The Role of SLPI Gene-Mediated Inflammation in Osteoarthritis
Abstract
1. Introduction
2. Osteoarthritis Overview
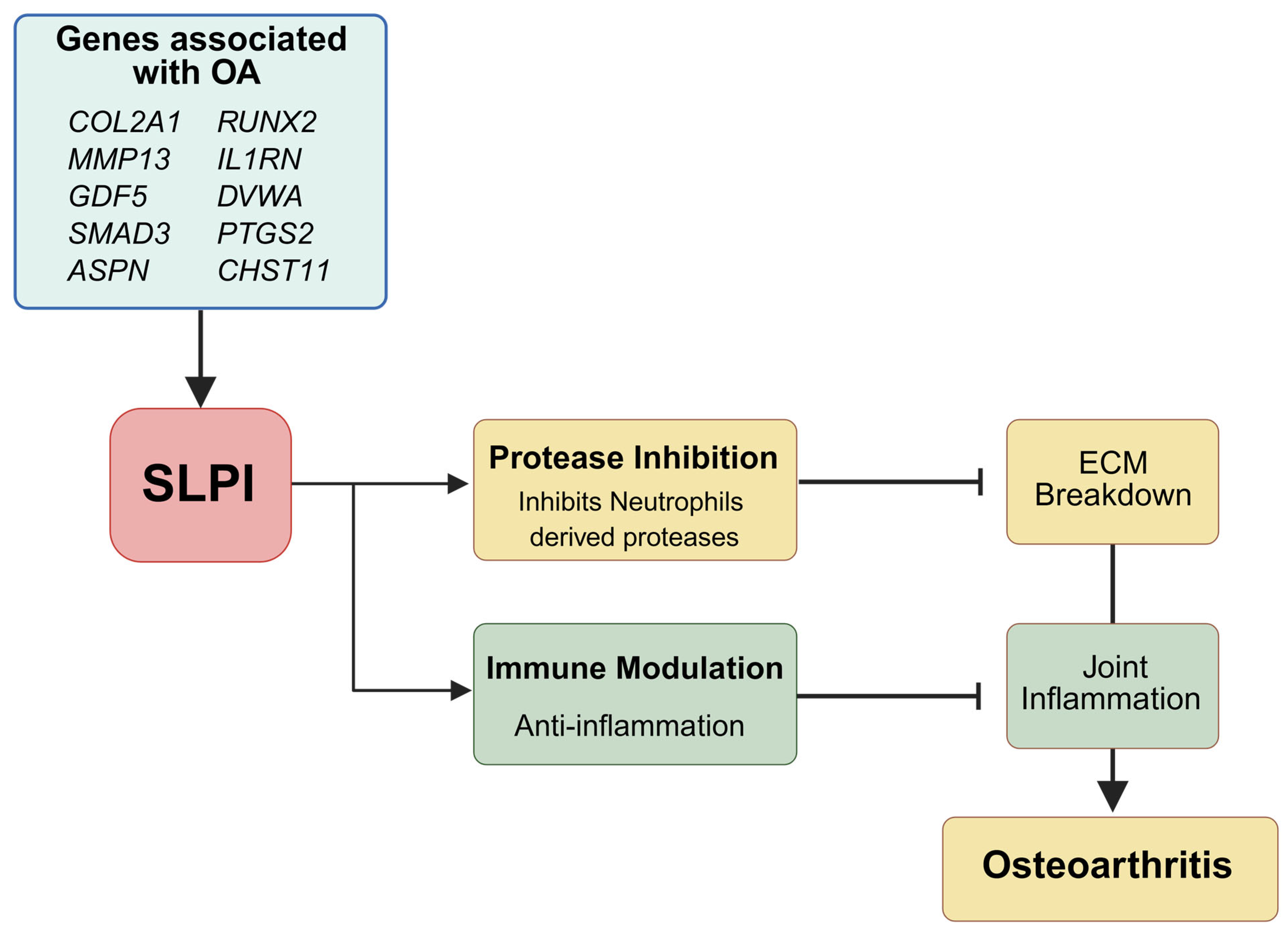
3. Molecular Basis of SLPI in Osteoarthritis (OA)
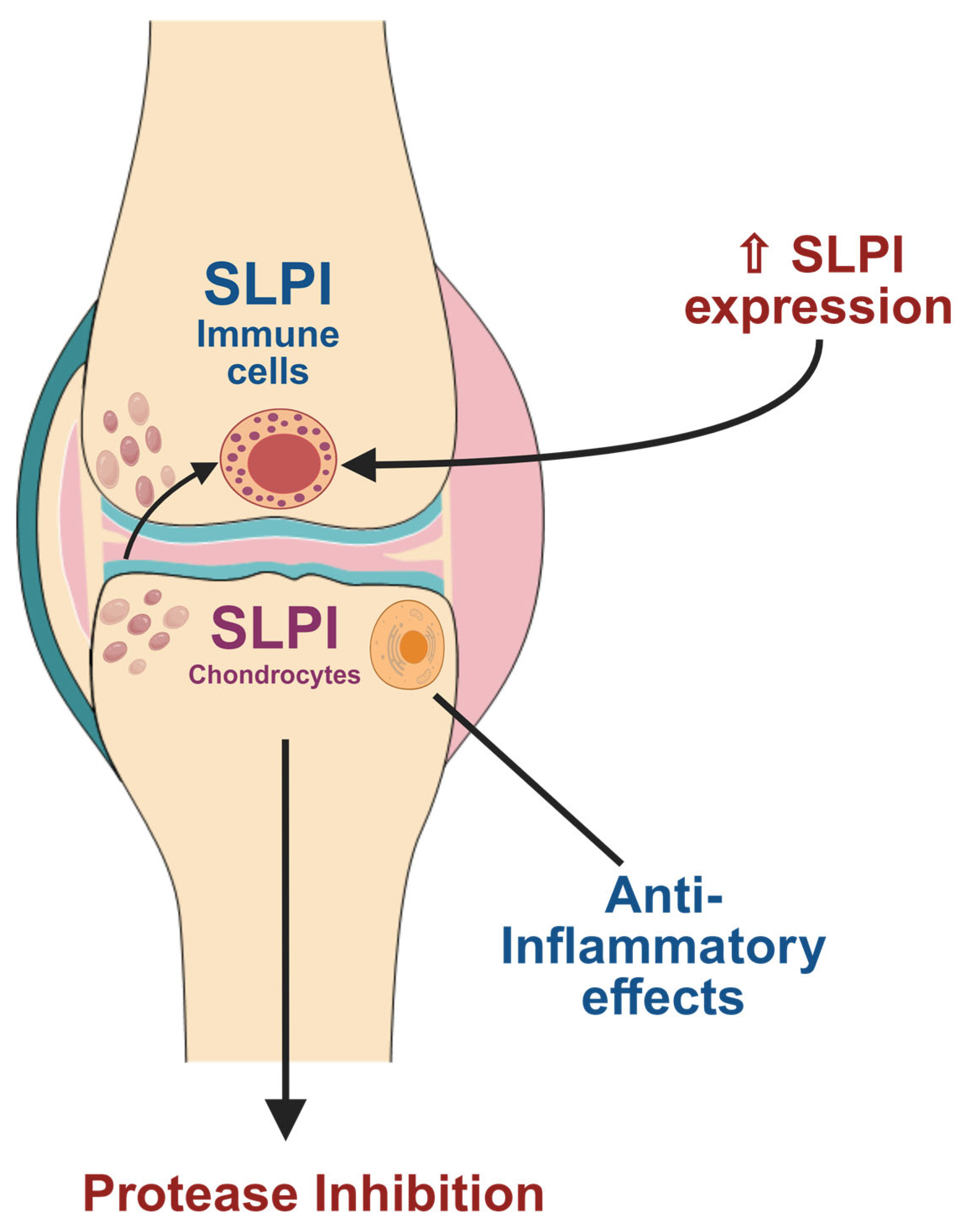
4. SLPI-Mediated Pathways in Osteoarthritis
5. Preclinical Evidence and Therapeutic Potential of SLPI in Osteoarthritis
5.1. SLPI Knockout Models: Functional Insights and Translational Relevance
5.2. Therapeutic Inference and Clinical Potential of SLPI
6. SLPI Expression in Human Osteoarthritis (OA) Samples
7. Comparative Insights: SLPI’s Role in Other Inflammatory Conditions
8. Emerging Therapies or Biotech Developments Involving SLPI
9. Discussion
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Delpachitra, S.N.; Dimitroulis, G. Osteoarthritis of the temporomandibular joint: A review of aetiology and pathogenesis. Br. J. Oral Maxillofac. Surg. 2022, 60, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Banjar, M.; Horiuchi, S.; Gedeon, D.N.; Yoshioka, H. Review of Quantitative Knee Articular Cartilage MR Imaging. Magn. Reson. Med. Sci. 2022, 21, 29–40. [Google Scholar] [CrossRef]
- Ho, J.; Mak, C.; Sharma, V.; To, K.; Khan, W. Mendelian Randomization Studies of Lifestyle-Related Risk Factors for Osteoarthritis: A PRISMA Review and Meta-Analysis. Int. J. Mol. Sci. 2022, 23, 11906. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, L.S.; Sousa, C.; Álvaro, A.R.; Cavadas, C.; Mendes, A.F. Common risk factors and therapeutic targets in obstructive sleep apnea and osteoarthritis: An unexpectable link? Pharmacol. Res. 2021, 164, 105369. [Google Scholar] [CrossRef]
- Mao, L.; Wu, W.; Wang, M.; Guo, J.; Li, H.; Zhang, S.; Xu, J.; Zou, J. Targeted treatment for osteoarthritis: Drugs and delivery system. Drug Deliv. 2021, 28, 1861–1876. [Google Scholar] [CrossRef]
- Aleem, A.W.; Rai, M.F.; Cai, L.; Brophy, R.H. Gene Expression in Glenoid Articular Cartilage Varies Across Acute Instability, Chronic Instability, and Osteoarthritis. J. Bone Jt. Surg. Am. 2023, 105, 990–1000. [Google Scholar] [CrossRef]
- Makarczyk, M.J.; Gao, Q.; He, Y.; Li, Z.; Gold, M.S.; Hochberg, M.C.; Bunnell, B.A.; Tuan, R.S.; Goodman, S.B.; Lin, H. Current Models for Development of Disease-Modifying Osteoarthritis Drugs. Tissue Eng. Part C Methods 2021, 27, 124–138. [Google Scholar] [CrossRef] [PubMed]
- Fu, K.; Si, S.; Jin, X.; Zhang, Y.; Duong, V.; Cai, Q.; Li, G.; Oo, W.M.; Zheng, X.; Boer, C.G.; et al. Exploring antidiabetic drug targets as potential disease-modifying agents in osteoarthritis. eBioMedicine 2024, 107, 105285. [Google Scholar] [CrossRef]
- Munadziroh, E.; Putri, G.A.; Ristiana, V.; Agustantina, T.H.; Nirwana, I.; Razak, F.A.; Surboyo, M.D.C. The Role of Recombinant Secretory Leukocyte Protease Inhibitor to CD163, FGF-2, IL-1 and IL-6 Expression in Skin Wound Healing. Clin. Cosmet. Investig. Dermatol. 2022, 15, 903–910. [Google Scholar] [CrossRef]
- Aubonnet, R.; Ramos, J.; Recenti, M.; Jacob, D.; Ciliberti, F.; Guerrini, L.; Gislason, M.K.; Sigurjonsson, O.; Tsirilaki, M.; Jónsson, H.; et al. Toward New Assessment of Knee Cartilage Degeneration. Cartilage 2023, 14, 351–374. [Google Scholar] [CrossRef]
- Brown, R.; Dougan, C.; Ferris, P.; Delaney, R.; Houston, C.J.; Rodgers, A.; Downey, D.G.; Mall, M.A.; Connolly, B.; Small, D.; et al. SLPI deficiency alters airway protease activity and induces cell recruitment in a model of muco-obstructive lung disease. Front. Immunol. 2024, 15, 1433642. [Google Scholar] [CrossRef] [PubMed]
- Song, X.Y.; Zeng, L.; Jin, W.; Thompson, J.; Mizel, D.E.; Lei, K.; Billinghurst, R.C.; Poole, A.R.; Wahl, S.M. Secretory leukocyte protease inhibitor suppresses the inflammation and joint damage of bacterial cell wall-induced arthritis. J. Exp. Med. 1999, 190, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-E.; Shin, Y.; Jung, I.J.; Yang, J.-I.; Chun, C.-H.; Kim, H.A.; Chun, J.-S. Overexpression of secretory leukocyte peptidase inhibitor (SLPI) does not modulate experimental osteoarthritis but may be a biomarker for the disease. Osteoarthr. Cartil. 2021, 29, 558–567. [Google Scholar] [CrossRef]
- Holden, M.A.; Nicolson, P.J.A.; Thomas, M.J.; Corp, N.; Hinman, R.S.; Bennell, K.L. Osteoarthritis year in review 2022: Rehabilitation. Osteoarthr. Cartil. 2023, 31, 177–186. [Google Scholar] [CrossRef]
- Cai, X.; Yuan, S.; Zeng, Y.; Wang, C.; Yu, N.; Ding, C. New Trends in Pharmacological Treatments for Osteoarthritis. Front. Pharmacol. 2021, 12, 645842. [Google Scholar] [CrossRef]
- Liew, J.W.; King, L.K.; Mahmoudian, A.; Wang, Q.; Atkinson, H.F.; Flynn, D.B.; Appleton, C.T.; Englund, M.; Haugen, I.K.; Lohmander, L.S.; et al. A scoping review of how early-stage knee osteoarthritis has been defined. Osteoarthr. Cartil. 2023, 31, 1234–1241. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yuan, T.; Wang, Y.; Liu, C.; Li, D.; Li, Z.; Sun, S. Osteoclasts and osteoarthritis: Novel intervention targets and therapeutic potentials during aging. Aging Cell 2024, 23, e14092. [Google Scholar] [CrossRef]
- Zhang, C.; Li, X.; Wen, P.; Li, Y. Ellagic acid improves osteoarthritis by inhibiting PGE2 production in M1 macrophages via targeting PTGS2. Clin. Exp. Pharmacol. Physiol. 2024, 51, e13918. [Google Scholar] [CrossRef]
- Horváth, E.; Sólyom, Á.; Székely, J.; Nagy, E.E.; Popoviciu, H. Inflammatory and Metabolic Signaling Interfaces of the Hypertrophic and Senescent Chondrocyte Phenotypes Associated with Osteoarthritis. Int. J. Mol. Sci. 2023, 24, 16468. [Google Scholar] [CrossRef]
- Jørgensen, A.E.M.; Agergaard, J.; Schjerling, P.; Heinemeier, K.M.; van Hall, G.; Kjaer, M. The regional turnover of cartilage collagen matrix in late-stage human knee osteoarthritis. Osteoarthr. Cartil. 2022, 30, 886–895. [Google Scholar] [CrossRef]
- Pirozzi, K.M. Histophysiology of Fibrocartilage. Clin. Podiatr. Med. Surg. 2022, 39, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Emanuel, K.S.; Kellner, L.J.; Peters, M.J.M.; Haartmans, M.J.J.; Hooijmans, M.T.; Emans, P.J. The relation between the biochemical composition of knee articular cartilage and quantitative MRI: A systematic review and meta-analysis. Osteoarthr. Cartil. 2022, 30, 650–662. [Google Scholar] [CrossRef] [PubMed]
- Kijowski, R. Standardization of Compositional MRI of Knee Cartilage: Why and How. Radiology 2021, 301, 433–434. [Google Scholar] [CrossRef]
- Moo, E.K.; Ebrahimi, M.; Sibole, S.C.; Tanska, P.; Korhonen, R.K. The intrinsic quality of proteoglycans, but not collagen fibres, degrades in osteoarthritic cartilage. Acta Biomater. 2022, 153, 178–189. [Google Scholar] [CrossRef]
- Shiozawa, J.; de Vega, S.; Yoshinaga, C.; Ji, X.; Negishi, Y.; Momoeda, M.; Nakamura, T.; Yoshida, H.; Kaneko, H.; Ishijima, M.; et al. Expression and regulation of recently discovered hyaluronidases, HYBID and TMEM2, in chondrocytes from knee osteoarthritic cartilage. Sci. Rep. 2022, 12, 17242. [Google Scholar] [CrossRef]
- Cui, J.; Zhang, J. Cartilage Oligomeric Matrix Protein, Diseases, and Therapeutic Opportunities. Int. J. Mol. Sci. 2022, 23, 9253. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Duan, M.; Zhang, D.; Xie, J. The role of mechano growth factor in chondrocytes and cartilage defects: A concise review. ABBS 2023, 55, 701–712. [Google Scholar] [CrossRef]
- Doumas, S.; Kolokotronis, A.; Stefanopoulos, P. Anti-Inflammatory and Antimicrobial Roles of Secretory Leukocyte Protease Inhibitor. Infect. Immun. 2005, 73, 1271–1274. [Google Scholar] [CrossRef]
- Geraghty, P.; Greene, C.M.; O’Mahony, M.; O’Neill, S.J.; Taggart, C.C.; McElvaney, N.G. Secretory Leucocyte Protease Inhibitor Inhibits Interferon-γ-induced Cathepsin S Expression. J. Biol. Chem. 2007, 282, 33389–33395. [Google Scholar] [CrossRef]
- Greene, C.M.; McElvaney, N.G.; O’Neill, S.J.; Taggart, C.C. Secretory Leucoprotease Inhibitor Impairs Toll-Like Receptor 2- and 4-Mediated Responses in Monocytic Cells. Infect. Immun. 2004, 72, 3684–3687. [Google Scholar] [CrossRef]
- Sano, C.; Shimizu, T.; Sato, K.; Kawauchi, H.; Tomioka, H. Effects of secretory leucocyte protease inhibitor on the production of the anti-inflammatory cytokines, IL-10 and transforming growth factor-beta (TGF-beta), by lipopolysaccharide-stimulated macrophages. Clin. Exp. Immunol. 2000, 121, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Messier, S.P.; Beavers, D.P.; Queen, K.; Mihalko, S.L.; Miller, G.D.; Losina, E.; Katz, J.N.; Loeser, R.F.; DeVita, P.; Hunter, D.J.; et al. Effect of Diet and Exercise on Knee Pain in Patients With Osteoarthritis and Overweight or Obesity: A Randomized Clinical Trial. JAMA 2022, 328, 2242–2251. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.K.; Mak, R.Y.; Kwok, T.S.; Tsang, J.S.; Leung, M.Y.; Funabashi, M.; Macedo, L.G.; Dennett, L.; Wong, A.Y. Prevalence, Incidence, and Factors Associated With Non-Specific Chronic Low Back Pain in Community-Dwelling Older Adults Aged 60 Years and Older: A Systematic Review and Meta-Analysis. J. Pain 2022, 23, 509–534. [Google Scholar] [CrossRef]
- Rakutt, M.J.; Mace, R.A.; Conley, C.E.W.; Stone, A.V.; Duncan, S.T.; Greenberg, J.; Landy, D.C.; Vranceanu, A.-M.; Jacobs, C.A. Association of Osteoarthritis and Functional Limitations With Cognitive Impairment Among Older Adults in the United States. J. Aging Health 2023, 35, 643–650. [Google Scholar] [CrossRef]
- Gulati, M.; Dursun, E.; Vincent, K.; Watt, F.E. The influence of sex hormones on musculoskeletal pain and osteoarthritis. Lancet Rheumatol. 2023, 5, e225–e238. [Google Scholar] [CrossRef]
- Wei, Q.; Zhu, X.; Wang, L.; Zhang, W.; Yang, X.; Wei, W. Extracellular matrix in synovium development, homeostasis and arthritis disease. Int. Immunopharmacol. 2023, 121, 110453. [Google Scholar] [CrossRef]
- Shi, D.; Mei, Y.; Hao, W.; Li, J.; Liu, S.; Lin, X. Biological functions and applications of LncRNAs in the regulation of the extracellular matrix in osteoarthritis. Front. Cell Dev. Biol. 2023, 11, 1330624. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Wilkinson, D.; Bou-Gharios, G. Targeting Dysregulation of Metalloproteinase Activity in Osteoarthritis. Calcif. Tissue Int. 2021, 109, 277–290. [Google Scholar] [CrossRef]
- Grillet, B.; Pereira, R.V.S.; Van Damme, J.; Abu El-Asrar, A.; Proost, P.; Opdenakker, G. Matrix metalloproteinases in arthritis: Towards precision medicine. Nat. Rev. Rheumatol. 2023, 19, 363–377. [Google Scholar] [CrossRef]
- Kulkarni, P.; Harsulkar, A.; Märtson, A.-G.; Suutre, S.; Märtson, A.; Koks, S. Mast Cells Differentiated in Synovial Fluid and Resident in Osteophytes Exalt the Inflammatory Pathology of Osteoarthritis. Int. J. Mol. Sci. 2022, 23, 541. [Google Scholar] [CrossRef]
- Wei, W.; He, S.; Wang, Z.; Dong, J.; Xiang, D.; Li, Y.; Ren, L.; Kou, N.; Lv, J. LINC01534 Promotes the Aberrant Metabolic Dysfunction and Inflammation in IL-1β-Simulated Osteoarthritic Chondrocytes by Targeting miR-140-5p. Cartilage 2021, 13, 898S–907S. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xie, L.; Zeng, H.; Wu, Y. PDK4 inhibits osteoarthritis progression by activating the PPAR pathway. J. Orthop. Surg. Res. 2024, 19, 109. [Google Scholar] [CrossRef]
- Elkhenany, H.A.; Linardi, R.L.; Ortved, K.F. Differential modulation of inflammatory cytokines by recombinant IL-10 in IL-1β and TNF-α—stimulated equine chondrocytes and synoviocytes: Impact of washing and timing on cytokine responses. BMC Vet. Res. 2024, 20, 546. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, D.; Deng, B.; Yan, L. Syringaresinol attenuates osteoarthritis via regulating the NF-κB pathway. Int. Immunopharmacol. 2023, 118, 109982. [Google Scholar] [CrossRef]
- Zhou, H.; Xie, Z.; Qian, Y.; Ni, W.; Cui, L.; Fang, X.; Wan, S.; Zhao, X.; Qin, A.; Fan, S.; et al. FTO-mediated SMAD2 m6A modification protects cartilage against Osteoarthritis. Exp. Mol. Med. 2024, 56, 2283–2295. [Google Scholar] [CrossRef]
- Wang, L.; He, C. Nrf2-mediated anti-inflammatory polarization of macrophages as therapeutic targets for osteoarthritis. Front. Immunol. 2022, 13, 967193. [Google Scholar] [CrossRef] [PubMed]
- Majchrzak-Gorecka, M.; Majewski, P.; Grygier, B.; Murzyn, K.; Cichy, J. Secretory leukocyte protease inhibitor (SLPI), a multifunctional protein in the host defense response. Cytokine Growth Factor Rev. 2016, 28, 79–93. [Google Scholar] [CrossRef]
- Wen, J.; Nikitakis, N.G.; Chaisuparat, R.; Greenwell-Wild, T.; Gliozzi, M.; Jin, W.; Adli, A.; Moutsopoulos, N.; Wu, T.; Warburton, G.; et al. Secretory Leukocyte Protease Inhibitor (SLPI) Expression and Tumor Invasion in Oral Squamous Cell Carcinoma. Am. J. Pathol. 2011, 178, 2866–2878. [Google Scholar] [CrossRef]
- Quillard, T.; Tesmenitsky, Y.; Croce, K.; Travers, R.; Shvartz, E.; Koskinas, K.C.; Sukhova, G.K.; Aikawa, E.; Aikawa, M.; Libby, P. Selective Inhibition of Matrix Metalloproteinase-13 Increases Collagen Content of Established Mouse Atherosclerosis. ATVB 2011, 31, 2464–2472. [Google Scholar] [CrossRef]
- Zani, M.; Baranger, K.; Guyot, N.; Dallet-Choisy, S.; Moreau, T. Protease inhibitors derived from elafin and SLPI and engineered to have enhanced specificity towards neutrophil serine proteases. Protein Sci. 2009, 18, 579–594. [Google Scholar] [CrossRef]
- Vandenbroucke, R.E.; Dejonckheere, E.; Van Hauwermeiren, F.; Lodens, S.; De Rycke, R.; Van Wonterghem, E.; Staes, A.; Gevaert, K.; López-Otin, C.; Libert, C. Matrix metalloproteinase 13 modulates intestinal epithelial barrier integrity in inflammatory diseases by activating TNF. EMBO Mol. Med. 2013, 5, 1000–1016. [Google Scholar] [CrossRef] [PubMed]
- Arai, Y.; Choi, B.; Kim, B.J.; Park, S.; Park, H.; Moon, J.J.; Lee, S.-H. Cryptic ligand on collagen matrix unveiled by MMP13 accelerates bone tissue regeneration via MMP13/Integrin α3/RUNX2 feedback loop. Acta Biomater. 2021, 125, 219–230. [Google Scholar] [CrossRef]
- Kwiecinska, P.; Santocki, M.; Skrzeczynska-Moncznik, J.; Sinkevich, I.; Piwowarczyk, K.; Majewski, P.; Grygier, B.; Majchrzak-Gorecka, M.; Czyz, J.; Kolaczkowska, E.; et al. SLPI controls neutrophil migration abilities and impacts neutrophil skin infiltration in experimental psoriasis. Cell Mol. Life Sci. 2025, 82, 74. [Google Scholar] [CrossRef] [PubMed]
- Migita, K.; Izumi, Y.; Jiuchi, Y.; Kozuru, H.; Kawahara, C.; Nakamura, M.; Nakamura, T.; Agematsu, K.; Masumoto, J.; Yasunami, M.; et al. Serum amyloid A induces NLRP-3-mediated IL-1β secretion in neutrophils. PLoS ONE 2014, 9, e96703. [Google Scholar] [CrossRef]
- Anthony, D.; Seow, H.J.; Uddin, M.; Thompson, M.; Dousha, L.; Vlahos, R.; Irving, L.B.; Levy, B.D.; Anderson, G.P.; Bozinovski, S. Serum amyloid A promotes lung neutrophilia by increasing IL-17A levels in the mucosa and γδ T cells. Am. J. Respir. Crit. Care Med. 2013, 188, 179–186. [Google Scholar] [CrossRef]
- Mongkolpathumrat, P.; Pikwong, F.; Phutiyothin, C.; Srisopar, O.; Chouyratchakarn, W.; Unnajak, S.; Nernpermpisooth, N.; Kumphune, S. The secretory leukocyte protease inhibitor (SLPI) in pathophysiology of non-communicable diseases: Evidence from experimental studies to clinical applications. Heliyon 2024, 10, e24550. [Google Scholar] [CrossRef]
- Filgueiras, L.R.; Brandt, S.L.; Wang, S.; Wang, Z.; Morris, D.L.; Evans-Molina, C.; Mirmira, R.G.; Jancar, S.; Serezani, C.H. Leukotriene B4-mediated sterile inflammation promotes susceptibility to sepsis in a mouse model of type 1 diabetes. Sci. Signal 2015, 8, ra10. [Google Scholar] [CrossRef] [PubMed]
- Attur, M.; Wang, H.-Y.; Kraus, V.B.; Bukowski, J.F.; Aziz, N.; Krasnokutsky, S.; Samuels, J.; Greenberg, J.; McDaniel, G.; Abramson, S.B.; et al. Radiographic severity of knee osteoarthritis is conditional on interleukin 1 receptor antagonist gene variations. Ann. Rheum. Dis. 2010, 69, 856–861. [Google Scholar] [CrossRef]
- Attur, M.; Zhou, H.; Samuels, J.; Krasnokutsky, S.; Yau, M.; Scher, J.U.; Doherty, M.; Wilson, A.G.; Bencardino, J.; Hochberg, M.; et al. Interleukin 1 receptor antagonist (IL1RN) gene variants predict radiographic severity of knee osteoarthritis and risk of incident disease. Ann. Rheum. Dis. 2020, 79, 400–407. [Google Scholar] [CrossRef]
- Attur, M.; Belitskaya-Lévy, I.; Oh, C.; Krasnokutsky, S.; Greenberg, J.; Samuels, J.; Smiles, S.; Lee, S.; Patel, J.; Al-Mussawir, H.; et al. Increased interleukin-1β gene expression in peripheral blood leukocytes is associated with increased pain and predicts risk for progression of symptomatic knee osteoarthritis. Arthritis Rheum. 2011, 63, 1908–1917. [Google Scholar] [CrossRef]
- Scott, A.; Weldon, S.; Taggart, C.C. SLPI and elafin: Multifunctional antiproteases of the WFDC family. Biochem. Soc. Trans. 2011, 39, 1437–1440. [Google Scholar] [CrossRef] [PubMed]
- Fransès, R.E.; McWilliams, D.F.; Mapp, P.I.; Walsh, D.A. Osteochondral angiogenesis and increased protease inhibitor expression in OA. Osteoarthr. Cartil. 2010, 18, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, S.S.; Ma, J.; Qu, W. Secretory leukocyte protease inhibitor (SLPI) in cancer pathophysiology: Mechanisms of action and clinical implications. Pathol. Res. Pract. 2023, 248, 154633. [Google Scholar] [CrossRef]
- Tang, R.; Botchway, B.O.A.; Meng, Y.; Zhang, Y.; Zhou, C.; Jiang, J.; Liu, X. The Inhibition of Inflammatory Signaling Pathway by Secretory Leukocyte Protease Inhibitor can Improve Spinal Cord Injury. Cell. Mol. Neurobiol. 2020, 40, 1067–1073. [Google Scholar] [CrossRef]
- Sun, K.; Jing, X.; Guo, J.; Yao, X.; Guo, F. Mitophagy in degenerative joint diseases. Autophagy 2021, 17, 2082–2092. [Google Scholar] [CrossRef]
- Molnar, V.; Matišić, V.; Kodvanj, I.; Bjelica, R.; Jeleč, Ž.; Hudetz, D.; Rod, E.; Čukelj, F.; Vrdoljak, T.; Vidović, D.; et al. Cytokines and Chemokines Involved in Osteoarthritis Pathogenesis. Int. J. Mol. Sci. 2021, 22, 9208. [Google Scholar] [CrossRef]
- Chen, B.Y.; Pathak, J.L.; Lin, H.Y.; Guo, W.Q.; Chen, W.J.; Luo, G.; Wang, L.J.; Sun, X.F.; Ding, Y.; Li, J.; et al. Inflammation Triggers Chondrocyte Ferroptosis in TMJOA via HIF-1α/TFRC. J. Dent. Res. 2024, 103, 712–722. [Google Scholar] [CrossRef]
- Barros, S.C.; Martins, J.A.; Marcos, J.C.; Cavaco-Paulo, A. Influence of secretory leukocyte protease inhibitor-based peptides on elastase activity and their incorporation in hyaluronic acid hydrogels for chronic wound therapy. Biopolymers 2012, 98, 576–590. [Google Scholar] [CrossRef] [PubMed]
- McGarry, N.; Greene, C.M.; McElvaney, N.G.; Weldon, S.; Taggart, C.C. The Ability of Secretory Leukocyte Protease Inhibitor to Inhibit Apoptosis in Monocytes Is Independent of Its Antiprotease Activity. J. Immunol. Res. 2015, 2015, 507315. [Google Scholar] [CrossRef]
- Chuluyan, E.; Ambrosi, N.; Fraunhoffer, N.; Caro, F.; Remolins, C.; Reiteri, M.; Guerrieri, D.; Soubeyran, P.; Gayet, O.; Roques, J.; et al. Secretory leukocyte proteinase inhibitor: A key player in the dialogue between the tumor and its microenvironment in pancreatic cancer patients. J. Clin. Oncol. 2022, 40, 592. [Google Scholar] [CrossRef]
- Kam, N.W.; Brentano, F.; Kyburz, D.; Gay, S.; Filer, A.; Buckley, C.; Pitzalis, C.; Bombardieri, M. A1.12 Endogenous SLPI released by rheumatoid synovial fibroblasts control BAFF-dependent-B cell activation in vitro and in the CIA and RA/SCID-arthritis models. Ann. Rheum. Dis. 2014, 73, A5. [Google Scholar] [CrossRef]
- Kikuchi, T.; Abe, T.; Yaekashiwa, M.; Tominaga, Y.; Mitsuhashi, H.; Satoh, K.; Nakamura, T.; Nukiwa, T. Secretory leukoprotease inhibitor augments hepatocyte growth factor production in human lung fibroblasts. Am. J. Respir. Cell Mol. Biol. 2000, 23, 364–370. [Google Scholar] [CrossRef]
- Jin, F.Y.; Nathan, C.; Radzioch, D.; Ding, A. Secretory leukocyte protease inhibitor: A macrophage product induced by and antagonistic to bacterial lipopolysaccharide. Cell 1997, 88, 417–426. [Google Scholar] [CrossRef]
- Müller, A.M.; Jun, E.; Conlon, H.; Sadiq, S.A. Inhibition of SLPI ameliorates disease activity in experimental autoimmune encephalomyelitis. BMC Neurosci. 2012, 13, 30. [Google Scholar] [CrossRef]
- Niu, W.; Zhang, T.; Ma, L. Correlation analysis between immune-related genes and cell infiltration revealed prostate cancer immunotherapy biomarkers linked to T cells gamma delta. Sci. Rep. 2023, 13, 2459. [Google Scholar] [CrossRef] [PubMed]
- Leelasukseree, R.; Chouyratchakarn, W.; Phutiyothin, C.; Pikwong, F.; Srisopar, O.; Baipaywad, P.; Udomsom, S.; Mongkolpathumrat, P.; Supanchart, C.; Kumphune, S. Recombinant human secretory leukocyte protease inhibitor (rhSLPI) coated titanium enhanced human osteoblast adhesion and differentiation. Sci. Rep. 2023, 13, 23013. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.-D.; Lee, S.-Y.; Jeong, S.-J.; Lim, D.-S.; Cha, H.-J.; Chung, W.-G.; Jeong, M.-J. Secretory leukocyte protease inhibitor promotes differentiation and mineralization of MC3T3-E1 preosteoblasts on a titanium surface. Mol. Med. Rep. 2016, 14, 1241–1246. [Google Scholar] [CrossRef]
- Sui, B.; Liu, J.; Zheng, C.; Dang, L.; Chen, J.; Cao, Y.; Zhang, K.; Liu, L.; Dang, M.; Zhang, L.; et al. Targeted inhibition of osteoclastogenesis reveals the pathogenesis and therapeutics of bone loss under sympathetic neurostress. Int. J. Oral. Sci. 2022, 14, 39. [Google Scholar] [CrossRef]
- Morimoto, A.; Kikuta, J.; Nishikawa, K.; Sudo, T.; Uenaka, M.; Furuya, M.; Hasegawa, T.; Hashimoto, K.; Tsukazaki, H.; Seno, S.; et al. SLPI is a critical mediator that controls PTH-induced bone formation. Nat. Commun. 2021, 12, 2136. [Google Scholar] [CrossRef]
- Jian, J.; Yu-Qing, L.; Rang-Yue, H.; Xia, Z.; Ke-Huan, X.; Ying, Y.; Li, W.; Rui-Zhi, T. Isorhamnetin ameliorates cisplatin-induced acute kidney injury in mice by activating SLPI-mediated anti-inflammatory effect in macrophage. Immunopharmacol. Immunotoxicol. 2024, 46, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Kozin, S.V.; Maimon, N.; Wang, R.; Gupta, N.; Munn, L.; Jain, R.K.; Garkavtsev, I. Secretory leukocyte protease inhibitor (SLPI) as a potential target for inhibiting metastasis of triple-negative breast cancers. Oncotarget 2017, 8, 108292–108302. [Google Scholar] [CrossRef] [PubMed]
- Ozaka, S.; Sonoda, A.; Kudo, Y.; Ito, K.; Kamiyama, N.; Sachi, N.; Chalalai, T.; Kagoshima, Y.; Soga, Y.; Ekronarongchai, S.; et al. Daikenchuto, a Japanese herbal medicine, ameliorates experimental colitis in a murine model by inducing secretory leukocyte protease inhibitor and modulating the gut microbiota. Front. Immunol. 2024, 15, 1457562. [Google Scholar] [CrossRef]
- Sriwattanapong, K.; Sa-Ard-Iam, N.; Boonprakong, L.; Subbalekha, K.; Trachoo, V.; Suratannon, N.; Porntaveetus, T.; Shotelersuk, V. Reduced ELANE and SLPI expression compromises dental pulp cell activity. Cell Prolif. 2021, 54, e13132. [Google Scholar] [CrossRef] [PubMed]
- Osbourn, M.; Rodgers, A.M.; Dubois, A.V.; Small, D.M.; Humphries, F.; Delagic, N.; Moynagh, P.N.; Weldon, S.; Taggart, C.C.; Ingram, R.J. Secretory Leucoprotease Inhibitor (SLPI) Promotes Survival during Acute Pseudomonas aeruginosa Infection by Suppression of Inflammation Rather Than Microbial Killing. Biomolecules 2022, 12, 1728. [Google Scholar] [CrossRef]
- Wada, H.; Kagoshima, M.; Ito, K.; Barnes, P.J.; Adcock, I.M. 5-Azacytidine suppresses RNA polymerase II recruitment to the SLPI gene. Biochem. Biophys. Res. Commun. 2005, 331, 93–99. [Google Scholar] [CrossRef]
- Munadziroh, E.; Askandar, M.G.; Yuliati, A.; Surboyo, M.D.C.; Wan Harun, W.H.A. The effect of secretory leukocyte protease inhibitor amnion membrane on incisional wound healing. J. Oral Biol. Craniofacial Res. 2022, 12, 358–362. [Google Scholar] [CrossRef]
- Katsoula, G.; Kreitmaier, P.; Zeggini, E. Insights into the molecular landscape of osteoarthritis in human tissues. Curr. Opin. Rheumatol. 2022, 34, 79–90. [Google Scholar] [CrossRef]
- Habgood, A.N.; Tatler, A.L.; Porte, J.; Wahl, S.M.; Laurent, G.J.; John, A.E.; Johnson, S.R.; Jenkins, G. Secretory leukocyte protease inhibitor gene deletion alters bleomycin-induced lung injury, but not development of pulmonary fibrosis. Lab. Invest. 2016, 96, 623–631. [Google Scholar] [CrossRef]
- Hritz, I.; Kuester, D.; Vieth, M.; Herszenyi, L.; Stolte, M.; Roessner, A.; Tulassay, Z.; Wex, T.; Malfertheiner, P. Secretory leukocyte protease inhibitor expression in various types of gastritis: A specific role of Helicobacter pylori infection. Eur. J. Gastroenterol. Hepatol. 2006, 18, 277–282. [Google Scholar] [CrossRef]
- Nugteren, S.; Goos, J.A.C.M.; Delis-van Diemen, P.M.; Simons-Oosterhuis, Y.; Lindenbergh-Kortleve, D.J.; van Haaften, D.H.; Sanders, J.; Meijer, G.A.; Fijneman, R.J.A.; Samsom, J.N. Expression of the immune modulator secretory leukocyte protease inhibitor (SLPI) in colorectal cancer liver metastases and matched primary tumors is associated with a poorer prognosis. Oncoimmunology 2020, 9, 1832761. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, S.K.; Shlomchik, M.J.; Glant, T.T.; Cao, Y.; Doodes, P.D.; Finnegan, A. Antigen-specific B cells are required as APCs and autoantibody-producing cells for induction of severe autoimmune arthritis. J. Immunol. 2005, 174, 3781–3788. [Google Scholar] [CrossRef] [PubMed]
- Reeves, E.P.; Banville, N.; Ryan, D.M.; O’Reilly, N.; Bergin, D.A.; Pohl, K.; Molloy, K.; McElvaney, O.J.; Alsaleh, K.; Aljorfi, A.; et al. Intracellular secretory leukoprotease inhibitor modulates inositol 1,4,5-triphosphate generation and exerts an anti-inflammatory effect on neutrophils of individuals with cystic fibrosis and chronic obstructive pulmonary disease. Biomed. Res. Int. 2013, 2013, 560141. [Google Scholar] [CrossRef]
- Grobmyer, S.R.; Barie, P.S.; Nathan, C.F.; Fuortes, M.; Lin, E.; Lowry, S.F.; Wright, C.D.; Weyant, M.J.; Hydo, L.; Reeves, F.; et al. Secretory leukocyte protease inhibitor, an inhibitor of neutrophil activation, is elevated in serum in human sepsis and experimental endotoxemia. Crit. Care Med. 2000, 28, 1276–1282. [Google Scholar] [CrossRef] [PubMed]
- Ozaka, S.; Sonoda, A.; Ariki, S.; Kamiyama, N.; Hidano, S.; Sachi, N.; Ito, K.; Kudo, Y.; Minata, M.; Saechue, B.; et al. Protease inhibitory activity of secretory leukocyte protease inhibitor ameliorates murine experimental colitis by protecting the intestinal epithelial barrier. Genes. Cells 2021, 26, 807–822. [Google Scholar] [CrossRef]
- Kwiecinska, P.; Grygier, B.; Morytko, A.; Sanecka-Duin, A.; Majchrzak-Gorecka, M.; Kwitniewski, M.; Kapinska-Mrowiecka, M.; Porebski, G.; Cichy, J. Secretory leukocyte protease inhibitor regulates nerve reflex-mediated skin barrier function in psoriasis. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 1266–1274. [Google Scholar] [CrossRef]
- Afacan, B.; Öztürk, V.Ö.; Emingil, G.; Köse, T.; Mitsakakis, K.; Bostanci, N. Salivary secretory leukocyte protease inhibitor levels in patients with stage 3 grade C periodontitis: A comparative cross-sectional study. Sci. Rep. 2022, 12, 21267. [Google Scholar] [CrossRef]
- Korkmaz, B.; Horwitz, M.S.; Jenne, D.E.; Gauthier, F. Neutrophil elastase, proteinase 3, and cathepsin G as therapeutic targets in human diseases. Pharmacol. Rev. 2010, 62, 726–759. [Google Scholar] [CrossRef]
- Cassuto, J.; Folestad, A.; Göthlin, J.; Malchau, H.; Kärrholm, J. Concerted actions by MMPs, ADAMTS and serine proteases during remodeling of the cartilage callus into bone during osseointegration of hip implants. Bone Rep. 2020, 13, 100715. [Google Scholar] [CrossRef]
- Zhang, Y.; DeWitt, D.L.; McNeely, T.B.; Wahl, S.M.; Wahl, L.M. Secretory leukocyte protease inhibitor suppresses the production of monocyte prostaglandin H synthase-2, prostaglandin E2, and matrix metalloproteinases. J. Clin. Invest. 1997, 99, 894–900. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Guo, Y.; Liu, Y.; Yang, H.; Gong, Z.; Du, Y.; Xu, R.; Gao, L.; Xu, Q.; Li, N. Guizhi Shaoyao Zhimu Decoction alleviates rheumatoid arthritis by inhibiting inflammation by targeting SLPI. Phytomedicine 2025, 139, 156471. [Google Scholar] [CrossRef] [PubMed]
- Ashcroft, G.S.; Lei, K.; Jin, W.; Longenecker, G.; Kulkarni, A.B.; Greenwell-Wild, T.; Hale-Donze, H.; McGrady, G.; Song, X.Y.; Wahl, S.M. Secretory leukocyte protease inhibitor mediates non-redundant functions necessary for normal wound healing. Nat. Med. 2000, 6, 1147–1153. [Google Scholar] [CrossRef] [PubMed]

| Disease | SLPI Expression Pattern | Protective Functions | Reference |
|---|---|---|---|
| Osteoarthritis (OA) | Upregulated in chondrocytes and synovial tissue but may not modulate disease progression | Potential biomarker; inhibits neutrophil elastase activity in cartilage | [13] |
| Rheumatoid Arthritis (RA) | Elevated in synovium and macrophages; induced by TNF-α and IL-1β | Protects against protease-mediated damage; inhibits NF-κB activation | [91] |
| Chronic Obstructive Pulmonary Disease (COPD) | Upregulated in airway epithelial cells and alveolar macrophages | Inhibits neutrophil elastase; mitigates airway inflammation | [92] |
| Cystic Fibrosis (CF) | Highly expressed but often inactivated by neutrophil elastase | Antimicrobial, anti-elastase; protects airway lining | [93] |
| Sepsis | Elevated in plasma during severe infection | Limits systemic inflammation; correlates with organ function | [93] |
| Inflammatory Bowel Disease (IBD) | Upregulated in colonic epithelium in active disease | Protects mucosa; suppresses cytokines; aids barrier repair | [94] |
| Psoriasis | Increased in keratinocytes; affects neutrophil responses | Reduces skin inflammation, dryness; regulates NETs | [95] |
| Periodontitis | High in gingivitis, decreased in chronic periodontitis | Inhibits local proteases and bacterial damage | [96] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shefa, M.S.; Kim, W. The Role of SLPI Gene-Mediated Inflammation in Osteoarthritis. Biomolecules 2025, 15, 909. https://doi.org/10.3390/biom15070909
Shefa MS, Kim W. The Role of SLPI Gene-Mediated Inflammation in Osteoarthritis. Biomolecules. 2025; 15(7):909. https://doi.org/10.3390/biom15070909
Chicago/Turabian StyleShefa, Mahmuda Siddika, and Wanil Kim. 2025. "The Role of SLPI Gene-Mediated Inflammation in Osteoarthritis" Biomolecules 15, no. 7: 909. https://doi.org/10.3390/biom15070909
APA StyleShefa, M. S., & Kim, W. (2025). The Role of SLPI Gene-Mediated Inflammation in Osteoarthritis. Biomolecules, 15(7), 909. https://doi.org/10.3390/biom15070909







