A Transcriptomics Approach to Unveil the Antioxidant Effects of Tryptophan on Oocyte Quality Under Oxidative Stress in Pigs
Abstract
1. Introduction
2. Material and Methods
2.1. Porcine Oocytes Collection
2.2. Oocyte Maturation and Treatment with H2O2 and Tryptophan
2.3. Oxidative Stress Biomarkers Determination
2.4. RNA Extraction and Assessment of Quality
2.5. Differential Analysis of Genes
2.6. GO Annotation and KEGG Pathway Enrichment Analysis
2.7. Protein–Protein Interaction (PPI) Analysis
2.8. Validation of DEGs Quantitative Real-Time PCR (qRT-PCR)
2.9. Statistical Analysis
3. Results
3.1. Tryptophan Improved the Quality of the Aged Oocytes
3.2. Tryptophan Supplementation Mitigates the Oxidative Stress
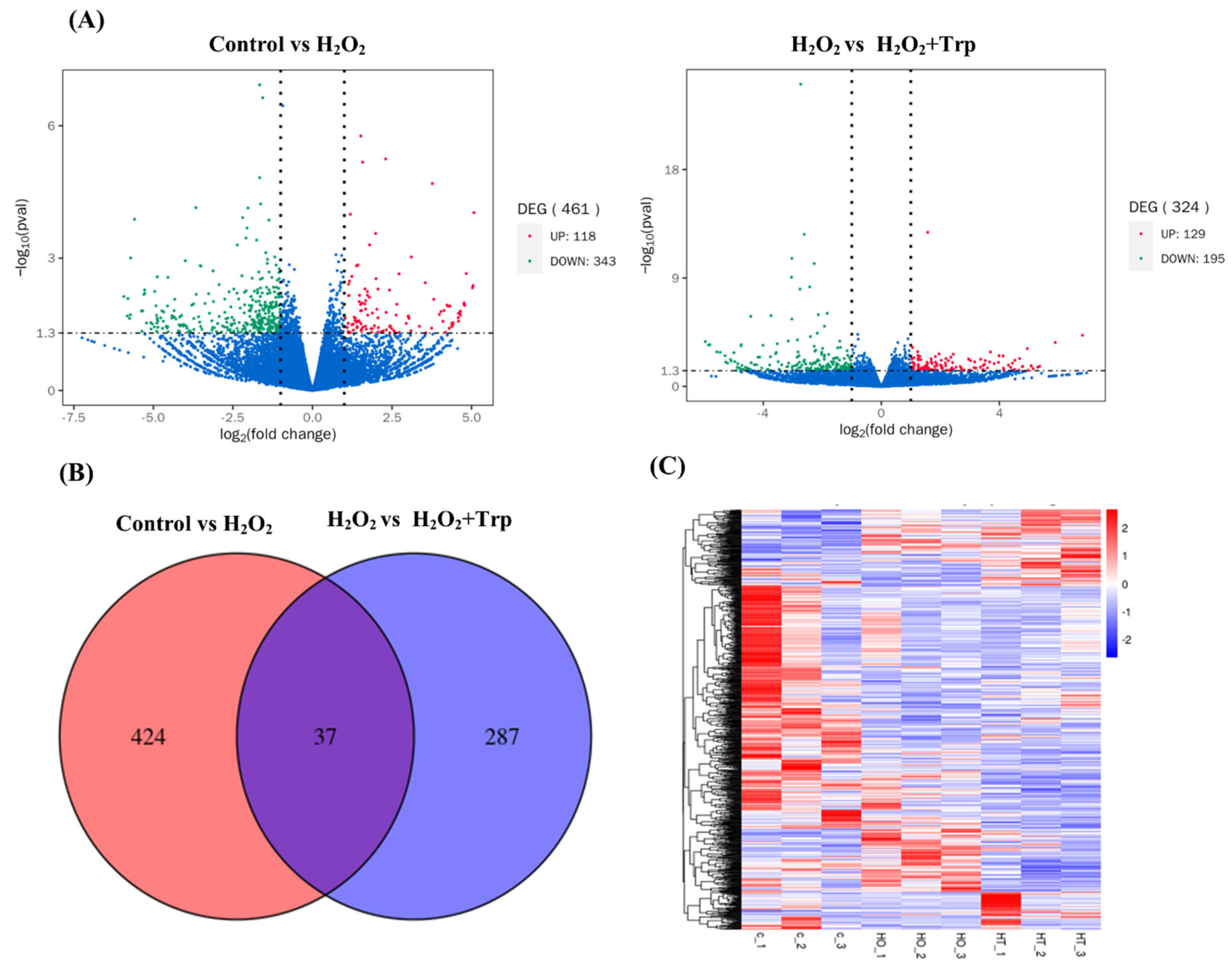
3.3. Differentially Expressed Genes (DEGs)
3.4. Comparison of GO Enrichment Analysis
3.5. KEGG Enrichment Analysis
3.6. Protein–Protein Interaction Network
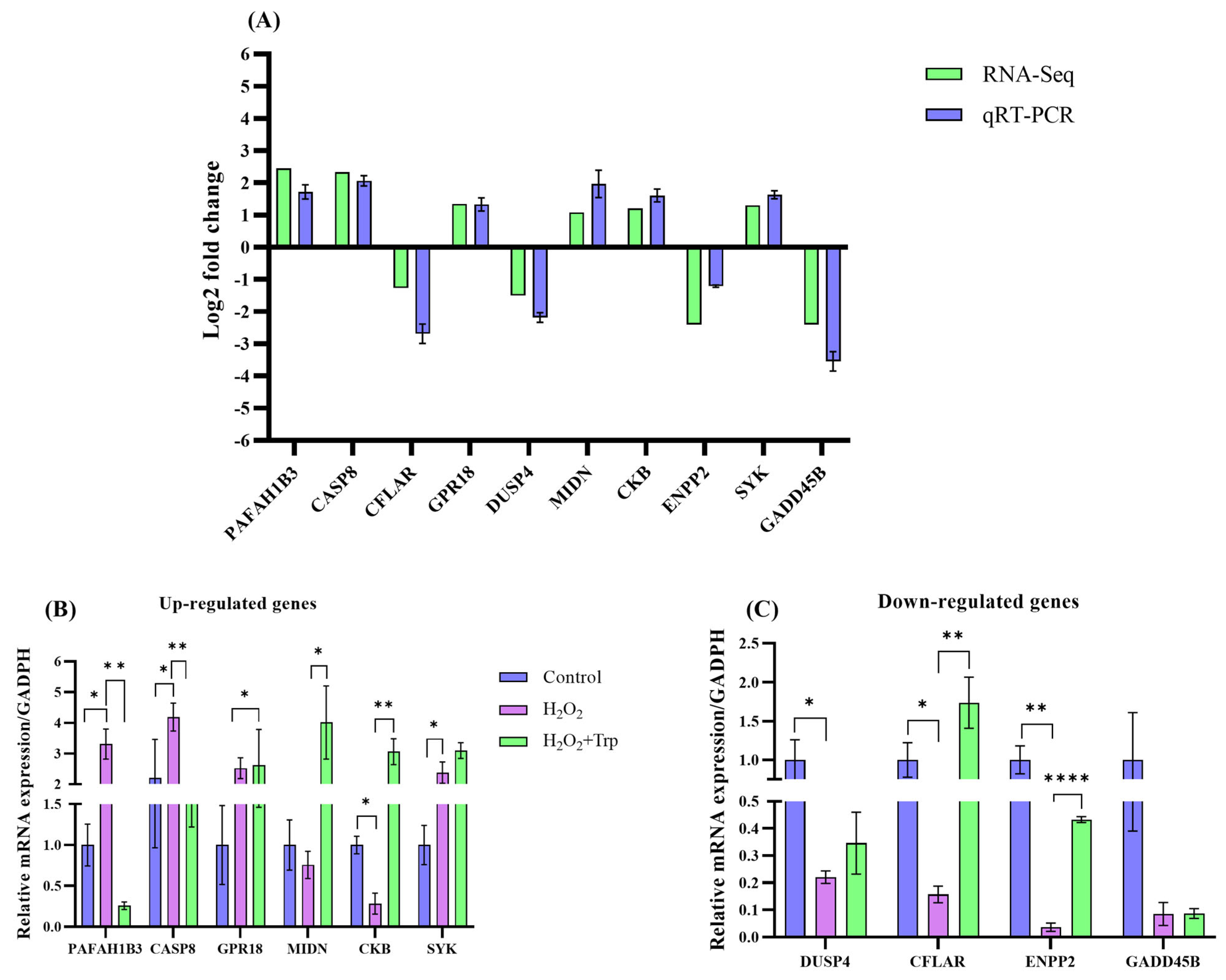
3.7. Confirmation of Sequencing Data Through qRT-PCR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, K.; Smith, G.W. Maternal Control of Early Embryogenesis in Mammals. Reprod. Fertil. Dev. 2015, 27, 880–896. [Google Scholar] [CrossRef] [PubMed]
- Harasimov, K.; Gorry, R.L.; Welp, L.M.; Penir, S.M.; Horokhovskyi, Y.; Cheng, S.; Takaoka, K.; Stützer, A.; Frombach, A.-S.; Taylor Tavares, A.L.; et al. The Maintenance of Oocytes in the Mammalian Ovary Involves Extreme Protein Longevity. Nat. Cell Biol. 2024, 26, 1124–1138. [Google Scholar] [CrossRef]
- Wang, L.; Tang, J.; Wang, L.; Tan, F.; Song, H.; Zhou, J.; Li, F. Oxidative Stress in Oocyte Aging and Female Reproduction. J. Cell. Physiol. 2021, 236, 7966–7983. [Google Scholar] [CrossRef]
- Sasaki, H.; Hamatani, T.; Kamijo, S.; Iwai, M.; Kobanawa, M.; Ogawa, S.; Miyado, K.; Tanaka, M. Impact of Oxidative Stress on Age-Associated Decline in Oocyte Developmental Competence. Front. Endocrinol. 2019, 10, 811. [Google Scholar] [CrossRef]
- Swain, J.E.; Pool, T.B. ART Failure: Oocyte Contributions to Unsuccessful Fertilization. Hum. Reprod. Update 2008, 14, 431–446. [Google Scholar] [CrossRef] [PubMed]
- Kashir, J.; Ganesh, D.; Jones, C.; Coward, K. Oocyte Activation Deficiency and Assisted Oocyte Activation: Mechanisms, Obstacles and Prospects for Clinical Application. Hum. Reprod. Open 2022, 2022, hoac003. [Google Scholar] [CrossRef]
- Rodríguez-Nuevo, A.; Torres-Sanchez, A.; Duran, J.M.; De Guirior, C.; Martínez-Zamora, M.A.; Böke, E. Oocytes Maintain ROS-Free Mitochondrial Metabolism by Suppressing Complex I. Nature 2022, 607, 756–761. [Google Scholar] [CrossRef] [PubMed]
- Ufer, C.; Wang, C.C. The Roles of Glutathione Peroxidases during Embryo Development. Front. Mol. Neurosci. 2011, 4, 12. [Google Scholar] [CrossRef]
- Mihalas, B.P.; Redgrove, K.A.; McLaughlin, E.A.; Nixon, B. Molecular Mechanisms Responsible for Increased Vulnerability of the Ageing Oocyte to Oxidative Damage. Oxid. Med. Cell. Longev. 2017, 2017, 4015874. [Google Scholar] [CrossRef]
- Silva, B.R.; Silva, J.R. V Mechanisms of Action of Non-Enzymatic Antioxidants to Control Oxidative Stress during in Vitro Follicle Growth, Oocyte Maturation, and Embryo Development. Anim. Reprod. Sci. 2023, 249, 107186. [Google Scholar] [CrossRef]
- Leem, J.; Lee, C.; Choi, D.Y.; Oh, J.S. Distinct Characteristics of the DNA Damage Response in Mammalian Oocytes. Exp. Mol. Med. 2024, 56, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Bahety, D.; Böke, E.; Rodríguez-Nuevo, A. Mitochondrial Morphology, Distribution and Activity during Oocyte Development. Trends Endocrinol. Metab. 2024, 35, 902–917. [Google Scholar] [CrossRef]
- Adhikari, D.; Lee, I.-W.; Yuen, W.S.; Carroll, J. Oocyte Mitochondria-Key Regulators of Oocyte Function and Potential Therapeutic Targets for Improving Fertility. Biol. Reprod. 2022, 106, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Tiwari, M.; Pandey, A.N.; Shrivastav, T.G.; Chaube, S.K. Impact of Stress on Oocyte Quality and Reproductive Outcome. J. Biomed. Sci. 2016, 23, 36. [Google Scholar] [CrossRef]
- van der Reest, J.; Nardini Cecchino, G.; Haigis, M.C.; Kordowitzki, P. Mitochondria: Their Relevance during Oocyte Ageing. Ageing Res. Rev. 2021, 70, 101378. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, S.; Pan, X. Research Progress on Mitochondrial Damage and Repairing in Oocytes: A Review. Mitochondrion 2024, 75, 101845. [Google Scholar] [CrossRef]
- Martin, J.H.; Nixon, B.; Cafe, S.L.; Aitken, R.J.; Bromfield, E.G.; Lord, T. OXIDATIVE STRESS AND REPRODUCTIVE FUNCTION: Oxidative Stress and In Vitro Ageing of the Post-Ovulatory Oocyte: An Update on Recent Advances in the Field. Reproduction 2022, 164, F109–F124. [Google Scholar] [CrossRef]
- Long, S.; Zheng, Y.; Deng, X.; Guo, J.; Xu, Z.; Scharffetter-Kochanek, K.; Dou, Y.; Jiang, M. Maintaining Mitochondrial DNA Copy Number Mitigates ROS-Induced Oocyte Decline and Female Reproductive Aging. Commun. Biol. 2024, 7, 1229. [Google Scholar] [CrossRef] [PubMed]
- Oehninger, S.; Acosta, A.A.; Veeck, L.L.; Simonetti, S.; Muasher, S.J. Delayed Fertilization during In Vitro Fertilization and Embryo Transfer Cycles: Analysis of Causes and Impact on Overall Results. Fertil. Steril. 1989, 52, 991–997. [Google Scholar] [CrossRef]
- He, M.; Zhang, T.; Yang, Y.; Wang, C. Mechanisms of Oocyte Maturation and Related Epigenetic Regulation. Front. Cell Dev. Biol. 2021, 9, 654028. [Google Scholar] [CrossRef]
- Di Nisio, V.; Antonouli, S.; Damdimopoulou, P.; Salumets, A.; Cecconi, S. In Vivo and In Vitro Postovulatory Aging: When Time Works against Oocyte Quality? J. Assist. Reprod. Genet. 2022, 39, 905–918. [Google Scholar] [CrossRef]
- Alexandru, I.; Nistor, D.; Motofelea, A.C.; Cadar, B.-A.; Crintea, A.; Tatu, C.; Pop, G.N.; Csep, A.N. Vitamins, Coenzyme Q10, and Antioxidant Strategies to Improve Oocyte Quality in Women with Gynecological Cancers: A Comprehensive Review. Antioxidants 2024, 13, 1567. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Varela, C.; Labarta, E. Clinical Application of Antioxidants to Improve Human Oocyte Mitochondrial Function: A Review. Antioxidants 2020, 9, 1197. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Dong, Z.; Liu, S.; Gao, F.; Zhang, J.; Peng, Z.; Wang, L.; Pan, X. Astaxanthin Improves the Development of the Follicles and Oocytes through Alleviating Oxidative Stress Induced by BPA in Cultured Follicles. Sci. Rep. 2022, 12, 7853. [Google Scholar] [CrossRef]
- Budani, M.C.; Tiboni, G.M. Effects of Supplementation with Natural Antioxidants on Oocytes and Preimplantation Embryos. Antioxidants 2020, 9, 612. [Google Scholar] [CrossRef]
- Yang, L.; Chen, Y.; Liu, Y.; Xing, Y.; Miao, C.; Zhao, Y.; Chang, X.; Zhang, Q. The Role of Oxidative Stress and Natural Antioxidants in Ovarian Aging. Front. Pharmacol. 2020, 11, 617843. [Google Scholar] [CrossRef]
- Lim, E.-S.; Lee, S.-E.; Park, M.-J.; Han, D.-H.; Lee, H.-B.; Ryu, B.; Kim, E.-Y.; Park, S.-P. Piperine Improves the Quality of Porcine Oocytes by Reducing Oxidative Stress. Free Radic. Biol. Med. 2024, 213, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Wang, Y.; Jiang, T.; Wen, K.; Cong, P.; Chen, Y.; He, Z. Quercetin Protects Porcine Oocytes from in Vitro Aging by Reducing Oxidative Stress and Maintaining the Mitochondrial Functions. Front. Cell Dev. Biol. 2022, 10, 915898. [Google Scholar] [CrossRef]
- Jia, B.-Y.; Xiang, D.-C.; Shao, Q.-Y.; Zhang, B.; Liu, S.-N.; Hong, Q.-H.; Quan, G.-B.; Wu, G.-Q. Inhibitory Effects of Astaxanthin on Postovulatory Porcine Oocyte Aging In Vitro. Sci. Rep. 2020, 10, 20217. [Google Scholar] [CrossRef]
- Paredes, S.D.; Barriga, C.; Reiter, R.J.; Rodríguez, A.B. Assessment of the Potential Role of Tryptophan as the Precursor of Serotonin and Melatonin for the Aged Sleep-Wake Cycle and Immune Function: Streptopelia Risoria as a Model. Int. J. Tryptophan Res. 2009, 2, 23–36. [Google Scholar] [CrossRef]
- Barik, S. The Uniqueness of Tryptophan in Biology: Properties, Metabolism, Interactions and Localization in Proteins. Int. J. Mol. Sci. 2020, 21, 8776. [Google Scholar] [CrossRef] [PubMed]
- Comai, S.; Bertazzo, A.; Brughera, M.; Crotti, S. Tryptophan in Health and Disease. Adv. Clin. Chem. 2020, 95, 165–218. [Google Scholar] [CrossRef]
- Friedman, M. Analysis, Nutrition, and Health Benefits of Tryptophan. Int. J. Tryptophan Res. 2018, 11, 1178646918802282. [Google Scholar] [CrossRef]
- Chibuye, B.; Singh, I.S.; Ramasamy, S.; Maseka, K.K. Natural Antioxidants: A Comprehensive Elucidation of Their Sources, Mechanisms, and Applications in Health. Next Res. 2024, 1, 100086. [Google Scholar] [CrossRef]
- Nimse, S.B.; Pal, D. Free Radicals, Natural Antioxidants, and Their Reaction Mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef]
- Xu, K.; Liu, G.; Fu, C. The Tryptophan Pathway Targeting Antioxidant Capacity in the Placenta. Oxid. Med. Cell. Longev. 2018, 2018, 1054797. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Yang, H.; Chen, Y.; Wang, H.; Wang, C.; Fan, J.; Chen, Y.; Li, Y.; Zhu, M. Formononetin Promotes Porcine Oocytes Maturation and Improves Embryonic Development by Reducing Oxidative Stress. Front. Cell Dev. Biol. 2025, 12, 1520429. [Google Scholar] [CrossRef]
- Khezri, M.R.; Jafari, R.; Yousefi, K.; Zolbanin, N.M. The PI3K/AKT Signaling Pathway in Cancer: Molecular Mechanisms and Possible Therapeutic Interventions. Exp. Mol. Pathol. 2022, 127, 104787. [Google Scholar] [CrossRef]
- Shcherbik, N.; Pestov, D.G. The Impact of Oxidative Stress on Ribosomes: From Injury to Regulation. Cells 2019, 8, 1379. [Google Scholar] [CrossRef]
- Fingleton, B. Matrix Metalloproteinases as Regulators of Inflammatory Processes. Biochim. Biophys. Acta-Mol. Cell Res. 2017, 1864, 2036–2042. [Google Scholar] [CrossRef]
- Pang, Y.; Jiang, X.; Zhao, S.; Huang, Z.; Zhu, H. Beneficial Role of Melatonin in Protecting Mammalian Gametes and Embryos from Oxidative Damage. J. Integr. Agric. 2018, 17, 2320–2335. [Google Scholar] [CrossRef]
- Sun, F.; Ali, N.N.; Londoño-Vásquez, D.; Simintiras, C.A.; Qiao, H.; Ortega, M.S.; Agca, Y.; Takahashi, M.; Rivera, R.M.; Kelleher, A.M.; et al. Increased DNA Damage in Full-Grown Oocytes Is Correlated with Diminished Autophagy Activation. Nat. Commun. 2024, 15, 9463. [Google Scholar] [CrossRef] [PubMed]
- Marei, W.F.A.; Leroy, J.L.M.R. Cellular Stress Responses in Oocytes: Molecular Changes and Clinical Implications. In Cell Biology and Translational Medicine, Volume 16: Stem Cells in Tissue Regeneration, Therapy and Drug Discovery; Turksen, K., Ed.; Springer International Publishing: Cham, Switzerland, 2022; pp. 171–189. ISBN 978-3-031-10638-5. [Google Scholar]
- Lin, J.; Wang, L. Oxidative Stress in Oocytes and Embryo Development: Implications for In Vitro Systems. Antioxid. Redox Signal. 2021, 34, 1394–1406. [Google Scholar] [CrossRef] [PubMed]
- Palego, L.; Betti, L.; Rossi, A.; Giannaccini, G. Tryptophan Biochemistry: Structural, Nutritional, Metabolic, and Medical Aspects in Humans. J. Amino Acids 2016, 2016, 8952520. [Google Scholar] [CrossRef]
- Khemaissa, S.; Sagan, S.; Walrant, A. Tryptophan, an Amino-Acid Endowed with Unique Properties and Its Many Roles in Membrane Proteins. Crystals 2021, 11, 1032. [Google Scholar] [CrossRef]
- Cosme, P.; Rodríguez, A.B.; Garrido, M.; Espino, J. Coping with Oxidative Stress in Reproductive Pathophysiology and Assisted Reproduction: Melatonin as an Emerging Therapeutical Tool. Antioxidants 2022, 12, 86. [Google Scholar] [CrossRef]
- Polyakova, V.; Medvedev, D.; Linkova, N.; Mushkin, M.; Muraviev, A.; Krasichkov, A.; Dyatlova, A.; Ivanova, Y.; Gullo, G.; Gorelova, A.A. Melatonin Receptors and Serotonin: Age-Related Changes in the Ovaries. J. Pers. Med. 2024, 14, 1009. [Google Scholar] [CrossRef]
- Azouzi, S.; Santuz, H.; Morandat, S.; Pereira, C.; Côté, F.; Hermine, O.; El Kirat, K.; Colin, Y.; Le Van Kim, C.; Etchebest, C.; et al. Antioxidant and Membrane Binding Properties of Serotonin Protect Lipids from Oxidation. Biophys. J. 2017, 112, 1863–1873. [Google Scholar] [CrossRef]
- Cruz, M.H.C.; Leal, C.L.V.; Cruz, J.F.; Tan, D.X.; Reiter, R.J. Essential Actions of Melatonin in Protecting the Ovary from Oxidative Damage. Theriogenology 2014, 82, 925–932. [Google Scholar] [CrossRef]
- Talpur, H.S.; Chandio, I.B.; Brohi, R.D.; Worku, T.; Rehman, Z.; Bhattarai, D.; Ullah, F.; JiaJia, L.; Yang, L. Research Progress on the Role of Melatonin and Its Receptors in Animal Reproduction: A Comprehensive Review. Reprod. Domest. Anim. 2018, 53, 831–849. [Google Scholar] [CrossRef]
- Liu, C.; Dernburg, A.F. Chemically Induced Proximity Reveals a Piezo-Dependent Meiotic Checkpoint at the Oocyte Nuclear Envelope. Science 2024, 386, eadm7969. [Google Scholar] [CrossRef]
- De Belly, H.; Paluch, E.K.; Chalut, K.J. Interplay between Mechanics and Signalling in Regulating Cell Fate. Nat. Rev. Mol. Cell Biol. 2022, 23, 465–480. [Google Scholar] [CrossRef]
- Zhong, X.; Rescorla, F.J. Cell Surface Adhesion Molecules and Adhesion-Initiated Signaling: Understanding of Anoikis Resistance Mechanisms and Therapeutic Opportunities. Cell. Signal. 2012, 24, 393–401. [Google Scholar] [CrossRef]
- Glaviano, A.; Foo, A.S.C.; Lam, H.Y.; Yap, K.C.H.; Jacot, W.; Jones, R.H.; Eng, H.; Nair, M.G.; Makvandi, P.; Geoerger, B.; et al. PI3K/AKT/MTOR Signaling Transduction Pathway and Targeted Therapies in Cancer. Mol. Cancer 2023, 22, 138. [Google Scholar] [CrossRef]
- Paul, R.; Luo, M.; Mo, X.; Lu, J.; Yeo, S.K.; Guan, J.-L. FAK Activates AKT-MTOR Signaling to Promote the Growth and Progression of MMTV-Wnt1-Driven Basal-like Mammary Tumors. Breast Cancer Res. 2020, 22, 59. [Google Scholar] [CrossRef]
- Dawson, T.R.; Weaver, A.M. Niche Tension Controls Exosome Production. Nat. Cell Biol. 2023, 25, 377–378. [Google Scholar] [CrossRef]
- Abbas, Z.; Tong, Y.; Zhang, J.; Sammad, A.; Wang, J.; Ahmad, B.; Wei, X.; Si, D.; Zhang, R. Transcriptomics and Microbiome Insights Reveal the Protective Mechanism of Mulberry-Derived Postbiotics against Inflammation in LPS-Induced Mice. Front. Immunol. 2025, 16, 1536694. [Google Scholar] [CrossRef]
- Deng, J.; Wei, R.-Q.; Zhang, W.-M.; Shi, C.-Y.; Yang, R.; Jin, M.; Piao, C. Crocin’s Role in Modulating MMP2/TIMP1 and Mitigating Hypoxia-Induced Pulmonary Hypertension in Mice. Sci. Rep. 2024, 14, 12716. [Google Scholar] [CrossRef]
- Arpino, V.; Brock, M.; Gill, S.E. The Role of TIMPs in Regulation of Extracellular Matrix Proteolysis. Matrix Biol. 2015, 44, 247–254. [Google Scholar] [CrossRef]
- Ries, C. Cytokine Functions of TIMP-1. Cell. Mol. Life Sci. 2014, 71, 659–672. [Google Scholar] [CrossRef]





| Sample | Raw Reads | Raw Bases | Clean Reads | Clean Bases | Error Rate | Q20 | Q30 | GC Content |
|---|---|---|---|---|---|---|---|---|
| Control | 82,062,904 | 12.3 G | 81,898,158 | 12.28 G | 0.03% | 96.96% | 93.15% | 51.04% |
| Control | 71,254,586 | 10.68 G | 71,111,626 | 10.67 G | 0.03% | 97.36% | 93.18% | 50.44% |
| Control | 63,043,680 | 9.45 G | 61,790,976 | 9.27 G | 0.03% | 94.72% | 88.21% | 47.34% |
| H2O2 | 91,947,906 | 13.79 G | 90,856,418 | 13.63 G | 0.03% | 95.40% | 89.33% | 48.77% |
| H2O2 | 1.13 × 108 | 17.02 G | 1.13 × 108 | 16.92 G | 0.03% | 96.00% | 90.36% | 48.86% |
| H2O2 | 1.02 × 108 | 15.27 G | 1.01 × 108 | 15.16 G | 0.03% | 95.75% | 89.98% | 48.70% |
| H2O2+Trp | 95,672,042 | 14.35 G | 94,854,498 | 14.23 G | 0.03% | 95.77% | 90.01% | 48.59% |
| H2O2+Trp | 1.28 × 108 | 19.2 G | 1.27 × 108 | 19.11 G | 0.03% | 96.64% | 91.38% | 49.43% |
| H2O2+Trp | 63,072,776 | 9.46 G | 61,947,186 | 9.29 G | 0.03% | 95.21% | 88.79% | 48.36% |
| Gene | Primer Sequence | Primer Length |
|---|---|---|
| GAPDH | F: 5′-GAACGGGAAGCTCACTGG-3′ R: 5′-GCCTGCTTCACCACCTTCT-3′ | 18, 18 |
| DUSP4 | F: 5′-TGCATCCCAGTGGAAGATAA-3′ R: 5′-GCAGTCCTTCACGGCATC-3′ | 20, 18 |
| PAFAH1B3 | F: 5′-CTGGGCTACACACCTGTTTGC-3′ R: 5′-GGAGAGTTTAATGTTGTGGGAAGG-3′ | 21, 24 |
| CASP-8 | F: 5′- GTTGTAGCAAGCCGAGATCA-3′ R: 5′-GTGGTCCATGAGTTGGTAGATT-3′ | 20, 21 |
| CFLAR | F: 5′-TGGAGAATGTGGTACGTTAG-3′ R: 5′-AGGAGTGGTGTGGTGGAAG-3′ | 20, 20 |
| GPR183 | F: 5’-ACCACCGCTTTGCCTACACGAA-3’ R: 5’-CACCACAGCAATGAAGCGGTCA-3’ | 22, 22 |
| MIDN | F: 5′-CCCCAACTGCCAGGATAGTA-3′ R: 5′-GGT AGTTTTGGGGGTGAGGT-3′ | 20, 20 |
| CKB | F: 5’-ATGCCTGCCCAGAAATGA-3’ R: 5’-GCACTGCCCAGGCAATAA-3’ | 18, 18 |
| ENFP2 | F: 5’-GCCCTGATGTCCGTGTATCT-3’ R: 5’-CGTTTGAAGGCAGGGTACAT-3’ | 20, 20 |
| GADD45B | F: 5′-TGACAACGACATCAACATC-3′ R: 5′-GTGACCAGAGACAATGCAG-3′ | 18, 18 |
| SYK | F: 5’ ACTCTGTGGCAGGTATTTCCG-3’ R: 5’ AATAAAGGAAGGCACAGGAGGG-3’ | 20, 22 |
| Top 10 Hub Genes in Control vs H2O2 Group Comparison | ||||
|---|---|---|---|---|
| Genes id | Genes Description | Genes Regulation | Log2FC | p-Value |
| RPLP1 | Ribosomal phosphoprotein1 | Down | −0.21973 | 0.35 |
| RPL34 | Ribosomal phosphoprotein34 | Down | −0.23755 | 0.33683 |
| RPL38 | Ribosomal phosphoprotein38 | Up | 0.026498 | 0.94114 |
| RPL28 | Ribosomal phosphoprotein28 | Down | −0.32671 | 0.28 |
| RPLP2 | Ribosomal phosphoprotein2 | Down | −1.4884 | 0.01 |
| RPL37A | Ribosomal phosphoprotein37A | Down | −1.1143 | 0.03 |
| RPL36A-HNRNPH2 | Ribosomal phosphoprotein36A- HNRNPH2 | down | −0.40596 | 0.21 |
| RPS16 | Ribosomal Protein S16 | Down | −1.0723 | 0.03 |
| RPS18 | Ribosomal ProteinS18 | Down | −1.208 | 0.02 |
| RPS29 | Ribosomal ProteinS29 | Down | −1.0404 | 0.02 |
| Top 10 hub genes in H2O2 vs H2O2+Trp group comparison | ||||
| TIMP1 | TIMP Metallopeptidase Inhibitor 1 | Down | −2.7308 | 8.45 × 10−26 |
| CCN2 | Cellular Communication Network Factor 2 | Down | −2.7744 | 0.009 |
| PLAT | Plasminogen activator | Down | −2.2715 | 6.45 × 10−11 |
| THBS1 | Thrombospondin 1 | Down | −1.1954 | 0.025 |
| SERPINE1 | Serine proteinase inhibitor 1 | Down | −2.6116 | 2.37 × 10−13 |
| PLAU | Urokinase plasminogen activator | Down | −1.6891 | 0.008 |
| MMP12 | Matrix Metallopeptidase 12 | Up | 5.3694 | 0.024 |
| COL9A2 | Type IX collagen | Down | −2.4929 | 0.031286 |
| SOX9 | SRY-Box Transcription Factor 9 | Down | −2.1862 | 0.008 |
| CCL5 | C-C Motif Chemokine Ligand 5 | Up | 1.9127 | 0.048 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Z.; Li, Y.; Fan, X.; Cai, S.; Li, S.; Wang, Y.; Wang, X.; Yang, F. A Transcriptomics Approach to Unveil the Antioxidant Effects of Tryptophan on Oocyte Quality Under Oxidative Stress in Pigs. Biomolecules 2025, 15, 949. https://doi.org/10.3390/biom15070949
Zhu Z, Li Y, Fan X, Cai S, Li S, Wang Y, Wang X, Yang F. A Transcriptomics Approach to Unveil the Antioxidant Effects of Tryptophan on Oocyte Quality Under Oxidative Stress in Pigs. Biomolecules. 2025; 15(7):949. https://doi.org/10.3390/biom15070949
Chicago/Turabian StyleZhu, Zhekun, Yanlong Li, Xinyin Fan, Shuang Cai, Siyu Li, Yutian Wang, Xinyu Wang, and Fengjuan Yang. 2025. "A Transcriptomics Approach to Unveil the Antioxidant Effects of Tryptophan on Oocyte Quality Under Oxidative Stress in Pigs" Biomolecules 15, no. 7: 949. https://doi.org/10.3390/biom15070949
APA StyleZhu, Z., Li, Y., Fan, X., Cai, S., Li, S., Wang, Y., Wang, X., & Yang, F. (2025). A Transcriptomics Approach to Unveil the Antioxidant Effects of Tryptophan on Oocyte Quality Under Oxidative Stress in Pigs. Biomolecules, 15(7), 949. https://doi.org/10.3390/biom15070949






