New Benzimidazole 3′-Deoxynucleosides: Synthesis and Antiherpes Virus Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. General Procedures
2.2. Enzymatic Reactions
2.3. Nucleosides Synthesis
2.3.1. 1-(β-D-3′-Deoxyribofuranosyl)benzimidazole (9)
2.3.2. 5,6-Difluoro-1-(β-D-3′-deoxyribofuranosyl)benzimidazole (10)
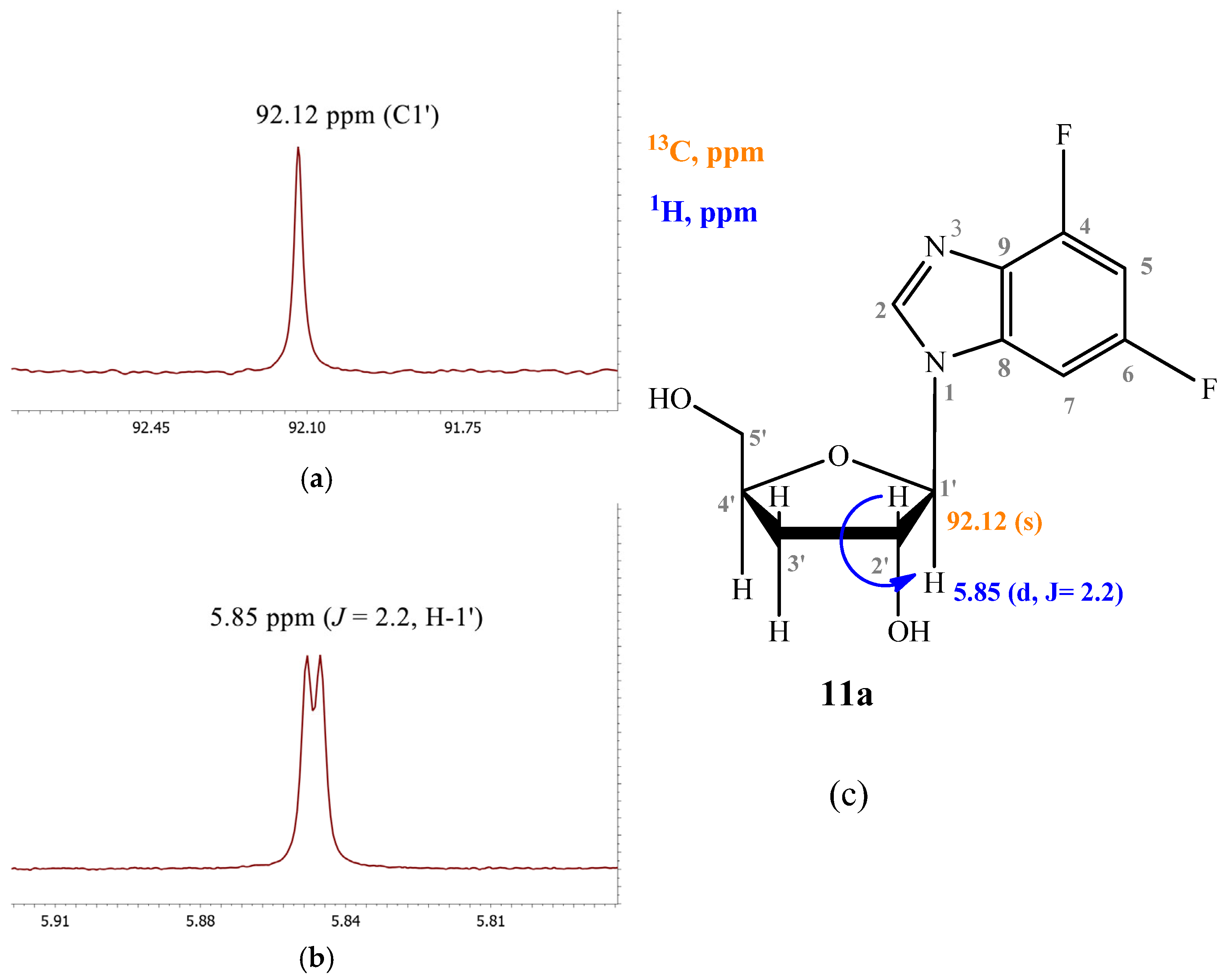
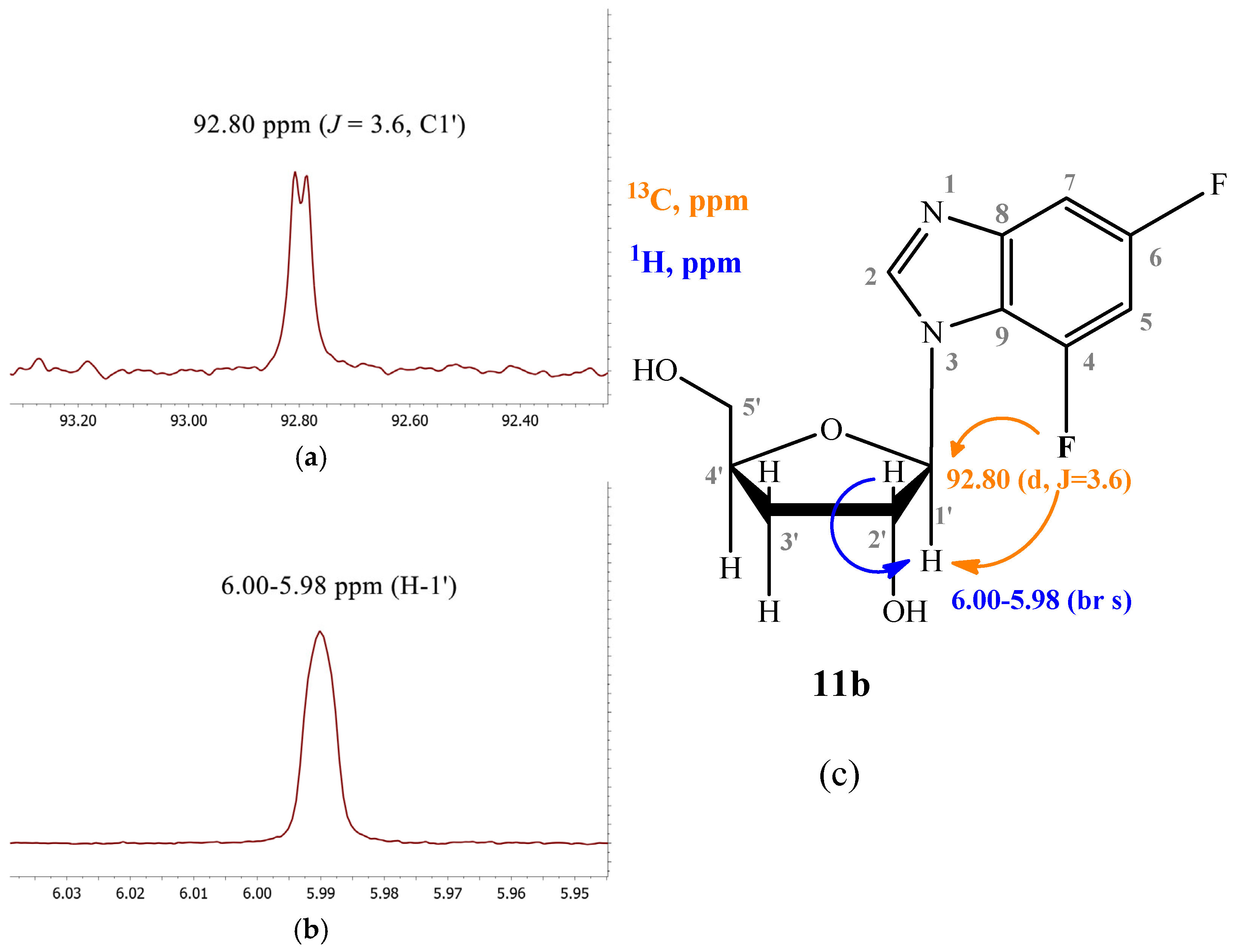
2.3.3. 4,6-Difluoro-1-(β-D-3′-deoxyribofuranosyl)benzimidazole (11)
2.3.4. 4,5,6-Trifluoro-1-(β-D-3′-deoxyribofuranosyl)benzimidazole (12)
2.3.5. 4,6-Difluoro-5-metoxy-1-(β-D-3′-deoxyribofuranosyl)benzimidazole (13)
2.3.6. 2-Amino-5,6-difluoro-1-(β-D-3′-deoxyribofuranosyl)benzimidazole (16)
2.4. Antiviral Activity and Cytotoxicity
3. Results and Discussion
3.1. Enzymatic Synthesis
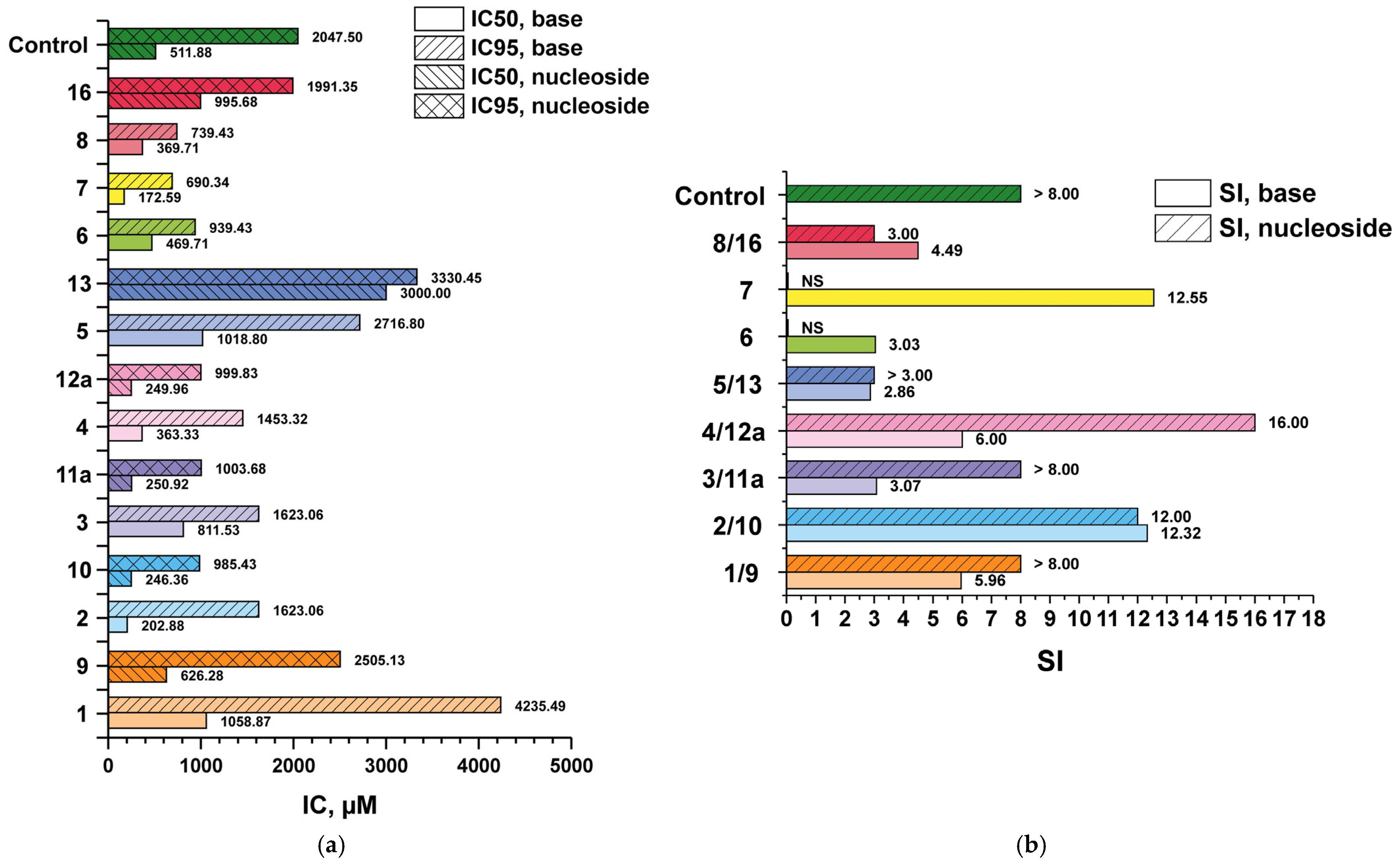
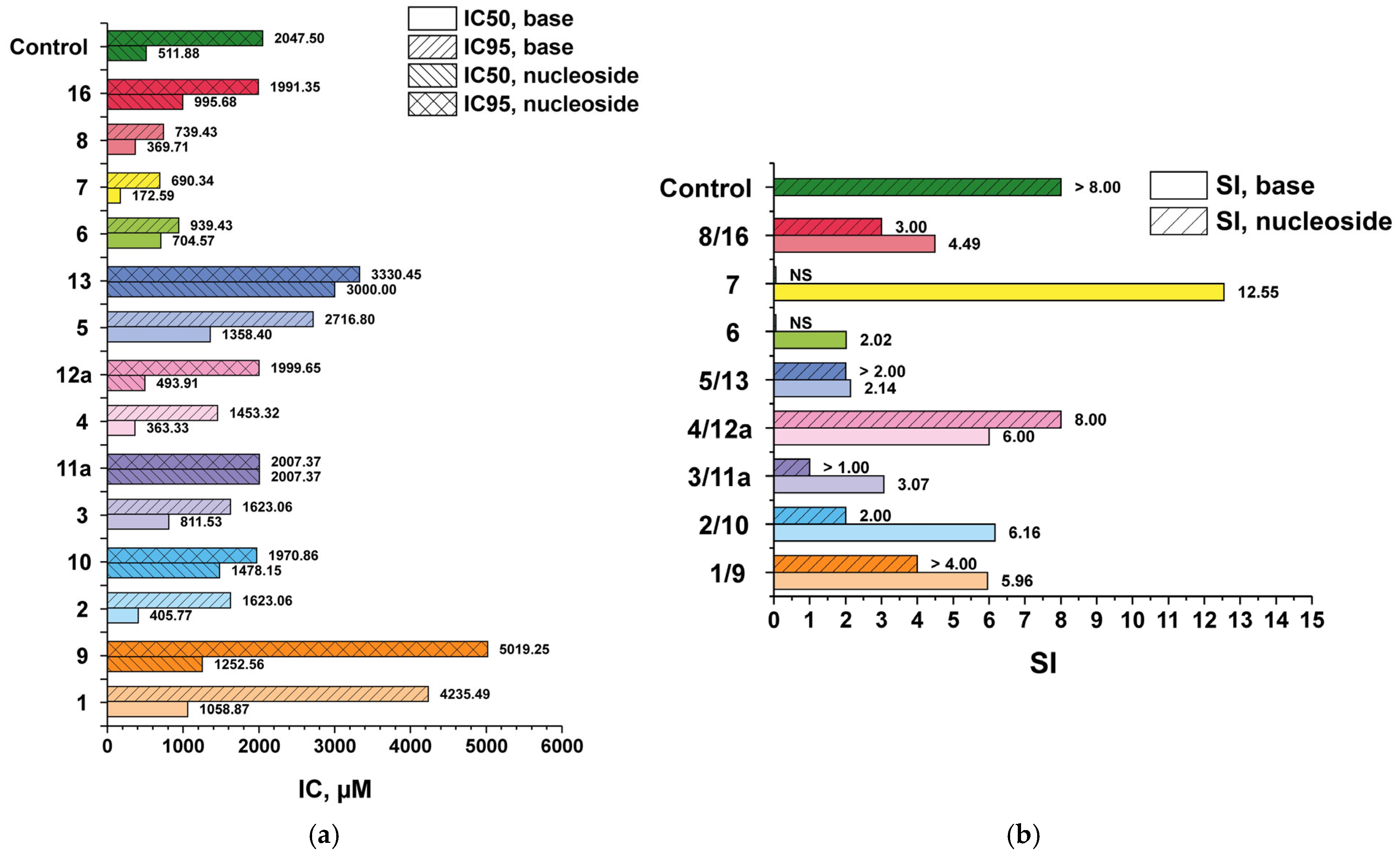
3.2. In Vitro Antiviral Activity of 3′-Deoxyribosides of 1–8
3.3. Antiviral Activity—Comparative Data
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PNP | purine nucleoside phosphorylase |
| WHO | World Health Organization |
| FDA | Food and Drug Administration |
| HSV | herpes simplex virus |
| HCMV | human cytomegalovirus |
| EBV | Epstein–Barr virus |
| DRB | 5,6-dichloro-1-(β-D-ribofuranosyl)benzimidazole |
| TCRB | 2,5,6-trichloro-1-(β-D-ribofuranosyl)benzimidazole |
| BDCRB | 2-bromo-5,6-dichloro-1-(β-D-ribofuranosyl)benzimidazole |
| MBV | Maribavir |
| DMSO | dimethyl sulfoxide |
| Hyp | hypoxantine |
| Pi | inorganic phosphate |
| TK | thymidine kinase |
| MOI | multiplicity of infection |
| SI | selectivity index |
| CPE | cytopathic effect |
References
- Herpes Simplex Virus. Available online: https://www.who.int/ru/news-room/fact-sheets/detail/herpes-simplex-virus (accessed on 5 April 2023).
- Fatahzadeh, M.; Schwartz, R.A. Human herpes simplex virus infections: Epidemiology, pathogenesis, symptomatology, diagnosis, and management. J. Am. Acad. Dermatol. 2007, 57, 737–763. [Google Scholar] [CrossRef] [PubMed]
- Brady, R.C.; Bernstein, D.I. Treatment of herpes simplex virus infections. Antivir. Res. 2004, 61, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Sadowski, L.A.; Upadhyay, R.; Greeley, Z.W.; Margulies, B.J. Current Drugs to Treat Infections with Herpes Simplex Viruses-1 and -2. Viruses 2021, 13, 1228. [Google Scholar] [CrossRef] [PubMed]
- Schalkwijk, H.H.; Snoeck, R.; Andrei, G. Acyclovir resistance in herpes simplex viruses: Prevalence and therapeutic alternatives. Biochem. Pharmacol. 2022, 206, 115322. [Google Scholar] [CrossRef]
- Sherif, S.H. Synthesis, Characterization and Antibacterial Activity of Benzimidazole Derivatives. Saudi J. Humanit. Soc. Sci. 2021, 4, 14–19. [Google Scholar] [CrossRef]
- Yadav, S.; Narasimhan, B.; Lim, S.M.; Ramasamy, K.; Vasudevan, M.; Shah, S.A.A.; Mathur, A. Synthesis and evaluation of antimicrobial, antitubercular and anticancer activities of benzimidazole derivatives. Egypt. J. Basic App. Sci. 2018, 5, 100–109. [Google Scholar] [CrossRef]
- Srivastava, R.; Gupta, S.K.; Naaz, F.; Singh, A.; Singh, V.K.; Verma, R.; Singh, R.K. Synthesis, antibacterial activity, synergistic effect, cytotoxicity, docking and molecular dynamics of benzimidazole analogues. Comp. Biol. Chem. 2018, 76, 1–16. [Google Scholar] [CrossRef]
- Fei, F.; Zhou, Z.M. New substituted benzimidazole derivatives: A patent review (2010–2012). Expert Opin. Ther. Pat. 2014, 23, 1157–1179. [Google Scholar] [CrossRef]
- Marty, F.M.; Ljungman, P.; Papanicolaou, G.A.; Winston, D.J.; Chemaly, R.F.; Strasfeld, L.; Young, J.A.; Rodriguez, T.; Maertens, J.; Schmitt, M.; et al. Maribavir prophylaxis for prevention of cytomegalovirus disease in recipients of allogeneic stem-cell transplants: A phase 3, double-blind, placebo-controlled, randomised trial. Lancet Infect. Dis. 2011, 11, 284–292. [Google Scholar] [CrossRef]
- Prichard, M.N.; Frederick, S.L.; Daily, S.; Borysko, K.Z.; Townsend, L.B.; Drach, J.C.; Kern, E.R. Benzimidazole analogs inhibit human herpesvirus 6. Antimicrob. Agents Chemother. 2011, 55, 2442–2445. [Google Scholar] [CrossRef]
- Biron, K.K. Antiviral drugs for cytomegalovirus diseases. Antivir. Res. 2006, 71, 154–163. [Google Scholar] [CrossRef] [PubMed]
- De Clercq, E. Looking back in 2009 at the dawning of antiviral therapy now 50 years ago an historical perspective. Adv. Virus Res. 2009, 73, 1–53. [Google Scholar] [CrossRef]
- Tamm, I.; Folkers, K.; Shunk, C.H.; Horsfall, F.L. Inhibition of influenza virus multiplication by N-glycosides of benzimidazoles-N. J. Exp. Med. 1954, 99, 227–250. [Google Scholar] [CrossRef]
- Tamm, I.; Sehgal, P.B. Halobenzimidazole ribosides and RNA synthesis of cells and viruses. Adv. Virus Res. 1978, 22, 187–258. [Google Scholar] [CrossRef]
- Tamm, I.; Overman, J.R. Relationship between structure of benzimidazole derivatives and inhibitory activity on vaccinia virus multiplication. Virology 1957, 3, 185–196. [Google Scholar] [CrossRef]
- Pothier, P.; Dru, A.; Beaud, G. The inhibition of vaccinia virus replication by 5,6-dichloro-1-beta-D-ribofuranosylbenzimidazole (DRB): An effect at the assembly stage. J. Gen. Virol. 1981, 55, 87–94. [Google Scholar] [CrossRef]
- Bucknall, R.A. The effects of substituted benzimidazoles on the growth of viruses and the nucleic acid metabolism of host cells. J. Gen. Virol. 1967, 1, 89–99. [Google Scholar] [CrossRef]
- Townsend, L.B.; Devivar, R.V.; Turk, S.R.; Nassiri, M.R.; Drach, J.C. Design, synthesis, and antiviral activity of certain 2,5,6-trihalo-1-(beta-D-ribofuranosyl)benzimidazoles. J. Med. Chem. 1995, 38, 4098–4105. [Google Scholar] [CrossRef]
- Alain, S.; Revest, M.; Veyer, D.; Essig, M.; Rerolles, J.P.; Rawlinson, W.; Mengelle, C.; Huynh, A.; Kamar, N.; Garrigue, I.; et al. Maribavir use in practice for cytomegalovirus infection in French transplantation centers. Transpl. Proc. 2013, 45, 1603–1607. [Google Scholar] [CrossRef]
- Takeda Announces Publication of Data from SOLSTICE, a Pivotal Phase 3 Trial for LIVTENCITY™ (Maribavir) in Post-Transplant Recipients with Cytomegalovirus (CMV) Infection (Refractory, With or Without Resistance). Available online: https://www.takeda.com/newsroom/newsreleases/2021/publication-of-data-from-solstice/ (accessed on 8 December 2021).
- Livtencity (Maribavir). An Overview of Livtencity and Why It Is Authorised in the EU. Available online: https://www.ema.europa.eu/en/documents/overview/livtencity-epar-medicine-overview_en.pdf (accessed on 9 November 2022).
- Kazimierczuk, Z.; Dudyu, L.; Stolarski, R.; Shugar, D. Preparation of 1-α-D arabinofuranosylbenzimidazole and its 5,6-dichloro derivative, and the direct bromination of benzimidazole nucleosides. Z. Naturforsch. 1980, 35, 30–35. [Google Scholar] [CrossRef]
- Kissman, H.M.; Child, R.G.; Weiss, M.J. Synthesis and biological properties of certain dichlorobenzimidazole ribosides. J. Am. Chem. Soc. 1957, 79, 1185–1188. [Google Scholar] [CrossRef]
- Zou, R.; Kawashima, E.; Freeman, G.A.; Koszalka, G.W.; Drach, J.C.; Townsend, L.B. Design, synthesis, and antiviral evaluation of 2-deoxy-D-ribosides of substituted benzimidazoles as potential agents for human cytomegalovirus infections. Nucleosides Nucleotides Nucleic Acids 2000, 19, 125–153. [Google Scholar] [CrossRef] [PubMed]
- Shinde, V.S.; Lawande, P.P.; Sontakke, V.A.; Khan, A. Synthesis of benzimidazole nucleosides and their anticancer activity. Carbohydr. Res. 2020, 498, 108178. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, L.C.; Fernandes, E.; Marques, M.M. Developments towards regioselective synthesis of 1,2-disubstituted benzimidazoles. Chemistry 2011, 17, 12544–12555. [Google Scholar] [CrossRef]
- Ojima, I. Fluorine in Medicinal Chemistry and Chemical Biology; Wiley: New York, NY, USA, 2009. [Google Scholar]
- Kharitonova, M.I.; Antonov, K.V.; Fateev, I.V.; Berzina, M.Y.; Kaushin, A.L.; Paramonov, A.S.; Kotovskaya, S.K.; Andronova, V.L.; Konstantinova, I.D.; Galegov, A.G.; et al. Chemoenzymatic Synthesis of Modified 2′-Deoxy-2′-fluoro-β-Darabinofuranosyl Benzimidazoles and Evaluation of Their Activity Against Herpes Simplex Virus Type 1. Synthesis 2017, 49, 1043–1052. [Google Scholar] [CrossRef]
- Denisova, A.O.; Tokunova, Y.A.; Fateev, I.V.; Breslav, A.A.; Leonov, V.N.; Dorofeeva, E.V.; Lutonina, O.I.; Muzyka, I.S.; Esipov, R.S.; Kayushin, A.L.; et al. The Chemoenzymatic Synthesis of 2-Chloro- and 2-Fluorocordycepins. Synthesis 2017, 49, 4853–4860. [Google Scholar] [CrossRef]
- Esipov, R.S.; Gurevich, A.I.; Chuvikovsky, D.V.; Chupova, L.A.; Muravyova, T.I.; Miroshnikov, A.I. Overexpression of Escherichia coli genes encoding nucleoside phosphorylases in the pET/Bl21(DE3) system yields active recombinant enzymes. Protein Expr. Purif. 2002, 24, 56–60. [Google Scholar] [CrossRef]
- Fernández-Lucas, J.; Condezo, L.A.; Martinez-Lagos, F.; Sinisterra, J.V. Synthesis of 2′-deoxyibosylnucleosides using new 2′-deoxyribosyltransferase microorganism producers. Enzym. Microb. Technol. 2007, 40, 1147–1155. [Google Scholar] [CrossRef]
- Fresco-Taboada, A.; De La Mata, I.; Arroyo, M.; Fernández-Lucas, J. New insights on nucleoside 2′-deoxyribosyltransferases: A versatile biocatalyst for one-pot one-step synthesis of nucleoside analogs. Appl. Microbiol. Biotechnol. 2013, 97, 3773–3785. [Google Scholar] [CrossRef]
- Kaminski, P.A. Functional cloning, heterologous expression, and purification of two different N-deoxyribosyltransferases from Lactobacillus helveticus. J. Biol. Chem. 2002, 277, 14400–14407. [Google Scholar] [CrossRef]
- Mikhailopulo, I.A.; Kazimierczuk, Z.; Zinchenko, A.I.; Barai, V.N.; Romanova, V.V.; Eroshevskaya, L.A. Benzimidazoles in The Reaction of Enzymatic Transglycosylation. Nucleosides Nucleotides 1995, 14, 477–480. [Google Scholar] [CrossRef]
- Koellner, G.; Luic, M.; Shugar, D.; Saenger, W.; Bzowska, A. Crystal structure of the ternary complex of E. coli purine nucleoside phosphorilase with formycin B a structural analogue of the substrate inosine and phosphate (sulphate) at 2.1 Ao resolution. J. Mol. Biol. 1998, 280, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Bzowska, A.; Kulikowska, E.; Shugar, D. Purine nucleoside phosphorylases: Properties, functions and clinical aspects. Pharmacol. Ther. 2000, 88, 349–425. [Google Scholar] [CrossRef] [PubMed]
- Kharitonova, M.I.; Denisova, A.O.; Andronova, V.L.; Kayushin, A.L.; Konstantinova, I.D.; Kotovskaya, S.K.; Galegov, G.A.; Charushin, V.N.; Miroshnikov, A.I. New modified 2-aminobenzimidazole nucleosides: Synthesis and evaluation of their activity against herpes simplex virus type 1. Bioorg. Med. Chem. Lett. 2017, 27, 2484–2487. [Google Scholar] [CrossRef]
- Ostermeier, L.; Rosario, O.; Roland, W. The multifaceted effects of DMSO and high hydrostatic pressure on the kinetic constants of hydrolysis reactions catalyzed by α-chymotrypsin. Phys. Chem. Chem. Phys. 2020, 22, 16325–16333. [Google Scholar] [CrossRef]
- Ferreira, J.C.; Fadl, S.; Ilter, M.; Pekel, H.; Rezgui, R.; Sensoy, O.; Rabeh, W.M. Dimethyl sulfoxide reduces the stability but enhances catalytic activity of the main SARS-CoV-2 protease 3CLpro. FASEB J. 2021, 35, e21774. [Google Scholar] [CrossRef]
- Faulds, C.B.; Pérez-Boada, M.; Martínez, T.A. Influence of organic co-solvents on the activity and substrate specificity of feruloyl esterases. Bioresour. Technol. 2011, 102, 4962–4967. [Google Scholar] [CrossRef]
- Tanaka, T.; Hiroshi, M.; Takahisa, O. Substrate specificity of aqualysin I altered by an organic solvent, DMSO. Biosci. Biotechnol. Biochem. 1999, 63, 446–448. [Google Scholar] [CrossRef]
- Koellner, G.; Bzowska, A.; Wielgus-Kutrowska, B.; Luić, M.; Steiner, T.; Saenger, W.; Stȩpiński, J. Open and closed conformation of the E. coli purine nucleoside phosphorylase active center and implications for the catalytic mechanism. J. Mol. Biol. 2002, 315, 351–371. [Google Scholar] [CrossRef]
- Kharitonova, M.I.; Fateev, I.V.; Kayushin, A.L.; Konstantinova, I.D.; Kotovskaya, S.K.; Leontʹeva, V.L.; Galegov, G.A.; Charushin, V.N.; Miroshnikov, A.I. Chemo-Enzymatic Syntheses and Antiviral Evaluation of 5-substituted 4,6-difluorobenzimidazoles Ribo- and 2′-Deoxyribo-Nucleosides. Synthesis 2016, 48, 394–406. [Google Scholar] [CrossRef]
- Underwood, M.R.; Harvey, R.J.; Stanat, S.C.; Hemphill, M.L.; Miller, T.; Drach, J.C.; Townsend, L.B.; Biron, K.K. Inhibition of human cytomegalovirus DNA maturation by a benzimidazole ribonucleoside is mediated through the UL89 gene product. J. Virol. 1998, 72, 717–725. [Google Scholar] [CrossRef]
- Sidwell, R.W.; Huffman, J.H.; Khare, G.P.; Allen, L.B.; Witkowski, J.T.; Robins, R.K. Broad-spectrum antiviral activity of Virazole: 1-beta-D-ribofuranosyl-1,2,4-triazole-3-carboxamide. Science 1972, 177, 705–706. [Google Scholar] [CrossRef] [PubMed]

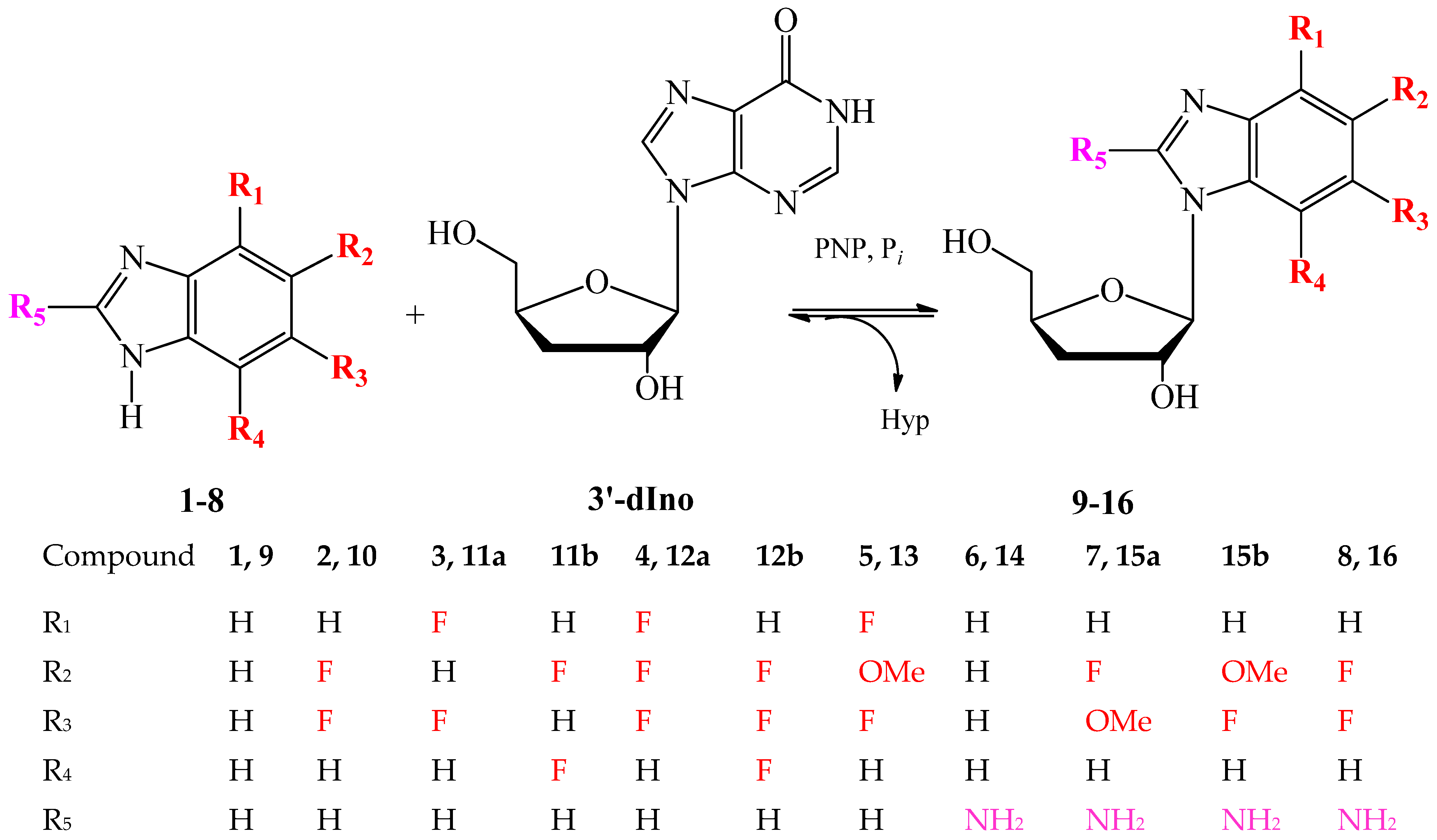
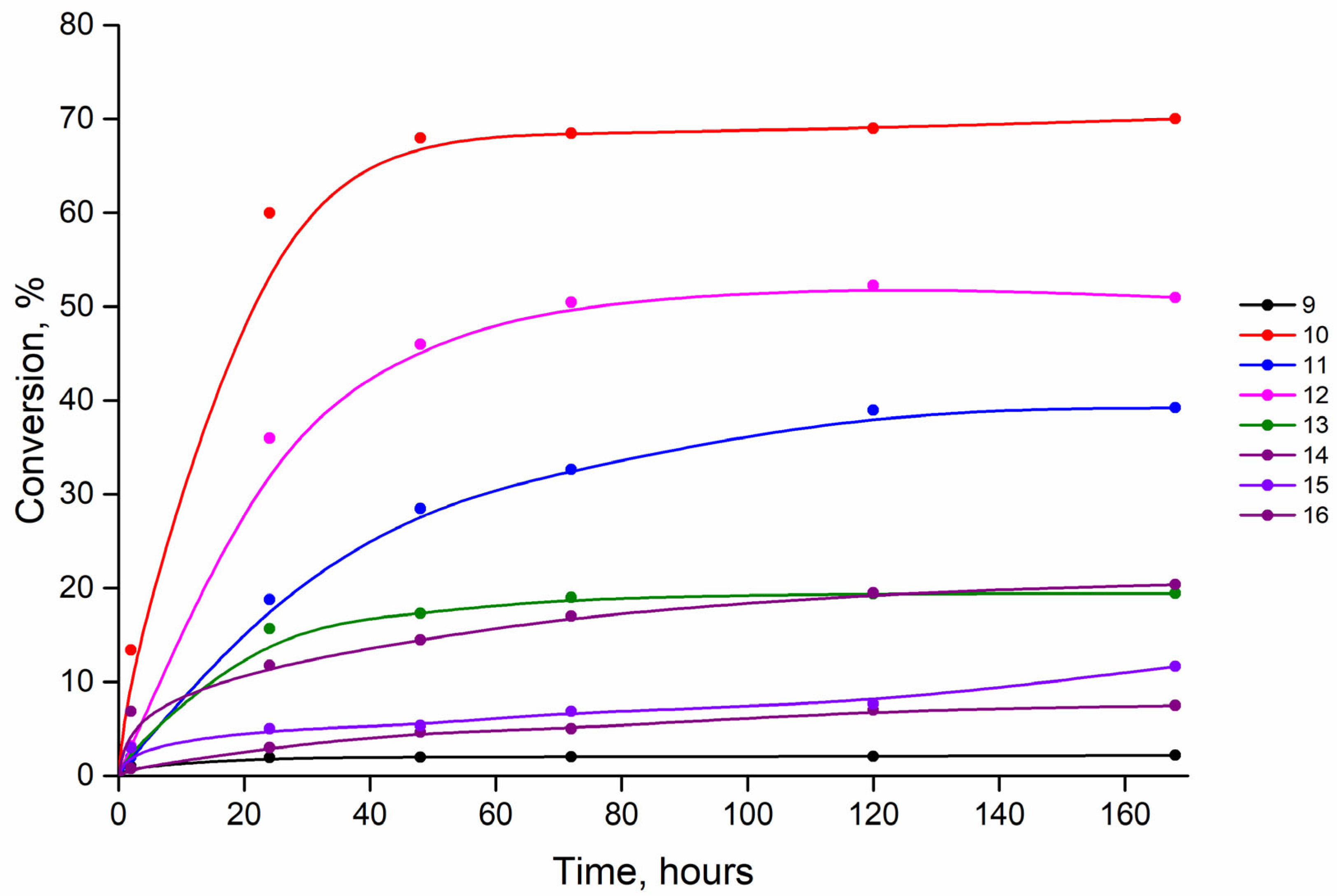
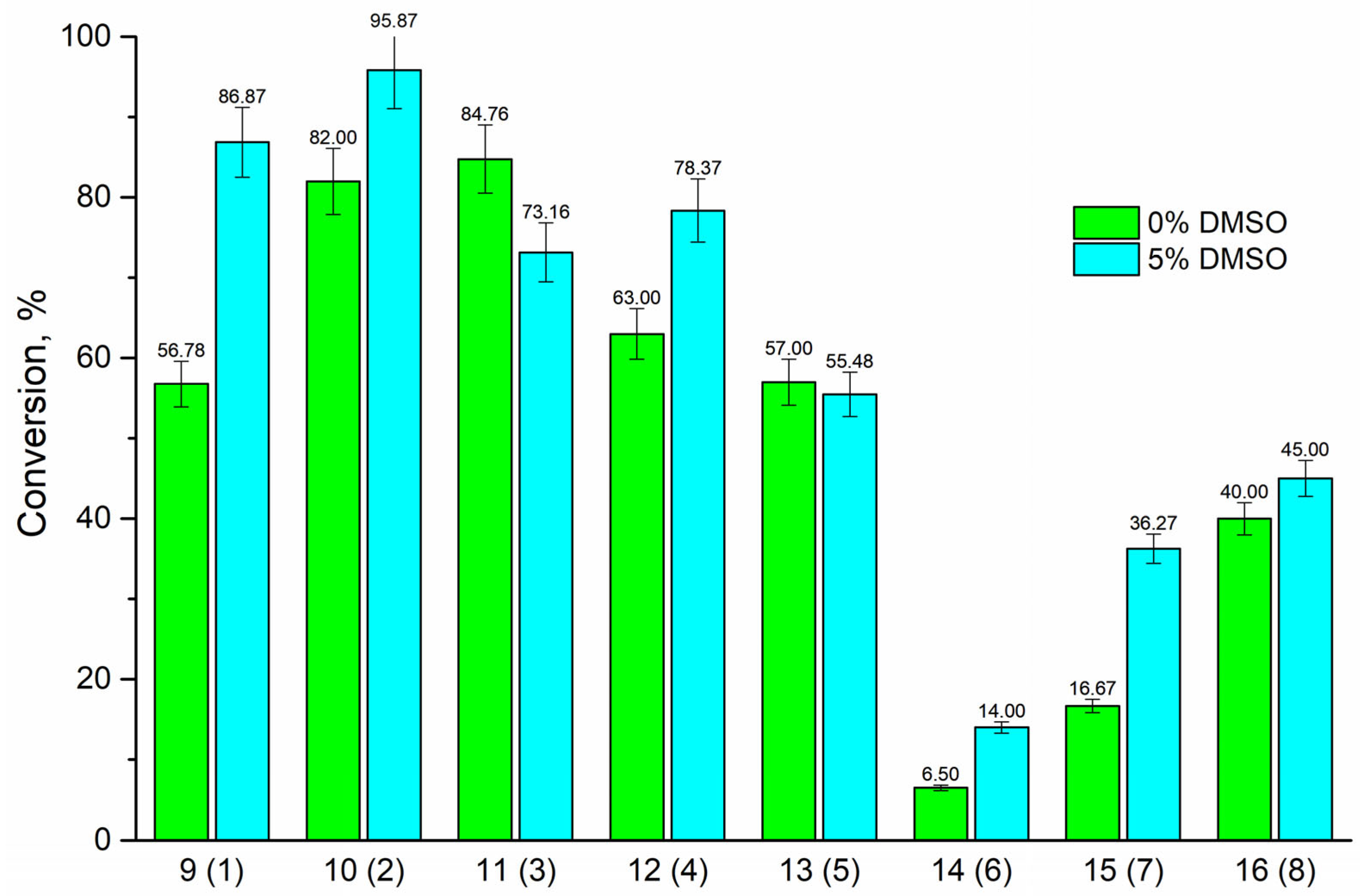


| Compound (Base) | Base Structure | Base: 3′-dIno Ratio (by Moles) | PNP Concentration (Units/mL) | Conversion, % in 168 h |
|---|---|---|---|---|
| 9 (1) | 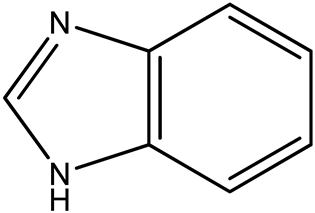 | 9:1 | 21 | 78 |
| 10 (2) | 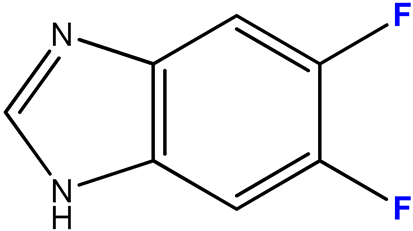 | 9:1 | 7 | 80 |
| 11 (3) | 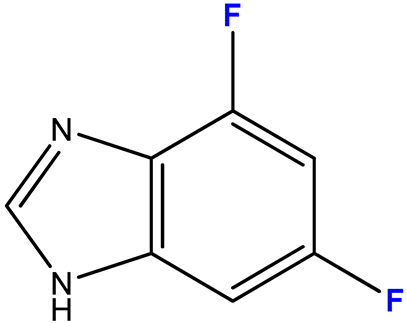 | 1:5 | 7 | 81 |
| 12 (4) | 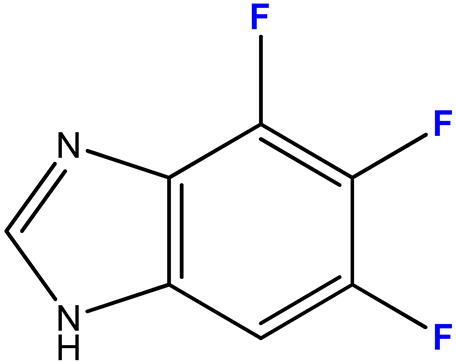 | 9:1 | 7 | 68 |
| 13 (5) | 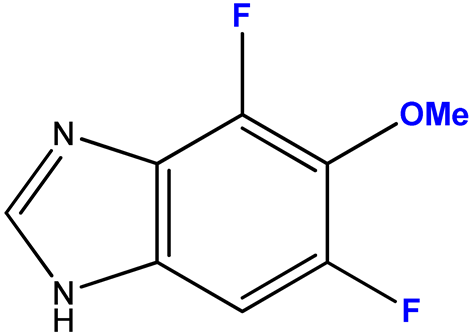 | 1:7 | 7 | 72 |
| 14 (6) | 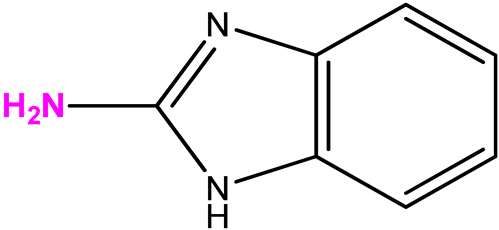 | 9:1 | 21 | 7 |
| 15 (7) | 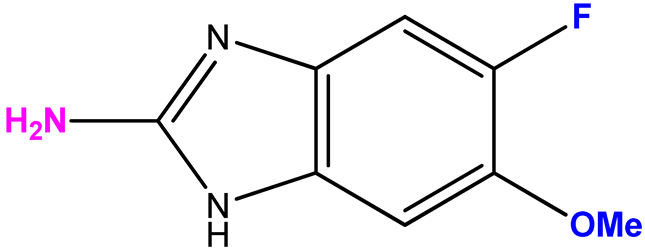 | 9:1 | 10.5 | 28 |
| 16 (8) | 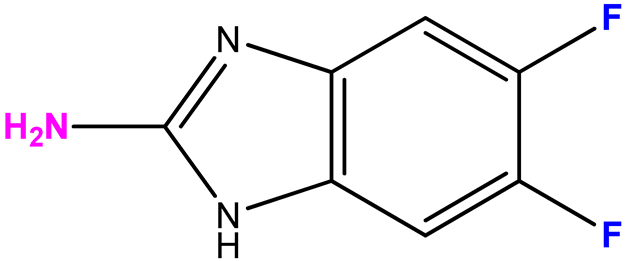 | 9:1 | 21 | 61 |
| Compound | Base, mg (mmol) | 3′-dIno, mg (mmol) | Ratio Base: 3′dIno (by Moles) | PNP, Units | Potassium Phosphate (pH 7.0)/Volume, mL | Time, Days | Isomer Ratio (HPLC Data) | Conversion/Yield, % (mg) | |
|---|---|---|---|---|---|---|---|---|---|
| N-1 | N-3 | ||||||||
| 9 | 1—271 (2.29) | 64 (0.25) | 9:1 | 4200 | 10 mM/200 | 20 | - | - | 54/27 (16) |
| 10 | 2—182.58 (1.18) | 100 (0.39) | 3:1 | 1400 | 10 mM/100 | 40 | - | - | 93/32 (33.2) |
| 11 | 3—25 (0.16) | 205(0.81) | 1:5 | 1400 | 6 mM/200 | 40 | 54 | 46 | 73/46 (7) |
| 12 | 4—110 (0.64) | 32.37 (0.13) | 5:1 | 1400 | 10 mM/100 | 40 | 73 | 27 | 79/46 (17) |
| 13 | 5—23 (0.12) | 215 (0.85) | 1:7 | 2100 | 6 mM/100 | 2 | - | - | 87/58 (20) |
| 16 | 8—601 (3.5) | 100 (0.4) | 9:1 | 3500 | 9 mM/200 | 40 | - | - | 62/8 (8.5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arnautova, A.O.; Aleksakhina, I.A.; Zorina, E.A.; Berzina, M.Y.; Fateev, I.V.; Eletskaya, B.Z.; Antonov, K.V.; Smirnova, O.S.; Paramonov, A.S.; Kayushin, A.L.; et al. New Benzimidazole 3′-Deoxynucleosides: Synthesis and Antiherpes Virus Properties. Biomolecules 2025, 15, 922. https://doi.org/10.3390/biom15070922
Arnautova AO, Aleksakhina IA, Zorina EA, Berzina MY, Fateev IV, Eletskaya BZ, Antonov KV, Smirnova OS, Paramonov AS, Kayushin AL, et al. New Benzimidazole 3′-Deoxynucleosides: Synthesis and Antiherpes Virus Properties. Biomolecules. 2025; 15(7):922. https://doi.org/10.3390/biom15070922
Chicago/Turabian StyleArnautova, Aleksandra O., Irina A. Aleksakhina, Ekaterina A. Zorina, Maria Ya. Berzina, Ilya V. Fateev, Barbara Z. Eletskaya, Konstantin V. Antonov, Olga S. Smirnova, Alexander S. Paramonov, Alexey L. Kayushin, and et al. 2025. "New Benzimidazole 3′-Deoxynucleosides: Synthesis and Antiherpes Virus Properties" Biomolecules 15, no. 7: 922. https://doi.org/10.3390/biom15070922
APA StyleArnautova, A. O., Aleksakhina, I. A., Zorina, E. A., Berzina, M. Y., Fateev, I. V., Eletskaya, B. Z., Antonov, K. V., Smirnova, O. S., Paramonov, A. S., Kayushin, A. L., Andronova, V. L., Galegov, G. A., Kostromina, M. A., Zayats, E. A., Karpenko, I. L., Kotovskaya, S. K., Charushin, V. N., Esipov, R. S., Miroshnikov, A. I., & Konstantinova, I. D. (2025). New Benzimidazole 3′-Deoxynucleosides: Synthesis and Antiherpes Virus Properties. Biomolecules, 15(7), 922. https://doi.org/10.3390/biom15070922









