β-Secosterol, an Oxyphytosterol Produced Through the Reaction of β-Sitosterol with Ozone, Demonstrates Different Cytotoxic Effects on BRL-3A and HTC Cells
Abstract
1. Introduction

2. Materials and Methods
2.1. Synthesis and Characterization of Oxyphytosterol βsec
2.2. Preparation of Compounds
2.3. Cell Maintenance and Cultivation
2.4. Cell Metabolic Activity—MTT Assay
2.5. Cell Viability—Trypan Blue Exclusion Assay
2.6. Cell Viability—Fluorescent Cell Staining with Hoechst 33342 and Propidium Iodide
2.7. Analysis of the Distribution of Cell Cycle Phases
2.8. Analysis of Morphology and Cytoskeletal Organization
2.9. Analysis of Actin Filaments and Focal Adhesion
2.10. Cell Recovery
2.11. Statistical Analysis
3. Results
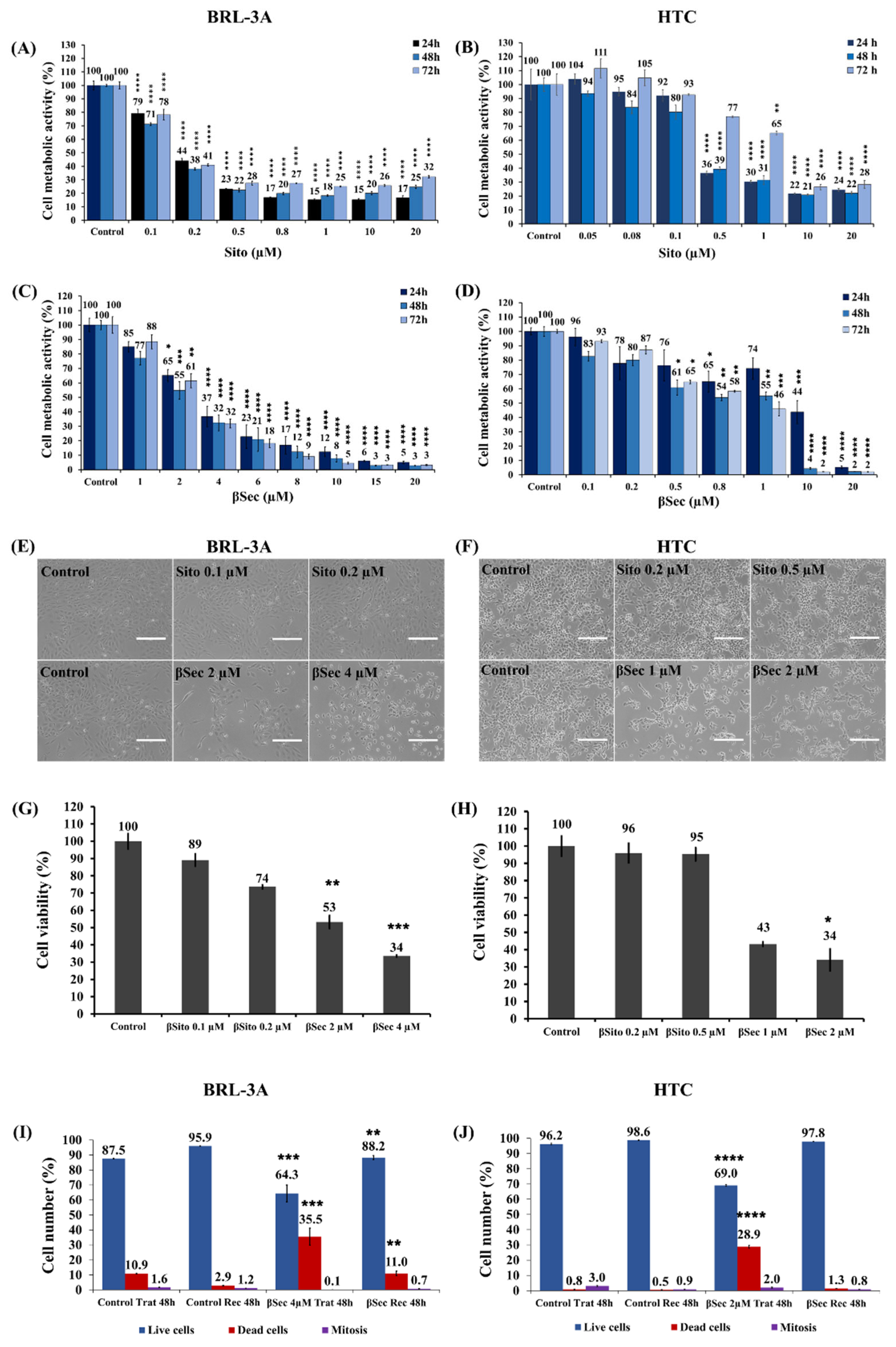
3.1. Analysis of Cell Metabolic Activity and Cell Viability
3.2. Analysis of the Cell Cycle
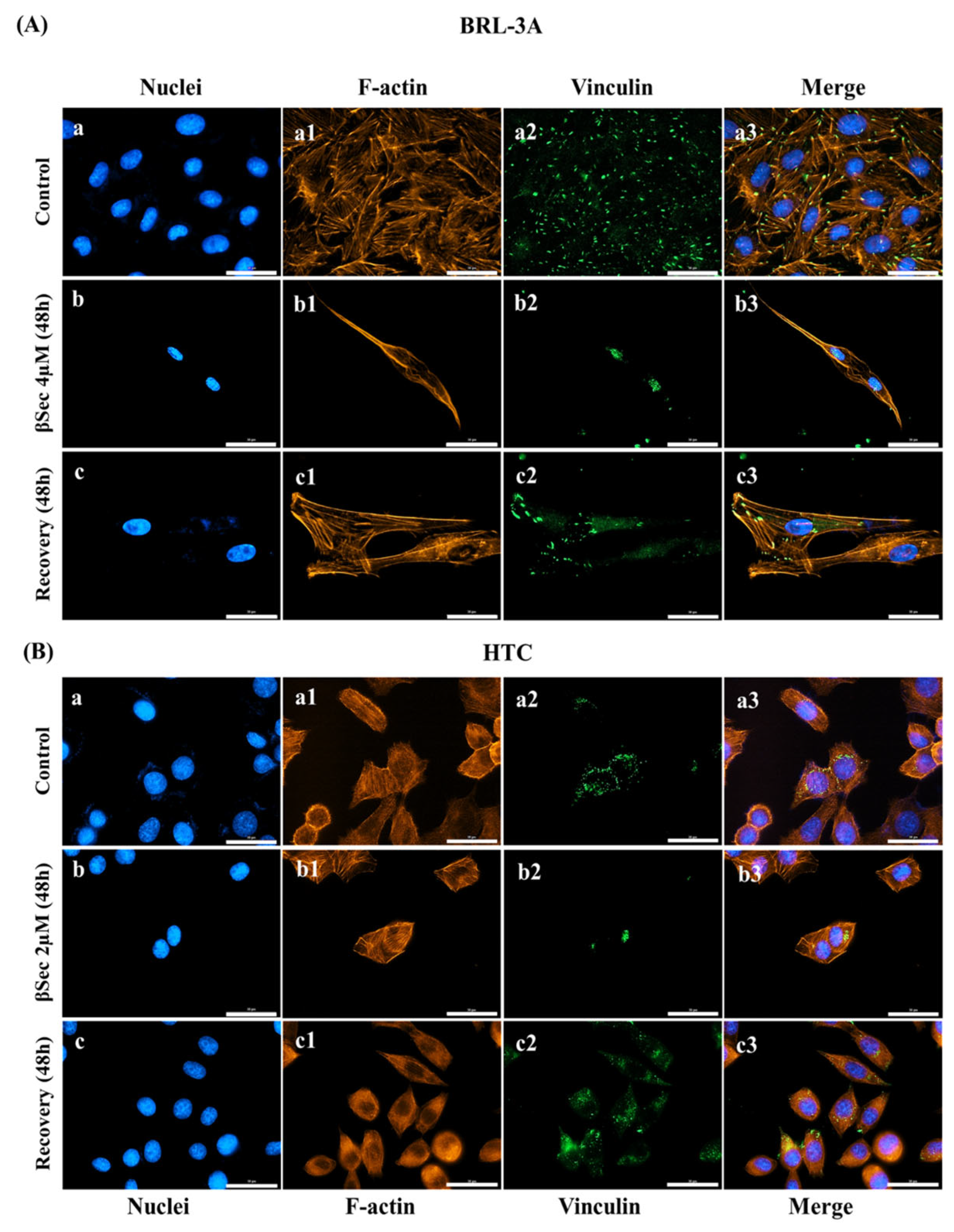
3.3. Analysis of Cell Morphology and Cytoskeletal Organization
3.4. Focal Adhesion
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moreau, R.A.; Nyström, L.; Whitaker, B.D.; Winkler-Moser, J.K.; Baer, D.J.; Gebauer, S.K.; Hicks, K.B. Phytosterols and Their Derivatives: Structural Diversity, Distribution, Metabolism, Analysis, and Health-Promoting Uses. Prog. Lipid Res. 2018, 70, 35–61. [Google Scholar] [CrossRef]
- Babu, S.; Jayaraman, S. An Update on β-Sitosterol: A Potential Herbal Nutraceutical for Diabetic Management. Biomed. Pharmacother. 2020, 131, 110702. [Google Scholar] [CrossRef]
- Liang, Y.T.; Wong, W.T.; Guan, L.; Tian, X.Y.; Ma, K.Y.; Huang, Y.; Chen, Z.-Y. Effect of Phytosterols and Their Oxidation Products on Lipoprotein Profiles and Vascular Function in Hamster Fed a High Cholesterol Diet. Atherosclerosis 2011, 219, 124–133. [Google Scholar] [CrossRef]
- Bao, X.; Zhang, Y.; Zhang, H.; Xia, L. Molecular Mechanism of β-Sitosterol and Its Derivatives in Tumor Progression. Front. Oncol. 2022, 12, 926975. [Google Scholar] [CrossRef]
- He, W.-S.; Zhu, H.; Chen, Z.-Y. Plant Sterols: Chemical and Enzymatic Structural Modifications and Effects on Their Cholesterol-Lowering Activity. J. Agric. Food Chem. 2018, 66, 3047–3062. [Google Scholar] [CrossRef]
- Khan, Z.; Nath, N.; Rauf, A.; Emran, T.B.; Mitra, S.; Islam, F.; Chandran, D.; Barua, J.; Khandaker, M.U.; Idris, A.M.; et al. Multifunctional Roles and Pharmacological Potential of β-Sitosterol: Emerging Evidence toward Clinical Applications. Chem.-Biol. Interact. 2022, 365, 110117. [Google Scholar] [CrossRef]
- Wang, R.; Tang, D.; Ou, L.; Jiang, J.; Wu, Y.; Tian, X. β-Sitosterol Alleviates the Malignant Phenotype of Hepatocellular Carcinoma Cells via Inhibiting GSK3B Expression. Hum. Cell 2024, 37, 1156–1169. [Google Scholar] [CrossRef]
- Scholz, B.; Guth, S.; Engel, K.H.; Steinberg, P. Phytosterol Oxidation Products in Enriched Foods: Occurrence, Exposure, and Biological Effects. Mol. Nutr. Food Res. 2015, 59, 1339–1352. [Google Scholar] [CrossRef]
- Ryan, L.; O’Callaghan, Y.C.; O’Brien, N.M. The Role of the Mitochondria in Apoptosis Induced by 7β-Hydroxycholesterol and Cholesterol-5β,6β-Epoxide. Br. J. Nutr. 2005, 94, 519–525. [Google Scholar] [CrossRef]
- Roussi, S.; Gossé, F.; Aoudé-Werner, D.; Zhang, X.; Marchioni, E.; Geoffroy, P.; Miesch, M.; Raul, F.; Roussi, S.; Gossé, F.; et al. Mitochondrial Perturbation, Oxidative Stress and Lysosomal Destabilization Are Involved in 7β-Hydroxysitosterol and 7β-Hydroxycholesterol Triggered Apoptosis in Human Colon Cancer Cells. Apoptosis 2007, 12, 87–96. [Google Scholar] [CrossRef]
- Plat, J.; Theuwissen, E.; Husche, C.; Lütjohann, D.; Gijbels, M.J.J.; Jeurissen, M.; Shiri-Sverdlov, R.; van der Made, I.; Mensink, R.P. Oxidised Plant Sterols as Well as Oxycholesterol Increase the Proportion of Severe Atherosclerotic Lesions in Female LDL Receptor+/− Mice. Br. J. Nutr. 2014, 111, 64–70. [Google Scholar] [CrossRef]
- Alemany, L.; Laparra, J.M.; Barberá, R.; Alegría, A. Relative Expression of Cholesterol Transport-Related Proteins and Inflammation Markers through the Induction of 7-Ketosterol-Mediated Stress in Caco-2 Cells. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2013, 56, 247–253. [Google Scholar] [CrossRef]
- dos Santos Souza Marinho, R.; Ramos, C.J.B.; Leite, J.P.G.; Teixeira, V.L.; de Palmer Paixão, I.C.N.; Belo, C.A.D.; Pereira, A.B.; Pinto, A.M.V. Antiviral Activity of 7-Keto-Stigmasterol Obtained from Green Antarctic Algae Prasiola Crispa against Equine Herpesvirus 1. J. Appl. Phycol. 2016, 29, 555–562. [Google Scholar] [CrossRef]
- Hovenkamp, E.; Demonty, I.; Plat, J.; Lütjohann, D.; Mensink, R.P.; Trautwein, E.A. Biological Effects of Oxidized Phytosterols: A Review of the Current Knowledge. Prog. Lipid Res. 2008, 47, 37–49. [Google Scholar] [CrossRef]
- Wang, M.; Lu, B. How Do Oxyphytosterols Affect Human Health? Trends Food Sci. Technol. 2018, 79, 148–159. [Google Scholar] [CrossRef]
- Baumgartner, S.; Lütjohann, D.; Husche, C.; Kerksiek, A.; Groen, A.K.; Mensink, R.P.; Plat, J. Plasma Oxyphytosterols Most Likely Originate from Hepatic Oxidation and Subsequent Spill-over in the Circulation. J. Steroid Biochem. Mol. Biol. 2022, 216, 106039. [Google Scholar] [CrossRef]
- Modesti, M.; Macaluso, M.; Taglieri, I.; Bellincontro, A.; Sanmartin, C. Ozone and Bioactive Compounds in Grapes and Wine. Foods 2021, 10, 2934. [Google Scholar] [CrossRef]
- Aziz, F.A.B.A.; Rahman, N.; Ali, J.M. Tropospheric Ozone Formation Estimation in Urban City, Bangi, Using Artificial Neural Network (ANN). Comput. Intell. Neurosci. 2019, 2019, 6252983. [Google Scholar] [CrossRef]
- Martins, I.R.; Onuki, J.; Miyamoto, S.; Uemi, M. Characterization of Oxyphytosterols Generated by β-Sitosterol Ozonization. Arch. Biochem. Biophys. 2020, 689, 108472. [Google Scholar] [CrossRef]
- Takayasu, B.S.; Martins, I.R.; Garnique, A.M.B.; Miyamoto, S.; Machado-Santelli, G.M.; Uemi, M.; Onuki, J. Biological Effects of an Oxyphytosterol Generated by β-Sitosterol Ozonization. Arch. Biochem. Biophys. 2020, 696, 108654. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- AAT Bioquest, Inc. Quest Graph™ IC50 Calculator. AAT Bioquest. Available online: https://www.aatbio.com/tools/ic50-calculator (accessed on 12 May 2023).
- Strober, W. Trypan Blue Exclusion Test of Cell Viability. In Current Protocols in Immunology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2001; Volume 21, pp. A.3B.1–A.3B.2. [Google Scholar]
- Chazotte, B. Labeling Nuclear DNA with Hoechst 33342. Cold Spring Harb. Protoc. 2011, 6, 83–86. [Google Scholar] [CrossRef]
- Krishan, A. Rapid Flow Cytofluorometric Analysis of Mammalian Cell Cycle by Propidium Iodide Staining. J. Cell Biol. 1975, 66, 188–193. [Google Scholar] [CrossRef]
- Ionta, M.; Ferreira-Silva, G.A.; Niero, E.L.; Costa, É.D.; Martens, A.A.; Rosa, W.; Soares, M.G.; Machado-Santelli, G.M.; Henrique, J.; Lago, G.; et al. 7-Epiclusianone, a Benzophenone Extracted from Garcinia Brasiliensis (Cusiaceae), Induces Cell Cycle Arrest in G1/S Transition in A549 Cells. Molecules 2015, 20, 12804–12816. [Google Scholar] [CrossRef]
- Koschutnig, K.; Heikkinen, S.; Kemmo, S.; Lampi, A.-M.; Piironen, V.; Wagner, K.-H. Cytotoxic and Apoptotic Effects of Single and Mixed Oxides of Beta-Sitosterol on HepG2-Cells. Toxicol. Vitr. 2009, 23, 755–762. [Google Scholar] [CrossRef]
- Vo, T.K.; Ta, Q.T.H.; Chu, Q.T.; Nguyen, T.T.; Vo, V.G. Anti-Hepatocellular-Cancer Activity Exerted by β-Sitosterol and β-Sitosterol-Glucoside from Indigofera Zollingeriana Miq. Molecules 2020, 25, 3021. [Google Scholar] [CrossRef]
- Ionta, M.; Ferreira, R.A.S.; Pfister, S.C.; Machado-Santelli, G.M. Exogenous Cx43 Expression Decrease Cell Proliferation Rate in Rat Hepatocarcinoma Cells Independently of Functional Gap Junction. Cancer Cell Int. 2009, 9, 22. [Google Scholar] [CrossRef]
- Sazonova, E.V.; Chesnokov, M.S.; Zhivotovsky, B.; Kopeina, G.S. Drug Toxicity Assessment: Cell Proliferation versus Cell Death. Cell Death Discov. 2022, 8, 417. [Google Scholar] [CrossRef]
- Rubis, B.; Polrolniczak, A.; Knula, H.; Potapinska, O.; Kaczmarek, M.; Rybczynska, M. Phytosterols in Physiological Concentrations Target Multidrug Resistant Cancer Cells. Med. Chem. 2010, 6, 184–190. [Google Scholar] [CrossRef]
- Matthews, H.K.; Bertoli, C.; De Bruin, R.A.M. Cell Cycle Control in Cancer. Nat. Rev. Mol. Cell Biol. 2022, 23, 74–88. [Google Scholar] [CrossRef]
- Pennycook, B.R.; Barr, A.R. Restriction Point Regulation at the Crossroads between Quiescence and Cell Proliferation. FEBS Lett. 2020, 594, 2046–2060. [Google Scholar] [CrossRef]
- Montalto, F.I.; De Amicis, F. Cyclin D1 in Cancer: A Molecular Connection for Cell Cycle Control, Adhesion and Invasion in Tumor and Stroma. Cells 2020, 9, 2648. [Google Scholar] [CrossRef]
- Neumeister, P.; Pixley, F.J.; Xiong, Y.; Xie, H.; Wu, K.; Ashton, A.; Cammer, M.; Chan, A.; Symons, M.; Stanley, E.R.; et al. Cyclin D1 Governs Adhesion and Motility of Macrophages V. Mol. Biol. Cell 2003, 14, 2005–2015. [Google Scholar] [CrossRef]
- Kohrman, A.Q.; Matus, D.Q. Divide or Conquer: Cell Cycle Regulation of Invasive Behavior. Trends Cell Biol. 2017, 27, 12–25. [Google Scholar] [CrossRef]
- Legátová, A.; Pelantová, M.; Rösel, D.; Brábek, J.; Škarková, A. The Emerging Role of Microtubules in Invasion Plasticity. Front. Oncol. 2023, 13, 1118171. [Google Scholar] [CrossRef]
- Fife, C.M.; McCarroll, J.A.; Kavallaris, M. Movers and Shakers: Cell Cytoskeleton in Cancer Metastasis. Br. J Pharmacol. 2014, 171, 5507–5523. [Google Scholar] [CrossRef]
- Mohammed, R.N.; Khosravi, M.; Rahman, H.S.; Adili, A.; Kamali, N.; Soloshenkov, P.P.; Thangavelu, L.; Saeedi, H.; Shomali, N.; Tamjidifar, R.; et al. Anastasis: Cell Recovery Mechanisms and Potential Role in Cancer. Cell Commun. Signal 2022, 20, 81. [Google Scholar] [CrossRef]
- Fontana, R.; Mestre-Farrera, A.; Yang, J. Update on Epithelial-Mesenchymal Plasticity in Cancer Progression. Annu. Rev. Pathol. Mech. Dis. 2024, 19, 133–156. [Google Scholar] [CrossRef]
- Wang, H.; Said, R.; Nguyen-Vigouroux, C.; Henriot, V.; Gebhardt, P.; Pernier, J.; Grosse, R.; Le Clainche, C. Talin and Vinculin Combine Their Activities to Trigger Actin Assembly. Nat. Commun. 2024, 15, 9497. [Google Scholar] [CrossRef]
- Wojnacki, J.; Quassollo, G.; Marzolo, M.-P.; Cáceres, A. Rho GTPases at the Crossroad of Signaling Networks in Mammals: Impact of Rho-GTPases on Microtubule Organization and Dynamics. Small GTPases 2014, 5, e28430. [Google Scholar] [CrossRef]
- Jaffe, A.B.; Hall, A. Rho GTPases: Biochemistry and Biology. Annu. Rev. Cell Dev. Biol. 2005, 21, 247–269. [Google Scholar] [CrossRef]
- Katoh, K. Signal Transduction Mechanisms of Focal Adhesions: Src and FAK-Mediated Cell Response. Front. Biosci. 2024, 29, 392. [Google Scholar] [CrossRef]
- Dantas, L.S.; Chaves-Filho, A.B.; Coelho, F.R.; Genaro-Mattos, T.C.; Tallman, K.A.; Porter, N.A.; Augusto, O.; Miyamoto, S. Cholesterol Secosterol Aldehyde Adduction and Aggregation of Cu,Zn-Superoxide Dismutase: Potential Implications in ALS. Redox Biol. 2018, 19, 105–115. [Google Scholar] [CrossRef]
- Miyamoto, S.; Lima, R.S.; Inague, A.; Viviani, L.G. Electrophilic Oxysterols: Generation, Measurement and Protein Modification. Free Radic. Res. 2021, 55, 416–440. [Google Scholar] [CrossRef]
- Dantas, L.S.; Viviani, L.G.; Inague, A.; Piccirillo, E.; de Rezende, L.; Ronsein, G.E.; Augusto, O.; Medeiros, M.H.G.; do Amaral, A.T.; Miyamoto, S. Lipid Aldehyde Hydrophobicity Affects Apo-SOD1 Modification and Aggregation. Free Radic. Biol. Med. 2020, 156, 157–167. [Google Scholar] [CrossRef]
- Wachtel, E.; Bach, D.; Epand, R.F.; Tishbee, A.; Epand, R.M. A Product of Ozonolysis of Cholesterol Alters the Biophysical Properties of Phosphatidylethanolamine Membranes. Biochemistry 2006, 45, 1345–1351. [Google Scholar] [CrossRef]
- Bach, D.; Wachtel, E.; Miller, I.R. Kinetics of Schiff Base Formation between the Cholesterol Ozonolysis Product 3β-Hydroxy-5-Oxo-5,6-Secocholestan-6-al and Phosphatidylethanolamine. Chem. Phys. Lipids 2009, 157, 51–55. [Google Scholar] [CrossRef]
- Bach, D.; Epand, R.F.; Epand, R.M.; Miller, I.R.; Wachtel, E. The Oxysterol 3β-Hydroxy-5-Oxo-5,6-Secocholestan-6-al Changes the Phase Behavior and Structure of Phosphatidylethanolamine–Phosphatidylcholine Mixtures. Chem. Phys. Lipids 2011, 164, 672–679. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takayasu, B.S.; Martins, I.R.; Uemi, M.; Onuki, J.; Machado-Santelli, G.M. β-Secosterol, an Oxyphytosterol Produced Through the Reaction of β-Sitosterol with Ozone, Demonstrates Different Cytotoxic Effects on BRL-3A and HTC Cells. Biomolecules 2025, 15, 939. https://doi.org/10.3390/biom15070939
Takayasu BS, Martins IR, Uemi M, Onuki J, Machado-Santelli GM. β-Secosterol, an Oxyphytosterol Produced Through the Reaction of β-Sitosterol with Ozone, Demonstrates Different Cytotoxic Effects on BRL-3A and HTC Cells. Biomolecules. 2025; 15(7):939. https://doi.org/10.3390/biom15070939
Chicago/Turabian StyleTakayasu, Bianca S., Igor R. Martins, Miriam Uemi, Janice Onuki, and Glaucia M. Machado-Santelli. 2025. "β-Secosterol, an Oxyphytosterol Produced Through the Reaction of β-Sitosterol with Ozone, Demonstrates Different Cytotoxic Effects on BRL-3A and HTC Cells" Biomolecules 15, no. 7: 939. https://doi.org/10.3390/biom15070939
APA StyleTakayasu, B. S., Martins, I. R., Uemi, M., Onuki, J., & Machado-Santelli, G. M. (2025). β-Secosterol, an Oxyphytosterol Produced Through the Reaction of β-Sitosterol with Ozone, Demonstrates Different Cytotoxic Effects on BRL-3A and HTC Cells. Biomolecules, 15(7), 939. https://doi.org/10.3390/biom15070939






