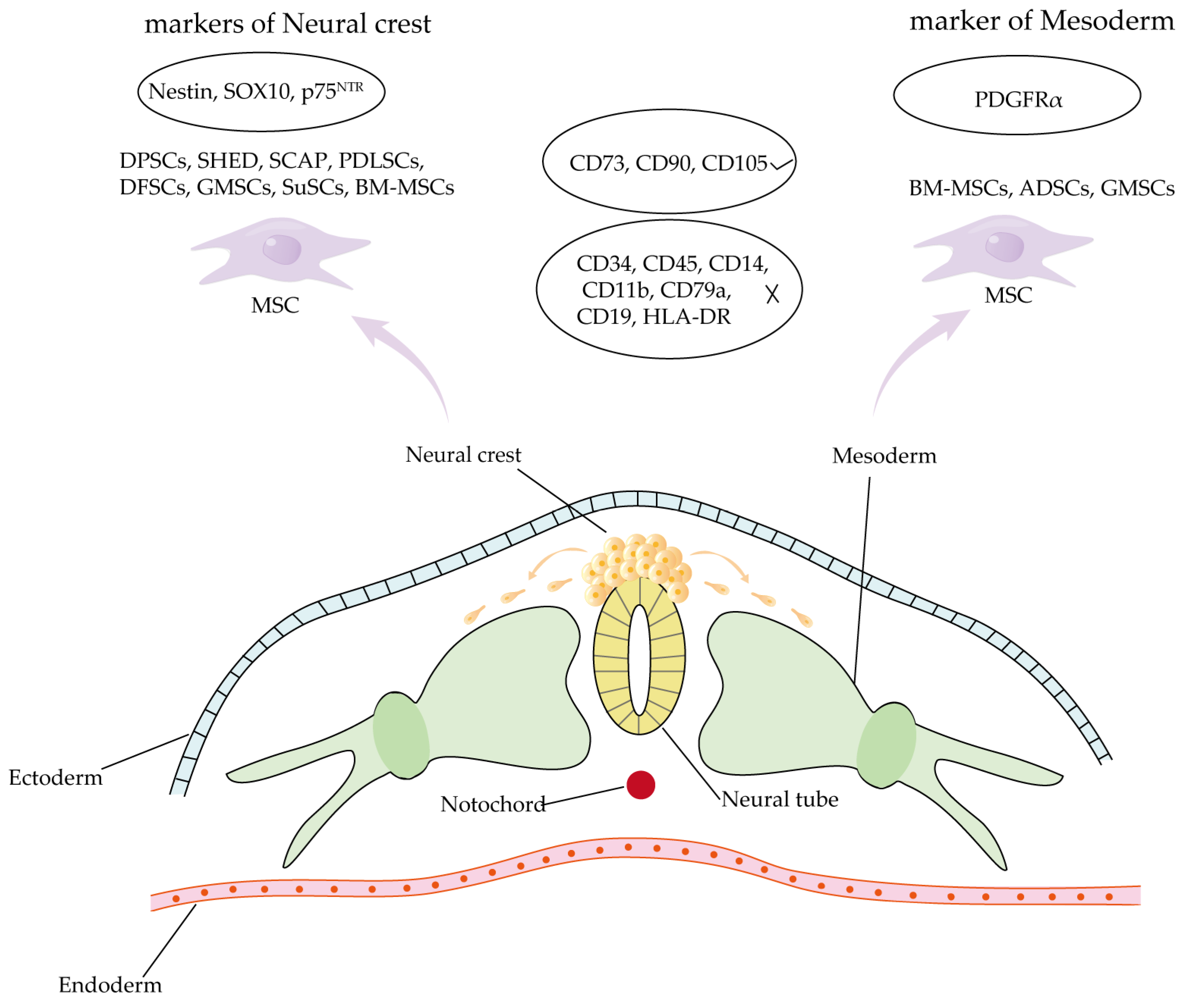
4.3. Comparison of Craniomaxillofacial-Derived Mesenchymal Stem Cells and Other Tissue-Specific Stem Cell Populations
5. Applications of Stem Cell Therapy in Congenital Craniofacial Defects
5.1. Cleft Lip and Palate
5.1.1. DPSCs in Cleft Lip and Palate Repair
5.1.2. SHED in Cleft Lip and Palate Repair
5.1.3. Additional Stem Cell Populations Derived from the Oral Cavity
5.2. Craniosynostosis (CS)
5.3. Tooth Agenesis
6. Therapeutic Limitations of Mesenchymal Stem Cells in Congenital Defects
7. Conclusions and Prospect
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MSCs | mesenchymal stem cells |
| ASCs | adult stem cells |
| BM-MSCs | bone marrow mesenchymal stem cells |
| ESCs | embryonic stem cells |
| iPSCs | induced pluripotent stem cells |
| SuSCs | suture stem cells |
| ADSCs | adipose-derived stem cells |
| DPSCs | dental pulp stem cells |
| PDLSCs | periodontal ligament stem cells |
| DFSCs | dental follicle stem cells |
| SHED | stem cells from human exfoliated deciduous teeth |
| SCAP | stem cells from apical papilla |
| GMSCs | gingival mesenchymal stem cells |
| DSCs | dental stem cells |
| MACS | magnetic activated cell sorting |
| MHC | major histocompatibility complex |
| HLA | human leukocyte antigen |
| CL/P | cleft lip with or without cleft palate |
| SHED-CM | SHED conditioned medium |
| BMP2 | bone morphogenetic protein 2 |
| EVs | extracellular vesicles |
| PDL | periodontal ligament |
| TDM | treated dentin matrix |
| SD | Sprague-Dawley |
| HA/TCP | hydroxyapatite tricalcium phosphate |
| mDCs | monocyte-derived dendritic cells |
| PGE2 | prostaglandin E2 |
| IDO | indoleamine-pyrrole 2,3-dioxygenase |
| IFN-γ | interferon-γ |
| TNF-α | tumor necrosis factor-α |
| TGF-β | transforming growth factor-β |
| IL | interleukin |
| CCL-2 | C-C motif chemokine ligand 2 |
| Arg1 | Arginase 1 |
| VEGF | vascular endothelial growth factor |
| HGF | hepatocyte growth factor |
| CRISPR | clustered regularly interspaced short palindromic repeats |
References
- Lopez, C.D.; Witek, L.; Torroni, A.; Flores, R.L.; Demissie, D.B.; Young, S.; Cronstein, B.N.; Coelho, P.G. The role of 3D printing in treating craniomaxillofacial congenital anomalies. Birth Defects Res. 2018, 110, 1055–1064. [Google Scholar] [CrossRef] [PubMed]
- Bueno, D.F.; Sunaga, D.Y.; Kobayashi, G.S.; Aguena, M.; Raposo-Amaral, C.E.; Masotti, C.; Cruz, L.A.; Pearson, P.L.; Passos-Bueno, M.R. Human stem cell cultures from cleft lip/palate patients show enrichment of transcripts involved in extracellular matrix modeling by comparison to controls. Stem Cell Rev. Rep. 2011, 7, 446–457. [Google Scholar] [CrossRef]
- Barbaro, V.; Bonelli, F.; Ferrari, S.; La Vella, G.; Di Iorio, E. Innovative Therapeutic Approaches for the Treatment of the Ocular Morbidities in Patients with EEC Syndrome. Cells 2023, 12, 495. [Google Scholar] [CrossRef]
- Szpalski, C.; Barr, J.; Wetterau, M.; Saadeh, P.B.; Warren, S.M. Cranial bone defects: Current and future strategies. Neurosurg. Focus 2010, 29, E8. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, J.; Dai, B.; Liu, W.; Wang, J.; Li, Q.; Wang, J.; Zhao, L.; Ngai, T. A Bilayer Membrane Doped with Struvite Nanowires for Guided Bone Regeneration. Adv. Healthc. Mater. 2022, 11, e2201679. [Google Scholar] [CrossRef]
- Tiffany, A.S.; Gray, D.L.; Woods, T.J.; Subedi, K.; Harley, B.A.C. The inclusion of zinc into mineralized collagen scaffolds for craniofacial bone repair applications. Acta Biomater. 2019, 93, 86–96. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, A.B.G.; Rahimnejad, M.; Oliveira, R.; Sikder, P.; Saavedra, G.; Bhaduri, S.B.; Gawlitta, D.; Malda, J.; Kaigler, D.; Trichês, E.S.; et al. Personalized bioceramic grafts for craniomaxillofacial bone regeneration. Int. J. Oral Sci. 2024, 16, 62. [Google Scholar] [CrossRef]
- Kawecki, F.; Clafshenkel, W.P.; Fortin, M.; Auger, F.A.; Fradette, J. Biomimetic Tissue-Engineered Bone Substitutes for Maxillofacial and Craniofacial Repair: The Potential of Cell Sheet Technologies. Adv. Healthc. Mater. 2018, 7, e1700919. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Wang, Z.; Huang, Y.; Bi, X.; Zhou, H.; Lin, M.; Yu, Z.; Wang, Y.; Ni, N.; Sun, J.; et al. Characterization of human ethmoid sinus mucosa derived mesenchymal stem cells (hESMSCs) and the application of hESMSCs cell sheets in bone regeneration. Biomaterials 2015, 66, 67–82. [Google Scholar] [CrossRef]
- Banimohamad-Shotorbani, B.; Karkan, S.F.; Rahbarghazi, R.; Mehdipour, A.; Jarolmasjed, S.; Saghati, S.; Shafaei, H. Application of mesenchymal stem cell sheet for regeneration of craniomaxillofacial bone defects. Stem Cell Res. Ther. 2023, 14, 68. [Google Scholar] [CrossRef]
- Schreurs, M.; Suttorp, C.M.; Mutsaers, H.A.M.; Kuijpers-Jagtman, A.M.; Von den Hoff, J.W.; Ongkosuwito, E.M.; Carvajal Monroy, P.L.; Wagener, F. Tissue engineering strategies combining molecular targets against inflammation and fibrosis, and umbilical cord blood stem cells to improve hampered muscle and skin regeneration following cleft repair. Med. Res. Rev. 2020, 40, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.T.; Phuong, T.N.T.; Tien, N.L.B.; Tran, D.K.; Thanh, V.V.; Quang, T.L.; Truong, D.T.; Pham, V.H.; Ngoc, V.T.N.; Chu-Dinh, T.; et al. An Update on the Progress of Isolation, Culture, Storage, and Clinical Application of Human Bone Marrow Mesenchymal Stem/Stromal Cells. Int. J. Mol. Sci. 2020, 21, 708. [Google Scholar] [CrossRef]
- Anil, S.; Thomas, N.G.; Chalisserry, E.P.; Dalvi, Y.B.; Ramadoss, R.; Vellappally, S. Isolation, Culture, and Characterization of Dental Pulp Stem Cells from Human Deciduous and Permanent Teeth. J. Vis. Exp. 2024, 207, e65767. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Raju, R.; Sui, S.; Hu, W.S. Stem cell culture engineering—Process scale up and beyond. Biotechnol. J. 2011, 6, 1317–1329. [Google Scholar] [CrossRef]
- Thomson, J.A.; Itskovitz-Eldor, J.; Shapiro, S.S.; Waknitz, M.A.; Swiergiel, J.J.; Marshall, V.S.; Jones, J.M. Embryonic stem cell lines derived from human blastocysts. Science 1998, 282, 1145–1147. [Google Scholar] [CrossRef]
- Yu, J.; Vodyanik, M.A.; Smuga-Otto, K.; Antosiewicz-Bourget, J.; Frane, J.L.; Tian, S.; Nie, J.; Jonsdottir, G.A.; Ruotti, V.; Stewart, R.; et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 2007, 318, 1917–1920. [Google Scholar] [CrossRef] [PubMed]
- Volarevic, V.; Markovic, B.S.; Gazdic, M.; Volarevic, A.; Jovicic, N.; Arsenijevic, N.; Armstrong, L.; Djonov, V.; Lako, M.; Stojkovic, M. Ethical and Safety Issues of Stem Cell-Based Therapy. Int. J. Med. Sci. 2018, 15, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Fus-Kujawa, A.; Mendrek, B.; Trybus, A.; Bajdak-Rusinek, K.; Stepien, K.L.; Sieron, A.L. Potential of Induced Pluripotent Stem Cells for Use in Gene Therapy: History, Molecular Bases, and Medical Perspectives. Biomolecules 2021, 11, 699. [Google Scholar] [CrossRef]
- Marei, H.E. Stem cell therapy: A revolutionary cure or a pandora’s box. Stem Cell Res. Ther. 2025, 16, 255. [Google Scholar] [CrossRef]
- Horwitz, E.M.; Le Blanc, K.; Dominici, M.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Deans, R.J.; Krause, D.S.; Keating, A. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy 2005, 7, 393–395. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Lin, C.S.; Ning, H.; Lin, G.; Lue, T.F. Is CD34 truly a negative marker for mesenchymal stromal cells? Cytotherapy 2012, 14, 1159–1163. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Xu, J. Immune modulation by mesenchymal stem cells. Cell Prolif. 2020, 53, e12712. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef]
- Estes, B.T.; Diekman, B.O.; Gimble, J.M.; Guilak, F. Isolation of adipose-derived stem cells and their induction to a chondrogenic phenotype. Nat. Protoc. 2010, 5, 1294–1311. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.J.; Bae, Y.K.; Kim, M.; Kwon, S.J.; Jeon, H.B.; Choi, S.J.; Kim, S.W.; Yang, Y.S.; Oh, W.; Chang, J.W. Comparative analysis of human mesenchymal stem cells from bone marrow, adipose tissue, and umbilical cord blood as sources of cell therapy. Int. J. Mol. Sci. 2013, 14, 17986–18001. [Google Scholar] [CrossRef]
- Sun, J.S.; Wu, S.Y.; Lin, F.H. The role of muscle-derived stem cells in bone tissue engineering. Biomaterials 2005, 26, 3953–3960. [Google Scholar] [CrossRef]
- Tamaki, T.; Uchiyama, Y.; Okada, Y.; Ishikawa, T.; Sato, M.; Akatsuka, A.; Asahara, T. Functional recovery of damaged skeletal muscle through synchronized vasculogenesis, myogenesis, and neurogenesis by muscle-derived stem cells. Circulation 2005, 112, 2857–2866. [Google Scholar] [CrossRef]
- In’t Anker, P.S.; Scherjon, S.A.; Kleijburg-van der Keur, C.; de Groot-Swings, G.M.; Claas, F.H.; Fibbe, W.E.; Kanhai, H.H. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells 2004, 22, 1338–1345. [Google Scholar] [CrossRef]
- Forraz, N.; McGuckin, C.P. The umbilical cord: A rich and ethical stem cell source to advance regenerative medicine. Cell Prolif. 2011, 44 (Suppl. 1), 60–69. [Google Scholar] [CrossRef]
- De Coppi, P.; Bartsch, G., Jr.; Siddiqui, M.M.; Xu, T.; Santos, C.C.; Perin, L.; Mostoslavsky, G.; Serre, A.C.; Snyder, E.Y.; Yoo, J.J.; et al. Isolation of amniotic stem cell lines with potential for therapy. Nat. Biotechnol. 2007, 25, 100–106. [Google Scholar] [CrossRef]
- Sun, Q.; Nakata, H.; Yamamoto, M.; Kasugai, S.; Kuroda, S. Comparison of gingiva-derived and bone marrow mesenchymal stem cells for osteogenesis. J. Cell. Mol. Med. 2019, 23, 7592–7601. [Google Scholar] [CrossRef]
- Bar, J.K.; Lis-Nawara, A.; Grelewski, P.G. Dental Pulp Stem Cell-Derived Secretome and Its Regenerative Potential. Int. J. Mol. Sci. 2021, 22, 12018. [Google Scholar] [CrossRef] [PubMed]
- Ben-David, U.; Mayshar, Y.; Benvenisty, N. Large-scale analysis reveals acquisition of lineage-specific chromosomal aberrations in human adult stem cells. Cell Stem Cell 2011, 9, 97–102. [Google Scholar] [CrossRef]
- Ben-David, U.; Benvenisty, N.; Mayshar, Y. Genetic instability in human induced pluripotent stem cells: Classification of causes and possible safeguards. Cell Cycle 2010, 9, 4603–4604. [Google Scholar] [CrossRef] [PubMed]
- Ben-David, U.; Benvenisty, N. The tumorigenicity of human embryonic and induced pluripotent stem cells. Nat. Rev. Cancer 2011, 11, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Miura, M.; Miura, Y.; Padilla-Nash, H.M.; Molinolo, A.A.; Fu, B.; Patel, V.; Seo, B.M.; Sonoyama, W.; Zheng, J.J.; Baker, C.C.; et al. Accumulated chromosomal instability in murine bone marrow mesenchymal stem cells leads to malignant transformation. Stem Cells 2006, 24, 1095–1103. [Google Scholar] [CrossRef]
- Franceschini, N.; Verbruggen, B.; Tryfonidou, M.A.; Kruisselbrink, A.B.; Baelde, H.; de Visser, K.E.; Szuhai, K.; Cleton-Jansen, A.M.; Bovée, J. Transformed Canine and Murine Mesenchymal Stem Cells as a Model for Sarcoma with Complex Genomics. Cancers 2021, 13, 1126. [Google Scholar] [CrossRef]
- Tarte, K.; Gaillard, J.; Lataillade, J.J.; Fouillard, L.; Becker, M.; Mossafa, H.; Tchirkov, A.; Rouard, H.; Henry, C.; Splingard, M.; et al. Clinical-grade production of human mesenchymal stromal cells: Occurrence of aneuploidy without transformation. Blood 2010, 115, 1549–1553. [Google Scholar] [CrossRef]
- Meng, Z.; Chen, G.; Chen, J.; Yang, B.; Yu, M.; Feng, L.; Jiang, Z.; Guo, W.; Tian, W. Tumorigenicity analysis of heterogeneous dental stem cells and its self-modification for chromosome instability. Cell Cycle 2015, 14, 3396–3407. [Google Scholar] [CrossRef]
- Mehler, V.J.; Burns, C.; Moore, M.L. Concise Review: Exploring Immunomodulatory Features of Mesenchymal Stromal Cells in Humanized Mouse Models. Stem Cells 2019, 37, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Ren, C.; Yang, L.; Zhu, Y.; Li, G.; Chang, Y.; Du, J.; Yang, Z.; Yuan, Y. Efficacy and Safety of Human Umbilical Cord Mesenchymal Stem Cells in Improving Fertility in Polycystic Ovary Syndrome Mice. Curr. Stem Cell Res. Ther. 2025, 20, 279–290. [Google Scholar] [CrossRef]
- Sudres, M.; Maurer, M.; Robinet, M.; Bismuth, J.; Truffault, F.; Girard, D.; Dragin, N.; Attia, M.; Fadel, E.; Santelmo, N.; et al. Preconditioned mesenchymal stem cells treat myasthenia gravis in a humanized preclinical model. J. Clin. Investig. 2017, 2, e89665. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, Y.; Mellado-Damas, N.; Olmedo-Moreno, L.; López, V.; Panadero-Morón, C.; Benito, M.; Guerrero-Cázares, H.; Márquez-Vega, C.; Martín-Montalvo, A.; Capilla-González, V. Preclinical Safety Evaluation of Intranasally Delivered Human Mesenchymal Stem Cells in Juvenile Mice. Cancers 2021, 13, 1169. [Google Scholar] [CrossRef] [PubMed]
- Weng, Z.; Wang, Y.; Ouchi, T.; Liu, H.; Qiao, X.; Wu, C.; Zhao, Z.; Li, L.; Li, B. Mesenchymal Stem/Stromal Cell Senescence: Hallmarks, Mechanisms, and Combating Strategies. Stem Cells Transl. Med. 2022, 11, 356–371. [Google Scholar] [CrossRef]
- Yang, Y.K.; Ogando, C.R.; Wang See, C.; Chang, T.Y.; Barabino, G.A. Changes in phenotype and differentiation potential of human mesenchymal stem cells aging in vitro. Stem Cell Res. Ther. 2018, 9, 131. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.P.; Wright, W.E.; Shay, J.W. Comparison of telomere length measurement methods. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2018, 373, 20160451. [Google Scholar] [CrossRef]
- Niemann, J.; Johne, C.; Schröder, S.; Koch, F.; Ibrahim, S.M.; Schultz, J.; Tiedge, M.; Baltrusch, S. An mtDNA mutation accelerates liver aging by interfering with the ROS response and mitochondrial life cycle. Free Radic. Biol. Med. 2017, 102, 174–187. [Google Scholar] [CrossRef]
- Kozlowski, M.; Ladurner, A.G. ATM, MacroH2A.1, and SASP: The Checks and Balances of Cellular Senescence. Mol. Cell 2015, 59, 713–715. [Google Scholar] [CrossRef]
- Hwang, E.S. Senescence suppressors: Their practical importance in replicative lifespan extension in stem cells. Cell. Mol. Life Sci. 2014, 71, 4207–4219. [Google Scholar] [CrossRef]
- D’Adda di Fagagna, F. Living on a break: Cellular senescence as a DNA-damage response. Nat. Rev. Cancer 2008, 8, 512–522. [Google Scholar] [CrossRef] [PubMed]
- Schofield, R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells 1978, 4, 7–25. [Google Scholar] [PubMed]
- Abu-Remaileh, M.; Wyant, G.A.; Kim, C.; Laqtom, N.N.; Abbasi, M.; Chan, S.H.; Freinkman, E.; Sabatini, D.M. Lysosomal metabolomics reveals V-ATPase- and mTOR-dependent regulation of amino acid efflux from lysosomes. Science 2017, 358, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Colacurcio, D.J.; Nixon, R.A. Disorders of lysosomal acidification-The emerging role of v-ATPase in aging and neurodegenerative disease. Ageing Res. Rev. 2016, 32, 75–88. [Google Scholar] [CrossRef]
- Wagner, W.; Horn, P.; Castoldi, M.; Diehlmann, A.; Bork, S.; Saffrich, R.; Benes, V.; Blake, J.; Pfister, S.; Eckstein, V.; et al. Replicative senescence of mesenchymal stem cells: A continuous and organized process. PLoS ONE 2008, 3, e2213. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Q.; Wang, Y.; Li, L.; Bu, H.; Bao, J. Senescence of mesenchymal stem cells (Review). Int. J. Mol. Med. 2017, 39, 775–782. [Google Scholar] [CrossRef]
- Sanidas, I.; Morris, R.; Fella, K.A.; Rumde, P.H.; Boukhali, M.; Tai, E.C.; Ting, D.T.; Lawrence, M.S.; Haas, W.; Dyson, N.J. A Code of Mono-phosphorylation Modulates the Function of RB. Mol. Cell 2019, 73, 985–1000.e1006. [Google Scholar] [CrossRef]
- Gil, J.; Peters, G. Regulation of the INK4b-ARF-INK4a tumour suppressor locus: All for one or one for all. Nat. Rev. Mol. Cell Biol. 2006, 7, 667–677. [Google Scholar] [CrossRef]
- Li, J.S.; Miralles Fusté, J.; Simavorian, T.; Bartocci, C.; Tsai, J.; Karlseder, J.; Lazzerini Denchi, E. TZAP: A telomere-associated protein involved in telomere length control. Science 2017, 355, 638–641. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Shi, Y.; Liu, Y.; Pan, X.H.; Zhang, K.X. Telomere shortening activates TGF-β/Smads signaling in lungs and enhances both lipopolysaccharide and bleomycin-induced pulmonary fibrosis. Acta Pharmacol. Sin. 2018, 39, 1735–1745. [Google Scholar] [CrossRef]
- Chen, R.J.; Wu, P.H.; Ho, C.T.; Way, T.D.; Pan, M.H.; Chen, H.M.; Ho, Y.S.; Wang, Y.J. P53-dependent downregulation of hTERT protein expression and telomerase activity induces senescence in lung cancer cells as a result of pterostilbene treatment. Cell Death Dis. 2017, 8, e2985. [Google Scholar] [CrossRef]
- Yamashita, S.; Ogawa, K.; Ikei, T.; Udono, M.; Fujiki, T.; Katakura, Y. SIRT1 prevents replicative senescence of normal human umbilical cord fibroblast through potentiating the transcription of human telomerase reverse transcriptase gene. Biochem. Biophys. Res. Commun. 2012, 417, 630–634. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Du, W.; Mancuso, A.; Wellen, K.E.; Yang, X. Reciprocal regulation of p53 and malic enzymes modulates metabolism and senescence. Nature 2013, 493, 689–693. [Google Scholar] [CrossRef]
- Wiley, C.D.; Velarde, M.C.; Lecot, P.; Liu, S.; Sarnoski, E.A.; Freund, A.; Shirakawa, K.; Lim, H.W.; Davis, S.S.; Ramanathan, A.; et al. Mitochondrial Dysfunction Induces Senescence with a Distinct Secretory Phenotype. Cell Metab. 2016, 23, 303–314. [Google Scholar] [CrossRef] [PubMed]
- So, A.Y.; Jung, J.W.; Lee, S.; Kim, H.S.; Kang, K.S. DNA methyltransferase controls stem cell aging by regulating BMI1 and EZH2 through microRNAs. PLoS ONE 2011, 6, e19503. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Wei, W.; Zhou, D.X. Histone Acetylation Enzymes Coordinate Metabolism and Gene Expression. Trends Plant Sci. 2015, 20, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Park, J.R.; Seo, M.S.; Roh, K.H.; Park, S.B.; Hwang, J.W.; Sun, B.; Seo, K.; Lee, Y.S.; Kang, S.K.; et al. Histone deacetylase inhibitors decrease proliferation potential and multilineage differentiation capability of human mesenchymal stem cells. Cell Prolif. 2009, 42, 711–720. [Google Scholar] [CrossRef]
- Squillaro, T.; Severino, V.; Alessio, N.; Farina, A.; Di Bernardo, G.; Cipollaro, M.; Peluso, G.; Chambery, A.; Galderisi, U. De-regulated expression of the BRG1 chromatin remodeling factor in bone marrow mesenchymal stromal cells induces senescence associated with the silencing of NANOG and changes in the levels of chromatin proteins. Cell Cycle 2015, 14, 1315–1326. [Google Scholar] [CrossRef]
- Al-Azab, M.; Safi, M.; Idiiatullina, E.; Al-Shaebi, F.; Zaky, M.Y. Aging of mesenchymal stem cell: Machinery, markers, and strategies of fighting. Cell. Mol. Biol. Lett. 2022, 27, 69. [Google Scholar] [CrossRef]
- Cheng, M.; Yuan, W.; Moshaverinia, A.; Yu, B. Rejuvenation of Mesenchymal Stem Cells to Ameliorate Skeletal Aging. Cells 2023, 12, 998. [Google Scholar] [CrossRef]
- Li, B.; Wang, Y.; Fan, Y.; Ouchi, T.; Zhao, Z.; Li, L. Cranial Suture Mesenchymal Stem Cells: Insights and Advances. Biomolecules 2021, 11, 1129. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Feng, J.; Ho, T.V.; Grimes, W.; Urata, M.; Chai, Y. The suture provides a niche for mesenchymal stem cells of craniofacial bones. Nat. Cell Biol. 2015, 17, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, T.; Jeong, J.; Sheu, T.J.; Hsu, W. Stem cells of the suture mesenchyme in craniofacial bone development, repair and regeneration. Nat. Commun. 2016, 7, 10526. [Google Scholar] [CrossRef]
- Aldawood, Z.A.; Mancinelli, L.; Geng, X.; Yeh, S.A.; Di Carlo, R.; Leite, T.C.; Gustafson, J.; Wilk, K.; Yozgatian, J.; Garakani, S.; et al. Expansion of the sagittal suture induces proliferation of skeletal stem cells and sustains endogenous calvarial bone regeneration. Proc. Natl. Acad. Sci. USA 2023, 120, e2120826120. [Google Scholar] [CrossRef] [PubMed]
- Debnath, S.; Yallowitz, A.R.; McCormick, J.; Lalani, S.; Zhang, T.; Xu, R.; Li, N.; Liu, Y.; Yang, Y.S.; Eiseman, M.; et al. Discovery of a periosteal stem cell mediating intramembranous bone formation. Nature 2018, 562, 133–139. [Google Scholar] [CrossRef]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef]
- Li, P.; Ou, Q.; Shi, S.; Shao, C. Immunomodulatory properties of mesenchymal stem cells/dental stem cells and their therapeutic applications. Cell. Mol. Immunol. 2023, 20, 558–569. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Y.; Tian, W.; Pan, J. Advances of tooth-derived stem cells in neural diseases treatments and nerve tissue regeneration. Cell Prolif. 2019, 52, e12572. [Google Scholar] [CrossRef]
- Heng, B.C.; Lim, L.W.; Wu, W.; Zhang, C. An Overview of Protocols for the Neural Induction of Dental and Oral Stem Cells In Vitro. Tissue Eng. Part B Rev. 2016, 22, 220–250. [Google Scholar] [CrossRef]
- Huang, G.T.; Gronthos, S.; Shi, S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: Their biology and role in regenerative medicine. J. Dent. Res. 2009, 88, 792–806. [Google Scholar] [CrossRef]
- Soliman, H.; Theret, M.; Scott, W.; Hill, L.; Underhill, T.M.; Hinz, B.; Rossi, F.M.V. Multipotent stromal cells: One name, multiple identities. Cell Stem Cell 2021, 28, 1690–1707. [Google Scholar] [CrossRef] [PubMed]
- Samsonraj, R.M.; Raghunath, M.; Nurcombe, V.; Hui, J.H.; van Wijnen, A.J.; Cool, S.M. Concise Review: Multifaceted Characterization of Human Mesenchymal Stem Cells for Use in Regenerative Medicine. Stem Cells Transl. Med. 2017, 6, 2173–2185. [Google Scholar] [CrossRef]
- Li, H.; Ghazanfari, R.; Zacharaki, D.; Lim, H.C.; Scheding, S. Isolation and characterization of primary bone marrow mesenchymal stromal cells. Ann. N. Y. Acad. Sci. 2016, 1370, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Mazini, L.; Rochette, L.; Amine, M.; Malka, G. Regenerative Capacity of Adipose Derived Stem Cells (ADSCs), Comparison with Mesenchymal Stem Cells (MSCs). Int. J. Mol. Sci. 2019, 20, 2523. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.J.; Fu, R.H.; Shyu, W.C.; Liu, S.P.; Jong, G.P.; Chiu, Y.W.; Wu, H.S.; Tsou, Y.A.; Cheng, C.W.; Lin, S.Z. Adipose-derived stem cells: Isolation, characterization, and differentiation potential. Cell Transplant. 2013, 22, 701–709. [Google Scholar] [CrossRef]
- Al Madhoun, A.; Sindhu, S.; Haddad, D.; Atari, M.; Ahmad, R.; Al-Mulla, F. Dental Pulp Stem Cells Derived From Adult Human Third Molar Tooth: A Brief Review. Front. Cell Dev. Biol. 2021, 9, 717624. [Google Scholar] [CrossRef]
- Anitua, E.; Troya, M.; Zalduendo, M. Progress in the use of dental pulp stem cells in regenerative medicine. Cytotherapy 2018, 20, 479–498. [Google Scholar] [CrossRef]
- Sabbagh, J.; Ghassibe-Sabbagh, M.; Fayyad-Kazan, M.; Al-Nemer, F.; Fahed, J.C.; Berberi, A.; Badran, B. Differences in osteogenic and odontogenic differentiation potential of DPSCs and SHED. J. Dent. 2020, 101, 103413. [Google Scholar] [CrossRef]
- Seo, B.M.; Miura, M.; Gronthos, S.; Bartold, P.M.; Batouli, S.; Brahim, J.; Young, M.; Robey, P.G.; Wang, C.Y.; Shi, S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 2004, 364, 149–155. [Google Scholar] [CrossRef]
- Liu, Q.; Gao, Y.; He, J. Stem Cells from the Apical Papilla (SCAPs): Past, Present, Prospects, and Challenges. Biomedicines 2023, 11, 2047. [Google Scholar] [CrossRef]
- Shetty, S.S.; Sowmya, S.; Pradeep, A.; Jayakumar, R. Gingival Mesenchymal Stem Cells: A Periodontal Regenerative Substitute. Tissue Eng. Regen. Med. 2025, 22, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Diar-Bakirly, S.; El-Bialy, T. Human gingival fibroblasts: Isolation, characterization, and evaluation of CD146 expression. Saudi J. Biol. Sci. 2021, 28, 2518–2526. [Google Scholar] [CrossRef]
- Xu, X.; Chen, C.; Akiyama, K.; Chai, Y.; Le, A.D.; Wang, Z.; Shi, S. Gingivae contain neural-crest- and mesoderm-derived mesenchymal stem cells. J. Dent. Res. 2013, 92, 825–832. [Google Scholar] [CrossRef]
- Saito, M.T.; Silvério, K.G.; Casati, M.Z.; Sallum, E.A.; Nociti, F.H., Jr. Tooth-derived stem cells: Update and perspectives. World J. Stem Cells 2015, 7, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Feng, J.; Seidel, K.; Shi, S.; Klein, O.; Sharpe, P.; Chai, Y. Secretion of shh by a neurovascular bundle niche supports mesenchymal stem cell homeostasis in the adult mouse incisor. Cell Stem Cell 2014, 14, 160–173. [Google Scholar] [CrossRef]
- Merckx, G.; Hosseinkhani, B.; Kuypers, S.; Deville, S.; Irobi, J.; Nelissen, I.; Michiels, L.; Lambrichts, I.; Bronckaers, A. Angiogenic Effects of Human Dental Pulp and Bone Marrow-Derived Mesenchymal Stromal Cells and their Extracellular Vesicles. Cells 2020, 9, 312. [Google Scholar] [CrossRef] [PubMed]
- Pilbauerova, N.; Soukup, T.; Suchankova Kleplova, T.; Schmidt, J.; Suchanek, J. The Effect of Cultivation Passaging on the Relative Telomere Length and Proliferation Capacity of Dental Pulp Stem Cells. Biomolecules 2021, 11, 464. [Google Scholar] [CrossRef]
- Miura, M.; Gronthos, S.; Zhao, M.; Lu, B.; Fisher, L.W.; Robey, P.G.; Shi, S. SHED: Stem cells from human exfoliated deciduous teeth. Proc. Natl. Acad. Sci. USA 2003, 100, 5807–5812. [Google Scholar] [CrossRef]
- Paiva, K.B.S.; Maas, C.S.; Dos Santos, P.M.; Granjeiro, J.M.; Letra, A. Extracellular Matrix Composition and Remodeling: Current Perspectives on Secondary Palate Formation, Cleft Lip/Palate, and Palatal Reconstruction. Front. Cell Dev. Biol. 2019, 7, 340. [Google Scholar] [CrossRef]
- Sonoyama, W.; Liu, Y.; Fang, D.; Yamaza, T.; Seo, B.M.; Zhang, C.; Liu, H.; Gronthos, S.; Wang, C.Y.; Wang, S.; et al. Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS ONE 2006, 1, e79. [Google Scholar] [CrossRef]
- Sonoyama, W.; Liu, Y.; Yamaza, T.; Tuan, R.S.; Wang, S.; Shi, S.; Huang, G.T. Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: A pilot study. J. Endod. 2008, 34, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Bakopoulou, A.; Leyhausen, G.; Volk, J.; Tsiftsoglou, A.; Garefis, P.; Koidis, P.; Geurtsen, W. Comparative analysis of in vitro osteo/odontogenic differentiation potential of human dental pulp stem cells (DPSCs) and stem cells from the apical papilla (SCAP). Arch. Oral Biol. 2011, 56, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.; Wang, W.; Liu, Y.; An, Y.; Zhang, C.; Shi, S.; Wang, S. Effect of cryopreservation on biological and immunological properties of stem cells from apical papilla. J. Cell. Physiol. 2010, 223, 415–422. [Google Scholar] [CrossRef]
- Wen, S.; Zheng, X.; Yin, W.; Liu, Y.; Wang, R.; Zhao, Y.; Liu, Z.; Li, C.; Zeng, J.; Rong, M. Dental stem cell dynamics in periodontal ligament regeneration: From mechanism to application. Stem Cel. Res. Ther. 2024, 15, 389. [Google Scholar] [CrossRef]
- Zhao, B.; Chen, Q.; Zhao, L.; Mao, J.; Huang, W.; Han, X.; Liu, Y. Periodontal Ligament Stem Cell-Derived Small Extracellular Vesicles Embedded in Matrigel Enhance Bone Repair Through the Adenosine Receptor Signaling Pathway. Int. J. Nanomed. 2022, 17, 519–536. [Google Scholar] [CrossRef]
- Mohebichamkhorami, F.; Fattahi, R.; Niknam, Z.; Aliashrafi, M.; Khakpour Naeimi, S.; Gilanchi, S.; Zali, H. Periodontal ligament stem cells as a promising therapeutic target for neural damage. Stem Cell Res. Ther. 2022, 13, 273. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Xu, W.; Chen, H.; Li, S.; Dou, R.; Shen, H.; Liu, X.; Liu, X.; Hong, Y.; He, J. Comparison of the differentiation of dental pulp stem cells and periodontal ligament stem cells into neuron-like cells and their effects on focal cerebral ischemia. Acta Biochim. Biophys. Sin. 2020, 52, 1016–1029. [Google Scholar] [CrossRef]
- Kotova, A.V.; Lobov, A.A.; Dombrovskaya, J.A.; Sannikova, V.Y.; Ryumina, N.A.; Klausen, P.; Shavarda, A.L.; Malashicheva, A.B.; Enukashvily, N.I. Comparative Analysis of Dental Pulp and Periodontal Stem Cells: Differences in Morphology, Functionality, Osteogenic Differentiation and Proteome. Biomedicines 2021, 9, 1606. [Google Scholar] [CrossRef]
- Yang, Y.; Alves, T.; Miao, M.Z.; Wu, Y.C.; Li, G.; Lou, J.; Hasturk, H.; Van Dyke, T.E.; Kantarci, A.; Wu, D. Single-Cell Transcriptomic Analysis of Dental Pulp and Periodontal Ligament Stem Cells. J. Dent. Res. 2024, 103, 71–80. [Google Scholar] [CrossRef]
- Zhang, Q.; Shi, S.; Liu, Y.; Uyanne, J.; Shi, Y.; Shi, S.; Le, A.D. Mesenchymal stem cells derived from human gingiva are capable of immunomodulatory functions and ameliorate inflammation-related tissue destruction in experimental colitis. J. Immunol. 2009, 183, 7787–7798. [Google Scholar] [CrossRef]
- El-Sayed, K.M.; Paris, S.; Graetz, C.; Kassem, N.; Mekhemar, M.; Ungefroren, H.; Fändrich, F.; Dörfer, C. Isolation and characterisation of human gingival margin-derived STRO-1/MACS(+) and MACS(-) cell populations. Int. J. Oral Sci. 2015, 7, 80–88. [Google Scholar] [CrossRef]
- Srithanyarat, S.S.; Choosiri, M.; Sa-Ard-Iam, N.; Petcharat, P.; Osathanon, T. Characteristics of mesenchymal stem cells from supracrestal gingival connective tissue. J. Periodontol. 2023, 94, 439–450. [Google Scholar] [CrossRef]
- Kim, D.; Lee, A.E.; Xu, Q.; Zhang, Q.; Le, A.D. Gingiva-Derived Mesenchymal Stem Cells: Potential Application in Tissue Engineering and Regenerative Medicine—A Comprehensive Review. Front. Immunol. 2021, 12, 667221. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Gao, L.N.; An, Y.; Hu, C.H.; Jin, F.; Zhou, J.; Jin, Y.; Chen, F.M. Comparison of mesenchymal stem cells derived from gingival tissue and periodontal ligament in different incubation conditions. Biomaterials 2013, 34, 7033–7047. [Google Scholar] [CrossRef]
- Dave, J.R.; Chandekar, S.S.; Behera, S.; Desai, K.U.; Salve, P.M.; Sapkal, N.B.; Mhaske, S.T.; Dewle, A.M.; Pokare, P.S.; Page, M.; et al. Human gingival mesenchymal stem cells retain their growth and immunomodulatory characteristics independent of donor age. Sci. Adv. 2022, 8, eabm6504. [Google Scholar] [CrossRef] [PubMed]
- Fonticoli, L.; Della Rocca, Y.; Rajan, T.S.; Murmura, G.; Trubiani, O.; Oliva, S.; Pizzicannella, J.; Marconi, G.D.; Diomede, F. A Narrative Review: Gingival Stem Cells as a Limitless Reservoir for Regenerative Medicine. Int. J. Mol. Sci. 2022, 23, 4135. [Google Scholar] [CrossRef] [PubMed]
- Fawzy El-Sayed, K.M.; Dörfer, C.E. Gingival Mesenchymal Stem/Progenitor Cells: A Unique Tissue Engineering Gem. Stem Cells Int. 2016, 2016, 7154327. [Google Scholar] [CrossRef]
- Meng, Z.; Liu, J.; Feng, Z.; Guo, S.; Wang, M.; Wang, Z.; Li, Z.; Li, H.; Sui, L. N-acetylcysteine regulates dental follicle stem cell osteogenesis and alveolar bone repair via ROS scavenging. Stem Cell Res. Ther. 2022, 13, 466. [Google Scholar] [CrossRef]
- Morsczeck, C.; Götz, W.; Schierholz, J.; Zeilhofer, F.; Kühn, U.; Möhl, C.; Sippel, C.; Hoffmann, K.H. Isolation of precursor cells (PCs) from human dental follicle of wisdom teeth. Matrix Biol. 2005, 24, 155–165. [Google Scholar] [CrossRef]
- Chen, Z.; Gan, L.; Chen, X.; Zheng, J.; Shi, S.; Wu, L.; Cao, Y. LncRNA HOTAIRM1 promotes dental follicle stem cell-mediated bone regeneration by regulating HIF-1α/KDM6/EZH2/H3K27me3 axis. J. Cell. Physiol. 2023, 238, 1542–1557. [Google Scholar] [CrossRef]
- Mayo, V.; Sawatari, Y.; Huang, C.Y.; Garcia-Godoy, F. Neural crest-derived dental stem cells—Where we are and where we are going. J. Dent. 2014, 42, 1043–1051. [Google Scholar] [CrossRef] [PubMed]
- Kaltschmidt, B.; Kaltschmidt, C.; Widera, D. Adult craniofacial stem cells: Sources and relation to the neural crest. Stem Cell Rev. Rep. 2012, 8, 658–671. [Google Scholar] [CrossRef] [PubMed]
- Chai, Y.; Jiang, X.; Ito, Y.; Bringas, P., Jr.; Han, J.; Rowitch, D.H.; Soriano, P.; McMahon, A.P.; Sucov, H.M. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development 2000, 127, 1671–1679. [Google Scholar] [CrossRef]
- Alperovich, M.; Tonello, C.; Mayes, L.C.; Kahle, K.T. Non-syndromic craniosynostosis. Nat. Rev. Dis. Primers 2025, 11, 24. [Google Scholar] [CrossRef]
- Srinivasan, A.; Teo, N.; Poon, K.J.; Tiwari, P.; Ravichandran, A.; Wen, F.; Teoh, S.H.; Lim, T.C.; Toh, Y.C. Comparative Craniofacial Bone Regeneration Capacities of Mesenchymal Stem Cells Derived from Human Neural Crest Stem Cells and Bone Marrow. ACS Biomater. Sci. Eng. 2021, 7, 207–221. [Google Scholar] [CrossRef]
- Zhang, C.; Han, X.; Liu, J.; Chen, L.; Lei, Y.; Chen, K.; Si, J.; Wang, T.Y.; Zhou, H.; Zhao, X.; et al. Single-cell Transcriptomic Analysis Reveals the Cellular Heterogeneity of Mesenchymal Stem Cells. Genom. Proteom. Bioinf. 2022, 20, 70–86. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.N.; Huang, W.K.; Li, X.L.; Bai, Y.Z.; Zhang, S.C. Sox10 Is a Specific Biomarker for Neural Crest Stem Cells in Immunohistochemical Staining in Wistar Rats. Dis. Markers 2020, 2020, 8893703. [Google Scholar] [CrossRef]
- Farahani, R.M.; Xaymardan, M. Platelet-Derived Growth Factor Receptor Alpha as a Marker of Mesenchymal Stem Cells in Development and Stem Cell Biology. Stem Cells Int. 2015, 2015, 362753. [Google Scholar] [CrossRef]
- Zhang, Y.; Xing, Y.; Jia, L.; Ji, Y.; Zhao, B.; Wen, Y.; Xu, X. An In Vitro Comparative Study of Multisource Derived Human Mesenchymal Stem Cells for Bone Tissue Engineering. Stem Cells Dev. 2018, 27, 1634–1645. [Google Scholar] [CrossRef]
- Winning, L.; El Karim, I.A.; Lundy, F.T. A Comparative Analysis of the Osteogenic Potential of Dental Mesenchymal Stem Cells. Stem Cells Dev. 2019, 28, 1050–1058. [Google Scholar] [CrossRef]
- Cao, C.; Maska, B.; Malik, M.A.; Tagett, R.; Kaigler, D. Immunomodulatory differences between mesenchymal stem cells from different oral tissues. Heliyon 2024, 10, e23317. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Yamada, Y.; Katagiri, W.; Sugito, T.; Ito, K.; Ueda, M. Stem cell proliferation pathways comparison between human exfoliated deciduous teeth and dental pulp stem cells by gene expression profile from promising dental pulp. J. Endod. 2009, 35, 1536–1542. [Google Scholar] [CrossRef] [PubMed]
- Abuarqoub, D.; Aslam, N.; Almajali, B.; Shajrawi, L.; Jafar, H.; Awidi, A. Neuro-regenerative potential of dental stem cells: A concise review. Cell Tissue Res. 2020, 382, 267–279. [Google Scholar] [CrossRef]
- Pagella, P.; Miran, S.; Neto, E.; Martin, I.; Lamghari, M.; Mitsiadis, T.A. Human dental pulp stem cells exhibit enhanced properties in comparison to human bone marrow stem cells on neurites outgrowth. FASEB J. 2020, 34, 5499–5511. [Google Scholar] [CrossRef]
- Ornoy, A. Craniofacial malformations and their association with brain development: The importance of a multidisciplinary approach for treatment. Odontology 2020, 108, 1–15. [Google Scholar] [CrossRef]
- Alkuraya, F.S.; Saadi, I.; Lund, J.J.; Turbe-Doan, A.; Morton, C.C.; Maas, R.L. SUMO1 haploinsufficiency leads to cleft lip and palate. Science 2006, 313, 1751. [Google Scholar] [CrossRef] [PubMed]
- Mossey, P.A.; Little, J.; Munger, R.G.; Dixon, M.J.; Shaw, W.C. Cleft lip and palate. Lancet 2009, 374, 1773–1785. [Google Scholar] [CrossRef]
- Martín-Del-Campo, M.; Rosales-Ibañez, R.; Rojo, L. Biomaterials for Cleft Lip and Palate Regeneration. Int. J. Mol. Sci. 2019, 20, 2176. [Google Scholar] [CrossRef]
- Siewert, A.; Hoeland, S.; Mangold, E.; Ludwig, K.U. Combining genetic and single-cell expression data reveals cell types and novel candidate genes for orofacial clefting. Sci. Rep. 2024, 14, 26492. [Google Scholar] [CrossRef]
- Parisi, L.; Rihs, S.; La Scala, G.C.; Schnyder, I.; Katsaros, C.; Degen, M. Discovery and characterization of heterogeneous and multipotent fibroblast populations isolated from excised cleft lip tissue. Stem Cell Res. Ther. 2022, 13, 469. [Google Scholar] [CrossRef]
- Zheng, C.; Chen, J.; Liu, S.; Jin, Y. Stem cell-based bone and dental regeneration: A view of microenvironmental modulation. Int. J. Oral Sci. 2019, 11, 23. [Google Scholar] [CrossRef] [PubMed]
- Alarcón-Apablaza, J.; Prieto, R.; Rojas, M.; Fuentes, R. Potential of Oral Cavity Stem Cells for Bone Regeneration: A Scoping Review. Cells 2023, 12, 1392. [Google Scholar] [CrossRef]
- Nobre Pacifico Pereira, K.H.; Cruz Dos Santos Correia, L.E.; Ritir Oliveira, E.L.; Bernardo, R.B.; Nagib Jorge, M.L.; Mezzena Gobato, M.L.; Ferreira de Souza, F.; Rocha, N.S.; Chiacchio, S.B.; Gomes Lourenço, M.L. Incidence of congenital malformations and impact on the mortality of neonatal canines. Theriogenology 2019, 140, 52–57. [Google Scholar] [CrossRef]
- Fakhouri, W.D.; Metwalli, K.; Naji, A.; Bakhiet, S.; Quispe-Salcedo, A.; Nitschke, L.; Kousa, Y.A.; Schutte, B.C. Intercellular Genetic Interaction Between Irf6 and Twist1 during Craniofacial Development. Sci. Rep. 2017, 7, 7129. [Google Scholar] [CrossRef] [PubMed]
- Leyendecker Junior, A.; Gomes Pinheiro, C.C.; Lazzaretti Fernandes, T.; Franco Bueno, D. The use of human dental pulp stem cells for in vivo bone tissue engineering: A systematic review. J. Tissue Eng. 2018, 9, 2041731417752766. [Google Scholar] [CrossRef] [PubMed]
- Stanton, E.; Feng, J.; Kondra, K.; Sanchez, J.; Jimenez, C.; Brown, K.S.; Skiles, M.L.; Urata, M.M.; Chai, Y.; Hammoudeh, J.A. A Calvarial Defect Model to Investigate the Osteogenic Potential of Umbilical Cord Stem Cells in Bone Regeneration. Plast. Reconstr. Surg. 2024, 153, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Ahlfeld, T.; Lode, A.; Richter, R.F.; Pradel, W.; Franke, A.; Rauner, M.; Stadlinger, B.; Lauer, G.; Gelinsky, M.; Korn, P. Toward Biofabrication of Resorbable Implants Consisting of a Calcium Phosphate Cement and Fibrin-A Characterization In Vitro and In Vivo. Int. J. Mol. Sci. 2021, 22, 1218. [Google Scholar] [CrossRef]
- Lee, A.E.; Choi, J.G.; Shi, S.H.; He, P.; Zhang, Q.Z.; Le, A.D. DPSC-Derived Extracellular Vesicles Promote Rat Jawbone Regeneration. J. Dent. Res. 2023, 102, 313–321. [Google Scholar] [CrossRef]
- Chamieh, F.; Collignon, A.M.; Coyac, B.R.; Lesieur, J.; Ribes, S.; Sadoine, J.; Llorens, A.; Nicoletti, A.; Letourneur, D.; Colombier, M.L.; et al. Accelerated craniofacial bone regeneration through dense collagen gel scaffolds seeded with dental pulp stem cells. Sci. Rep. 2016, 6, 38814. [Google Scholar] [CrossRef]
- Lin, C.Y.; Kuo, P.J.; Chin, Y.T.; Weng, I.T.; Lee, H.W.; Huang, H.M.; Lin, H.Y.; Hsiung, C.N.; Chan, Y.H.; Lee, S.Y. Dental Pulp Stem Cell Transplantation with 2,3,5,4′-Tetrahydroxystilbene-2-O-β-D-glucoside Accelerates Alveolar Bone Regeneration in Rats. J. Endod. 2019, 45, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Jahanbin, A.; Rashed, R.; Alamdari, D.H.; Koohestanian, N.; Ezzati, A.; Kazemian, M.; Saghafi, S.; Raisolsadat, M.A. Success of Maxillary Alveolar Defect Repair in Rats Using Osteoblast-Differentiated Human Deciduous Dental Pulp Stem Cells. J. Oral Maxillofac. Surg. 2016, 74, 829.e1–829.e9. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Kim, H.Y.; Park, J.S.; Lee, D.J.; Zhang, S.; Green, D.W.; Okano, T.; Hong, J.H.; Jung, H.S. Developing palatal bone using human mesenchymal stem cell and stem cells from exfoliated deciduous teeth cell sheets. J. Tissue Eng. Regen. Med. 2019, 13, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Hiraki, T.; Kunimatsu, R.; Nakajima, K.; Abe, T.; Yamada, S.; Rikitake, K.; Tanimoto, K. Stem cell-derived conditioned media from human exfoliated deciduous teeth promote bone regeneration. Oral Dis. 2020, 26, 381–390. [Google Scholar] [CrossRef]
- Tanikawa, D.Y.S.; Pinheiro, C.C.G.; Almeida, M.C.A.; Oliveira, C.; Coudry, R.A.; Rocha, D.L.; Bueno, D.F. Deciduous Dental Pulp Stem Cells for Maxillary Alveolar Reconstruction in Cleft Lip and Palate Patients. Stem Cells Int. 2020, 2020, 6234167. [Google Scholar] [CrossRef] [PubMed]
- Kandalam, U.; Kawai, T.; Ravindran, G.; Brockman, R.; Romero, J.; Munro, M.; Ortiz, J.; Heidari, A.; Thomas, R.; Kuriakose, S.; et al. Predifferentiated Gingival Stem Cell-Induced Bone Regeneration in Rat Alveolar Bone Defect Model. Tissue Eng. Part A 2021, 27, 424–436. [Google Scholar] [CrossRef]
- Bueno, D.F.; Kerkis, I.; Costa, A.M.; Martins, M.T.; Kobayashi, G.S.; Zucconi, E.; Fanganiello, R.D.; Salles, F.T.; Almeida, A.B.; do Amaral, C.E.; et al. New source of muscle-derived stem cells with potential for alveolar bone reconstruction in cleft lip and/or palate patients. Tissue Eng. Part A 2009, 15, 427–435. [Google Scholar] [CrossRef]
- Bariana, M.; Dwivedi, P.; Ranjitkar, S.; Kaidonis, J.A.; Losic, D.; Anderson, P.J. Biological response of human suture mesenchymal cells to Titania nanotube-based implants for advanced craniosynostosis therapy. Colloids Surf. B Biointerfaces 2017, 150, 59–67. [Google Scholar] [CrossRef]
- Wu, X.; Gu, Y. Signaling Mechanisms Underlying Genetic Pathophysiology of Craniosynostosis. Int. J. Biol. Sci. 2019, 15, 298–311. [Google Scholar] [CrossRef]
- Twigg, S.R.; Wilkie, A.O. A Genetic-Pathophysiological Framework for Craniosynostosis. Am. J. Hum. Genet. 2015, 97, 359–377. [Google Scholar] [CrossRef]
- Swanson, W.B.; Omi, M.; Zhang, Z.; Nam, H.K.; Jung, Y.; Wang, G.; Ma, P.X.; Hatch, N.E.; Mishina, Y. Macropore design of tissue engineering scaffolds regulates mesenchymal stem cell differentiation fate. Biomaterials 2021, 272, 120769. [Google Scholar] [CrossRef]
- Teng, C.S.; Ting, M.C.; Farmer, D.T.; Brockop, M.; Maxson, R.E.; Crump, J.G. Altered bone growth dynamics prefigure craniosynostosis in a zebrafish model of Saethre-Chotzen syndrome. Elife 2018, 7, e37024. [Google Scholar] [CrossRef]
- Fearon, J.A.; Barrientos, S.; Ditthakasem, K.; Herbert, M. Optic Nerve Atrophy in Syndromic Craniosynostosis. Plast. Reconstr. Surg. 2022, 150, 381e–386e. [Google Scholar] [CrossRef]
- Hermann, C.D.; Hyzy, S.L.; Olivares-Navarrete, R.; Walker, M.; Williams, J.K.; Boyan, B.D.; Schwartz, Z. Craniosynostosis and Resynostosis: Models, Imaging, and Dental Implications. J. Dent. Res. 2016, 95, 846–852. [Google Scholar] [CrossRef]
- Matrongolo, M.J.; Ang, P.S.; Wu, J.; Jain, A.; Thackray, J.K.; Reddy, A.; Sung, C.C.; Barbet, G.; Hong, Y.K.; Tischfield, M.A. Piezo1 agonist restores meningeal lymphatic vessels, drainage, and brain-CSF perfusion in craniosynostosis and aged mice. J. Clin. Investig. 2023, 134, e171468. [Google Scholar] [CrossRef] [PubMed]
- Azoury, S.C.; Reddy, S.; Shukla, V.; Deng, C.X. Fibroblast Growth Factor Receptor 2 (FGFR2) Mutation Related Syndromic Craniosynostosis. Int. J. Biol. Sci. 2017, 13, 1479–1488. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Ma, L.; Yuan, Y.; Ye, X.; Montagne, A.; He, J.; Ho, T.V.; Wu, Y.; Zhao, Z.; Sta Maria, N.; et al. Cranial Suture Regeneration Mitigates Skull and Neurocognitive Defects in Craniosynostosis. Cell 2021, 184, 243–256.e18. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, T.; Stevens, R.; Boka, A.; DiRienzo, L.; Chang, C.; Yu, H.I.; Nishimori, K.; Morrison, C.; Hsu, W. BMPR1A maintains skeletal stem cell properties in craniofacial development and craniosynostosis. Sci. Transl. Med. 2021, 13, eabb4416. [Google Scholar] [CrossRef]
- Bok, S.; Yallowitz, A.R.; Sun, J.; McCormick, J.; Cung, M.; Hu, L.; Lalani, S.; Li, Z.; Sosa, B.R.; Baumgartner, T.; et al. A multi-stem cell basis for craniosynostosis and calvarial mineralization. Nature 2023, 621, 804–812. [Google Scholar] [CrossRef]
- Zhang, H.; Gong, X.; Xu, X.; Wang, X.; Sun, Y. Tooth number abnormality: From bench to bedside. Int. J. Oral Sci. 2023, 15, 5. [Google Scholar] [CrossRef]
- Wu, Y.; Lai, L.; Chen, J.; Li, X.; Hou, J. Genotypic and phenotypic correlations in tooth agenesis: Insights from WNT10A and EDA mutations in syndromic and non-syndromic forms. Hum. Genet. 2024, 143, 1253–1264. [Google Scholar] [CrossRef]
- Jiang, C.; Yu, K.; Shen, Y.; Wang, F.; Dai, Q.; Wu, Y. The phenotype and genotype of PAX9 mutations causing tooth agenesis. Clin. Oral Investig. 2023, 27, 4369–4378. [Google Scholar] [CrossRef]
- Fournier, B.P.; Bruneau, M.H.; Toupenay, S.; Kerner, S.; Berdal, A.; Cormier-Daire, V.; Hadj-Rabia, S.; Coudert, A.E.; de La Dure-Molla, M. Patterns of Dental Agenesis Highlight the Nature of the Causative Mutated Genes. J. Dent. Res. 2018, 97, 1306–1316. [Google Scholar] [CrossRef] [PubMed]
- Matalova, E.; Fleischmannova, J.; Sharpe, P.T.; Tucker, A.S. Tooth agenesis: From molecular genetics to molecular dentistry. J. Dent. Res. 2008, 87, 617–623. [Google Scholar] [CrossRef]
- Liu, H.; Yue, Y.; Xu, Z.; Guo, L.; Wu, C.; Zhang, D.; Luo, L.; Huang, W.; Chen, H.; Yang, D. mTORC1 signaling pathway regulates tooth repair. Int. J. Oral Sci. 2023, 15, 14. [Google Scholar] [CrossRef]
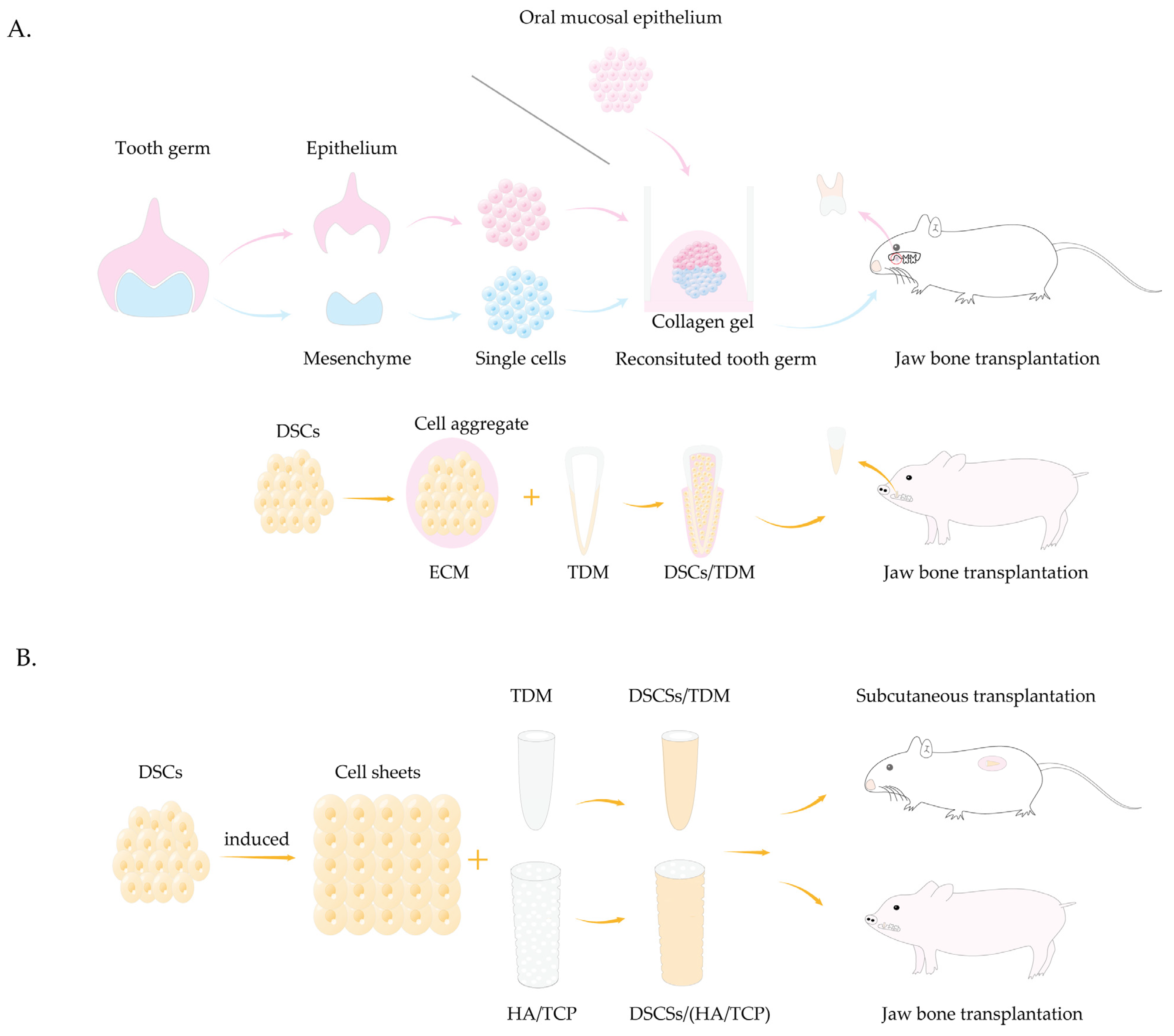
- Sui, B.D.; Zheng, C.X.; Zhao, W.M.; Xuan, K.; Li, B.; Jin, Y. Mesenchymal condensation in tooth development and regeneration: A focus on translational aspects of organogenesis. Physiol. Rev. 2023, 103, 1899–1964. [Google Scholar] [CrossRef] [PubMed]
- Nakao, K.; Morita, R.; Saji, Y.; Ishida, K.; Tomita, Y.; Ogawa, M.; Saitoh, M.; Tomooka, Y.; Tsuji, T. The development of a bioengineered organ germ method. Nat. Methods 2007, 4, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, E.; Morita, R.; Nakao, K.; Ishida, K.; Nakamura, T.; Takano-Yamamoto, T.; Ogawa, M.; Mizuno, M.; Kasugai, S.; Tsuji, T. Fully functional bioengineered tooth replacement as an organ replacement therapy. Proc. Natl. Acad. Sci. USA 2009, 106, 13475–13480. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, F.; Fan, Z.; Wu, T.; He, J.; Wang, J.; Zhang, C.; Wang, S. Whole-Tooth Regeneration by Allogeneic Cell Reassociation in Pig Jawbone. Tissue Eng. Part A 2019, 25, 1202–1212. [Google Scholar] [CrossRef]
- Volponi, A.A.; Pang, Y.; Sharpe, P.T. Stem cell-based biological tooth repair and regeneration. Trends Cell Biol. 2010, 20, 715–722. [Google Scholar] [CrossRef]
- Nakagawa, E.; Itoh, T.; Yoshie, H.; Satokata, I. Odontogenic potential of post-natal oral mucosal epithelium. J. Dent. Res. 2009, 88, 219–223. [Google Scholar] [CrossRef]
- Son, Y.B.; Bharti, D.; Kim, S.B.; Jo, C.H.; Bok, E.Y.; Lee, S.L.; Kang, Y.H.; Rho, G.J. Comparison of Pluripotency, Differentiation, and Mitochondrial Metabolism Capacity in Three-Dimensional Spheroid Formation of Dental Pulp-Derived Mesenchymal Stem Cells. Biomed. Res. Int. 2021, 2021, 5540877. [Google Scholar] [CrossRef] [PubMed]
- Suga, T.; Usui, M.; Onizuka, S.; Sano, K.; Sato, T.; Nakazawa, K.; Ariyoshi, W.; Nishihara, T.; Nakashima, K. Characterization and Study of Gene Expression Profiles of Human Periodontal Mesenchymal Stem Cells in Spheroid Cultures by Transcriptome Analysis. Stem Cells Int. 2021, 2021, 5592804. [Google Scholar] [CrossRef] [PubMed]
- Xuan, K.; Li, B.; Guo, H.; Sun, W.; Kou, X.; He, X.; Zhang, Y.; Sun, J.; Liu, A.; Liao, L.; et al. Deciduous autologous tooth stem cells regenerate dental pulp after implantation into injured teeth. Sci. Transl. Med. 2018, 10, eaaf3227. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Li, B.; Wu, M.; Zhao, W.; He, X.; Sui, B.; Dong, Z.; Wang, L.; Shi, S.; Huang, X.; et al. Odontogenesis-related developmental microenvironment facilitates deciduous dental pulp stem cell aggregates to revitalize an avulsed tooth. Biomaterials 2021, 279, 121223. [Google Scholar] [CrossRef]
- Yang, X.; Ma, Y.; Guo, W.; Yang, B.; Tian, W. Stem cells from human exfoliated deciduous teeth as an alternative cell source in bio-root regeneration. Theranostics 2019, 9, 2694–2711. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Chen, G.; Li, J.; Zou, Q.; Xie, D.; Chen, Y.; Wang, H.; Zheng, X.; Long, J.; Tang, W.; et al. Tooth root regeneration using dental follicle cell sheets in combination with a dentin matrix—Based scaffold. Biomaterials 2012, 33, 2449–2461. [Google Scholar] [CrossRef]
- Gao, Z.H.; Hu, L.; Liu, G.L.; Wei, F.L.; Liu, Y.; Liu, Z.H.; Fan, Z.P.; Zhang, C.M.; Wang, J.S.; Wang, S.L. Bio-Root and Implant-Based Restoration as a Tooth Replacement Alternative. J. Dent. Res. 2016, 95, 642–649. [Google Scholar] [CrossRef]
- Zhang, Z.; Hu, H.; Xu, Z.; Shan, C.; Chen, H.; Xie, K.; Wang, K.; Wang, Y.; Zhu, Q.; Yin, Y.; et al. A Chemically Defined Culture for Tooth Reconstitution. Adv. Sci. 2025, 12, e2404345. [Google Scholar] [CrossRef]
- Alghadeer, A.; Hanson-Drury, S.; Patni, A.P.; Ehnes, D.D.; Zhao, Y.T.; Li, Z.; Phal, A.; Vincent, T.; Lim, Y.C.; O’Day, D.; et al. Single-cell census of human tooth development enables generation of human enamel. Dev. Cell 2023, 58, 2163–2180.e9. [Google Scholar] [CrossRef]
- Gao, X.; Wu, Y.; Liao, L.; Tian, W. Oral Organoids: Progress and Challenges. J. Dent. Res. 2021, 100, 454–463. [Google Scholar] [CrossRef]
- Wu, Y.; Li, X.; Liu, H.; Yang, X.; Li, R.; Zhao, H.; Shang, Z. Organoids in the oral and maxillofacial region: Present and future. Int. J. Oral Sci. 2024, 16, 61. [Google Scholar] [CrossRef] [PubMed]
- Hermans, F.; Hemeryck, L.; Bueds, C.; Torres Pereiro, M.; Hasevoets, S.; Kobayashi, H.; Lambrechts, D.; Lambrichts, I.; Bronckaers, A.; Vankelecom, H. Organoids from mouse molar and incisor as new tools to study tooth-specific biology and development. Stem Cell Rep. 2023, 18, 1166–1181. [Google Scholar] [CrossRef]
- Rosowski, J.; Bräunig, J.; Amler, A.K.; Strietzel, F.P.; Lauster, R.; Rosowski, M. Emulating the early phases of human tooth development in vitro. Sci. Rep. 2019, 9, 7057. [Google Scholar] [CrossRef]
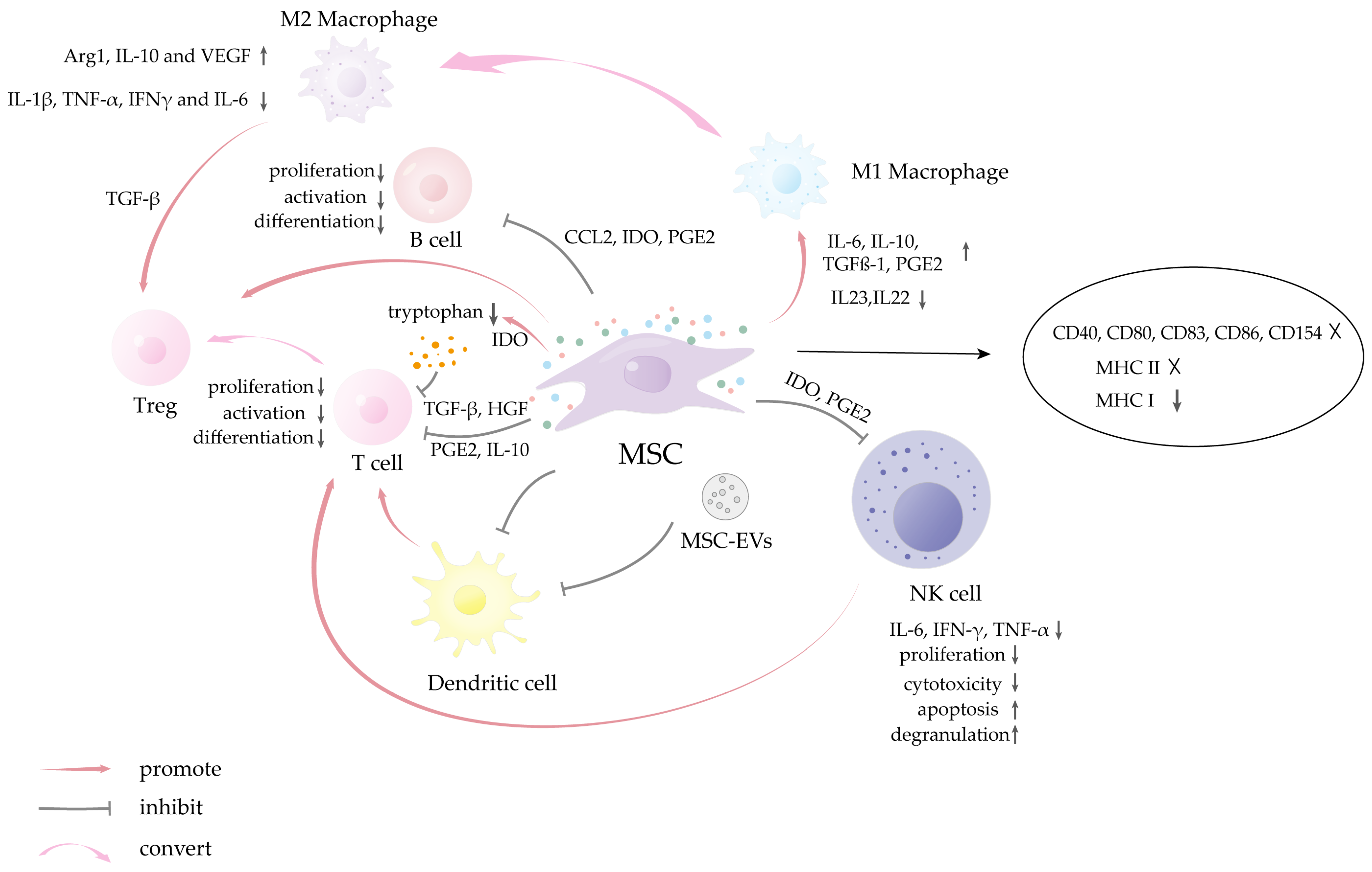
- Shah, K.; Shah, N.; Ghassemi, F.; Ly, C.; George, T.; Lutz, C.; Sumer, H. Alloreactivity of Allogeneic Mesenchymal Stem/Stromal Cells and Other Cellular Therapies: A Concise Review. Stem Cells Int. 2022, 2022, 9589600. [Google Scholar] [CrossRef]
- Gebler, A.; Zabel, O.; Seliger, B. The immunomodulatory capacity of mesenchymal stem cells. Trends Mol. Med. 2012, 18, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wu, Q.; Tam, P.K.H. Immunomodulatory Mechanisms of Mesenchymal Stem Cells and Their Potential Clinical Applications. Int. J. Mol. Sci. 2022, 23, 10023. [Google Scholar] [CrossRef]
- Abbasi, B.; Shamsasenjan, K.; Ahmadi, M.; Beheshti, S.A.; Saleh, M. Mesenchymal stem cells and natural killer cells interaction mechanisms and potential clinical applications. Stem Cell Res. Ther. 2022, 13, 97. [Google Scholar] [CrossRef] [PubMed]
- Moloudizargari, M.; Govahi, A.; Fallah, M.; Rezvanfar, M.A.; Asghari, M.H.; Abdollahi, M. The mechanisms of cellular crosstalk between mesenchymal stem cells and natural killer cells: Therapeutic implications. J. Cell. Physiol. 2021, 236, 2413–2429. [Google Scholar] [CrossRef]
- Abumaree, M.H.; Al Jumah, M.A.; Kalionis, B.; Jawdat, D.; Al Khaldi, A.; Abomaray, F.M.; Fatani, A.S.; Chamley, L.W.; Knawy, B.A. Human placental mesenchymal stem cells (pMSCs) play a role as immune suppressive cells by shifting macrophage differentiation from inflammatory M1 to anti-inflammatory M2 macrophages. Stem Cell Rev. Rep. 2013, 9, 620–641. [Google Scholar] [CrossRef]
- Hyvärinen, K.; Holopainen, M.; Skirdenko, V.; Ruhanen, H.; Lehenkari, P.; Korhonen, M.; Käkelä, R.; Laitinen, S.; Kerkelä, E. Mesenchymal Stromal Cells and Their Extracellular Vesicles Enhance the Anti-Inflammatory Phenotype of Regulatory Macrophages by Downregulating the Production of Interleukin (IL)-23 and IL-22. Front. Immunol. 2018, 9, 771. [Google Scholar] [CrossRef]
- Dang, J.; Yang, J.; Yu, Z.; Chen, L.; Zhang, Z.; Wang, K.; Tang, J.; Yi, C. Bone marrow mesenchymal stem cells enhance angiogenesis and promote fat retention in fat grafting via polarized macrophages. Stem Cell Res. Ther. 2022, 13, 52. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Hua, J. Interactions between mesenchymal stem cells and the immune system. Cell. Mol. Life Sci. 2017, 74, 2345–2360. [Google Scholar] [CrossRef]
- Podestà, M.A.; Remuzzi, G.; Casiraghi, F. Mesenchymal Stromal Cells for Transplant Tolerance. Front. Immunol. 2019, 10, 1287. [Google Scholar] [CrossRef] [PubMed]
- Giacomini, C.; Granéli, C.; Hicks, R.; Dazzi, F. The critical role of apoptosis in mesenchymal stromal cell therapeutics and implications in homeostasis and normal tissue repair. Cell. Mol. Immunol. 2023, 20, 570–582. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Cai, C.; Xu, D.; Liu, Q.; Zheng, S.; Liu, L.; Li, G.; Zhang, X.; Li, X.; Ma, Y.; et al. Human Mesenchymal Stem Cell-Treated Regulatory CD23(+)CD43(+) B Cells Alleviate Intestinal Inflammation. Theranostics 2019, 9, 4633–4647. [Google Scholar] [CrossRef]
- Reis, M.; Mavin, E.; Nicholson, L.; Green, K.; Dickinson, A.M.; Wang, X.N. Mesenchymal Stromal Cell-Derived Extracellular Vesicles Attenuate Dendritic Cell Maturation and Function. Front. Immunol. 2018, 9, 2538. [Google Scholar] [CrossRef]
- Ma, S.; Xie, N.; Li, W.; Yuan, B.; Shi, Y.; Wang, Y. Immunobiology of mesenchymal stem cells. Cell Death Differ. 2014, 21, 216–225. [Google Scholar] [CrossRef]
- Zangi, L.; Margalit, R.; Reich-Zeliger, S.; Bachar-Lustig, E.; Beilhack, A.; Negrin, R.; Reisner, Y. Direct imaging of immune rejection and memory induction by allogeneic mesenchymal stromal cells. Stem Cells 2009, 27, 2865–2874. [Google Scholar] [CrossRef]
- Wang, F.; Li, R.; Xu, J.Y.; Bai, X.; Wang, Y.; Chen, X.R.; Pan, C.; Chen, S.; Zhou, K.; Heng, B.C.; et al. Downregulating human leucocyte antigens on mesenchymal stromal cells by epigenetically repressing a β2-microglobulin super-enhancer. Nat. Biomed. Eng. 2024, 8, 1682–1699. [Google Scholar] [CrossRef]
- Zheng, D.; Wang, X.; Zhang, Z.; Li, E.; Yeung, C.; Borkar, R.; Qin, G.; Wu, Y.; Xu, R.H. Engineering of human mesenchymal stem cells resistant to multiple natural killer subtypes. Int. J. Biol. Sci. 2022, 18, 426–440. [Google Scholar] [CrossRef]
- Zha, S.; Tay, J.C.; Zhu, S.; Li, Z.; Du, Z.; Wang, S. Generation of Mesenchymal Stromal Cells with Low Immunogenicity from Human PBMC-Derived β2 Microglobulin Knockout Induced Pluripotent Stem Cells. Cell Transplant. 2020, 29, 963689720965529. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, S.; Shi, Y.; Galipeau, J.; Krampera, M.; Leblanc, K.; Martin, I.; Nolta, J.; Phinney, D.G.; Sensebe, L. Mesenchymal stem versus stromal cells: International Society for Cell & Gene Therapy (ISCT®) Mesenchymal Stromal Cell committee position statement on nomenclature. Cytotherapy 2019, 21, 1019–1024. [Google Scholar] [PubMed]
- Yan, K.; Ma, F.; Song, X.; Wang, H.; Liu, P.; Zhang, J.; Jin, X.; Han, P.; Zuo, X.; Kang, Y.J. Unveiling distinctions between mesenchymal stromal cells and stem cells by single-cell transcriptomic analysis. Heliyon 2025, 11, e42311. [Google Scholar] [CrossRef]
- Pinkhasov, I.; Kabakov, L.; Nemcovsky, C.E.; Weinreb, M.; Schlesinger, P.; Bender, O.; Gal, M.; Bar, D.Z.; Weinberg, E. Single-cell transcriptomic analysis of oral masticatory and lining mucosa-derived mesenchymal stromal cells. J. Clin. Periodontol. 2023, 50, 807–818. [Google Scholar] [CrossRef]
- FDA Approves First Mesenchymal Stromal Cell Therapy to Treat Steroid-Refractory Acute Graft-Versus-Host Disease. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-mesenchymal-stromal-cell-therapy-treat-steroid-refractory-acute-graft-versus-host (accessed on 18 December 2024).
- Chęciński, M.; Chęcińska, K.; Turosz, N.; Kamińska, M.; Nowak, Z.; Sikora, M.; Chlubek, D. Autologous Stem Cells Transplants in the Treatment of Temporomandibular Joints Disorders: A Systematic Review and Meta-Analysis of Clinical Trials. Cells 2022, 11, 2709. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.; Seagroves, J.T.; Chen, C.; Shah, K.; Aghaloo, T.; Wu, B.M.; Bencharit, S.; Moshaverinia, A. Dental and orofacial mesenchymal stem cells in craniofacial regeneration: The prosthodontist’s point of view. J. Prosthet. Dent. 2017, 118, 455–461. [Google Scholar] [CrossRef]
- Ivanovski, S.; Han, P.; Peters, O.A.; Sanz, M.; Bartold, P.M. The Therapeutic Use of Dental Mesenchymal Stem Cells in Human Clinical Trials. J. Dent. Res. 2024, 103, 1173–1184. [Google Scholar] [CrossRef]
- Shan, Z.; Zhao, Y.; Chen, X.; Zhan, G.; Huang, J.; Yang, X.; Xu, C.; Guo, N.; Xiong, Z.; Wu, F.; et al. KMT2D deficiency leads to cellular developmental disorders and enhancer dysregulation in neural-crest-containing brain organoids. Sci. Bull. 2024, 69, 3533–3546. [Google Scholar] [CrossRef]
- Luo, E.; Liu, H.; Zhao, Q.; Shi, B.; Chen, Q. Dental-craniofacial manifestation and treatment of rare diseases. Int. J. Oral Sci. 2019, 11, 9. [Google Scholar] [CrossRef]
| MSC Type | Markers | References |
|---|---|---|
| BM-MSCs | CD73, CD90, CD105, CD146, CD29, CD44, OCT4, Nanog, STRO-1, CD49a, PDGFR-α/β, CD271 | [81,82,83] |
| ADSCs | CD29, CD49e, CD44, CD144, CD13, CD73, CD90, CD105, CD146, CD10, CD36, CD106 | [84,85] |
| DPSCs | CD13, CD29, CD44, CD59, CD73, CD90, CD105, CD146, OCT4, STRO-1, CD151, CD166 | [80,86,87] |
| SHED | CD44, CD90, CD105, CD73, CD146, OCT4, STRO-1 | [80,88] |
| PDLSCs | CD13, CD29, CD44, CD59, CD90, CD105, STRO-1 | [80,89] |
| SCAP | CD13, CD44, CD24, CD29, CD73, CD90, CD105, CD106, CD146, STRO-1, OCT4, CD166 | [80,90] |
| GMSCs | CD13, CD29, CD44, CD54, CD73, CD90, CD105, CD166, STRO-1 | [91,92,93] |
| DFSCs | CD13, CD29, CD44, CD59, CD73, CD90, CD105, STRO-1 | [80,94] |
| Title | Trial Number | MSCs Source | Phase | Enrollment | Primary Purpose | Scaffold | Study Type |
|---|---|---|---|---|---|---|---|
| Use of Mesenchymal Stem Cells for Alveolar Bone Tissue Engineering for Cleft Lip and Palate Patients | NCT01932164 | SHED | Phase 1, pilot | 5 | Treatment | Geistlich Bio-Oss®♦, III ▲ | Interventional |
| Bone Tissue Engineering with Dental Pulp Stem Cells for Alveolar Cleft Repair | NCT03766217 | SHED | Phase 3, pivotal | 62 | Treatment | Hydroxyapatite/collagen, III | Interventional |
| Tissue Engineered Constructs for Alveolar Cleft Repair | NCT03563495 | BM-MSCs | Phase 1, pilot | 10 | Treatment | None | Interventional |
| The Effect of Bone Marrow Stem Cells Harvested from the Iliac Crest Versus Mandibular Ramus in Alveolar Cleft Regeneration | NCT06636643 | BM-MSCs | Phase 1, pilot | 12 | N/A | Collagen sponge and nanohydroxyapatite, III | Observational |
| Validation of a Production Method of Stem Cell Isolated from the Nasal Cavity for an Innovative Cell Therapy of Cleft Palate | NCT02900014 | Nasal MSCs | N/A * | 2 | Basic Science | None | Interventional |
| Cell Therapy for Craniofacial Bone Defects | NCT01616953 | BM-MSCs | Phase 1 Phase 2, pilot | 18 | Treatment | None | Interventional |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, X.; Peng, L.; Bian, Z.; Yin, W. Craniomaxillofacial-Derived MSCs in Congenital Defect Reconstruction. Biomolecules 2025, 15, 953. https://doi.org/10.3390/biom15070953
Song X, Peng L, Bian Z, Yin W. Craniomaxillofacial-Derived MSCs in Congenital Defect Reconstruction. Biomolecules. 2025; 15(7):953. https://doi.org/10.3390/biom15070953
Chicago/Turabian StyleSong, Xiaona, Linlin Peng, Zhuan Bian, and Wei Yin. 2025. "Craniomaxillofacial-Derived MSCs in Congenital Defect Reconstruction" Biomolecules 15, no. 7: 953. https://doi.org/10.3390/biom15070953
APA StyleSong, X., Peng, L., Bian, Z., & Yin, W. (2025). Craniomaxillofacial-Derived MSCs in Congenital Defect Reconstruction. Biomolecules, 15(7), 953. https://doi.org/10.3390/biom15070953





