Adeno-Associated Virus Vectors in Retinal Gene Therapy: Challenges, Innovations, and Future Directions
Abstract
1. Introduction
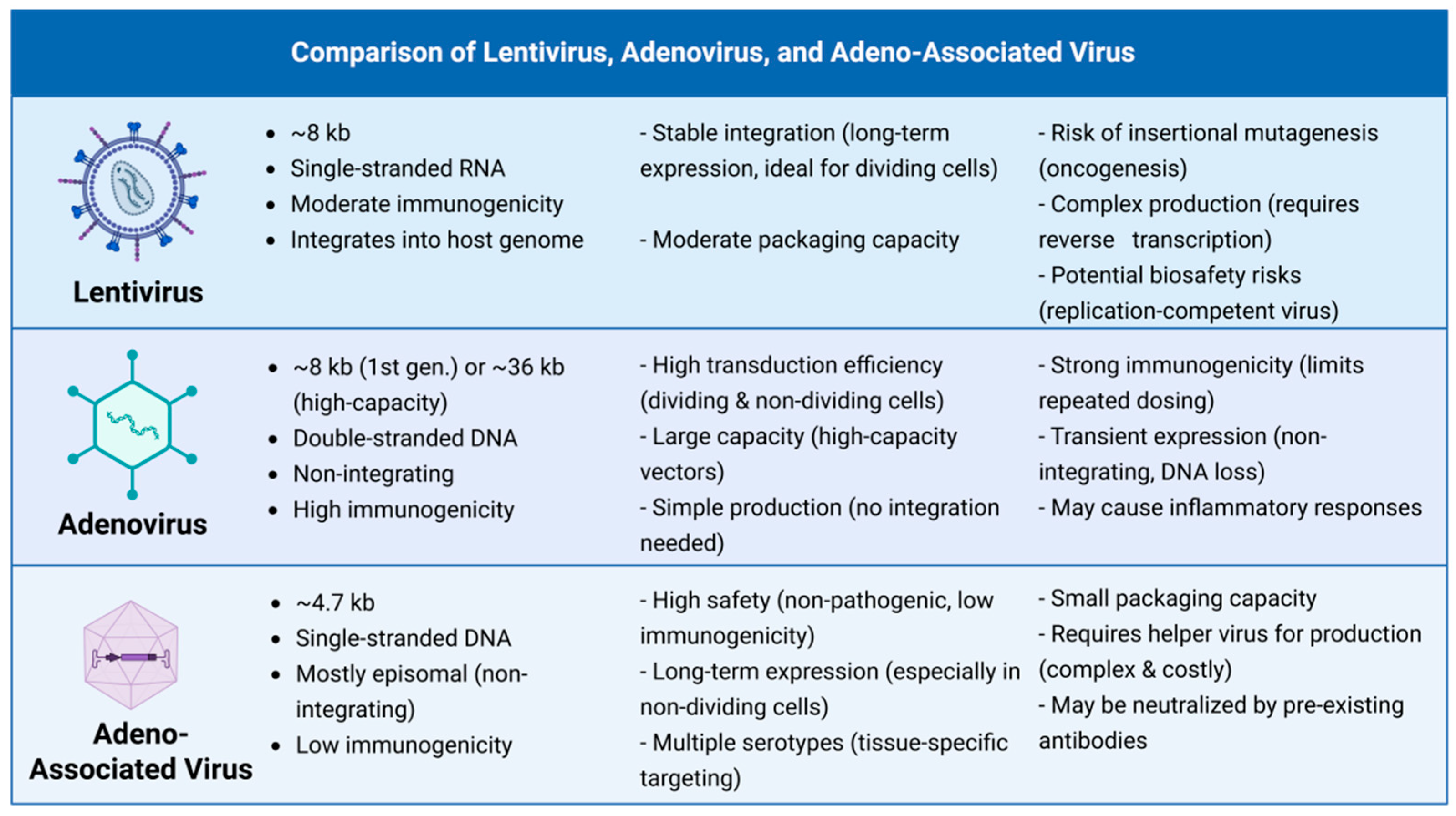
2. AAV Vector Fundamentals: A Concise Toolkit for Retinal Therapy
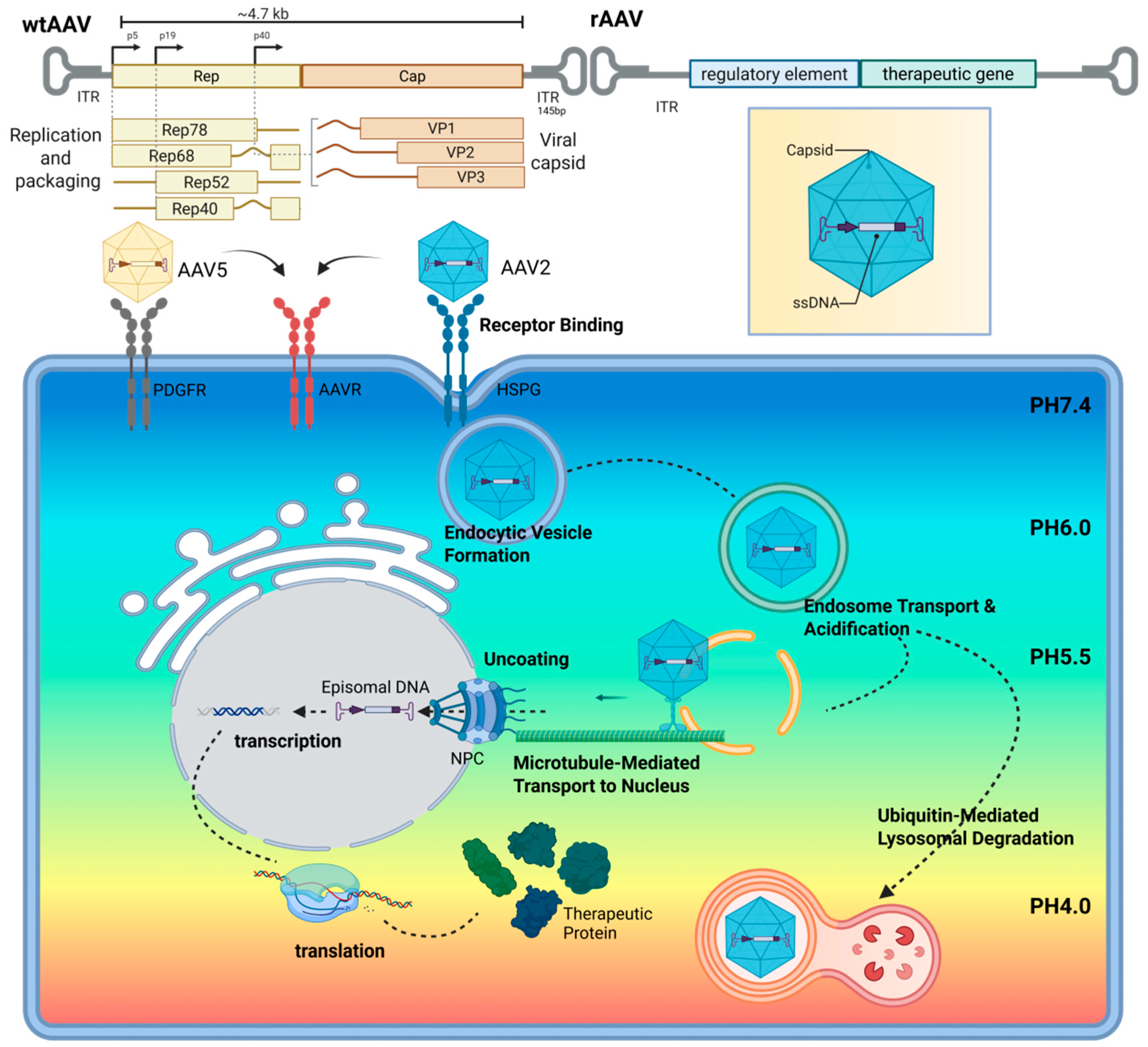
2.1. AAV Structural Basis
2.2. AAV Infection Process
3. The Delivery Dilemma: Reconciling Safety, Efficacy, and Disease-Specific Applicability
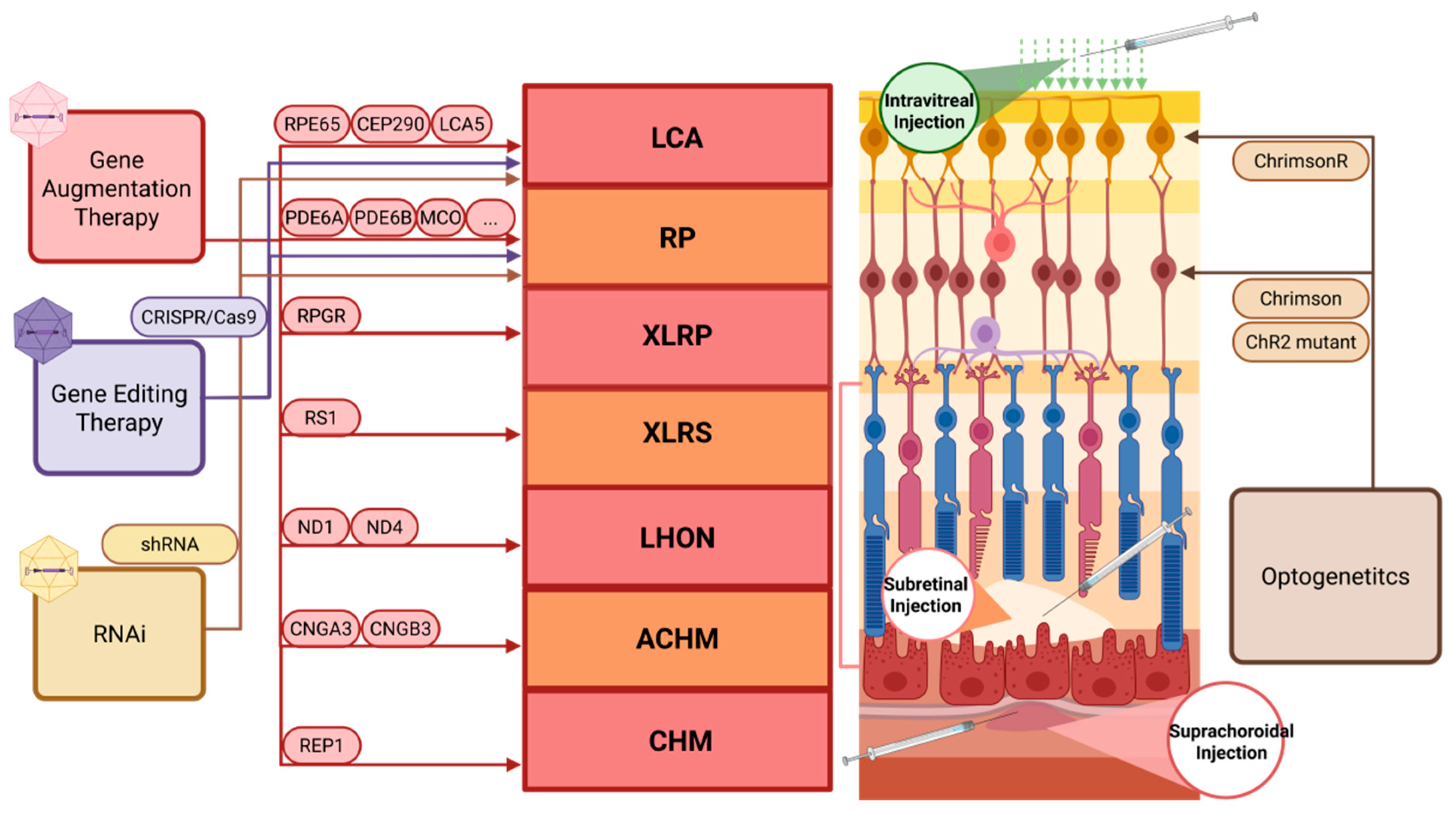
3.1. Subretinal Delivery: Precision at a Cost
3.2. Intravitreal Injection: Accessibility with Trade-Offs
3.3. Strategic Considerations: Disease Context Matters
4. Inherited Retinal Diseases: AAV-Based Emerging Therapeutic Strategies
4.1. AAV-Mediated Gene Augmentation Therapy
4.2. AAV-Mediated Gene Editing
4.3. AAV-Mediated Optogenetics
5. Large-Gene Delivery: Overcoming AAV Packaging Limits Through Advanced Vector Design
| Disease Name | Gene Name | cDNA Size (kb) | Affected Cell Types | Current Therapeutic Progress | Strategies to Overcome Size Limits |
|---|---|---|---|---|---|
| Stargardt | ABCA4 | ~6.8 | PRs | ABO-504: Validated in murine/porcine models; preclinical phase [84] VG-801: High efficiency at transcript/protein levels [85] OCU-410ST: Phase I/II trial [86,87] MCO-010: Phase II trial [82,88] SAR422459: Phase I/II trial [89] | 1. Dual AAV systems ABO-504: Intein-mediated dual-AAVVG-801: REVeRT technology 2. Non-gene replacement OCU-410ST: AAV5-hRORA (encodes RORA) MCO-010: Optogenetic therapy 3. Alternative vectors SAR422459: Lentiviral vector carrying ABCA4 |
| LCA10 | CEP290 | ~7.4 | PRs (connecting cilium) | EDIT-101: Phase I/II clinical trial [68] miniCEP290: demonstrated efficacy in mouse models [90] sepofarsen (QR-110): Phase II/III clinical trial [91,92,93] | 1. In situ gene editing (CRISPR/Cas9) EDIT-101: CRISPR-Cas9-based exon skipping/correction of the endogenous CEP290 gene in vivo 2. AON-mediated exon skipping sepofarsen (QR-110): uses antisense oligonucleotides to restore correct CEP290 pre-mRNA splicing 3. Micro-gene construction miniCEP290 |
| UsherI | MYO7A | ~6.7 | PRs and inner ear hair cells | AAVB-081: Phase I/II clinical trial [94] | 1. Dual AAV vector constructs AAVB-081: dual AAV8 delivering full-length MYO7A |
| PCDH15 | ~5.7 | Dual AAV system: demonstrated efficacy in mouse models [95] miniPCDH15: demonstrated efficacy in mouse models [96] | 1. Dual AAV system DNA-level trans-splicing 2. Micro-gene construction miniPCDH15 | ||
| Usher II | USH2A | ~15.7 | eSpCas9: corrected USH2A mutations in iPSCs [97] Ultevursen: Phase II/III clinical trial [98,99] pS/MAR-USH2A: preliminary exploration in zebrafish models [100] Minigene-4: demonstrated efficacy in mouse models [101] | 1. Gene editing eSpCas9: using CRISPR/eSpCas9-mediated genome editing to correct the two most common USH2A mutations in patient-derived iPSCs 2. AON-mediated exon skipping Ultevursen: antisense oligonucleotide prevents aberrant USH2A pre-mRNA splicing 3. Use of non-AAV vectors pS/MAR-USH2A: DNA plasmid |
5.1. DNA Level
5.2. Protein Level
5.3. mRNA Level
5.4. Capacity Expansion Methods Other than Multiple AAV
6. Acquired Retinal Diseases: Novel Therapeutic Strategies for Multifactorial Pathogenesis
6.1. Diabetic Retinopathy
6.2. Age-Related Macular Degeneration
7. The Accessibility Frontier: From Bench to Bedside
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BRB | Blood–Retinal Barrier |
| AAV | Adeno-Associated Virus |
| ssDNA | Single-Stranded DNA |
| wtAAV | Wild-Type Adeno-Associated Virus |
| AAP | Assembly Associated Protein |
| MAAP | Membrane-Associated Accessory Protein |
| rAAV | Recombinant Adeno-Associated Virus |
| ITRs | Inverted Terminal Repeats |
| HSPG | Heparan Sulfate Proteoglycan |
| AAVR | Adeno-Associated Virus Receptor |
| CLIC/GEEC | CLathrin-Independent Carrier/GPI-Enriched Endocytic Compartment pathway |
| SR Injection | Subretinal Injection |
| PRs | Photoreceptors |
| RPE | Retinal Pigment Epithelium |
| IRDs | Inherited Retinal Diseases |
| BSS | Balanced Salt Solution |
| MI-OCT | Multimodal Imaging-Optical Coherence Tomography |
| DR | Diabetic Retinopathy |
| DME | Diabetic Macular Edema |
| RGCs | Retinal Ganglion Cells |
| ILM | Internal Limiting Membrane |
| nAMD | Neovascular Age-Related Macular Degeneration |
| XLRP | X-Linked Retinitis Pigmentosa |
| GTAU | Gene Therapy-Associated Uveitis |
| TLR | Toll-Like Receptor |
| SCI | Suprachoroidal Injection |
| LCA | Leber Congenital Amaurosis |
| BCVA | Best Corrected Visual Acuity |
| RP | Retinitis Pigmentosa |
| DSBs | Double-Strand Breaks |
| HDR | Homology-Directed Repair |
| NHEJ | Non-Homologous End Joining |
| BE | Base Editing |
| PE | Prime Editing |
| NHPs | Non-Human Primates |
| LLVA | Low Luminance Visual Acuity |
| ecDHFR | Escherichia coli Dihydrofolate Reductase |
| NPDR | Non-Proliferative Diabetic Retinopathy |
| PDR | Proliferative Diabetic Retinopathy |
| DRSS | Diabetic Retinopathy Severity Scale |
| AMD | Age-Related Macular Degeneration |
| dAMD | Dry Age-Related Macular Degeneration |
| CNV | Choroidal Neovascularization |
| GA | Geographic Atrophy |
References
- Leclercq, B.; Mejlachowicz, D.; Behar-Cohen, F. Ocular Barriers and Their Influence on Gene Therapy Products Delivery. Pharmaceutics 2022, 14, 998. [Google Scholar] [CrossRef] [PubMed]
- Nisanov, A.M.; de Jesús, J.A.R.; Schaffer, D.V. Advances in AAV Capsid Engineering: Integrating Rational Design, Directed Evolution and Machine Learning. Mol. Ther. 2025, 33, 1937–1945. [Google Scholar] [CrossRef]
- Lopes, V.S.; Boye, S.E.; Louie, C.M.; Boye, S.; Dyka, F.; Chiodo, V.; Fofo, H.; Hauswirth, W.W.; Williams, D.S. Retinal Gene Therapy with a Large MYO7A cDNA Using Adeno-Associated Virus. Gene Ther. 2013, 20, 824–833. [Google Scholar] [CrossRef]
- Lopes, V.S.; Diemer, T.; Williams, D.S. Assessment of Different Virus-Mediated Approaches for Retinal Gene Therapy of Usher 1B. Adv. Exp. Med. Biol. 2014, 801, 725–731. [Google Scholar] [CrossRef]
- Cheng, S.-Y.; Punzo, C. Ocular Inflammation with Anti-Vascular Endothelial Growth Factor Treatments. Hum. Gene Ther. 2021, 32, 639–641. [Google Scholar] [CrossRef]
- Bulcha, J.T.; Wang, Y.; Ma, H.; Tai, P.W.L.; Gao, G. Viral Vector Platforms within the Gene Therapy Landscape. Signal Transduct. Target. Ther. 2021, 6, 53. [Google Scholar] [CrossRef]
- Geoffroy, M.-C.; Salvetti, A. Helper Functions Required for Wild Type and Recombinant Adeno-Associated Virus Growth. Curr. Gene Ther. 2005, 5, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Calcedo, R.; Vandenberghe, L.H.; Gao, G.; Lin, J.; Wilson, J.M. Worldwide Epidemiology of Neutralizing Antibodies to Adeno-Associated Viruses. J. Infect. Dis. 2009, 199, 381. [Google Scholar] [CrossRef]
- Hastie, E.; Samulski, R.J. Adeno-Associated Virus at 50: A Golden Anniversary of Discovery, Research, and Gene Therapy Success—A Personal Perspective. Hum. Gene Ther. 2015, 26, 257–265. [Google Scholar] [CrossRef]
- Matsuzaka, Y.; Yashiro, R. Therapeutic Application and Structural Features of Adeno-Associated Virus Vector. Curr. Issues Mol. Biol. 2024, 46, 8464–8498. [Google Scholar] [CrossRef]
- Kuz, C.A.; McFarlin, S.; Qiu, J. The Expression and Function of the Small Nonstructural Proteins of Adeno-Associated Viruses (AAVs). Viruses 2024, 16, 1215. [Google Scholar] [CrossRef] [PubMed]
- Galibert, L.; Hyvönen, A.; Eriksson, R.A.E.; Mattola, S.; Aho, V.; Salminen, S.; Albers, J.D.; Peltola, S.K.; Weman, S.; Nieminen, T.; et al. Functional Roles of the Membrane-Associated AAV Protein MAAP. Sci. Rep. 2021, 11, 21698. [Google Scholar] [CrossRef]
- Samulski, R.J.; Chang, L.S.; Shenk, T. A Recombinant Plasmid from Which an Infectious Adeno-Associated Virus Genome Can Be Excised In Vitro and Its Use to Study Viral Replication. J. Virol. 1987, 61, 3096. [Google Scholar] [CrossRef]
- Large, E.E.; Silveria, M.A.; Zane, G.M.; Weerakoon, O.; Chapman, M.S. Adeno-Associated Virus (AAV) Gene Delivery: Dissecting Molecular Interactions upon Cell Entry. Viruses 2021, 13, 1336. [Google Scholar] [CrossRef]
- Suarez-Amaran, L.; Song, L.; Tretiakova, A.P.; Mikhail, S.A.; Samulski, R.J. AAV Vector Development, Back to the Future. Mol. Ther. 2025, 33, 1903–1936. [Google Scholar] [CrossRef]
- Romanovsky, D.; Scherk, H.; Föhr, B.; Babutzka, S.; Bogedein, J.; Lu, Y.; Reschigna, A.; Michalakis, S. Heparan Sulfate Proteoglycan Affinity of Adeno-Associated Virus Vectors: Implications for Retinal Gene Delivery. Eur. J. Pharm. Sci. 2025, 206, 107012. [Google Scholar] [CrossRef]
- El-Sayed, A.; Harashima, H. Endocytosis of Gene Delivery Vectors: From Clathrin-Dependent to Lipid Raft-Mediated Endocytosis. Mol. Ther. 2013, 21, 1118–1130. [Google Scholar] [CrossRef]
- Goldmann, O.; Medina, E. Revisiting Pathogen Exploitation of Clathrin-Independent Endocytosis: Mechanisms and Implications. Cells 2025, 14, 731. [Google Scholar] [CrossRef]
- Nonnenmacher, M.; Weber, T. Adeno-Associated Virus 2 Infection Requires Endocytosis through the CLIC/GEEC Pathway. Cell Host Microbe 2011, 10, 563–576. [Google Scholar] [CrossRef]
- Stahnke, S.; Lux, K.; Uhrig, S.; Kreppel, F.; Hösel, M.; Coutelle, O.; Ogris, M.; Hallek, M.; Büning, H. Intrinsic Phospholipase A2 Activity of Adeno-Associated Virus Is Involved in Endosomal Escape of Incoming Particles. Virology 2011, 409, 77–83. [Google Scholar] [CrossRef]
- Gonzalez-Sandoval, A.; Pekrun, K.; Tsuji, S.; Zhang, F.; Hung, K.L.; Chang, H.Y.; Kay, M.A. The AAV Capsid Can Influence the Epigenetic Marking of rAAV Delivered Episomal Genomes in a Species Dependent Manner. Nat. Commun. 2023, 14, 2448. [Google Scholar] [CrossRef] [PubMed]
- Loeb, E.J.; Havlik, P.L.; Elmore, Z.C.; Rosales, A.; Fergione, S.M.; Gonzalez, T.J.; Smith, T.J.; Benkert, A.R.; Fiflis, D.N.; Asokan, A. Capsid-Mediated Control of Adeno-Associated Viral Transcription Determines Host Range. Cell Rep. 2024, 43, 113902. [Google Scholar] [CrossRef]
- Xia, X.; Guo, X. Adeno-Associated Virus Vectors for Retinal Gene Therapy in Basic Research and Clinical Studies. Front. Med. 2023, 10, 1310050. [Google Scholar] [CrossRef] [PubMed]
- Wiley, L.A.; Boyce, T.M.; Meyering, E.E.; Ochoa, D.; Sheehan, K.M.; Stone, E.M.; Mullins, R.F.; Tucker, B.A.; Han, I.C. The Degree of Adeno-Associated Virus-Induced Retinal Inflammation Varies Based on Serotype and Route of Delivery: Intravitreal, Subretinal, or Suprachoroidal. Hum. Gene Ther. 2023, 34, 530. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.L.; Gregori, N.Z.; MacLaren, R.E.; Lam, B.L. Surgical Technique for Subretinal Gene Therapy in Humans with Inherited Retinal Degeneration. Retina 2019, 39 (Suppl. S1), S2–S8. [Google Scholar] [CrossRef]
- Tripepi, D.; Jalil, A.; Ally, N.; Buzzi, M.; Moussa, G.; Rothschild, P.-R.; Rossi, T.; Ferrara, M.; Romano, M.R. The Role of Subretinal Injection in Ophthalmic Surgery: Therapeutic Agent Delivery and Other Indications. Int. J. Mol. Sci. 2023, 24, 10535. [Google Scholar] [CrossRef]
- Simunovic, M.P.; Prime, Z.J.; Chow, R.C.; Shao, E.H.; Madanat, Z.; Osaadon, P.; Yeo, T.H.; Oo, K.T.; Too, L.K. The 1-Step Versus 2-Step Subretinal Injection Trial (1,2-SIT)-A Randomized Controlled Trial to Compare Drug Reflux Following Subretinal Injection. Am. J. Ophthalmol. 2025, 274, 149–162. [Google Scholar] [CrossRef]
- L’Abbate, D.; Prescott, K.; Geraghty, B.; Kearns, V.R.; Steel, D.H.W. Biomechanical Considerations for Optimising Subretinal Injections. Surv. Ophthalmol. 2024, 69, 722–732. [Google Scholar] [CrossRef]
- Aglyamov, S.R.; Larin, K.V. Optical Coherence Tomography for Noninvasive Monitoring of Drug Delivery. Adv. Drug Deliv. Rev. 2025, 220, 115571. [Google Scholar] [CrossRef]
- Vasconcelos, H.M.; Lujan, B.J.; Pennesi, M.E.; Yang, P.; Lauer, A.K. Intraoperative Optical Coherence Tomographic Findings in Patients Undergoing Subretinal Gene Therapy Surgery. Int. J. Retin. Vitr. 2020, 6, 13. [Google Scholar] [CrossRef]
- Gray, A.P.; Sato, Y.; Meyer, T.; Stoner, K.; Beltran, W.A. Surgical Procedure and Applicability of the Orbit Subretinal Delivery System (SDS)TM in the Normal Adult Canine Eye. Investig. Ophthalmol. Vis. Sci. 2022, 63, 4118-F0355. [Google Scholar]
- Mi, H.; MacLaren, R.E.; Cehajic-Kapetanovic, J. Robotising Vitreoretinal Surgeries. Eye 2025, 39, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.L.; Edwards, T.L.; O’Hare, F.; Hickey, D.G.; Wang, J.-H.; Liu, Z.; Ayton, L.N. Gene Therapy for Inherited Retinal Diseases: Progress and Possibilities. Clin. Exp. Optom. 2021, 104, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Frederick, A.; Sullivan, J.; Liu, L.; Adamowicz, M.; Lukason, M.; Raymer, J.; Luo, Z.; Jin, X.; Rao, K.N.; O’Riordan, C. Engineered Capsids for Efficient Gene Delivery to the Retina and Cornea. Hum. Gene Ther. 2020, 31, 756–774. [Google Scholar] [CrossRef]
- Boye, S.L.; Choudhury, S.; Crosson, S.; Di Pasquale, G.; Afione, S.; Mellen, R.; Makal, V.; Calabro, K.R.; Fajardo, D.; Peterson, J.; et al. Novel AAV44.9-Based Vectors Display Exceptional Characteristics for Retinal Gene Therapy. Mol. Ther. 2020, 28, 1464–1478. [Google Scholar] [CrossRef]
- Mével, M.; Pichard, V.; Bouzelha, M.; Alvarez-Dorta, D.; Lalys, P.-A.; Provost, N.; Allais, M.; Mendes, A.; Landagaray, E.; Ducloyer, J.-B.; et al. Mannose-Coupled AAV2: A Second-Generation AAV Vector for Increased Retinal Gene Therapy Efficiency. Mol. Ther. Methods Clin. Dev. 2024, 32, 101187. [Google Scholar] [CrossRef]
- Ross, M.; Ofri, R. The Future of Retinal Gene Therapy: Evolving from Subretinal to Intravitreal Vector Delivery. Neural Regen. Res. 2021, 16, 1751–1759. [Google Scholar] [CrossRef]
- Bennett, A.; Keravala, A.; Makal, V.; Kurian, J.; Belbellaa, B.; Aeran, R.; Tseng, Y.-S.; Sousa, D.; Spear, J.; Gasmi, M.; et al. Structure Comparison of the Chimeric AAV2.7m8 Vector with Parental AAV2. J. Struct. Biol. 2020, 209, 107433. [Google Scholar] [CrossRef]
- Khabou, H.; Desrosiers, M.; Winckler, C.; Fouquet, S.; Auregan, G.; Bemelmans, A.-P.; Sahel, J.-A.; Dalkara, D. Insight into the Mechanisms of Enhanced Retinal Transduction by the Engineered AAV2 Capsid Variant -7m8. Biotechnol. Bioeng. 2016, 113, 2712–2724. [Google Scholar] [CrossRef]
- 4D Molecular Therapeutics. 4D Molecular Therapeutics Presents Interim Results from the Ongoing 4D-125 Phase 1/2 Clinical Trial in Patients with Advanced X-Linked Retinitis Pigmentosa at the ASRS Annual Meeting. Available online: https://www.globenewswire.com/news-release/2021/10/10/2311484/0/en/4D-Molecular-Therapeutics-Presents-Interim-Results-from-the-Ongoing-4D-125-Phase-1-2-Clinical-Trial-in-Patients-with-Advanced-X-linked-Retinitis-Pigmentosa-at-the-ASRS-Annual-Meeti.html (accessed on 4 June 2025).
- 4D Molecular Therapeutics. 4DMT Presents Positive 52-Week Results from Phase 2b Cohort in PRISM Wet AMD and Long-Term Durability Data Supporting the 4D-150 4FRONT Global Registrational Program. Available online: https://ir.4dmoleculartherapeutics.com/news-releases/news-release-details/4dmt-presents-positive-52-week-results-phase-2b-cohort-prism-wet/ (accessed on 4 June 2025).
- 4D Molecular Therapeutics. 4DMT Announces Positive Interim Data from 4D-150 SPECTRA Clinical Trial in DME and Alignment with FDA on Registration Path. Available online: https://ir.4dmoleculartherapeutics.com/news-releases/news-release-details/4dmt-announces-positive-interim-data-4d-150-spectra-clinical/ (accessed on 4 June 2025).
- Zeng, Y.; Qian, H.; Wu, Z.; Marangoni, D.; Sieving, P.A.; Bush, R.A. AAVrh-10 Transduces Outer Retinal Cells in Rodents and Rabbits Following Intravitreal Administration. Gene Ther. 2019, 26, 386–398. [Google Scholar] [CrossRef]
- Buckley, T.; MacLaren, R.E.; Cehajic Kapetanovic, J. Meta-Analysis of Gene Therapy Associated Uveitis (GTAU). Investig. Ophthalmol. Vis. Sci. 2024, 65, 5312. [Google Scholar]
- Purdy, R.; John, M.; Bray, A.; Clare, A.J.; Copland, D.A.; Chan, Y.K.; Henderson, R.H.; Nerinckx, F.; Leroy, B.P.; Yang, P.; et al. Gene Therapy-Associated Uveitis (GTAU): Understanding and Mitigating the Adverse Immune Response in Retinal Gene Therapy. Prog. Retin. Eye Res. 2025, 106, 101354. [Google Scholar] [CrossRef] [PubMed]
- Barnes, C.; Scheideler, O.; Schaffer, D. Engineering the AAV Capsid to Evade Immune Responses. Curr. Opin. Biotechnol. 2019, 60, 99–103. [Google Scholar] [CrossRef]
- Bentler, M.; Hardet, R.; Ertelt, M.; Rudolf, D.; Kaniowska, D.; Schneider, A.; Vondran, F.W.; Schoeder, C.T.; Delphin, M.; Lucifora, J.; et al. Modifying Immune Responses to Adeno-Associated Virus Vectors by Capsid Engineering. Mol. Ther. Methods Clin. Dev. 2023, 30, 576–592. [Google Scholar] [CrossRef]
- Chan, Y.K.; Wang, S.K.; Chu, C.J.; Copland, D.A.; Letizia, A.J.; Verdera, H.C.; Chiang, J.J.; Sethi, M.; Wang, M.K.; Neidermyer, W.J., Jr.; et al. Engineering adeno-associated viral vectors to evade innate immune and inflammatory responses. Sci. Transl. Med. 2021, 13, eabd3438. [Google Scholar] [CrossRef]
- Bertolini, T.B.; Shirley, J.L.; Zolotukhin, I.; Li, X.; Kaisho, T.; Xiao, W.; Kumar, S.R.P.; Herzog, R.W. Effect of CpG Depletion of Vector Genome on CD8+ T Cell Responses in AAV Gene Therapy. Front. Immunol. 2021, 12, 672449. [Google Scholar] [CrossRef]
- Chung, S.H.; Mollhoff, I.N.; Mishra, A.; Sin, T.-N.; Ngo, T.; Ciulla, T.; Sieving, P.; Thomasy, S.M.; Yiu, G. Host Immune Responses after Suprachoroidal Delivery of AAV8 in Nonhuman Primate Eyes. Hum. Gene Ther. 2021, 32, 682–693. [Google Scholar] [CrossRef]
- AbbVie. A Phase 2, Randomized, Dose-Escalation, Ranibizumab-Controlled Study to Evaluate the Efficacy, Safety, and Tolerability of RGX-314 Gene Therapy Delivered Via One or Two Suprachoroidal Space (SCS) Injections in Participants with Neovascular Age-Related Macular De-generation (nAMD) (AAVIATE); AbbVie: North Chicago, IL, USA, 2024. Available online: https://clinicaltrials.gov/study/NCT04514653 (accessed on 4 June 2025).
- AbbVie. A Phase 2, Open-Label Study to Explore the Pharmacodynamics of Two Doses in Two Formulations of RGX-314 Gene Therapy Administered Via Subretinal Delivery in Participants with Neovascular Age-Related Macular Degeneration; AbbVie: North Chicago, IL, USA, 2024. Available online: https://clinicaltrials.gov/study/NCT04832724 (accessed on 4 June 2025).
- German Society of Ophthalmology (Deutsche Ophthalmologische Gesellschaft, DOG); German Retina Society e. V. (Retinolo-gische Gesellschaft e. V., RG); Professional Association of German Ophthalmologists (Berufsverband der Augenärzte Deutsch-lands e. V., BVA). Statement of the DOG, the RG, and the BVA on the Therapeutic Use of Voretigene Neparvovec (LuxturnaTM) in Ophthalmology. English Version: January 2019. Ophthalmologe 2020, 117, 16–24. [Google Scholar] [CrossRef]
- Gemayel, M.C.; Bhatwadekar, A.D.; Ciulla, T. RNA Therapeutics for Retinal Diseases. Expert Opin. Biol. Ther. 2020, 21, 603. [Google Scholar] [CrossRef]
- Ameri, H.; Kesavamoorthy, N.; Bruce, D.N. Frequency and Pattern of Gene Therapy Clinical Trials for Inherited Retinal Diseases. Adv. Exp. Med. Biol. 2025, 1468, 89–93. [Google Scholar] [CrossRef]
- Zhai, Y.; Xu, M.; Radziwon, A.; Dimopoulos, I.S.; Crichton, P.; Mah, R.; MacLaren, R.E.; Somani, R.; Tennant, M.T.; MacDonald, I.M. AAV2-Mediated Gene Therapy for Choroideremia: 5-Year Results and Alternate Anti-Sense Oligonucleotide Therapy. Am. J. Ophthalmol. 2023, 248, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Igoe, J.M.; Lam, B.L.; Gregori, N.Z. Update on Clinical Trial Endpoints in Gene Therapy Trials for Inherited Retinal Diseases. J. Clin. Med. 2024, 13, 5512. [Google Scholar] [CrossRef] [PubMed]
- Tuohy, G.P.; Megaw, R. A Systematic Review and Meta-Analyses of Interventional Clinical Trial Studies for Gene Therapies for the Inherited Retinal Degenerations (IRDs). Biomolecules 2021, 11, 760. [Google Scholar] [CrossRef]
- Confalonieri, F.; La Rosa, A.; Ottonelli, G.; Barone, G.; Ferraro, V.; Di Maria, A.; Romano, M.; Randazzo, A.; Vallejo-Garcia, J.L.; Vinciguerra, P.; et al. Retinitis Pigmentosa and Therapeutic Approaches: A Systematic Review. J. Clin. Med. 2024, 13, 4680. [Google Scholar] [CrossRef]
- Segurado, O.G.; Jiang, R.; Pipe, S.W. Challenges and Opportunities When Transitioning from In Vivo Gene Replacement to In Vivo CRISPR/Cas9 Therapies—A Spotlight on Hemophilia. Expert Opin. Biol. Ther. 2022, 22, 1091–1098. [Google Scholar] [CrossRef]
- Hustedt, N.; Durocher, D. The Control of DNA Repair by the Cell Cycle. Nat. Cell Biol. 2016, 19, 1–9. [Google Scholar] [CrossRef]
- Bohrer, L.R.; Wiley, L.A.; Burnight, E.R.; Cooke, J.A.; Giacalone, J.C.; Anfinson, K.R.; Andorf, J.L.; Mullins, R.F.; Stone, E.M.; Tucker, B.A. Correction of NR2E3 Associated Enhanced S-Cone Syndrome Patient-Specific iPSCs Using CRISPR-Cas9. Genes 2019, 10, 278. [Google Scholar] [CrossRef]
- Overlack, N.; Goldmann, T.; Wolfrum, U.; Nagel-Wolfrum, K. Gene Repair of an Usher Syndrome Causing Mutation by Zinc-Finger Nuclease Mediated Homologous Recombination. Investig. Ophthalmol. Vis. Sci. 2012, 53, 4140–4146. [Google Scholar] [CrossRef]
- Cai, Y.; Cheng, T.; Yao, Y.; Li, X.; Ma, Y.; Li, L.; Zhao, H.; Bao, J.; Zhang, M.; Qiu, Z.; et al. In Vivo Genome Editing Rescues Photoreceptor Degeneration via a Cas9/RecA-Mediated Homology-Directed Repair Pathway. Sci. Adv. 2019, 5, eaav3335. [Google Scholar] [CrossRef]
- Cox, D.B.T.; Platt, R.J.; Zhang, F. Therapeutic Genome Editing: Prospects and Challenges. Nat. Med. 2015, 21, 121. [Google Scholar] [CrossRef]
- Suzuki, K.; Tsunekawa, Y.; Hernandez-Benitez, R.; Wu, J.; Zhu, J.; Kim, E.J.; Hatanaka, F.; Yamamoto, M.; Araoka, T.; Li, Z.; et al. In Vivo Genome Editing via CRISPR/Cas9 Mediated Homology-Independent Targeted Integration. Nature 2016, 540, 144. [Google Scholar] [CrossRef] [PubMed]
- Maeder, M.L.; Stefanidakis, M.; Wilson, C.J.; Baral, R.; Barrera, L.A.; Bounoutas, G.S.; Bumcrot, D.; Chao, H.; Ciulla, D.M.; DaSilva, J.A.; et al. Development of a Gene-Editing Approach to Restore Vision Loss in Leber Congenital Amaurosis Type 10. Nat. Med. 2019, 25, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Editas Medicine, Inc. Open-Label, Single Ascending Dose Study to Evaluate the Safety, Tolerability, and Efficacy of EDIT-101 in Adult and Pediatric Participants with Leber Congenital Amaurosis Type 10 (LCA10), with Centrosomal Protein 290 (CEP290)-Related Retinal Degeneration Caused by a Compound Heterozygous or Homozygous Mutation Involving c.2991+1655A>G in Intron 26 (IVS26) of the CEP290 Gene ("LCA10-IVS26”); Editas Medicine, Inc.: Cambridge, MA, USA, 2022. Available online: https://clinicaltrials.gov (accessed on 4 June 2025).
- Choi, E.H.; Suh, S.; Foik, A.T.; Leinonen, H.; Newby, G.A.; Gao, X.D.; Banskota, S.; Hoang, T.; Du, S.W.; Dong, Z.; et al. In Vivo Base Editing Rescues Cone Photoreceptors in a Mouse Model of Early-Onset Inherited Retinal Degeneration. Nat. Commun. 2022, 13, 1830. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.; Jo, D.H.; Cho, C.S.; Shin, J.H.; Seo, J.H.; Yu, G.; Gopalappa, R.; Kim, D.; Cho, S.-R.; Kim, J.H.; et al. Application of Prime Editing to the Correction of Mutations and Phenotypes in Adult Mice with Liver and Eye Diseases. Nat. Biomed. Eng. 2022, 6, 181–194. [Google Scholar] [CrossRef]
- Anzalone, A.V.; Koblan, L.W.; Liu, D.R. Genome Editing with CRISPR-Cas Nucleases, Base Editors, Transposases and Prime Editors. Nat. Biotechnol. 2020, 38, 824–844. [Google Scholar] [CrossRef]
- Kurt, I.C.; Zhou, R.; Iyer, S.; Garcia, S.P.; Miller, B.R.; Langner, L.M.; Grünewald, J.; Joung, J.K. CRISPR C-to-G Base Editors for Inducing Targeted DNA Transversions in Human Cells. Nat. Biotechnol. 2020, 39, 41. [Google Scholar] [CrossRef]
- Zhao, D.; Li, J.; Li, S.; Xin, X.; Hu, M.; Price, M.A.; Rosser, S.J.; Bi, C.; Zhang, X. Glycosylase Base Editors Enable C-to-A and C-to-G Base Changes. Nat. Biotechnol. 2021, 39, 35–40. [Google Scholar] [CrossRef]
- Suh, S.; Choi, E.H.; Leinonen, H.; Foik, A.T.; Newby, G.A.; Yeh, W.-H.; Dong, Z.; Kiser, P.D.; Lyon, D.C.; Liu, D.R.; et al. Restoration of Visual Function in Adult Mice with an Inherited Retinal Disease via Adenine Base Editing. Nat. Biomed. Eng. 2020, 5, 169–178. [Google Scholar] [CrossRef]
- Hansen, S.; McClements, M.E.; Corydon, T.J.; MacLaren, R.E. Future Perspectives of Prime Editing for the Treatment of Inherited Retinal Diseases. Cells 2023, 12, 440. [Google Scholar] [CrossRef]
- Simunovic, M.P.; Shen, W.; Lin, J.Y.; Protti, D.A.; Lisowski, L.; Gillies, M.C. Optogenetic Approaches to Vision Restoration. Exp. Eye Res. 2019, 178, 15–26. [Google Scholar] [CrossRef]
- GenSight Biologics. A Phase 1/2a, Open-Label, Non-Randomized, Dose-Escalation Study to Evaluate the Safety and Tolerability of GS030 in Subjects with Retinitis Pigmentosa; GenSight Biologics: Paris, France, 2022. Available online: https://clinicaltrials.gov/study/NCT03326336 (accessed on 4 June 2025).
- Sahel, J.-A.; Boulanger-Scemama, E.; Pagot, C.; Arleo, A.; Galluppi, F.; Martel, J.N.; Esposti, S.D.; Delaux, A.; de Saint Aubert, J.-B.; de Montleau, C.; et al. Partial Recovery of Visual Function in a Blind Patient after Optogenetic Therapy. Nat. Med. 2021, 27, 1223–1229. [Google Scholar] [CrossRef] [PubMed]
- Lindner, M.; Gilhooley, M.J.; Hughes, S.; Hankins, M.W. Optogenetics for Visual Restoration: From Proof of Principle to Translational Challenges. Prog. Retin. Eye Res. 2022, 91, 101089. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, K.P.; Pham, A.; Werblin, F.S. Differential Targeting of Optical Neuromodulators to Ganglion Cell Soma and Dendrites Allows Dynamic Control of Center-Surround Antagonism. Neuron 2011, 69, 713–720. [Google Scholar] [CrossRef]
- Rodgers, J.; Hughes, S.; Ebrahimi, A.S.; Allen, A.E.; Storchi, R.; Lindner, M.; Peirson, S.N.; Badea, T.C.; Hankins, M.W.; Lucas, R.J. Enhanced Restoration of Visual Code after Targeting ON Bipolar Cells Compared with Retinal Ganglion Cells with Optogenetic Therapy. Mol. Ther. 2025, 33, 1264–1281. [Google Scholar] [CrossRef]
- Nanoscope Therapeutics Inc. A Phase 2a, Open Label Multicenter Clinical Trial to Evaluate the Safety and Effects of a Single Intravitreal Injection of vMCO-010 Optogenetic Therapy in Subjects with Stargardt Disease; Nanoscope Therapeutics Inc.: Dallas, TX, USA, 2023. Available online: https://clinicaltrials.gov/study/NCT05417126 (accessed on 4 June 2025).
- Tornabene, P.; Trapani, I. Can Adeno-Associated Viral Vectors Deliver Effectively Large Genes? Hum. Gene Ther. 2020, 31, 47–56. [Google Scholar] [CrossRef]
- Abeona Therapeutics Announces Update on AAV Ophthalmology Program. Available online: https://investors.abeonatherapeutics.com/press-releases/detail/246/abeona-therapeutics-announces-update-on-aav-ophthalmology (accessed on 4 June 2025).
- ViGeneron. ViGeneron Announces FDA Clearance of IND for Novel mRNA Trans-Splicing Gene Therapy VG801 to Treat Stargardt Disease and Other ABCA4-Linked Retinal Dystrophies. Available online: https://veongen.com/press/vigeneron-announces-fda-clearance-of-ind-for-novel-mrna-trans-splicing-gene-therapy-vg801-to-treat-stargardt-disease-and-other-abca4-linked-retinal-dystrophies/ (accessed on 4 June 2025).
- Ocugen. A Phase 1/2 Study to Assess the Safety and Efficacy of OCU410ST for Stargardt Disease; Ocugen: Malvern, PA, USA, 2025. Available online: https://clinicaltrials.gov/study/NCT05956626 (accessed on 4 June 2025).
- Akula, M.; McNamee, S.; Chan, N.P.M.; DeAngelis, M.M.; Haider, N.B. RORA Modifier Gene Therapy Rescues Retinal Degeneration in a Juvenile AMD Mouse Model of Stargardt Disease. Investig. Ophthalmol. Vis. Sci. 2023, 64, 3842. [Google Scholar]
- Nanoscope Therapeutics Inc. A Phase I/IIa Open Label, Dose-Escalation Study to Evaluate the Safety and Tolerability of Intravitreal vMCO-I in Patients with Advanced Retinitis Pigmentosa; Nanoscope Therapeutics Inc.: Dallas, TX, USA, 2021. Available online: https://clinicaltrials.gov/study/NCT04919473 (accessed on 4 June 2025).
- Sanofi. An Open Label Study to Determine the Long Term Safety, Tolerability and Biological Activity of SAR422459 in Patients with Stargardt’s Macular Degeneration; Sanofi: Paris, France, 2024. Available online: https://clinicaltrials.gov/study/NCT01736592 (accessed on 4 June 2025).
- Zhang, W.; Li, L.; Su, Q.; Gao, G.; Khanna, H. Gene Therapy Using a miniCEP290 Fragment Delays Photoreceptor Degeneration in a Mouse Model of Leber Congenital Amaurosis. Hum. Gene Ther. 2018, 29, 42–50. [Google Scholar] [CrossRef]
- Garanto, A.; Chung, D.C.; Duijkers, L.; Corral-Serrano, J.C.; Messchaert, M.; Xiao, R.; Bennett, J.; Vandenberghe, L.H.; Collin, R.W.J. In Vitro and In Vivo Rescue of Aberrant Splicing in CEP290-Associated LCA by Antisense Oligonucleotide Delivery. Hum. Mol. Genet. 2016, 25, 2552–2563. [Google Scholar] [CrossRef]
- Leroy, B.P.; Birch, D.G.; Duncan, J.L.; Lam, B.L.; Koenekoop, R.K.; Porto, F.B.O.; Russell, S.R.; Girach, A. Leber Congenital Amaurosis Due to Cep290 Mutations-Severe Vision Impairment with a High Unmet Medical Need: A Review. Retina 2021, 41, 898–907. [Google Scholar] [CrossRef]
- Laboratoires Thea. An Open-Label, Multiple Dose, Dose Escalation Study to Evaluate the Safety and Tolerability of QR-110 in Subjects with Leber’s Congenital Amaurosis (LCA) Due to c.2991+1655A>G Mutation (p.Cys998X) in the CEP290 Gene; Laboratoires Thea: Auvergne, France, 2024. Available online: https://clinicaltrials.gov/study/NCT03140969 (accessed on 4 June 2025).
- AAVantgarde Bio Srl. A Phase 1/2 Multicenter, Open-Label, Dose Escalation, Safety and Efficacy Study of Subretinal Administration of Dual AAV8.MYO7A, AAVB-081 in Subjects with Usher Syndrome Type IB (USH1B) Retinitis Pigmentosa; AAVantgarde Bio Srl: Milan, Italy, 2025. Available online: https://clinicaltrials.gov/study/NCT06591793 (accessed on 4 June 2025).
- Riaz, S.; Sethna, S.; Duncan, T.; Naeem, M.A.; Redmond, T.M.; Riazuddin, S.; Riazuddin, S.; Carvalho, L.S.; Ahmed, Z.M. Dual AAV-Based PCDH15 Gene Therapy Achieves Sustained Rescue of Visual Function in a Mouse Model of Usher Syndrome 1F. Mol. Ther. 2023, 31, 3490–3501. [Google Scholar] [CrossRef]
- Ivanchenko, M.V.; Hathaway, D.M.; Klein, A.J.; Pan, B.; Strelkova, O.; De-la-Torre, P.; Wu, X.; Peters, C.W.; Mulhall, E.M.; Booth, K.T.; et al. Mini-PCDH15 Gene Therapy Rescues Hearing in a Mouse Model of Usher Syndrome Type 1F. Nat. Commun. 2023, 14, 2400. [Google Scholar] [CrossRef] [PubMed]
- Sanjurjo-Soriano, C.; Erkilic, N.; Baux, D.; Mamaeva, D.; Hamel, C.P.; Meunier, I.; Roux, A.-F.; Kalatzis, V. Genome Editing in Patient iPSCs Corrects the Most Prevalent USH2A Mutations and Reveals Intriguing Mutant mRNA Expression Profiles. Mol. Ther. Methods Clin. Dev. 2020, 17, 156–173. [Google Scholar] [CrossRef] [PubMed]
- Laboratoires Thea. A Double-Masked, Randomized, Controlled, Multiple-Dose Study to Evaluate the Efficacy, Safety and Tolerability of Ultevursen in Subjects with Retinitis Pigmentosa (RP) Due to Mutations in Exon 13 of the USH2A Gene (Sirius); Laboratoires Thea: Auvergne, France, 2024. Available online: https://clinicaltrials.gov/study/NCT05158296 (accessed on 4 June 2025).
- Laboratoires Thea. A Two-Year Double-Masked, Randomized, Sham-Controlled Study to Evaluate the Efficacy, Safety and Tolerability of Ultevursen in Subjects with Retinitis Pigmentosa (RP) Due to Mutations in Exon 13 of the USH2A Gene; Laboratoires Thea: Auvergne, France, 2024. Available online: https://clinicaltrials.gov/study/NCT06627179 (accessed on 4 June 2025).
- Toms, M.; Toualbi, L.; Almeida, P.V.; Harbottle, R.; Moosajee, M. Successful Large Gene Augmentation of USH2A with Non-Viral Episomal Vectors. Mol. Ther. 2023, 31, 2755–2766. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Vandenberghe, L.H. Miniaturization of Usher Syndrome Type 2A Gene for AAV Mediated Gene Therapy. Investig. Ophthalmol. Vis. Sci. 2021, 62, 1194. [Google Scholar]
- Yan, Z.; Zhang, Y.; Duan, D.; Engelhardt, J.F. Trans-Splicing Vectors Expand the Utility of Adeno-Associated Virus for Gene Therapy. Proc. Natl. Acad. Sci. USA 2000, 97, 6716–6721. [Google Scholar] [CrossRef]
- Duan, D.; Yue, Y.; Yan, Z.; Engelhardt, J.F. A New Dual-Vector Approach to Enhance Recombinant Adeno-Associated Virus-Mediated Gene Expression through Intermolecular Cis Activation. Nat. Med. 2000, 6, 595–598. [Google Scholar] [CrossRef]
- Duan, D.; Yue, Y.; Engelhardt, J.F. Expanding AAV Packaging Capacity with Trans-Splicing or Overlapping Vectors: A Quantitative Comparison. Mol. Ther. 2001, 4, 383–391. [Google Scholar] [CrossRef]
- Ghosh, A.; Yue, Y.; Lai, Y.; Duan, D. A Hybrid Vector System Expands Adeno-Associated Viral Vector Packaging Capacity in a Transgene-Independent Manner. Mol. Ther. 2008, 16, 124–130. [Google Scholar] [CrossRef]
- Ferla, R.; Dell’Aquila, F.; Doria, M.; Ferraiuolo, M.; Noto, A.; Grazioli, F.; Ammendola, V.; Testa, F.; Melillo, P.; Iodice, C.; et al. Efficacy, Pharmacokinetics, and Safety in the Mouse and Primate Retina of Dual AAV Vectors for Usher Syndrome Type 1B. Mol. Ther. Methods Clin. Dev. 2023, 28, 396–411. [Google Scholar] [CrossRef]
- Carvalho, L.S.; Turunen, H.T.; Wassmer, S.J.; Luna-Velez, M.V.; Xiao, R.; Bennett, J.; Vandenberghe, L.H. Evaluating Efficiencies of Dual AAV Approaches for Retinal Targeting. Front. Neurosci. 2017, 11, 503. [Google Scholar] [CrossRef]
- Maddalena, A.; Tornabene, P.; Tiberi, P.; Minopoli, R.; Manfredi, A.; Mutarelli, M.; Rossi, S.; Simonelli, F.; Naggert, J.K.; Cacchiarelli, D.; et al. Triple Vectors Expand AAV Transfer Capacity in the Retina. Mol. Ther. 2018, 26, 524–541. [Google Scholar] [CrossRef] [PubMed]
- Tornabene, P.; Trapani, I.; Minopoli, R.; Centrulo, M.; Lupo, M.; de Simone, S.; Tiberi, P.; Dell’Aquila, F.; Marrocco, E.; Iodice, C.; et al. Intein-Mediated Protein Trans-Splicing Expands Adeno-Associated Virus Transfer Capacity in the Retina. Sci. Transl. Med. 2019, 11, eaav4523. [Google Scholar] [CrossRef] [PubMed]
- Trapani, I.; Colella, P.; Sommella, A.; Iodice, C.; Cesi, G.; de Simone, S.; Marrocco, E.; Rossi, S.; Giunti, M.; Palfi, A.; et al. Effective Delivery of Large Genes to the Retina by Dual AAV Vectors. EMBO Mol. Med. 2014, 6, 194–211. [Google Scholar] [CrossRef]
- Levy, J.M.; Yeh, W.-H.; Pendse, N.; Davis, J.R.; Hennessey, E.; Butcher, R.; Koblan, L.W.; Comander, J.; Liu, Q.; Liu, D.R. Cytosine and Adenine Base Editing of the Brain, Liver, Retina, Heart and Skeletal Muscle of Mice via Adeno-Associated Viruses. Nat. Biomed. Eng. 2020, 4, 97–110. [Google Scholar] [CrossRef]
- Zhi, S.; Chen, Y.; Wu, G.; Wen, J.; Wu, J.; Liu, Q.; Li, Y.; Kang, R.; Hu, S.; Wang, J.; et al. Dual-AAV Delivering Split Prime Editor System for In Vivo Genome Editing. Mol. Ther. 2022, 30, 283–294. [Google Scholar] [CrossRef]
- Kuang, J.; Lyu, Q.; Wang, J.; Cui, Y.; Zhao, J. Advances in Base Editing with an Emphasis on an AAV-Based Strategy. Methods 2021, 194, 56–64. [Google Scholar] [CrossRef]
- Jo, D.H.; Jang, H.-K.; Cho, C.S.; Han, J.H.; Ryu, G.; Jung, Y.; Bae, S.; Kim, J.H. Visual Function Restoration in a Mouse Model of Leber Congenital Amaurosis via Therapeutic Base Editing. Mol. Ther. Nucleic Acids 2023, 31, 16–27. [Google Scholar] [CrossRef]
- Wu, Y.; Wan, X.; Zhao, D.; Chen, X.; Wang, Y.; Tang, X.; Li, J.; Li, S.; Sun, X.; Bi, C.; et al. AAV-Mediated Base-Editing Therapy Ameliorates the Disease Phenotypes in a Mouse Model of Retinitis Pigmentosa. Nat. Commun. 2023, 14, 4923. [Google Scholar] [CrossRef]
- Tornabene, P.; Trapani, I.; Centrulo, M.; Marrocco, E.; Minopoli, R.; Lupo, M.; Iodice, C.; Gesualdo, C.; Simonelli, F.; Surace, E.M.; et al. Inclusion of a Degron Reduces Levelsof Undesired Inteins after AAV-Mediated Proteintrans-Splicing in the Retina. Mol. Ther. Methods Clin. Dev. 2021, 23, 448–459. [Google Scholar] [CrossRef]
- Kolesnik, V.V.; Nurtdinov, R.F.; Oloruntimehin, E.S.; Karabelsky, A.V.; Malogolovkin, A.S. Optimization Strategies and Advances in the Research and Development of AAV-based Gene Therapy to Deliver Large Transgenes. Clin. Transl. Med. 2024, 14, e1607. [Google Scholar] [CrossRef]
- Riedmayr, L.M.; Hinrichsmeyer, K.S.; Thalhammer, S.B.; Mittas, D.M.; Karguth, N.; Otify, D.Y.; Böhm, S.; Weber, V.J.; Bartoschek, M.D.; Splith, V.; et al. mRNA Trans-Splicing Dual AAV Vectors for (Epi)Genome Editing and Gene Therapy. Nat. Commun. 2023, 14, 6578. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.-E.; Wong, T.Y. Diabetic Retinopathy: Looking Forward to 2030. Front. Endocrinol. 2022, 13, 1077669. [Google Scholar] [CrossRef] [PubMed]
- Rowe, L.W.; Ciulla, T.A. Gene Therapy for Non-Hereditary Retinal Disease: Age-Related Macular Degeneration, Diabetic Retinopathy, and Beyond. Genes 2024, 15, 720. [Google Scholar] [CrossRef] [PubMed]
- Adverum Biotechnologies, Inc. A Long-Term Follow-Up Study of ADVM-022 in Diabetic Macular Edema-INFINITY Extension; Adverum Biotechnologies, Inc.: Redwood, AC, USA, 2025. Available online: https://clinicaltrials.gov/study/NCT05607810 (accessed on 4 June 2025).
- REGENXBIO. REGENXBIO Presents Positive One-Year Data from Phase II ALTITUDE® Trial of ABBV-RGX-314 Delivered via Suprachoroidal Administration for Diabetic Retinopathy. Available online: https://regenxbio.gcs-web.com/news-releases/news-release-details/regenxbio-presents-positive-one-year-data-phase-ii-altituder/ (accessed on 24 November 2024).
- Frontera Therapeutics. A Dose-Escalation and Dose-Expanded Phase I/II Clinical Study to Evaluate the Safety, and Efficacy of FT-003 in Subjects with DME; Frontera Therapeutics: Bedford, MA, USA, 2024. Available online: https://clinicaltrials.gov/study/NCT06492876 (accessed on 4 June 2025).
- Zhang, X.; Das, S.K.; Passi, S.F.; Uehara, H.; Bohner, A.; Chen, M.; Tiem, M.; Archer, B.; Ambati, B.K. AAV2 Delivery of Flt23k Intraceptors Inhibits Murine Choroidal Neovascularization. Mol. Ther. 2015, 23, 226–234. [Google Scholar] [CrossRef]
- Bainbridge, J.W.B.; Mistry, A.; De Alwis, M.; Paleolog, E.; Baker, A.; Thrasher, A.J.; Ali, R.R. Inhibition of Retinal Neovascularisation by Gene Transfer of Soluble VEGF Receptor sFlt-1. Gene Ther. 2002, 9, 320–326. [Google Scholar] [CrossRef]
- Wang, J.-H.; Roberts, G.E.; Liu, G.-S. Updates on Gene Therapy for Diabetic Retinopathy. Curr. Diabetes Rep. 2020, 20, 22. [Google Scholar] [CrossRef]
- Pfaller, A.M.; Kaplan, L.; Carido, M.; Grassmann, F.; Díaz-Lezama, N.; Ghaseminejad, F.; Wunderlich, K.A.; Glänzer, S.; Bludau, O.; Pannicke, T.; et al. The Glucocorticoid Receptor as a Master Regulator of the Müller Cell Response to Diabetic Conditions in Mice. J. Neuroinflamm. 2024, 21, 33. [Google Scholar] [CrossRef]
- Barboni, M.T.S.; Vaillend, C.; Joachimsthaler, A.; Liber, A.M.P.; Khabou, H.; Roux, M.J.; Vacca, O.; Vignaud, L.; Dalkara, D.; Guillonneau, X.; et al. Rescue of Defective Electroretinographic Responses in Dp71-Null Mice with AAV-Mediated Reexpression of Dp71. Investig. Ophthalmol. Vis. Sci. 2020, 61, 11. [Google Scholar] [CrossRef]
- Buck, T.M.; Wijnholds, J. Recombinant Adeno-Associated Viral Vectors (rAAV)-Vector Elements in Ocular Gene Therapy Clinical Trials and Transgene Expression and Bioactivity Assays. Int. J. Mol. Sci. 2020, 21, 4197. [Google Scholar] [CrossRef]
- REGENXBIO. REGENXBIO Presents Positive Data from Phase II Study of Subretinal ABBV-RGX-314 in Bilateral Wet AMD Patients at AAO 2024. Available online: https://regenxbio.gcs-web.com/news-releases/news-release-details/regenxbio-presents-positive-data-phase-ii-study-subretinal-abbv/ (accessed on 5 June 2025).
- REGENXBIO. REGENXBIO Announces Positive Interim Data from Phase II AAVIATE® Trial of Suprachoroidal ABBV-RGX-314 for Wet AMD. Available online: https://regenxbio.gcs-web.com/news-releases/news-release-details/regenxbio-announces-positive-interim-data-phase-ii-aaviater/ (accessed on 5 June 2025).
- Adverum Biotechnologies Announces Positive 52-Week LUNA and 4-Year OPTIC Results, and Provides Key Pivotal Program Design Elements. Available online: https://investors.adverum.com/press_releases/news-details/2024/Adverum-Biotechnologies-Announces-Positive-52-Week-LUNA-and-4-Year-OPTIC-Results-and-Provides-Key-Pivotal-Program-Design-Elements/default.aspx (accessed on 5 June 2025).
- Khanani, A.M.; Boyer, D.S.; Wykoff, C.C.; Regillo, C.D.; Busbee, B.G.; Pieramici, D.; Danzig, C.J.; Joondeph, B.C.; James, C.; Major, J.; et al. Safety and Efficacy of Ixoberogene Soroparvovec in Neovascular Age-Related Macular Degeneration in the United States (OPTIC): A Prospective, Two-Year, Multicentre Phase 1 Study. EClinicalMedicine 2023, 67, 102394. [Google Scholar] [CrossRef]
- HuidaGene Therapeutics Announces First Patient Dosed Of The World’s First Novel CRISPR/Cas13 RNA-Editing Therapy HG202 For Neovascular Age-Related Macular Degeneration-Press Release-HUIDAGENE. Available online: https://www.huidagene.com/new/news/43 (accessed on 5 June 2025).
- de Guimaraes, T.A.C.; Varela, M.D.; Georgiou, M.; Michaelides, M. Treatments for Dry Age-Related Macular Degeneration: Therapeutic Avenues, Clinical Trials and Future Directions. Br. J. Ophthalmol. 2022, 106, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Gyroscope Therapeutics Ltd. HORIZON: A Phase II, Open-Label, Outcomes-Assessor Masked, Multicentre, Randomised, Controlled Study to Evaluate the Safety and Efficacy of Two Doses of GT005 Administered as a Single Subretinal Injection in Subjects with Geographic Atrophy Secondary to Dry Age-Related Macular Degeneration; Gyroscope Therapeutics Ltd.: London, UK, 2024. Available online: https://clinicaltrials.gov/study/NCT04566445 (accessed on 4 June 2025).
- Calton, M.A. Preclinical Characterization of 4D-175, a Novel AAV-Based Investigational Intravitreal Gene Therapy for Geographic Atrophy. Investig. Ophthalmol. Vis. Sci. 2023, 65, 6164. [Google Scholar]
- Janssen Research & Development, LLC. A Phase 1, Open-Label, Multi-Center, Dose-Escalating, Safety and Tolerability Study of a Single Intravitreal Injection of AAVCAGsCD59 in Patients with Advanced Non-Exudative (Dry) Age-Related Macular Degeneration with Geographic Atrophy; Janssen Research & Development, LLC: Raritan, NJ, USA, 2021. Available online: https://clinicaltrials.gov/study/NCT03144999 (accessed on 4 June 2025).
- Janssen Research & Development, LLC. A Phase 1 Proof of Concept Study Evaluating Intravitreal AAVCAGsCD59 for the Treatment of Wet Age-Related Macular Degeneration (AMD); Janssen Research & Development, LLC: Raritan, NJ, USA, 2021. Available online: https://clinicaltrials.gov/study/NCT03585556 (accessed on 4 June 2025).
- Ocugen. A Phase 1/2 Study to Assess the Safety And Efficacy Of OCU410 For Geographic Atrophy Secondary To Dry Age-Related Macular Degeneration; Ocugen: Malvern, PA, USA, 2024. Available online: https://clinicaltrials.gov/study/NCT06018558 (accessed on 4 June 2025).
- Britten-Jones, A.C.; McGuinness, M.B.; Chen, F.K.; Grigg, J.R.; Mack, H.G.; Ayton, L.N. A Multinational Survey of Potential Participant Perspectives on Ocular Gene Therapy. Gene Ther. 2024, 31, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Tan, F.; Dong, Y.; Qi, J.; Yu, W.; Chai, R. Artificial Intelligence-Based Approaches for AAV Vector Engineering. Adv. Sci. 2025, 12, 2411062. [Google Scholar] [CrossRef]
- Asaad, W.; Volos, P.; Maksimov, D.; Khavina, E.; Deviatkin, A.; Mityaeva, O.; Volchkov, P. AAV Genome Modification for Efficient AAV Production. Heliyon 2023, 9, e15071. [Google Scholar] [CrossRef]

| Disease | Major Affected Cell Types | Therapeutic Target Cell Types | Target Genes | Sponsor | Phase | Interventions and Vector | Delivery | NCT number |
|---|---|---|---|---|---|---|---|---|
| RPE65 Mutation-associated Retinal Dystrophy | RPEs; secondary PRs | RPEs | RPE65 | Novartis Pharmaceuticals | III | Voretigene neparvovec-rzyl (AAV2) | SR | NCT04516369 |
| Innostellar Biotherapeutics Co. | I/II | LX101 (AAV2) | SR | NCT06196827 | ||||
| NCT06212297 | ||||||||
| Frontera Therapeutics | I/II | FT-001 (AAV2) | SR | NCT05858983 | ||||
| LCA | PRs; RPEs | RPE65 | Spark Therapeutics | I | Voretigene neparvovec-rzyl (rAAV2) | SR | NCT00516477 | |
| NCT01208389 | ||||||||
| III | NCT01208389 | |||||||
| Applied Genetic Technologies Corp | I/II | rAAV2-CB-hRPE65 | SR | NCT00749957 | ||||
| MeiraGTx UK II Ltd. | I/II | MGT003 (rAAV2) | SR | NCT02781480 | ||||
| Hadassah Medical Organization | I | rAAV2-hRPE65 | SR | NCT00821340 | ||||
| Nantes University Hospital | I/II | rAAV2/4.hRPE65 | SR | NCT01496040 | ||||
| University of Pennsylvania | I | rAAV2-CBSB-hRPE65 | SR | NCT00481546 | ||||
| HuidaGene Therapeutics Co. | I/II | HG004 (rAAV9) | SR | NCT05906953 | ||||
| University College, London | I/II | tgAAG76 (rAAV2/2) | SR | NCT00643747 | ||||
| PRs | CEP290 | Ocugen | I/II | OCU400 (rAAV5) | SR | NCT05203939 | ||
| CEP290 | Editas Medicine | I/II | EDIT-101 (rAAV5) | SR | NCT03872479 | |||
| GUCY2D | Atsena Therapeutics | I/II | ATSN-101 (rAAV5) | SR | NCT03920007 | |||
| RP | Rods, secondary Cones and RPEs | PRs | PDE6A | STZ eyetrial | I/II | Raav8.hPDE6A | SR | NCT04611503 |
| PDE6B | eyeDNA Therapeutics | I/II | rAAV2/5-hPDE6B | SR | NCT03328130 | |||
| Cones | RHO, PDE6A or PDE6B | SparingVision | I/II | SPVN06 (rAAV) | SR | NCT05748873 | ||
| RPEs | RLBP1 | Novartis Pharmaceuticals | I/II | CPK850 (rAAV8) | SR | NCT03374657 | ||
| Bipolar cells | MCO | Nanoscope Therapeutics Inc. | I/II | vMCO-I (rAAV2) | IVT | NCT04919473 | ||
| II | MCO-010 (rAAV2) | IVT | NCT04945772 | |||||
| RGCs | ChrimsonR | GenSight Biologics | I/II | GS030 (rAAV2.7m8) | IVT | NCT03326336 | ||
| XLRP | Rods, secondary Cones and RPEs | PRs | RPGR | Beacon Therapeutics | I/II | AGTC-501 (rAAV2tYF) | SR | NCT03316560 |
| II | NCT06333249 | |||||||
| II | NCT06275620 | |||||||
| II/III | NCT04850118 | |||||||
| Janssen Pharmaceutical K.K. | II | rAAV2/5-hRKp.RPGR | SR | NCT06646289 | ||||
| III | NCT04671433 | |||||||
| NCT04794101 | ||||||||
| NCT05926583 | ||||||||
| Biogen (NightstaRx Ltd., a Biogen Company) | I/II/III | BIIB112 (rAAV8-RPGR) | SR | NCT03116113 | ||||
| III | BIIB111 (rAAV2-REP1); BIIB112 (rAAV8-RPGR) | SR | NCT03584165 | |||||
| MeiraGTx UK II Ltd. | I/II | rAAV2/5-RPGR | SR | NCT03252847 | ||||
| Frontera Therapeutics | I/II | FT-002 (rAAV) | SR | NCT06492850 | ||||
| 4D Molecular Therapeutics | I/II | 4D-125 (rAAV.R100) | IVT | NCT04517149 | ||||
| XLRS | PRs; Bipolar cells | PRs; Bipolar cells | RS1 | Atsena Therapeutics Inc. | I/II | ATSN-201 (AAV.SPR-hGRK1-hRS1syn) | SR | NCT05878860 |
| Applied Genetic Technologies Corp | I/II | rAAV2tYF-CB-hRS1 | IVT | NCT02416622 | ||||
| VegaVect | I/II | rAAV8-scRS/IRBPhRS | IVT | NCT02317887 | ||||
| LHON | RGCs | RGCs | ND1 | Neurophth Therapeutics Inc. | I/II | NFS-02 (rAAV2-ND1) | IVT | NCT05820152 |
| ND4 | Neurophth Therapeutics Inc. | I/II | NR082 (rAAV-ND4) | IVT | NCT05293626 | |||
| Wuhan Neurophth Biotechnology Limited Company | II/III | NCT04912843 | ||||||
| GenSight Biologics | I/II | GS010 (rAAV2/2-ND4) | IVT | NCT02064569 | ||||
| III | NCT03406104 | |||||||
| NCT03293524 | ||||||||
| NCT02652780 | ||||||||
| NCT02652767 | ||||||||
| Huazhong University of Science and Technology | II/III | rAAV2-ND4 | IVT | NCT03153293 | ||||
| Byron Lam | I | scAAV2-P1ND4v2 | IVT | NCT02161380 | ||||
| ACHM | Cones | Cones | CNGA3 | STZ eyetrial | I/II | rAAV.hCNGA3 | SR | NCT02610582 |
| MeiraGTx UK II Ltd. | I/II | rAAV2/8-hG1.7p.coCNGA3 | SR | NCT03758404 | ||||
| CNGB3 | I/II | rAAV2/8-hG1.7p.coCNGB3 | SR | NCT03001310 | ||||
| CNGA3 and CNGB3 | I/II | rAAV2/8-hCARp.hCNGB3 and rAAV2/8-hG1.7p.coCNGA3 | SR | NCT03278873 | ||||
| CNGB3 | Applied Genetic Technologies Corp | I/II | rAAV2tYF-PR1.7-hCNGB3 | SR | NCT02599922 | |||
| CHM | RPEs; secondary PRs | RPEs | REP1 | Biogen | II | BIIB111 (rAAV2-REP1) | SR | NCT03507686 |
| III | BIIB111 (rAAV2-REP1) | SR | NCT03496012 | |||||
| Biogen (NightstaRx Ltd., a Biogen Company) | III | BIIB111 (rAAV2-REP1); BIIB112 (AAV8-RPGR) | SR | NCT03584165 | ||||
| University of Oxford | I/II | rAAV2.REP1 | SR | NCT01461213 | ||||
| II | rAAV2.REP1 | SR | NCT02407678 | |||||
| Byron Lam | II | rAAV2-REP1 | SR | NCT02553135 | ||||
| University of Alberta | I/II | rAAV2.REP1 | SR | NCT02077361 | ||||
| STZ eyetrial | II | rAAV2.REP1 | SR | NCT02671539 | ||||
| Spark Therapeutics | I/II | rAAV2-hCHM | SR | NCT02341807 | ||||
| 4D Molecular Therapeutics | I | 4D-110 (rAAV.R100) | IVT | NCT04483440 |
| Mechanism of Action | Interventions and Vector | Sponsor | Disease | Phase | Delivery | NCT number |
|---|---|---|---|---|---|---|
| Expressing aflibercept | ADVM-022 (AAV.7m8) | Adverum Biotechnologies | DME | II | IVT | NCT05607810 |
| DME | II | IVT | NCT04418427 | |||
| nAMD | I | IVT | NCT03748784 | |||
| nAMD | II | IVT | NCT05536973 | |||
| nAMD | II | IVT | NCT04645212 | |||
| Expressing both aflibercept and a VEGF-C inhibitory RNAi | 4D-150(AAV.R100) | 4D Molecular Therapeutics | DME | II | IVT | NCT05930561 |
| nAMD | I/II | IVT | NCT05197270 | |||
| Encoding a ranibizumab-like anti-VEGF monoclonal antibody fragment | ABBV-RGX-314 (AAV8) | REGENXBIO Inc. | nAMD | I/II | SR | NCT03066258 |
| AbbVie | DME | II | SCS | NCT04567550 | ||
| nAMD | II | SR | NCT04832724 | |||
| nAMD | II | SR | NCT03999801 | |||
| nAMD | II/III | SR | NCT04704921 | |||
| nAMD | III | SR | NCT05407636 | |||
| nAMD | II | SCS | NCT04514653 | |||
| Encoding a confidential anti-VEGF protein | FT-003 | Frontera Therapeutics | DME | I | IVT | NCT05916391 |
| DME | I/II | IVT | NCT06492876 | |||
| nAMD | I | IVT | NCT05611424 | |||
| nAMD | I/II | IVT | NCT06492863 | |||
| Encoding a confidential anti-VEGF protein | SKG0106 | Skyline Therapeutics (US) Inc. | nAMD | I/II | IVT | NCT05986864 |
| Wang Min | DME | I | IVT | NCT06237777 | ||
| Youxin Chen | nAMD | I | IVT | NCT06213038 | ||
| Encoding a confidential anti-VEGF protein | KH658 | Chengdu Origen Biotechnology Co. | nAMD | I | SCS | NCT05657301 |
| Expressing human VEGF receptor fusion protein with binding affinity to VEGF-A, VEGF-B, and PlGF | KH631 | nAMD | I/II | SR | NCT05672121 | |
| Encoding a confidential anti-VEGF protein | LX102 | Innostellar Biotherapeutics Co. | nAMD | I | SR | NCT06198413 |
| nAMD | II | SR | NCT06196840 | |||
| Encoding a confidential anti-VEGF protein | RRG001 | Shanghai Refreshgene Technology Co. | nAMD | I/II | SR | NCT06141460 |
| Encoding a soluble VEGF receptor | rAAV.sFlt-1 | Lions Eye Institute, Perth, Western Australia | nAMD | I/II | SR | NCT01494805 |
| AAV2-sFLT01 | Genzyme, a Sanofi Company | nAMD | I | IVT | NCT01024998 | |
| Encoding an angiopoietin domain and VEGF receptor (ABD-VEGFR) fusion protein | EXG102-031 | Exegenesis Bio | nAMD | I | SR | NCT05903794 |
| Targeted degradation of retinal VEGF-A mRNA via high-fidelity CRISPR/Cas13Y (hfCas13Y) system. | HG202 | HuidaGene | nAMD | I | SR | NCT06623279 |
| Complement pathway modulation: encoding a soluble form of CD59 (sCD59) | JNJ-1887 (AAV2) | Janssen Research and Development, LLC | nAMD | I | IVT | NCT03585556 |
| dAMD | II | IVT | NCT05811351 | |||
| I | IVT | NCT03144999 | ||||
| II | IVT | NCT06635148 | ||||
| II | IVT | NCT04358471 | ||||
| Complement pathway modulation: encoding encoding human CFI | GT005 (AAV2) | Gyroscope Therapeutics Limited | dAMD | II | SR | NCT05481827 |
| Anti-inflammatory: encoding RORα | OCU410 (AAV5) | Ocugen | dAMD | I/II | SR | NCT06018558 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Li, J.; Xu, X.; Li, K. Adeno-Associated Virus Vectors in Retinal Gene Therapy: Challenges, Innovations, and Future Directions. Biomolecules 2025, 15, 940. https://doi.org/10.3390/biom15070940
Huang J, Li J, Xu X, Li K. Adeno-Associated Virus Vectors in Retinal Gene Therapy: Challenges, Innovations, and Future Directions. Biomolecules. 2025; 15(7):940. https://doi.org/10.3390/biom15070940
Chicago/Turabian StyleHuang, Jiayu, Jiajun Li, Xiangzhong Xu, and Keran Li. 2025. "Adeno-Associated Virus Vectors in Retinal Gene Therapy: Challenges, Innovations, and Future Directions" Biomolecules 15, no. 7: 940. https://doi.org/10.3390/biom15070940
APA StyleHuang, J., Li, J., Xu, X., & Li, K. (2025). Adeno-Associated Virus Vectors in Retinal Gene Therapy: Challenges, Innovations, and Future Directions. Biomolecules, 15(7), 940. https://doi.org/10.3390/biom15070940





