Ultrastructural Aspects of Physiological Mineralization: A Comparative Study in Different Hard Tissues
Abstract
1. Introduction
2. Materials and Methods
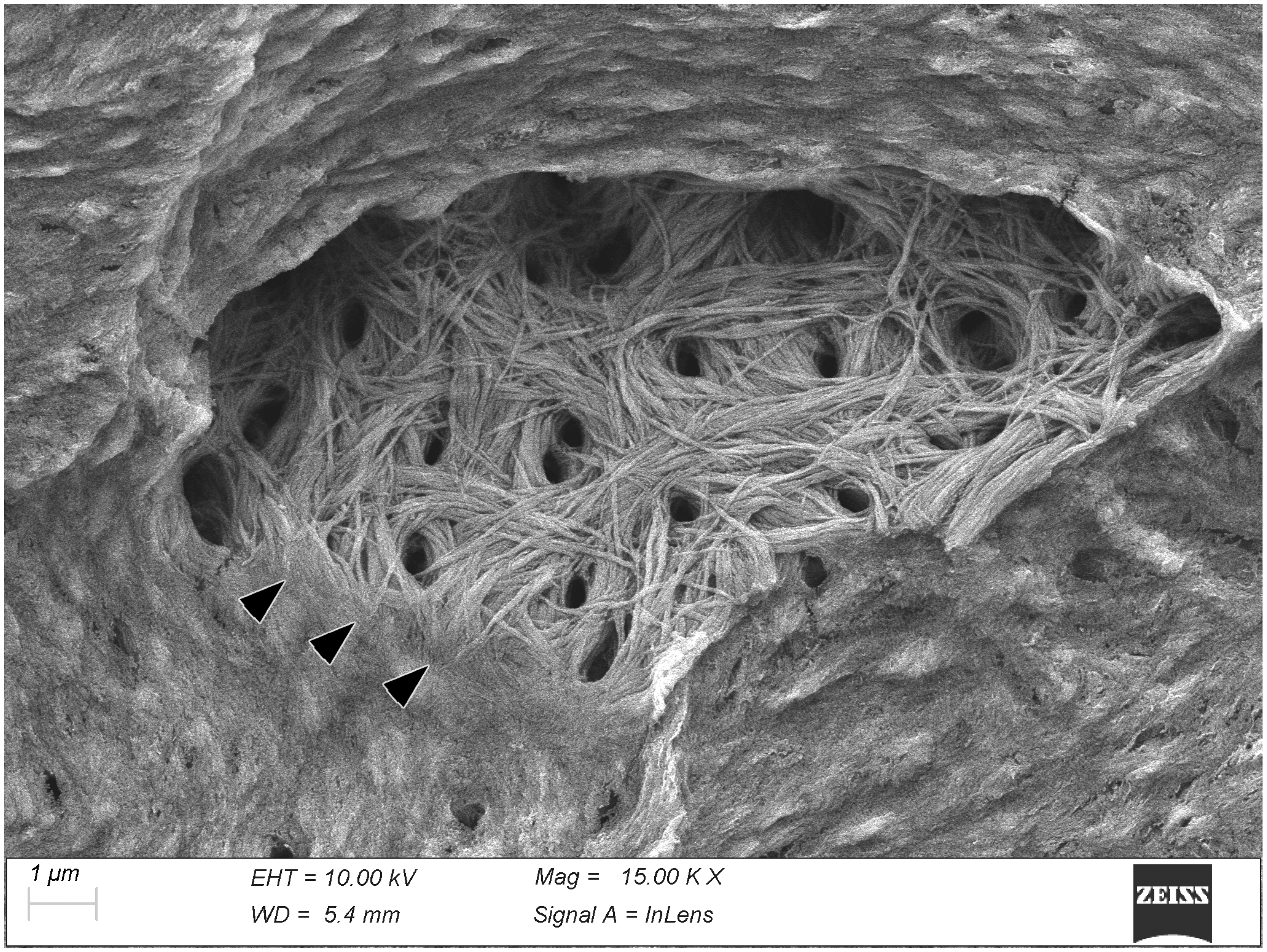
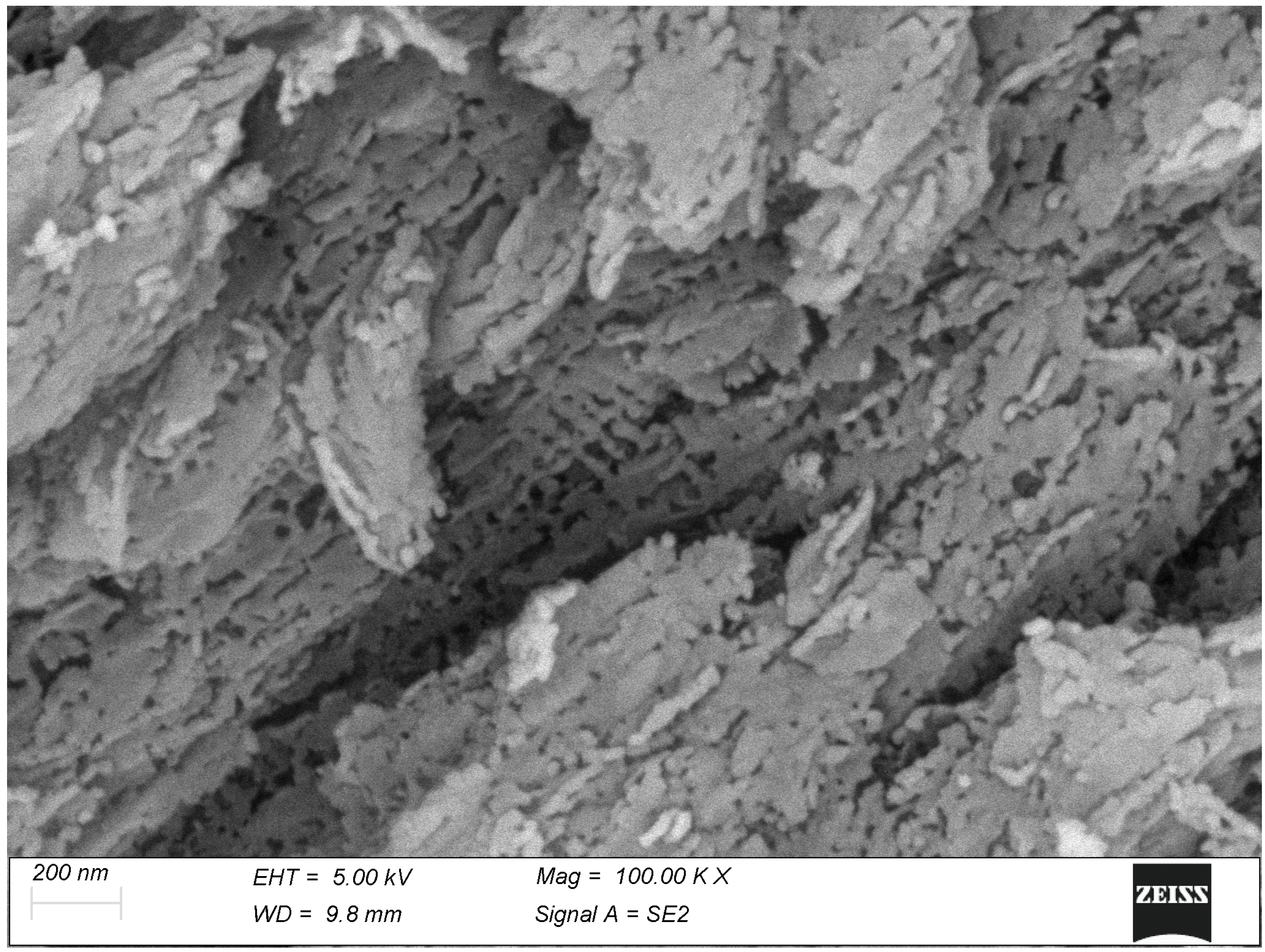
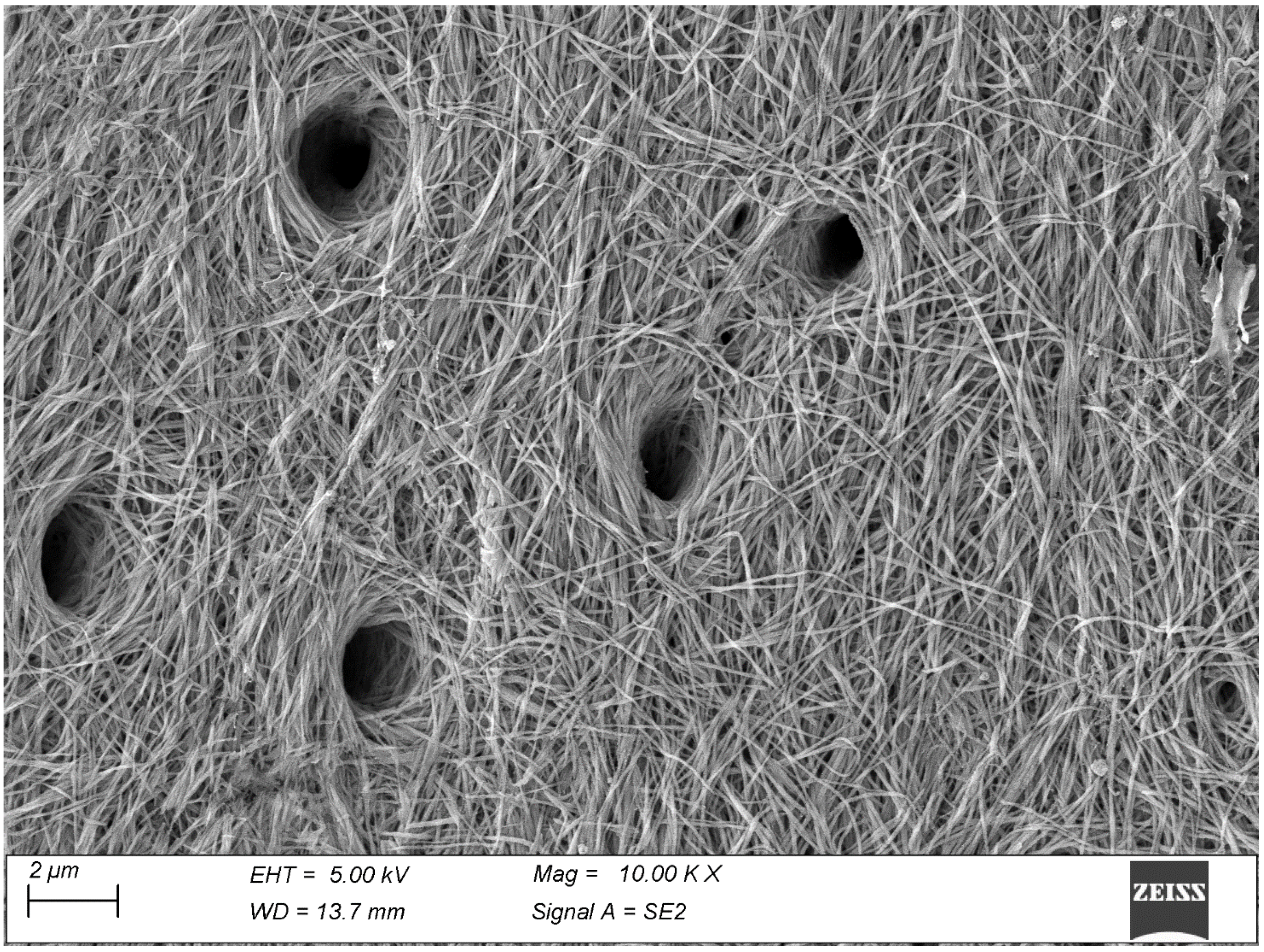
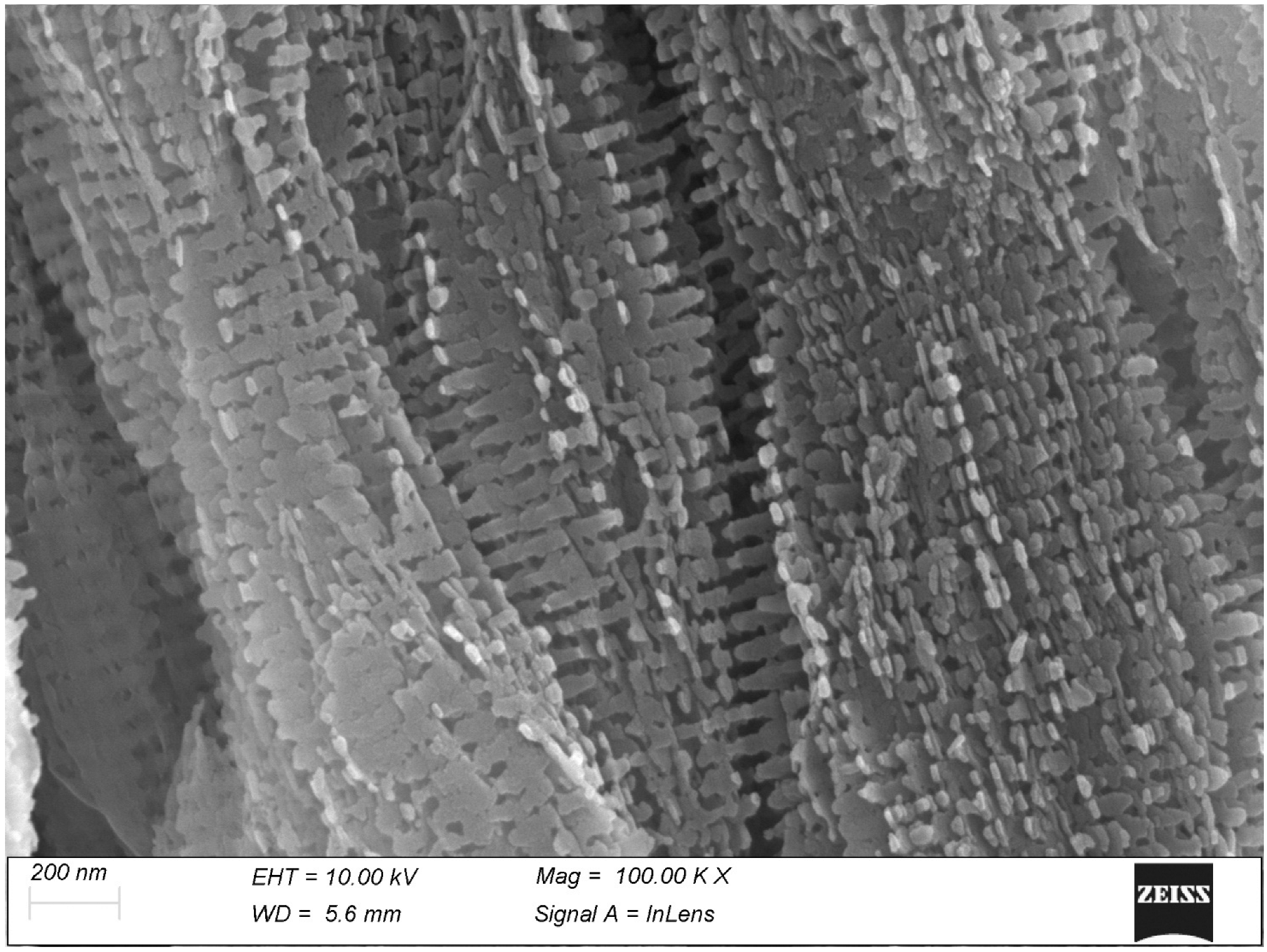
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shahar, R.; Weiner, S. Open questions on the 3D structures of collagen containing vertebrate mineralized tissues: A perspective. J. Struct. Biol. 2018, 201, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Lausch, A.J.; Quan, B.C.; Miklas, J.W.; Sone, E.D. Extracellular Matrix Control of Collagen Mineralization In Vitro. Adv. Funct. Mater. 2013, 23, 4906–4912. [Google Scholar] [CrossRef]
- Reznikov, N.; Hoac, B.; Buss, D.J.; Addison, W.N.; Barros, N.M.T.; McKee, M.D. Biological stenciling of mineralization in the skeleton: Local enzymatic removal of inhibitors in the extracellular matrix. Bone 2020, 138, 115447. [Google Scholar] [CrossRef] [PubMed]
- Oosterlaken, B.M.; Vena, M.P.; de With, G. In Vitro Mineralization of Collagen. Adv. Mater. 2021, 33, 2004418. [Google Scholar] [CrossRef]
- Christoffersen, J.; Landis, W.J. A contribution with review to the description of mineralization of bone and other calcified tissues in vivo. Anat. Rec. 1991, 230, 435–450. [Google Scholar] [CrossRef]
- Landis, W.J.; Hodgens, K.J.; McKee, M.D.; Nanci, A.; Song, M.J.; Kivonaga, S.; Arena, J.; McEwen, B.F. Extracellular vesiscles of calcifying turkey leg tendon characterized by immunocytochemistry and high voltage electron microscopic tomography and 3D graphic image reconstruction. Bone Miner. 1992, 17, 237–241. [Google Scholar] [CrossRef]
- Mohr, W.; Görz, E. Does arteriosclerotic calcinosis of vessel walls imitate osteogenesis? Pathomorphological studies of arteriosclerotic plaque. Z. Kardiol. 2002, 91, 212–232. [Google Scholar] [CrossRef]
- Kirsch, T. Determinants of pathological mineralization. Curr. Opin. Rheumatol. 2006, 18, 174–180. [Google Scholar] [CrossRef]
- Duer, M.J.; Friscić, T.; Proudfoot, D.; Reid, D.G.; Schoppet, M.; Shanahan, C.M.; Skepper, J.N.; Wise, E.R. Mineral surface in calcified plaque is like that of bone: Further evidence for regulated mineralization. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 2030–2034. [Google Scholar] [CrossRef]
- Kim, K.M. Apoptosis and Calcification. Scanning Microsc. 1995, 9, 1137–1175. [Google Scholar]
- Li, M.; Wang, Z.W.; Fang, L.J.; Cheng, S.Q.; Wang, X.; Liu, N.F. Programmed cell death in atherosclerosis and vascular calcification. Cell Death Dis. 2022, 13, 467. [Google Scholar] [CrossRef]
- Bonetti, A.; Marchini, M.; Ortolani, F. Ectopic mineralization in heart valves: New insights from in vivo and in vitro procalcific models and promising perspectives on noncalcifiable bioengineered valves. J. Thorac. Dis. 2019, 11, 2126–2143. [Google Scholar] [CrossRef] [PubMed]
- Bonetti, A.; Contin, M.; Marchini, M.; Ortolani, F. Ultrastructural and Immunohistochemical Detection of Hydroxyapatite Nucleating Role by rRNA and Nuclear Chromatin Derivatives in Aortic Valve Calcification: In Vitro and In Vivo Pro-Calcific Animal Models and Actual Calcific Disease in Humans. Int. J. Mol. Sci. 2023, 24, 2667. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, K.; Buchanan, A.V.; Weiss, K.M. Biomineralization in Humans: Making the Hard Choices in Life. Annu. Rev. Genet. 2009, 43, 119–142. [Google Scholar] [CrossRef] [PubMed]
- Landis, W.J. Mineral characterization in calcifying tissues: Atomic, molecular and macromolecular perspectives. Connect. Tissue Res. 1996, 34, 239–246. [Google Scholar] [CrossRef]
- Reznikov, N.; Bilton, M.; Lari, L.; Stevens, M.M.; Kröger, R. Fractal-like hierarchical organization of bone begins at the nanoscale. Science 2018, 360, 507. [Google Scholar] [CrossRef]
- Xu, Y.F.; Nudelman, F.; Eren, E.D.; Wirix, M.J.M.; Cantaert, B.; Nijhuis, W.H.; Hermida-Merino, D.; Portale, G.; Bomans, P.H.H.; Ottmann, C.; et al. Intermolecular channels direct crystal orientation in mineralized collagen. Nat. Commun. 2020, 11, 5068. [Google Scholar] [CrossRef]
- Landis, W.J.; Song, M.J.; Leith, A.; McEwen, L.; McEwen, B.F. Mineral and organic matrix interaction in normally calcifying tendon visualized in three dimensions by high-voltage electron microscopic tomography and graphic image reconstruction. J. Struct. Biol. 1993, 110, 39–54. [Google Scholar] [CrossRef]
- Siperko, L.M.; Landis, W.J. Aspects of mineral structure in normally calcifying avian tendon. J. Struct. Biol. 2001, 135, 313–320. [Google Scholar] [CrossRef]
- Landis, W.J.; Silver, F.H. The structure and function of normally mineralizing avian tendons. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2002, 133, 1135–1157. [Google Scholar] [CrossRef]
- Landis, W.J.; Hodgens, K.J.; Arena, J.; Song, M.J.; McEwen, B.F. Structural relations between collagen and mineral in bone as determined by high voltage electron microscopic tomography. Micros. Res. Tech. 1996, 33, 192–202. [Google Scholar] [CrossRef]
- Sorokina, L.; Shahbazian-Yassar, R.; Shokhufar, T. Collagen biomineralization: Pathways, mechanisms and thermodynamics. Emergent Mater. 2021, 4, 1025–1224. [Google Scholar] [CrossRef]
- Buss, D.J.; Rechav, K.; Reznikov, N.; McKee, M.D. Mineral tessellation in mouse enthesis fibrocartilage, Achilles tendon, and Hyp calcifying enthesopathy: A shared 3D mineralization pattern. Bone 2023, 174, 116818. [Google Scholar] [CrossRef] [PubMed]
- McKee, M.D.; Buss, D.J.; Reznikov, N. Mineral tessellation in bone and the stenciling principle for extracellular matrix mineralization. J. Struct. Biol. 2022, 214, 107823. [Google Scholar] [CrossRef]
- Micheletti, C.; Hurley, A.; Gourrier, A.; Palmquist, A.; Tang, T.; Shah, F.A.; Grandfield, K. Bone mineral organization at the mesoscale: A review of mineral ellipsoids in bone and at bone interfaces. Acta Biomater. 2022, 142, 1–13. [Google Scholar] [CrossRef]
- Kirsch, T.; Nah, H.-D.; Shapiro, I.M.; Pacifici, M. Regulated Production of Mineralization-competent Matrix Vesicles in Hypertrophic Chondrocytes. J. Cell Biol. 1997, 137, 1149–1160. [Google Scholar] [CrossRef]
- Kirsch, T.; Claassen, H. Matrix vesicles mediate mineralization of human thyroid cartilage. Calcif. Tissue Int. 2000, 66, 292–297. [Google Scholar] [CrossRef]
- Azoidis, I.; Cox, S.C.; Davies, O.G. The role of extracellular vesicles in biomineralisation: Current perspective and application in regenerative medicine. Tissue Eng. 2018, 9, 2041731418810130. [Google Scholar] [CrossRef]
- Yi, G.; Ma, Y.; Chen, Y.; Yang, X.; Yang, B.; Tian, W. A review of the functions of matrix vesicles in periodontal tissues. Stem Cells Dev. 2021, 30, 165–176. [Google Scholar] [CrossRef]
- Boyan, B.D.; Asmussen, N.C.; Lin, Z.; Schwartz, Z. The Role of Matrix-Bound Extracellular Vesicles in the Regulation of Endochondral Bone Formation. Cells 2022, 11, 1619. [Google Scholar] [CrossRef]
- Iwayama, T.; Bhongsatiern, P.; Takedachi, M.; Murakami, S. Matrix Vesicle–Mediated Mineralization and Potential Applications. J. Dent. Res. 2022, 101, 1554–1562. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, T.; Hongo, H.; Yamamoto, T.; Abe, M.; Yoshino, H.; Haraguchi-Kitakamae, M.; Ishizu, H.; Shimizu, T.; Iwasaki, N.; Amizuka, N. Matrix Vesicle-Mediated Mineralization and Osteocytic Regulation of Bone Mineralization. Int. J. Mol. Sci. 2022, 23, 9941. [Google Scholar] [CrossRef] [PubMed]
- Veschi, E.A.; Bolean, M.; da Silva Andrilli, L.H.; Gobbi Sebinelli, H.; Strzelecka-Kiliszek, A.; Bandorowicz-Pikula, J.; Pikula, S.; Granjon, T.; Mebarek, S.; Magne, D.; et al. Mineralization Profile of Annexin A6-Harbouring Proteoliposomes: Shedding Light on the Role of Annexin A6 on Matrix Vesicle-Mediated Mineralization. Int. J. Mol. Sci. 2022, 23, 8945. [Google Scholar] [CrossRef] [PubMed]
- Jaroszewicz, J.; Bazarnik, P.; Osiecka-Iwan, A.; Hyc, A.; Choinska, E.; Chlanda, A.; Swieszkowski, W.; Moskalewski, S. From Matrix Vesicles to Miniature Rocks: Evolution of Calcium Deposits in Calf Costochondral Junctions. Cartilage 2021, 13, 326S–335S. [Google Scholar] [CrossRef] [PubMed]
- Zecca, P.A.; Reguzzoni, M.; Protasoni, M.; Raspanti, M. The chondro-osseous junction of articular cartilage. Tissue Cell 2023, 80, 101993. [Google Scholar] [CrossRef]
- Jaroszewicz, J.; Kosowska, A.; Hutmacher, D.; Swieszkowski, W.; Moskalewski, S. Insight into characteristic features of cartilage growth plate as a physiological template for bone formation. J. Biomed. Mater. Res. A 2016, 104, 357–366. [Google Scholar] [CrossRef]
- Zecca, P.A.; Reguzzoni, M.; Borgese, M.; Protasoni, M.; Filibian, M.; Raspanti, M. Investigating the Interfaces of the Epiphyseal Plate: An Integrated Approach of Histochemistry, Microtomography and SEM. J. Anat. 2023, 243, 870–877. [Google Scholar] [CrossRef]
- Lories, R.J.; Luyten, F.P. The bone-cartilage unit in osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 43–49. [Google Scholar] [CrossRef]
- Zhou, X.; Cao, H.; Yuan, Y.; Wu, W. Biochemical Signals Mediate the Crosstalk between Cartilage and Bone in Osteoarthritis. BioMed Res. Int. 2020, 6, 5720360. [Google Scholar] [CrossRef]
- Raspanti, M.; Guizzardi, S.; De Pasquale, V.; Martini, D.; Ruggeri, A. Ultrastructure of heat-deproteinated compact bone. Biomaterials 1994, 15, 433–437. [Google Scholar] [CrossRef]
- Raspanti, M.; Cesari, C.; De Pasquale, V.; Ottani, V.; Strocchi, R.; Zucchelli, G.; Ruggeri, A. A histological and electron-microscopic study of the architecture and ultrastructure of human periodontal tissues. Arch. Oral. Biol. 2000, 45, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, H. The functional significance of dentin sialoprotein-phosphophoryn and dentin sialoprotein (Review). Int. J. Oral. Sci. 2018, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- Anada, R.; Satoshi Hara, E.; Nagaoka, N.; Okada, M.; Kamioka, H.; Matsumoto, T. Important roles of odontoblast membrane phospholipids in early dentin mineralization. J. Mater. Chem. B 2023, 11, 657–666. [Google Scholar] [CrossRef]
- Silver, F.H.; Landis, W.J. Deposition of apatite in mineralizing vertebrate extracellular matrices: A model of possible nucleation sites on type I collagen. Connect. Tissue Res. 2011, 52, 242–254. [Google Scholar] [CrossRef] [PubMed]
- Ortolani, F.; Giordano, M.; Marchini, M. A model for type II collagen fibrils. Distinctive D-band patterns in native and reconstituted fibrils compared with sequence data for helix and telopeptide domains. Biopolymers 2000, 54, 448–463. [Google Scholar] [CrossRef]
- Antipova, O.; Orgel, J.P.R.O. In Situ D-periodic Molecular Structure of Type II Collagen. J. Biol. Chem. 2010, 285, 7087–7096. [Google Scholar] [CrossRef]
- Müller, K.H.; Hayward, R.; Rajan, R.; Whitehead, M.; Cobb, A.M.; Ahmad, S.; Sun, M.; Goldberga, I.; Li, R.; Bashtanova, U.; et al. Poly(ADP-Ribose) Links the DNA Damage Response and Biomineralization. Cell Rep. 2019, 27, 3124–3138. [Google Scholar] [CrossRef]
- Zecca, M.A.; Greer, H.F.; Duer, M.J. Poly(ADP-ribose) binding sites on collagen I fibrils for nucleating intrafibrillar bone mineral. bioRxiv 2024. [Google Scholar] [CrossRef]
- Gordon, M.K.; Gerecke, D.R.; Dublet, B.; van der Rest, M.; Olsen, B. Type XII Collagen: A Large Multidomain Molecule With Partial Homology to Type IX Collagen. J. Biol. Chem. 1989, 264, 19772–19778. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borgese, M.; Raspanti, M.; Protasoni, M.; Zecca, P.A.; Ortolani, F.; Reguzzoni, M. Ultrastructural Aspects of Physiological Mineralization: A Comparative Study in Different Hard Tissues. Biomolecules 2025, 15, 932. https://doi.org/10.3390/biom15070932
Borgese M, Raspanti M, Protasoni M, Zecca PA, Ortolani F, Reguzzoni M. Ultrastructural Aspects of Physiological Mineralization: A Comparative Study in Different Hard Tissues. Biomolecules. 2025; 15(7):932. https://doi.org/10.3390/biom15070932
Chicago/Turabian StyleBorgese, Marina, Mario Raspanti, Marina Protasoni, Piero Antonio Zecca, Fulvia Ortolani, and Marcella Reguzzoni. 2025. "Ultrastructural Aspects of Physiological Mineralization: A Comparative Study in Different Hard Tissues" Biomolecules 15, no. 7: 932. https://doi.org/10.3390/biom15070932
APA StyleBorgese, M., Raspanti, M., Protasoni, M., Zecca, P. A., Ortolani, F., & Reguzzoni, M. (2025). Ultrastructural Aspects of Physiological Mineralization: A Comparative Study in Different Hard Tissues. Biomolecules, 15(7), 932. https://doi.org/10.3390/biom15070932






